Cytokine Regulation and Oxidative Stress in Helicobacter Pylori-Associated Gastric Adenocarcinoma at Different Stages: Insights from a Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Baseline Patient Characteristics
2.2. Study of the Cytokine Link in Patients with Gastric Cancer at Different Stages of the Disease
2.3. Indicators of LPO–AP in Gastric Cancer Depending on the Stage of the Disease
2.4. Regression Analysis of the Studied Cytokines and LPO-AOP Indices in Gastric Cancer
3. Discussion
4. Materials and Methods
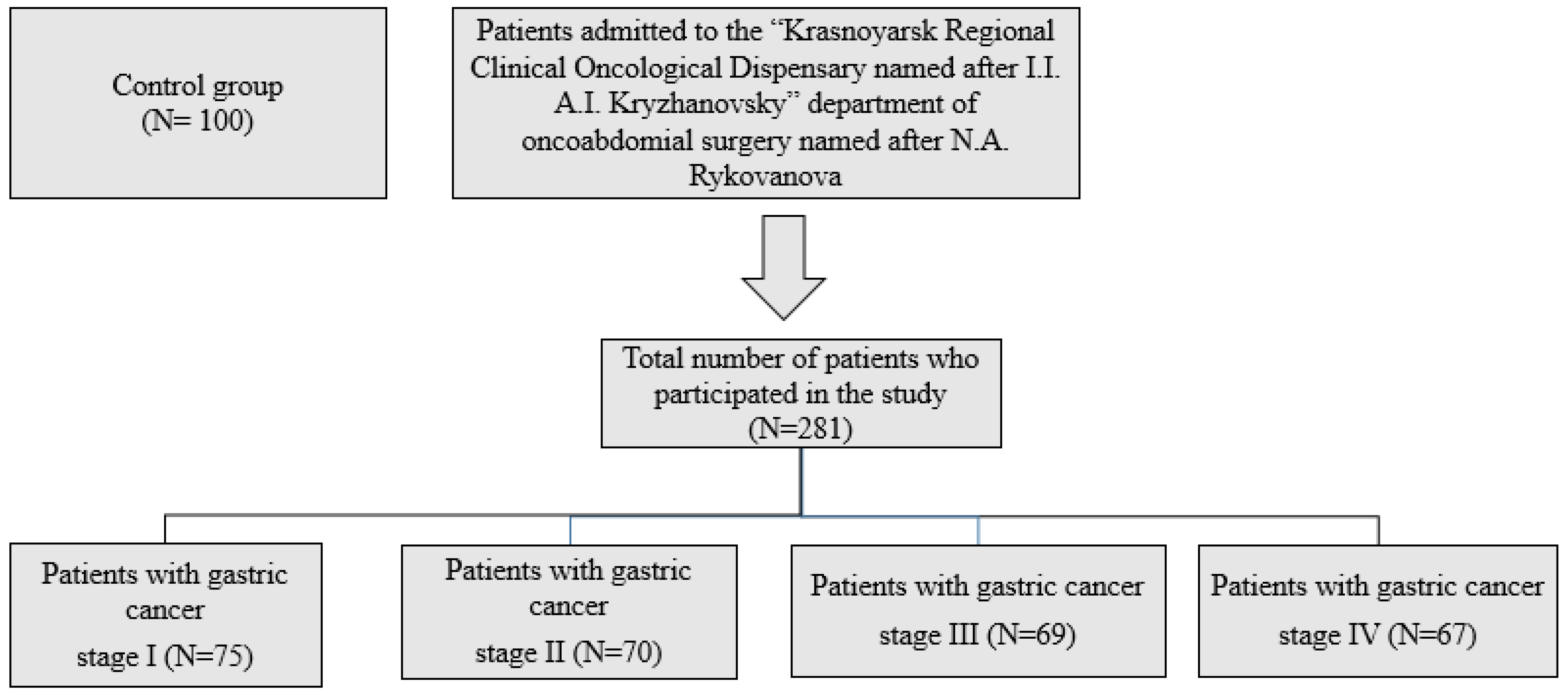
4.1. Subjects
4.2. Endoscopic Examination, Histologic Examination, H. pylori Test, and Gastric Juice Sampling
4.3. Determination of Cytokines by ELISA
4.4. Lipid Peroxidation and Antioxidants
4.5. Determination of Malondialdehyde Content
4.6. Determination of Superoxide Dismutase Activity
4.7. Determination of Catalase Activity
4.8. Determination of Glutathione-S-Transferase Activity
4.9. Determination of Glutathione Peroxidase Activity
4.10. Determination of Oxidative Stress Index (OSR)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Bhagat, G.; Abrams, J.A.; Marache, F.; Good, P.; Lee, M.D.; Lee, Y.; Friedman, R.; Asfaha, S.; Dubeykovskaya, Z.; et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, E.; Noorbakhsh, P.; Foroughi, E.; Farahnak, S.; Nasiri, R.; Neamatzadeh, H. A Survey on the Role of Interleukin-10 in Breast Cancer: A Narrative. Rep. Biochem. Mol. Biol. 2018, 7, 30–37. [Google Scholar] [PubMed]
- Salkeni, M.A.; Naing, A. Interleukin-10 in cancer immunotherapy: From bench to bedside. Trends Cancer 2023, 9, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.M.; Davies, M.J.; Mapstone, N.P.; Dixon, M.F.; Schorah, C.J.; White, K.L.; Chalmers, D.M.; Axon, A.T. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 1996, 17, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, R.; Agarwal, S.; Kotlov, N.; Plotnikova, O.; Nomie, K.; Huang, D.W.; Wright, G.W.; Smith, G.A.; Li, M.; Takata, K.; et al. Compromised counterselection by FAS creates an aggressive subtype of germinal center lymphoma. J. Exp. Med. 2021, 218, e20201173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Liu, Y.-G.; Shi, X.-J.; Chen, X.-W.; Zhou, D.; Zhu, D.-J. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A meta-analysis. Int. J. Surg. 2015, 21, 84–91. [Google Scholar] [CrossRef]
- Jung, K.; Heishi, T.; Incio, J.; Huang, Y.; Beech, E.Y.; Pinter, M.; Ho, W.W.; Kawaguchi, K.; Rahbari, N.N.; Chung, E.; et al. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 10455–10460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Han, R.; Liu, C.; Qin, X.; Li, P.; Gu, R. Signaling pathways of oxidative stress response: The potential therapeutic targets in gastric cancer. Front. Immunol. 2023, 14, 1139589. [Google Scholar] [CrossRef] [PubMed]
- Ustyanovska Avtenyuk, N.; Choukrani, G.; Ammatuna, E.; Niki, T.; Cendrowicz, E.; Lourens, H.J.; Huls, G.; Wiersma, V.R.; Bremer, E. Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines 2021, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Barbariga, M.; Curnis, F.; Spitaleri, A.; Andolfo, A.; Zucchelli, C.; Lazzaro, M.; Magnani, G.; Musco, G.; Corti, A.; Alessio, M. Oxidation-induced structural changes of ceruloplasmin foster NGR motif deamidation that promotes integrin binding and signaling. J. Biol. Chem. 2014, 289, 3736–3748. [Google Scholar] [CrossRef] [PubMed]
- Barbariga, M.; Zanardi, A.; Curnis, F.; Conti, A.; Boselli, D.; Di Terlizzi, S.; Alessio, M. Ceruloplasmin oxidized and deamidated by Parkinson’s disease cerebrospinal fluid induces epithelial cells proliferation arrest and apoptosis. Sci. Rep. 2020, 10, 15507. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative Stress Resulting From Helicobacter pylori Infection Contributes to Gastric Carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Heverly-Coulson, G.S.; Boyd, R.J. Reduction of hydrogen peroxide by glutathione peroxidase mimics: Reaction mechanism and energetics. J. Phys. Chem. A 2010, 114, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Zinatullina, K.M.; Kasaikina, O.T.; Kuz’min, V.A.; Khrameeva, N.P. Interaction of Glutathione with Hydrogen Peroxide: A Kinetic Model. Kinet. Catal. 2019, 60, 266–272. [Google Scholar] [CrossRef]
- Nasier-Hussain, M.; Samanje, J.N.; Mokhtari, K.; Nabi-Afjadi, M.; Fathi, Z.; Hoseini, A.; Bahreini, E. Serum levels of oxidative stress, IL-8, and pepsinogen I/II ratio in Helicobacter pylori and gastric cancer patients: Potential diagnostic biomarkers. BMC Gastroenterol. 2025, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Bockerstett, K.A.; DiPaolo, R.J. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Green, S.B. How Many Subjects Does It Take to Do a Regression Analysis. Multivariate Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Control Group N = 100 (1) | Stomach Cancer Stage I N = 75 (2) | Stomach Cancer Stage II N = 70 (3) | Stomach Cancer Stage III N = 69 (4) | Stomach Cancer Stage IV N = 67 (5) | p-Value |
|---|---|---|---|---|---|---|
| Gender (n,%) Male Female | 0.325 | |||||
| 58 | 35 | 26 | 22 | 18 | ||
| 42 | 14 | 19 | 18 | 17 | ||
| Age, (y) | 47.54 ± 12.65 | 49.3 ± 9.65 | 53.87 ± 8.34 | 55.4 ± 6.34 | 56.1 ± 4.34 | 0.414 |
| Weight, (kg) | 58.4 (±5.3) | 56.1 (±3.2) | 53.2 (±4.1) | 57.4 (±4.3) | 54.3 (±3.2) | 0.256 |
| Height, (m) | 1.75 (±0.05) | 1.7 (±0.08) | 1.69 (±0.05) | 1.72 (±0.09) | 1.67 (±0.08) | 0.465 |
| BMI, (kg/m2) | 22.69 ± 3.71 | 19.52 ± 3.33 | 17.22 ± 3.89 | 18.52 ± 4.2 | 16.32 ± 3.9 | 0.398 |
| Alcohol drink 1. Never 2. Past 3. Current | 0.472 | |||||
| 16 (15.3) | 5 (10.9) | 7 (13.8) | 10 (13.1) | 5 (9.2) | ||
| 41 (17.4) | 20 (20.7) | 23 (11.8) | 16 (12.8) | 20 (23.1) | ||
| 43 (71.7) | 24 (65.5) | 15 (82.4) | 14 (71.3) | 10 (14.3) | ||
| Smoking 1. Never 2. Past 3. Current | 0.371 | |||||
| 50 (44.1) | 12 (18.3) | 11 (33,1) | 16 (27.1) | 13 (23.2) | ||
| 18 (43.5) | 7 (47.1) | 11 (37.9) | 14 (25.2) | 10 (30.8) | ||
| 32 (21.7) | 20 (23.5) | 8 (20.7) | 10 (19.4) | 12 (21.7) | ||
| Indicators | Control Group N = 100 (1) | Stomach Cancer Stage I N = 75 (2) | Stomach Cancer Stage II N = 70 (3) | Stomach Cancer Stage III N = 69 (4) | Stomach Cancer Stage IV N = 67 (5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | |
| TNF-α (pg/mL) | 3.54 | 0.8–7.87 | 43.2 | 40.1–60.9 | 48.9 | 37.4–55.7 | 38.1 | 32.3–43.4 | 0.3 | 0.1–0.4 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001; p2–5 < 0.001; p3–5 < 0.001; p4–5 < 0.001 | |||||||
| IL-2 (pg/mL) | 1.1 | 0.5–3.05 | 5.7 | 3.6–10.3 | 4.9 | 3.8–9.5 | 5.2 | 3.0–8.7 | 8.4 | 7.3–15.4 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001 | |||||||
| IL-8 (pg/mL) | 7.1 | 5.5–10.1 | 80.5 | 75.5–97.2 | 78.1 | 75.5–87.3 | 67.1 | 62.2–79.5 | 1.2 | 0.8–1.3 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 = 0.01; p2–5 < 0.001; p3–5 < 0.001; p4–5 < 0.001 | |||||||
| IFNγ (U/mL) | 6.6 | 4.2–7.4 | 2.9 | 2.2–4.0 | 3.2 | 2.3–4.8 | 4.4 | 3.3–6.9 | 12.4 | 10.5–14.9 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001; p2–4 = 0.02 | p1–5 < 0.001; p2–5 < 0.001; p3–5 < 0.001; p4–5 < 0.001 | |||||||
| TNF-β (pg/mL) | 3.54 | 0.8–7.87 | 43.2 | 40.1–60.9 | 48.9 | 37.4–55.7 | 38.1 | 32.3–43.4 | 0.3 | 0.1–0.4 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001; p2–5 < 0.001; p3–5 < 0.001; p4–5 < 0.001 | |||||||
| IL-17A (pg/mL) | 2.8 | 1.4–3.1 | 9.4 | 8.2–11.3 | 8.6 | 7.7–9.3 | 8.1 | 7.4–8.2 | 7.4 | 7.2–8.1 |
| p1–2 = 0.03 | p1–3 = 0.03 | p1–4 = 0.03 | p1–5 = 0.03 | |||||||
| IL-6 (pg/mL) | 3.2 | 1.3–4.8 | 60.5 | 59.1–67.8 | 68.4 | 66.3–72.1 | 75.1 | 72.4–81.1 | 88.1 | 79.4–97.1 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001 | |||||||
| IL-10 (pg/mL) | 5.4 | 2.2–8.9 | 22.3 | 19.1–57.2 | 38.5 | 32.4–45.3 | 47.2 | 40.6–53.1 | 56.7 | 48.4–61.2 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001 | |||||||
| IL-4 (pg/mL) | 4.1 | 3.6–5.5 | 86.8 | 76.8–103.5 | 91.4 | 73.2–112.3 | 93.3 | 68.6–122.1 | 2.2 | 1.1–3.4 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 = 0.03; p2–5 < 0.001; p3–5 < 0.001; p4–5 < 0.001 | |||||||
| Indicators | Control Group N = 100 (1) | Stomach Cancer Stage I N = 75 (2) | Stomach Cancer Stage II N = 70 (3) | Stomach Cancer Stage III N = 69 (4) | Stomach Cancer Stage IV N = 67 (5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | Me | C25–C75 | |
| MDA, µmol/1 g protein | 1.6 | 0.96–2.24 | 45.3 | 33.1–86.2 | 56.35 | 32.46–101.74 | 76.4 | 69.2–123.3 | 91.3 | 82.4–167.1 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001; p2–5 = 0.03 | |||||||
| SOD, units/min/1 g protein | 204.41 | 151.05–250.32 | 231.2 | 176.1–256.9 | 235.2 | 133.7–462.27 | 174.1 | 169.9–200.1 | 157.2 | 148.1–189.3 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 = 0.03 | p1–5 < 0.001 | |||||||
| CAT, µmol/s/1 g protein | 0.27 | 0.2–0.39 | 0.16 | 0.15–0.28 | 0.14 | 0.13–0.18 | 0.15 | 0.13–0.19 | 0.1 | 0.08–0.17 |
| p1–2 = 0.04 | p1–3 = 0.04 | p1–4 = 0.04 | p1–5 = 0.003 | |||||||
| GST, mmol/min/1 g protein | 41.3 | 37.7–42.64 | 72.86 | 46.32–80.4 | 83.5 | 79.3–110.6 | 95.1 | 76.9–91.1 | 103.4 | 79.3–110.9 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001 | |||||||
| GPO µmol/1 g protein | 105.9 | 81.19–162.38 | 148.33 | 120.4–201.5 | 168.6 | 158.7–211.5 | 210.39 | 183.4–221.6 | 223.2 | 191.2–254.3 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001; p2–4 = 0.03; p3–4 = 0.03 | p1–5 < 0.001; p2–5 = 0.03; p3–5 = 0.03 | |||||||
| CP mg/L | 192.5 | 157.5–227.5 | 368.1 | 276.3–400.7 | 375.8 | 282.9–826.06 | 389.8 | 280.1–412.5 | 400.1 | 299.1–453.1 |
| p1–2 < 0.001 | p1–3 < 0.001 | p1–4 < 0.001 | p1–5 < 0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, O.; Sinyakov, A.; Kasparov, E. Cytokine Regulation and Oxidative Stress in Helicobacter Pylori-Associated Gastric Adenocarcinoma at Different Stages: Insights from a Cross-Sectional Study. Int. J. Mol. Sci. 2025, 26, 7609. https://doi.org/10.3390/ijms26157609
Smirnova O, Sinyakov A, Kasparov E. Cytokine Regulation and Oxidative Stress in Helicobacter Pylori-Associated Gastric Adenocarcinoma at Different Stages: Insights from a Cross-Sectional Study. International Journal of Molecular Sciences. 2025; 26(15):7609. https://doi.org/10.3390/ijms26157609
Chicago/Turabian StyleSmirnova, Olga, Aleksander Sinyakov, and Eduard Kasparov. 2025. "Cytokine Regulation and Oxidative Stress in Helicobacter Pylori-Associated Gastric Adenocarcinoma at Different Stages: Insights from a Cross-Sectional Study" International Journal of Molecular Sciences 26, no. 15: 7609. https://doi.org/10.3390/ijms26157609
APA StyleSmirnova, O., Sinyakov, A., & Kasparov, E. (2025). Cytokine Regulation and Oxidative Stress in Helicobacter Pylori-Associated Gastric Adenocarcinoma at Different Stages: Insights from a Cross-Sectional Study. International Journal of Molecular Sciences, 26(15), 7609. https://doi.org/10.3390/ijms26157609







