Abstract
Ribulose bisphosphate carboxylase (RuBisCO) is the primary regulator of carbon fixation in the plant kingdom. Although the large subunit (RBCL) is the site of catalysis, RuBisCO efficiency is also influenced by the sequence divergence of the small subunit (RBCS). This project compared the RBCS gene family in C3 and C4 grasses to identify genetic targets for improved crop photosynthesis. Triticeae/Aveneae phylogeny groups exhibited a syntenic tandem duplication array averaging 326.1 Kbp on ancestral chromosomes 2 and 3, with additional copies on other chromosomes. Promoter analysis revealed a paired I-box element promoter arrangement in chromosome 5 RBCS of H. vulgare, S. cereale, and A. tauschii. The I-box pair was associated with significantly enhanced expression, suggesting functional adaptation of specific RBCS gene copies in Triticaeae. H. vulgare-derived pan-transcriptome data showed that RBCS expression was 50.32% and 28.44% higher in winter-type accessions compared to spring types for coleoptile (p < 0.05) and shoot, respectively (p < 0.01). Molecular dynamics simulations of a mutant H. vulgare Rubisco carrying a C4-like amino acid substitution (G59C) in RBCS significantly enhanced the stability of the Rubisco complex. Given the known structural efficiency of C4 Rubisco complexes, G59C could serve as an engineering target for enhanced RBCS in economically crucial crop species which, in comparison, possess less efficient Rubisco complexes.
1. Introduction
As the sole carbon-fixing catalyst in the Tree of Life, ribulose bisphosphate carboxylase (RuBisCO) is responsible for converting most of the atmosphere’s inorganic carbon (over 90%) into biomass []. Consequently, RuBisCO is the only food producer sustaining the global food chain and is arguably one of the most essential enzymes on Earth. RuBisCO acts as a processing enzyme in the initial step of the light-independent reactions of photosynthesis, encompassing the Calvin cycle []. Its catalytic action facilitates the incorporation of one carbon from atmospheric CO2 into a five-carbon substrate, ribulose-1,5-bisphosphate (RuBP), resulting in the production of two molecules of 3-phosphoglyceric acid (3PGA), each being a three-carbon product []. These molecules then participate in reactions that lead to the eventual generation of more complex sugar molecules (i.e., glucose), which can be used as an energy source [].
RuBisCO is the entry point for inorganic carbon in subsequent processing reactions and constitutes the rate-limiting step of carbon fixation. It is thus the central determinant of an organism’s carbon-fixation efficacy []. Activation of the RuBisCO complex for catalysis involves both carbamylation and stabilisation of the active site via magnesium ion binding []. Without these processes, enzymatic activity is blocked—a likely safeguard against energy wastage resulting from RuBisCO inefficiency []. The inefficiency of RuBisCO is unusual, given its ubiquity and necessity as the prime inorganic carbon assimilator. Its double affinity for both CO2 and O2 poses substantial challenges to the enzyme’s carbon fixation efficiency, which is exacerbated by increases in temperature []. As temperatures rise, the reduced solubility of CO2 relative to O2 results in increased oxygen concentration in the leaf cellular space. This, combined with a higher specificity toward oxygen at elevated temperatures, leads to the preferential binding of oxygen by RuBisCO and subsequent ATP wastage in photorespiration [,].
RuBisCO operates as a moderately complex molecular machine, with the predominant Form I RuBisCO making up a substantial enzymatic complex of approximately 550 kilodaltons (kDa). This complex comprises eight small (RBCS) and eight large subunits (RBCL), forming a hexadecameric structure []. The catalytic activity occurs in the large subunits, which pair as dimers and create an α/β barrel-shaped arrangement at the C-terminal domain [,]. However, the observed diversity of RBCL is minimal across plant genera, exhibiting a high degree of conservation even among distantly related groups. This is demonstrated by species with group 1B RuBisCOs (from higher plants) showing an average sequence identity of 90% or more in the large subunit [,]. In contrast, sequence identity of as little as 30% is observed among plant RBCS, with copy numbers ranging from 1 to over 20 in some species [,].
Carbon fixation capacity variability is prominent among plant RuBisCOs [,,,]. This has been partly attributed to sequence divergence of the small subunit. The impact of specific RBCS amino acid substitutions on RuBisCO catalysis has provided promising evidence that RBCS mutagenesis drives RuBisCO evolution. The result is an improved capacity for RuBisCO to function in ‘rate-limiting’ environments across select plant taxa. One such study recently assessed the impact of hybridisation between RBCL derived from rice and RBCS derived from Sorghum bicolor, a C4 crop known for its high photosynthetic efficacy at elevated temperatures. The observed effect of hybridisation resulted in a higher catalytic rate (Kcat), attributed to residue L102 in the βC-βD Hairpin of sorghum-derived RBCS, leading to modified flexibility of the 60S loop in RBCL []. These results support the notion that RBCS sequence identity can fine-tune the catalytic turnover of RuBisCO, providing accelerated pathways to achieve bioengineering targets for enhanced biomass generation.
Despite C3 plants dominating a significant portion of the global market, their RuBisCOs are considerably more susceptible to the harmful effects of photorespiration. This is both due to the reduced catalytic efficiency of the RuBisCO complex and the absence of C4-adapted cellular compartmentalization to mitigate O2 specificity issues [,,,,]. Given the known catalytic advantages of C4 RuBisCOs, this project aimed to investigate the evolutionary and structural characteristics of true grass RuBisCO small subunit (RBCS) gene families (n = 16) to identify novel targets for photosynthetic improvement. The successful engineering of RuBisCO complexes in agriculturally significant crop species could offer a promising avenue to boost productivity targets in a future likely to be characterised by extensive global warming events.
2. Results
2.1. The RBCS Gene Family Exhibits a Distinct Copy Number Separation Between Phylogenetic Groupings Strongly Correlated with Genome Size
Phylogenetic comparison of RBCS gene sequences in the study species indicated the presence of a multigene family across all representatives except S. bicolor, which possessed a single RBCS gene copy (Figure 1A and Figure 2A). The most basal grass lineage in our study (i.e., P. latifolius) similarly possessed a low RBCS copy number (n = 2) along with the basal Bambusoideae subfamily (with 4 and 3 RBCS gene copies identified for O. latifolia and R. guianensis, respectively). This potentially suggests that an ancestral low RBCS copy number state existed in the grasses, that generally expanded as species radiation occurred over time (Figure 1A and Figure 2A). A somewhat noticeable divergence in RBCS copy numbers was identified between C3 and C4 species, with average RBCS copy numbers of 6.6 and 3.17, respectively (Figure 2C). When classifying RBCS copy number by climate type, warm climate-adapted species exhibited an average RBCS copy number of 3.3. In contrast, cold climate-adapted species in this study, of which the majority of species (83.4%) consisted of Triticeae/Aveneae tribe members, possessed an average copy number of 8.67 (Figure 2B). A large expansion of the RBCS gene family was apparent for members of the Triticeae and Aveneae tribes, with a minimum copy number ranging from 7 (observed for A. tauschii) to 11 copies (observed in S. cereale and A. atlantica). All other grass representatives in our study possessed an RBCS copy number less than or equal to 5 (Figure 1A). RBCS gene copies in A. atlantica (group IV; Figure 1A) formed a separate cluster from those in the Triticeae tribe. This observation indicated two separate RBCS gene family expansion events occurred, and these likely happened following the divergence of the Triticeae and Aveneae. Furthermore, three distinct RBCS gene family clusters were present within the Triticeae phylogenetic group. Unique clusters for RBCS on chromosome 2 were observed for all Triticeae members (group I; Figure 1A), RBCS on chromosome 1 for S. cereale and H. vulgare (group II; Figure 1A) and RBCS on chr5 for all Triticaeae, these being S. cereale, H. vulgare, T. urartu, and A. tauschii (Group III; Figure 1A). These observations, in turn, suggested that the expanded RBCS gene family was inherited via a common ancestor before diversification of Triticeae species members. Additional RBCS copies on chr7 were observed for S. cereale in the group I cluster (Figure 1A).
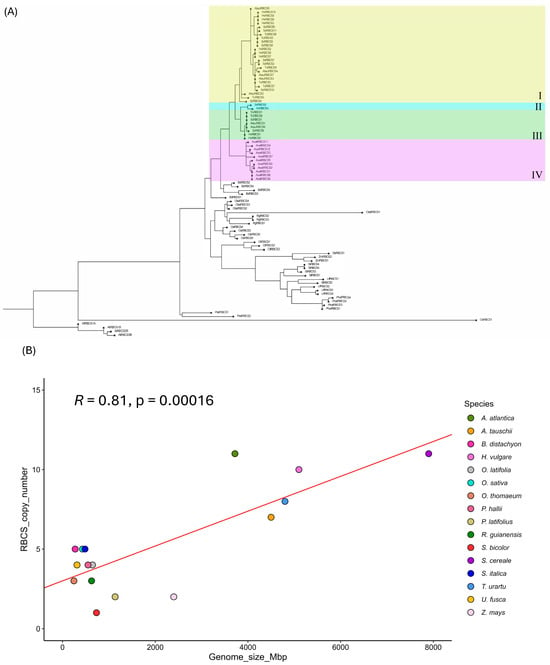
Figure 1.
(A) Phylogenetic tree based on protein sequence similarity of RBCS sequences. Groups in Triticeae and Aveneae are highlighted. Group I: all RBCS on chr2 (including RBCS on chr7 in S. cereale), Group II: all RBCS on chr1 (H. vulgare and S. cereale only), Group III: all RBCS on chr5, Group IV: all oat RBCS (diverged separately from Triticeae). Gene code identifiers for the respective gene(s) are in Supplementary File S1. (B) Linear regression plot of RBCS copy number (Y-axis) and genome size (X-axis) in 16 members of the true grasses. Included are the Pearson correlation coefficient (R) and its p-value and linear regression line (red).
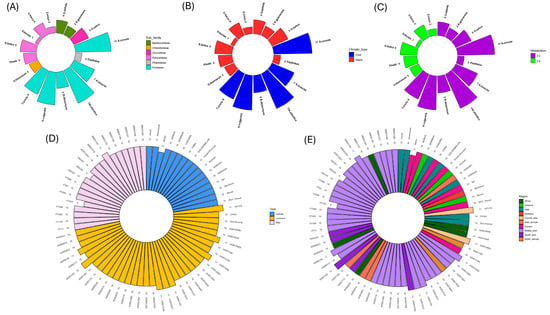
Figure 2.
Polar bar plots show copy number variation in RBCS sequences between true grass species (n = 16) and are colourised based on sub-family (A), climate type (B), and metabolism (C). Pangenome copy number variation is plotted and colourised based on domestication status (D) and geographic region (E).
To further assess the source of RBCS gene family expansion in members of the Triticeae/Aveneae tribes, Pearson correlation analysis was carried out to determine the extent of correlation between genome size (Mbp) and RBCS copy number. Overall, genome size was highly positively correlated with RBCS copy number in the species compared (R = 0.81, p < 0.001), suggesting that RBCS expansion in the Triticaeae/Aveneae could be attributed to genome duplication events at some point in evolutionary history (Figure 1B). However, Z. mays, which possesses a relatively large genome compared to other members of the C4 group, exhibits a somewhat conflicting relationship between genome size and RBCS copy number. In this instance, despite Z. mays possessing a 2400 Mbp genome (which is only ~35% smaller than the genome size of A. atlantica (3720 Mbp)), the Z. mays RBCS copy number (n = 2) is ~5.5 times smaller than that of the RBCS gene family possessed by A. atlantica. This may suggest that RBCS copy number does not substantially affect photosynthetic processes in some C4 grass lineages, perhaps due to possessing enzymes with high catalytic efficacy. This observation may also substantiate the low RBCS copy number prevalent in S. bicolor. Despite possessing a somewhat equivalent genome size (732 Mbp) to other species in this study, such as R. guianensus (626 Mbp, RBCS copy number = 3) and O. latifolia (646 Mbp, RBCS copy number = 4), S. bicolor possessed a drastically reduced RBCS gene family size, consisting of a single gene copy.
Expanding into the H. vulgare (barley) pangenome, RBCS copy number was mostly uniform across accessions, with 83% of varieties exhibiting an RBCS copy number of 10 (Figure 2D,E). When comparing accessions based on domestication status, only one cultivar (Barke) possessed a divergent RBCS copy number (n = 9) (Figure 2D). No landraces possessed an RBCS copy number lower than the dominant 10 RBCS copies. However, seven accessions exhibited one extra RBCS copy—these being 10TJ18, HOR10892, HOR13594, HOR1702, HOR18321, HOR19184 and HOR8148 (Figure 2D). In addition, one landrace accession (HOR7552) showed an RBCS copy number of 12—the highest observed in the pangenome varieties studied. Wild barley accessions also showed some diversity in RBCS copy number, with the lowest copy number recorded for FT333 (n = 8), followed by HID249 (n = 9), and WBDC103 exhibiting the greatest copy number (n = 11) (Figure 2D). When classified based on geographic origin, the greatest RBCS copy number diversity was observed for the Middle East accessions (ranging from 8 to 11 copies). Finally, geographic regions with accessions exhibiting predominantly high RBCS copy numbers included south Asia (with 40% of varieties possessing RBCS copy number > 10) and Central Asia, of which both representatives possessed an RBCS copy number of 11 (Figure 2E).
2.2. The Expanded RBCS Gene Family Shows Syntenic Associations Between Representatives of the Triticeae/Aveneae
Given the phylogenetic relationships observed between the Triticae/Aveneae RBCS gene families (covered in Section 2.1), synteny analysis was carried out to establish a clearer understanding of RBCS gene family expansion in H. vulgare (barley), T. urartu (diploid wheat), S. cereale (rye), A. tauschii (Aegilops/Tausch’s Goatgrass), and A. atlantica (diploid oat). Initial assessment of RBCS gene loci revealed that for those gene copies located on chromosome 2 in the Triticeae (and the syntenic chromosome 3 in A. atlantica), the expanded RBCS gene family was present as a compact tandem duplication array. The array spanned an average of 326.1 Kbp, and presented as small as 52.4 Kbp in A. tauschii. The proximity of RBCS gene copies in turn suggested that these arrays were likely expressed together in the respective species. Following the assessment of macrosynteny blocks, we determined the most likely chromosomal origin of the large RBCS gene family in Triticeae/Aveneae members. Synteny analysis revealed that RBCS copies on Chr1 were syntenic between H. vulgare and S. cereale, and RBCS copies on Chr5 were syntenic between H. vulgare, T. urartu, S. cereale, and A. tauschii (Figure 3A–C). The syntenic relationships between RBCS gene copies in Triticeae species on Chr1 and Chr5, echoed the pattern of phylogenetic clustering observed between group II and III RBCS gene groupings (covered in Section 2.1).
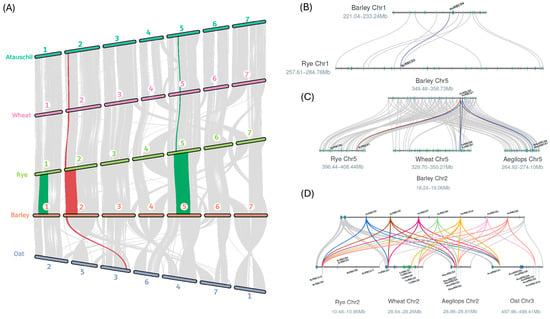
Figure 3.
(A) Macrosynteny map showing syntenic blocks (colourised) containing RBCS genes shared between members of the Triticeae (H. vulgare (barley), S. cereale (rye), A. tauschii (Tausch’s goatgrass), T. urartu (diploid wheat)) and Aveneae (represented by Avena atlantica, a diploid oat). The red block indicates the region comprising syntenic RBCS gene copies shared across both members of the Aveneae and Triticeae tribes. The chromosome number of each species are labelled above. Microsynteny maps (right) display the syntenic relationship between barley and rye RBCS gene copies on chromosome 1 (highlighted in purple) (B), barley gene copies on selected regions of chromosome 5 (highlighted in blue and brown) with Rye, diploid wheat (T. urartu) and Tausch’s goatgrass/Aegilops (A. tauschii) (C), and finally between barley gene copies on selected regions of chromosome 2 (blue, brown, magenta, green, yellow, pink, and orange) with Rye (S. cereale), diploid wheat (T. urartu), diploid oat (A. atlantica) and A. tauschii (D). Syntenic RBCS copies are labelled above, noting that all maps use the barley chromosome as the reference for mapping. Numbers (presented as megabases, (Mb)) in the macrosynteny maps, denote the chromosome region being illustrated in the respective genomes.
RBCS gene copies on chromosome 2 of H. vulgare exhibited syntenic relationships between all members of the Triticeae, in addition to A. atlantica of the Aveneae tribe. As shown by Figure 3D, all species demonstrated a syntenic link between at least two RBCS gene copies on chr2/3 with RBCS copies present in the tandem duplication array on chromosome 2 in H. vulgare. The combined results of synteny analysis suggested that diversification of the RBCS gene family in the Triticeae/Aveneae, was achieved via the expansion of RBCS gene copies from an ancestral arrangement of two RBCS copies on chromosome 2/3, in a common progenitor grass species to both lineages.
2.3. Cis-Regulatory Element Profiles of Grass Family RBCS Are Dominated by a Light- and Drought-Receptive Landscape
A cross-comparison of cis-regulatory element (CRE) configurations among the true grasses showed highly analogous CRE composition. RBCS promotors were primarily dominated by drought-inducible elements in most species studied, representing on average approximately 26.4% of CRE function (Figure 4). RBCS promoters additionally exhibited a high prevalence of light-inducible CREs, constituting approximately 20.6% of CRE function. Light-inducible elements were the dominant CRE detected in RBCS promoters of the C4 members Z. mays, U. fusca, and O. thomaeum, potentially suggesting evolutionary trends towards enhanced light-dependent regulation in these species (Figure 4B). Other prominent CRE identified in the RBCS promoter landscape include those related to ABA-response, Methyl Jasmonate (MeJA) response, and multiple stress response pathways, representing on average 7.7%, 6.7% and 8.34% of CRE function, respectively, across species possessing the CRE type(s). A more defined comparison of relative CRE composition across species showed some unique trends. Circadian CREs predominated the RBCS promoters of C3 grasses, being present in at least one RBCS gene promoter in all members of the Triticeae (with the exception of S. cereale) (Figure 4A). CREs with strong prevalence across all studied RBCS grass promoters included MYC (absent only from AvatRBCS6 and RgRBCS1), MYB (absent only from BdRBCS4 and RgRBCS1), and STRE, further highlighting that RBCS gene families are regulated by a variety of stress response pathways. The light-associated G-box and I-box elements were also prevalent in the majority of RBCS promoters, present in 90% and 73.4% of upstream gene regions, respectively. The low-temperature response element (LTR) was identified in the RBCS promoters of all species except P. latifolius and was present in 63.4% of all RBCS gene promoters studied, showing no clear differentiation between the cool and warm climate grasses. LTR element distribution likely highlights an intrinsic interplay between light intensity and, thus, temperature sensitivity in regulating RBCS expression. ARE elements involved in gene expression under low oxygen conditions were present at high levels across all species studied, with a prevalence of 87.8%, indicating an intrinsic relationship between light-sensitive RBCS genes and control of anaerobic respiration. Finally, CREs involved in wound healing had a notable prevalence across RBCS promoters such as WUN, with the WRE3 elements being present in the RBCS gene family of all grass species in this study. This in turn conveyed a relationship between regulation of carbon assimilation and wound healing. CRE function and distribution in the RBCS gene family of the true grasses paint a landscape defined by drought and light receptivity, with links to stress-associated pathways and those bound to wound healing and anoxic gene expression. This is a pattern not too unconventional, considering the role of RBCS in biomass accumulation during photosynthesis.
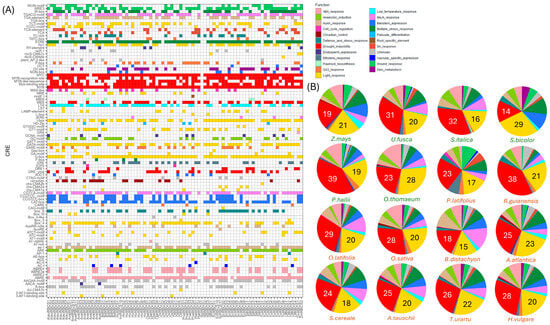
Figure 4.
Presence-absence matrix displaying cis regulatory elements (CREs) present in RBCS gene family members derived from 16 true grass species, arranged by gene and colourised by CRE function (A). To the right (B) are pie charts demonstrating the relative percentages of CREs (based on function) that are present in the RBCS gene family of each species. A legend of CRE function colours is provided above.
2.4. Unique Distribution of Terminal Cis-Regulatory Elements in the RBCS Promoters of Select Members of the Triticeae Exhibit Concordantly Enhanced Expression
This study investigated potential associations between cis-regulatory element configuration and RBCS gene family expression. Isolating six major CREs frequently reported in RBCS promoters, we sought to identify expression profiles that may be influenced by CRE arrangements. Following CRE mapping of the 2000 bp upstream region of RBCS gene(s), we identified a region close to the start of the gene in RBCS gene copies located on chromosome 5 in H. vulgare (HvRBCS1 and HvRBCS5), S. cereale (ScRBCS1 and ScRBCS6), and A. tauschii (AtauRBCS1 and AtauRBCS6), containing an I-box element pair with elements spaced ~25 bp apart (Figure 5; Supplementary File S5 Figure S1)). In all promoter regions containing the pair, it was located no further than 168 bp from the gene start site. As shown by the phylogenetic tree in Figure 5, all gene copies possessing the terminal I-box pair were closely related, reflecting the likely inheritance of the pair from a common ancestor shared by the three Triticeae members. Following transcriptomic analysis of RBCS gene expression in H. vulgare, S. cereale and A. tauschii, the chromosome 5 RBCS showed clearly differentiated expression, with a transcript abundance representing at least over 20% of total gene expression per chromosome five gene copy, and up to 48.7% of total RBCS transcript abundance in A. tauschii for a single gene copy (AtauRBCS1) (Figure 5). In comparison, all other RBCS gene family copies per species, each represented under 10% of total RBCS transcript abundance when considered individually, with ScRBCS2 representing as little as 0.05% of the total RBCS transcript abundance in S. cereale (Figure 5). Our observations indicated that a likely ancestrally derived expression profile has been retained in H. vulgare, S. cereale and A. tauschii, with RBCS copies on chromosome 5 dominating most of the transcript output. The remaining chromosome copies, despite altogether representing about half of the total RBCS transcript abundance in the three species, each exhibit, on average, about 4.36% of the total RBCS transcript abundance. These trends suggest a divergent function of RBCS expression in a chromosome-specific pattern, which may allow enhanced fine-tuning of RBCS expression via the regulation of gene subsets for select members of the Triticeae.
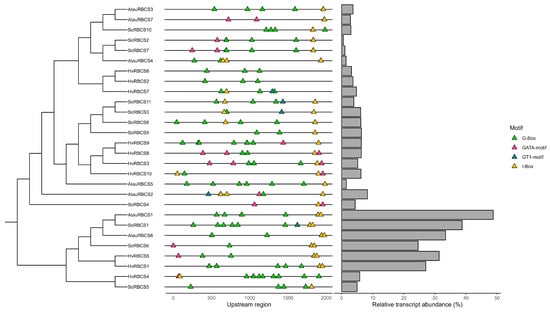
Figure 5.
Panel figure demonstrating the relationship between specific promoter arrangements for RBCS gene family copies present in three members of the Triticeae (H. vulgare, A. tauschii, and S. cereale) (middle) and relative transcript abundance (given as a percentage) of RBCS gene copies (right). RBCS genes are grouped based on phylogenetic relationship, as shown by the cladogram on the left.
2.5. Divergent RBCS Expression Profiles Are Observed Between H. vulgare Pangenome Accessions Possessing a Spring and Winter-Type Growth Habit
Utilising data provided by the barley pangenome consortium, we investigated RBCS transcript expression for 19 barley varieties to determine any trends between transcript abundance and growth habit over five tissue types (Caryopsis, Coleoptile, Inflorescence, Root and Shoot). As expected for a photosynthetic gene, expression levels were highest in the coleoptile and shoot, with average ‘transcripts per million’ (TPM) of 7102 and 9539, respectively, for winter-type varieties (n = 5) and 3529 and 6826 for spring-type varieties (n = 14) (Figure 6). Both expression profiles for the coleoptile and shoot showed a statistically significant difference in expression, with winter-type varieties exhibiting 28.44% higher transcript levels in the shoot (p < 0.01) and 50.32% higher transcript levels in the coleoptile versus their spring-type counterparts (p < 0.05) (Figure 6). Winter type expression also exceeded spring type expression in the caryopsis (with 24.8% higher expression) (p < 0.05) and inflorescence, with 17% higher expression—however, the difference in transcript abundance observed for the inflorescence was not statistically significant. The difference in root expression between the spring and winter type varieties was minimal (10%), with highly reduced expression for both growth habits (TPM value < 4) (Figure 6). The above expression data is suggestive that adaptive regulation of the RBCS gene family is present in barley varieties with a winter-type growth habit, perhaps as a compensatory mechanism due to exposure to low sunlight environments.
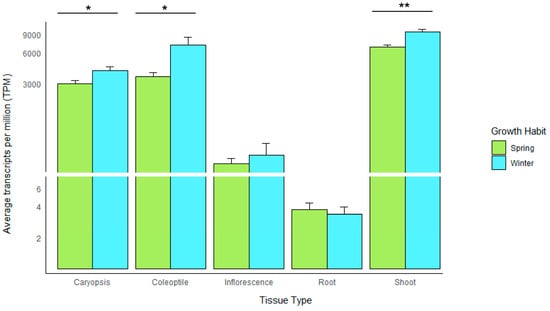
Figure 6.
Barplot showing the average transcript abundance (TPM) for the RBCS gene family across five tissues for 19 barley pangenome accessions categorised by growth habit. Error bars are plotted and significance levels indicated for groups where there was a significant difference as determined by Student’s t-test. * = p < 0.05, ** = p < 0.01.
2.6. Barley Mutant Rubisco Possessing Amino Acid Substitution Derived from Wildtype Maize RBCS Exhibits Superior Protein Stability
Molecular dynamics analysis was completed in this study to better understand the perceived differences in RuBisCO efficiency observed between different grass species. Given that the small subunit exhibits substantially higher diversity than RBCL, we investigated the effect of two amino acid substitutions on the RBCS sequence of H. vulgare, L83Y and G59C, noting that both represent the native amino acid at each position in the C4 crop Z. mays (Figure 7A,B). The selection of the candidate amino acid substitutions was a multi-step process. First, candidate residues in the small subunit were identified within 4 angstrom units of the large subunit. An amino acid alignment of the RBCS sequences from the 16 true grass species was then compared to determine the nature of amino acid sequence divergence at the identified sites. Following alignment, the L83Y substitution was identified as a desirable candidate. This was because 100% of C4 species in this study possessed the aromatic Y residue at this position versus only one of the C3 representatives (O. latifolia), with all other C3 RBCS sequences instead primarily possessing the aliphatic L or I residue. The C residue at position 59 showed prevalence only among C4 representatives, with Z. mays, S. bicolor, and S. italica possessing the G59C substitution (versus all other grass representatives possessing G at position 59). The protein alignment thus showed that cysteine (C) at position 59 was absent in all C3 photosynthesizing grasses included in this study. In addition, G59C was an amino acid substitution of interest identified following proximity-based selection of candidate RBCS residues close to the large subunit.
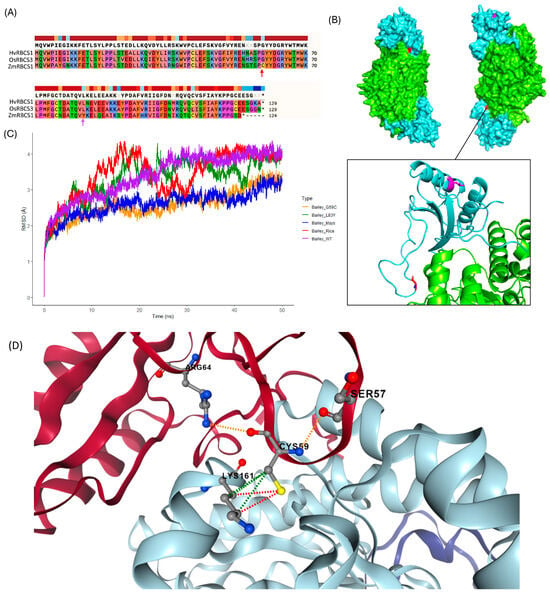
Figure 7.
Protein alignment of Barley, Mays and Rice RBCS sequences, with residues of interest highlighted (arrows). Asterisks indicate termination of the protein sequence(s) (stop codon) (A). Residues at position 59 (red) and 83 (magenta) were selected due to combined close-proximity to the large subunit, distribution of amino acid types at these positions among the C3 and C4 grasses in this study, and the predicted impact of an amino acid substitution on stability of the barley RuBisCO protein (using structural and sequence-based predictors). Position numbering is based on RBCS derived from spinach (S. oleracea). (B) shows a protein surface model of the wildtype barley RuBisCO complex, consisting of two small subunits (cyan) and two large subunits (green), used in molecular dynamics simulations. Target residues covered in (A) are highlighted in red and magenta, representing G59C and L83Y substitutions, respectively (noting that these are the native residues at the selected positions in wildtype mays RBCS). A zoomed view of the G59 residue (Red) and the L83 residue (magenta) with their locations in the βA-Bβ loop (G59) and αA helice (L83) of the small subunit, is also provided in the plot above. (C) shows a line plot of relative Root Mean Square Deviation (RMSD) values (in Angstrom units (Å)) recorded over a 50-nanosecond timescale following molecular dynamics simulations of various hybrid RuBisCO complexes. Hybrid complexes were generated in silico using AlphaFold2 (ColabFold version 1.5.5) and PyMOL (software version 2.5.5) and include Barley RBCL complexed with Mays RBCS (Barley_Mays), Rice RBCS (Barley_Rice) and wildtype barley RBCS (Barley_WT). Two in silico mutants were also generated, with the production of Barley RuBisCO complexes possessing the single amino acid substitution L83Y (Barley_L83Y) and G59C (Barley_G59C) in the small subunit of RuBisCO, respectively. Molecular dynamics simulations were carried out using GROMACS version 2023.3. (D) shows a zoomed view of the G59C substitution (CYS59) displayed in DynaMut2. The red-coloured subunit is RBCS, whilst the light blue subunit represents RBCL. Interacting residues (with CYS59) are also labelled above. Dashed lines indicate polar (orange) and hydrophobic (green) interactions, whereas hydrogen bonds are represented in red.
Selected residues were then assessed with the Sorting Intolerant From Tolerant (SIFT) sequence-based predictor. Given that SIFT scores ranging from 0.0 to 0.05 are predicted to be deleterious to protein function, and those >0.05 up to 1.0 are tolerated, the G59C substitution was predicted to be tolerated, with a score of 0.07, and the L83Y substitution was predicted impact protein function with a score of 0.04. It should be noted that due to the low nature of the SIFT score for the G59C substitution (0.07—near the 0.05 threshold), we additionally chose to assess impacts on protein stability for this substitution. DynaMut2 results indicated that both substitutions somewhat impact stability, with the L83Y (ΔΔG Stability: −1.45 kcal/mol) substitution predicted to have a more significant impact on protein stability than the G59C substitution (ΔΔG Stability: −0.56 kcal/mol) (in conjunction with the SIFT score results).
Next, we completed molecular dynamics analysis of the wildtype RBCS (complexed with RBCL) derived from H. vulgare. We also modelled H. vulgare RBCL complexed with a C3-derived RBCS from O. sativa and a C4-derived RBCS from Z. mays. Finally, two additional in silico generated RBCS mutants were modelled using the H. vulgare sequence—one with the G59C substitution and the other with L83Y. Figure 7C above shows the plotted Root Mean Square Deviation (RMSD) value of the protein complexes, modelled over a 50 ns timescale. Reflecting the SIFT score and DynaMut2 result, RBCS bearing the L83Y substitution negatively impacted stability, resulting in a high degree of variability in the RMSD value before plateauing to approximately 3.9 Å at 46 ns. Low stability (relative to the other modelled protein complexes) was additionally observed for the RuBisCO complex containing the O. sativa RBCS, plateauing to approximately 3.98 Å at 34 ns. The wildtype RuBisCO complex derived from H. vulgare exhibited similar stability to the complex containing O. sativa-derived RBCS, except with fewer fluctuations to the RMSD value over the 50 ns timescale, plateauing to approximately 3.93 Å at the 27 ns timepoint. Modelling of the wildtype Z. mays RBCS complexed with H. vulgare RBCL, revealed a comparatively lower RMSD value throughout the simulation, maintaining an average value of 2.89 Å after 30 ns. Interestingly, modelling the H. vulgare RuBisCO complex containing the mays-derived G59C mutation revealed comparable stability to the complex containing the wildtype maize RBCS, with an average value of 2.97 Å after 30 ns. Figure 7D, shows the G59C substitution in the βA-βB loop of the small subunit, as generated by DynaMut2. The output showed that a hydrogen bond forms between the βA-βB loop and a large subunit of RBCL, which may substantiate the observed increase in stability for H. vulgare-derived RBCS bearing the G59C mutation. The combined results of molecular dynamics analysis indicate that C3-derived RBCS generally imparted lower stability on the rubisco complex versus the sequence of C4 RBCS and highlights Cysteine at position 59 as a potential contributor to enhanced RuBisCO stability.
2.7. Natural Selection Analysis Reveals Distinct Selection Pressures in the RBCS Gene Family Lineages of Distinct Phylogenetic Groupings
Phylogenetic analysis by maximum likelihood (PAML) was performed to detect selection pressures across RBCS lineages. First, we aimed to elucidate if there was any specific selection pressure towards residues in the panicoid (C4) RBCS branch versus the other grasses in this study. Second, we aimed to determine if there was increased selection pressure on the Chr5 RBCS lineage compared to the Chr2 RBCS lineage in the Triticeae. In turn, four phylogeny groups were compared—C4 RBCS (ωC4), Chr5 RBCS in the Triticeae (ωCh5), Chr2 RBCS in the Triticeae (ωCh2) and all other grass RBCS sequences (ωrest). Branch-specific tests were performed to identify the dN/dS ratio (ω) (ratio of synonymous to non-synonymous mutations) of select phylogeny groups under differing assumptions. As shown in Table 1, under the one ratio model (which assumes ω is the same for all phylogeny groups), the dN/dS ratio was 0.04908. When we expanded the model to two branches, ω (dN/dS) was 0.0479 (ωCh5 = ωCh2 = ωrest) and 0.1999 (ωC4), indicating that despite relatively strong negative selection observed for both branches, there is more relaxed selection pressure in the C4 RBCS lineage. Under the three-branch model, we compared relative selection pressures for Triticeae RBCS on Chr5 and Triticeae RBCS on Chr2. From the results of the 3-branch model, it was deduced that more stringent selection pressure is applied to the chr2 RBCS branch (ω = 0.02328) versus the chr5/1 branch (ω = 0.40993). This is interesting given that the chr5 copies are the most highly expressed in the Triticeae, as observed for rye, Aegilops and barley (Figure 5). This result could indicate that the tandem duplication array in Triticeae species serves a specific accessory function to photosynthesis, despite comparatively lower expression levels, as evidenced by the increased stringency on amino acid sequence conservation. Finally, we completed a 4-branch model analysis, where all four phylogeny groups were considered under different selection pressures. Under the four-branch model, ω (dN/dS) was 0.04755 (ωrest), 0.20165 (ωC4), 0.02307 (ωCh2), and 0.44535 (ωCh5) (Table 1).

Table 1.
PAML (Phylogenetic Analysis by Maximum Likelihood) results of branch and branch-site-specific models, performed as part of the analysis of selection pressures in the RBCS gene family across select phylogenetic groups of true grass species. From left to right, the specified model, its number of parameters (np), likelihood value (lnL), the parameter value(s), and amino acid sites under positive selection following likelihood ratio tests (LRTs), are provided. Phylogenetic groups were defined as C4 group RBCSs (ωC4), Chr5 Triticeae RBCSs and relatives (ωCh5), Chr2 Triticeae RBCSs and relatives (ωCh2), and finally all other C3 group RBCS (ωrest). Two branch and site-specific models were implemented with C4 branch RBCS and Chr5 Triticeae RBCS defined as the foreground groups, respectively. The dn/ds ratios are represented as ‘ω’. Model A was defined to include four site classes: class I (ω0) and class II (ω1), representing conserved selection and neutral selection across RBCS lineages. Under the last two site classes, background lineages were defined to either possess ω0 or ω1 representing conserved or neutral selection, with the foreground lineage(s) possessing ω2, indicating positive selection pressure. The Null Model was defined by class I and II being the same as model A and the final site class differing, hence being defined by conserved or neutral selection across all lineages (ω1 = ω2). ‘P’ symbols indicate the relative proportions of site classes defined prior (e.g., p0 represents the proportion for ω0 and so on). Amino acid number positions are based on the barley reference sequence HORVU.MOREX.r3.5HG0468970. Analysis and table structure are based on the natural selection analysis method outlined in Jia et al. [].
Next, we further investigated the C4 RBCS, Chr2 Triticeae RBCS, and Chr5 Triticeae RBCS lineages to identify residues that may be under positive selection. Likelihood ratio tests (LRTs) were performed to determine the best model for running subsequent site and branch models in downstream analysis. Comparing the one-branch to the two-branch model and the two-branch to the four-branch model, the two-branch model (with C4 RBCS sequences as the foreground lineage) was determined to be the best-fit model for analysis, with a p-value of 0.0041. The results of the LRTs thus indicated that the C4 lineage is subjected to a different selection pressure relative to the C3 lineages (i.e., ωC4 ≠ ωCh5 = ωCh2 = ωrest). Site-branch models were used to investigate whether the C4, Chr2 and Chr5 groups were under positive selection. For the Chr2 group RBCS sequences, the branch and site-specific model revealed no residues under positive selection (Supplementary File S4). In contrast, the model results for the Chr5 group Triticaeae RBCS sequences revealed that residue 103A was under positive selection, with a probability of 0.999 (Table 1). Interestingly, residue 103A was only specific to the chromosome 5 group RBCS and relatives, with all chromosome 2 copies possessing serine at this position in select Triticaeae members, including H. vulgare, S. cereale, A. tauschii, and T. urartu. This may suggest a divergent function between the two groups, potentially explaining the differential expression patterns observed in upstream analysis (Section 2.4). Site-branch test results for the C4 group RBCS sequences, revealed a large number of sites under selection, including 70T (in the αA helice), and 102N, 103A, and 111R, which are located in the catalytically relevant βA-βB loop. Another residue of interest was 143A, which was within βC in the βC-βD loop. Interestingly, this residue is conserved across 100% of C3 representatives in this study, but with high diversity in the C4 clade. O. thomaeum has C at this position, P. hallii H or N, U. fusca C and S. italica A or C. The final step of natural selection analysis was the completion of LRTs to determine if ModelA was significantly better than its null hypothesis, thus indicating the identified amino acid sites are indeed under selection. As expected, the LRT was insignificant for the Chr2 group, where no sites were identified as under selection (p = 1.00). LRTs for the Chr5 group RBCS and C4 RBCS branches were significant, with p values of 0.0367 and p < 0.0001, respectively. The above analysis indicated that C4 group RBCS sequences may be under greater evolutionary change than other C3 grass RBCS sequences, which, in turn, allows for the greater adaptability of C4 rubisco complexes over time. In addition, several residues in the βA-βB loop were identified as under selection in the C4 group and Ch5 group RBCS sequences, which indicated that catalytically pivotal regions were targeted by positive selection. Finally, these observations may suggest that the divergence in sequence at position 103 between Chr2 and Chr5 copies may serve a specific functional purpose in the RBCS gene family possessed by Triticeae members.
3. Discussion
3.1. Expanded RBCS Arrays in the Triticeae/Aveneae May Compensate for Reduced Catalytic Efficacy and/or Enhance Adaptability to Temperate Environments
In this study, we observed large variability in RBCS gene family size across grass lineages, with a greatly expanded RBCS family observed in members of the Triticeae/Aveneae (as covered in Section 2.1). Genome expansion/duplication in the Triticeae is a well-documented phenomenon. Triticeae members possess large and highly dynamic genomes compared to their relatives, consisting of over 80% transposable element sequences which assist in continuous modification through time [,]. Given the high degree of correlation between genome size and RBCS copy number (R = 0.81, p < 0.001), genome duplication event(s) in part likely contributed to expanded RBCS copy number in representatives of the Triticeae/Aveneae. We deduced that this occurred prior to the split of these lineages in a common ancestral species due to the high degree of synteny observed between RBCS copies on all chromosomes in H. vulgare, T. urartu, A. tauschii, S. cereale and A. atlantica (Figure 3B–D).
The proximity of chromosome 2 genes also supports the hypothesis that all chromosome 2 RBCS expansions in the aforementioned species may have resulted from a tandem duplication event. Specifically, the location of RBCS gene(s) at the terminal end of chromosome 2 (chromosome 3 in A. atlantica) indicates that sub-telomeric duplication events may have facilitated a more rapid expansion of the gene family, thereby enhancing the environmental adaptation of photosynthetic efficiency []. A study by Hanada et al. [] found that the nature of gene duplication events in Arabidopsis reflected the adaptive patterns of the duplicate gene copies. Tandem duplications were associated with environmental stress response, while other duplication types correlated more with intracellular regulation []. Supporting this notion, a high proportion of cis-regulatory elements linked to drought, stress, and light conditions was observed in the promoters of the grass species studied, with light and drought response elements comprising over 30% of CRE composition across all lineages (and up to 59% in some instances) (Figure 4A). The authors also noted that the tandem expansion of gene families was lineage-specific, attributed to changes in distinct species that reflect their unique environmental exposures []. Given that members of Triticeae examined in this study are generally adapted to temperate regions, we suggest that the observed tandem expansions in H. vulgare, T. urartu, A. tauschii, S. Cereale, and A. atlantica likely occurred independently. This is likely due to an increased need to upregulate photosynthesis, caused by the lower sunlight intensity seen at 40–60° latitudes [,]. Furthermore, all RBCS copies retained an expression profile in H. vulgare, S. Cereale, and A. Tauschii (Figure 5) and were also retained in the expression of all members of the barley pan-transcriptome (Supplementary File S3). The absence of pseudogene development across lineages suggests an adaptive functionality of the expanded array, potentially to enhance photosynthesis in temperate environments or to better regulate photosynthetic response through differential expression mechanisms [,].
Finally, we observed higher expression in winter-type barley pangenome accessions for both the coleoptile and shoot (primary regions where photosynthetic processes occur). This further highlighted the association between plants adapted to cool regions that modulate their photosynthetic response to maximise biomass generation under low sunlight intensities (Figure 6). This trend is echoed in other studies, such as that of Huner et al. [], which reported that winter-adapted plant cultivars exhibit higher efficacy of the photosystem apparatus and greater resistance of photosynthetic processes to reduced light intensities. Another study demonstrated the high yield capacity of the winter wheat cultivar Weimai 8, resulting from superior performance of photosystem II compared to the control []. Despite the positive associations reported between winter adapted crops and photosynthetic traits, the impact of enhanced RBCS expression observed in the winter type barley pangenome accessions remains elusive without further investigation. It is widely known that winter-type barley accessions generally exhibit higher yield stability than their spring-type counterparts, which shows promise for an association of cold adaptation with photosynthetic vigour []. However, such studies primarily attribute phenology differences (i.e., earlier maturing of winter type cultivars) to superior winter-type biomass accumulation, and improved yield stability [,]. Hence, a thorough assessment is needed of relative RuBisCO catalytic efficacy in spring- and winter-type varieties and their correlation with RBCS expression levels and biomass accumulation. The findings may then better elucidate a relationship between cool climate adaptation and photosynthetic efficiency. A final consideration is the relative sensitivity of winter-type varieties to climatic conditions, and the potential of other environmental triggers (e.g., low temperature and/or low light induced gene pathways) affecting winter-type RBCS expression []. Overall, the combined phylogenetic and transcriptome-based observations of the Triticeae in our study suggest a photosynthetic apparatus temporally adapted to cool climatic conditions through tandem expansion and modulation of expression. High winter-type RBCS expression in the barley pan-transcriptome is compelling, yet we recognise the potential caveats of small sample size in the winter-type group, which may conflate observed trends []. The identification of regulatory mechanisms surrounding enhanced winter-type RBCS expression would be highly beneficial. Such findings may be adapted to spring-type barley genotypes, which have been identified to perform more robustly in a future shaped by climate change [].
3.2. Exploring the Terminal I Box Element Pair as a Potential Enhancer of Rubisco Expression and Modulator of Photosynthetic Rate
Light-induced I-box elements are a well-known feature of RBCS promoters, with numerous studies documenting their effects on RBCS expression. Historical studies involving tomato (Solanum lycopersicum) and Arabidopsis have shown that I-box elements are required to maintain high levels of RBCS expression [,]. Furthermore, cotton RBCS promoters containing I-box elements exhibit constitutive expression levels similar to those of the Cauliflower mosaic virus (CaMV) []. However, a more recent study found that native wheat RBCS promoters are less efficient than CaMV promoters based on GUS reporter gene expression. This may indicate that other factors, such as species-specific promoter CRE arrangements, may also influence the effectiveness of RBCS expression []. Our study observed that all highly expressed RBCS gene copies in representatives of the Triticeae possessed a terminal I-box element pair, separated by a spacer sequence approximately 26 bp in length in most sequences (Figure 5). Thus, there is potential that the spatial arrangement and frequency of I-box elements at the terminal region of RBCS promoters may greatly enhance RBCS expression and, consequently, the rates of photosynthesis in these gene copies. Studies in tomato have shown that the spatial arrangement of I and G box elements is critical for maintaining gene expression []. Additionally, the characterisation of conserved modular array 5 (CMA5) in RBCS8B (derived from Nicotiana plumbaginifolia) showed that the expression of some RBCS family members relies on the presence of specific light-reactive CREs (in this case, an I- and G-box arrangement) []. In fact, one study demonstrated that the distance between the I and G box elements in CMA5 is critical for ensuring adequate strength of the transcription signal []. We know that RuBisCO large subunit expression is influenced by the relative transcript levels of RBCS, and thus RBCS expression somewhat directs the rate of photosynthesis. The identified terminal I-box element arrangement in this study may thus serve as a pathway to modify promoter composition of accessory RBCS copies on chromosomes 2/3 in barley and rye [,]. Such modifications may enhance global RBCS expression, subsequently improving photosynthetic efficacy and biomass generation []. Overall, the terminal I-box element pair arrangement may present a promising avenue for enhancing photosynthesis in cereal crop species of nutritional relevance. However, given that our current evidence for I-box induced expression is computational, we establish this inference in a cautionary manner. As such, before targeted modifications can occur, functional characterisation experiments will need to be completed to confirm that the terminal I-box pair does indeed impart high RBCS expression levels. Validation of I-box associated enhancement of RBCS expression via CRISPR-induced removal of the terminal I-box pair would serve an appropriate validation approach [].
3.3. Native C4 Polymorphisms in the βA-βB Loop Serve as Novel Polymorphisms for the Targeted Improvement of C3 Rubisco Complexes
An assessment of the C4 grass clade reveals a somewhat contrasting pattern in terms of copy number. Given the current understanding of C4 type RuBisCOs and their enhanced catalytic efficacy, C4 grasses may not have necessitated the development of a large RBCS gene family to support photosynthetic processes []. In this study, the observed capacity for enhanced RuBisCO complex stabilisation in C4 RBCS was demonstrated through in-silico molecular dynamics. Following the in-silico analysis, we found that wildtype RBCS derived from the C4 crop Z. mays provided the greatest stability (Figure 7). These observations somewhat echo previously reported results from hybrid RuBisCO experiments. Matsumura et al. [] demonstrated that rice RBCL complexed with RBCS derived from S. bicolor exhibited superior catalytic performance compared to the wildtype RuBisCO complex, likely attributable to specific residues in the βC-βD hairpin of S. bicolor RBCS. This research further supports the idea that RBCS, despite lacking any catalytic components, is adapted to influence the overall quality of the RuBisCO complex. In our study, we identified a C4-specific amino acid substitution, G59C, in the βA-βB loop region of RBCS, which significantly enhanced the capacity of barley RBCS to stabilise the RuBisCO complex (Figure 7). The βA-βB loop is recognised as a site of catalytic importance in RBCS. Firstly, the length of the βA-βB loop is believed to regulate the size and width of the solvent channel [,]. In addition, mutations of residues present in the loop region, especially those near RBCL, influence the kinetic activity of RuBisCO, particularly its specificity for the CO2 substrate []. Our results from PAML analysis also highlighted several residues in the βA-βB loop that were under positive selection in the C4 lineage, emphasising the loop region’s critical role in the evolution of RuBisCO catalytic efficiency (Table 1). Among the residues under positive selection were 102N and 103A (55N and 56A based on amino acid sequence position in S. oleracea, respectively), which were only three and two residues away, respectively, from the cysteine residue ‘59C’ naturally occurring in Z. mays RBCS (Table 1; Figure 7). Finally, Dynamut2 revealed that the G59C substitution leads to the formation of a hydrogen bond with Lysine at position 161 in the large subunit of H. vulgare (Figure 7D), likely contributing to enhanced stability of the assembled RuBisCO complex []. Given these observations, we have identified the G59C substitution in the βA-βB loop region as a potential candidate for improving the catalytic potential of C3 crop RBCS. However, given the current lack of experimental validation of this novel protein substitution, our inferences remain conservative at this stage. Future functional analyses will be required to understand the true impact of the G59C substitution on the assembled rubisco complex. Potential validation protocols include differential scanning calorimetry (DSC) to assess reactive protein stability following thermal exposure. In addition, investigation of enzyme efficiency (Kcat) may be completed following CRISPR-induced knockout of the RBCS multigene family in barley and subsequent assembly of a modified barley rubisco complex comprising wildtype HvRBCL and HvRBCS possessing the novel G59C substitution [,].
3.4. Specific Selection Pressures in the Expanded RBCS Gene Family Are Suggestive of Potential Adaptive Divergence of RBCS Copies in the Triticeae
Adaptive divergence in the RBCS gene family has previously been observed in other species [,,]. For example, authors have found that rice OsRBCS1, located on Chromosome 2, exhibits expression in the root, anther, culm and leaf sheath but no expression in the leaf blade (the primary site of photosynthesis) []. Given the lower CO2 specificity of OsRBCS1 compared to its gene family relatives, it was proposed that OsRBCS1 expression may produce a more efficient RuBisCO complex in regions like the leaf sheath, which exhibit higher CO2 concentrations []. In our study, it was found that selection pressures were prevalent upon comparison of Chr5/1 and Chrs2 RBCS lineages in the Triticeae, with specific selection pressure identified for residue 103A in the HvRBCS5 reference sequence (Table 1). Given that all Chr5 RBCS copies possess the A residue at this position (in contrast to Chr2 RBCS possessing residue S), this could indicate a specific evolutionary pressure for A residue maintenance at position 103. In addition, given the observed high expression of the Chr5 RBCS gene copies in S. cereale, H. vulgare, and A. tauschii in this study, there exists a potential evolutionary favouritism of Chr5 RBCS functionality in the Triticeae. This may have led to observed shifts in expression such that Chr5 copies express the highest by a substantial degree. This pattern of favoured expression of specific RBCS copies echoes across various plant lineages, including A. thaliana, O. sativa, S. lycopersicum and S. tubersum []. However, little understanding can be gained as to why specific RBCS gene copies are preferentially expressed over others—especially given the high sequence homology observed between them []. One explanation for the combined low expression of tandem array copies in Triticaeae may be that they serve to supplement the full photosynthetic effect or otherwise may assist in the fine-tuning of environmentally sensitive responses [,]. For example, the expanded RBCS Chr2 copies observed in the Triticeae may ramp up more under specific stress-induced pressures to assist in photosynthetic efficacy—a notion further substantiated by our observation of unique promoter composition across gene family members at the species level (Figure 4A).
Another study that investigated the sequence composition of ancestral plant RuBisCOs noted the difficulty in isolating key RBCS residues that may improve catalytic efficiency. Authors observed unique residues reported in an efficient potato RBCS were not present in any of the high Kcat ancestral RBCS sequences [,]. In turn, this highlights that adaptation of RBCS catalysis across species results from multiple instances of differing sequence composition, due to temporal exposure to varied environments. Hence, diversity in RBCS evolution across species may provide a rich source of potential for future modification and catalytic fine-tuning of rubiscos in commercial crops. Ultimately, further work is needed to gain a better understanding of the RBCS gene family’s function across species and the roles of specific RBCS sequences in photosynthesis and beyond.
4. Materials and Methods
4.1. Phylogenetic Characterisation of the RBCS Gene Family and Assessment of Copy Number Variation Across True Grass Genera
A total of 17 plant species were selected for analysis, including 16 members of the true grasses (family Poaceae) and Arabidopsis thaliana as the outgroup species. All selected species possessed a diploid genome arrangement to deconvolute any potential associations of observed RBCS copy number with ploidy level for any given species. Genome assemblies were retrieved from various sources, summarised in Table 2 below. The study incorporated true grass representatives from both C3 (n = 10) and C4 (n = 6) metabolising classes, including basal members from both the BOP (subfamilies Bamusoideae, Pharoideae) and PACMAD clades (subfamily Chloridoideae).

Table 2.
Species selected for bioinformatic interrogation in this study. Included in the table are the metabolic type, subfamily and source of genome assembly for each species.
OrthoFinder version 2.5.5 was used to identify RBCS orthologues through homology-based search of species amino acid sequences. Identified orthologues were then further screened with conserved domain batch search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, accessed on 29 April 2023) for the presence of RBCS-associated domains, namely pfam12338 and pfam00101. Any severely truncated orthologues with insufficient domain architecture were subsequently removed before any downstream analysis. Following the filtering step, identified RBCS sequences were assessed for phylogenetic relationships between species lineages. First, an amino acid alignment of RBCS sequences (n = 89) was generated with ClustalO (https://www.ebi.ac.uk/jdispatcher/msa/clustalo, accessed on 5 May 2023) with an MBED-like clustering iteration, and default parameter settings for combined number of iterations (0), max HMM iterations, and max guide tree iterations. The alignment was then passed to raxmlGUI version 2.0 for phylogenetic tree construction, using the JTT amino acid model with ML + rapid bootstrap analysis and gamma substitution rates for 100 repetitions []. The resulting tree file was then ported to FigTree version 1.4.4 for subsequent gene label annotation and production of the finalised tree graphic. Correlation analysis of RBCS copy number versus genome size was carried out using the Pearson correlation method as part of the ggpubr R software package (version 0.6.0). Protein FASTA files and data frames used for analysis (including the master file containing all gene identifiers and their gene names), are available in Supplementary File S1.
4.2. Investigation of Relative Copy Number of the RBCS Gene Family in the Expanded Barley Pangenome
The genome assemblies of 71 newly sequenced barley (H. vulgare) pangenome varieties were obtained from Jayakodi et al. []. The Gene Model Mapper (GeMoMa) pipeline version 1.9 was selected to run projections for newly sequenced pangenome accessions, to generate gene annotations for use in downstream analysis []. Projections were run remotely on the Setonix supercomputer using a research allocation provided by the Pawsey Supercomputing Research Centre in Kensington, Western Australia. Pangenome annotations generated as part of this project can be found in Supplementary File S2. FASTA files of protein sequences for each barley accession were then generated with gffread version 0.12.7 in preparation for sequence homology search []. Using the Many-against-Many sequence searching (MMseqs2) software suite (release version 15-6f452), a combined protein database derived from the barley pangenome varieties was searched using the RBCS gene copy HORVU.MOREX.r3.1HG0037500.1 from the MorexV3 reference sequence as the query, with thresholds of 90% identity and 80% coverage [,]. Copy number counts were then assimilated from the MMseq2 sequence hits and polar bar plots generated with the count data using the ggplot2 version 3.5.1 package in RStudio (version 4.4.1).
4.3. Synteny Analysis of the Expanded RBCS Gene Family in the Triticaeae/Aveneae
The JCVI toolkit python library (https://github.com/tanghaibao/jcvi, accessed on 18 June 2023) was used to identify syntenic blocks comprising RBCS gene family copies, shared between members of the Triticeae and Aveneae tribe []. Macrosynteny and microsynteny plots were then generated with JCVI, highlighting syntenic RBCS genes identified on chromosomes 1, 2 and 5, respectively, and using the barley (H. vulgare) chromosome as the reference for mapping.
4.4. Extraction of Upstream RBCS Gene Regions and Identification of Cis-Regulatory Elements in the True Grasses and the Barley Pangenome
The sequence region located 2000 bp upstream of the gene start site was extracted from the genome assemblies of both the true grass family members and the barley pangenome accessions using BEDtools version 2.31.0. Extracted upstream regions were then submitted to the PlantCARE online database (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 20 September 2023) to determine the cis-regulatory element (CRE) profile of the query sequences []. Identified promoter elements were then filtered for CREs commonly associated with the regulation of rubisco small subunit expression using information derived from a combined literature search, yielding six CREs of interest [,,]. Promoter element maps of the filtered results were then created with TB tools (version 2.0) using the BioSequence structure illustrator []. Input files required to generate the promoter element map included a list of the upstream RBCS promoter sequences and their lengths (2000 bp), a four-column ‘Input ID list’ with Gene code, motif start position, motif end position and motif name, and phylogenetic tree of RBCS sequences (Newick format). A presence-absence matrix was additionally created for true grass family species via processing of the unfiltered PlantCARE output with pandas (version 2.0.3) and plotted with ggplot2. Plotted matrix CREs were colourised based on the CRE function. Pie charts depicting relative percentages for each CRE function type were additionally generated using ggplot2.
4.5. Investigation of RBCS Gene Family Copy Relative Expression Observed in H. vulgare, A. tauschii and S. cereale
RNA sequencing (RNAseq) data derived from H. vulgare, A. tauschii and S. cereale, was retrieved to determine which gene copies exhibit the greatest levels of RBCS transcript expression. Expression data for H. vulgare was retrieved from the BarleyExpDB (http://barleyexp.com/index.html, accessed on 15 September 2023) using Bioproject PRJEB14349: RNA-seq of 16 developmental stages of barley—and taking epidermis tissue expression (EPI) only []. RNAseq data for A. tauschii was obtained from Bioproject accession PRJNA748580 and treatment run SRR15206207 (https://www.ncbi.nlm.nih.gov/sra/SRX11512710[accn], accessed on 15 September 2023), pertaining to wheat rust resistant Aegilops tauschii that had not been inoculated with the rust pathogen []. S. cereale RNAseq data was retrieved from the European Nucleotide Archive (ENA), under experiment accession ERX3662813 and run number ERR3671408 (https://www.ebi.ac.uk/ena/browser/view/ERR3671408, accessed on 15 September 2023). For H. vulgare expression data, RNAseq results were provided directly as Fragments Per Kilobase Per Million mapped fragments (FPKM) following a database search of MorexV3 assembly RBCS gene queries. Further processing of raw RNAseq values (provided in paired layout), was required for A. tauschii and S. cereale, and this was achieved using Kallisto software version 0.5.0 to generate estimated transcript abundance values (as transcripts per million, or TPM) for each species []. The relative transcript abundance was then calculated as a percentage (%) for each species and plotted against a phylogenetic tree of the RBCS sequences and CRE map (see Section 4.4 for how CRE maps were generated). The treeio (version 1.28.0), ggtree (version 3.12.0), and aplot (version 0.2.3) packages were used for plot creation in RStudio version 4.4.1 [,,].
4.6. Assessment of Relative RBCS Expression in the Barley Pan-Transcriptome
Relative transcript abundance was compared for RBCS gene copies obtained from novel transcriptome data derived from 20 barley accessions comprising the original v1.0 barley pangenome collection and provided by the Western Crop Genetics Alliance for analysis []. Raw data reads can be accessed from the European Nucleotide Archive (ENA), under bioproject accessions PRJEB64639 and PRJEB64637 (https://www.ebi.ac.uk/ena/browser/search, accessed on 20 November 2023). Before the interrogation of genotype-specific reference transcript datasets (GsRTDs), homologous RBCS gene copies were first identified from the pan-transcriptome annotations via blastp of the MorexV3 RBCS protein sequences (HORVU.MOREX.r3.5HG0468970, HORVU.MOREX.r3.5HG0469020) against protein databases derived from the pan-transcriptome accession annotation data. Transcript abundance (TPM) was calculated using the sum of raw TPM data values across the RBCS gene families of pangenome varieties possessing a spring-type growth habit (n = 14) and winter-type growth habit (n = 5) and plotted as a simple bar chart with ggplot2. TPM values for each variety are provided in Supplementary File S3. Student t-tests were then performed for the distribution of summed raw TPM value(s) across all RBCS gene copies, between the spring and winter type varieties, for three biological replicates collected from five tissue types—Inflorescence (In), Coleoptile (Co), Caryopsis (Ca), Root (Ro), and Shoot (Sh) [].
4.7. Molecular Dynamics Analysis of in Silico Rubisco Hybrid Complexes
Before molecular dynamics (MD) analysis, a combination of methods was used to select amino acid substitutions for in silico mutagenesis in the RBCS of H. vulgare. First, an amino acid alignment (generated with ClustalO: see Section 4.1) for all true grass RBCS included in this study, was interrogated using SnapGene software version 7.0 to identify dominant amino acid residues in the C3 photosynthesising grasses and the C4 photosynthesising grasses. Any residues exhibiting substantial amino acid divergence between the C3 and C4 grasses (for example, being present in at least half of the C4 group species and not present in any representatives of the C3 group at the same position) were considered potential candidates for mutagenesis. The experimentally verified protein structure file (PDB: 6KYI) of wildtype O. sativa generated by Matsumura et al. [] was then retrieved from the Protein Data Bank (https://www.rcsb.org/search, accessed on 25 September 2023) and imported to PyMOL software (version 2.5.5). PyMOL was used to identify amino acid residues in the RBCS chains within 4 angstrom (Å) units of the large subunit molecule(s) that may influence RBCL catalysis. Residues identified near the large subunit and homologous between the C3 species H. vulgare and O. sativa (but differed in Z. mays) at the same position were considered for upcoming interrogation with sequence-based predictor software. The next step involved the use of the Sorting Intolerant From Tolerant (SIFT) engine (https://sift.bii.a-star.edu.sg/www/SIFT_seq_submit2.html, accessed on 29 September 2023) to query the predicted impact of selected substitutions of interest in the protein sequence of HORVU.MOREX.r3.5HG0469020.1. SIFT parameters included a median sequence conservation of 3.00, and the selected database was the UniProt-SwissProt 2010_09. The DynaMut2 structural-based predictor (https://biosig.lab.uq.edu.au/dynamut2/, accessed on 29 September 2023) was also used to discern amino acid substitution impacts on protein function, using an H. vulgare wildtype rubisco complex Protein Data Bank (PDB) file generated with AlphaFold2 (ColabFold version 1.5.5) and using HORVU.MOREX.r3.5HG0469020.1 (HvRBCS1) as the RBCS amino acid backbone sequence [].
Additional hybrid protein PDB files were created with AlphaFold2, including H. vulgare RBCL complexed with Z. mays RBCS1 (Zm00001d052595) and H. vulgare RBCL complexed with O. sativa RBCS3 (Os12g0291100). AlphaFold2 parameters included selection of the alphafold2_multimer_v3 model (generally selected for modelling of complex multimers), utilising a greedy pairing strategy for enhanced prediction of complex protein structure. The model was run with 3 recycles, and a maximum of 200 iterations. Based on outcomes of the combined RBCS protein alignment, sequence-based predictor and structure-based predictor analyses, two in silico H. vulgare rubisco mutants (one possessing the L83Y substitution in RBCS, and the other possessing the G59C substitution in RBCS, using the spinach (S. oleracea) RBCS as the reference sequence for amino acid position) were generated using the PyMOL mutagenesis wizard. Substitutions involving a change from an aliphatic (L) to aromatic (Y) residue, may substantially alter protein properties, such as stability, in part due to the increased hydrophobicity imparted by the aromatic ring structure []. L83Y was thus considered an intriguing target for modelling analysis, combined with the knowledge that L83 is a highly conserved residue across plant representatives []. The G59C substitution was primarily considered an appropriate candidate for modelling, given its prevalence among C4 grass in our study, and its location in the βA-βB loop region (a critical region known to impact rubisco catalysis) []. All PDB files were then utilised in the subsequent molecular dynamics simulation(s) using GROMACS version 2023.3. RuBisCO protein complexes were modelled utilising the OPLS-AA/L (all-atom optimised potentials for liquid simulations (long chain)) forcefield and the SPC/E (extended simple point charge) 3-point water model, which implements the ideal shape of water molecules (109.47 degrees) []. The selection of the OPLS-AA/L forcefield was justified based on its suitability for large biomolecule modelling, and its excellent balance of accuracy and efficiency, which was important for a moderate simulation time []. Methods utilised for MD analysis were adapted based on the protocols developed by Lemkul (2019) []. First, imported PDBs were stripped of crystal water molecules, and the parameters of a simple cubic box were defined as the system for MD simulation, ensuring a minimum distance of 1 nm between the modelled protein and box edge (to satisfy periodic boundary conditions). The system was then solvated with water, and any net charge present in the protein solute was balanced by adding ions to generate an electroneutral system suitable for subsequent energy minimisation. Systems producing negative potential energy with an order of approximately 105–106 and a maximum force not exceeding 1000 kJ mol−1 nm−1 were determined not to possess abnormal/interfering atomic arrangements (steric clashes) and were thus suitable for equilibration temperature and pressure. Equilibration of temperature (utilising a reference temperature of 300 K) and pressure (utilising a reference pressure and 1 bar) was completed for 100 picoseconds (ps) while imparting position restraints on system-heavy atoms to allow for system stabilisation without substantial structural changes to the protein []. Equilibration was considered successful following the observed plateau of temperature fluctuations around the 300 K reference value and an observed stable density fluctuation sustained around 1008 kg m−3 (the expected density of an SPC/E modelled system) []. Finally, MD runs were completed for all equilibrated systems over a 50 nanosecond (ns) timescale, a common simulation length for complex protein modelling []. Parameters specified for the MD runs included implementation of a modified Berendsen theromostat (tcoupl = V-rescale) combined with rapid thermal coupling parameters (tau_t = 0.1 ps) and independent equilibration of temperature baths for protein and non-protein regions of the system, to assist with artefact reduction (tc-grps = Protein Non-Protein). Pressure related controls included use of the Parrinello-Rahman barostat combined with slowed pressure coupling (tau_p = 2.0 ps). H-bonds were constrained in our system and subsequently accommodated by the LINCs algorithm and 2 femtosecond timestep (dt = 0.002). Simulations were run concurrently on five Nimbus cloud compute instances, each comprising 16 VCPUs and 64 GB of RAM, utilising an allocation provided by the Pawsey Supercomputing Research Centre.
Following dynamics, computation trajectories were corrected to compensate for spurious protein behaviour resulting from system periodic boundary conditions []. The relative stability of the modelled protein complexes was then compared by plotting the root mean square deviation (RMSD) values (Å) over the 50 ns timescale.
4.8. Natural Selection Analysis of RBCS Sequences in the True Grasses
Natural selection analysis was performed with Phylogenetic Analysis by Maximum Likelihood (PAML) software (version 4.9) utilising an alignment of RBCS CDS sequences generated with MEGA (version 11), and using HORVU.MOREX.r3.5HG0468970 (HvRBCS5) from H. vulgare as the reference sequence [,]. Analysis was performed on four phylogeny groups—RBCS sequences from C4 grasses (ωC4), sequences comprising chromosome 5 RBCS in the Triticeae (ωCh5), sequences comprising chromosome 2 RBCS in the Triticeae (ωCh2) and all other grass species RBCS sequences grouped as ωrest. Branch-specific models revealed the ratio of non-synonymous to synonymous amino acid substitutions (omega (dN/dS)) for specified phylogenetic protein groupings. Specification of branch-specific model parameters included the one-branch model—assuming ω is the same for all branches (ωC4 = ωCh5 = ωCh2 = ωrest), two-branch model—assuming ω (C4 branch RBCS sequences) does not the equal ω of all other the RBCS sequences studied (C3 members) (ωC4 ≠ ωCh5 = ωCh2 = ωrest), the 3-branch model, assuming that ω (Chr5 and Chr2 Triticeae RBCS sequences) does not equal ω of the other phylogeny groups (ωCh5 ≠ ωC4 = ωrest ≠ ωCh2) and the four-branch model which compared all four phylogenetic groups (assumed ωC4 ≠ ωCh5 ≠ ωCh2 ≠ ωrest). Codeml parameters for all branch-specific models included implementation of codon analysis (Seqtype = 1) utilising a standard genetic code conversion table (icode = 0), and the F3X4 model (CodonFreq = 2), which takes into account codon position bias, thus improving the accuracy of dN/dS estimates. The model = 0 parameter was set for the one-branch model, and the model = 2 parameter was set for models assessing two or more branches. Files used in the analysis, including code parameter files, are in Supplementary File S4. For each set of model analyses, a phylogenetic tree (with branches pertinent to analysis labelled) was provided, along with an alignment file of the RBCS sequences.
Likelihood ratio tests (LRTs) were then completed to determine the most suitable branch model based on the value of 2ΔL. First, we derived the value of 2ΔL, utilising the following equation:
2ΔL = 2(lnL[model1] − lnL[model2])
The p-value was then determined from both 2ΔL and the DF value, where
DF = np [model1] − np [model2]
The site and branch-specific tests were carried out with two specified foreground lineages (C4 branch RBCS and Chr5 Triticaeae RBCS) to identify potential residues under positive selection pressure. Two separate models were ran and compared for the respective foreground lineages. Model A assumed positive selection pressure in foreground lineage(s), and conserved or neutral selection in background lineage(s) (model = 2, NSites = 2, fix_omega = 0, omega = 1.5). Model A null assumed conserved or neutral selection pressure for all lineage(s), fixing omega (ω) to a value of 1 (model = 2, Nsites = 2, fix_omega = 1, omega = 1). LRTs were subsequently performed to compare model A and model A null, to determine whether residues were in fact subjected to positive selection pressure based on the statistical significance threshold (p < 0.05). The site and branch-specific LRTs performed here were carried out utilising the same protocol listed above for the calculation of the 2ΔL and the DF value. The above protocol was adapted from the methodology developed by Jia et al. [].
5. Conclusions
Our study revealed diverse RBCS gene family compositions across various true grass species. The Triticeae tribe exhibited a significant expansion of the gene family, which greatly contrasted with the C4 members Z. mays and S. bicolor (which historically demonstrate greater catalytic efficiency of the rubisco complex). Extensive synteny of the RBCS gene family was observed in the Triticeae, with syntenic relationships observed spanning multiple chromosomes. We identified chromosome 2 RBCS as the likely ancestral copy, given the syntenic relationships shared between both Triticeae and Aveneae RBCS sequences. The promoter composition of the RBCS gene family showcased predominant light and drought-responsive elements, followed by other stress-associated CREs, reflecting the primary function of RBCS in photosynthetic processes. Furthermore, it suggests that rubisco expression is transient with environmental flux. Our observation that winter-adapted barley varieties exhibited higher global RBCS expression than their spring-adapted counterparts further suggests an environmental impact on RuBisCO evolution. Lastly, we identified 59C, located in the βA-βB loop region of RBCS, as a specific residue of interest for the future targeted modification of RuBisCO function, due to the observed positive effect of the G59C substitution on barley RuBisCO stability. Natural selection analysis led to the identification of residues under positive selection pressure in the βA-βB loop (of which a subset was in close proximity to the G59C substitution region). The results of this study help develop a clearer picture of RuBisCO evolution in the grasses, revealing novel promoter and sequence regions as potential targets for enhancing photosynthesis. Such targets may significantly enhance catalysis in C3 rubisco complexes and warrant future functional validation, with the possibility for superior crops with improved biomass generation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157424/s1.
Author Contributions
B.C.T. devised the research concept, completed the research and wrote the manuscript. C.L., Y.J. and T.H. assisted in project development and provided research guidance and advice on the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was completed whilst B.C.T. was supported by a Research Training Program (RTP) Scholarship provided by the Commonwealth of Australia and a Grains Research and Development Corporation (GRDC) scholarship provided by the GRDC (grant number: GRS; contract code: UMU2302-007RSX).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Relevant data supporting this study is provided within the Supplementary Materials Files and outlined in the article. Further inquiries regarding data can be directed to the authors.
Acknowledgments
B.C.T. would like to express gratitude to T.H. and C.L. for their insightful advice regarding various aspects of the research direction and plan. B.C.T. additionally extends tremendous gratitude to Yong Jia, who provided much-appreciated guidance and support during the research process.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| RBCS | Rubisco Small Subunit |
| RBCL | Rubisco Large Subunit |
References
- Erb, T.J.; Zarzycki, J. A Short History of Rubisco: The Rise and Fall (?) of Nature’s Predominant CO2 Fixing Enzyme. Curr. Opin. Biotechnol. 2018, 49, 100–107. [Google Scholar] [CrossRef]
- Andralojc, P.J.; Carmo-Silva, E.; Degen, G.E.; Parry, M.A.; Gutteridge, S. Increasing Metabolic Potential: C-Fixation. Essays Biochem. 2018, 62, 109–118. [Google Scholar] [CrossRef]
- Meloni, M.; Gurrieri, L.; Fermani, S.; Velie, L.; Sparla, F.; Crozet, P.; Henri, J.; Zaffagnini, M. Ribulose-1,5-Bisphosphate Regeneration in the Calvin-Benson-Bassham Cycle: Focus on the Last Three Enzymatic Steps that Allow the Formation of Rubisco Substrate. Front. Plant Sci. 2023, 14, 1130430. [Google Scholar] [CrossRef]
- Chen, J.-H.; Tang, M.; Jin, X.-Q.; Li, H.; Chen, L.-S.; Wang, Q.-L.; Sun, A.-Z.; Yi, Y.; Guo, F.-Q. Regulation of Calvin—Benson Cycle Enzymes under High Temperature Stress. aBIOTECH 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Jensen, R.G. Activation of Rubisco Regulates Photosynthesis at High Temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 12937–12938. [Google Scholar] [CrossRef]
- Lorimer, G.H.; Miziorko, H.M. Carbamate Formation on the.Epsilon.-Amino Group of a Lysyl Residue as the Basis for the Activation of Ribulosebisphosphate Carboxylase by Carbon Dioxide and Magnesium(2+). Biochemistry 1980, 19, 5321–5328. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.; Lobo, A.K.M.; Carmo-Silva, E. Regulation of Rubisco Activity in Crops. New Phytol. 2023, 241, 35–51. [Google Scholar] [CrossRef]
- Brooks, A.; Farquhar, G.D. Effect of Temperature on the CO2/O2 Specificity of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase and the Rate of Respiration in the Light. Planta 1985, 165, 397–406. [Google Scholar] [CrossRef]
- Cavanagh, A.P.; Slattery, R.; Kubien, D.S.; Sharwood, R. Temperature-Induced Changes in Arabidopsis Rubisco Activity and Isoform Expression. J. Exp. Bot. 2022, 74, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Sapir, Y.; Shapira, M. A Conserved Mechanism Controls Translation of Rubisco Large Subunit in Different Photosynthetic Organisms. Plant Physiol. 2006, 141, 1089–1097. [Google Scholar] [CrossRef]
- Whitney, S.M.; Houtz, R.L.; Alonso, H. Advancing Our Understanding and Capacity to Engineer Nature’s CO2-Sequestering Enzyme, Rubisco. Plant Physiol. 2010, 155, 27–35. [Google Scholar] [CrossRef]
- Liu, D.; Ramya, R.C.S.; Mueller-Cajar, O. Surveying the Expanding Prokaryotic Rubisco Multiverse. FEMS Microbiol. Lett. 2017, 364, fnx156. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Catherall, E.; Díaz-Ramos, A.; Greiff, G.R.L.; Azinas, S.; Gunn, L.; McCormick, A.J.; Sharwood, R. The Small Subunit of Rubisco and Its Potential as an Engineering Target. J. Exp. Bot. 2022, 74, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.; Popilka, L.; Hartl, F.U.; Hayer-Hartl, M. Role of Auxiliary Proteins in Rubisco Biogenesis and Function. Nat. Plants 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. Variation in the Kcat of Rubisco in C3 and C4 Plants and Some Implications for Photosynthetic Performance at High and Low Temperature. J. Exp. Bot. 2002, 53, 609–620. [Google Scholar] [CrossRef]
- Whitney, S.M.; Sharwood, R.E.; Orr, D.; White, S.J.; Alonso, H.; Galmés, J. Isoleucine 309 Acts as a C4 Catalytic Switch that Increases Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Carboxylation Rate in Flaveria. Proc. Natl. Acad. Sci. USA 2011, 108, 14688–14693. [Google Scholar] [CrossRef]
- Galmés, J.; Capó-Bauçà, S.; Niinemets, Ü.; Iñiguez, C. Potential Improvement of Photosynthetic CO2 Assimilation in Crops by Exploiting the Natural Variation in the Temperature Response of Rubisco Catalytic Traits. Curr. Opin. Plant Biol. 2019, 49, 60–67. [Google Scholar] [CrossRef]
- Bouvier, J.W.; Emms, D.M.; Kelly, S. Rubisco is Evolving for Improved Catalytic Efficiency and CO2 Assimilation in Plants. Proc. Natl. Acad. Sci. USA 2024, 121, e2321050121. [Google Scholar] [CrossRef]
- Matsumura, H.; Shiomi, K.; Yamamoto, A.; Taketani, Y.; Kobayashi, N.; Yoshizawa, T.; Tanaka, S.; Yoshikawa, H.; Endo, M.; Fukayama, H. Hybrid Rubisco with Complete Replacement of Rice Rubisco Small Subunits by Sorghum Counterparts Confers C4 Plant-like High Catalytic Activity. Mol. Plant 2020, 13, 1570–1581. [Google Scholar] [CrossRef]
- Gowik, U.; Westhoff, P. The Path from C3 to C4 Photosynthesis. Plant Physiol. 2010, 155, 56–63. [Google Scholar] [CrossRef]
- Galmés, J.; Conesa, M.À.; Díaz-Espejo, A.; Mir, A.; Perdomo, J.A.; Niinemets, Ü.; Flexas, J. Rubisco Catalytic Properties Optimized for Present and Future Climatic Conditions. Plant Sci. 2014, 226, 61–70. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the Global Number and Distribution of Maize and Wheat Farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, M.; Hu, H.; Chapman, B.; Watt, C.; Buerte, B.; Han, N.; Zhu, M.; Bian, H.; Li, C.; et al. Comparative Gene Retention Analysis in Barley, Wild Emmer, and Bread Wheat Pangenome Lines Reveals Factors Affecting Gene Retention Following Gene Duplication. BMC Biol. 2023, 21, 25. [Google Scholar] [CrossRef]
- Mascher, M.; Wicker, T.; Jenkins, J.; Plott, C.; Lux, T.; Koh, C.S.; Ens, J.; Gundlach, H.; Boston, L.B.; Tulpová, Z.; et al. Long-Read Sequence Assembly: A Technical Evaluation in Barley. Plant Cell 2021, 33, 1888–1906. [Google Scholar] [CrossRef]
- Wicker, T.; Gundlach, H.; Spannagl, M.; Uauy, C.; Borrill, P.; Ramírez-González, R.H.; De Oliveira, R.; International Wheat Genome Sequencing Consortium; Mayer, K.F.X.; Paux, E.; et al. Impact of Transposable Elements on Genome Structure and Evolution in Bread Wheat. Genome Biol. 2018, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Linardopoulou, E.V.; Williams, E.M.; Fan, Y.; Friedman, C.; Young, J.M.; Trask, B.J. Human Subtelomeres are Hot Spots of Interchromosomal Recombination and Segmental Duplication. Nature 2005, 437, 94–100. [Google Scholar] [CrossRef]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.-H. Importance of Lineage-Specific Expansion of Plant Tandem Duplicates in the Adaptive Response to Environmental Stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Desta, K.T.; Choi, Y.-M.; Yoon, H.; Lee, S.; Yi, J.; Jeon, Y.; Wang, X.; Park, J.-C.; Kim, K.-M.; Shin, M.-J. Comprehensive Characterization of Global Barley (Hordeum vulgare L.) Collection Using Agronomic Traits, β-Glucan Level, Phenolic Content, and Antioxidant Activities. Plants 2024, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Blackman, G.E.; Wilson, G.L. Physiological and Ecological Studies in the Analysis of Plant Environment. Ann. Bot. 1951, 15, 373–408. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, L.-K.; Zhang, K.; Chen, S.-M.; Hu, J.-B.; Cheng, F. The Impact of Tandem Duplication on Gene Evolution in Solanaceae Species. J. Integr. Agric. 2022, 21, 1004–1014. [Google Scholar] [CrossRef]
- Cai, H.; Des Marais, D.L. Expression variability following gene duplication facilitates gene retention. bioXiv 2024, 11, 622370. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, Photoinhibition and Low Temperature Acclimation in Cold Tolerant Plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef]
- Sui, N.; Li, M.; Meng, Q.-W.; Tian, J.-C.; Zhao, S.-J. Photosynthetic Characteristics of a Super High Yield Cultivar of Winter Wheat during Late Growth Period. Agric. Sci. China 2010, 9, 346–354. [Google Scholar] [CrossRef]
- Yiğit, A.; Chmielewski, F.-M. A Deeper Insight into the Yield Formation of Winter and Spring Barley in Relation to Weather and Climate Variability. Agronomy 2024, 14, 1503. [Google Scholar] [CrossRef]
- Chen, J.; Manevski, K.; Lærke, P.E.; Jørgensen, U. Biomass yield, Yield Stability and Soil Carbon and Nitrogen Content Under Cropping Systems Destined for Biorefineries. Soil Tillage Res. 2022, 221, 105397. [Google Scholar] [CrossRef]
- del Río, Á.R.; Monteagudo, A.; Contreras-Moreira, B.; Kiss, T.; Mayer, M.; Karsai, I.; Igartua, E.; Casas, A.M. Diversity of Gene Expression Responses to Light Quality in Barley. Sci. Rep. 2023, 13, 17143. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M. Sample Size, Power and Effect Size Revisited: Simplified and Practical Approaches in Pre-Clinical, Clinical and Laboratory Studies. Biochem. Medica 2021, 31, 27–53. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Safonov, T.A.; Malko, A.M.; Bocharov, G.A.; Goncharov, S.V. Climate Change Expected to Increase Yield of Spring Cereals and Reduce Yield of Winter Cereals in the Western Siberian Grain Belt. Field Crop. Res. 2023, 302, 109038. [Google Scholar] [CrossRef]
- Donald, R.G.; Cashmore, A.R. Mutation of Either G Box or I Box Sequences Profoundly Affects Expression from the Arabidopsis rbcS-1A Promoter. EMBO J. 1990, 9, 1717–1726. [Google Scholar] [CrossRef]
- Rose, A.; Meier, I.; Wienand, U. The Tomato I-Box Binding Factor LeMYBI is a Member of a Novel Class of Myb-Like Proteins. Plant J. 1999, 20, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Heinen, J.L.; Burns, T.H.; Allen, R.D. Expression of Two Tissue-Specific Promoters in Transgenic Cotton Plants. J. Cotton Sci. 2000, 4, 217–223. [Google Scholar]
- Mukherjee, S.; Stasolla, C.; Brûlé-Babel, A.; Ayele, B.T. Isolation and Characterization of Rubisco Small Subunit Gene Promoter from Common Wheat (Triticum aestivum L.). Plant Signal. Behav. 2015, 10, e989033. [Google Scholar] [CrossRef][Green Version]
- Martínez-Hernández, A.; López-Ochoa, L.; Argüello-Astorga, G.; Herrera-Estrella, L. Functional Properties and Regulatory Complexity of a MinimalRbcs Light-Responsive Unit Activated by Phytochrome, Cryptochrome, and Plastid Signals. Plant Physiol. 2002, 128, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- López-Ochoa, L.; Acevedo-Hernández, G.; Martínez-Hernández, A.; Argüello-Astorga, G.; Herrera-Estrella, L. Structural Relationships between Diverse Cis-Acting Elements are Critical for the Functional Properties of a Rbcs Minimal Light Regulatory Unit. J. Exp. Bot. 2007, 58, 4397–4406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakabayashi, K.; Yoshizawa, R.; Mae, T.; Makino, A. Differences in Expression of the RBCS Multigene Family and Rubisco Protein Content in Various Rice Plant Tissues at Different Growth Stages. Plant Cell Physiol. 2009, 50, 1851–1855. [Google Scholar] [CrossRef]
- Suzuki, Y.; Makino, A. Availability of Rubisco Small Subunit Up-Regulates the Transcript Levels of Large Subunit for Stoichiometric Assembly of Its Holoenzyme in Rice. Plant Physiol. 2012, 160, 533–540. [Google Scholar] [CrossRef]
- Liang, F.; Lindblad, P. Synechocystis PCC 6803 Overexpressing Rubisco Grow Faster with Increased Photosynthesis. Metab. Eng. Commun. 2017, 4, 29–36. [Google Scholar] [CrossRef]
- Ludwig, Y.; Dueñas, C.; Arcillas, E.; Macalalad-Cabral, R.J.; Kohli, A.; Reinke, R.; Slamet-Loedin, I.H. CRISPR-Mediated Promoter Editing of a Cis-Regulatory Element of OSNAS2 Increases ZN Uptake/Translocation and Plant Yield in Rice. Front. Genome Ed. 2024, 5, 1308228. [Google Scholar] [CrossRef]
- Sharwood, R.E.; Ghannoum, O.; Whitney, S.M. Prospects for improving CO2 Fixation in C3-Crops through Understanding C4-Rubisco Biogenesis and Catalytic Diversity. Curr. Opin. Plant Biol. 2016, 31, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J. Role of the Small Subunit in Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Arch. Biochem. Biophys. 2003, 414, 141–149. [Google Scholar] [CrossRef]
- Valegård, K.; Hasse, D.; Andersson, I.; Gunn, L.H. Structure of Rubisco from Arabidopsis thalianain Complex with 2-Carboxyarabinitol-1,5-Bisphosphate. Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 1–9. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of Hydrogen Bonds to Protein Stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Li, G.; Mao, H.; Ruan, X.; Xu, Q.; Gong, Y.; Zhang, X.; Zhao, N. Association of Heat-Induced Conformational Change with Activity Loss of Rubisco. Biochem. Biophys. Res. Commun. 2002, 290, 1128–1132. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Schwender, J.; Ohlrogge, J.B. The Capacity of Green Oilseeds to Utilize Photosynthesis to Drive Biosynthetic Processes. Plant Physiol. 2004, 136, 2700–2709. [Google Scholar] [CrossRef]
- Whitney, S.M.; Andrews, T.J. The Gene for the Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Small Subunit Relocated to the Plastid Genome of Tobacco Directs the Synthesis of Small Subunits That Assemble into Rubisco. Plant Cell 2001, 13, 193–205. [Google Scholar] [CrossRef]
- Pottier, M.; Gilis, D.; Boutry, M. The Hidden Face of Rubisco. Trends Plant Sci. 2018, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Hatanaka, T.; Misoo, S.; Fukayama, H. Unusual Small Subunit That Is Not Expressed in Photosynthetic Cells Alters the Catalytic Properties of Rubisco in Rice. Plant Physiol. 2013, 164, 69–79. [Google Scholar] [CrossRef]
- Khumsupan, P.; Kozlowska, M.A.; Orr, D.J.; Andreou, A.I.; Nakayama, N.; Patron, N.; Carmo-Silva, E.; McCormick, A.J.; Sharwood, R. Generating and Characterizing Single- and Multigene Mutants of the Rubisco Small Subunit Family in Arabidopsis. J. Exp. Bot. 2020, 71, 5963–5975. [Google Scholar] [CrossRef] [PubMed]
- Meier, I.; Callan, K.L.; Fleming, A.J.; Gruissem, W. Organ-Specific Differential Regulation of a Promoter Subfamily for the Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Small Subunit Genes in Tomato. Plant Physiol. 1995, 107, 1105–1118. [Google Scholar] [CrossRef][Green Version]
- Martin-Avila, E.; Lim, Y.-L.; Birch, R.; Dirk, L.M.A.; Buck, S.; Rhodes, T.; Sharwood, R.E.; Kapralov, M.V.; Whitney, S.M. Modifying Plant Photosynthesis and Growth via Simultaneous Chloroplast Transformation of Rubisco Large and Small Subunits. Plant Cell 2020, 32, 2898–2916. [Google Scholar] [CrossRef]
- Lin, M.T.; Salihovic, H.; Clark, F.K.; Hanson, M.R. Improving the Efficiency of Rubisco by Resurrecting its Ancestors in the Family Solanaceae. Sci. Adv. 2022, 8, eabm6871. [Google Scholar] [CrossRef]
- Maughan, P.J.; Lee, R.; Walstead, R.; Vickerstaff, R.J.; Fogarty, M.C.; Brouwer, C.R.; Reid, R.R.; Jay, J.J.; Bekele, W.A.; Jackson, E.W.; et al. Genomic Insights from the First Chromosome-Scale Assemblies of Oat (Avena spp.) Diploid Species. BMC Biol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-H.; Ma, P.-F.; Yang, G.-Q.; Hu, J.-Y.; Liu, Y.-L.; Xia, E.-H.; Zhong, M.-C.; Zhao, L.; Sun, G.-L.; Xu, Y.-X.; et al. Genome Sequences Provide Insights into the Reticulate Origin and Unique Traits of Woody Bamboos. Mol. Plant 2019, 12, 1353–1365. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D.; Matschiner, M. raxmlGUI 2.0: A Graphical Interface and Toolkit for Phylogenetic Analyses Using Raxml. Methods Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Jayakodi, M.; Lu, Q.; Pidon, H.; Rabanus-Wallace, M.T.; Bayer, M.; Lux, T.; Guo, Y.; Jaegle, B.; Badea, A.; Bekele, W.; et al. Structural Variation in the Pangenome of Wild and Domesticated Barley. Nature 2024, 636, 654–662. [Google Scholar] [CrossRef]
- Keilwagen, J.; Hartung, F.; Grau, J. GEMOMA: Homology-Based Gene Prediction Utilizing Intron Position Conservation and RNA-Seq Data. Methods Mol. Biol. 2019, 1962, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Research 2020, 9, 304. [Google Scholar] [CrossRef]
- Steinegger, M.; Söding, J. MMSEQS2 Enables Sensitive Protein Sequence Searching for the Analysis of Massive Data Sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Tang, H.; Krishnakumar, V.; Zeng, X.; Xu, Z.; Taranto, A.; Lomas, J.S.; Zhang, Y.; Huang, Y.; Wang, Y.; Yim, W.C.; et al. JCVI: A Versatile Toolkit for Comparative Genomics Analysis. iMeta 2024, 3, e211. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Baloch, F.S.; Bakhsh, A.; Asim, M. RuBisCo Small Subunit as Strong Green Tissue Specific Promoter. Curr. Opin. Biotechnol. 2011, 22, S139. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.; Shangguan, H.; Bian, J.; Luo, R.; Tian, Y.; Li, Z.; Nie, X.; Cui, L. BarleyExpDB: An Integrative Gene Expression Database for Barley. BMC Plant Biol. 2023, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Dorostkar, S.; Dadkhodaie, A.; Ebrahimie, E.; Heidari, B.; Ahmadi-Kordshooli, M. Comparative Transcriptome Analysis of Two Contrasting Resistant and Susceptible Aegilops Tauschii Accessions to Wheat Leaf Rust (Puccinia triticina) Using RNA-Sequencing. Sci. Rep. 2022, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Wang, L.-G.; Lam, T.T.-Y.; Xu, S.; Dai, Z.; Zhou, L.; Feng, T.; Guo, P.; Dunn, C.W.; Jones, B.R.; Bradley, T.; et al. Treeio: An R Package for Phylogenetic Tree Input and Output with Richly Annotated and Associated Data. Mol. Biol. Evol. 2019, 37, 599–603. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Luo, X.; Chen, M.; Tang, W.; Zhan, L.; Dai, Z.; Lam, T.T.; Guan, Y.; Yu, G. Ggtree: A Serialized Data Object for Visualization of a Phylogenetic Tree and Annotation Data. iMeta 2022, 1, e56. [Google Scholar] [CrossRef]
- Yu, G. Aplot: Decorate a “ggplot” with Associated Information, R Package Version 0.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://CRAN.R-project.org/package=aplot (accessed on 1 July 2025).
- Waugh, R.; Guo, W.; Schreiber, M.; Marosi, V.; Bagnaresi, P.; Chalmers, K.; Chapman, B.; Dang, V.; Dockter, C.; Fiebig, A.; et al. A barley pan-transcriptome reveals layers of genotype-dependent transcriptional complexity. Nat. Genet. 2024, 57, 441–450. [Google Scholar] [CrossRef]
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. Dynamut2: Assessing Changes in Stability and Flexibility upon Single and Multiple Point Missense Mutations. Protein Sci. 2020, 30, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, M.L.; Bowler, B.E. Propensities of Aromatic Amino Acids versus Leucine and Proline to Induce Residual Structure in the Denatured-State Ensemble of ISO-1-Cytochrome C. J. Mol. Biol. 2010, 403, 495–504. [Google Scholar] [CrossRef]
- Park, C.; Robinson, F.; Kim, D. On the Choice of Different Water Model in Molecular Dynamics Simulations of Nanopore Transport Phenomena. Membranes 2022, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sweere, A.J.; Fraaije, J.G. Accuracy Test of the OPLS-AA Force Field for Calculating Free Energies of Mixing and Comparison with Pac-Mac. J. Chem. Theory Comput. 2017, 13, 1911–1923. [Google Scholar] [CrossRef]
- Lemkul, J. From Proteins to Perturbed Hamiltonians: A Suite of Tutorials for the Gromacs-2018 Molecular Simulation Package [Article V1.0]. Living J. Comput. Mol. Sci. 2019, 1, 5068. [Google Scholar] [CrossRef]
- Rampogu, S.; Son, M.; Baek, A.; Park, C.; Rana, R.M.; Zeb, A.; Parameswaran, S.; Lee, K.W. Targeting Natural Compounds Against HER2 Kinase Domain as Potential Anticancer Drugs Applying Pharmacophore Based Molecular Modelling Approaches. Comput. Biol. Chem. 2018, 74, 327–338. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. Mega11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).