H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel
Abstract
1. Introduction
2. Major Topics
- (1)
- The Hv1 channel as an analogue of the VSD.
- (2)
- There is evidence from sodium channels that gating is slowed by D2O.
- (3)
- Certain mutations in the gate region make a huge difference.
- (4)
- Proton concentrations at the gate can be very high.
- (5)
- Wallace and coworkers found, in a bacterial sodium channel, NavAb, that hinge motions of the pore helix S6 and the C-terminal domain were involved with gating, but not S4 motion.
- (6)
- T1, the intracellular segment of the channel protein, matters.
- (7)
- The piquito, a brief pulse at the start of the channel opening sequence—proton tunneling?
- (8)
- Much of the evidence for the standard mechanical model is derived from experiments in which arginine was swapped for cysteine.
- (9)
- Other residues could be mutated as well.
- (10)
- The interactions that exist in the gate include ion–ion and hydrogen bonds.
- (11)
- Many molecular dynamics calculations on ion channels have been published.
- (12)
- Confined water, especially in channels.
- (13)
- Hydration dynamics.
- (14)
- In addition to the prolines at the gate, there is another class of conserved residues.
- (15)
- The EAG channels are also potassium channels.
- (16)
- Vibrations and rotations.
- (17)
- Thermodynamic behavior.
- (18)
- The probable importance of protons.
- (19)
- Experiments that appear to be more consistent with the standard model.
- (20)
- Energetics.
- (21)
- Omissions from this review.
2.1. The Hv1 Channel as an Analogue of the VSD
2.2. There Is Evidence from Sodium Channels That Gating Was Slowed by D2O [24,25,26]
2.3. Certain Mutations in the Gate Region Make a Huge Difference
2.4. Proton Concentration at the Gate Can Be Very High
2.5. Wallace and Coworkers Found, in a Bacterial Sodium Channel, NavAb, That Hinge Motions of the Pore Helix S6 and the C-Terminal Domain Were Involved with Gating, but Not S4 Motion
2.6. T1, the Intracellular Segment of the Channel Protein, Matters
2.7. The Piquito, a Brief Pulse at the Start of the Channel Opening Sequence—Proton Tunneling?
2.8. Much of the Evidence for the Standard Mechanical Model Is Derived from Experiments in Which Arginine Was Swapped for Cysteine
2.9. Other Residues Could Be Mutated as Well
2.10. The Interactions That Exist in the Gate Include Ion–Ion and Hydrogen Bonds
2.11. Many Molecular Dynamics Calculations on Ion Channels Have Been Published
2.12. Confined Water, Especially in Channels
2.13. Hydration Dynamics
2.14. In Addition to the Prolines at the Gate, There Is Another Class of Conserved Residues

2.15. The EAG Channels Are Also Potassium Channels
2.16. Vibrations and Rotations
2.17. Thermodynamic Behavior
2.18. The Probable Importance of Protons
2.19. Experiments That Appear to Be More Consistent with the Standard Model
2.20. Energetics
2.21. Omissions from This Review
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef]
- Green, M.E. Electrorheological effects and gating of membrane channels. J. Theor. Biol. 1989, 138, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Caution is required in interpretation of mutations in the voltage sensing domain of voltage gated channels as evidence for gating mechanisms. Int. J. Mol. Sci. 2015, 16, 1627–1643. [Google Scholar] [CrossRef]
- Kariev, A.M.; Green, M.E. Quantum Calculation of Proton and Other Charge Transfer Steps in Voltage Sensing in the Kv1.2 Channel. J. Phys. Chem. B 2019, 123, 7984–7998. [Google Scholar] [CrossRef]
- Kariev, A.M.; Green, M.E. Water, Protons, and the Gating of Voltage Gated Potassium Channels. Membranes 2024, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate. Membranes 2022, 12, 718. [Google Scholar] [CrossRef]
- Cherny, V.V.; Morgan, D.; DeCoursey, T.E.; Musset, B.; Chaves, G.; Smith, S.M.E. Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J. Gen. Physiol. 2015, 146, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cherny, V.V.; Morgan, D.; Thomas, S.; Smith, S.M.E.; DeCoursey, T.E. Histidine168 is crucial for ΔpH-dependent gating of the human voltage-gated proton channel, hHV1. J. Gen. Physiol. 2018, 150, 851–862. [Google Scholar] [CrossRef]
- Decoursey, T.E. Voltage-gated proton channels. Compr. Physiol. 2012, 2, 1355–1385. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kurokawa, T.; Takeshita, K.; Kobayashi, M.; Okochi, Y.; Nakagawa, A.; Okamura, Y. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat. Commun. 2012, 3, 816. [Google Scholar] [CrossRef]
- Okamura, Y.; Fujiwara, Y.; Sakata, S. Gating mechanisms of voltage-gated proton channels. Annu. Rev. Biochem. 2015, 84, 685–709. [Google Scholar] [CrossRef]
- Boonamnaj, P.; Sompornpisut, P. Effect of Ionization State on Voltage-Sensor Structure in Resting State of the Hv1 Channel. J. Phys. Chem. B 2019, 123, 2864–2873. [Google Scholar] [CrossRef]
- De La Rosa, V.; Ramsey, I.S. Gating Currents in the Hv1 Proton Channel. Biophys. J. 2018, 114, 2844–2854. [Google Scholar] [CrossRef]
- Decoursey, T.E. Voltage and pH sensing by the voltage-gated proton channel, HV1. J. R. Soc. Interface 2018, 15, 20180108. [Google Scholar] [CrossRef] [PubMed]
- Gianti, E.; Delemotte, L.; Klein, M.L.; Carnevale, V. On the role of water density fluctuations in the inhibition of a proton channel. Proc. Natl. Acad. Sci. USA 2016, 113, E8359. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Okochi, Y. Molecular mechanisms of coupling to voltage sensors in voltage-evoked cellular signals. Proc. Jpn. Acad. Ser. B 2019, 95, 111–135. [Google Scholar] [CrossRef]
- Randolph, A.L.; Mokrab, Y.; Bennett, A.L.; Sansom, M.S.; Ramsey, I.S. Proton currents constrain structural models of voltage sensor activation. Elife 2016, 5, e18017. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Cherny, V.V.; Ayuyan, A.G.; DeCoursey, T.E. Analysis of an electrostatic mechanism for ΔpH dependent gating of the voltage-gated proton channel, Hv1, supports a contribution of protons to gating charge. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148480. [Google Scholar] [CrossRef]
- Islas, L.D. The acid test for pH-dependent gating in cloned HV1 channels. J. Gen. Physiol. 2018, 150, 781–782. [Google Scholar] [CrossRef] [PubMed]
- De Coursey, T.E. CrossTalk proposal: Proton permeation through HV1 requires transient protonation of a conserved aspartate in the S1 transmembrane helix. J. Physiol. 2017, 595, 6793–6795. [Google Scholar] [CrossRef]
- Banh, R.; Cherny, V.V.; Morgan, D.; Musset, B.; Thomas, S.; Kulleperuma, K.; Smith, S.M.E.; Pomès, R.; DeCoursey, T.E. Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 2019, 116, 18951–18961. [Google Scholar] [CrossRef]
- Cherny, V.V.; Musset, B.; Morgan, D.; Thomas, S.; Smith, S.M.E.; Decoursey, T.E. Engineered high-affinity zinc binding site reveals gating configurations of a human proton channel. J. Gen. Physiol. 2020, 152, e202012664. [Google Scholar] [CrossRef] [PubMed]
- Gelenter, M.D.; Mandala, V.S.; Niesen, M.J.M.; Sharon, D.A.; Dregni, A.J.; Willard, A.P.; Hong, M. Water orientation and dynamics in the closed and open influenza B virus M2 proton channels. Commun. Biol. 2021, 4, 338. [Google Scholar] [CrossRef]
- Schauf, C.L.; Bullock, J.O. Solvent substitution as a probe of channel gating in Myxicola: Differential effects of D2O on some components of membrane conductance. Biophys. J. 1980, 30, 295–306. [Google Scholar] [CrossRef]
- Schauf, C.L.; Bullock, J.O. Solvent substitution as a probe of channel gating in Myxicola. Biophys. J. 1982, 37, 441–452. [Google Scholar] [CrossRef]
- Schauf, C.L.; Bullock, J.O. Modifications of sodium channel gating in Myxicola giant axons by deuterium oxide, temperature, and internal cations. Biophys. J. 1979, 27, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.D.; Starkus, J.G. The steady-state distribution of gating charge in crayfish giant axons. Biophys. J. 1989, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alicata, D.A.; Rayner, M.D.; Starkus, J.G. Sodium channel activation mechanisms. Insights from deuterium oxide substitution. Biophys. J. 1990, 57, 745–758. [Google Scholar] [CrossRef]
- Starkus, J.G.; Rayner, M.D. Gating current “fractionation” in crayfish giant axons. Biophys. J. 1991, 60, 1101–1119. [Google Scholar] [CrossRef]
- Phan, L.X.; Lynch, C.I.; Crain, J.; Sansom, M.S.P.; Tucker, S.J. Influence of effective polarization on ion and water interactions within a biomimetic nanopore. Biophys. J. 2022, 121, 2014–2026. [Google Scholar] [CrossRef]
- Szanto, T.G.; Gaal, S.; Karbat, I.; Varga, Z.; Reuveny, E.; Panyi, G. Shaker-IR K+ channel gating in heavy water: Role of structural water molecules in inactivation. J. Gen. Physiol. 2021, 153, e202012742. [Google Scholar] [CrossRef]
- Szanto, T.G.; Papp, F.; Zakany, F.; Varga, Z.; Deutsch, C.; Panyi, G. Molecular rearrangements in S6 during slow inactivation in Shaker-IR potassium channels. J. Gen. Physiol. 2023, 155, e202313352. [Google Scholar] [CrossRef]
- Sukhareva, M.; Hackos, D.H.; Swartz, K.J. Constitutive activation of the shaker Kv channel. J. Gen. Physiol. 2003, 122, 541–556. [Google Scholar] [CrossRef]
- Diaz-Franulic, I.; Sepulveda, R.V.; Navarro-Quezada, N.; Gonzalez-Nilo, F.; Naranjo, D. Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels. J. Gen. Physiol. 2015, 146, 133–146. [Google Scholar] [CrossRef]
- Hackos, D.H.; Chang, T.H.; Swartz, K.J. Scanning the intracellular s6 activation gate in the Shaker K+ channel. J. Gen. Physiol. 2002, 119, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.-X.; de Groot, B.L. Central cavity dehydration as a gating mechanism of potassium channels. Nat. Commun. 2023, 14, 2178. [Google Scholar] [CrossRef]
- McCusker, E.C.; Bagneris, C.; Naylor, C.E.; Cole, A.R.; D’Avanzo, N.; Nichols, C.G.; Wallace, B.A. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat. Commun. 2012, 3, 1102. [Google Scholar] [CrossRef] [PubMed]
- Montini, G.; Booker, J.; Sula, A.; Wallace, B.A. Comparisons of voltage-gated sodium channel structures with open and closed gates and implications for state-dependent drug design. Biochem. Soc. Trans. 2018, 46, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.L., Jr.; Lin, Y.-F.; Mobley, B.C.; Avelar, A.; Jan, Y.N.; Jan, L.Y.; Berger, J.M. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell 2000, 102, 657–670. [Google Scholar] [CrossRef]
- Cushman, S.J.; Nanao, M.H.; Jahng, A.W.; DeRubeis, D.; Choe, S.; Pfaffinger, P.J. Voltage dependent activation of potassium channels is coupled to T1 domain structure. Nat. Struct. Biol. 2000, 7, 403–407. [Google Scholar]
- Stefani, E.; Sigg, D.; Bezanilla, F. Correlation between the early component of gating current and total gating current in Shaker K channels. Biophys. J. 2000, 78, 7A. [Google Scholar]
- Sigg, D.; Bezanilla, F.; Stefani, E. Fast gating in the Shaker K+ channel and the energy landscape of activation. Proc. Natl. Acad. Sci. USA 2003, 100, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Schiff, L.I. Quantum Mechanics, 2nd ed.; McGraw Hill: New York, NY, USA, 1955. [Google Scholar]
- Chen, X.; Wang, Q.; Ni, F.; Ma, J. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci. USA 2010, 107, 11352–11357. [Google Scholar] [CrossRef]
- Asamoah, O.K.; Wuskell, J.P.; Loew, L.M.; Bezanilla, F. A Fluorometric Approach to Local Electric Field Measurements in a Voltage-Gated Ion Channel. Neuron 2003, 37, 85–97. [Google Scholar] [CrossRef]
- Yang, N.; Horn, R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 1995, 15, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Starace, D.M.; Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 2004, 427, 548–553. [Google Scholar] [CrossRef]
- Yang, N.; George, A.L.; Horn, R. Probing the outer mouth of the ‘S4 channel’ of a voltage-gated sodium channel. Biophys. J. 1997, 72, A262. [Google Scholar]
- Gosselin-Badaroudine, P.; Delemotte, L.; Moreau, A.; Klein, M.L.; Chahine, M. Gating pore currents and the resting state of Nav1.4 voltage sensor domains. Proc. Natl. Acad. Sci. USA 2012, 109, 19250–19255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Horn, R. Movement and crevices around a sodium channel S3 segment. J. Gen. Physiol. 2002, 120, 419–436. [Google Scholar] [CrossRef]
- Gonzalez-Perez, V.; Stack, K.; Boric, K.; Naranjo, D. Reduced voltage sensitivity in a K+-channel voltage sensor by electric field remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 5178–5183. [Google Scholar] [CrossRef]
- Gonzalez, C.; Rosenman, E.; Bezanilla, F.; Alvarez, O.; Latorre, R. Modulation of the Shaker K+ channel gating kinetics by the S3-S4 linker. J. Gen. Physiol. 2000, 115, 193–207. [Google Scholar] [CrossRef]
- Weinhold, F. Anti-Electrostatic Pi-Hole Bonding: How Covalency Conquers Coulombics. Molecules 2022, 27, 377. [Google Scholar] [CrossRef]
- Weinhold, F.; Klein, R.A. Anti-Electrostatic Hydrogen Bonds. Angew. Chem. Int. Ed. 2014, 53, 11214–11217. [Google Scholar] [CrossRef]
- Weinhold, F. Resonance character of hydrogen-bonding interactions in water and other H-bonded species. Adv. Protein Chem. 2006, 72, 121–155. [Google Scholar] [CrossRef]
- Al-Sheakh, L.; Fritsch, S.; Appelhagen, A.; Villinger, A.; Ludwig, R. Thermodynamically Stable Cationic Dimers in Carboxyl-Functionalized Ionic Liquids: The Paradoxical Case of “Anti-Electrostatic” Hydrogen Bonding. Molecules 2022, 27, 366. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Russo, J.; Tanaka, H. Common microscopic structural origin for water’s thermodynamic and dynamic anomalies. J. Chem. Phys. 2018, 149, 224502. [Google Scholar] [CrossRef] [PubMed]
- Nucci, N.V.; Vanderkooi, J.M. Effects of salts of the Hofmeister series on the hydrogen bond network of water. J. Mol. Liq. 2008, 143, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.N.; Nucci, N.V.; Vanderkooi, J.M. Changes in Water Structure Induced by the Guanidinium Cation and Implications for Protein Denaturation. J. Phys. Chem. A 2008, 112, 10939–10948. [Google Scholar] [CrossRef]
- Cobar, E.A.; Horn, P.R.; Bergman, R.G.; Head-Gordon, M. Examination of the hydrogen-bonding networks in small water clusters (n = 2-5, 13, 17) using absolutely localized molecular orbital energy decomposition analysis. Phys. Chem. Chem. Phys. 2012, 14, 15328–15339. [Google Scholar] [CrossRef]
- Onsager, L. Deviations from Ohm’s Law in Weak Electrolytes. J. Chem. Phys. 1934, 2, 599–615. [Google Scholar] [CrossRef]
- Lasham, J.; Djurabekova, A.; Zickermann, V.; Vonck, J.; Sharma, V. Role of Protonation States in the Stability of Molecular Dynamics Simulations of High-Resolution Membrane Protein Structures. J. Phys. Chem. B 2024, 128, 2304–2316. [Google Scholar] [CrossRef]
- Min, J.; Britt, M.; Brooks, B.R.S.; Sukharev, S.; Klauda, J.B. Thermodynamics of Arginine Interactions with Organic Phosphates. Biophys. J. 2025, 124, 2176–2194. [Google Scholar] [CrossRef]
- Lynch, C.I.; Rao, S.; Sansom, M.S.P. Water in Nanopores and Biological Channels: A Molecular Simulation Perspective. Chem. Rev. 2020, 120, 10298–10335. [Google Scholar] [CrossRef]
- Geissler, P.L.; Dellago, C.; Chandler, D.; Hutter, J.; Parinello, M. Autoionization in liquid water. Science 2001, 291, 2121–2124. [Google Scholar] [CrossRef]
- Hassanali, A.; Prakash, M.K.; Eshet, H.; Parrinello, M. On the recombination of hydronium and hydroxide ions in water. Proc. Natl. Acad. Sci. USA 2011, 108, 20410–20415. [Google Scholar] [CrossRef]
- Zaleski, R.; Gorgol, M.; Kierys, A.; Maheshwari, P.; Pietrow, M.; Pujari, P.K.; Zgardzinska, B. Unraveling the Phase Behavior of Water Confined in Nanochannels through Positron Annihilation. J. Phys. Chem. C 2022, 126, 5916–5926. [Google Scholar] [CrossRef]
- Galitskaya, E.A.; Zavorotnaya, U.M.; Ryzhkin, I.A.; Sinitsyn, V.V. Model of confined water self-diffusion and its application to proton-exchange membranes. Ionics 2021, 27, 2717–2721. [Google Scholar] [CrossRef]
- Tan, H.; Duan, M.; Xie, H.; Zhao, Y.; Liu, H.; Yang, M.; Liu, M.; Yang, J. Fast collective motions of backbone in transmembrane α helices are critical to water transfer of aquaporin. Sci. Adv. 2024, 10, eade9520. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, H.T.; Watkins, L.C.; Mravic, M.; Thomaston, J.L.; Nicoludis, J.M.; Somberg, N.H.; Liu, L.; Hong, M.; Voth, G.A.; DeGrado, W.F. Transient water wires mediate selective proton transport in designed channel proteins. Nat. Chem. 2023, 15, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.D.E.; Hewage, K.S.K.; Eitel, A.R.; Struts, A.V.; Weerasinghe, N.; Perera, S.M.D.C.; Brown, M.F. Hydration-mediated G-protein-coupled receptor activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2117349119. [Google Scholar] [CrossRef]
- Reiter, G.; Burnham, C.; Homouz, D.; Platzman, P.M.; Mayers, J.; Abdul-Redah, T.; Moravsky, A.P.; Li, J.C.; Loong, C.K.; Kolesnikov, A.I. Anomalous Behavior of Proton Zero Point Motion in Water Confined in Carbon Nanotubes. Phys. Rev. Lett. 2006, 97, 247801. [Google Scholar] [CrossRef]
- Reiter, G.F.; Kolesnikov, A.I.; Paddison, S.J.; Platzman, P.M.; Moravsky, A.P.; Adams, M.A.; Mayers, J. Evidence for an anomalous quantum state of protons in nanoconfined water. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 045403. [Google Scholar] [CrossRef]
- Reiter, G.F.; Deb, A.; Sakurai, Y.; Itou, M.; Kolesnikov, A.I. Quantum Coherence and Temperature Dependence of the Anomalous State of Nanoconfined Water in Carbon Nanotubes. J. Phys. Chem. Lett. 2016, 7, 4433–4437. [Google Scholar] [CrossRef]
- Kolesnikov, A.I.; Reiter, G.F.; Choudhury, N.; Prisk, T.R.; Mamontov, E.; Podlesnyak, A.; Ehlers, G.; Seel, A.G.; Wesolowski, D.J.; Anovitz, L.M. Quantum tunneling of water in beryl: A new state of the water molecule. Phys. Rev. Lett. 2016, 116, 167802. [Google Scholar] [CrossRef]
- Yoon, H.; Yoon, B.J. Observation of the thermal influenced quantum behaviour of water near a solid interface. Sci. Rep. 2018, 8, 7016. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.N.; Sokolov, A.P. Quantum effects in dynamics of water and other liquids of light molecules. Eur. Phys. J. E Soft Matter Biol. Phys. 2017, 40, 57. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkin, M.I.; Ryzhkin, I.A.; Kashin, A.M.; Zavorotnaya, U.M.; Sinitsyn, V.V. Quantum Protons in One-Dimensional Water. J. Phys. Chem. C 2022, 126, 8100–8106. [Google Scholar] [CrossRef]
- Pradhan, M.R.; Nguyen, M.N.; Kannan, S.; Fox, S.J.; Kwoh, C.K.; Lane, D.P.; Verma, C.S. Characterization of Hydration Properties in Structural Ensembles of Biomolecules. J. Chem. Inf. Model. 2019, 59, 3316–3329. [Google Scholar] [CrossRef]
- Setny, P. Conserved internal hydration motifs in protein kinases. Proteins Struct. Funct. Bioinf. 2020, 88, 1578–1591. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Proton transfer reactions: From photochemistry to biochemistry and bioenergetics. BBA Adv. 2023, 3, 100085. [Google Scholar] [CrossRef]
- Sobhia, M.E.; Ghosh, K.; Kumar, G.S.; Sivangula, S.; Laddha, K.; Kumari, S.; Kumar, H. The Role of Water Network Chemistry in Proteins: A Structural Bioinformatics Perspective in Drug Discovery and Development. Curr. Top. Med. Chem. 2022, 22, 1636–1653. [Google Scholar] [CrossRef]
- Carugo, O. Statistical survey of the buried waters in the Protein Data Bank. Amino Acids 2016, 48, 193–202. [Google Scholar] [CrossRef]
- Gaber, M.; Fayed, T.A.; El-Nahass, M.N.; Diab, H.A.; El-Gamil, M.M. Synthesis, spectroscopic characterization and biological evaluation of a novel chemosensor with different metal ions. Appl. Organomet. Chem. 2019, 33, e5133. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Maurer, M.; Oostenbrink, C. Water in protein hydration and ligand recognition. J. Mol. Recognit. 2019, 32, e2810. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mukherjee, S.; Bagchi, B. Protein Hydration Dynamics: Much Ado about Nothing? J. Phys. Chem. Lett. 2017, 8, 4878–4882. [Google Scholar] [CrossRef]
- Caminiti, L.; Taddei, M.; Catalini, S.; Bartolini, P.; Taschin, A.; Torre, R. Protein Crowding Effects on Hydration Water Dynamics. J. Phys. Chem. Lett. 2025, 16, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Perspective: Structure and ultrafast dynamics of biomolecular hydration shells. Struct. Dyn. 2017, 4, 044018. [Google Scholar] [CrossRef]
- Brahma, R.; Raghuraman, H. Novel insights in linking solvent relaxation dynamics and protein conformations utilizing red edge excitation shift approach. Emerg. Top. Life Sci. 2021, 5, 89–101. [Google Scholar] [CrossRef]
- Lang, X.; Shi, L.; Zhao, Z.; Min, W. Probing the structure of water in individual living cells. Nat. Commun. 2024, 15, 5271. [Google Scholar] [CrossRef]
- Biswas, R.; Bagchi, B. Anomalous water dynamics at surfaces and interfaces: Synergistic effects of confinement and surface interactions. J. Phys. Condens. Matter 2018, 30, 013001. [Google Scholar] [CrossRef]
- Mondal, S.; Bagchi, B. From structure and dynamics to biomolecular functions: The ubiquitous role of solvent in biology. Curr. Opin. Struct. Biol. 2022, 77, 102462. [Google Scholar] [CrossRef] [PubMed]
- Moron, M.C. Protein hydration shell formation: Dynamics of water in biological systems exhibiting nanoscopic cavities. J. Mol. Liq. 2021, 337, 116584. [Google Scholar] [CrossRef]
- Wilkins, D.M.; Manolopoulos, D.E.; Pipolo, S.; Laage, D.; Hynes, J.T. Nuclear Quantum Effects in Water Reorientation and Hydrogen-Bond Dynamics. J. Phys. Chem. Lett. 2017, 8, 2602–2607. [Google Scholar] [CrossRef]
- Ceriotti, M.; Fang, W.; Kusalik, P.G.; McKenzie, R.H.; Michaelides, A.; Morales, M.A.; Markland, T.E. Nuclear Quantum Effects in Water and Aqueous Systems: Experiment, Theory, and Current Challenges. Chem. Rev. 2016, 116, 7529–7550. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Guo, J.-P.; Gao, J.; Song, B.; Jiang, L. A physical derivation of high-flux ion transport in biological channel via quantum ion coherence. Nat. Commun. 2024, 15, 7189. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Zhan, C.; Zhang, H.; Yin, M.; Lu, W.; Xu, B. A Nanoconfined Water-Ion Coordination Network for Flexible Energy-Dissipation Devices. Adv. Mater. 2023, 35, 2303759. [Google Scholar] [CrossRef]
- Kim, K.; Choi, S.; Zhang, Z.; Bai, L.; Chung, S.; Jang, J. Molecular Features of Hydration Layers: Insights from Simulation, Microscopy, and Spectroscopy. J. Phys. Chem. C 2022, 126, 8967–8977. [Google Scholar] [CrossRef]
- Menendez, C.A.; Accordino, S.R.; Loubet, N.A.; Appignanesi, G.A. Study of Protein Hydration Water with the V4S Structural Index: Focus on Binding Site Description. J. Phys. Chem. B 2024, 128, 11865–11875. [Google Scholar] [CrossRef]
- Iwamoto, M.; Oiki, S. Counting ion and water molecules in a streaming file through the open-filter structure of the K channel. J. Neurosci. 2011, 31, 12180–12188. [Google Scholar] [CrossRef]
- Bysack, A.; Jash, C.; Raghuraman, H. Structural Dynamics of the Slide Helix of Inactive/Closed Conformation of KirBac1.1 in Micelles and Membranes: A Fluorescence Approach. J. Membr. Biol. 2025, 258, 97–112. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1379. [Google Scholar] [CrossRef]
- Mähler, J.; Persson, I. A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution. Inorg. Chem. 2012, 51, 425–438. [Google Scholar] [CrossRef]
- Åqvist, J.; Luzhkov, V. Ion permeation mechanism of the potassium channel. Nature 2000, 404, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, C. Dehydration governs electric-field-driven ion transport through Ångstrom-scale pores. Acad. Nano Sci. Mater. Technol. 2025, 2, 1–11. [Google Scholar] [CrossRef]
- Chowdhury, S.; Haehnel, B.M.; Chanda, B. Interfacial gating triad is crucial for electromechanical transduction in voltage-activated potassium channels. J. Gen. Physiol. 2014, 144, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Pardo, L.A.; Dominguez, P.; Sierra, L.M.; De la Pena, P. New structures and gating of voltage-dependent potassium (Kv) channels and their relatives: A multi-domain and dynamic question. Int. J. Mol. Sci. 2019, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marino, A.I.; Tan, X.F.; Bae, C.; Huffer, K.; Jiang, J.; Swartz, K.J. Inactivation of the Kv2.1 channel through electromechanical coupling. Nature 2023, 622, 410–417. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, Y.; Pei, D. Mechanism underlying delayed rectifying in human voltage-mediated activation Eag2 channel. Nat. Commun. 2023, 14, 1470. [Google Scholar] [CrossRef]
- Mandala, V.S.; MacKinnon, R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl. Acad. Sci. USA 2022, 119, e2214151119. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Ghose, R.; Green, M.E. Voltage gating and anions, especially phosphate: A model system. Biochem. Biophys. Acta 2005, 1717, 97–103. [Google Scholar] [CrossRef]
- Malak, O.A.; Gluhov, G.S.; Grizel, A.V.; Kudryashova, K.S.; Sokolova, O.S.; Loussouarn, G. Voltage-dependent activation in EAG channels follows a ligand-receptor rather than a mechanical-lever mechanism. J. Biol. Chem. 2019, 294, 6506–6522. [Google Scholar] [CrossRef]
- Barros, F.; de la Pena, P.; Dominguez, P.; Sierra, L.M.; Pardo, L.A. The EAG voltage-dependent K+ channel subfamily: Similarities and differences in structural organization and gating. Front. Pharmacol. 2020, 11, 00411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, B.; Zhang, Q.-L. Collective Vibration Decoupling of Confined Water in Membrane Channels. J. Phys. Chem. B 2025, 129, 4432–4437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-L.; Zhou, T.; Chang, C.; Gu, S.-Y.; Wang, Y.-J.; Liu, Q.; Zhu, Z. Ultrahigh-Flux Water Nanopumps Generated by Asymmetric Terahertz Absorption. Phys. Rev. Lett. 2024, 132, 184003. [Google Scholar] [CrossRef] [PubMed]
- Çetiner, U.; Raz, O.; Britt, M.; Sukharev, S. Dissipation during the Gating Cycle of the Bacterial Mechanosensitive Ion Channel Approaches the Landauer Limit. Entropy 2023, 25, 779. [Google Scholar] [CrossRef]
- Costa, F.; Guardiani, C.; Giacomello, A. Molecular dynamics simulations suggest possible activation and deactivation pathways in the hERG channel. Commun. Biol. 2022, 5, 165. [Google Scholar] [CrossRef]
- Fologea, D.; Krueger, E.; Mazur, Y.I.; Stith, C.; Okuyama, Y.; Henry, R.; Salamo, G.J. Bi-stability, hysteresis, and memory of voltage-gated lysenin channels. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2933–2939. [Google Scholar] [CrossRef]
- Jones, D.K. Hysteretic hERG channel gating current recorded at physiological temperature. Sci. Rep. 2022, 12, 5950. [Google Scholar] [CrossRef]
- Pustovoit, M.A.; Berezhkovskii, A.M.; Bezrukov, S.M. Analytical theory of hysteresis in ion channels: Two-state model. J. Chem. Phys. 2006, 125, 194907. [Google Scholar] [CrossRef]
- Semaan, M.T.; Crutchfield, J.P. Homeostatic and adaptive energetics: Nonequilibrium fluctuations beyond detailed balance in voltage-gated ion channels. Phys. Rev. E 2022, 106, 044410. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic behavior in voltage-gated channels. Front. Pharmacol. 2020, 11, 579596. [Google Scholar] [CrossRef]
- Cowgill, J.; Chanda, B. Charge-voltage curves of Shaker potassium channel are not hysteretic at steady state. J. Gen. Physiol. 2023, 155, e202112883. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F.; Bezanilla, F.; Eisenberg, R.S. Gating current noise produced by Brownian models of a voltage sensor. Biophys. J. 2021, 120, 3983–4001. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J. Hydrophobic gating in bundle-crossing ion channels: A case study of TRPV4. Commun. Biol. 2023, 6, 1094. [Google Scholar] [CrossRef]
- Kasimova, M.A.; Yazici, A.; Yudin, Y.; Granata, D.; Klein, M.L.; Rohacs, T.; Carnevale, V. Ion Channel Sensing: Are Fluctuations the Crux of the Matter? J. Phys. Chem. Lett. 2018, 9, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Warshel, A. Equilibrium fluctuation relations for voltage coupling in membrane proteins. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2985–2997. [Google Scholar] [CrossRef]
- Lan, T.-H.; Xi, H.; Lin, J.-R. Correlation character of ionic current fluctuations: Analysis of ion current through a voltage-dependent potassium single channel. Biophys. Chem. 2005, 117, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Analytical modeling of the ion number fluctuations in biological ion channels. J. Nanosci. Nanotechnol. 2012, 12, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, G.; Brown, F.L.H. A consistent model for thermal fluctuations and protein-induced deformations in lipid bilayers. Biophys. J. 2006, 90, 1501–1520. [Google Scholar] [CrossRef]
- Foreman-Ortiz, I.U.; Liang, D.; Laudadio, E.D.; Calderin, J.D.; Wu, M.; Keshri, P.; Zhang, X.; Schwartz, M.P.; Hamers, R.J.; Rotello, V.M.; et al. Anionic nanoparticle-induced perturbation to phospholipid membranes affects ion channel function. Proc. Natl. Acad. Sci. USA 2020, 117, 27854–27861. [Google Scholar] [CrossRef]
- Wolf, S.; Freier, E.; Gerwert, K. A Delocalized Proton-Binding Site within a Membrane Protein. Biophys. J. 2014, 107, 174–184. [Google Scholar] [CrossRef]
- Prisk, T.R.; Hoffmann, C.; Kolesnikov, A.I.; Mamontov, E.; Podlesnyak, A.A.; Wang, X.; Kent, P.R.C.; Anovitz, L.M. Fast Rotational Diffusion of Water Molecules in a 2D Hydrogen Bond Network at Cryogenic Temperatures. Phys. Rev. Lett. 2018, 120, 196001. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Quantum Effects in a Simple Ring with Hydrogen Bonds. J. Phys. Chem. B 2015, 119, 5962–5969. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.-Z.; Peng, J.; Wang, E.-G.; Jiang, Y. Atomic-scale investigation of nuclear quantum effects of surface water: Experiments and theory. Prog. Surf. Sci. 2017, 92, 203–239. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Z.; Guo, J.; Chen, J.; Wang, E.-G.; Jiang, Y.; Li, X.-Z. The collective and quantum nature of proton transfer in the cyclic water tetramer on NaCl(001). J. Chem. Phys. 2018, 148, 102329. [Google Scholar] [CrossRef]
- Lin, L.; Morrone, J.A.; Car, R. Correlated Tunneling in Hydrogen Bonds. J. Stat. Phys. 2011, 145, 365–384. [Google Scholar] [CrossRef]
- McKenzie, R.H.; Bekker, C.; Athokpam, B.; Ramesh, S.G. Effect of quantum nuclear motion on hydrogen bonding. J. Chem. Phys. 2014, 140, 174508. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Senesi, R.; Krzystyniak, M.; Romanelli, G.; Fernandez-Alonso, F. Experimental studies of nuclear quantum effects in condensed matter: The case of water. Riv. Nuovo Cimento Soc. Ital. Fis. 2018, 41, 291–340. [Google Scholar] [CrossRef]
- Andreani, C.; Colognesi, D.; Pietropaolo, A.; Senesi, R. Ground state proton dynamics in stable phases of water. Chem. Phys. Lett. 2011, 518, 1–6. [Google Scholar] [CrossRef]
- Moid, M.; Finkelstein, Y.; Moreh, R.; Maiti, P.K. Microscopic Study of Proton Kinetic Energy Anomaly for Nanoconfined Water. J. Phys. Chem. B 2020, 124, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Morrone, J.A.; Car, R.; Parrinello, M. Momentum distribution, vibrational dynamics, and the potential of mean force in ice. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 220302. [Google Scholar] [CrossRef]
- Doron, D.; Weitman, M.; Vardi-Kilshtain, A.; Azuri, A.; Engel, H.; Major, D.T. Multiscale quantum-classical simulations of enzymes. Isr. J. Chem. 2014, 54, 1108–1117. [Google Scholar] [CrossRef]
- Li, X.-Z.; Walker, B.; Michaelides, A. Quantum nature of the hydrogen bond. Proc. Natl. Acad. Sci. USA 2011, 108, 6369–6373. [Google Scholar] [CrossRef]
- Ryzhkin, I.A.; Ryzhkin, M.I. Quantum Symmetrization of Hydrogen Bonds in Ice. JETP Lett. 2021, 113, 461–465. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, D.; Gurung, A.; Warmuth, R.; Kuroda, D.G.; Wang, L. pKa Matching Enables Quantum Proton Delocalization in Acid-1-Methylimidazole Binary Mixtures. J. Chem. Inf. Model. 2025, 65, 798–810. [Google Scholar] [CrossRef]
- Polakowski, M.; Panfil, M. Quantum features of the transport through ion channels in the soft knock-on model. Phys. Biol. 2025, 22, 016007. [Google Scholar] [CrossRef]
- Yarov-Yarovoy, V.; DeCaen, P.G.; Westenbroek, R.E.; Pan, C.-Y.; Scheuer, T.; Baker, D.; Catterall, W.A. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. USA 2012, 109, E93–E102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, W.; DeCaen, P.; Yan, C.; Tao, X.; Tang, L.; Wang, J.; Hasegawa, K.; Kumasaka, T.; He, J.; et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 2012, 486, 130–134. [Google Scholar] [CrossRef] [PubMed]
- DeCaen, P.G.; Yarov-Yarovoy, V.; Zhao, Y.; Scheuer, T.; Catterall, W.A. Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation. Proc. Natl. Acad. Sci USA 2008, 105, 15142–15147. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhao, H.; Dai, Y.; Wang, Y.; Lo, Y.-h.; Jan, L.Y.; Lee, C.-H. Activation and closed-state inactivation mechanisms of the human voltage-gated KV4 channel complexes. Mol. Cell 2022, 82, 2427–2442.e4. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.M.; Sigg, D.; Bezanilla, F. Voltage gating of Shaker K+ channels: The effect of temperature on ionic and gating currents. J. Gen. Physiol. 1998, 112, 223–242. [Google Scholar] [CrossRef]
- Pinto-Anwandter, B.I.; Bassetto, C.A.Z., Jr.; Latorre, R.; Bezanilla, F. Energy landscape of a Kv channel revealed by temperature steps while perturbing its electromechanical coupling. Nat. Commun. 2025, 16, 3379. [Google Scholar] [CrossRef] [PubMed]
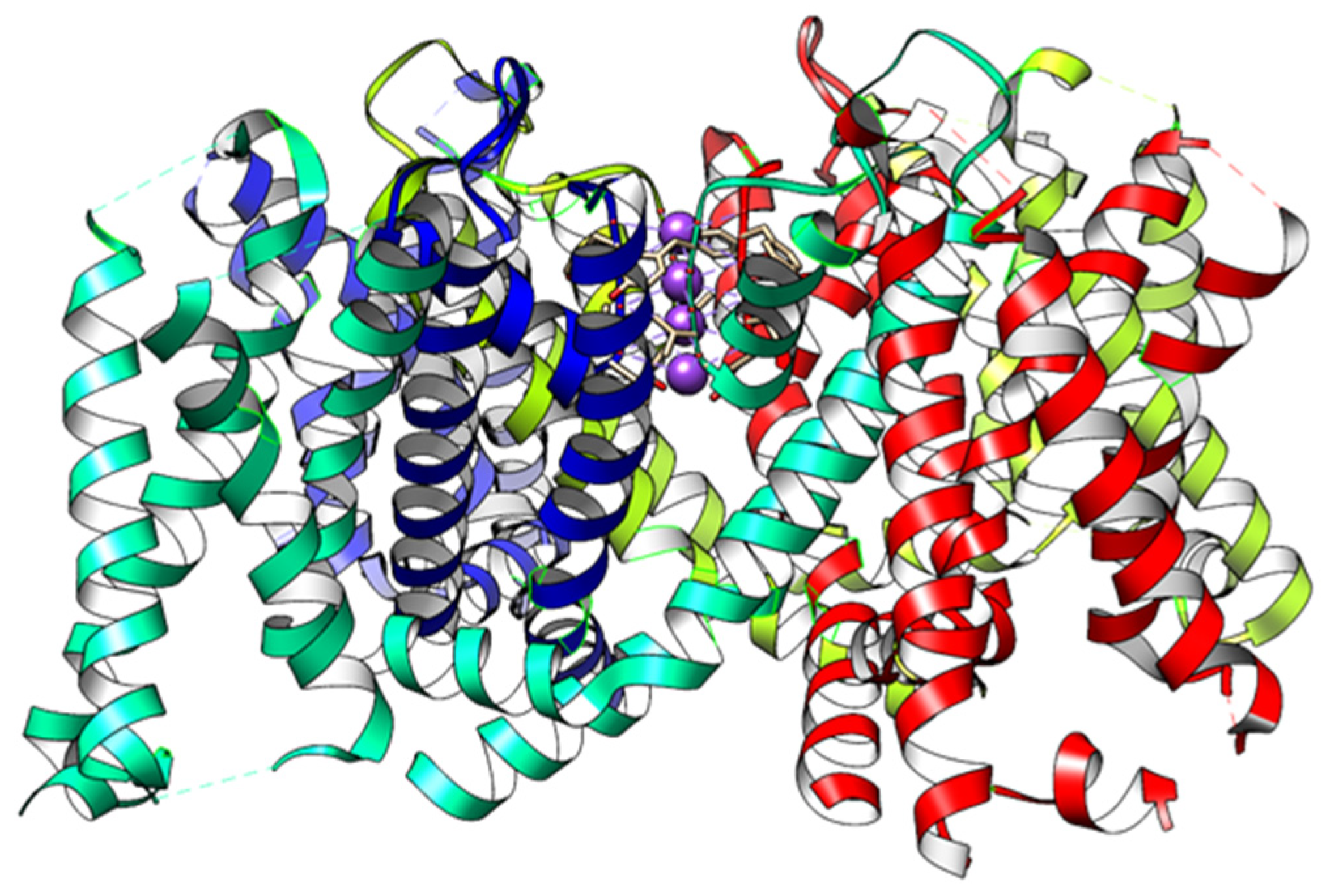
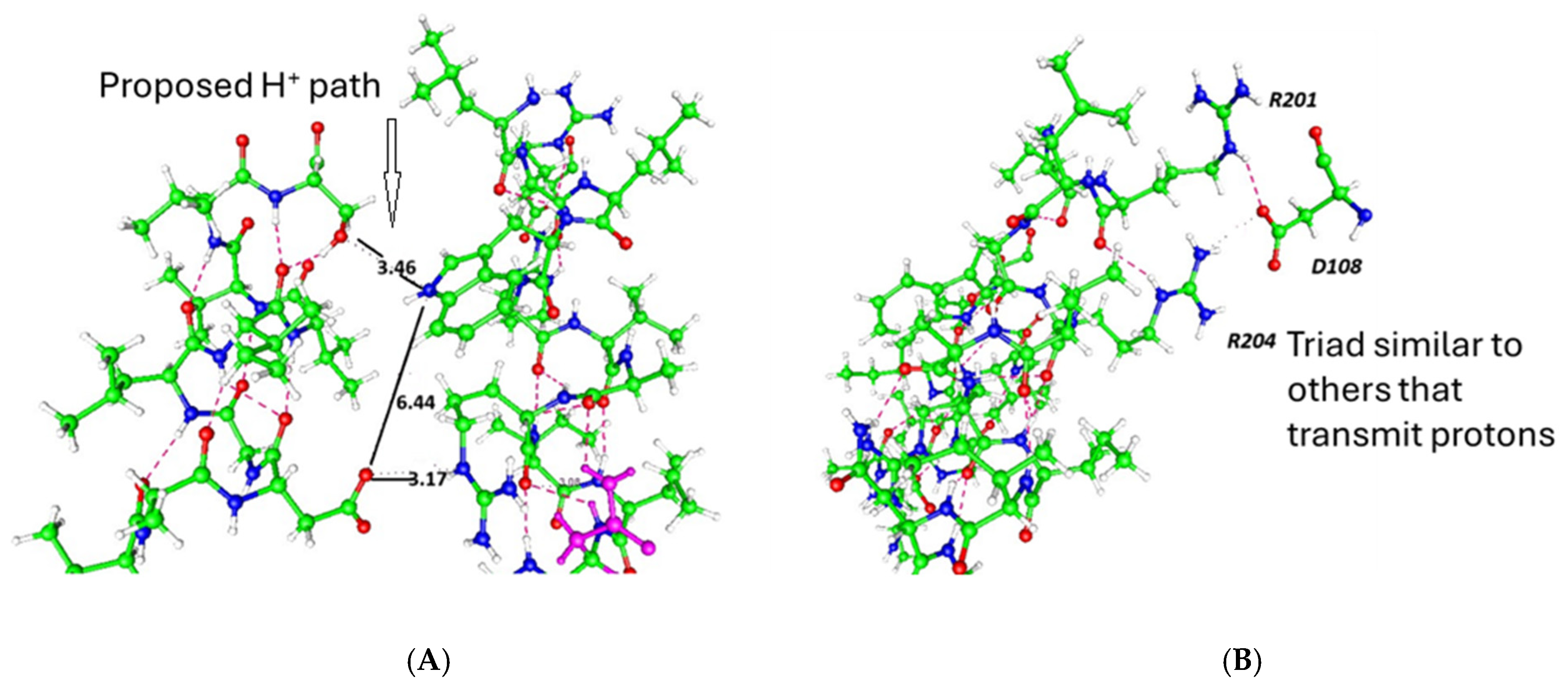
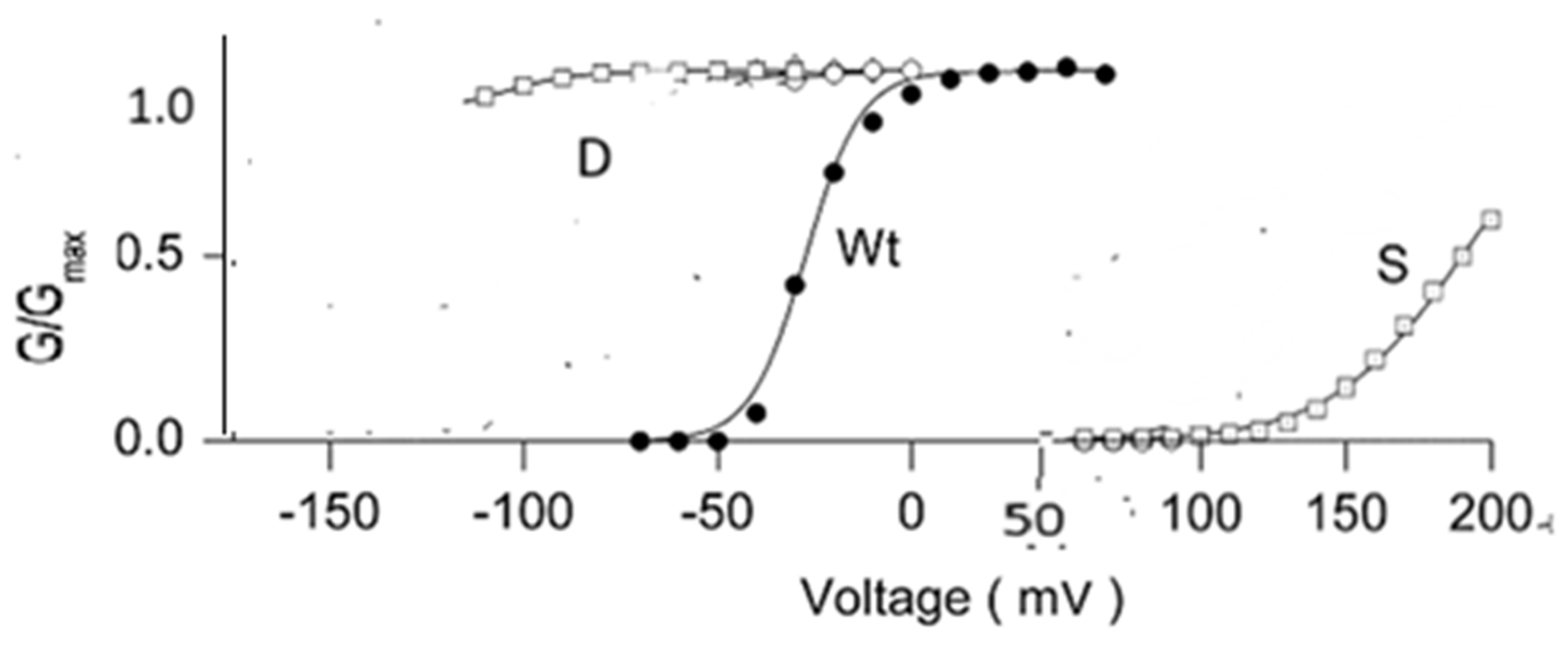
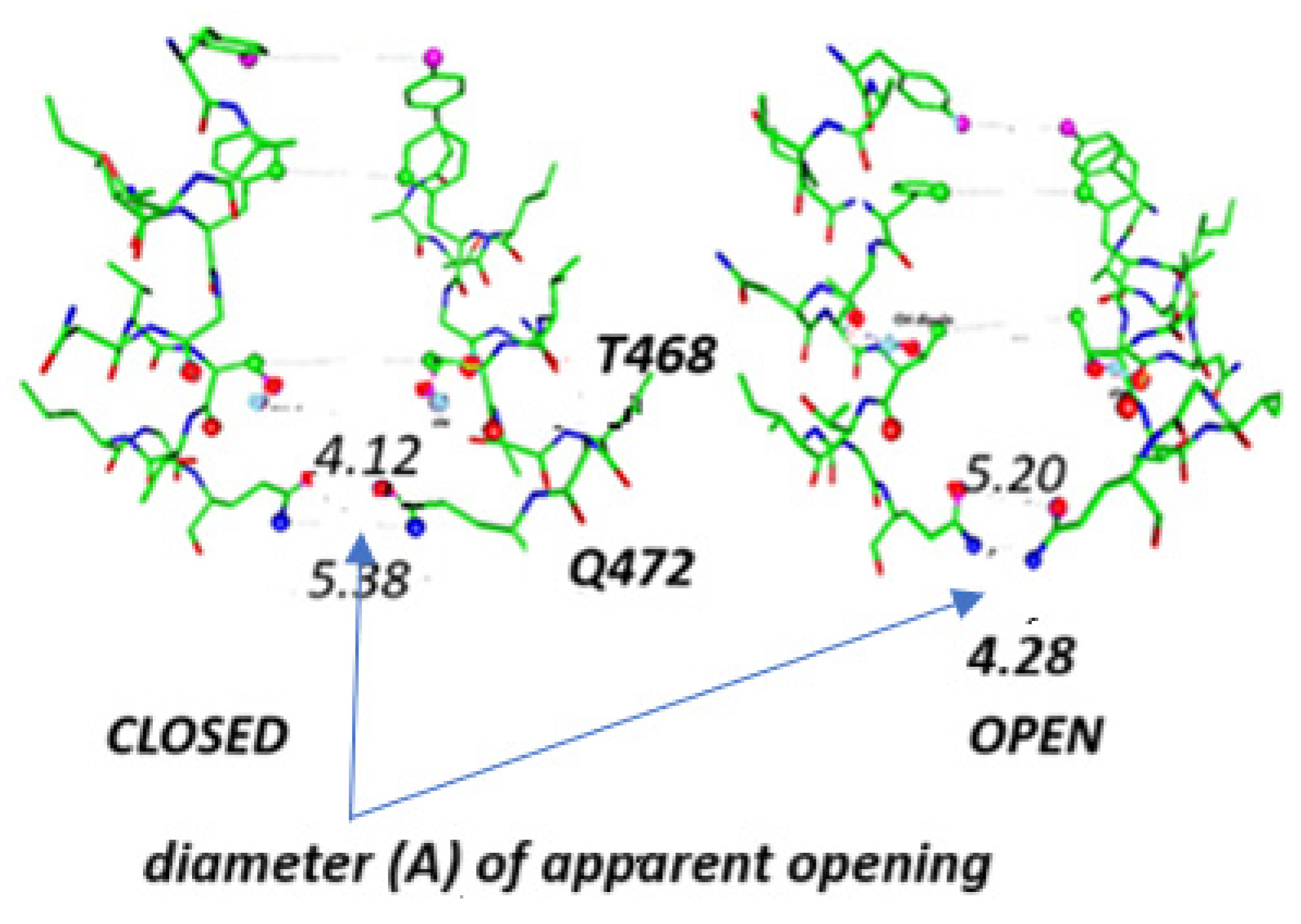
| Channel | Amino Acids—Residue Number | pdb Code |
|---|---|---|
| Kv1.2 | N-412; S-411; N-414 | 3Lut |
| Kv2.1 | S413; N410; S413 (next domain) | 8SD3 |
| Shaker | N480; S479; N482 | 7SIP |
| Hv1 | Q219; S215; Q98 | 3WKV |
| Y157; D170; R207 | ||
| Bacteriorhodopsin | Y57; R82; Q194 | 1FBB |
| Y185; W86; R212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariev, A.M.; Green, M.E. H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel. Int. J. Mol. Sci. 2025, 26, 7325. https://doi.org/10.3390/ijms26157325
Kariev AM, Green ME. H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel. International Journal of Molecular Sciences. 2025; 26(15):7325. https://doi.org/10.3390/ijms26157325
Chicago/Turabian StyleKariev, Alisher M., and Michael E. Green. 2025. "H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel" International Journal of Molecular Sciences 26, no. 15: 7325. https://doi.org/10.3390/ijms26157325
APA StyleKariev, A. M., & Green, M. E. (2025). H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel. International Journal of Molecular Sciences, 26(15), 7325. https://doi.org/10.3390/ijms26157325






