A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy
Abstract
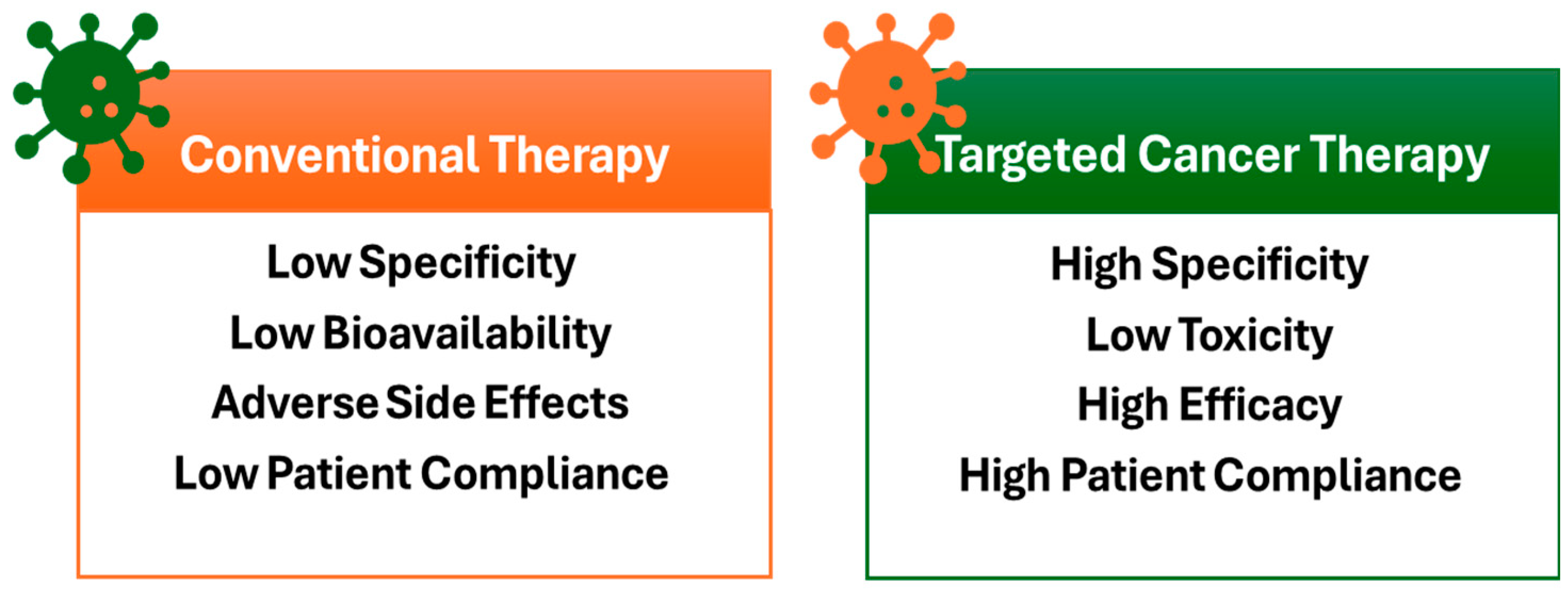
1. Introduction
2. Temperature as a Triggering Mechanism
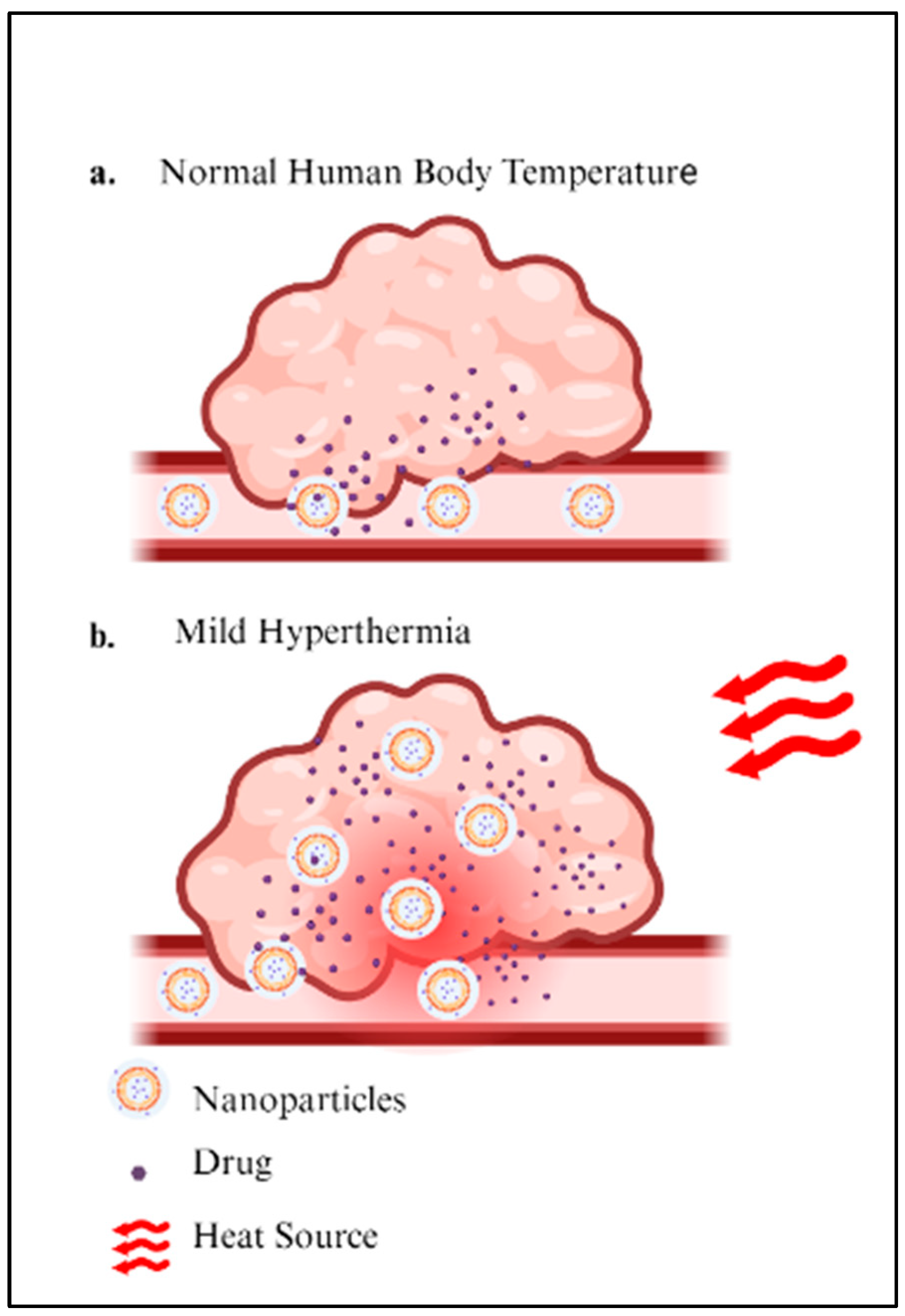
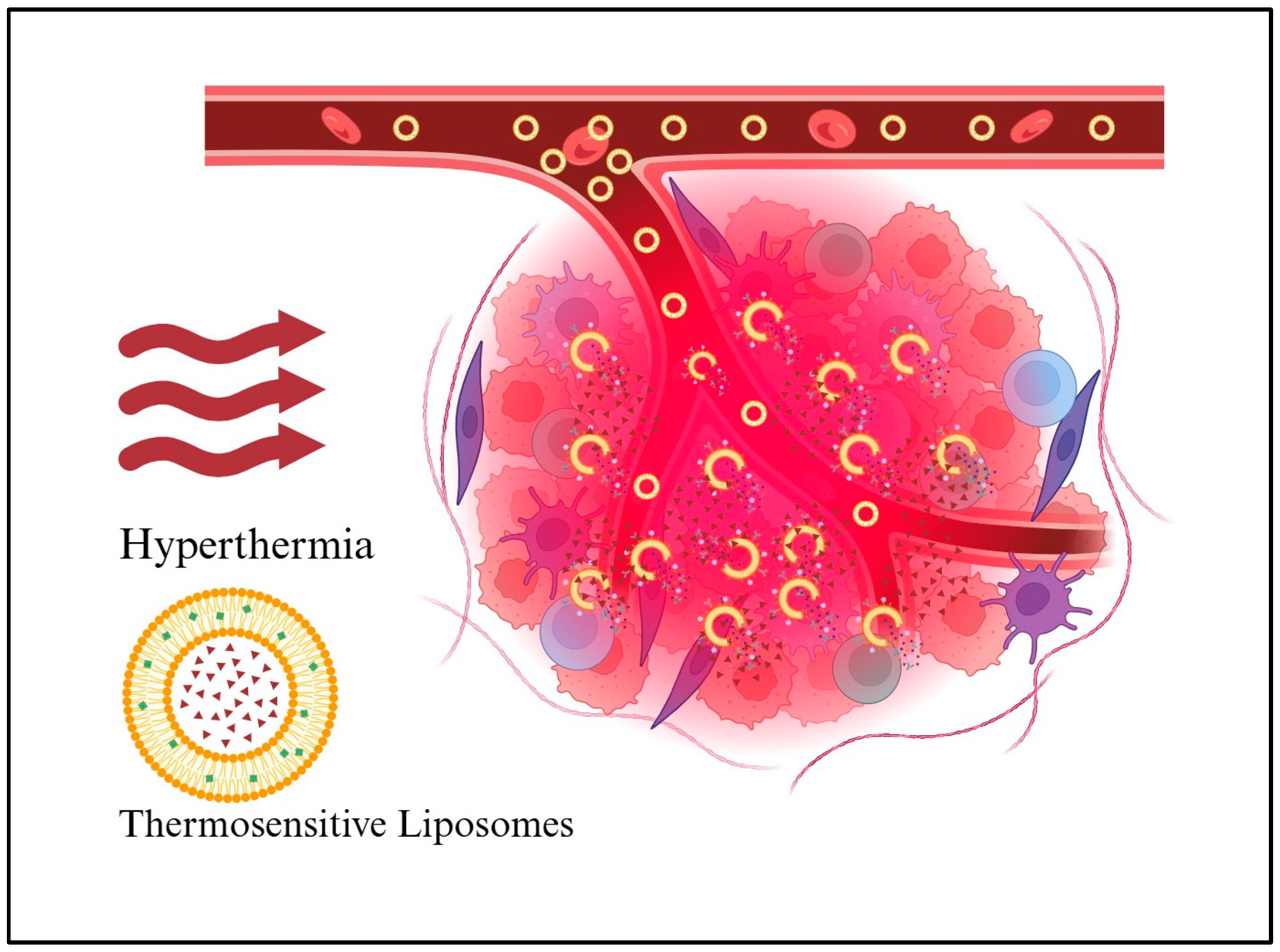
2.1. Hyperthermia Cancer Therapy
2.2. Hyperthermia-Induced Drug Release
3. Thermosensitive Nanoparticles
3.1. Polymer-Based Thermosensitive Nanocarriers
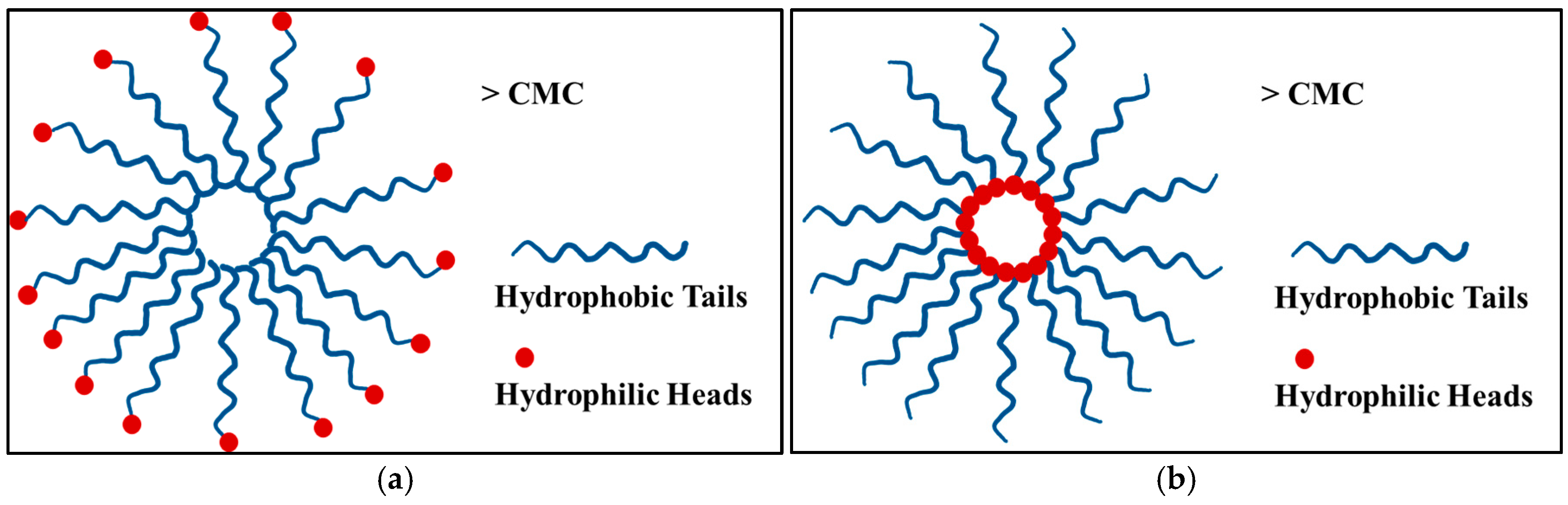
3.1.1. Thermosensitive Micelles
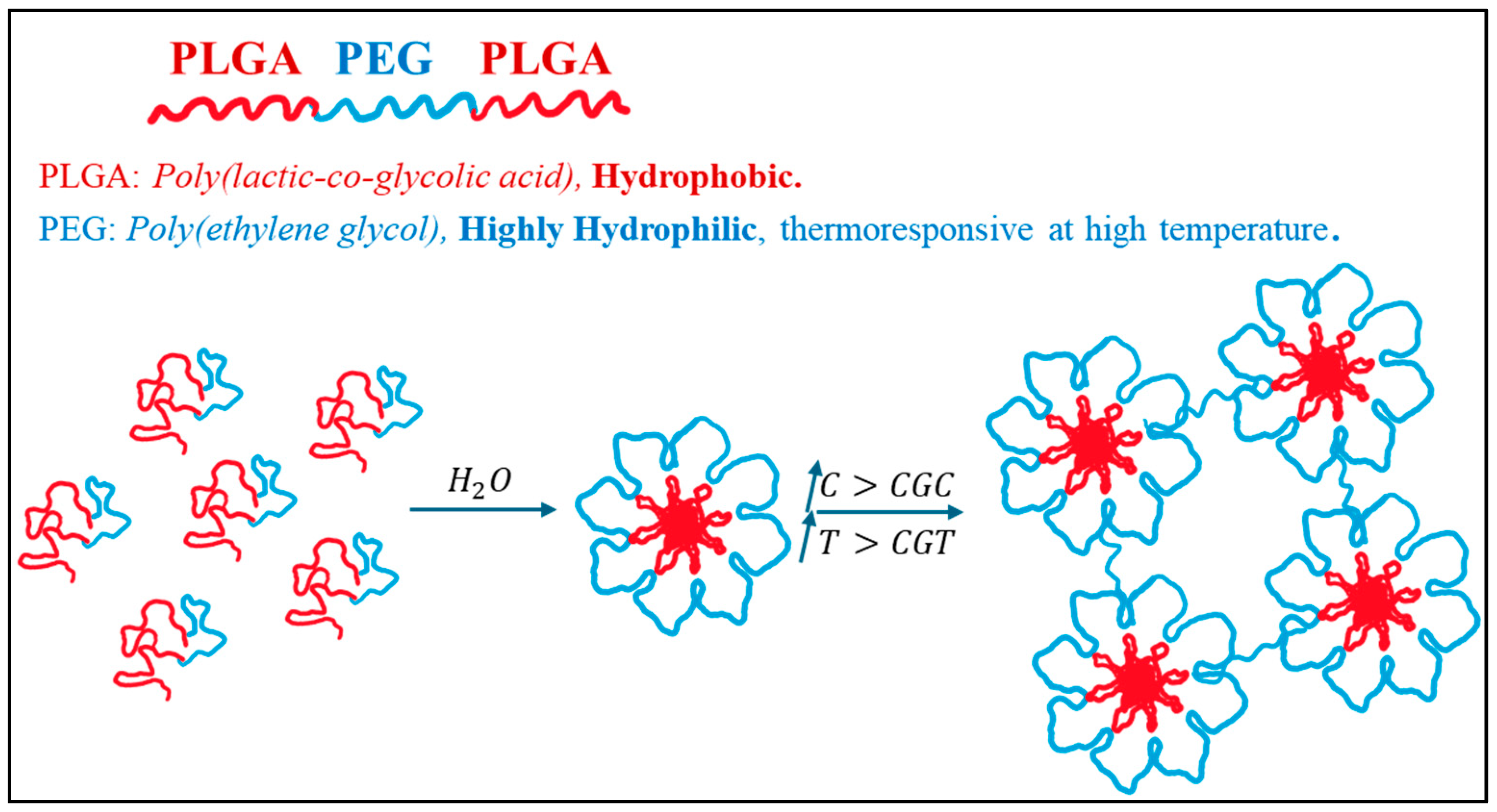
3.1.2. Thermosensitive Hydrogel
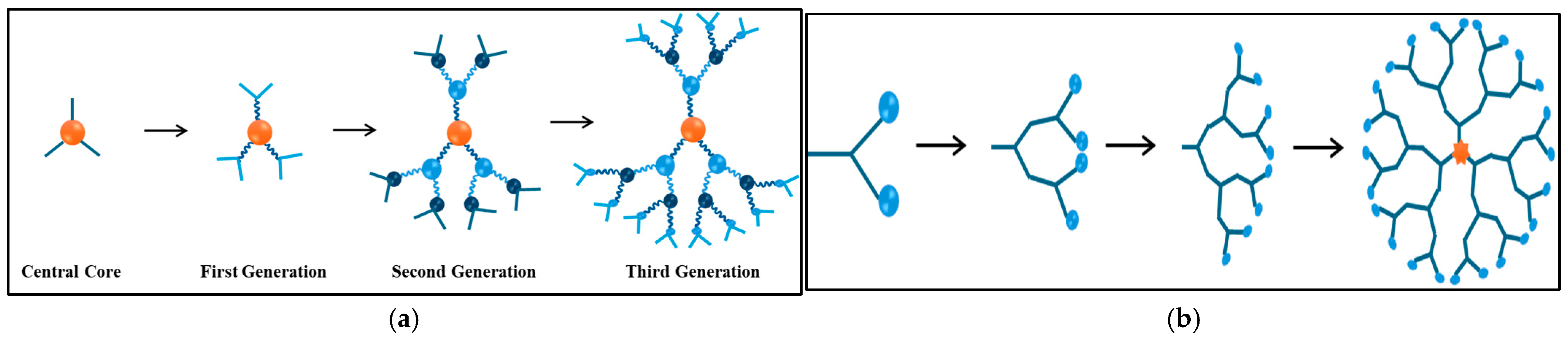
3.1.3. Thermosensitive Dendrimers
3.2. Lipid-Based Thermosensitive Nanocarriers
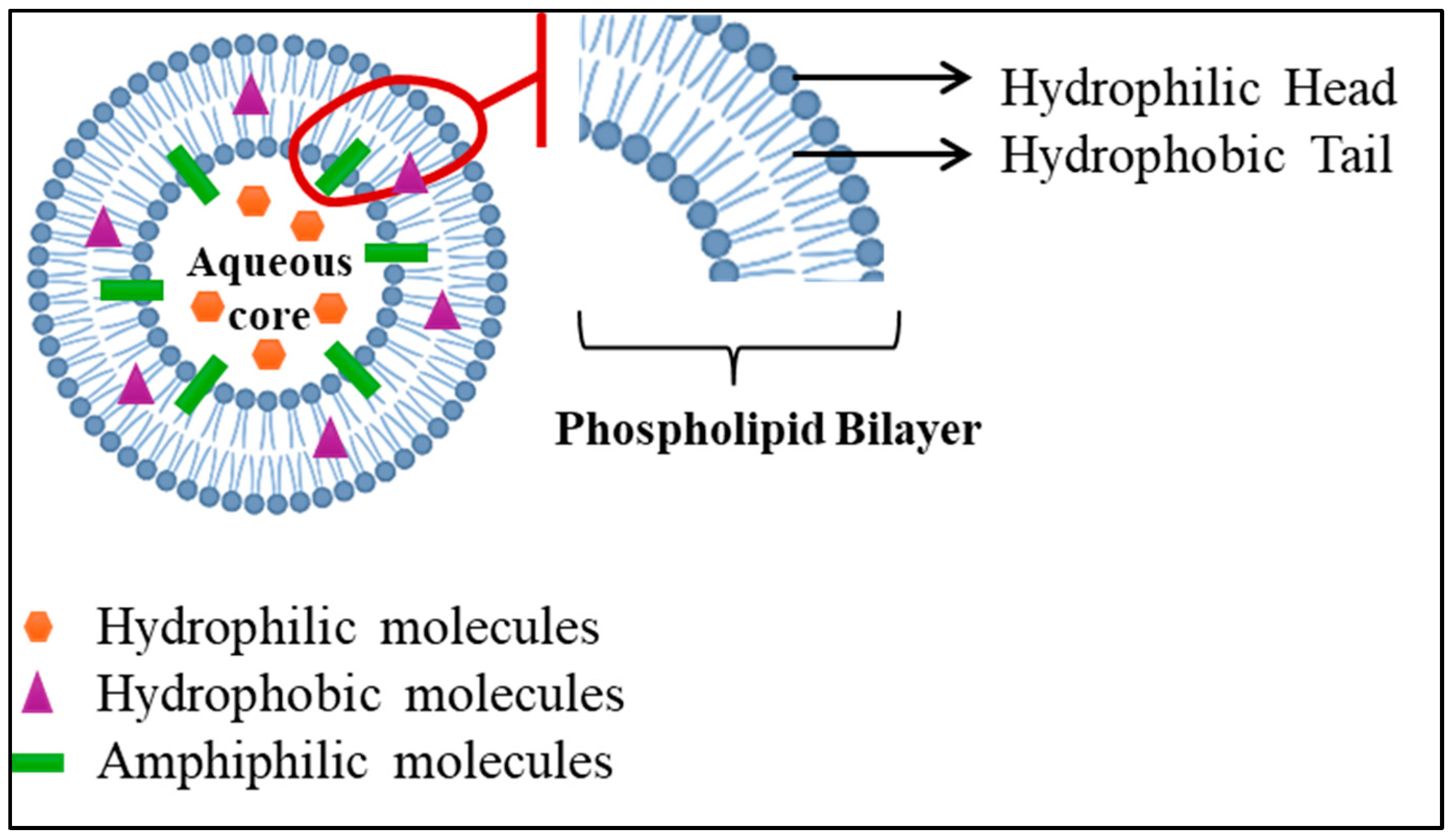
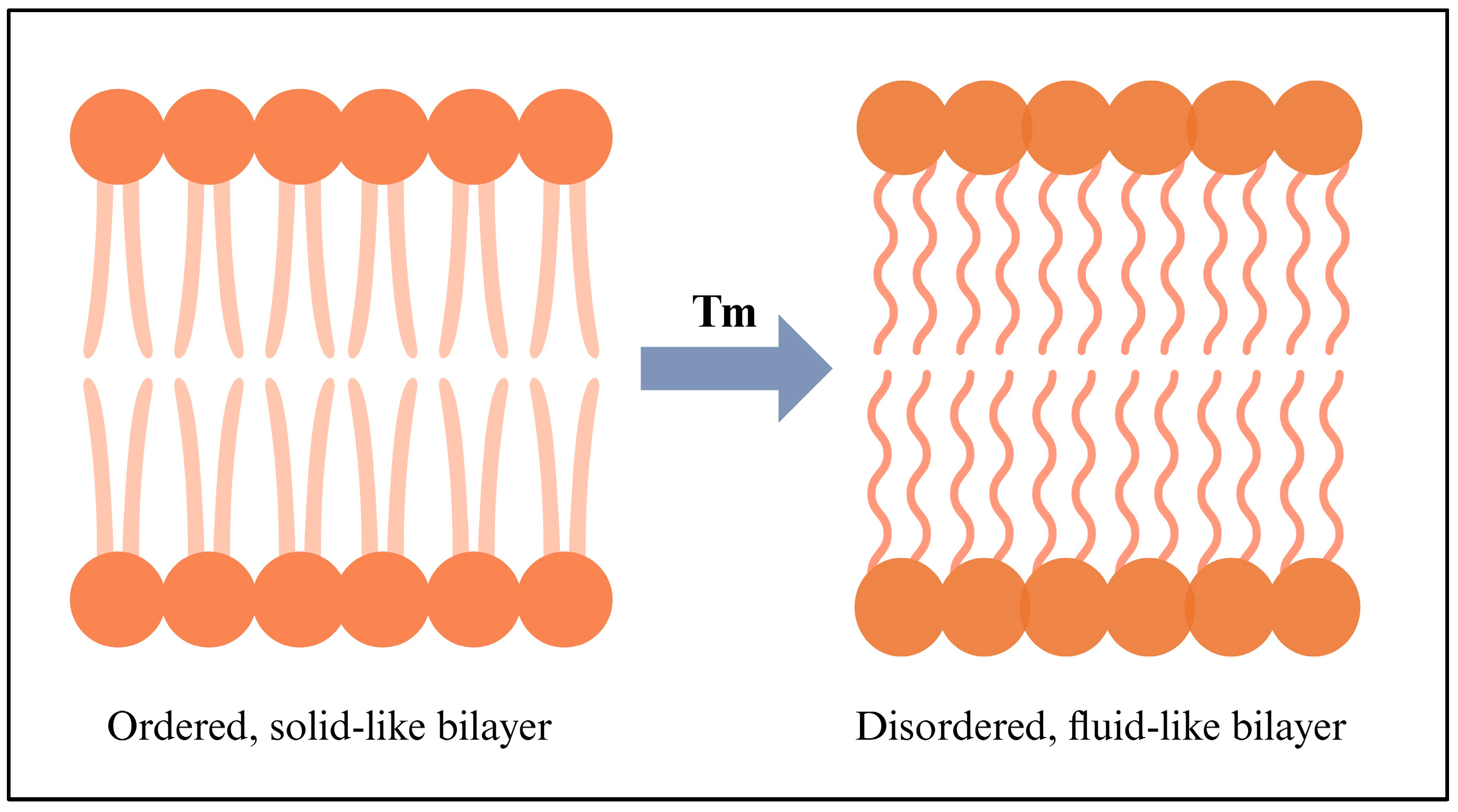
3.2.1. Thermosensitive Liposomes
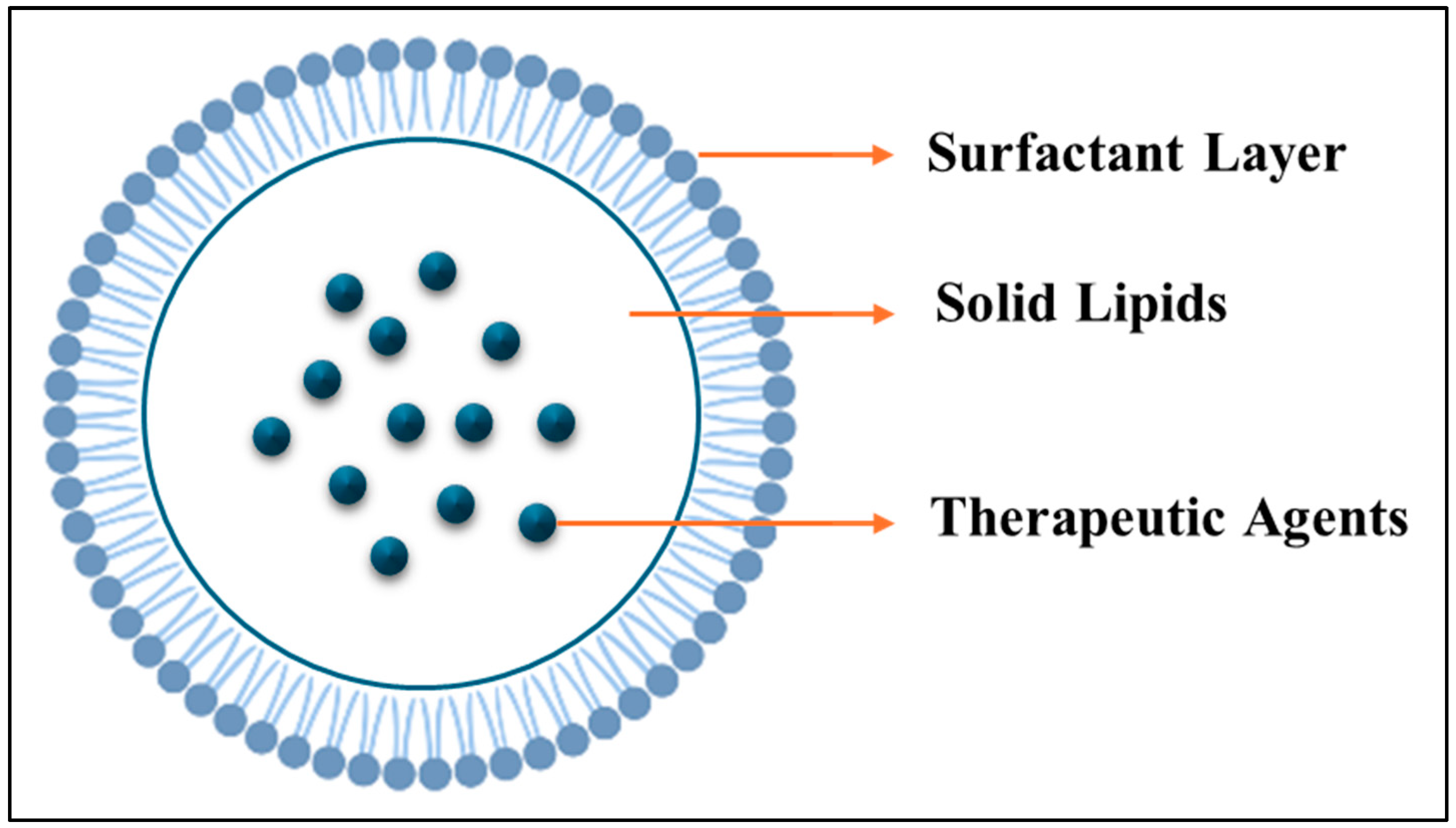
3.2.2. Thermosensitive Solid Lipid Nanoparticles (SLNs)
- Solvent-based methods:
- 1.
- Solvent Emulsification–Evaporation Method: dissolves lipids and drugs in water-immiscible organic solvents, emulsifies in an aqueous faze, and evaporates the solvent, yielding SLNs with nano-sized distribution (100 mL); however, it requires the removal of toxic solvents [134].
- 2.
- Solvent Emulsification–Diffusion Method: includes saturating water and organic solvents, preparing nanoemulsions, diluting with water, and then disposing of the solvents. However, this method results in low concentrations of SLNs [135].
- 3.
- Solvent Injection Method: The oil phase containing lipids and drugs is rapidly injected into an aqueous phase, which leads to direct droplet formation and SLN stabilization. However, this method requires exact control during the injection [136].
- Non-Solvent-based methods:
- 1.
- 2.
- 3.
- 4.
- The phase inversion temperature method uses temperature-dependent surfactants to create emulsions by heating above a specific temperature and then cooling to produce SLNs. The downside of this method is that it can lead to low stability of the molecules [143].
- 5.
- Other methods:
- 1.
- The supercritical fluid-based method uses supercritical fluids like CO2 to facilitate SLN production but requires expensive fluids [146].
- 2.
- The double emulsion method forms a water/oil/water double emulsion. This method is effective for hydrophilic drugs but is prone to high drug loss and large sizes [146].
4. Preclinical and Clinical Applications of Thermosensitive Nanoparticles for Cancer Therapy
5. Challenges and Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDDs | Smart Drug Delivery Systems |
| NPs | Nanoparticles |
| SCF | Supercritical Fluid |
| EPR | Enhanced Permeability and Retention |
| DDSs | Drug Delivery Systems |
| DNA | Deoxyribonucleic acid |
| TNPs | Temperature-responsive Nanoparticles |
| SDS | Sodium Dodecyl Sulphate |
| CST | Critical Solution Temperature |
| UCST | Upper Critical Solution Temperature |
| LCST | Lower Critical Solution Temperature |
| PNIPAAM | Poly(N-isopropyl acrylamide) |
| PEG | Polyethylene glycol |
| PHPMA | Poly(N-(2-hydroxypropyl) methacrylamide) |
| PLA | Poly(L, D-lactide) |
| ATRP | Atom Transfer Radical Polymerization |
| RAFT | Reversible Addition-fragmentation Chain-transfer |
| PVCL | Poly(N-vinylcaprolactam) |
| ROP | Ring-opening Polymerization |
| CMRP | Cobalt-mediated Radical Polymerization |
| CMC | Critical Micelle Concentration |
| PMs | Polymer Micelles |
| PPO | Polypropylene Oxide |
| PEO | Polyethylene Oxide |
| SSPMs | Stimuli-sensitive Polymer Micelles |
| NAAMe | N-acryloyl-Ala-methylester |
| NAβAMe | N-acryloyl-βAla-methylester |
| mPEG | Monomethoxy Poly(ethylene glycol) |
| DC | Deoxycholic Acid |
| PCL | Poly(ε-caprolactone) |
| PVS | Poly(vinyl stearate) |
| PVL | Poly(vinyl laurate) |
| DOX | Doxorubicin |
| HEK 293 T | Human Embryonic Kidney 293 T Cells |
| HeLa | Human Cervical Cancer Cells |
| Alg | Alginic Acid |
| DP | Dipyridamole |
| p(AAm-co-AN) | Poly(acrylamide-co-acrylonitrile) |
| LEN | Lenvatinib |
| AcD | Acridine |
| NIR-II | Near-infrared Second-region |
| SPLI | SP94-PEG-p(AAm-co-AN)/LEN/IR-1061-AcD |
| PBnCL | Poly(γ-benzyloxy-ε-caprolactone) |
| PPhCL | Poly(γ-phenyl- ε-caprolactone) |
| PEtOPhCL | Poly(γ-(4-ethoxyphenyl)-ε-caprolactone) |
| PME3CL | γ-tri(ethylene glycol) Functionalized |
| Chit5 | Chitosan Oligosaccharide Lactate 5 kDa |
| OA | Oleic Acid |
| SA | Stearic Acid |
| LA | Lipoic Acid |
| MUA | 11-mercaptoundecanoic Acid |
| A549 | Adenocarcinomic Human Alveolar Basal Epithelial Cell Lines |
| ZnPP | Zinc Protoporphyrin |
| PNOG | Poly(N-octylglycine) |
| PNAG | Poly(N-allylglycine) |
| PNMG | Poly(N-methylglycine) |
| Cip | Ciprofoxacin |
| CAC | Critical Aggregation Concentration |
| PLGA | Poly(lactic-co-glycolic acid) |
| CGC | Critical Gelation Concentration |
| CGT | Critical Gelation Temperature |
| FDA | Food and Drug Administration |
| CTLA-4 | Cytotoxic T-lymphocyte-associated |
| TME | Tumor Microenvironment |
| PEU | Poly(ether urethane) |
| GEM | Gemcitabine |
| CpG-ODN | Cytosine-phosphate-guanine Oligonucleotide |
| PDLLA | Poly (D, L-lactide) |
| PLEL | PDLLA-PEG-PDLLA |
| CTX | Cabazitaxel |
| RMPs | Irradiated tumor cell-derived microparticles |
| Mn2+ | Manganese Ions |
| mPEG2k | Methoxy Poly (ethylene glycol)2000 |
| Ala | Alanine |
| Cy7 | Cyanine Dyes |
| EGF | Epidermal Growth Factor |
| HTPM | Halofuginone-loaded D-alpha Tocopherol Acid Polyethylene Glycol succinate (TPGS) Polymer Micelles |
| HTPM & AgNPs-gel | HTPM composite silver nanoparticle thermosensitive gel |
| AgNPs | Silver Nanoparticles |
| HF | Halofuginone Hydrobromide |
| C6 | Coumarin 6 |
| Ag+ | Silver Ion |
| DSF | Disulfiram |
| FCDL | Glycyrrhizic Acid-Cu |
| Cu2+ | Copper(II) Ion |
| DSF-SE | DSF Submicroemulsion |
| DOPA-rGO | Dopamine-reduced Graphene Oxide |
| DOPA-rGO@PC-gel | Pluronic F127/Chitosan injectable in situ forming hydrogel loaded with DOPA-rGO |
| NHDF | Normal Human Dermal Fibroblasts |
| MCF-7 | Michigan Cancer Foundation-7 |
| PAMAMs | Poly(amidoamine) dendrimers |
| G4.0 PAMAM | The dendrimers of the 4th generation |
| G2.0 PAMAM | The dendrimers of the 2nd generation |
| PPI | Poly (Propylene Imine) |
| OEG | Oligo (ethylene glycol) |
| IBAM | Isobutyramide |
| Suc | Succinic Anhydride |
| Phe | Phenylalanine |
| MCE | Magnetocaloric Effect |
| Fe49Rh51 | FeRh alloy |
| CRY | Chrysin |
| PSMA | Prostate-specific Membrane Antigen |
| PD | Polyamidoamine Dendrimer |
| CTT1298 | Irreversible PSMA Ligand |
| PCa | Prostate Cancer |
| Cabo | Cabozantinib |
| SPAAC | Strain-promoted Azide–alkyne Cycloaddition |
| NDs | Nanodiamonds |
| DXL | Docetaxel |
| Tc | Transition Temperature |
| Tm | Melting Temperature |
| TSL | Temperature-sensitive Liposomes |
| TT | Tissue Transit Time |
| AUC | Area Under the Concentration-time Curve |
| MTD | Maximum Tolerated Dose |
| DPPC | Dipalmitoylphosphatidylcholine |
| MSPC | Myristoylstearoylphosphatidylcholine |
| RFA | Radiofrequency Thermal Ablation |
| HIFU | High-intensity Focused Ultrasound |
| DSPE-mPEG2000 | 1,2-distearyl-sn-glycero-3-phosphoethanolamine-N-[amino-(polyethyleneglycol)-2000] |
| LTLD | lyso-thermosensitive liposomes |
| mEHT | Modulated Electro-hyperthermia |
| PLD | PEGylated liposomal DOX |
| ThermoDox® | Liposomal Encapsulation of Doxorubicin |
| FUS | Focused Ultrasound |
| FeNP | Fe3O4 NPs |
| Gel | Gelatin |
| PGA | Polyglutamic Acid |
| Dox-Lipo | Thermosensitive Liposomes Encapsulating Dox |
| EOMA | Mouse Microvascular Endothelial Cell Line |
| rGECs | Rat Glomerular Endothelial Cells |
| BDNF | Neurotrophin Brain-Derived Neurotrophic Factor |
| LTSL | Low Temperature Sensitive Liposomes |
| HITSLLS | Homing Peptide |
| cRGD | cyclic RGD |
| GFB | Glomerular Filtration Barrier |
| BBR | Berberine |
| PTA | Photothermal Agent |
| ICG | Indocyanine Green |
| BI-LP | BBR and ICG Dual-Loaded Liposome |
| FA | Folic Acid |
| BI-FA-LP | BBR and ICG were Loaded into FA Modified Liposomes |
| PC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| BiNSs | Bismuthene Nanosheets |
| 5-FU | 5-fluorouracil |
| Met | Metformin |
| CF | Carboxy Fluorescein |
| AuNPRs | Gold Nanoprisms |
| BioSi@NPs | Silica Nanoparticles |
| TNBC | Triple-negative Breast Cancer |
| RT | Radiation Therapy |
| Ht | Hyperthermia |
| NiPTZ | Organometallic Photosensitizer |
| NIR-II | Second Near-infrared |
| GA | Gambogic Acid |
| PTAs | Photothermal Agents |
| PAI | Photoacoustic Imaging |
| PTT | Photothermal Therapy |
| SLNs | Solid Lipid Nanoparticles |
| DRTH | Double-reverse Thermosensitive Hydrogel |
| PAC | Paclitaxel |
| CUR | Curcumin |
| HPH | High-Pressure Homogenization |
| BBS | Lauric Acid |
| COPA | β-Caryophyllene |
| PDI | Polydispersity Index |
| HD | Hydrodynamic Diameter |
| HGF | Human Gingival Fibroblast |
| SPIONs | Superparamagnetic Iron Oxide Nanoparticles |
| HTT | Hyperthermic tumor therapy |
| IFP | Interstitial Fluid Pressure |
| ROS | Reactive Oxygen Species |
| EM | Electromagnetic |
| CT | Computed Tomography |
| TSMLPs | Thermosensitive Small Multilamellar Lipid Particles |
| HAS | Human Serum Albumin |
| DSPC | Distearoyl Phosphatidylcholine |
| DPPG2 | Dipalmitoyl-sn-glycerophosphatidyldiglycerol |
| FU-TSL DDS | Focused Ultrasound-targeted DDS |
| ICD | Immunogenic Cell Death |
| PDT | Photodynamic Therapy |
| MTX-TSL | Mitoxantrone Thermosensitive Liposome |
| PFP | Perfluoropentane |
| HCC | Hepatocellular Carcinoma |
| MLHT | Mild Local Hyperthermia |
| CWR | Chest Wall Recurrences |
| NLCs | Nanostructured Lipid Carriers |
References
- Al-Shamsi, H.O.; Abdelwahab, S.I.; Albasheer, O.; Taha, M.M.E.; Alqassim, A.Y.; Alharbi, A.; Farasani, A.; Altraifi, A.A.A.; Medani, I.E.; Hakami, N.; et al. Cancer research in the United Arab Emirates from birth to present: A bibliometric analysis. Heliyon 2024, 10, e27201. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A.; Cancer, H.O. Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Bryszewska, M.; Klajnert-Maculewicz, B.; Zablocka, M.; Majoral, J. Advances in Combination Therapies Based on Nanoparticles for Efficacious Cancer Treatment: An Analytical Report. Biomacromolecules 2015, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef]
- Khan, I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.M.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Kandula, S.; Singh, P.K.; Kaur, G.A.; Tiwari, A. Trends in smart drug delivery systems for targeting cancer cells. Mater. Sci. Eng. B 2023, 297, 116816. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Moros, M.; Idiago-López, J.; Asín, L.; Moreno-Antolín, E.; Beola, L.; Grazú, V.; Fratila, R.M.; Gutiérrez, L.; de la Fuente, J.M. Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Adv. Drug Deliv. Rev. 2019, 138, 326–343. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Zhang, L.; Dai, T.; Sun, Z.; Shi, Y.; Li, S.; Yu, N. Application of high intensity focused ultrasound combined with nanomaterials in anti-tumor therapy. Drug Deliv. 2024, 31, 2342844. [Google Scholar] [CrossRef]
- Qiu, G.; Zhou, W.; Liu, Y.; Meng, T.; Yu, F.; Jin, X.; Lian, K.; Zhou, X.; Yuan, H.; Hu, F. NIR-Triggered Thermosensitive Nanoreactors for Dual-Guard Mechanism-Mediated Precise and Controllable Cancer Chemo-Phototherapy. Biomacromolecules 2024, 25, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; ten Hagen, T.L.M. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, M.; Gong, Y.; Tang, H.; Li, J.; Cao, Y. Thermoresponsive Polymers with Lower Critical Solution Temperature- or Upper Critical Solution Temperature-Type Phase Behaviour Do Not Induce Toxicity to Human Endothelial Cells. Basic Clin. Pharmacol. Toxicol. 2017, 120, 79–85. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- Gas, P. Essential Facts on the History of Hyperthermia and their Connections with Electromedicine. arXiv 2017, arXiv:1710.00652. [Google Scholar] [CrossRef]
- Hahn, G.M. Hyperthermia and Cancer; Springer Science & Business Media: New York, VY, USA, 2012. [Google Scholar]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Bala, V.-M.; Lampropoulou, D.I.; Grammatikaki, S.; Kouloulias, V.; Lagopati, N.; Aravantinos, G.; Gazouli, M. Nanoparticle-Mediated Hyperthermia and Cytotoxicity Mechanisms in Cancer. Int. J. Mol. Sci. 2024, 25, 296. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Myerson, R.J.; Moros, E.G.; Diederich, C.J.; Haemmerich, D.; Hurwitz, M.D.; Hsu, I.J.; McGough, R.J.; Nau, W.H.; Straube, W.L.; Turner, P.F.; et al. Components of a hyperthermia clinic: Recommendations for staffing, equipment, and treatment monitoring. Int. J. Hyperth. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Van Rhoon, G.C.; Paulides, M.M.; van Holthe, J.M.L.; Franckena, M. Hyperthermia by electromagnetic fields to enhanced clinical results in oncology. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar] [CrossRef]
- Kok, H.P.; Navarro, F.; Strigari, L.; Cavagnaro, M.; Crezee, J. Locoregional hyperthermia of deep-seated tumours applied with capacitive and radiative systems: A simulation study. Int. J. Hyperth. 2018, 34, 714–730. [Google Scholar] [CrossRef]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef]
- ter Haar, G.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperth. 2007, 23, 89–104. [Google Scholar] [CrossRef]
- Hijnen, N.; Langereis, S.; Grüll, H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 65–81. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef]
- Staruch, R.M.; Hynynen, K.; Chopra, R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: Therapeutic effect in rabbit Vx2 tumours. Int. J. Hyperth. 2015, 31, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Kheirolomoom, A.; Lai, C.; Tam, S.M.; Mahakian, L.M.; Ingham, E.S.; Watson, K.D.; Ferrara, K.W. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J. Control. Release 2013, 172, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Diederich, C.J.; Seegenschmiedt, M.H. Interstitial heating technologies. In Thermoradiotherapy and Thermochemotherapy: Biology, Physiology, Physics; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Kaplan, I.D.; Svensson, G.K.; Hansen, M.S.; Hynynen, K. Feasibility and patient tolerance of a novel transrectal ultrasound hyperthermia system for treatment of prostate cancer. Int. J. Hyperth. 2001, 17, 31–37. [Google Scholar] [CrossRef]
- Petrovich, Z.; Ameye, F.; Boyd, M.; Pike, S.; Baert, L. Relationship of response to transurethral hyperthermia and prostate volume in BPH patients. Urology 1992, 40, 317–321. [Google Scholar] [CrossRef]
- Curto, S.; Faridi, P.; Shrestha, T.B.; Pyle, M.; Maurmann, L.; Troyer, D.; Bossmann, S.H.; Prakash, P. An integrated platform for small-animal hyperthermia investigations under ultra-high-field MRI guidance. Int. J. Hyperth. 2018, 34, 341–351. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia Enables Tumor-specific Nanoparticle Delivery: Effect of Particle Size1. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Horsman, M.R.; Murata, R.; Overgaard, J. Improving local tumor control by combining vascular targeting drugs, mild hyperthermia and radiation. Acta Oncol. 2001, 40, 497–503. [Google Scholar] [CrossRef]
- Wood, B.J.; Poon, R.T.; Locklin, J.K.; Dreher, M.R.; Ng, K.K.; Eugeni, M.; Seidel, G.; Dromi, S.; Neeman, Z.; Kolf, M.; et al. Phase I Study of Heat-Deployed Liposomal Doxorubicin during Radiofrequency Ablation for Hepatic Malignancies. J. Vasc. Interv. Radiol. 2012, 23, 248–255.e7. [Google Scholar] [CrossRef]
- Poon, R.T.P.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: A novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- May, J.P.; Li, S.D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013, 10, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Wang, B.; Wang, Y.; Li, J.; Zhang, Y. The application of thermosensitive nanocarriers in controlled drug delivery. J. Nanomater. 2011, 2011, 389640. [Google Scholar] [CrossRef]
- Fernandez-Nieves, A.; Wyss, H.; Mattsson, J.; Weitz, D.A. Microgel Suspensions: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ghosh Dastidar, D.G.; Chakrabarti, G. Chapter 6—Thermoresponsive drug delivery systems, characterization and application. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef]
- Najafi, M.; Hebels, E.; Hennink, W.E.; Vermonden, T. Poly(N-isopropylacrylamide): Physicochemical Properties and Biomedical Applications. In Temperature-Responsive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–34. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Thermoresponsive Multiblock Copolymers: Chemistry, Properties and Applications. In Temperature-Responsive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 35–65. [Google Scholar] [CrossRef]
- Tenkovtsev, A.V.; Amirova, A.I.; Filippov, A.P. In Star-shaped Poly(2-alkyl-2-oxazolines): Synthesis and Properties. In Temperature-Responsive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 67–92. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Kharlampieva, E. Poly(N-vinylcaprolactam): From Polymer Synthesis to Smart Self-assemblies. In Temperature-Responsive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 93–120. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Rana, S.; Bhattacharjee, J.; Barick, K.C.; Verma, G.; Hassan, P.A.; Yakhmi, J.V. Chapter 7—Interfacial engineering of nanoparticles for cancer therapeutics. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. In Nanomedicine in Cancer; Jenny Stanford Publishing: New York, NY, USA, 2017; pp. 47–98. [Google Scholar] [CrossRef]
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Chapter 3—Mechanism for development of nanobased drug delivery system. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papavasileiou, K.D.; Papadopoulos, M.G.; Xenakis, A. Reverse Micelles as Antioxidant Carriers: An Experimental and Molecular Dynamics Study. Langmuir 2017, 33, 5077–5085. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Li, S.; Deratani, A. Reverse micelles prepared from amphiphilic polylactide-b-poly(ethylene glycol) block copolymers for controlled release of hydrophilic drugs. Int. J. Pharm. 2015, 495, 154–161. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef]
- Han, J.O.; Lee, H.J.; Jeong, B. Thermosensitive core-rigid micelles of monomethoxy poly(ethylene glycol)-deoxy cholic acid. Biomater. Res. 2022, 26, 16. [Google Scholar] [CrossRef]
- de Moraes, R.M.; de Carvalho, L.T.; Teixeira, A.J.R.M.; Medeiros, S.F.; dos Santos, A.M. Well-defined amphiphilic poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) and thermosensitive micelles formulation. Iran. Polym. J. 2023, 32, 1627–1641. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Chen, X.; Ullah, A. Tunable self-assembly of lipid-based block polymeric micelles with temperature-sensitive poly(vinylcaprolactam) shell for effective anticancer drug delivery. Eur. Polym. J. 2024, 206, 112795. [Google Scholar] [CrossRef]
- Manna, K.; Dey, S.; Phukan, A.; Dey, S.K.; Pal, S. Alginic acid-based pH and thermo responsive reversible switched polymeric micelle via RAFT polymerization. Polymer 2024, 291, 126581. [Google Scholar] [CrossRef]
- Du, Y.; Shan, C.; You, Y.; Chen, M.; Zhu, L.; Shu, G.; Han, G.; Wu, L.; Ji, J.; Yu, H.; et al. NIR-II fluorescence imaging-guided hepatocellular carcinoma treatment via IR-1061-acridine and lenvatinib co-loaded thermal-sensitive micelles and anti-PD-1 combinational therapy. Chem. Eng. J. 2023, 454, 140437. [Google Scholar] [CrossRef]
- Wang, H.; Polara, H.; Bhadran, A.; Shah, T.; Babanyinah, G.K.; Ma, Z.; Calubaquib, E.L.; Miller, J.T.; Biewer, M.C.; Stefan, M.C. Effect of aromatic substituents on thermoresponsive functional polycaprolactone micellar carriers for doxorubicin delivery. Front. Pharmacol. 2024, 15, 1356639. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart pH-and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef]
- Bagheri-Meyabad, M.; Motasadizadeh, H.; Norouzi, P.; Fatahi, Y.; Asadi, H.; Varshochian, R.; Ghazi-Khansari, M.; Dinarvand, R. Self-assembled NIPAM–PEG–NIPAM polymeric nanomicelles for the delivery of zinc protoporphyrin: A potential stimuli-triggered cancer treatment approach. J. Mater. Sci. 2024, 59, 3049–3065. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, D.; Shen, X.; Xu, L.; Yang, K.; Feng, L.; Jiang, Y.; Qiao, Y.; Wen, J.; Lu, J.; et al. Synthesis and characterization of micelles from thermal-responsive amphiphilic triblock polypeptoids as drug carrier. J. Polym. Res. 2024, 31, 237. [Google Scholar] [CrossRef]
- Khan, B.; Arbab, A.; Khan, S.; Fatima, H.; Bibi, I.; Chowdhry, N.P.; Ansari, A.Q.; Ursani, A.A.; Kumar, S.; Hussain, J. Recent progress in thermosensitive hydrogels and their applications in drug delivery area. MedComm–Biomater. Appl. 2023, 2, e55. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Pre-clinical and clinical applications of thermoreversible hydrogels in biomedical engineering: A review. Polym. Int. 2021, 70, 1433–1448. [Google Scholar] [CrossRef]
- Patel, P.; Mandal, A.; Gote, V.; Pal, D.; Mitra, A.K. Thermosensitive hydrogel-based drug delivery system for sustained drug release. J. Polym. Res. 2019, 26, 131. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Atabaki, R.; Behrouzi, S.; Mohamadpour, F.; Kamali, H. The recent advancement in the PLGA-based thermo-sensitive hydrogel for smart drug delivery. Int. J. Pharm. 2022, 631, 122484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for anti-tumor immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M. Thermosensitive Micellar Hydrogels as Vehicles to Deliver Drugs with Different Wettability. Front. Bioeng. Biotechnol. 2020, 8, 708. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.Y.; Dai, L.Q.; Shi, K.; Hao, Y.; Chu, B.Y.; Hu, D.R.; Bei, Z.W.; Yuan, L.P.; Pan, M.; et al. Intravesical chemotherapy synergize with an immune adjuvant by a thermo-sensitive hydrogel system for bladder cancer. Bioact. Mater. 2024, 31, 315–332. [Google Scholar] [CrossRef]
- Huang, J.; Yue, B.; Sun, J.; Xu, T.; Zhou, J.; Lu, L.; Yan, Y.; Lovell, J.F.; Wan, C.; Zhu, M.; et al. Injectable thermosensitive hydrogels loaded with irradiated tumor cell-derived microparticles and manganese activate anti-tumor immunity. Nano Today 2024, 58, 102455. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, Y.; Kim, J.; Kim, T.; Yu, S.; Seo, D.; Sung, D.; Kim, H. Tumor cell loaded thermosensitive hydrogel for photodynamic therapy associated tumor antigens release. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2024, 1868, 130703. [Google Scholar] [CrossRef]
- Piao, L.; Xiang, P.; Zhou, Y.; Zhao, W.; Yang, T.; Xia, S.; Gao, G.; Chen, K.; Li, D. Thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of EGF to inhibit cervical cancer recurrence. Colloids Surf. B Biointerfaces 2024, 236, 113795. [Google Scholar] [CrossRef]
- Zuo, R.; Gong, J.; Gao, X.; Nepovimova, E.; Zhang, J.; Jiang, S.; Kuca, K.; Wu, W.; Guo, D. Injectable nano-in situ-thermosensitive-hydrogels based on halofuginone and silver for postoperative treatment against triple-negative breast cancer. Int. J. Pharm. 2024, 661, 124384. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, L.; Pang, W.; Jiang, S. Halofuginone-guided nano-local therapy: Nano-thermosensitive hydrogels for postoperative metastatic canine mammary carcinoma with scar removal. Int. J. Pharm. X 2024, 7, 100241. [Google Scholar] [CrossRef]
- Wang, P.; Liu, B.; Wang, Q.; Wang, Y.; Gao, X.; Gou, J.; He, H.; Zhang, Y.; Yin, T.; Jin, X.; et al. Enhanced localized therapeutic precision: A face-to-face folate-targeted Cu2+-mediated nanotherapy with thermosensitive sustained-release system. Int. J. Pharm. 2024, 658, 124213. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, L.; Shi, K.; Pan, M.; Yuan, L.; Qian, Z. Cabazitaxel-Loaded Thermosensitive Hydrogel System for Suppressed Orthotopic Colorectal Cancer and Liver Metastasis. Adv. Sci. 2024, 11, 2404800. [Google Scholar] [CrossRef]
- Pouso, M.R.; Melo, B.L.; Gonçalves, J.J.; Mendonça, A.G.; Correia, I.J.; de Melo-Diogo, D. Development of dual-crosslinked Pluronic F127/Chitosan injectable hydrogels incorporating graphene nanosystems for breast cancer photothermal therapy and antibacterial applications. Eur. J. Pharm. Biopharm. 2024, 203, 114476. [Google Scholar] [CrossRef]
- Sarode, R.J.; Mahajan, H.S. Dendrimers for drug delivery: An overview of its classes, synthesis, and applications. J. Drug Deliv. Sci. Technol. 2024, 98, 105896. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Szota, M.; Szwedowicz, U.; Rembialkowska, N.; Janicka-Klos, A.; Doveiko, D.; Chen, Y.; Kulbacka, J.; Jachimska, B. Dendrimer Platforms for Targeted Doxorubicin Delivery—Physicochemical Properties in Context of Biological Responses. Int. J. Mol. Sci. 2024, 25, 7201. [Google Scholar] [CrossRef] [PubMed]
- Yabbarov, N.G.; Posypanova, G.A.; Vorontsov, E.A.; Popova, O.N.; Severin, E.S. Targeted delivery of doxorubicin: Drug delivery system based on PAMAM dendrimers. Biochem. Mosc. 2013, 78, 884–894. [Google Scholar] [CrossRef]
- Bacha, K.; Chemotti, C.; Mbakidi, J.; Deleu, M.; Bouquillon, S. Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review. Macromol 2023, 3, 343–370. [Google Scholar] [CrossRef]
- Tamaki, M.; Kojima, C. pH-Switchable LCST/UCST-type thermosensitive behaviors of phenylalanine-modified zwitterionic dendrimers. RSC Adv. 2020, 10, 10452–10460. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2016, 8, 220–232. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef]
- Haba, Y.; Kojima, C.; Harada, A.; Kono, K. Comparison of thermosensitive properties of poly(amidoamine) dendrimers with peripheral N-isopropylamide groups and linear polymers with the same groups. Angew. Chem. Int. Ed. Engl. 2006, 46, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Tono, Y.; Kojima, C.; Haba, Y.; Takahashi, T.; Harada, A.; Yagi, S.; Kono, K. Thermosensitive properties of poly(amidoamine) dendrimers with peripheral phenylalanine residues. Langmuir 2006, 22, 4920–4922. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Fukushima, D.; Kojima, C. Dual pH-sensitive and UCST-type thermosensitive dendrimers: Phenylalanine-modified polyamidoamine dendrimers with carboxyl termini. RSC Adv. 2018, 8, 28147–28151. [Google Scholar] [CrossRef]
- Wu, W.; Driessen, W.; Jiang, X. Oligo (ethylene glycol)-Based Thermosensitive Dendrimers and Their Tumor Accumulation and Penetration. J. Am. Chem. Soc. 2014, 136, 3145–3155. [Google Scholar] [CrossRef]
- Haba, Y.; Harada, A.; Takagishi, T.; Kono, K. Rendering Poly(amidoamine) or Poly(propylenimine) Dendrimers Temperature Sensitive. J. Am. Chem. Soc. 2004, 126, 12760–12761. [Google Scholar] [CrossRef]
- Zheng, K.; Ren, J.; He, J. Thermally Responsive Unimolecular Nanoreactors from Amphiphilic Dendrimer-Like Copolymer Prepared via Anionic Polymerization and Cross Metathesis Reaction. Macromolecules 2019, 52, 6780–6791. [Google Scholar] [CrossRef]
- Amirov, A.A.; Permyakova, E.S.; Yusupov, D.M.; Savintseva, I.V.; Murliev, E.K.; Rabadanov, K.S.; Popov, A.L.; Chirkova, A.M.; Aliev, A.M. Thermoresponsive PNIPAM/FeRh smart composite activated by magnetic field for doxorubicin release. ACS Appl. Eng. Mater. 2025, 3, 410–418. [Google Scholar] [CrossRef]
- Bekhradnia, S.; Zhu, K.; Knudsen, K.D.; Sande, S.A.; Nyström, B. Structure; swelling, and drug release of thermoresponsive poly(amidoamine) dendrimer–poly(N-isopropylacrylamide) hydrogels. J. Mater. Sci. 2014, 49, 6102–6110. [Google Scholar] [CrossRef]
- Rai, D.B.; Solanki, R.; Patel, S.; Pooja, D.; Kulhari, H. Designing of fucosylated dendrimers as a biocompatible carrier for the targeted delivery of chrysin to human lung cancer cells. Next Mater. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Dhull, A.; Wei, J.; Pulukuri, A.J.; Rani, A.; Sharma, R.; Mesbahi, N.; Yoon, H.; Savoy, E.A.; Xaivong Vi, S.; Goody, K.J.; et al. PSMA-targeted dendrimer as an efficient anticancer drug delivery vehicle for prostate cancer. Nanoscale 2024, 16, 5634–5652. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.M.; Samadi Zadeh, M.; Ahmad Panahi, H.; Hesami Tackallou, S.; Safaeijavan, R. Surface modification of nanodiamond with pH/thermo dual responsive polymer and hyper-branched dendrimer as a near-infrared photothermal-triggered drug delivery for cancer therapy. J. Mol. Liq. 2023, 390, 123155. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.; Lyon, P.; Coussios, C.; Carlisle, R. Thermosensitive liposomes: A promising step toward localised chemotherapy. Expert Opin. Drug Deliv. 2022, 19, 899–912. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Stein, W.D.; Litman, T. Chapter 1—Structural basis of movement across cell membranes. In Channels, Carriers, and Pumps, 2nd ed.; Stein, W.D., Litman, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Haemmerich, D.; Ramajayam, K.K.; Newton, D.A. Review of the Delivery Kinetics of Thermosensitive Liposomes. Cancers 2023, 15, 398. [Google Scholar] [CrossRef]
- Hagen, T.L.M.T.; Dreher, M.R.; Zalba, S.; Seynhaeve, A.L.B.; Amin, M.; Li, L.; Haemmerich, D. Drug transport kinetics of intravascular triggered drug delivery systems. Commun. Biol. 2021, 4, 920. [Google Scholar] [CrossRef] [PubMed]
- Ramajayam, K.K.; Wolfe, A.M.; Motamarry, A.; Nahhas, G.J.; Yost, J.; Yost, M.J.; Haemmerich, D. Untargeted Large Volume Hyperthermia Reduces Tumor Drug Uptake from Thermosensitive Liposomes. IEEE Open J. Eng. Med. Biol. 2021, 2, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Motamarry, A.; Negussie, A.H.; Rossmann, C.; Small, J.; Wolfe, A.M.; Wood, B.J.; Haemmerich, D. Real-time fluorescence imaging for visualization and drug uptake prediction during drug delivery by thermosensitive liposomes. Int. J. Hyperth. 2019, 36, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Regenold, M.; Bannigan, P.; Evans, J.C.; Waspe, A.; Temple, M.J.; Allen, C. Turning down the heat: The case for mild hyperthermia and thermosensitive liposomes. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102484. [Google Scholar] [CrossRef]
- Sawpari, R.; Samanta, S.; Banerjee, J.; Das, S.; Dash, S.S.; Ahmed, R.; Giri, B.; Dash, S.K. Recent advances and futuristic potentials of nano-tailored doxorubicin for prostate cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 81, 104212. [Google Scholar] [CrossRef]
- Borys, N.; Dewhirst, M.W. Drug development of lyso-thermosensitive liposomal doxorubicin: Combining hyperthermia and thermosensitive drug delivery. Adv. Drug Deliv. Rev. 2021, 178, 113985. [Google Scholar] [CrossRef]
- Aloss, K.; Bokhari, S.M.Z.; Leroy Viana, P.H.; Giunashvili, N.; Schvarcz, C.A.; Szénási, G.; Bócsi, D.; Koós, Z.; Storm, G.; Miklós, Z.; et al. Modulated Electro-Hyperthermia Accelerates Tumor Delivery and Improves Anticancer Activity of Doxorubicin Encapsulated in Lyso-Thermosensitive Liposomes in 4T1-Tumor-Bearing Mice. Int. J. Mol. Sci. 2024, 25, 3101. [Google Scholar] [CrossRef]
- Spiers, L.; Gray, M.; Lyon, P.; Sivakumar, S.; Bekkali, N.; Scott, S.; Collins, L.; Carlisle, R.; Wu, F.; Middleton, M.; et al. Clinical trial protocol for PanDox: A phase I study of targeted chemotherapy delivery to non-resectable primary pancreatic tumours using thermosensitive liposomal doxorubicin (ThermoDox®) and focused ultrasound. BMC Cancer 2023, 23, 896. [Google Scholar] [CrossRef]
- Koehler, J.K.; Schmager, S.; Schnur, J.; Gedda, L.; Edwards, K.; Heerklotz, H.; Massing, U. Novel thermosensitive small multilamellar lipid nanoparticles with promising release characteristics made by dual centrifugation. Eur. J. Pharm. Sci. 2025, 206, 106999. [Google Scholar] [CrossRef]
- Einolghasi, F.B.; Zeinali, B.; Vafai, K.; Mojra, A. Development of a prediction model for hyperthermia-enhanced drug delivery using thermosensitive nanoparticles. J. Therm. Biol. 2025, 129, 104124. [Google Scholar] [CrossRef]
- Sun, R.; Chen, H.; Wang, M.; Yoshitomi, T.; Takeguchi, M.; Kawazoe, N.; Yang, Y.; Chen, G. Smart composite scaffold to synchronize magnetic hyperthermia and chemotherapy for efficient breast cancer therapy. Biomaterials 2024, 307, 122511. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, M.; Espinoza, M.I.M.; Zennaro, C.; Bossi, F.; Lonati, C.; Oldoni, S.; Castellano, G.; Alfieri, C.; Messa, P.; et al. Brain-Derived Neurotrophic Factor-Loaded Low-Temperature-Sensitive liposomes as a drug delivery system for repairing podocyte damage. Int. J. Pharm. 2024, 660, 124322. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lan, J.; Liu, L.; Wang, Y.; Chen, L.; Zeng, R.; Gu, D.; Hu, R.; Zhang, T.; Ding, Y. Versatile Thermo-Sensitive liposomes with HSP inhibition and Anti-Inflammation for synergistic Chemo-Photothermal to inhibit breast cancer metastasis. Int. J. Pharm. 2024, 664, 124583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, X.; Li, B.; Yan, C.; Wu, C.; He, L.; Cao, J.; Lu, F.; Chen, H.; Li, W. Potent cancer therapy by liposome microstructure tailoring with active-to-passive targeting and shell-to-core thermosensitive features. Mater. Today Bio 2024, 26, 101035. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, H.; Raza, F.; Zafar, H.; Ge, Y.; Wang, N.; Zheng, R.; Zhang, D.; Yang, Y. Thermosensitive drug-loaded liposomes for photothermal and chemotherapeutic treatment of colon cancer. Mater. Adv. 2024, 5, 2456–2469. [Google Scholar] [CrossRef]
- Rubio-Camacho, M.; Cuestas-Ayllón, C.; Torres-Herrero, B.; José Martínez-Tomé, M.; Fuente, J.M.d.l.; Reyes Mateo, C. Harnessing the power of thermosensitive liposomes with gold nanoprisms and silica for controlled drug delivery in combined chemotherapy and phototherapy. RSC Adv. 2024, 14, 23073–23082. [Google Scholar] [CrossRef]
- Wang, X.; Allen, C. Synergistic effects of thermosensitive liposomal doxorubicin, mild hyperthermia, and radiotherapy in breast cancer management: An orthotopic mouse model study. Drug Deliv. Transl. Res. 2025, 15, 1011–1022. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, K.; He, X.; Han, H.; Li, L.; Li, M.; Shen, Q. Nanoscale Gambogic Acid Liposomes for NIR-II Photoacoustic Imaging and Photothermal-Chemo Tumor Therapy. ACS Appl. Nano Mater. 2024, 7, 17688–17696. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Abd-Algaleel, S.A.; Metwally, A.A.; Abdel-Bar, H.M.; Kassem, D.H.; Hathout, R.M. Synchronizing In Silico, In Vitro, and In Vivo Studies for the Successful Nose to Brain Delivery of an Anticancer Molecule. Mol. Pharm. 2021, 18, 3763–3776. [Google Scholar] [CrossRef]
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int. J. Pharm. 2003, 257, 153–160. [Google Scholar] [CrossRef]
- Schubert, M.A.; Müller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles—Evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Zielinska, A.; Silva, A.M. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharm. Dev. Technol. 2019, 24, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Esposito, E.; Luca, G.; Nastruzzi, C. Production of lipospheres as carriers for bioactive compounds. Biomaterials 2002, 23, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Gaur, P.K.; Puri, D.; Shehkar, P.; Kumar, S.S. Solid Lipid Nanoparticles Approach for Lymphatic Targeting through Intraduodenal Delivery of Quetiapine Fumarate. Curr. Drug Deliv. 2018, 15, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Garcia, M.J.; Azemar, N.; Solans, C. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef]
- Charcosset, C.; El-Harati, A.; Fessi, H. Preparation of solid lipid nanoparticles using a membrane contactor. J. Control. Release 2005, 108, 112–120. [Google Scholar] [CrossRef]
- Khayata, N.; Abdelwahed, W.; Chehna, M.F.; Charcosset, C.; Fessi, H. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef]
- Nguyen, T.; Duong, V. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Mathur, V.; Satrawala, Y.; Rajput, M.S.; Kumar, P.; Shrivastava, P.; Vishvkarma, A. Solid lipid nanoparticles in cancer therapy. Int. J. Drug Deliv. 2010, 2, 192–199. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Mühlen, A.Z.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Caputo, O.; Gasco, M.R. Preparation and characterization of solid lipid nanospheres containing paclitaxel. Eur. J. Pharm. Sci. 2000, 10, 305–309. [Google Scholar] [CrossRef]
- Cavalli, R.; Caputo, O.; Gasco, M.R. Solid lipospheres of doxorubicin and idarubicin. Int. J. Pharm. 1993, 89, R9–R12. [Google Scholar] [CrossRef]
- Ugazio, E.; Cavalli, R.; Gasco, M.R. Incorporation of cyclosporin A in solid lipid nanoparticles (SLN). Int J Pharm 2002, 241, 341–344. [Google Scholar] [CrossRef]
- Sivadasan, D.; Ramakrishnan, K.; Mahendran, J.; Ranganathan, H.; Karuppaiah, A.; Rahman, H. Solid Lipid Nanoparticles: Applications and Prospects in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6199. [Google Scholar] [CrossRef]
- Din, F.U.; Jin, S.G.; Choi, H. Particle and Gel Characterization of Irinotecan-Loaded Double-Reverse Thermosensitive Hydrogel. Polymers 2021, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, S.G.; Ambade, A.V. Poly(N-vinylcaprolactam) containing solid lipid polymer hybrid nanoparticles for controlled delivery of a hydrophilic drug gemcitabine hydrochloride††Electronic supplementary information (ESI) available. RSC Adv. 2022, 12, 17621–17628. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Chon, J.; Kim, Y.; Lee, H.; Oh, D.; Lee, H.; Han, C.; Kim, D.; Park, C. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int. J. Nanomed. 2019, 14, 5381–5396. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid lipid nanoparticles for thermoresponsive targeting: Evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int. J. Nanomed. 2017, 12, 8325–8336. [Google Scholar] [CrossRef]
- Li, M.; Fang, G.; Zahid, F.; Saleem, R.; Ishrat, G.; Ali, Z.; Naeem, M.; Din, F.u. Co-delivery of paclitaxel and curcumin loaded solid lipid nanoparticles for improved targeting of lung cancer: In vitro and in vivo investigation. Heliyon 2024, 10, e30290. [Google Scholar] [CrossRef]
- da Silva, M.J.F.; Rodrigues, A.M.; Costa, M.C.P.; Camara, A.L.; Cabral, L.M.; Ricci Junior, E.; Vanzan, D.F.; Matos, A.P.d.S.; da Silva Honorio, T.; Borges, A.C.R. Solid Lipid Nanoparticles Based on Babassu Oil and Copaiba Oleoresin: A Promising Approach for Prostate Cancer Therapy. Nanomaterials 2024, 14, 1014. [Google Scholar] [CrossRef]
- German-Cortés, J.; Vilar-Hernández, M.; Rafael, D.; Abasolo, I.; Andrade, F. Solid Lipid Nanoparticles: Multitasking Nano-Carriers for Cancer Treatment. Pharmaceutics 2023, 15, 831. [Google Scholar] [CrossRef]
- An, R.; Zhang, Q.; Du, D.; Sun, C.; Zhang, S.; Wu, J.; Younis, M.A.; Ge, H.; Wang, K.; Younis, M.R.; et al. Ultrasmall polylysine dendrimer nanodots for glutathione-responsive targeted chemotherapy and enhanced photodynamic/immunotherapy of metastatic cancer. Chem. Eng. J. 2025, 519, 165522. [Google Scholar] [CrossRef]
- Ahmed, Y.W.; Wu, T.; Tsai, H.; Candra, A.; Kitaw, S.L.; Anley, B.E.; Thankachan, D.; Hong, Z.X.; Chuang, S.; Khan, M.H.; et al. Synergistic Local Delivery of Gemcitabine and Resiquimod (R848) via Janus Micelles Encapsulated in a Dual-Responsive Hydrogel for Subcutaneous Glioblastoma Treatment Models. J. Drug Deliv. Sci. Technol. 2025, 112, 107255. [Google Scholar] [CrossRef]
- Casadidio, C.; Fens, M.H.A.M.; Fliervoet, L.A.L.; Censi, R.; Vermonden, T. Injectable thermosensitive hydrogel for local and controlled delivery of siRNA-STAT3 polyplexes to treat advanced-stage ovarian cancer. J. Control. Release 2025, 384, 113890. [Google Scholar] [CrossRef]
- Wang, F.; Lai, W.; Xie, D.; Zhou, M.; Wang, J.; Xu, R.; Zhang, R.; Li, G. Nanoparticle-mediated celastrol ER targeting delivery amplify immunogenic cell death in melanoma. J. Adv. Res. 2025, 71, 585–601. [Google Scholar] [CrossRef]
- Gao, Y.; Wen, T.; Shan, B.; Meng, M.; Bai, J.; Tian, W.; Li, C. Effects of the mitoxantrone thermosensitive liposome nanodelivery system on prostate cancer in vivo and in vitro. Sci. Rep. 2025, 15, 15940. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Duan, H.; Shi, Y.; Zhou, Z.; Wei, Z.; Qian, X.; Lu, J.; Jiang, Y.; Mao, F.; Xue, N.; et al. Ultrasound-mediated paclitaxel-loaded EGFR nanoparticles for targeted therapy in breast cancer. RSC Adv. 2025, 15, 23136–23145. [Google Scholar] [CrossRef] [PubMed]
- Tak, W.Y.; Lin, S.; Wang, Y.; Zheng, J.; Vecchione, A.; Park, S.Y.; Chen, M.H.; Wong, S.; Xu, R.; Peng, C.; et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin. Cancer Res. 2018, 24, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zagar, T.M.; Vujaskovic, Z.; Formenti, S.; Rugo, H.; Muggia, F.; O’Connor, B.; Myerson, R.; Stauffer, P.; Hsu, I.; Diederich, C.; et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperth. 2014, 30, 285–294. [Google Scholar] [CrossRef]
- de Maar, J.S.; Suelmann, B.B.M.; Braat, M.N.G.J.A.; Diest, P.J.v.; Vaessen, H.H.B.; Witkamp, A.J.; Linn, S.C.; Moonen, C.T.W.; Wall, E.v.d.; Deckers, R. Phase I feasibility study of Magnetic Resonance guided High Intensity Focused Ultrasound-induced hyperthermia, Lyso-Thermosensitive Liposomal Doxorubicin and cyclophosphamide in de novo stage IV breast cancer patients: Study protocol of the i-GO study. BMJ Open 2020, 10, e040162. [Google Scholar] [CrossRef]
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef]
- Tian, M.; Dong, B.; Li, W.; Wang, L.; Yu, H. Applications of Novel Microscale and Nanoscale Materials for Theranostics: From Design to Clinical Translation. Pharmaceutics 2024, 16, 1339. [Google Scholar] [CrossRef]
- Sinha, S.; Tripathi, A.K.; Pandey, A.; Naik, P.; Pandey, A.; Verma, V.S. Self-assembled PEGylated micelles for precise and targeted drug delivery: Current challenges and future directions. Biocatal. Agric. Biotechnol. 2024, 60, 103296. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Koland, M.; Narayanan Vadakkepushpakath, A.; John, A.; Tharamelveliyil Rajendran, A.; Raghunath, I. Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery. Gels 2022, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, V.C.; Martin, L.; Bica, M.; Vasile, R.C.; Gresita, A.; Bunescu, M.; Ruscu, M.A.; Aldea, M.; Rotaru-Zavaleanu, A.D. Hydrogel-Based Innovations in Carpal Tunnel Syndrome: Bridging Pathophysiological Complexities and Translational Therapeutic Gaps. Gels 2025, 11, 52. [Google Scholar] [CrossRef]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef]
- Khatik, A.S.; Kurdhane, S.; Batheja, S.; Gupta, U. Chapter 10—Dendrimers: Promises and challenges in drug delivery. In Molecular Pharmaceutics and Nano Drug Delivery; Gupta, U., Goyal, A.K., Eds.; Academica Press: Cambridge, MA, USA, 2024. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Needham, D.; Dewhirst, M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef]
- Wang, Y.; Grainger, D.W. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Front. Drug Deliv. 2022, 2, 901281. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M.; et al. Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. pH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef]
- Kim, J.; Choi, W.; Park, E.; Kang, Y.; Lee, K.J.; Kim, H.H.; Kim, W.J.; Kim, C. Real-Time Photoacoustic Thermometry Combined with Clinical Ultrasound Imaging and High-Intensity Focused Ultrasound. IEEE Trans. Biomed. Eng. 2019, 66, 3330–3338. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.; Kanase, A.; Siewert, K.; Rosenkranz, D.; Singh, R.; Laux, P.; Luch, A. Machine-Learning-Based Approach to Decode the Influence of Nanomaterial Properties on Their Interaction with Cells. ACS Appl. Mater. Interfaces 2021, 13, 1943–1955. [Google Scholar] [CrossRef]



| Polymer Name | Applications | Polymerization Methods | Properties |
|---|---|---|---|
| Poly(N-isopropyl acrylamide) (PNIPAAm) | Drug and gene delivery, bioseparation, and cell culture | Free radical polymerization, living radical polymerization | Temperature-responsive when mixed with water and organic solvents. Solvent–polymer interactions, coexistence of LCST and UCST, concentration-dependent behavior, LCST stability, amphiphilic end groups influence the LCST, LCST increases with ionic surfactants, polyelectrolyte behavior, entropy of counterions, the LCST of PNIPAM is influenced by the type of salt present in the solution. |
| Multi-block copolymers (ABA, BAB, etc.) | Drug delivery, tissue engineering, and injectable gels | Atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain-transfer (RAFT), oxyanionic | Self-assemble and stabilize in solution. It can promote microphase separation and facilitate the microphase separation within gels (either physical or covalently linked gels). |
| Star-shaped poly (2-alkyl-2- oxazolines) | Sensors, rheological additives, and multiple biological applications, including drug delivery | Divergent (“core-first”) and the convergent (“arm-first”) | Self-association in solution depends on solvent composition, temperature, and the microscopy analysis. |
| Poly(N-vinylcaprolactam) (PVCL) | Biosensing, controlled drug delivery, and stimuli-dependent targeting | RAFT, ring-opening polymerization (ROP) combined with ATRP, and cobalt-mediated radical polymerization (CMRP) | The phase transition in solution occurs above 35 °C upon heating. The temperature response is dependent on its molecular weight, polymer concentration, polymer chemical composition, cosolvent, ionic strength, and surfactants. |
| Components | Payload | Cancer Cell Line | Synthesize Method | Temperature-Triggered Release | Ref. |
|---|---|---|---|---|---|
| PVS-b-PNVCL PVL-b-PNVCL | DOX | HEK 293 T HeLa | RAFT | PVS18-b-PNVCL35 released 46–69% within 24 h and 53–90% within 72 h at different temperatures (25–37 °C). DOX release from PVS18-b-PNVCL95 was 78% within 72 h. Free DOX was released at 64.2% in 2 h and approximately 90% in 10 h. | [63] |
| Alg-g-PNIPAAm | Dipyridamole (DP) | HEK293T HeLa HCT116 | RAFT | The transmittance value decreased to 50% of its initial value at 32 °C. | [64] |
| SP94-PEG-p(AAm-co-AN)/LEN/IR-1061-AcD, SPLI | Lenvatinib (LEN) | H22 AML-12 Luc-H22 | Free Radical Polymerization | H22 cells treated with Nile red co-loaded micelles showed increased fluorescence under NIR laser, indicating micelle destruction and enhanced cellular release of Nile red. SPLI/Nile red exhibited higher fluorescence intensity, demonstrating better uptake by the cells. | [65] |
| PME3CL-b-PBnCL PME3CL-b-PPhCL PME3CL-b-PEtOPhCL | DOX | MDA-MB-231 | ROP | The initial release rate of PME3CL-b-PEtOPhCL micelles was the highest. (60% after 8 h). A polymer with a lower LCST shows a faster release. | [66] |
| Chit5-SA-20 Chit5-OA-20 Chit5-MUA-20 Chit5-LA-20 | DOX | A549 HEK293T | Not specified | The rate of Dox release increases by 1.5–3 times with temperature increases from 25 °C to 42 °C. The intensity of all peaks, especially N–H and O–H fluctuations, increases in the temperature range of 22–45 °C. | [67] |
| PNIPAM–PEG–PNIPAM | Zinc Protoporphyrin (ZnPP) | PC3 | RAFT | The ZnPP release was higher at 27 °C than at 37 °C. The ZnPP release was 48.2% and 11.35% within 0.5 h at 27 °C and 37 °C, respectively. The ZnPP release was 90.4% and 59.6% within 48 h and 93.1% and 62.45% within 72 h at 27 and 37 °C, respectively. | [68] |
| PNOG-b-PNAG-b-PNMG | Ciprofoxacin (Cip) | NCTC clone 929 (L-929) | ROP | The release rate of Cip without polymer was 100% in 3 h. The drug release rate with polymer wrapping was 56% at 20 h. | [69] |
| Components | Payload | Cancer Cell Line/Animal Model | Synthesize Method | Release Main Findings | Ref. |
|---|---|---|---|---|---|
| PDLLA-PEG-PDLLA, PLEL | Gemcitabine (GEM) Cytosine-phosphate-guanine oligonucleotide (CpG-ODN) | MB49 C57BL6 | Ring-Opening Copolymerization | The GEM release rates at 24 h and 10 d were 57.7% and 90.8%, respectively. The CpG release at 24 h and 96 h was 60.8% and 94.2%, respectively. Both were released entirely from PLEL on day 7. | [77] |
| mPEG2k-PAla32-block-PAsp5 | RMPs@Mn2+ | B16-F10 H22 C57BL/6 BALB/c | Not specified | At pH 6.8, Mn2+ release reached 82.6%.At pH 7.4, Mn2+ release reached 60.3%.The free Mn2+ rapidly accumulated in the liver only 15 min after injection. Gel@Mn2+ slowly released Mn2+. | [78] |
| Cy7-Cell@hydrogel (CT26-loaded Pluronic® F-127/gelatin) | Cyanine dyes (Cy7) | CT26 | Physical Crosslinking | (40 μg/mL) Cy7 exhibited a significant temperature increase (45 °C) at a laser power density of 0.9 W/cm2. The Cy7-Cell@hydrogel system can efficiently convert light into heat energy for up to 80 min. | [79] |
| PLGA-PEG-PLGA | Epidermal growth factor (EGF) | NCG HeLa | Ring-opening Polymerization | The tumor grew significantly faster in the free EGF group than in the hydrogel-EGF group. The weight and volume of tumors from the control group were significantly larger than those in the hydrogel-EGF group. HeLa cells showed more substantial growth potential in free EGF than in hydrogel-EGF. Hydrogels’ sustained EGF release behavior could effectively inhibit tumor growth. | [80] |
| HTPM and AgNPs-gel C6/HTPM and AgNPs-gel | Halofuginone hydrobromide (HF) Ag+ Coumarin 6 (C6) | HUVEC MDA-MB-231 MDA-MB-231-luc Eph4-ev BALB/c | Physical Swelling Method | Compared with the 60% release of the HF loaded into the HTPM-gel, the release pattern of the HF loaded into the HTPM and AgNPs-gel was slow and constant over 24 h. Compared with the 40% release of the AgNPs loaded into the AgNPs-gel, the release pattern of the AgNPs-gel loaded into HTPM and AgNPs-gel was slow and constant over 12 h. | [81] |
| Halofuginone-loaded D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) polymer micelles nano-thermosensitive hydrogels (HTPM-gel) | Halofuginone | CMT-U27Eph4-evMDCK | Physical Swelling Method | Within 24 h, under a pH 6.5 environment, the CRP of halofuginone from HTPM rose to 64.48%. The CRP of halofuginone from HTPM-gel was approximately 71.18% within 24 h. | [82] |
| FCDL and DSF-SE@G | Cu2+ Disulfiram (DSF) Glycyrrhizic acid-Cu (FCDL) | MGC-803 Balb/C | Reverse-phase Evaporation method | Cu2+ and DSF release in the FCDL and DSF-SE@G was 53% and 46%, respectively, which results in sustained drug release. | [83] |
| PDLLA-PEG-PDLLA (PLEL) | Cabazitaxel (CTX) | HCT-15 HCT-116 Balb/c-nu | Ring-opening Polymerization | At 48 h, the drug release rate of CTX/PLEL was approximately 11.4%. At 96 h, the release rate was only approximately 16.8%. The loading of PLEL significantly slowed the in vitro drug release of CTX. | [84] |
| DOPA-rGO@PC-gel | Dopamine-reduced graphene oxide (DOPA-rGO; photothermal nanoagent) | NHDF MCF-7 | Dual-crosslinking Method | DOPA-rGO@PC-gel could produce a similar photothermal heating (ΔT ≈ 22 °C) at a considerably lower concentration and laser intensity (99.94 µg/mL of DOPA-rGO; 1.7 W/cm2), but it required a slightly longer irradiation time (10 min). These results further confirm the good photothermal capacity of the DOPA-rGO@PC-gel. | [85] |
| Components | Payload | Cancer Cell Line | Synthesize Method | Release Main Findings | Ref. |
|---|---|---|---|---|---|
| Fucosylated PAMAM Dendrimers | Chrysin (CRY) | A549 (Human Lung Cancer) | Fucosylation via Schiff’s base formation with fucose | At 37 °C, CRY showed sustained release in two media: pH 5 (endolysosomal conditions): 87.6% released after 24 h pH 7.4 (plasma conditions): 93.6% released after 24 h. | [103] |
| PSMA-Targeted Dendrimer Nanoplatform (PD-CTT1298-Cabo) | Cabozantinib (Cabo) | Prostate-specific membrane antigen (PSMA). PSMA-positive PC3-PIP cells and PSMA-negative PC3 cells. PSMA-positive PC3-PIP tumor xenograft mouse model | Strain-promoted azide–alkyne cycloaddition (SPAAC) chemistry | The release rates were monitored through incubation at 37 °C: Plasma-like conditions (pH 7.4, phosphate-buffered saline): Drug release occurred gradually under these neutral pH conditions. Simulates systemic circulation where slower release is favorable. Intratumoral conditions (pH 5.5, citrate buffer, containing esterase): Drug release was significantly faster under these acidic conditions. Mimics the tumor microenvironment with lower pH and enzyme activity, promoting rapid drug delivery. | [104] |
| Nanodiamonds (NDs) modified with PNIPAM Hyper-branched dendrimers | Docetaxel (DXL) | Breast cancer | Surface functionalization Polymer grafting Dendrimer attachment Drug adsorption | 99.78% release at 45 °C (pH 5.6) in 6 h 69.58% release at 45 °C (pH 7.4) in 6 h 90.97% release in 20 min under NIR laser irradiation (808 nm, 1 W/cm2) Faster release due to polymer phase transition (LCST~32 °C). | [105] |
| PNIPAM/FeRh composite. | DOX | Not specified (general cancer model) | Solvent casting of PNIPAM on FeRh alloy; laser modification to create drug wells | Triggered by LCST (~32 °C) using magnetic field-induced cooling. | [101] |
| Amphiphilic dendrimer-like copolymer with a hydrophobic poly(styrene) core and hydrophilic PEO shell | Benzyl halide (benzyl chloride, benzyl bromide) | Not mentioned | An iterative divergent process involving anionic polymerization, hydrosilylation, chlorosilane coupling, and olefin cross-metathesis reaction | Release occurs due to the thermal responsiveness of the PEO segments forming the hydrophilic shell. LCST of PEO segments (~65 °C): Below LCST: PEO segments are hydrated, forming stable unimolecular micelles. Above LCST: PEO segments shrink, leading to aggregation and potential release of encapsulated hydrophobic molecules. | [100,101] |
| Components | Payload | Cancer Cell Line/Animal Model | Synthesize Method | Release Main Findings | Ref. |
|---|---|---|---|---|---|
| Dox-Lipo/Gel/PGA/FeNP | DOX | MDA-MB-231-Luc | Solvothermal method and thin-film hydration method | DOX was released from the liposomes, while the liposomes were not released from the scaffold during incubation in the magnetic hyperthermia environment. | [124] |
| BDNF-LTSL-cRGD or BDNF-LTSL-HIT | Neurotrophin Brain-Derived Neurotrophic Factor (BDNF) | Mouse microvascular endothelial cell line (EOMA), Rat glomerular endothelial cell (rGECs), 7-week-old Sprague Dawley rats | Lipid film hydration and extrusion method | BDNF delivery and permeation across the co-culture filter were achieved by pre-heating BDNF-LTSL-cRGD at 42 °C for 30 min, which enabled the thermoresponsive release of the payload. | [125] |
| BI-FA-LP | Berberine (BBR), ICG (an FDA-approved photothermal agent (PTA)) | 4 T1 and RAW264.7 cell lines, Female BALB/C mice (4–5 weeks old) | pH gradient method | After irradiation with an 808 nm laser, 47.26 ± 0.53% of BBR and 56.39 ± 3.39% of ICG were released from BI-FA-LP. The thermosensitive drug release ability of BI-FA-LP provided the basis for sustained release and long circulation of liposomes in vivo. | [126] |
| PC + Mal-PEG-DSPE PC + Mal-PEG-DSPE + K3 PC + Mal-PEG-DSPE + 09JA PC + Mal-PEG-DSPE + K3 + 09JA Anti-HER-2-Fab modified liposome | DOX | The human stomach cancer cell N87, Balb/c nude mice, female, 4–6 weeks, ~20 g | Not specified | Compared to DOX, the suspension exhibited a rapid increase in drug release over time and eventually reached a plateau. No significant leakage of DOX from samples a, b, c, and d was observed after incubation for 60 min at 37 °C. The release of free DOX molecules was examined as a control group, which showed a complete cumulative release within 12 h. The releases of liposomes c and d were potentiated at 42 °C and exhibited thermo-triggered burst release of DOX. | [127] |
| BiNSs/Met/5-FU@TSL | 5-fluorouracil (5-FU) and metformin (Met) | HT29 Healthy female Nod/scid mice (5–6 weeks old, 15–18 g) | Reverse phase evaporation method | In vitro drug release behavior of BiNSs/Met/5-FU@TSL is temperature-dependent. The release of the drugs is significantly accelerated at higher temperatures, resulting in a greater cumulative release within the specified time frame. | [128] |
| BioSi@NPs + TSLs + AuNPRs | Carboxy fluorescein (CF) | Not specified | Thin-film hydration method | A clear relationship was demonstrated between the release percentage and the applied power or exposure time, allowing for precise regulation of the fluorescent probe’s release kinetics. | [129] |
| LTLD + mEHT | DOX | The 4T1 triple-negative breast cancer (TNBC) cell line Six–eight-week-old female BALB/c mice | Not specified | LTLD releases 80% of DOX into the bloodstream in the heated tumor. mEHT did not enhance the DOX accumulation from PLD in the tumor. Strong proliferation inhibition was observed when DOX was combined with mEHT. LTLD caused stronger inhibition of proliferation than PLD. | [120] |
| ThermoDXR | DOX | 4T1 cells female BALB/c mice at the age of 6–8 weeks | Thin film hydration method | The combination of the thermosensitive liposome formulation of doxorubicin and radiation therapy (RT), together with hyperthermia at reduced doses of both the drug and RT, not only induced a strong therapeutic effect but also reduced treatment-related toxicities. | [130] |
| NIR-II Photoexcited Lip(NiPTZ-GA) | Gambogic acid (GA) | Five- or six-week-old BALB/c mice 4T1 tumor–bearing mice | Not specified | Liposomes composed of GA-PEG, DSPE-PEG2000, and DPPC decomposed under 1064 nm laser irradiation, releasing NiPTZ. NiPTZ generated elevated temperatures for efficient NIR-II photoacoustic imaging (PAI) and photothermal therapy (PTT). Lip(NiPTZ-GA) demonstrated a high PTT conversion efficiency of 49%. The combination of PTT and chemotherapy achieved optimal therapeutic outcomes in vivo studies. | [131] |
| Components | Payload | Cancer Cell Line | Synthesize Method | Main Findings | Ref. |
|---|---|---|---|---|---|
| PAC-CUR-SLNs | Paclitaxel (PAC) Curcumin (CUR) | A549 (Lung Cancer) BALB/c Mice | High-Pressure Homogenization (HPH) | In vitro Release (37 °C): Sustained release observed: ~41% PAC, ~29% CUR released in 6 h. ~97–98% released over 96 h. | [158] |
| SLN-BBS-COPA | Lauric acid (BBS) β-Caryophyllene (COPA) | PC-3: Androgen-independent human prostate cancer cell line DU-145: Androgen-independent human prostate cancer cell line | Emulsification–ultrasonication | Refrigerated samples (8 °C) maintained stability over 60 days, while samples at 25 °C showed increased hydrodynamic diameter (HD) and PDI over time. | [159] |
| Lauric acid + Oleic/Linoleic acid | 5-Fluorouracil | Human gingival fibroblast (HGF; PCS-201-108) Human breast adenocarcinoma cells (MDA-MB-231, HTB-26) | High-pressure homogenization | At 37 °C (normal physiological conditions): Sustained drug release observed. At 40–42 °C (mimicking tumor or hyperthermic conditions): Increased drug release, improving drug availability in targeted regions. | [157] |
| Superparamagnetic iron oxide NPs (SPIONs) | Dox | Not specified | Emulsification and solvent evaporation | At 37 °C: Minimal drug release under normal conditions. At 40–45 °C: Triggered release of doxorubicin due to temperature rise, synergistically enhanced by external magnetic fields and acidic tumor. | [160] |
| Nanoparticle Type | Targeting Mechanism | Animal Model | Remarks | Ref. |
|---|---|---|---|---|
| Ultrasmall dendrimer nanodots | Near-infrared (NIR) laser activation for photodynamic therapy (PDT). | Orthotopic 4T1 breast tumor model in mice. | 99% inhibition of primary tumor growth. Induced strong immunogenic cell death (ICD): CRT exposure, ATP/HMGB1 release, dendritic cell maturation. 98.5% suppression of lung metastasis Enhanced CD8+ T-cell infiltration and systemic antitumor immunity. | [161] |
| PLGA-core nanoparticle with DSPE-PEG modified by p-tosylethylenediamine (TSE-CEL/NP) | Triggered by external ultrasound. | C57BL/6 mice bearing B16F10 melanoma (primary, bilateral, and lung metastasis models). | Preferential accumulation in endoplasmic reticulum (ER) leads to strong ER stress (↑ p-IRE1α, ATF6, p-eIF2α; ↑ ATF4, GRP78, XBP1). Enhanced ICD markers: CRT exposure, HMGB1 and ATP release. Boosted immune response: ↑ immature and mature DCs, CD4+ and CD8+ T-cell infiltration, IFN-γ+/TNF-α+ CD8+ T-cells. Superior antitumor activity: strongest tumor inhibition across all models, including distant tumors and lung metastases. Excellent biocompatibility, with no significant body weight changes or organ toxicity. (↑ indicates an upregulation or increased expression/infiltration of the listed molecules or cell types as a result of treatment. For example, ER stress markers (IRE1α, ATF6, etc.) and immune components (DCs, CD4⁺, CD8⁺ T-cells) were elevated, reflecting activation of stress response and enhanced antitumor immunity.) | [164] |
| Mitoxantrone thermosensitive liposome (MTX-TSL) | Local hyperthermia at 41 °C, applied just before drug administration. External water-bath heating (localized heating). | BDF1 mice bearing RM-1 prostate tumors. | Hyperthermia-enhanced tumor accumulation of MTX-TSL. Significant tumor growth suppression in MTX-TSL + heat group vs. free drug ~80 % drug release within 30 min at 41 °C; ~96 % at 45 min. | [165] |
| EGFR-targeted PLGA nanoparticles encapsulating paclitaxel and perfluoropentane (PFP) | External ultrasound. | In vivo triple-negative breast cancer (TNBC) xenograft model in mice (MDA-MB-231). | PTX TNPs + ultrasound achieved the most potent tumor growth inhibition (tumor volume ~2.66 ± 1.72 vs. ≥5 in other groups). Marked reduction in microvessel density (CD31) and proliferation marker (Ki-67). Significant induction of apoptosis, with minimal systemic toxicity (no elevated ALT/AST or organ histopathology). | [166] |
| Thermosensitive hydrogel | Passive thermosensitive gelation (body heat triggered). | Orthotopic SKOV3-luc ovarian cancer model in athymic nude (BALB/c) mice via intraperitoneal inoculation. | Sustained release: Hydrogel erosion over 7 days with progressive siRNA accumulation in nodules. Tumor growth delay: Significant tumor suppression in treatment group (single injection), sustained through day 56 post-inoculation. Safety: No systemic toxicity or histopathological damage in major organs. | [163] |
| Janus micelles encapsulated within a thermosensitive hydrogel | Thermosensitive gelation: The micelle–hydrogel system transitions into a gel at physiological temperature (~37 °C). | Subcutaneous GBM model in mice. | Tumor volume reduction: 89.5 ± 3.34%. Immunostimulatory effects: Increased expression of CD80, NF-κB, IFN-γ, and TNF-α in both tumor and spleen tissues. Mechanism: Sequential release led to enhanced early apoptosis and reduced necrosis; dual-drug delivery amplified chemo-immunotherapy response. | [162] |
| Nanocarrier Type | Formulation | Payload | Indication | Trial Phase | NCT | Ref. |
|---|---|---|---|---|---|---|
| Lysolipid-based thermosensitive liposome | ThermoDox® | DOX | Hepatocellular carcinoma (HCC) | Phase III | NCT02112656 | [167] |
| Thermosensitive liposomes | ThermoDox® (TARDOX) | DOX | Liver tumors | Phase I | NCT02181075 | [33] |
| Thermosensitive liposomes | ThermoDox® | DOX | Recurrent chest wall breast cancer (CWR) | Phase I/II | NCT00826085 | [168] |
| Lyso-thermosensitive liposome | ThermoDox® | DOX | Metastatic breast cancer | Phase I | NCT03749850 | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaramiri, A.; Asalh, R.A.; Asalh, M.A.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 7322. https://doi.org/10.3390/ijms26157322
Yaramiri A, Asalh RA, Asalh MA, AlSawaftah N, Abuwatfa WH, Husseini GA. A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(15):7322. https://doi.org/10.3390/ijms26157322
Chicago/Turabian StyleYaramiri, Atena, Rand Abo Asalh, Majd Abo Asalh, Nour AlSawaftah, Waad H. Abuwatfa, and Ghaleb A. Husseini. 2025. "A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy" International Journal of Molecular Sciences 26, no. 15: 7322. https://doi.org/10.3390/ijms26157322
APA StyleYaramiri, A., Asalh, R. A., Asalh, M. A., AlSawaftah, N., Abuwatfa, W. H., & Husseini, G. A. (2025). A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy. International Journal of Molecular Sciences, 26(15), 7322. https://doi.org/10.3390/ijms26157322






