Circulating miRNA Profile in Inflammatory Bowel Disease Patients with Stress, Anxiety, and Depression
Abstract
1. Introduction
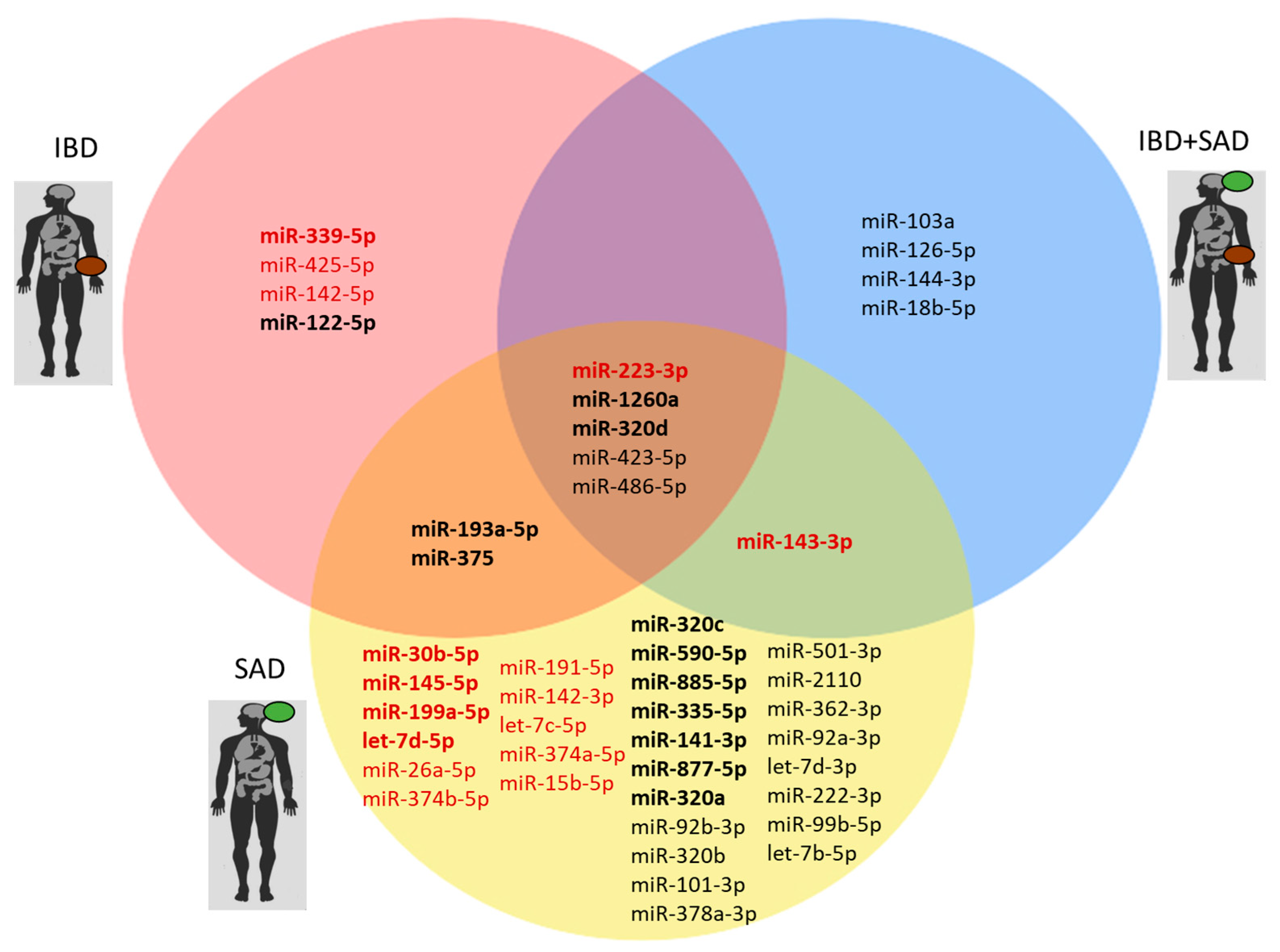
2. Results
| miRNA | Our Results (Plasma) | Author, Year | Biological Sample | Evidence |
|---|---|---|---|---|
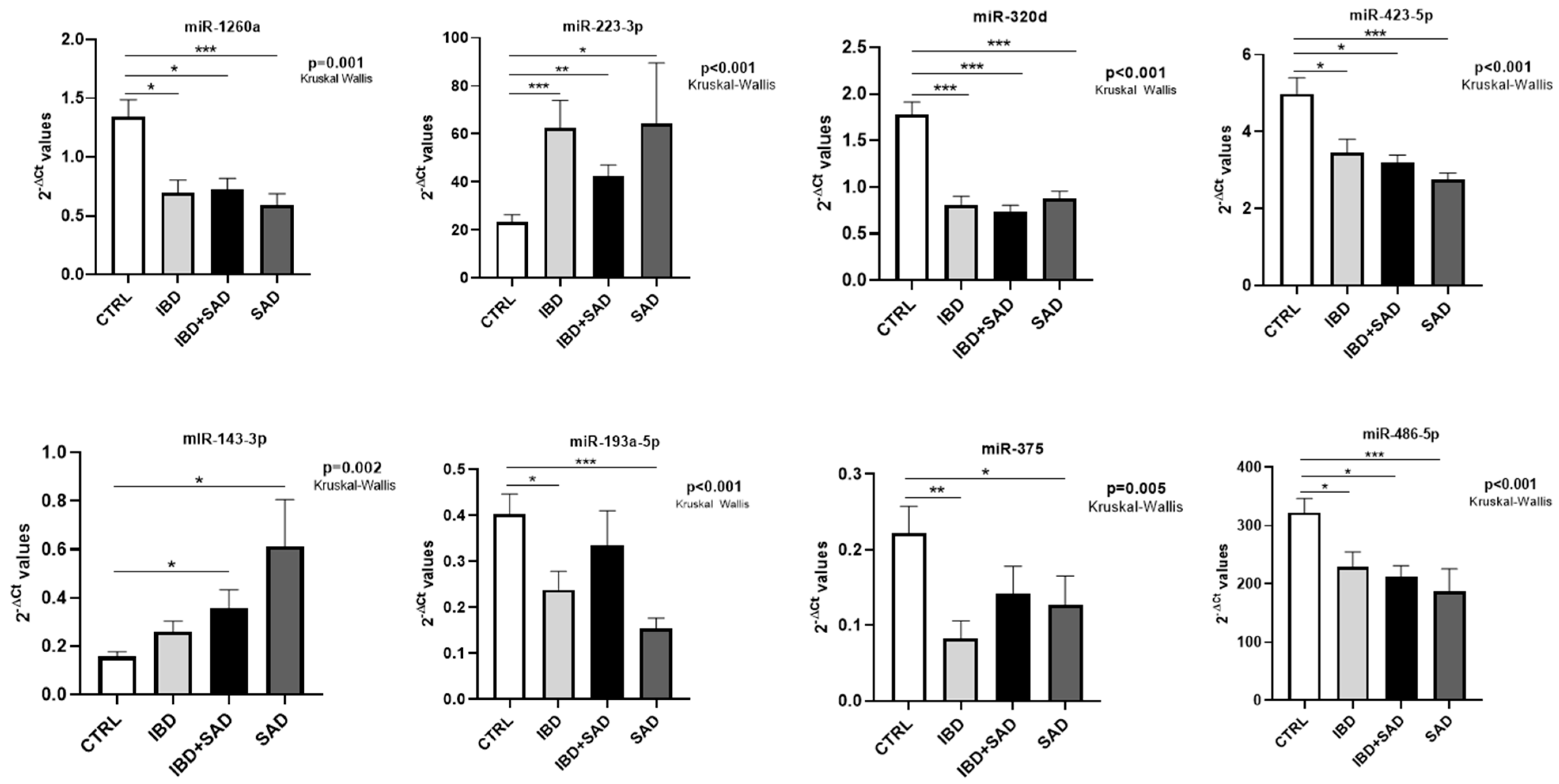
| miR-223-3p | ↑ IBD vs. CTRL FR = 2.67 p = 0.001 ↑ IBD + SAD vs. CTRL FR = 1.81 p = 0.002 ↑ SAD vs. CTRL FR = 2.74 p = 0.027 | Zhang J et al., 2023 [34] | Serum Intestinal mucosa Feces | ↑ CD vs. CTRL |
| Camkurt MH et al., 2015 [35] | Plasma | ↑ MDD vs. CTRL | ||
| miR-1260a | ↓ IBD vs. CTRL FR = −1.93 p = 0.012 ↓ IBD + SAD vs. CTRL FR = −1.87 p = 0.029 ↓ SAD vs. CTRL FR = −2.27 p = 0.001 | No data available | ||
| miR-320d | ↓ IBD vs. CTRL FR = −2.19 p < 0.001 ↓ IBD + SAD vs. CTRL FR = −2.41 p < 0.001 ↓ SAD vs. CTRL FR = −2.04 p < 0.001 | Ibrahim P et al., 2024 [36] | Brain EVs | ↓ MDD vs. CTRL |
| miR-423-5p | ↓ IBD + SAD vs. CTRL FR = −1.56 p = 0.012 ↓ SAD vs. CTRL FR = −1.81 p < 0.001 | Yoshino Y et al., 2021 [37] | dlPFC | ↑ MDD vs. CTRL |
| miR-486-5p | ↓ IBD + SAD vs. CTRL FR = −1.52 p = 0.015 ↓ SAD vs. CTRL FR = −1.73 p < 0.001 | No data available | ||
| miR-193a-5p | ↓ IBD vs. CTRL FR = −1.69 p = 0.022 ↓ SAD vs. CTRL FR = −2.61 p < 0.001 | Zhou et al., 2014 [38] | PBMCs | ↓ PTSD vs. CTRL |
| miR-375-5p | ↓ IBD vs. CTRL FR = −2.68 p = 0.005 ↓ SAD vs. CTRL FR = −1.75 p = 0.039 | Peck BCE et al., 2015 [39] | Colonic mucosa | ↓ IBD vs. CTRL |
| Wan Y et al., 2015 [40] | CSF | ↑ MDD vs. CTRL | ||
| miR-143-3p | ↑ IBD + SAD vs. CTRL FR = 2.28 p = 0.023 ↑ SAD vs. CTRL FR = 3.91 p = 0.002 | Li SQ et al., 2022 [41] | Serum [41] | ↓ MDD in association with risk of future relapse |
| miRNA Significant in SAD | PSS (Pearson Corr; p-Value) | HAMA (Pearson Corr; p-Value) | BDI (Pearson Corr; p-Value) |
|---|---|---|---|
| miR-486-5p | −0.350; 0.001 | ns | ns |
| miR-320d | ns | −0.311; 0.005 | ns |
| miR-26a-5p | ns | 0.364; 0.001 | 0.339; 0.002 |
| let-7b-5p | −0.317; 0.004 | −0.421; <0.001 | −0.394; <0.001 |
| miR-590-5p | ns | −0.332; 0.002 | −0.353; 0.001 |
| miR-320a | −0.358; 0.001 | −0.383; <0.001 | −0.377; 0.001 |
| miR-142-3p | ns | ns | 0.358; 0.001 |
| let-7d-3p | −0.351; 0.001 | −0.377; 0.001 | −0.318; 0.004 |
| miR-30b-5p | ns | ns | 0.378; 0.001 |
| let-7d-5p | ns | ns | 0.312; 0.005 |
| miR-378a-3p | ns | ns | 0.326; 0.003 |
| miR-501-3p | −0.324; 0.003 | ns | ns |
| miR-199a-5p | ns | ns | 0.446; <0.001 |
| miR-92a-3p | −0.313; 0.004 | ns | ns |
| miR-374a-5p | ns | ns | 0.384; <0.001 |
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Scale Description
4.3. miRNA Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| GI | Gastrointestinal |
| SAD | Stress, Anxiety, and Depression |
| HAM-A | Hamilton Anxiety Scale |
| BDI | Becks Depression Inventory |
| PSS | Perceived Stress Scale |
References
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. United Eur. Gastroenterol. J. 2022, 10, 1113. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, Z.; Qu, Y.; Zhao, J.; Wang, L.; Zhou, X.; Xu, Z.; Zhang, D.; Jiang, P.; et al. Targeting Programmed Cell Death in Inflammatory Bowel Disease through Natural Products: New Insights from Molecular Mechanisms to Targeted Therapies. Phytother. Res. 2025, 39, 1776–1807. [Google Scholar] [CrossRef]
- Cadwell, K.; Loke, P. Gene–Environment Interactions Shape the Host–Microbial Interface in Inflammatory Bowel Disease. Nat. Immunol. 2025, 26, 1023–1035. [Google Scholar] [CrossRef]
- Chhibba, T.; Gros, B.; King, J.A.; Windsor, J.W.; Gorospe, J.; Leibovitzh, H.; Xue, M.; Turpin, W.; Croitoru, K.; Ananthakrishnan, A.N.; et al. Environmental Risk Factors of Inflammatory Bowel Disease: Toward a Strategy of Preventative Health. J. Crohns Colitis 2025, 19, jjaf042. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yue, N.; Hu, P.; Tian, C.; Kong, C.; Zhao, H.; Zhang, Y.; Yao, J.; Wei, Y.; Li, D.; Wang, L. Dissecting Innate and Adaptive Immunity in Inflammatory Bowel Disease: Immune Compartmentalization, Microbiota Crosstalk, and Emerging Therapies. J. Inflamm. Res. 2024, 17, 9987–10014. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Meek, B.; Doi, Y.; Muramatsu, M.; Chiba, T.; Honjo, T.; Fagarasan, S. Aberrant Expansion of Segmented Filamentous Bacteria in IgA-Deficient Gut. Proc. Natl. Acad. Sci. USA 2004, 101, 1981. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Panea, C.; Nakato, G.; Cebula, A.; Lee, C.; Diez, M.G.; Laufer, T.M.; Ignatowicz, L.; Ivanov, I.I. Segmented Filamentous Bacteria Antigens Presented by Intestinal Dendritic Cells Drive Mucosal Th17 Cell Differentiation. Immunity 2014, 40, 594–607. [Google Scholar] [CrossRef]
- Nieuwenhuis, E.E.S.; Matsumoto, T.; Lindenbergh, D.; Willemsen, R.; Kaser, A.; Simons-Oosterhuis, Y.; Brugman, S.; Yamaguchi, K.; Ishikawa, H.; Aiba, Y.; et al. Cd1d-Dependent Regulation of Bacterial Colonization in the Intestine of Mice. J. Clin. Investig. 2009, 119, 1241–1250. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Coates, M.D.; Clarke, K.; Williams, E.; Jeganathan, N.; Yadav, S.; Giampetro, D.; Gordin, V.; Smith, S.; Vrana, K.; Bobb, A.; et al. Abdominal Pain in Inflammatory Bowel Disease: An Evidence-Based, Multidisciplinary Review. Crohns Colitis 360 2023, 5, otad055. [Google Scholar] [CrossRef]
- Malik, T.F.; Aurelio, D.M. Extraintestinal Manifestations of Inflammatory Bowel Disease; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marinelli, C.; Savarino, E.; Inferrera, M.; Lorenzon, G.; Rigo, A.; Ghisa, M.; Facchin, S.; D’Incà, R.; Zingone, F. Factors Influencing Disability and Quality of Life during Treatment: A Cross-Sectional Study on IBD Patients. Gastroenterol. Res. Pract. 2019, 2019, 5354320. [Google Scholar] [CrossRef]
- Román, A.L.S.; Muñoz, F. Comorbidity in Inflammatory Bowel Disease. World J. Gastroenterol. 2011, 17, 2723–2733. [Google Scholar] [CrossRef]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory Bowel Disease: Clinical Aspects and Treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Zhang, N.; Wei, H.; Wang, B. Gut-Brain Axis Interacts with Immunomodulation in Inflammatory Bowel Disease. Biochem. Pharmacol. 2024, 219, 115949. [Google Scholar] [CrossRef] [PubMed]
- Fairbrass, K.M.; Lovatt, J.; Barberio, B.; Yuan, Y.; Gracie, D.J.; Ford, A.C. Bidirectional Brain–Gut Axis Effects Influence Mood and Prognosis in IBD: A Systematic Review and Meta-Analysis. Gut 2022, 71, 1773–1780. [Google Scholar] [CrossRef]
- Hill, E.; Nguyen, N.H.; Qian, A.S.; Patel, S.; Chen, P.L.; Tse, C.-S.; Singh, S. Impact of Comorbid Psychiatric Disorders on Healthcare Utilization in Patients with Inflammatory Bowel Disease: A Nationally Representative Cohort Study. Dig. Dis. Sci. 2022, 67, 4373–4381. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of Symptoms of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Graff, L.A.; Walker, J.R.; Bernstein, C.N. Depression and Anxiety in Inflammatory Bowel Disease: A Review of Comorbidity and Management. Inflamm. Bowel Dis. 2009, 15, 1105–1118. [Google Scholar] [CrossRef]
- Regueiro, M.; Greer, J.B.; Szigethy, E. Etiology and Treatment of Pain and Psychosocial Issues in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 430–439.e4. [Google Scholar] [CrossRef] [PubMed]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.-B.; von Känel, R.; Swiss IBD Cohort Study Group. Symptoms of Depression and Anxiety Are Independently Associated with Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological Disorders in Gastrointestinal Disease: Epiphenomenon, Cause or Consequence? Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2014, 27, 224. [Google Scholar]
- Gong, W.; Guo, P.; Li, Y.; Liu, L.; Yan, R.; Liu, S.; Wang, S.; Xue, F.; Zhou, X.; Yuan, Z. Role of the Gut-Brain Axis in the Shared Genetic Etiology Between Gastrointestinal Tract Diseases and Psychiatric Disorders: A Genome-Wide Pleiotropic Analysis. JAMA Psychiatry 2023, 80, 360–370. [Google Scholar] [CrossRef]
- Person, H.; Keefer, L. Psychological Comorbidity in Gastrointestinal Diseases: Update on the Brain-Gut-Microbiome Axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 107, 110209. [Google Scholar] [CrossRef]
- Mules, T.C.; Swaminathan, A.; Hirschfeld, E.; Borichevsky, G.; Frampton, C.; Day, A.S.; Gearry, R.B. The Impact of Disease Activity on Psychological Symptoms and Quality of Life in Patients with Inflammatory Bowel Disease-Results from the Stress, Anxiety and Depression with Disease Activity (SADD) Study. Aliment. Pharmacol. Ther. 2022, 55, 201–211. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pavlidis, P.; Norton, C.; Norton, S.; Pariante, C.; Hayee, B.; Powell, N. Depressive Symptoms in Inflammatory Bowel Disease: An Extraintestinal Manifestation of Inflammation? Clin. Exp. Immunol. 2019, 197, 308. [Google Scholar] [CrossRef]
- Tarricone, I.; Regazzi, M.G.; Bonucci, G.; Rizzello, F.; Carini, G.; Muratori, R.; Poggioli, G.; Campieri, M. Prevalence and Effectiveness of Psychiatric Treatments for Patients with IBD: A Systematic Literature Review. J. Psychosom. Res. 2017, 101, 68–95. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Chen, Y.; Wang, C. Depression and Anxiety Disorders in Patients with Inflammatory Bowel Disease. Front. Psychiatry 2021, 12, 714057. [Google Scholar] [CrossRef]
- Karaca Dogan, B.; Salman Yilmaz, S.; Izgi, G.N.; Ozen, M. Circulating Non-Coding RNAs as a Tool for Liquid Biopsy in Solid Tumors. Epigenomics 2025, 17, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.; Capobianco, I.; Magrì, C.; Marafini, I.; Petito, V.; Scaldaferri, F. MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 7991. [Google Scholar] [CrossRef]
- Grosu, Ș.A.; Dobre, M.; Milanesi, E.; Hinescu, M.E. Blood-Based MicroRNAs in Psychotic Disorders—A Systematic Review. Biomedicines 2023, 11, 2536. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating MicroRNA in Body Fluid: A New Potential Biomarker for Cancer Diagnosis and Prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Wang, Z.; Zhu, W.; Li, Q. Fecal MiR-223 Is a Noninvasive Biomarker for Estimating Crohn’s Disease Activity. Immun. Inflamm. Dis. 2023, 11, e1131. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Acar, Ş.; Coşkun, S.; Güneş, M.; Güneş, S.; Yilmaz, M.F.; Görür, A.; Tamer, L. Comparison of Plasma MicroRNA Levels in Drug Naive, First Episode Depressed Patients and Healthy Controls. J. Psychiatr. Res. 2015, 69, 67–71. [Google Scholar] [CrossRef]
- Ibrahim, P.; Denniston, R.; Mitsuhashi, H.; Yang, J.; Fiori, L.M.; Żurawek, D.; Mechawar, N.; Nagy, C.; Turecki, G. Profiling Small RNA From Brain Extracellular Vesicles in Individuals with Depression. Int. J. Neuropsychopharmacol. 2024, 27, 13. [Google Scholar] [CrossRef]
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Differential and Unique Patterns of Synaptic MiRNA Expression in Dorsolateral Prefrontal Cortex of Depressed Subjects. Neuropsychopharmacology 2020, 46, 900–910. [Google Scholar] [CrossRef]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in MicroRNA Expression Is Associated with Alterations in Immune Functions in Combat Veterans with Post-Traumatic Stress Disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef]
- Peck, B.C.E.; Weiser, M.; Lee, S.E.; Gipson, G.R.; Iyer, V.B.; Sartor, R.B.; Herfarth, H.H.; Long, M.D.; Hansen, J.J.; Isaacs, K.L.; et al. MicroRNAs Classify Different Disease Behavior Phenotypes of Crohn’s Disease and May Have Prognostic Utility. Inflamm. Bowel Dis. 2015, 21, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, Y.; Wang, X.; Wu, J.; Liu, K.; Zhou, J.; Liu, L.; Zhang, C. Identification of Differential MicroRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder. PLoS ONE 2015, 10, e0121975. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Galbraith, D.; Morrison, R.L.; Trivedi, M.H.; Drevets, W.C. Circulating MicroRNA Associated with Future Relapse Status in Major Depressive Disorder. Front. Psychiatry 2022, 13, 937360. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, D.; Ponti, D.; Bassani, B.; Bruno, A.; Pulze, L.; Akkihal, S.A.; George-William, J.N.; Gundamaraju, R.; Campomenosi, P. MiR-223-3p in Cancer Development and Cancer Drug Resistance: Same Coin, Different Faces. Int. J. Mol. Sci. 2024, 25, 8191. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Is MiR-223 Upregulation in Inflammatory Bowel Diseases a Protective Response? Front. Biosci.-Elite 2023, 15, 5. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Günes, S.; Coskun, S.; Findikli, E. Peripheral Signatures of Psychiatric Disorders: MicroRNAs. Clin. Psychopharmacol. Neurosci. 2017, 15, 313. [Google Scholar] [CrossRef]
- Ghafour, A.A.; Odemis, D.A.; Tuncer, S.B.; Kurt, B.; Saral, M.A.; Erciyas, S.K.; Erdogan, O.S.; Celik, B.; Saip, P.; Yazici, H. High Expression Level of MiR-1260 Family in the Peripheral Blood of Patients with Ovarian Carcinoma. J. Ovarian Res. 2021, 14, 131. [Google Scholar] [CrossRef]
- Yufeng, Z.; Ming, Q.; Dandan, W. MiR-320d Inhibits Progression of EGFR-Positive Colorectal Cancer by Targeting TUSC3. Front. Genet. 2021, 12, 738559. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Pan, B.; He, B.; Chen, X.; Zeng, K.; Xu, M.; Pan, Y.; Sun, H.; Xu, T.; et al. Circulating MiR-1290 and MiR-320d as Novel Diagnostic Biomarkers of Human Colorectal Cancer. J. Cancer 2019, 10, 43–50. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Zhao, Y.Q.; Wang, J.P. IL-21 Mediates MicroRNA-423-5p /Claudin-5 Signal Pathway and Intestinal Barrier Function in Inflammatory Bowel Disease. Aging 2020, 12, 16099–16110. [Google Scholar] [CrossRef]
- MiR-486-5p Suppresses Gastric Cancer Cell Growth and Migration Through Downregulation of Fibroblast Growth Factor 9. Available online: https://www.spandidos-publications.com/10.3892/mmr.2021.12411 (accessed on 14 July 2025).
- Baghbanzadeh, A.; Baghbani, E.; Hajiasgharzadeh, K.; Noorolyai, S.; Khaze, V.; Mansoori, B.; Shirmohamadi, M.; Baradaran, B.; Mokhtarzadeh, A. MicroRNA-193a-5p Suppresses the Migratory Ability of Human KATO III Gastric Cancer Cells through Inhibition of Vimentin and MMP-9. Adv. Pharm. Bull. 2022, 12, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, K.C.; Kim, J.G.; Moon, S.J.; Kang, S.B.; Lee, D.S.; Sul, H.J.; Ji, J.S.; Jeong, H.Y. Dysregulation of MicroRNA-196b-5p and MicroRNA-375 in Gastric Cancer. J. Gastric Cancer 2016, 16, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.C.E.; Mah, A.T.; Pitman, W.A.; Ding, S.; Lund, P.K.; Sethupathy, P. Functional Transcriptomics in Diverse Intestinal Epithelial Cell Types Reveals Robust MicroRNA Sensitivity in Intestinal Stem Cells to Microbial Status. J. Biol. Chem. 2017, 292, 2586–2600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-Alpha-Induced Microglia Activation Requires MiR-342: Impact on NF-KB Signaling and Neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Brás, J.P.; Guillot de Suduiraut, I.; Zanoletti, O.; Monari, S.; Meijer, M.; Grosse, J.; Barbosa, M.A.; Santos, S.G.; Sandi, C.; Almeida, M.I. Stress-Induced Depressive-like Behavior in Male Rats Is Associated with Microglial Activation and Inflammation Dysregulation in the Hippocampus in Adulthood. Brain Behav. Immun. 2022, 99, 397–408. [Google Scholar] [CrossRef]
- Brás, J.P.; Pinto, S.; von Doellinger, O.; Prata, J.; Coelho, R.; Barbosa, M.A.; Almeida, M.I.; Santos, S.G. Combining Inflammatory MiRNA Molecules as Diagnostic Biomarkers for Depression: A Clinical Study. Front. Psychiatry 2023, 14, 1227618. [Google Scholar] [CrossRef] [PubMed]
- Gecys, D.; Dambrauskiene, K.; Simonyte, S.; Patamsyte, V.; Vilkeviciute, A.; Musneckis, A.; Butkute-Sliuoziene, K.; Lesauskaite, V.; Zemaitis, L.; Usaite, D.; et al. Circulating Hsa-Let-7e-5p and Hsa-MiR-125a-5p as Possible Biomarkers in the Diagnosis of Major Depression and Bipolar Disorders. Dis. Markers 2022, 2022, 3004338. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]

| IBD (N = 20) | IBD + SAD (N = 19) | SAD (N = 20) | CTRL (N = 22) | |
|---|---|---|---|---|
| Sex (N, %) | F (10; 50.0%) M (10; 50.0%) | F (11; 57.9%) M (8; 42.1%) | F (13; 65.0%) M (7; 35.0%) | F (11; 50.0%) M (11; 50.0%) |
| Age (mean ± SD) | 38.85 ± 14.80 (22.00–70.00) | 42.57 ± 16.22 (21.00–74.00) | 37.95 ± 11.91 (23.00–63.00) | 43.86 ± 6.99 (25.00–53.00) |
| HAM-A (N, %) | Mild (20; 100%) Mild to moderate (0; 0%) Moderate to severe (0; 0%) | Mild (13; 68.42%) Mild to moderate (3; 15.79%) Moderate to severe (3; 15.79%) | Mild (11; 55%) Mild to moderate (3; 15%) Moderate to severe (6; 30%) | Mild (22; 100%) Mild to moderate (0; 0%) Moderate to severe (0; 0%) |
| BDI (N, %) | Normal (19; 95%) Mild mood disturbance (1; 5%) Borderline (0; 0%) Moderate (0; 0%) Severe (0; 0%) Extreme (0; 0%) | Normal (12; 63.17%) Mild mood disturbance (3; 15.79%) Borderline (1; 5.26%) Moderate (1; 5.26%) Severe (2; 10.52%) Extreme (0; 0%) | Normal (1; 5%) Mild mood disturbance (5; 25%) Borderline (2; 10%) Moderate (10; 50%) Severe (1; 5%) Extreme (1; 5%) | Normal (22; 100%) Mild mood disturbance (0; 0%) Borderline (0; 0%) Moderate (0; 0%) Severe (0; 0%) Extreme (0; 0%) |
| PSS (N, %) | Low stress (18; 90%) Moderate stress (2; 10%) High stress (0; 0%) | Low stress (2; 10.53%) Moderate stress (13; 68.42%) High stress (4; 21.05%) | Low stress (4; 20%) Moderate stress (10; 50%) High stress (6; 30%) | Low stress (11; 50%) Moderate stress (11; 50%) High stress (0; 0%) |
| IBD Tot (N = 39) | |
|---|---|
| Sex (N, %) | F (21; 53.8%) M (18; 46.2%) |
| Age (mean ± SD) | 40.66 ± 15.42 |
| Disease type | UC (16; 41.0%) CD (23; 59.0%) |
| Disease duration (mean ± SD) | 9.04 ± 7.23 |
| Disease state (N, %) | Remission (27; 69.2%); Active (12; 30.8%) |
| Treatment type (N, %) | Biological (35; 89.7%); Other (4; 10.3%) |
| UC Montreal classification | E1: Proctitis (N = 0) E2: Left-sided colitis (N = 9) E3: Extensive colitis (N = 7) |
| Location of Crohn’s | Location of Crohn’s (N = 23) L1: Ileal (N = 3) L2: Colonic (N = 7) L3: Ileocolonic (N = 13) Upper GI involvement (N = 1) |
| Crohn’s behavior | B1: Inflammatory (N = 10) B2: Stricturing (N = 9) B3: Penetrating (N = 4) Perianal involvement (N = 6) |
| CRP, mg/L (mean ± SD) (min–max) | 9.20 ± 11.70 (0.50–45.30) |
| Fecal calprotectin, μg/g (mean ± SD) (min–max) | 689.51 ± 974.42 (5.00–4223.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobre, M.; Manuc, T.E.; Manuc, M.; Matei, I.-C.; Dobre, A.-M.; Dragne, A.-D.; Maffioletti, E.; Pelisenco, I.A.; Milanesi, E. Circulating miRNA Profile in Inflammatory Bowel Disease Patients with Stress, Anxiety, and Depression. Int. J. Mol. Sci. 2025, 26, 7321. https://doi.org/10.3390/ijms26157321
Dobre M, Manuc TE, Manuc M, Matei I-C, Dobre A-M, Dragne A-D, Maffioletti E, Pelisenco IA, Milanesi E. Circulating miRNA Profile in Inflammatory Bowel Disease Patients with Stress, Anxiety, and Depression. International Journal of Molecular Sciences. 2025; 26(15):7321. https://doi.org/10.3390/ijms26157321
Chicago/Turabian StyleDobre, Maria, Teodora Ecaterina Manuc, Mircea Manuc, Ioan-Costin Matei, Anastasia-Maria Dobre, Andrei-Daniel Dragne, Elisabetta Maffioletti, Iulia Andreea Pelisenco, and Elena Milanesi. 2025. "Circulating miRNA Profile in Inflammatory Bowel Disease Patients with Stress, Anxiety, and Depression" International Journal of Molecular Sciences 26, no. 15: 7321. https://doi.org/10.3390/ijms26157321
APA StyleDobre, M., Manuc, T. E., Manuc, M., Matei, I.-C., Dobre, A.-M., Dragne, A.-D., Maffioletti, E., Pelisenco, I. A., & Milanesi, E. (2025). Circulating miRNA Profile in Inflammatory Bowel Disease Patients with Stress, Anxiety, and Depression. International Journal of Molecular Sciences, 26(15), 7321. https://doi.org/10.3390/ijms26157321






