Environmental Hazards and Glial Brain Tumors: Association or Causation?
Abstract
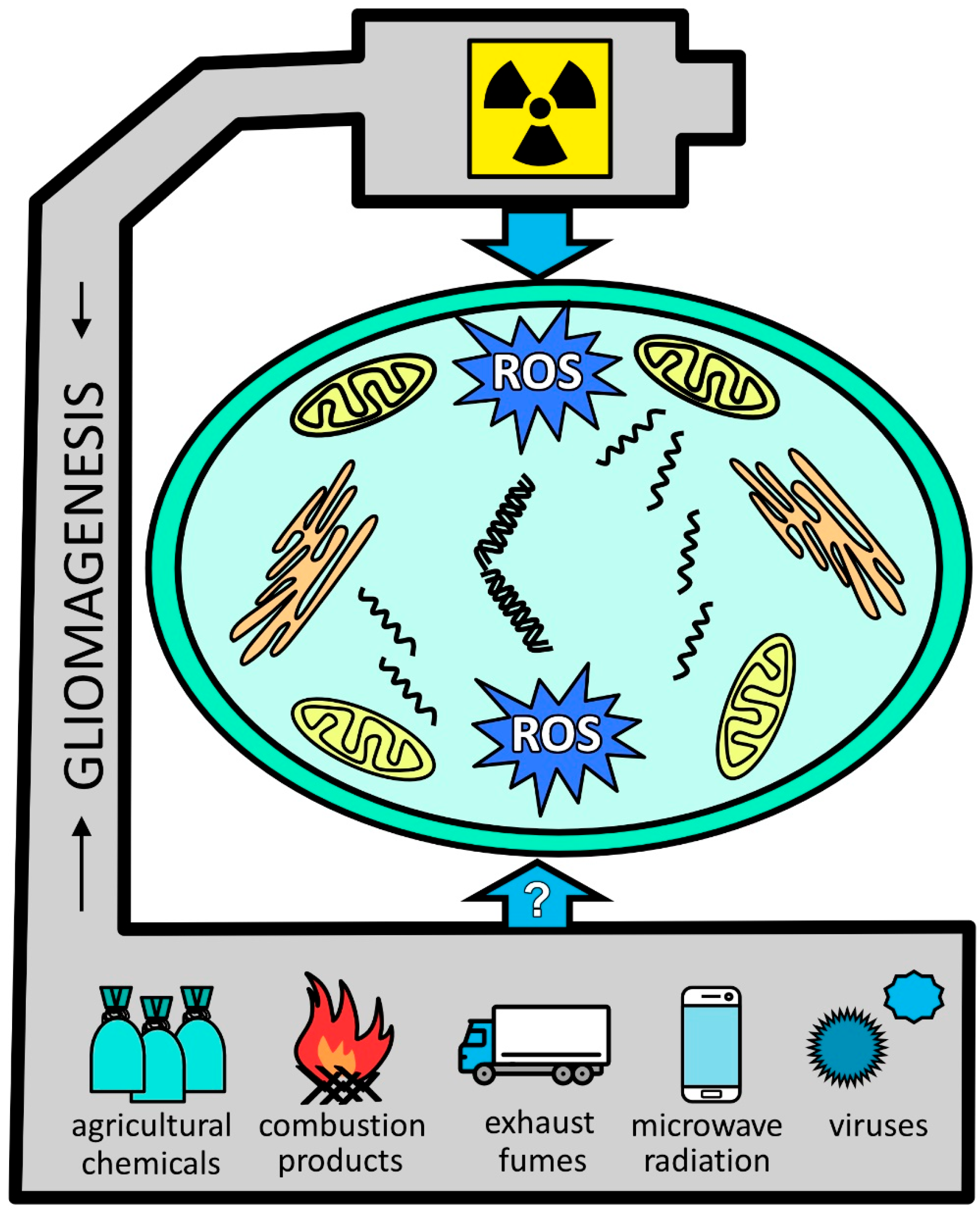
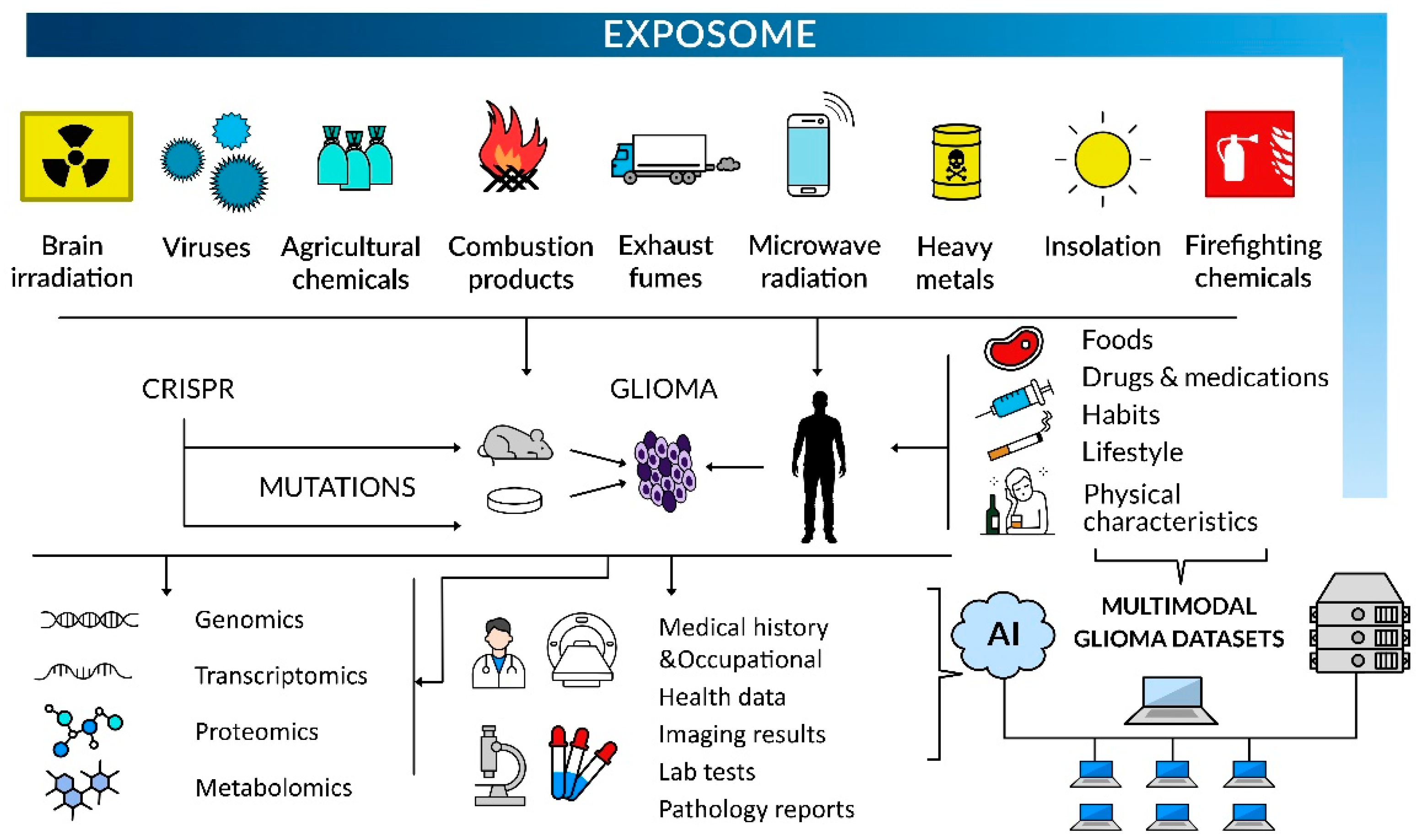
1. Introduction
2. Literature Search
2.1. Brain Irradiation
2.2. Agricultural Chemicals
2.3. Electromagnetic Fields
2.4. Diet and Lifestyle
2.5. Impact of Combustion Products
2.6. Ultraviolet
2.7. Heavy Metals
2.8. Geography, Demography, and Environmental Cues
2.9. Infectious Diseases
2.10. Recreational Substances and Habits
2.11. Intake of Drugs
3. Discussion
| Environmental/Lifestyle Factors Implicated in Glioma Initiation and/or Progression | Level of Evidence | References | Proposed Mechanisms |
|---|---|---|---|
| Agricultural chemicals, pesticides | 3b | [51] | Oxidative stress, DNA damage, carcinogenic organic compound formation [63] |
| 3a | [68] | ||
| Brain irradiation | 2a | [41] | DNA damage leading to oncogene amplifications and homozygous deletions of tumor suppressor genes [48] |
| Combustion products and air pollutants | 2b | [118] | Oxidative stress, DNA damage [119] |
| 3b | [123] | ||
| Firefighting chemicals (haloalkanes) | 3b | [127] | Mutations characterized by SBS42 signature [127] |
| Heavy metals | 2b | [135] | Oxidative stress, DNA damage, impaired DNA repair gene expression, OGG1 [133,138] |
| 3b | [136] | ||
| Microwave radiation | 3a | [93] | Induction of oxidative and nitrosative stress in the brain [78,79] |
| 3b | [92] | ||
| 4 | [81] | ||
| 5 | [218] | ||
| Oral contraceptives | 3b | [209] | Interaction of synthetic hormones with sex hormone receptors in brain cells [219,220] |
| Poverty | 2b | [169] | Increased exposures to environmental hazards and limited access to healthcare [169] |
| 2b | [168] | ||
| 2b | [170] | ||
| Smoking tobacco | 4 | [198] | Increased production of neurocarcinogens and ROS [204,205] |
| 3a | [21] | ||
| Viruses | 3a | [186] | Activation of oncogenic pathways and angiogenesis [189,190] |
| 5 | [185] |
Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimov, D.; Brainman, D.; Berger, B.; Coras, R.; Grote, A.; Simon, M. The role of cytoreductive surgery in multifocal/multicentric glioblastomas. J. Neurooncol. 2023, 164, 447–459. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; He, Z.; Pucko, E.B.; Matyja, E. Hemorrhage in brain tumor—An unresolved issue. Brain Hemorrhages 2022, 3, 98–102. [Google Scholar] [CrossRef]
- Chang, C.; Chavarro, V.S.; Gerstl, J.V.E.; Blitz, S.E.; Spanehl, L.; Dubinski, D.; Valdes, P.A.; Tran, L.N.; Gupta, S.; Esposito, L.; et al. Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms. Int. J. Mol. Sci. 2024, 25, 6733. [Google Scholar] [CrossRef]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef] [PubMed]
- Central Nervous System Tumours: Who Classification of Tumours, 5th ed.; IARC Publications: Lyon, France, 2022; Volume 6, p. 584.
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Leske, H.; Blakstad, H.; Lund-Iversen, M.; Skovholt, E.K.; Niehusmann, P.; Ramm-Pettersen, J.-T.; Skogen, K.; Kongelf, G.; Sprauten, M.; Magelssen, H.; et al. Astrocytoma (CNS WHO grade 4), IDH-mutant with co-occurrence of BRAF p.V600E mutation, and homozygous loss of CDKN2A. Neuropathology 2023, 43, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Alshiekh Nasany, R.; de la Fuente, M.I. Therapies for IDH-Mutant Gliomas. Curr. Neurol. Neurosci. Rep. 2023, 23, 225–233. [Google Scholar] [CrossRef]
- Deacu, M.; Docu Axelerad, A.; Popescu, S.; Topliceanu, T.S.; Aschie, M.; Bosoteanu, M.; Cozaru, G.C.; Cretu, A.M.; Voda, R.I.; Orasanu, C.I. Aggressiveness of Grade 4 Gliomas of Adults. Clin. Pract. 2022, 12, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Hertler, C.; Felsberg, J.; Gramatzki, D.; Le Rhun, E.; Clarke, J.; Soffietti, R.; Wick, W.; Chinot, O.; Ducray, F.; Roth, P.; et al. Long-term survival with IDH wildtype glioblastoma: First results from the ETERNITY Brain Tumor Funders’ Collaborative Consortium (EORTC 1419). Eur. J. Cancer 2023, 189, 112913. [Google Scholar] [CrossRef]
- Ceglie, G.; Del Baldo, G.; Agolini, E.; Rinelli, M.; Cacchione, A.; Del Bufalo, F.; Vinci, M.; Carta, R.; Boccuto, L.; Miele, E.; et al. Cancer Predisposition Syndromes Associated With Pediatric High-Grade Gliomas. Front. Pediatr. 2020, 8, 561487. [Google Scholar] [CrossRef]
- Dipro, S.; Al-Otaibi, F.; Alzahrani, A.; Ulhaq, A.; Al Shail, E. Turcot syndrome: A synchronous clinical presentation of glioblastoma multiforme and adenocarcinoma of the colon. Case Rep. Oncol. Med. 2012, 2012, 720273. [Google Scholar] [CrossRef]
- Pellerino, A.; Caccese, M.; Padovan, M.; Cerretti, G.; Lombardi, G. Epidemiology, risk factors, and prognostic factors of gliomas. Clin. Transl. Imaging 2022, 10, 467–475. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. International patterns and trends in the brain cancer incidence and mortality: An observational study based on the global burden of disease. Heliyon 2023, 9, e18222. [Google Scholar] [CrossRef] [PubMed]
- Stadler, C.; Gramatzki, D.; Le Rhun, E.; Hottinger, A.F.; Hundsberger, T.; Roelcke, U.; Läubli, H.; Hofer, S.; Seystahl, K.; Wirsching, H.-G.; et al. Glioblastoma in the oldest old: Clinical characteristics, therapy, and outcome in patients aged 80 years and older. Neuro-Oncol. Pract. 2023, 11, 132–141. [Google Scholar] [CrossRef]
- Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. The incidence of major subtypes of primary brain tumors in adults in England 1995–2017. Neuro Oncol. 2021, 23, 1371–1382. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, L.-C.; Gao, T.-Y.; Luo, J.; Zhang, C. The burden of brain and central nervous system cancers in Asia from 1990 to 2019 and its predicted level in the next twenty-five years. BMC Public Health 2023, 23, 2522. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Seyedmirzaei, H.; Shahrokhi Nejad, S.; Nejadghaderi, S.A. Epidemiology and socioeconomic correlates of brain and central nervous system cancers in Asia in 2020 and their projection to 2040. Sci. Rep. 2024, 14, 21936. [Google Scholar] [CrossRef]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995–2015 Suggests an Adverse Environmental or Lifestyle Factor. J. Environ. Public Health 2018, 2018, 7910754. [Google Scholar] [CrossRef]
- Zumel-Marne, A.; Castano-Vinyals, G.; Kundi, M.; Alguacil, J.; Cardis, E. Environmental Factors and the Risk of Brain Tumours in Young People: A Systematic Review. Neuroepidemiology 2019, 53, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Spallek, J.; Schüz, J.; Schlehofer, B.; Böhler, E.; Schlaefer, K.; Hettinger, I.; Kunna-Grass, K.; Wahrendorf, J.; Blettner, M. Occupational exposure to radio frequency/microwave radiation and the risk of brain tumors: Interphone Study Group, Germany. Am. J. Epidemiol. 2006, 164, 538–548. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Savarese, B.; Avilia, G.; Giarra, A.; Pagano, G.; Marano, A.; Trifuoggi, M.; Bifulco, M.; et al. Impacts of Environmental Pollution on Brain Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 5045. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez-Andrade, M.; Quezada-Maldonado, E.M.; Rivera-Pineda, A.; Chirino, Y.I.; García-Cuellar, C.M.; Sánchez-Pérez, Y. The Road to Malignant Cell Transformation after Particulate Matter Exposure: From Oxidative Stress to Genotoxicity. Int. J. Mol. Sci. 2023, 24, 1782. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Asthana, S.; Kumar, D. Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer. Cancers 2023, 15, 4920. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Ambel, E.T.; Naumowicz, M.; Velasco, G. Cellular stress responses as modulators of drug cytotoxicity in pharmacotherapy of glioblastoma. Biochim. Biophys. Acta-Rev. Cancer 2024, 1879, 189054. [Google Scholar] [CrossRef]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef]
- Rosendahl Huber, A.; Van Hoeck, A.; Van Boxtel, R. The Mutagenic Impact of Environmental Exposures in Human Cells and Cancer: Imprints Through Time. Front. Genet. 2021, 12, 760039. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef]
- Liu, S.; Dong, L.; Shi, W.; Zheng, Z.; Liu, Z.; Meng, L.; Xin, Y.; Jiang, X. Potential targets and treatments affect oxidative stress in gliomas: An overview of molecular mechanisms. Front. Pharmacol. 2022, 13, 921070. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Guo, X.; Wang, X.; Han, X.; Kanwore, K.; Hong, X.; Zhou, H.; Gao, D. Hypoxia-induced ROS aggravate tumor progression through HIF-1α-SERPINE1 signaling in glioblastoma. J. Zhejiang Univ. Sci. B 2023, 24, 32–49. [Google Scholar] [CrossRef]
- Elguindy, M.; Young, J.S.; Mondal, I.; Lu, R.O.; Ho, W.S. Glioma-Immune Cell Crosstalk in Tumor Progression. Cancers 2024, 16, 308. [Google Scholar] [CrossRef]
- Krawczynski, K.; Godlewski, J.; Bronisz, A. Oxidative Stress-Part of the Solution or Part of the Problem in the Hypoxic Environment of a Brain Tumor. Antioxidants 2020, 9, 747. [Google Scholar] [CrossRef]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.; Dai, Z.; Liu, J.; Zhang, H.; Wen, J.; Zhang, N.; Zhang, J.; Luo, P.; Liu, Z.; et al. Oxidative stress is involved in immunosuppression and macrophage regulation in glioblastoma. Clin. Immunol. 2024, 258, 109802. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Duerinck, J.; Neyns, B.; Movahedi, K.; Van Ginderachter, J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 2020, 9, e52176. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caffo, M.; Minutoli, L.; Marini, H.; Abbritti, R.V.; Squadrito, F.; Trichilo, V.; Valenti, A.; Barresi, V.; Altavilla, D.; et al. ROS and Brain Gliomas: An Overview of Potential and Innovative Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 984. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, Y.; Zheng, R.; Cheng, Y. Immune cell infiltration and drug response in glioblastoma multiforme: Insights from oxidative stress-related genes. Cancer Cell Int. 2024, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Zhu, Y.; Sun, S.-J.; Zhao, C.-J.; Bai, Y.; Wang, J.; Ma, L.-T. ROS regulation in gliomas: Implications for treatment strategies. Front. Immunol. 2023, 14, 1259797. [Google Scholar] [CrossRef] [PubMed]
- Ecemis, G.C.; Atmaca, A.; Meydan, D. Radiation-associated secondary brain tumors after conventional radiotherapy and radiosurgery. Expert Rev. Neurother. 2013, 13, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, R.; Vardon, A.; Akpalu, J.; Tampourlou, M.; Spiliotis, I.; Sbardella, E.; Lynch, J.; Shankaran, V.; Mavilakandy, A.; Gagliardi, I.; et al. Risk of second brain tumour after radiotherapy for pituitary adenoma or craniopharyngioma: A retrospective, multicentre, cohort study of 3679 patients with long-term imaging surveillance. Lancet Diabetes Endocrinol. 2022, 10, 581–588. [Google Scholar] [CrossRef]
- Todorova, P.K.; Fletcher-Sananikone, E.; Mukherjee, B.; Kollipara, R.; Vemireddy, V.; Xie, X.J.; Guida, P.M.; Story, M.D.; Hatanpaa, K.; Habib, A.A.; et al. Radiation-Induced DNA Damage Cooperates with Heterozygosity of TP53 and PTEN to Generate High-Grade Gliomas. Cancer Res. 2019, 79, 3749–3761. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Anikin, A.; Pearson, D.D. Chapter 33—Environmental Sources of Ionizing Radiation and Their Health Consequences. In Genome Stability; Kovalchuk, I., Kovalchuk, O., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 569–581. [Google Scholar]
- Meagher, M.J. Exposure of patients to ionizing radiation. What are the risks? Hawaii J. Med. Public Health 2012, 71, 309. [Google Scholar]
- Camacho, C.V.; Todorova, P.K.; Hardebeck, M.C.; Tomimatsu, N.; Gil del Alcazar, C.R.; Ilcheva, M.; Mukherjee, B.; McEllin, B.; Vemireddy, V.; Hatanpaa, K.; et al. DNA double-strand breaks cooperate with loss of Ink4 and Arf tumor suppressors to generate glioblastomas with frequent Met amplification. Oncogene 2015, 34, 1064–1072. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Whitehouse, J.P.; Howlett, M.; Federico, A.; Kool, M.; Endersby, R.; Gottardo, N.G. Defining the molecular features of radiation-induced glioma: A systematic review and meta-analysis. Neurooncol. Adv. 2021, 3, vdab109. [Google Scholar] [CrossRef]
- López, G.Y.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Clarke, J.; Oberheim Bush, N.A.; Taylor, J.; Chang, S.; et al. The genetic landscape of gliomas arising after therapeutic radiation. Acta Neuropathol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.H.; Chen, Z.H.; Liu, X.; Wu, Y.W.; Li, J.X.; Cao, Y.; Hei, T.K.; Tong, J. Oxidative damage in various tissues of rats exposed to radon. J. Toxicol. Environ. Health Part A 2012, 75, 694–699. [Google Scholar] [CrossRef]
- Abbasi, A.; Zakaly, H.M.H.; Hessien, M.M. Radon concentration in compressed natural gas and liquefied petroleum gas and its release range in residential houses. Radiochim. Acta 2021, 109, 793–798. [Google Scholar] [CrossRef]
- Lee, W.J.; Colt, J.S.; Heineman, E.F.; McComb, R.; Weisenburger, D.D.; Lijinsky, W.; Ward, M.H. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup. Environ. Med. 2005, 62, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Ruder, A.M.; Waters, M.A.; Butler, M.A.; Carreón, T.; Calvert, G.M.; Davis-King, K.E.; Schulte, P.A.; Sanderson, W.T.; Ward, E.M.; Connally, L.B.; et al. Gliomas and farm pesticide exposure in men: The upper midwest health study. Arch. Environ. Health 2004, 59, 650–657. [Google Scholar] [CrossRef]
- Gatto, N.M.; Ogata, P.; Lytle, B. Farming, Pesticides, and Brain Cancer: A 20-Year Updated Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Stowell, L. Pesticides in Farmed Animal Feed (A Study of World Animal Protection & Center for Biological Diversity). Available online: https://faunalytics.org/pesticides-in-farmed-animal-feed/# (accessed on 28 July 2025).
- Baldi, I.; De Graaf, L.; Bouvier, G.; Gruber, A.; Loiseau, H.; Meryet-Figuiere, M.; Rousseau, S.; Fabbro-Peray, P.; Lebailly, P. Occupational exposure to pesticides and central nervous system tumors: Results from the CERENAT case-control study. Cancer Causes Control 2021, 32, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Blair, A.; Hoppin, J.A.; Lubin, J.H.; Rusiecki, J.A.; Sandler, D.P.; Dosemeci, M.; Alavanja, M.C. Cancer incidence among pesticide applicators exposed to chlorpyrifos in the Agricultural Health Study. J. Natl. Cancer Inst. 2004, 96, 1781–1789. [Google Scholar] [CrossRef]
- Piel, C.; Pouchieu, C.; Carles, C.; Béziat, B.; Boulanger, M.; Bureau, M.; Busson, A.; Grüber, A.; Lecluse, Y.; Migault, L.; et al. Agricultural exposures to carbamate herbicides and fungicides and central nervous system tumour incidence in the cohort AGRICAN. Environ. Int. 2019, 130, 104876. [Google Scholar] [CrossRef]
- Purwanhono, A.; Tartila, J.; Firdaus, J. The Impact of Chronic Exposure to Organophosphate Pesticides on the Incidence of Primary Brain Tumors in Farmers: A Narrative Review. AKSONA 2024, 4, 43–53. [Google Scholar] [CrossRef]
- Bull, S.; Fletcher, K.; Boobis, A.R.; Battershill, J.M. Evidence for genotoxicity of pesticides in pesticide applicators: A review. Mutagenesis 2006, 21, 93–103. [Google Scholar] [CrossRef]
- Kapka-Skrzypczak, L.; Cyranka, M.; Skrzypczak, M.; Kruszewski, M. Biomonitoring and biomarkers of organophosphate pesticides exposure—State of the art. Ann. Agric. Environ. Med. 2011, 18, 294–303. [Google Scholar]
- Petit, P.; Gandon, G.; Chabardès, S.; Bonneterre, V. Agricultural activities and risk of central nervous system tumors among French farm managers: Results from the TRACTOR project. Int. J. Cancer 2022, 151, 1737–1749. [Google Scholar] [CrossRef]
- Yousefi, F.; Asadikaram, G.; Karamouzian, S.; Abolhassani, M.; Pourghadamyari, H.; Moazed, V.; Khanjani, N.; Paydar, P. Organochlorine and organophosphorus pesticides may induce brain cancer through oxidative stress. Toxicol. Ind. Health 2022, 38, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.B.; Islam, A.U.; Hassan, S.; Tarfeen, N.; Nisa, K.U.; Nisar, K.; Hassan, T.; Ganiee, S.A.; Bhat, A.R.; Ganai, B.A. Unraveling the toxic link between pesticides and brain cancer: A review on molecular mechanisms, signaling pathways and future research trends. Nucleus 2025, 1–16. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.K.; Rawat, S.S.; Jain, D.K.; Ghosh, S. Use of pesticides in agriculture and livestock animals and its impact on environment of India. Asian J. Environ. Sci. 2013, 8, 51–57. [Google Scholar]
- Natala, A.J.; Ochoje, O.S. Survey of pesticides used in the control of ectoparasites on farm animals in Kaduna State, Northern Nigeria. J. Anim. Plant Sci. 2009, 4, 276–280. [Google Scholar]
- Booth, B.J.; Jones, R.R.; Turyk, M.E.; Freels, S.; Patel, D.M.; Stayner, L.T.; Ward, M.H. Livestock and poultry density and childhood cancer incidence in nine states in the USA. Environ. Res. 2017, 159, 444–451. [Google Scholar] [CrossRef]
- Chen, S.; Gu, S.; Wang, Y.; Yao, Y.; Wang, G.; Jin, Y.; Wu, Y. Exposure to pyrethroid pesticides and the risk of childhood brain tumors in East China. Environ. Pollut. 2016, 218, 1128–1134. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef]
- Fetoui, H.; Feki, A.; Salah, G.B.; Kamoun, H.; Fakhfakh, F.; Gdoura, R. Exposure to lambda-cyhalothrin, a synthetic pyrethroid, increases reactive oxygen species production and induces genotoxicity in rat peripheral blood. Toxicol. Ind. Health 2015, 31, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.-A.; Rodríguez, J.-L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Anadón, A.; Ares, I. Oxidative stress and related gene expression effects of cyfluthrin in human neuroblastoma SH-SY5Y cells: Protective effect of melatonin. Environ. Res. 2019, 177, 108579. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Rácz, G.; Matsumoto, T.; Ádány, R.; Ádám, B. Evaluation of the genotoxicity of the pyrethroid insecticide phenothrin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 770, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, B.; Kong, B.; Wei, L.; Wang, R.; Zhou, C.; Shao, Y.; Lin, J.; Jin, Y.; Fu, Z. β-Cypermethrin and its metabolite 3-phenoxybenzoic acid exhibit immunotoxicity in murine macrophages. Acta Biochim. Biophys. Sin. 2017, 49, 1083–1091. [Google Scholar] [CrossRef]
- Doğanlar, O.; Doğanlar, Z.B.; Kurtdere, A.K.; Chasan, T.; Ok, E.S. Chronic exposure of human glioblastoma tumors to low concentrations of a pesticide mixture induced multidrug resistance against chemotherapy agents. Ecotoxicol. Environ. Saf. 2020, 202, 110940. [Google Scholar] [CrossRef]
- Fallahi, P.; Elia, G.; Foddis, R.; Cristaudo, A.; Antonelli, A. High risk of brain tumors in military personnel: A case control study. La Clin. Ter. 2017, 168, e376–e379. [Google Scholar] [CrossRef]
- Fereidouni, F.; Mohammadi, S.T.; Faramarzi Shahraki, V.; Jahantigh, F. Human Health Risk Assessment of 4-12 GHz Radar Waves using CST STUDIO SUITE Software. J. Biomed. Phys. Eng. 2022, 12, 285–296. [Google Scholar] [CrossRef]
- Peleg, M.; Berry, E.M.; Deitch, M.; Nativ, O.; Richter, E. On radar and radio exposure and cancer in the military setting. Environ. Res. 2023, 216, 114610. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Jourdan, N.; Aguida, B.; Dahon, C.; Baouz, S.; Terry, C.; Raad, H.; Ahmad, M. Exposure to 1.8 GHz radiofrequency field modulates ROS in human HEK293 cells as a function of signal amplitude. Commun. Integr. Biol. 2022, 15, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.; Deshmukh, P.S.; Banerjee, B.D.; Tripathi, A.K.; Ahmed, R.; Abegaonkar, M.P. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neurotoxicology 2015, 51, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Sawa, T.; Akaike, T. 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: Formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid. Redox Signal. 2006, 8, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Copp, T.R.A. Study Finds Higher Cancer Rates Among U.S. Military Airmen and Ground Crews. Available online: https://www.pbs.org/newshour/show/study-finds-higher-cancer-rates-among-u-s-military-airmen-and-ground-crews (accessed on 28 July 2025).
- Szmigielski, S. Cancer morbidity in subjects occupationally exposed to high frequency (radiofrequency and microwave) electromagnetic radiation. Sci. Total Environ. 1996, 180, 9–17. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.-Y.; Zhang, Y.; Park, S.-J.; Gu, J. Carbon-Based Radar Absorbing Materials toward Stealth Technologies. Adv. Sci. 2023, 10, 2303104. [Google Scholar] [CrossRef]
- Setua, D.K.; Mordina, B.; Srivastava, A.K.; Roy, D.; Eswara Prasad, N. Chapter 18—Carbon nanofibers-reinforced polymer nanocomposites as efficient microwave absorber. In Fiber-Reinforced Nanocomposites: Fundamentals and Applications; Han, B., Sharma, S., Nguyen, T.A., Longbiao, L., Bhat, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–430. [Google Scholar]
- Falcioni, L.; Bua, L.; Tibaldi, E.; Lauriola, M.; De Angelis, L.; Gnudi, F.; Mandrioli, D.; Manservigi, M.; Manservisi, F.; Manzoli, I.; et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ. Res. 2018, 165, 496–503. [Google Scholar] [CrossRef]
- Alkis, M.E.; Bilgin, H.M.; Akpolat, V.; Dasdag, S.; Yegin, K.; Yavas, M.C.; Akdag, M.Z. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn. Biol. Med. 2019, 38, 32–47. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. DNA single-strand break repair and human genetic disease. Trends Cell Biol. 2022, 32, 733–745. [Google Scholar] [CrossRef]
- Kloeber, J.A.; Lou, Z. Critical DNA damaging pathways in tumorigenesis. Semin. Cancer Biol. 2022, 85, 164–184. [Google Scholar] [CrossRef]
- Nassour, J.; Abbadie, C. A novel role for DNA single-strand breaks in senescence and neoplastic escape of epithelial cells. Mol. Cell Oncol. 2016, 3, e1190885. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; Pucko, E.B. Harnessing oxidative stress for anti-glioma therapy. Neurochem. Int. 2022, 154, 105281. [Google Scholar] [CrossRef]
- Little, M.P.; Rajaraman, P.; Curtis, R.E.; Devesa, S.S.; Inskip, P.D.; Check, D.P.; Linet, M.S. Mobile phone use and glioma risk: Comparison of epidemiological study results with incidence trends in the United States. BMJ 2012, 344, e1147. [Google Scholar] [CrossRef]
- Schüz, J.; Böhler, E.; Berg, G.; Schlehofer, B.; Hettinger, I.; Schlaefer, K.; Wahrendorf, J.; Kunna-Grass, K.; Blettner, M. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am. J. Epidemiol. 2006, 163, 512–520. [Google Scholar] [CrossRef]
- Yang, M.; Guo, W.; Yang, C.; Tang, J.; Huang, Q.; Feng, S.; Jiang, A.; Xu, X.; Jiang, G. Mobile phone use and glioma risk: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175136. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.L.; Miller, A.B.; Sasco, A.; Davis, D.L. Mobile phone radiation causes brain tumors and should be classified as a probable human carcinogen (2A) (Review). Int. J. Oncol. 2015, 46, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E. The Number of Smartphone Plans Has Doubled to 6 Billion in Last 5 Years. Available online: https://www.pcmag.com/news/smartphone-subs-have-doubled-to-6-billion-in-last-five-years (accessed on 28 July 2025).
- Silver, D.J.; Roversi, G.A.; Bithi, N.; Wang, S.Z.; Troike, K.M.; Neumann, C.K.A.; Ahuja, G.K.; Reizes, O.; Brown, J.M.; Hine, C.; et al. Severe consequences of a high-lipid diet include hydrogen sulfide dysfunction and enhanced aggression in glioblastoma. J. Clin. Investig. 2021, 131, e138276. [Google Scholar] [CrossRef]
- Kuan, A.S.; Green, J.; Kitahara, C.M.; De González, A.B.; Key, T.; Reeves, G.K.; Floud, S.; Balkwill, A.; Bradbury, K.; Liao, L.M.; et al. Diet and risk of glioma: Combined analysis of 3 large prospective studies in the UK and USA. Neuro-Oncology 2019, 21, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ward, M.H.; Tucker, K.L.; Graubard, B.I.; McComb, R.D.; Potischman, N.A.; Weisenburger, D.D.; Heineman, E.F. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control 2002, 13, 647–655. [Google Scholar] [CrossRef]
- Shu, L.; Yu, D.; Jin, F. Healthy dietary patterns, foods, and risk of glioma: A systematic review and meta-analysis of observational studies. Front. Nutr. 2022, 9, 1077452. [Google Scholar] [CrossRef] [PubMed]
- Almasi, F.; Nemati, M.; Aminianfar, A. Dietary Recommendations for Glioma: A Mini-Review. Curr. Nutr. Rep. 2024, 13, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhong, X.; Xu, L.; Han, W. Association between Dietary Vitamin A Intake and the Risk of Glioma: Evidence from a Meta-analysis. Nutrients 2015, 7, 8897–8904. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Kang, X.; Wang, C.; Chen, F.; Kang, Z.; Yang, S.; Zhang, R.; Peng, Y.; Li, W. Association between dietary minerals and glioma: A case-control study based on Chinese population. Front. Nutr. 2023, 10, 1118997. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, J.; Kang, X.; Wang, C.; Chen, F.; Zhang, B.; Li, S.; Huang, S.; Li, W. Dietary B vitamins and glioma: A case-control study based on Chinese population. Front. Nutr. 2023, 10, 1122540. [Google Scholar] [CrossRef]
- Cote, D.J.; Bever, A.M.; Wilson, K.M.; Smith, T.R.; Smith-Warner, S.A.; Stampfer, M.J. A prospective study of tea and coffee intake and risk of glioma. Int. J. Cancer 2020, 146, 2442–2449. [Google Scholar] [CrossRef]
- Creed, J.H.; Smith-Warner, S.A.; Gerke, T.A.; Egan, K.M. A prospective study of coffee and tea consumption and the risk of glioma in the UK Biobank. Eur. J. Cancer 2020, 129, 123–131. [Google Scholar] [CrossRef]
- Holick, C.N.; Smith, S.G.; Giovannucci, E.; Michaud, D.S. Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol. Biomark. Prev. 2010, 19, 39–47. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Jin, Y.; Guo, J. Association between tea and coffee consumption and brain cancer risk: An updated meta-analysis. World J. Surg. Oncol. 2019, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, J.; Markiewicz-Żukowska, R. The Influence of Nutritional and Lifestyle Factors on Glioma Incidence. Nutrients 2020, 12, 1812. [Google Scholar] [CrossRef]
- Shao, C.; Tang, H.; Wang, X.; He, J.; Wang, P.; Wu, N. Body mass index and glioma risk: A prospective multicenter study. Front. Endocrinol. 2022, 13, 933921. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.K.H.; Brunborg, C.; Di Ieva, A.; Lindemann, K.; Johannesen, T.B.; Vatten, L.; Helseth, E.; Zwart, J.A. The impact of body mass index and height on the risk for glioblastoma and other glioma subgroups: A large prospective cohort study. Neuro-Oncology 2016, 19, 976–985. [Google Scholar] [CrossRef]
- Velásquez-Meléndez, G.; Martins, I.S.; Cervato, A.M.; Fornés, N.S.; Marucci, M.F.; Coelho, L.T. Relationship between stature, overweight and central obesity in the adult population in São Paulo, Brazil. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 639–644. [Google Scholar] [CrossRef]
- Kabat, G.C.; Rohan, T.E. Adiposity at different periods of life and risk of adult glioma in a cohort of postmenopausal women. Cancer Epidemiol. 2018, 54, 71–74. [Google Scholar] [CrossRef]
- Niedermaier, T.; Behrens, G.; Schmid, D.; Schlecht, I.; Fischer, B.; Leitzmann, M.F. Body mass index, physical activity, and risk of adult meningioma and glioma. Neurology 2015, 85, 1342–1350. [Google Scholar] [CrossRef]
- Ahn, S.; Han, K.; Lee, J.E.; Jeun, S.S.; Park, Y.M.; Yang, S.H. Associations of General and Abdominal Obesity with the Risk of Glioma Development. Cancers 2021, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Li, S.; Huang, M.; Zhang, R.; Chen, Y.; Kang, Z.; Li, W. Causal effect between circulating metabolic markers and glioma: A bidirectional, two-sample, Bayesian weighted Mendelian randomization. Discov. Oncol. 2025, 16, 315. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Pedraza-Chaverri, J.; Huerta-Yepez, S. Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sci. 2020, 10, 381. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Szema, A. Burn Pits Exposure and Chronic Respiratory Illnesses among Iraq and Afghanistan Veterans. J. Environ. Health Sci. 2019, 5, 13–14. [Google Scholar] [CrossRef]
- Weichenthal, S.; Olaniyan, T.; Christidis, T.; Lavigne, E.; Hatzopoulou, M.; Van Ryswyk, K.; Tjepkema, M.; Burnett, R. Within-city Spatial Variations in Ambient Ultrafine Particle Concentrations and Incident Brain Tumors in Adults. Epidemiology 2020, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hassanipour, S.; Nikbakht, H.A.; Amrane, A.; Arab-Zozani, M.; Shojaie, L.; Rostami, S.; Badeenezhad, A. The Relationship between Air Pollution and Brain Cancer: A Systematic Review and Meta-Analysis. Ann. Glob. Health 2023, 89, 45. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Glass, D.C.; Reid, A.; de Klerk, N.; Armstrong, B.K.; Kellie, S.; Ashton, L.J.; Milne, E.; Fritschi, L. Parental occupational exposure to engine exhausts and childhood brain tumors. Int. J. Cancer 2013, 132, 2975–2979. [Google Scholar] [CrossRef]
- Liati, A.; Schreiber, D.; Arroyo Rojas Dasilva, Y.; Dimopoulos Eggenschwiler, P. Ultrafine particle emissions from modern Gasoline and Diesel vehicles: An electron microscopic perspective. Environ. Pollut. 2018, 239, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T. Diesel Exhaust and Lung Cancer-Aftermath of Becoming an IARC Group 1 Carcinogen. Am. J. Epidemiol. 2018, 187, 1149–1152. [Google Scholar] [CrossRef]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Geels, C.; Christensen, J.H.; Raaschou-Nielsen, O. Intracranial tumors of the central nervous system and air pollution—A nationwide case-control study from Denmark. Environ. Health 2020, 19, 81. [Google Scholar] [CrossRef]
- Gómez-Budia, M.; Konttinen, H.; Saveleva, L.; Korhonen, P.; Jalava, P.I.; Kanninen, K.M.; Malm, T. Glial smog: Interplay between air pollution and astrocyte-microglia interactions. Neurochem. Int. 2020, 136, 104715. [Google Scholar] [CrossRef]
- Bunin, G.R.; Buckley, J.D.; Boesel, C.P.; Rorke, L.B.; Meadows, A.T. Risk factors for astrocytic glioma and primitive neuroectodermal tumor of the brain in young children: A report from the Children’s Cancer Group. Cancer Epidemiol. Biomark. Prev. 1994, 3, 197–204. [Google Scholar]
- Ji, X.; Alakel, A.; Ghazawi, F.M.; Tsang, M.; Zubarev, A.; Lasry, O.J.; Litvinov, I.V. Investigation of incidence and geographic distribution of gliomas in Canada from 1992 to 2010: A national population-based study highlighting the importance of exposure to airport operations. Front. Oncol. 2023, 13, 1190366. [Google Scholar] [CrossRef]
- Cannataro, V.L.; Bracci, P.M.; Taylor, J.W.; McCoy, L.; Rice, T.; Hansen, H.M.; Heffernan, A.E.; Wiemels, J.; Wiencke, J.; Wrensch, M.; et al. Glioma mutational signatures associated with haloalkane exposure are enriched in firefighters. Cancer 2025, 131, e35732. [Google Scholar] [CrossRef]
- Pronin, S.; Koh, C.H.; Hughes, M. Cytotoxicity of ultraviolet-C radiation on a heterogeneous population of human glioblastoma multiforme cells: Meta-analysis. Photodiagn. Photodyn. Ther. 2018, 24, 158–163. [Google Scholar] [CrossRef]
- Karipidis, K.K.; Benke, G.; Sim, M.R.; Kauppinen, T.; Giles, G. Occupational exposure to ionizing and non-ionizing radiation and risk of glioma. Occup. Med. 2007, 57, 518–524. [Google Scholar] [CrossRef]
- Efird, J.T. Season of Birth and Risk for Adult Onset Glioma. Int. J. Environ. Res. Public Health 2010, 7, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Caffo, M.; Caruso, G.; Fata, G.L.; Barresi, V.; Visalli, M.; Venza, M.; Venza, I. Heavy metals and epigenetic alterations in brain tumors. Curr. Genom. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Caruso, G.; Nanni, A.; Curcio, A.; Lombardi, G.; Somma, T.; Minutoli, L.; Caffo, M. Impact of Heavy Metals on Glioma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 15432. [Google Scholar] [CrossRef]
- Singh, P.; Mitra, P.; Goyal, T.; Sharma, S.; Sharma, P. Blood lead and cadmium levels in occupationally exposed workers and their effect on markers of DNA damage and repair. Environ. Geochem. Health 2021, 43, 185–193. [Google Scholar] [CrossRef]
- Ahn, J.; Park, M.Y.; Kang, M.Y.; Shin, I.S.; An, S.; Kim, H.R. Occupational Lead Exposure and Brain Tumors: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3975. [Google Scholar] [CrossRef]
- Anttila, A.; Heikkilä, P.; Nykyri, E.; Kauppinen, T.; Pukkala, E.; Hernberg, S.; Hemminki, K. Risk of nervous system cancer among workers exposed to lead. J. Occup. Environ. Med. 1996, 38, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Dosemeci, M.; Heineman, E.F. Brain cancer and occupational exposure to lead. J. Occup. Environ. Med. 1998, 40, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.E.; Turner, M.C.; Lavoué, J.; Richard, H.; Figuerola, J.; Kincl, L.; Richardson, L.; Benke, G.; Blettner, M.; Fleming, S.; et al. Lifetime occupational exposure to metals and welding fumes, and risk of glioma: A 7-country population-based case-control study. Environ. Health 2017, 16, 90. [Google Scholar] [CrossRef]
- Wätjen, W.; Beyersmann, D. Cadmium-induced apoptosis in C6 glioma cells: Influence of oxidative stress. Biomet. Int. J. Role Met. Ions Biol. Biochem. Med. 2004, 17, 65–78. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Huang, G.-L.; Lin, Z.-Y.; Sun, X.-F.; Wu, C.-C.; Liu, Y.-W.; Liu, L.-Y.; Zeng, E.Y. Insufficient evidence to link human exposure to heavy metals with biomarkers of glioma. J. Hazard. Mater. 2023, 447, 130779. [Google Scholar] [CrossRef]
- Aaseth, J.; Hilt, B.; Bjørklund, G. Mercury exposure and health impacts in dental personnel. Environ. Res. 2018, 164, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Vivarelli, F.; Morosini, C.; Canistro, D.; Paolini, M.; Fairclough, L.C. Toxicological Aspects Associated with Consumption from Electronic Nicotine Delivery System (ENDS): Focus on Heavy Metals Exposure and Cancer Risk. Int. J. Mol. Sci. 2024, 25, 2737. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, A.; Vujotić, L.; Rovčanin, B.; Borković-Mitić, S.; Gavrović-Jankulović, M.; Manojlović, D. Assessment of trace metal alterations in the blood, cerebrospinal fluid and tissue samples of patients with malignant brain tumors. Sci. Rep. 2020, 10, 3816. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Wu, J.; Kong, S.; Yan, Y.; Cheng, Y.; Yan, Q.; Liu, D.; Wang, S.; Zhang, X.; Qi, S. The toxicity emissions and spatialized health risks of heavy metals in PM2.5 from biomass fuels burning. Atmos. Environ. 2022, 284, 119178. [Google Scholar] [CrossRef]
- Mohr, S.B.; Gorham, E.D.; Garland, C.F.; Grant, W.B.; Garland, F.C. Low ultraviolet B and increased risk of brain cancer: An ecological study of 175 countries. Neuroepidemiology 2010, 35, 281–290. [Google Scholar] [CrossRef]
- Olsson, F.; Sarri, N.; Papadopoulos, N.; Lennartsson, J.; Norlin, M. Effects of 1α,25-dihydroxyvitamin D(3) and tacalcitol on cell signaling and anchorage-independent growth in T98G and U251 glioblastoma cells. Biochem. Biophys. Rep. 2022, 31, 101313. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Xu, H.; Qin, Z. Geographic Variations in the Incidence of Glioblastoma and Prognostic Factors Predictive of Overall Survival in US Adults from 2004–2013. Front. Aging Neurosci. 2017, 9, 352. [Google Scholar] [CrossRef]
- Campen, C.J.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis Type 1. J. Child Neurol. 2018, 33, 73–81. [Google Scholar] [CrossRef]
- da Silva, B.; Fine, H.A. Optic nerve activity promotes the growth of optic pathway gliomas: Shedding light on the glioma microenvironment. Cancer Cell 2021, 39, 1056–1058. [Google Scholar] [CrossRef]
- Pan, Y.; Hysinger, J.D.; Barron, T.; Schindler, N.F.; Cobb, O.; Guo, X.; Yalçın, B.; Anastasaki, C.; Mulinyawe, S.B.; Ponnuswami, A.; et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 2021, 594, 277–282. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Orešković, D.; Kaštelančić, A.; Raguž, M.; Dlaka, D.; Predrijevac, N.; Matec, D.; Matec, M.; Tomac, D.; Jeleč, V.; Marinović, T.; et al. The vicious interplay between disrupted sleep and malignant brain tumors: A narrative review. Croat. Med. J. 2021, 62, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Kawamura, M.; Iima, M.; Ueda, D.; Ito, R.; Saida, T.; Kurokawa, R.; Takumi, K.; Sakata, A.; Ide, S.; et al. The glymphatic system in oncology: From the perspective of a radiation oncologist. J. Radiat. Res. 2025, 66, 343–353. [Google Scholar] [CrossRef]
- Central_Intelligence_Agency. The World Factbook; CIA: Langley, VA, USA, 2018. [Google Scholar]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the brain and CNS: Global patterns and trends in incidence. Neuro-Oncology 2016, 19, 270–280. [Google Scholar] [CrossRef]
- Alkire, S.C.M.; Conconi, A.; Seth, S.; Vaz, A. Poverty in Rural and Urban Areas. Available online: https://ophi.org.uk/global-mpi/2014 (accessed on 28 July 2025).
- Suttie, D. Overview: Rural Poverty in Developing Countries: Issues, Policies and Challenges. Available online: https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/03/Suttie-Paper.pdf (accessed on 28 July 2025).
- Vera-Toscano, E.; Shucksmith, M.; Brown, D.L.; Brown, H. The rural–urban poverty gap in England after the 2008 financial crisis: Exploring the effects of budgetary cuts and welfare reforms. Reg. Stud. 2024, 58, 1264–1281. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. Brief to the State of Food Security and Nutrition in the World 2024—Financing to End Hunger, Food Insecurity and Malnutrition in All Its Forms; FAO: Rome, Italy, 2024. [Google Scholar]
- Parker, M.A.; Weinberger, A.H.; Eggers, E.M.; Parker, E.S.; Villanti, A.C. Trends in Rural and Urban Cigarette Smoking Quit Ratios in the US From 2010 to 2020. JAMA Netw. Open 2022, 5, e2225326. [Google Scholar] [CrossRef]
- Tagkas, C.F.; Rizos, E.C.; Markozannes, G.; Karalexi, M.A.; Wairegi, L.; Ntzani, E.E. Fertilizers and Human Health—A Systematic Review of the Epidemiological Evidence. Toxics 2024, 12, 694. [Google Scholar] [CrossRef]
- Moses-Otutu, I.M.; Ojo, N.F.; Nzoputam, O.J.; Nzoputam, C.I. Seroprevalence of Human Cytomegalovirus Infection among HIV Patients in Edo State, Southern Nigeria. Venereology 2023, 2, 164–172. [Google Scholar] [CrossRef]
- Sapienza, A.; Lítlá, M.; Lehmann, S.; Alessandretti, L. Exposure to urban and rural contexts shapes smartphone usage behavior. PNAS Nexus 2023, 2, pgad357. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Ostrom, Q.T.; Gittleman, H.; Duncan, K.R.; CreveCoeur, T.S.; Kruchko, C.; Smith, T.R.; Stampfer, M.J.; Barnholtz-Sloan, J.S. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer 2019, 125, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Gorenflo, M.P.; Shen, A.; Murphy, E.S.; Cullen, J.; Yu, J.S. Area-level socioeconomic status is positively correlated with glioblastoma incidence and prognosis in the United States. Front. Oncol. 2023, 13, 1110473. [Google Scholar] [CrossRef]
- Bower, A.; Hsu, F.C.; Weaver, K.E.; Yelton, C.; Merrill, R.; Wicks, R.; Soike, M.; Hutchinson, A.; McTyre, E.; Laxton, A.; et al. Community economic factors influence outcomes for patients with primary malignant glioma. Neurooncol. Pract. 2020, 7, 453–460. [Google Scholar] [CrossRef]
- Söderlund, M.; Almqvist, C.; Sjöström, O.; Dahlin, A.M.; Sjöström, S.; Numan Hellquist, B.; Melin, B.; Sandström, M. The impact of socioeconomic status on glioma survival: A retrospective analysis. Cancer Causes Control 2025, 36, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Arsene, D.E.; Milanesi, E.; Dobre, M. Viral oncogenesis in tumours of the central nervous system: Reality or random association? A retrospective study on archived material. J. Cell. Mol. Med. 2022, 26, 1413–1420. [Google Scholar] [CrossRef]
- Gorriceta, J.H.; Lopez Otbo, A.; Uehara, G.; Posadas Salas, M.A. BK viral infection: A review of management and treatment. World J. Transpl. 2023, 13, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, T.; Enchev, Y.; Tsekov, I.; Simeonov, P.; Kalvatchev, Z.; Encheva, E. JC polyomavirus in the aetiology and pathophysiology of glial tumours. Neurosurg. Rev. 2016, 39, 47–53. [Google Scholar] [CrossRef]
- Abend, J.R.; Jiang, M.; Imperiale, M.J. BK virus and human cancer: Innocent until proven guilty. Semin. Cancer Biol. 2009, 19, 252–260. [Google Scholar] [CrossRef]
- Akhtar, S.; Vranic, S.; Cyprian, F.S.; Al Moustafa, A.-E. Epstein–Barr Virus in Gliomas: Cause, Association, or Artifact? Front. Oncol. 2018, 8, 123. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Faranoush, M.; Naderi, A.; Kiani, S.J.; Sadeghipour, A.; Javanmard, D.; Farahmand, M.; Ghorbani, S.; Sedaghati, F.; et al. Molecular Investigation of Human Cytomegalovirus and Epstein-Barr virus in Glioblastoma Brain Tumor: A Case-Control Study in Iran. Iran. Biomed. J. 2021, 25, 426–433. [Google Scholar] [CrossRef]
- Limam, S.; Missaoui, N.; Mestiri, S.; Yacoubi, M.T.; Krifa, H.; Selmi, B.; Mokni, M. Epstein-Barr virus infection in gliomas. Curr. Res. Transl. Med. 2019, 67, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, S.; Jha, H.C. Glial cell response to Epstein-Barr Virus infection: A plausible contribution to virus-associated inflammatory reactions in the brain. Virology 2021, 559, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Vega, S.; Palma-Lara, I.; Ortega-Soto, E.; Trejo-Solis, C.; de Arellano, I.T.; Ucharima-Corona, L.E.; Garcia-Chacón, G.; Ochoa, S.A.; Xicohtencatl-Cortes, J.; Cruz-Córdova, A.; et al. Role of Epstein-Barr Virus in Glioblastoma. Crit. Rev. Oncog. 2019, 24, 307–338. [Google Scholar] [CrossRef]
- Amirian, E.S.; Scheurer, M.E. Chromosomally-integrated human herpesvirus 6 in familial glioma etiology. Med. Hypotheses 2012, 79, 193–196. [Google Scholar] [CrossRef]
- Vidone, M.; Alessandrini, F.; Marucci, G.; Farnedi, A.; de Biase, D.; Ricceri, F.; Calabrese, C.; Kurelac, I.; Porcelli, A.M.; Cricca, M.; et al. Evidence of association of human papillomavirus with prognosis worsening in glioblastoma multiforme. Neuro-Oncology 2014, 16, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, S.; Li, X.; Chen, F.; Li, W. Viral infection and glioma: A meta-analysis of prognosis. BMC Cancer 2020, 20, 549. [Google Scholar] [CrossRef]
- Fernanda, M.; Balatti, V.; Francesco, L.; Massimo, G.; Tognon, M. Glioblastoma in a Patient with Professional Exposure to the Oncogenic Polyomavirus SV40. J. Cancer Mol. 2007, 3, 29–32. [Google Scholar]
- Rotondo, J.C.; Mazzoni, E.; Bononi, I.; Tognon, M.; Martini, F. Association Between Simian Virus 40 and Human Tumors. Front. Oncol. 2019, 9, 670. [Google Scholar] [CrossRef]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; McGregor Dallas, S.R.; Smit, M.; Soroceanu, L.; Cobbs, C.S. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology 2012, 14, 246–255. [Google Scholar] [CrossRef]
- Farias, K.P.R.A.; Moreli, M.L.; Floriano, V.G.; da Costa, V.G. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch. Virol. 2019, 164, 1249–1257. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Srinivasan, V.M.; Ghali, M.G.; Heimberger, A.B. Cytomegalovirus-targeted immunotherapy and glioblastoma: Hype or hope? Immunotherapy 2016, 8, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E. Cytomegalovirus and glioblastoma; controversies and opportunities. J. Neurooncol. 2015, 123, 465–471. [Google Scholar] [CrossRef]
- Krenzlin, H.; Behera, P.; Lorenz, V.; Passaro, C.; Zdioruk, M.; Nowicki, M.O.; Grauwet, K.; Zhang, H.; Skubal, M.; Ito, H.; et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Investig. 2019, 129, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Krenzlin, H.; Zdioruk, M.; Nowicki, M.O.; Finkelberg, T.; Keric, N.; Lemmermann, N.; Skubal, M.; Chiocca, E.A.; Cook, C.H.; Lawler, S.E. Cytomegalovirus infection of glioblastoma cells leads to NF-κB dependent upregulation of the c-MET oncogenic tyrosine kinase. Cancer Lett. 2021, 513, 26–35. [Google Scholar] [CrossRef]
- Ahn, J.; Shin, C.; Kim, Y.S.; Park, J.S.; Jeun, S.S.; Ahn, S. Cytomegalovirus-Specific Immunotherapy for Glioblastoma Treatments. Brain Tumor Res. Treat. 2022, 10, 135–143. [Google Scholar] [CrossRef]
- Yang, T.; Liu, D.; Fang, S.; Ma, W.; Wang, Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. J. Clin. Med. 2022, 11, 5221. [Google Scholar] [CrossRef]
- Lehrer, S. Anopheles mosquito transmission of brain tumor. Med. Hypotheses 2010, 74, 167–168. [Google Scholar] [CrossRef]
- Chauhan, L.; Matthews, E.; Piquet, A.L.; Henao-Martinez, A.; Franco-Paredes, C.; Tyler, K.L.; Beckham, D.; Pastula, D.M. Nervous System Manifestations of Arboviral Infections. Curr. Trop. Med. Rep. 2022, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Hatiboglu, M.A. Can COVID-19 induce glioma tumorogenesis through binding cell receptors? Med. Hypotheses 2020, 144, 110009. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, V.; Chinot, O.; Cabaniols, C.; Giorgi, R.; Alla, P.; Lehucher-Michel, M.P. Occupational and environmental risk factors for brain cancer: A pilot case-control study in France. Presse Med. 2010, 39, e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ramiro, A.; Ramírez-Ortega, D.; Pérez de la Cruz, V.; Hérnandez-Pedro, N.Y.; González-Esquivel, D.F.; Sotelo, J.; Pineda, B. Role of Redox Status in Development of Glioblastoma. Front. Immunol. 2016, 7, 156. [Google Scholar] [CrossRef]
- Shao, C.; Zhao, W.; Qi, Z.; He, J. Smoking and Glioma Risk: Evidence From a Meta-Analysis of 25 Observational Studies. Medicine 2016, 95, e2447. [Google Scholar] [CrossRef]
- Rumrich, I.K.; Viluksela, M.; Vähäkangas, K.; Gissler, M.; Surcel, H.M.; Hänninen, O. Maternal Smoking and the Risk of Cancer in Early Life—A Meta-Analysis. PLoS ONE 2016, 11, e0165040. [Google Scholar] [CrossRef]
- Nguyen-Grozavu, F.T.; Pierce, J.P.; Sakuma, K.K.; Leas, E.C.; McMenamin, S.B.; Kealey, S.; Benmarhnia, T.; Emery, S.L.; White, M.M.; Fagan, P.; et al. Widening disparities in cigarette smoking by race/ethnicity across education level in the United States. Prev. Med. 2020, 139, 106220. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Xiaochen, D.; Emmanuela, G.; Alan, D.L. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control 2022, 31, 129. [Google Scholar] [CrossRef]
- Ahn, S.; Han, K.D.; Park, Y.M.; Bae, J.M.; Kim, S.U.; Jeun, S.S.; Yang, S.H. Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study. Cancers 2020, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Hajdusianek, W.; Żórawik, A.; Waliszewska-Prosół, M.; Poręba, R.; Gać, P. Tobacco and Nervous System Development and Function-New Findings 2015–2020. Brain Sci. 2021, 11, 797. [Google Scholar] [CrossRef]
- Seo, Y.S.; Park, J.M.; Kim, J.H.; Lee, M.Y. Cigarette Smoke-Induced Reactive Oxygen Species Formation: A Concise Review. Antioxidants 2023, 12, 1732. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Wu, K.; El Zowalaty, A.E.; Sayin, V.I.; Papagiannakopoulos, T. The pleiotropic functions of reactive oxygen species in cancer. Nat. Cancer 2024, 5, 384–399. [Google Scholar] [CrossRef]
- Andersen, L.; Friis, S.; Hallas, J.; Ravn, P.; Kristensen, B.W.; Gaist, D. Hormonal contraceptive use and risk of glioma among younger women: A nationwide case-control study. Br. J. Clin. Pharmacol. 2015, 79, 677–684. [Google Scholar] [CrossRef]
- You, Q.; Liang, F.; Wu, G.; Cao, F.; Liu, J.; He, Z.; Wang, C.; Zhu, L.; Chen, X.; Yang, Y. The Landscape of Biomimetic Nanovesicles in Brain Diseases. Adv. Mater. 2023, 36, e2306583. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; You, Q.; Ye, H.; Fu, W.; Ma, X.; Tan, J.; Ma, Y.; Wang, C.; Yang, Y.; He, Z.; et al. Nanomaterials for visualized tumor surgical navigation and postoperative recurrence inhibition. Nano Res. 2023, 16, 13226–13249. [Google Scholar] [CrossRef]
- Wei, Y.; Song, X.; Gao, Y.; Gao, Y.; Li, Y.; Gu, L. Iron toxicity in intracerebral hemorrhage: Physiopathological and therapeutic implications. Brain Res. Bull. 2022, 178, 144–154. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, L.; Wang, C.; Yang, Y.; He, Z. BSA-MnO2-SAL multifunctional nanoparticle-mediated M(1) macrophages polarization for glioblastoma therapy. RSC Adv. 2021, 11, 35331–35341. [Google Scholar] [CrossRef]
- Devaraji, M.; Thanikachalam, P.V.; Elumalai, K. The potential of copper oxide nanoparticles in nanomedicine: A comprehensive review. Biotechnol. Notes 2024, 5, 80–99. [Google Scholar] [CrossRef]
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Ohno, M.; Miyakita, Y.; Takahashi, M.; Yanagisawa, S.; Omura, T.; Yoshida, A.; Kubo, Y.; Igaki, H.; Ichimura, K.; et al. Early Diagnosis and Surgical Intervention Within 3 Weeks from Symptom Onset Are Associated With Prolonged Survival of Patients With Glioblastoma. Neurosurgery 2022, 91, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Szmigielski, S. Cancer risks related to low-level RF/MW exposures, including cell phones. Electromagn. Biol. Med. 2013, 32, 273–280. [Google Scholar] [CrossRef]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of sex in the prevalence and progression of glioblastomas: The role of gonadal steroid hormones. Biol. Sex Differ. 2021, 12, 28. [Google Scholar] [CrossRef]
- Rossi, J.; Zedde, M.; Napoli, M.; Pascarella, R.; Pisanello, A.; Biagini, G.; Valzania, F. Impact of Sex Hormones on Glioblastoma: Sex-Related Differences and Neuroradiological Insights. Life 2024, 14, 1523. [Google Scholar] [CrossRef] [PubMed]
- Travers, S.; Litofsky, N.S. Daily Lifestyle Modifications to Improve Quality of Life and Survival in Glioblastoma: A Review. Brain Sci. 2021, 11, 533. [Google Scholar] [CrossRef]
- Kampers, L.F.C.; Metselaar, D.S.; Vinci, M.; Scirocchi, F.; Veldhuijzen van Zanten, S.; Eyrich, M.; Biassoni, V.; Hulleman, E.; Karremann, M.; Stücker, W.; et al. The Complexity of Malignant Glioma Treatment. Cancers 2025, 17, 879. [Google Scholar] [CrossRef]
- Sojka, C.; Sloan, S.A. Gliomas: A reflection of temporal gliogenic principles. Commun. Biol. 2024, 7, 156. [Google Scholar] [CrossRef]
- Wishart, D. Metabolomics and the Multi-Omics View of Cancer. Metabolites 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Kucab, J.E.; Zou, X.; Morganella, S.; Joel, M.; Nanda, A.S.; Nagy, E.; Gomez, C.; Degasperi, A.; Harris, R.; Jackson, S.P.; et al. A Compendium of Mutational Signatures of Environmental Agents. Cell 2019, 177, 821–836.e816. [Google Scholar] [CrossRef]
- Thong, T.; Forté, C.A.; Hill, E.M.; Colacino, J.A. Environmental exposures, stem cells, and cancer. Pharmacol. Ther. 2019, 204, 107398. [Google Scholar] [CrossRef]
- Stingone, J.A.; Geller, A.M.; Hood, D.B.; Makris, K.C.; Mouton, C.P.; States, J.C.; Sumner, S.J.; Wu, K.L.; Rajasekar, A.K. Community-level exposomics: A population-centered approach to address public health concerns. Exposome 2023, 3, osad009. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, D.; Marquès, M.; Pons-Escoda, A.; Vidal, N.; Bruna, J.; Restrepo-Montes, E.; López-Serna, R.; García-Sayago, F.; Majos, C.; Gago-Ferrero, P.; et al. Tumoral and normal brain tissue extraction protocol for wide-scope screening of organic pollutants. MethodsX 2023, 10, 102069. [Google Scholar] [CrossRef]
- Young, A.S.; Mullins, C.E.; Sehgal, N.; Vermeulen, R.C.H.; Kolijn, P.M.; Vlaanderen, J.; Rahman, M.L.; Birmann, B.M.; Barupal, D.; Lan, Q.; et al. The need for a cancer exposome atlas: A scoping review. JNCI Cancer Spectr. 2025, 9, pkae122. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.I.; Wuest, A.N.; Dong, K.; Johnson, G.A.; Hsu, A.; Narendra, V.K.; Atwa, O.; Levine, S.S.; Liu, D.R.; Sánchez Rivera, F.J. High-throughput evaluation of genetic variants with prime editing sensor libraries. Nat. Biotechnol. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Gould, S.I.; Sánchez-Rivera, F.J. Deconstructing cancer with precision genome editing. Biochem. Soc. Trans. 2024, 52, 803–819. [Google Scholar] [CrossRef] [PubMed]
- States, J.C.; Ouyang, M.; Helm, C.W. Systems approach to identify environmental exposures contributing to organ-specific carcinogenesis. Cancer Epidemiol. 2014, 38, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Mamun, A.A.; Alghamdi, B.S.; Tewari, D.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M. Epigenetics of glioblastoma multiforme: From molecular mechanisms to therapeutic approaches. Semin. Cancer Biol. 2022, 83, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Jażdżewska, A.; Kozłowski, J.; Zacny, A.; Lorenc, T.; Olejarz, W. Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5774. [Google Scholar] [CrossRef]
- Sakata, T.; Tanikawa, M.; Yamada, H.; Fujinami, R.; Nishikawa, Y.; Yamada, S.; Mase, M. Minimally invasive treatment for glioblastoma through endoscopic surgery including tumor embolization when necessary: A technical note. Front. Neurol. 2023, 14, 1170045. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowski, R.P.; Acewicz, A.; He, Z.; Pucko, E.B.; Godlewski, J. Environmental Hazards and Glial Brain Tumors: Association or Causation? Int. J. Mol. Sci. 2025, 26, 7425. https://doi.org/10.3390/ijms26157425
Ostrowski RP, Acewicz A, He Z, Pucko EB, Godlewski J. Environmental Hazards and Glial Brain Tumors: Association or Causation? International Journal of Molecular Sciences. 2025; 26(15):7425. https://doi.org/10.3390/ijms26157425
Chicago/Turabian StyleOstrowski, Robert P., Albert Acewicz, Zhaohui He, Emanuela B. Pucko, and Jakub Godlewski. 2025. "Environmental Hazards and Glial Brain Tumors: Association or Causation?" International Journal of Molecular Sciences 26, no. 15: 7425. https://doi.org/10.3390/ijms26157425
APA StyleOstrowski, R. P., Acewicz, A., He, Z., Pucko, E. B., & Godlewski, J. (2025). Environmental Hazards and Glial Brain Tumors: Association or Causation? International Journal of Molecular Sciences, 26(15), 7425. https://doi.org/10.3390/ijms26157425






