Abstract
In women, gonadal hormones play a crucial regulatory role in body fat distribution and glucose–lipidic homeostasis, which are closely associated with the hepatic steatogenesis and intrahepatic inflammatory pathways. Accumulating evidence supports the idea that hepatic health is closely linked to endocrine ovarian function through hormonal, metabolic, and immunological communications, collectively known as the “ovary–liver axis”. This review presents the molecular mechanisms involved in sex hormone synthesis, metabolism, and signaling pathways along the ovary–liver axis, focusing on dysregulated mechanisms that may contribute to common disorders and, specifically to hepatic derangements in the context of altered ovarian function. Additionally, we analyzed epidemiological evidence supporting the ovary–liver axis, specifically examining meta-analytic studies exploring the connection between polycystic ovary syndrome and metabolic dysfunction-associated steatotic liver disease (MASLD). We also discuss studies linking hypogonadism with liver health, with a specific focus on Turner syndrome and MASLD. Furthermore, we explore the impact of menopause on liver health. Our integrated molecular and epidemiological approach identifies important clinical and public health implications, aiming to uncover potentially innovative interventions and effective strategies for managing disease progression. However, unexplored areas within the ovary–liver axis highlight the need for further research on causal pathways.
1. Introduction
The concept of a functional axis connecting the ovary to the liver was first proposed in the early 1980s [1]. In recent years, the ovary–liver axis has gained significant attention as a crucial factor in women’s health. This axis involves hormonal, metabolic, and immunological interactions that connect ovarian endocrine function to liver health [2]. Estrogens and other ovarian hormones can profoundly influence glucose metabolism, lipid homeostasis, intrahepatic droplet formation, and inflammatory pathways in the liver. Meanwhile, liver function critically modulates the metabolism of these hormones, subsequently affecting ovarian processes such as folliculogenesis and ovulation [2]. Alterations in this dual interplay have been associated with conditions like polycystic ovary syndrome (PCOS), metabolic dysfunction-associated steatotic liver disease (MASLD), and metabolic syndrome (MetS), highlighting the significant burden these closely intertwined conditions can impose on overall public health [3,4,5]. While previous studies have independently explored the mutual and bi-directional influence of reproductive hormones on liver disease and the impact of hepatic dysfunction on ovarian health, a comprehensive understanding to fully explain the epidemiological patterns is still lacking.
Against this backdrop, the present review aims to first integrate current molecular insights regarding hormone synthesis, metabolism, and signaling pathways along the ovary–liver axis, with particular attention to dysregulated mechanisms that may underlie common disorders and liver disease associated with endocrine ovarian dysfunction. Second, it seeks to summarize epidemiological studies, ranging from cross-sectional surveys to population-based cohorts, and synthesize data on prevalence, risk factors, and outcomes associated with disrupted ovarian and hepatic functions. By doing so, the review will identify underexplored areas in this domain and underscore the need for further research on causal pathways. Finally, it intends to highlight the clinical and public health implications derived from combining molecular and epidemiological findings, laying out potential directions for interventions and strategies to mitigate disease progression. The present article does not cover the epidemiological and clinical underpinnings of infertility and fertility-related issues occurring in women with chronic liver disease.
For this review, a systematic literature search was conducted in major scientific databases such as PubMed, Scopus, and Web of Science. Targeted keywords included “ovary-liver axis”, “estrogen metabolism”, “reproductive hormones”, “hepatic metabolism”, “PCOS”, nonalcoholic fatty liver disease (“NAFLD”), nonalcoholic steatohepatitis (“NASH”), “MASLD”, “MASH”, metabolic dysfunction-associated steatohepatitis (“MALFD”), and “epidemiology.” Peer-reviewed articles, reviews, and meta-analyses published primarily in the past two decades were included to capture recent molecular discoveries and advanced epidemiological methods. The studies were screened for relevance based on their focus on the interplay between ovarian and liver functions. Entries without a clear link to either molecular mechanisms or epidemiological outcomes were excluded. The key data on study design, population characteristics, molecular markers, and clinical endpoints were extracted, with particular attention to the methodological robustness of epidemiological contributions. To ensure a transparent and reproducible selection process, we followed the PRISMA guidelines in screening, assessing, and reporting the findings [6] (Figure 1). Cross references and review articles were also considered when appropriate. Additional and detailed information on the strategy of bibliographic research can be found under Section 3, Section 4 and Section 5.
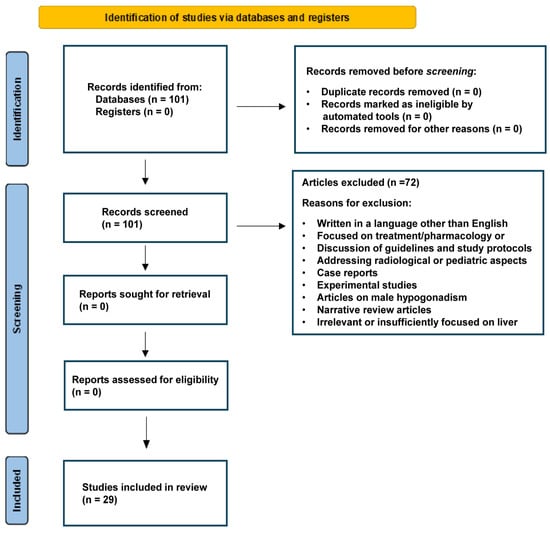
Figure 1.
PRISMA flow diagram depicting the study screening and selection process. The diagram was prepared following proposed recommendations [6].
2. Molecular Mechanisms of Action of Ovarian Hormones and Role of the Ovary in Maintaining Metabolic and Liver Health
Ovarian hormones, particularly estrogens like estradiol, play a crucial role in coordinating various physiological processes that link reproductive health and systemic metabolic regulation [7]. At the molecular level, these hormones exert their effects through distinct pathways, both classical and non-classical, that collectively influence gene transcription, enzyme activity, signal transduction, and cellular metabolism. Estrogen receptors (ERs), including estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), function as nuclear transcription factors when bound by their ligands. They form receptor-hormone complexes that bind to estrogen response elements (EREs) on target genes [8]. This interaction leads to the recruitment of co-regulators such as co-activators and co-repressors, ultimately altering chromatin structure and influencing gene expression patterns. Through these genomic actions, estrogens regulate a variety of metabolic pathways, from glucose homeostasis and lipid metabolism to inflammatory signaling in multiple tissues, with the liver being one of the most responsive and significant targets [9].
In hepatocytes, ERα is particularly abundant and crucial for regulating processes that maintain metabolic health. Under normal physiological conditions, estrogen binding to ERα promotes balanced lipid metabolism by regulating the expression of lipogenic enzymes, lipoprotein receptors, and proteins involved in cholesterol homeostasis [8]. Additionally, estrogens can increase pathways responsible for lipid oxidation, decrease triglyceride accumulation, and reduce overall lipotoxic stress in liver cells [2,9]. Apart from direct gene regulation, estrogens also participate in non-classical, membrane-initiated actions by binding to G protein-coupled estrogen receptor (GPER) or by interacting with membrane-localized ERs [10]. These rapid signaling events can activate second messenger pathways, such as phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK), that regulate enzymatic activities, glucose uptake, and inflammatory mediators in real time, highlighting the versatility and effectiveness of estrogen signaling [11,12,13].
In addition to the general regulatory framework for lipid metabolism in hepatocytes, estrogen signaling via ERα exerts precise control over key regulatory nodes such as sterol regulatory element-binding protein 1c (SREBP-1c) and members of the peroxisome proliferator-activated receptor family (PPARs) [14,15,16]. SREBP-1 is a transcription factor that controls the synthesis of lipids from glucose in the liver [17]. Under classical signaling, estrogen-ERα complexes bind to estrogen response elements with high affinity on target genes in response to E2 [18]. Estrogen stimulates an increase in SREBP-1 expression [14], promoting de novo lipogenesis and impacting carbohydrate metabolism [19,20]. Furthermore, estrogen and PPARs exhibit complex interactions. On one side, estrogen can suppress the activity of PPARs, and, conversely, PPARs can affect estrogen-signaling pathways that promote fatty acid catabolism, suggesting a complex crosstalk exists between PPARs and ERs [21]. This balances lipid uptake, storage, and oxidation, ultimately curbing triglyceride accumulation. In concert with the non-classical pathways involving GPERs, these pathways modulate glucose uptake, insulin sensitivity, and inflammatory signaling, thereby orchestrating metabolic gene expression.
Moreover, the liver plays a crucial role in regulating estrogen metabolism (Figure 2). It conjugates and breaks down estrogens to estrogenically inactive metabolites for excretion, maintaining stable blood hormone concentrations [22]. Low estrogen concentrations and impaired estrogen signaling are associated with liver pathology in both females and males [12].
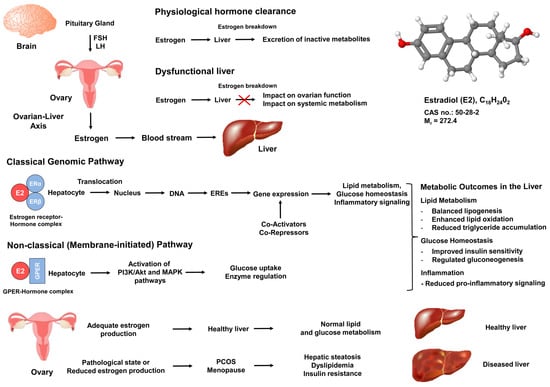
Figure 2.
Estrogen-signaling pathways and the ovary–liver axis in metabolic regulation. Estrogen signals originating from the ovary impact metabolic and inflammatory pathways in the liver through classic (genomic) and non-classic (membrane-initiated) mechanisms. Gonadotropins (FSH and LH) regulate estrogen release from the ovary, which then circulates to hepatocytes. Estradiol (E2), the most biologically active form of estrogen, binds to estrogen receptor alpha (ERα) or estrogen receptor beta (ERβ), forming hormone-receptor complexes that translocate to the nucleus and bind estrogen response elements (EREs) to regulate gene transcription. Additionally, estradiol can bind to the G protein-coupled estrogen receptor (GPER) on cell membranes, activating rapid signaling cascades (e.g., PI3K, MAPK) that control processes like glucose uptake, lipid oxidation, and inflammation. Key metabolic outcomes are depicted, such as improved lipid metabolism, reduced triglyceride accumulation, and decreased pro-inflammatory cytokine signaling, demonstrating how estrogens support healthy liver function and overall systemic balance.
Therefore, when hepatic function is compromised, hormone clearance can be affected, leading to either low or high hormone concentrations. Both scenarios can negatively impact ovarian function and overall metabolic regulation. The ovary is responsible for synthesizing and releasing estrogen and progesterone in response to follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland [23]. This cyclical regulation forms the basis of the menstrual cycle, with distinct phases (follicular, ovulatory, and luteal) characterized by changing hormone concentrations that prepare the body for potential pregnancy [23]. Estrogen plays a key role in folliculogenesis and ovulation as well as in maintaining systemic functions like insulin sensitivity, energy utilization, and lipid handling [24].
By controlling hormone secretion, the ovary ensures that target tissues including the liver receive optimal hormonal signals for maintaining metabolic homeostasis. The action of estrogen protects against insulin resistance (IR) and chronic inflammation, which are integral in the pathobiology of MetS [24]. Clinical observations show that—compared to postmenopausal women and men of similar age—premenopausal women have lower rates of metabolic disorders like dyslipidemia and MASLD [25]. This difference is attributed to ongoing ovarian hormone production, which specifically supports liver health in the context of systemic metabolic and immune function. Disruptions in ovarian hormone production, as seen in conditions like PCOS, premature ovarian insufficiency, or menopause, can lead to hormonal imbalances that perturb metabolic processes and impair energy and gluco-lipidic metabolism [26,27]. Elevated androgens can worsen IR in the liver and peripheral tissues [28], promoting hepatic steatosis and inflammation. Lower estrogen concentrations reduce the capacity of the liver to counteract these negative effects.
PCOS and MASLD have been correlated with infertility through overlapped pathophysiological mechanisms [29]. In women with PCOS, hyperandrogenemia can negatively impact hepatic lipid metabolism, increasing the risk of MASLD and other metabolic disorders [30]. Additionally, IR and central adiposity, common in PCOS, further strain liver function. Even in asymptomatic, otherwise healthy individuals, such as postmenopausal women, an insufficient estrogen environment can lead to weight gain, dyslipidemia, hepatic steatosis, and increased diabetes risk [31,32]. These findings emphasize the importance of maintaining ovarian function and drive research into hormone replacement therapies to protect women from hepatic and metabolic complications as they age.
In summary, the intricate relationship between ovarian hormones and metabolic/liver health underscores a complex system that regulates reproductive and metabolic balance. Estrogens play a central role in gene expression and signaling pathways, connecting energy balance, intrahepatic lipid content, and liver inflammation. The precise hormone secretion by the ovary ensures that the liver and other tissues receive the right hormonal environment, preserving metabolic health and reducing chronic disease risk. Any disruption in hormone synthesis, receptor function, or hepatic metabolism can trigger a chain reaction of disease risk, highlighting the interconnectedness of ovarian function and hepatic health. Further research into these pathways will enhance our ability to diagnose, prevent, and manage gynecological and metabolic conditions, ultimately improving health outcomes for women at all stages of life.
3. The Liver and PCOS
Given the abundance of published epidemiological studies, we decided to focus on meta-analytical evidence supporting an association between PCOS and NAFLD/MASLD. Our research strategy ((liver[Title/Abstract]) AND (PCOS[Title/Abstract])) AND (meta-analysis[Title/Abstract]) retrieved 17 results. Of these, nine were discarded because they focused on treatment, discussed guidelines and study protocols, were narrative review articles, or were irrelevant regarding the association of NAFLD & PCOS. The remaining eight studies are summarized in Table 1 [33,34,35,36,37,38,39,40].

Table 1.
Meta-analytical evidence supporting associations between PCOS and NAFLD/MASLD.
Most studies have found an increased risk of NAFLD/MASLD among women with PCOS compared to PCOS-free, healthy controls [33,34,35,36,39]. These studies collectively show an association between NAFLD/MASLD and PCOS, with estimates of the strength of the association ranging from an odds ratio (OR) of 2.25 [35] to an OR of 3.93 [33]. Several mechanisms underlie this nexus, which can be both metabolic and hormonal. Studies using multivariate analysis have shown that serum androgen concentrations strongly predict NAFLD among PCOS women regardless of confounding factors [34]. Shengir et al. [36] found that IR and MetS were more common among PCOS women than among controls. Manzano-Nunez et al. [37] identified body mass index (BMI), waist circumference (WC), alanine transaminase (ALT), homeostasis model of IR (HOMA-IR), free androgen index (FAI), hyperandrogenemia (HA), and triglycerides as variables associated with significantly higher risk of NAFLD among women with PCOS. These findings have been confirmed by others [40].
While epidemiological association does not prove causality, Liu et al. [38] conducted a bidirectional two-sample Mendelian Randomization analysis using the United Kingdom Biobank genome-wide association study database. They demonstrated that genetically predicted NAFLD is causally associated with higher odds of PCOS while PCOS may not necessarily be a risk factor for NAFLD. This conclusion conflicts with clinical wisdom that generally suggests addressing the association in either direction. In 2015, the Italian Association for the Study of the Liver was the first to suggest that “In young women, changes in the menstrual cycle and hirsutism may suggest the presence of polycystic ovary syndrome, frequently associated with NAFLD” [41]. Conversely, evidence that NAFLD may follow a particularly aggressive course in women with PCOS [42] recommends careful assessment of liver health status in all individuals with PCOS [43]. In conclusion, additional studies are requested to ascertain whether the NAFLD-PCOS relationship is mutual and bi-directional or uni-directional such as suggested by the study published by Liu [38].
Notably, young women are typically spared from NAFLD/MASLD [44] and more advanced disease stages, including fibrosis NASH/MASH. Therefore, hyperandrogenemic PCOS impairs this typical age- and sex-related protection, making the liver more susceptible not only to fat infiltration but also to more rapid progression of inflammatory and fibrosing disease [37,45] with the inherent risks of premature mortality and dangerous hepatic and extra-hepatic outcomes [46,47]. To prevent these risks, the diagnostic strategy must be refined, also considering the lessons learned from the PCOS studies. Hyperandrogenemia plays a major role in the pathogenesis of NAFLD/MASLD in PCOS through an extended half-life of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) via inhibited LDL-receptor expression leading to elevated concentrations of LDL, triglycerides and increased IR [39]. This pathogenic mechanism may be leveraged clinically using serum testosterone concentrations greater than 3.0 nmol/L as a cut-off value for defining PCOS women at a higher NAFLD risk [48]. At the same time, standard ultrasonography has sufficient sensitivity to detect fatty changes as low as 5% of hepatocytes [49] and, given its low cost and universal availability, remains a first-line assessment tool in this field.
Regarding the methodological limitations of the studies listed in Table 1, different diagnostic criteria have been used to identify both PCOS and NAFLD/MASLD. Liver biopsy was not utilized in these studies, and the MASLD nomenclature was not employed. Importantly, the diagnosis of PCOS relies on a mix of Rotterdam/NIH/AES criteria, which calls for additional and more standardized investigations. There is also evidence of differences among various ethnicities [36]. NAFLD exhibits a different prevalence in the general population across continents, with the highest values, around 30% for each region, being found in South America and the Middle East [27]. Gene variants like the rs738409 G allele of the patatin-like phospholipase domain-containing protein 3 gene are deemed to account for the elevated NAFLD prevalence in South America even though these individuals consume fewer daily calories than in the USA and Europe [27]. Publication bias is another concern [39]. Collectively, these drawbacks call for better designed and adequately sized additional studies.
4. The Liver in Hypogonadal Women
Compared to the extensive literature on PCOS, the published studies on hypogonadism and liver in women are much less numerous and there is no published meta-analytic review assessing the risk of liver disease among hypogonadic women. Therefore, a dual approach was taken to gather relevant epidemiological studies on this topic. Initially, the research strategy ((liver[Title/Abstract]) AND (hypogonadism[Title/Abstract])) AND (female[Title/Abstract]) yielded 55 results. Out of these 55 results, 7 were selected after excluding reviews, case reports, articles in languages other than English, experimental studies, articles on male hypogonadism, pharmacology of gonadotropins, radiological or pediatric aspects, and articles insufficiently focused on the liver or hypogonadism. These articles were combined with four additional articles found using the research query (ovarian insufficiency[Title/abstract]) and (Liver[Title/Abstract[) after excluding reviews, experimental articles, case reports, articles not in English, articles on menopause, retracted articles, and articles on therapeutics. Together, these eleven retrieved articles are summarized in Table 2 [50,51,52,53,54,55,56,57,58,59,60].

Table 2.
Epidemiological evidence supporting the association between female hypogonadism and liver disease.
There seems to be a two-way relationship between chronic liver disease (CLD), particularly cirrhosis, and hypogonadism. It has long been known that cirrhosis is often accompanied by hypogonadism which, in turn, increases the risk of osteoporosis and bone fractures [50]. More recently, researchers have focused on liver health in Turner syndrome (TS). TS is a rare genetic disorder caused by a partial or complete loss of one X chromosome [61]. Its prevalence is estimated to be 1/2500 female live births [62] and its primary clinical features include short stature and premature or primary ovarian insufficiency. Other complications include heart, aortic, thyroid, and kidney diseases, neurocognitive deficiency, skeletal deformities, and impaired hearing [62].
A substantial proportion of women with TS exhibit elevated liver enzymes, most often raised gamma-glutamyl transferase (GGT) [51,54]. These altered liver tests are associated with specific karyotypic abnormalities, cardiac valve disease [51], and hormonal imbalances [59,60] in the context of a dysmetabolic systemic environment featuring the MetS and its individual components (e.g., atherogenic dyslipidemia), IR, and subclinical chronic inflammation [59,60]. A wide range of histological changes related to the NAFLD spectrum sustain these enzyme alterations, including steatosis in most cases and liver fibrosis and cirrhosis in a variable fraction of subjects regardless of obesity [54,57,58]. Non-invasive indices of fibrosis, such as fibrosis-4 (FIB-4) and aspartate transaminase to platelet ratio index (APRI), are significantly altered compared to controls [58,60]. Methodological limitations of these studies—that should be addressed in future studies—include retrospective analysis, limited sample size, and failure to assess multi-ethnic populations.
Another clinical model used to determine the association of hypogonadism with CLD involves individuals with pituitary adenomas who undergo transsphenoidal adenectomy [55]. However, this disease paradigm is complex and difficult to decipher due to multiple hormonal insufficiencies that can result in severe fibrosing CLD [63]. This may explain why a large study failed to show a statistically significant association between the prevalence of NAFLD among hypogonadal vs. eugonadal subjects with non-functioning pituitary adenoma who underwent transsphenoidal adenectomy [55]. Therefore, additional studies are needed to address this research question.
Hereditary hemochromatosis (HH) also includes features of hypogonadism and CLD [64,65]. Both conditions arise from the accumulation of excess iron in tissues, leading to end-stage functional failure of the gonads and liver unless the excess iron is removed [66]. Because both the ovaries and the liver are impacted by the primary pathogenic insult (iron accumulation and its associated oxidative stress burden), this disease model may not be entirely suitable and probably not the ideal approach for evaluating the role of hypogonadism in the development of CLD.
Alström syndrome (AS) is a monogenic form of obesity caused by recessive mutations in ALMS1 (chromosome 2q13). It also features progressive retinal dystrophy, sensory hearing loss, cardiomyopathy, type 2 diabetes, hypertriglyceridemia, and progressive hepatic and renal dysfunction in late childhood and adulthood [52]. AS is rare with an estimated prevalence of 1 to 10 in 1,000,000 people and about 700 published cases [52]. Although incompletely defined, ALMS1 is deemed to play a role in ciliary function, cell cycle regulation, endosomal trafficking, cell migration, and extracellular matrix production [52]. About half of the individuals with AS are hypogonadal, and women often have alopecia, elevated testosterone, hirsutism, oligomenorrhea and amenorrhea in adulthood [52]. As predicted by the physiology of the ovary–liver axis, subjects with AS are insulin-resistant and have higher liver enzymes and intrahepatic fat content regardless of age, sex, ethnicity, and BMI [52]. The rarity of AS makes this disease entity scarcely suitable to conduct large studies on the association of ovarian function and CLD.
5. The Liver in Menopause and Ovarian Senescence
Menopause, caused by the loss of ovarian follicular function and associated with a decline in estrogen concentrations, marks the end of a woman’s reproductive years. It is characterized by the absence of menstruation and typically occurs in most women between the ages of 45 and 55 as a part of normal aging [67]. In 1947, the Danish clinician Jersild, based on his observation of an outbreak of subacute liver atrophy among post-menopausal women initially attributed to viral hepatitis, was the first to suggest the notion that estrogens may play a protective effect on the liver’s health. This implies that, in post-menopausal women, the physiological decline in estrogen concentrations may make them more susceptible to severe liver disease [68]. However, much progress has been made in understanding this phenomenon since Jersild’s pioneering report. A bibliographic search using the term (LIVER[TITLE]) AND (MENOPAUSE[Title]) yielded 25 results, of which 10 were deemed relevant after excluding articles in languages other than English, reviews, and articles on therapy. Table 3 summarizes the findings of studies that assess the role of menopause in liver disease [69,70,71,72,73,74,75,76,77,78].

Table 3.
Epidemiological studies assessing the risk of chronic liver disease in menopause.
Although not all published studies shown in Table 3 agree, the majority strongly support the notion that menopause exposes women to more severe CLD due to the fibrosing progression of chronic hepatitis C [69] and of NAFLD/NASH and MAFLD/MASLD [70,71,73,75,77]. Additionally, subclinical coronary artery disease identified through coronary artery calcification typically occurs in women over the age of 65. This likely indicates prolonged estrogen deficiency rather than aging itself and, unlike men, is probably not strongly related to MASLD specifically [78]. Menopause typically involves body fat redistribution with a preferential accumulation of visceral adipose tissue depots which leads to an increased risk of hepatic steato-fibrotic changes due to the increased risk of MetS and its inherent alteration in glucose tolerance and lipid profile [72]. These concepts are relevant for understanding the risk of progressive NAFLD/MASLD in the context of hormone-modulating treatments such as ovariectomy [71] and tamoxifen [79].
A recent study [80] has investigated the use of Anti-Müllerian hormone (AMH) as a marker of ovarian reserve. AMH is a glycoprotein produced by ovarian follicles that gradually decreases as women age and eventually becomes undetectable at menopause. Ovarian reserve refers to the number and quality of oocytes remaining in a woman’s ovaries and is closely linked to ovarian senescence, the aging process of the ovaries [81]. In their study, Maldonado, and colleagues utilized the NASH Clinical Research Network to examine the relationship between AMH concentrations and histological outcomes of NAFLD in 205 premenopausal women, 20% of whom had PCOS. The data revealed a negative correlation between higher AMH concentrations and NASH, with these associations being more pronounced in premenopausal women without PCOS [80], suggesting that low AMH concentrations not only indicate reproductive aging but also signify worsening cardiometabolic risk profiles [82]. Further investigation is necessary to determine the clinical significance of AMH measurements in hepatological practice and research.
A comprehensive analysis of the effects of menopausal hormonal replacement therapy (MRT) on liver health is beyond the scope of this review. A recent retrospective analysis of 368 post-menopausal women exposed to MRT suggests that transdermal estrogen can be beneficial in terms of NAFLD progression [83].
6. Molecular Pathogenesis of Liver Disease Associated with Ovarian Senescence, Menopause, PCOS, and Female Hypogonadism
The molecular pathogenesis of liver disease-associated ovarian senescence, menopause, PCOS, and female hypogonadism is intricately connected to the intersecting endocrine and metabolic disturbances that arise from altered hepatic function and its impact on the hypothalamic–pituitary–ovarian axis [38,84]. In healthy individuals, the liver plays a pivotal role in the metabolism, conjugation, and clearance of sex hormones, most notably estrogens and androgens, as well as in the regulation of insulin signaling and systemic nutrient balance [2,9].
Elevated pro-inflammatory cytokines such as TNF-α, released by diseased liver tissue, are known to promote androgen production in ovarian granulosa cells. This disruption affects gonadotropin-releasing hormone (GnRH) pulsatility, leading to changes in LH and FSH secretion [85]. These changes contribute to an imbalance between estrogen and androgens, favoring ovulatory dysfunction, hyperandrogenism, and compromising folliculogenesis [86]. Additionally, sex hormone-binding globulin (SHBG), a glycoprotein primarily produced by the liver, plays a crucial role in binding and affecting the bioavailability of sex hormones, such as testosterone and estrogen, impacting ovarian functionality [87]. A decrease in SHBG production during hepatic insult or metabolic dysfunction results in fewer hormones being bound, leading to an increase in free active androgens, promoting hyperandrogenism and insulin resistance [87,88]. This can impair folliculogenesis, increase oxidative stress, accelerate ovarian senescence, and cause various menstrual cycle and reproductive system disturbances [87]. Furthermore, SHBG levels are significantly lower in patients with NAFLD and type 2 diabetes [89]. On the contrary, a prospective study has indicated that basal serum SHBG is negatively associated with NAFLD development but positively associated with NAFLD regression, suggesting a more complex role of SHBG in the pathogenesis of NAFLD and insulin resistance [90].
These events highlight the critical role of a dysfunctional liver in creating a cycle of hormone imbalance, inflammatory signaling, and ovarian dysfunction that contributes to conditions like PCOS, early menopause, and female hypogonadism (Figure 3).
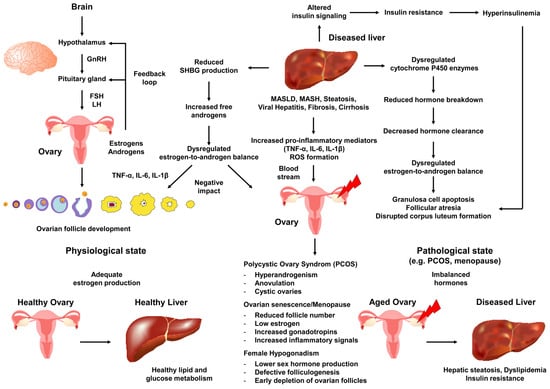
Figure 3.
Hepato-ovarian crosstalk in the molecular pathogenesis of female reproductive disorders. The diagram displays the bidirectional influences between the liver and ovaries in the context of chronic liver disease. A liver affected by conditions such as MASLD, cirrhosis, or viral hepatitis undergoes changes in cytochrome P450 enzyme activity, reduced hormone-metabolizing capacity, increased inflammatory cytokines, and insulin resistance. These changes impact the concentrations of circulating estrogens and androgens, while also favoring subclinical systemic inflammation. The hypothalamic–pituitary–ovarian (HPO) axis transmits signals through gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) receiving distorted feedback signals due to abnormal hormone ratios (e.g., higher androgen-to-estrogen concentrations) and inflammatory mediators circulating through the bloodstream to various organs. The ovary transitions with aging into pathophysiological states such as PCOS, early menopause, or hypogonadism (indicated by a red arrow). Key disruptions caused by the diseased liver affecting the ovary include hyperinsulinemia driving hyperandrogenism, reduced sex hormone-binding globulin (SHBG) production, and compromised folliculogenesis, all contributing to increased oxidative stress and follicular atresia. The bottom panels emphasize how sustained hepatic insults accelerate ovarian senescence, highlighting how impaired clearance by the liver and metabolic dysregulation of hormones can worsen ovarian dysfunction, creating a vicious cycle that may contribute to the development of PCOS, reproductive decline, or hypogonadism. Abbreviations are as follows: IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Moreover, estrogen signaling intersects with inflammatory pathways, as elevated mediators like TNF-α and IL-6 can disrupt ER function in hepatocytes, promoting lipogenesis and contributing to steatosis [91]. Conversely, ovarian hormones, especially estrogens, help limit hepatic lipid droplet formation by suppressing lipogenic factors such as SREBP-1c and boosting fatty acid oxidation via PPARα, thus enhancing insulin sensitivity [14,15,16]. In line with this, hepatic steatosis has been reported in ERα-deficient male mice after sexual maturation [91]. Additionally, epigenetic factors such as smoking and the decreased hepatic capacity to clear estrogens after menopause further alter systemic hormone levels, potentially exacerbating inflammation, lipotoxicity, and metabolic dysregulation [92]. These mechanisms underscore the complex interplay between estrogen signaling, inflammatory mediators, and liver metabolism during the reproductive transition.
Chronic liver impairment, whether induced by viral hepatitis, alcohol-related MASLD, or autoimmune hepatopathies, can disrupt these tightly regulated processes by altering hepatic enzyme expression and activity, affecting hormone clearance, and intensifying inflammation and oxidative stress, all of which can have downstream consequences for ovarian tissue [93]. One major factor contributing to this cascade is the dysregulation of cytochrome P450 enzymes in the liver, which typically break down estrogens and other steroid hormones into inactive metabolites for elimination [22]. When these enzymes are compromised, either through reduced expression or functionality, circulating hormone concentrations can deviate significantly from normal ranges. Excess estrogen can trigger an abnormal feedback loop at the hypothalamus and pituitary level, leading to changes in gonadotropin release, while inadequate estrogen, or an elevated androgen-to-estrogen ratio, can accelerate ovarian aging and anovulatory cycles seen in hypogonadal women or with premature menopause. Moreover, abnormal expression of estrogen and its receptors has been associated not only with ovarian disorders but also with malignant tumors [93]. Concurrently, inflammatory cytokines such as TNF-α, IL-6, and IL-1β, often elevated in liver disease, can enter systemic circulation and target various tissues, including the ovaries. There, they can trigger stress responses that impede follicle development, disrupt corpus luteum formation, and promote apoptotic pathways in granulosa cells, potentially driving ovarian cancer development [94]. Over time, these inflammatory injuries contribute to the gradual decline of ovarian follicles, reflected in clinical manifestations of early menopause or reduced ovarian reserve in younger women.
An intricate relationship between hepatic insulin regulation and ovarian function also plays a role in the molecular pathogenesis of liver disease-related ovarian conditions such as PCOS [95]. IR, a characteristic of many chronic liver diseases, can either initiate or worsen hepatic steatosis, leading to hyperinsulinemia [96]. This can boost androgen production in the gonads and suppress hepatic production of SHBG [97]. Reduced SHBG allows free testosterone to accumulate, shifting the hormonal balance towards hyperandrogenism, a key feature of PCOS [98]. This hyperandrogenic environment disrupts follicular growth and ovulation in the ovaries, while also triggering a persistent low-grade systemic subclinical inflammatory state. Elevated free fatty acids, originating from insulin-resistant adipose tissue and the diseased liver, further perpetuate this inflammatory environment by releasing pro-inflammatory substances and activating stress signals in ovarian theca cells [99]. Additionally, high insulin concentrations and hepatic dysfunction may converge on ovarian tissues through the mammalian target of rapamycin (mTOR) pathways [100]. It is worth noting that mTOR inhibitors can enhance cell growth and potentially contribute to ovarian cyst development [101]. In such conditions, excess reactive oxygen species (ROS) produced during liver and ovarian inflammation can harm mitochondrial structure, DNA, and function, thereby exacerbating the aging and apoptotic decay of oocytes [102]. Consequently, while PCOS is typically characterized by hyperandrogenism and chronic anovulation, prolonged disease duration and compounded stressors can lead to a decline in overall ovarian reserve, pushing women towards earlier ovarian failure or a more severe hypogonadal state [43].
Menopause, either premature or physiological, is influenced by these pathophysiological overlaps. Pre-menopause and menopause show a gradual decline in ovarian estrogen production to concentrations equal to those of age-matched men, which removes a protective buffer against metabolic, oxidative, and inflammatory insults in the liver and other tissues [103]. While menopause can occur independently of liver disease as part of natural aging, chronic hepatitis or cirrhosis can accelerate follicular atresia by increasing catabolic and inflammatory pathways that damage ovarian tissue. In particular, there is a unique hepato-ovarian link in which the liver and the ovaries develop similar metabolic abnormalities during the pathogenesis of MASLD and PCOS [104]. In menopause, the hepatic metabolism of estrogens, already reduced by the declining hormonal production of the ovaries, may be further compromised by cirrhotic changes that impede blood flow and impair enzyme function. Therefore, menopausal women with underlying liver disease may face a heightened risk of metabolic comorbidities, bone density loss, and cardiovascular complications [105]. Additionally, the combination of low estrogen concentrations, systemic inflammation, and potential hyperandrogenism can exacerbate IR, creating a vicious cycle where the decline in hepatic and ovarian health mutually reinforces each other. This interdependence is particularly evident in individuals with genetic predispositions to autoimmune disorders or lifestyles that increase metabolic risks, such as excessive alcohol consumption, cigarette smoking, obesity, or prolonged exposure to environmental toxins, all of which can intensify the cascade leading to liver disease [106,107]. This can potentially lead to gonadal senescence and metabolic collapse due to the aforementioned ovary–liver connection.
Female hypogonadism, whether primary (due to intrinsic ovarian pathology) or secondary (stemming from pituitary or hypothalamic dysfunction), is another manifestation that can emerge from or be exacerbated by liver disease processes [106]. Reduced hepatic clearance of toxins and byproducts of metabolism may lead to an altered endocrine environment around the hypothalamus, interfering with the pulsatile secretion of GnRH that is required for the cyclical release of LH and FSH [108]. Over time, this dysregulated gonadotropin profile impedes the ovarian production of sex steroids, potentially leading to a hypogonadal scenario. Additionally, hepatic complications can impede the normal synthesis of steroid precursor proteins and transport molecules, such as albumin and hormone-binding globulins, leading to circulating imbalances in estradiol, progesterone, and androgens [109]. The resulting hormonal disequilibrium impairs ovulatory cycles and fosters detrimental feedback loops that can lock the ovaries into a state of persistent dysfunction and accelerate follicular depletion. Furthermore, chronic alterations in amino acid metabolism and micronutrient absorption, both of which are frequently encountered in advanced liver disease, may limit the availability of essential substrates for steroidogenesis, compounding the hypogonadal state. Consequently, high macronutrient intakes with low micronutrient content are associated with the development of overweight and obesity [110], again impacting the ovary–liver axis.
Ultimately, ovarian senescence, menopause, PCOS, and female hypogonadism represent partially overlapping endpoints on a continuum of disrupted inter-organ communication. Hepatic insufficiency and inflammation derail the finely tuned hormonal networks that govern reproductive function. From a molecular standpoint, the chronic supply of pro-inflammatory cytokines triggering dysregulated steroidogenic pathways, and the buildup of oxidative and metabolic stress in both the liver and the ovaries play a central role in this deterioration [93]. As negative feedback loops multiply over time, the depletion of functional follicles occurs, pushing women towards premature ovarian senescence. Recent whole-organ imaging conducted in middle-aged mice has demonstrated that vascular aging in the ovaries is a significant factor associated with ovarian aging and decreased fertility [111].
Understanding these molecular underpinnings not only sheds light on the etiology of gynecological disorders in the context of liver disease but also provides a rationale for targeted interventions to intercept or slow the path towards endocrine failure [112]. These interventions may include anti-inflammatory agents, insulin sensitizers, antioxidants, carefully balanced hormone replacement therapies to prevent liver damage, or novel strategies aimed at restoring the normal hepatic metabolism of sex hormones. Furthermore, ongoing research into biomarkers such as altered concentrations of bone turnover biomarkers, hormone concentrations, growth hormone, markers of ovarian reserve such as AMH, and many others that capture the earliest shifts in liver–ovary crosstalk holds the promise of enabling clinicians to identify at-risk women well before profound ovarian senescence or hypogonadism develop, allowing for preventive or mitigative measures [106]. This work has recently shown that there are several shared markers between NAFLD and PCOS related to circadian rhythm disruption [113].
There are several potential strategies that could be highly beneficial in disrupting the mutual connection between NAFLD and PCOS. Initial therapeutic interventions such as lifestyle modifications including diet, weight loss and exercise are considered the simplest ones [114]. However, there are also different pharmacologic strategies, include antioxidant therapies aimed at reducing oxidative stress and hepatocellular damage, insulin sensitizers (e.g., metformin, thiazolidinediones, glucagon-like peptide-1 receptor agonists) that restore metabolic homeostasis and improve gonadal hormone balance, and mTOR inhibitors such as miR-615-5p that could attenuate hyperproliferative signals, inflammatory cascades, and decrease lipid droplet accumulation [30,115,116,117]. Exploring these strategies will enhance our understanding, highlighting not only their potential in reducing liver injury but also their relevance in addressing ovarian dysfunction and mitigating reproductive decline.
7. Conclusions
A mutual and bi-directional interaction occurs between liver health and ovarian function. While the consequences of underlying CLD on female fertility have been examined elsewhere [118], here, we have adopted a combined analysis of molecular science and epidemiological evidence to specifically identify the scope of the ovary–liver axis across a spectrum of disorders and conditions. These range from physiological and premature menopause to PCOS and menopause. By understanding the molecular intricacies of how a diseased liver can undermine ovarian function, and conversely, how disrupted endocrine ovarian function may eventually impair liver health, clinicians and researchers can develop more comprehensive approaches to women’s health. These approaches ensure that interventions are holistically designed to maintain not only reproductive capability but, importantly, also metabolic health, duration and quality of life. This knowledge not only informs clinical science but also shapes public health interventions and policies.
Author Contributions
Conceptualization, A.L. and R.W.; resources, A.L. and R.W.; data curation, AL. and R.W.; writing—original draft preparation, A.L. and R.W.; writing—review and editing, A.L. and R.W.; visualization, A.L. and R.W.; supervision, A.L. and R.W.; project administration, A.L. and R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This article is entirely original, written and edited by the two authors. AI was only used for English editing, and the result was then manually re-edited by RW and AL. Therefore, the authors take full responsibility for the content of this publication.
Conflicts of Interest
RW declares no conflicts of interest. AL serves as a member of the Editorial Board of IJMS. However, he was not involved in any stage of the editorial processing of this manuscript, from peer review to the final editorial decision, as all these steps were handled independently by the journal.
Abbreviations
The following abbreviations are used in this manuscript.
| APRI | AST to Platelet Ratio Index |
| AS | Alström syndrome |
| BMI | Body mass index |
| E2 | Estradiol |
| ERα | Estrogen receptor α |
| ERβ | Estrogen receptor β |
| ER(s) | Estrogen receptor(s) |
| ERE(s) | Estrogen response element(s) |
| FIB-4 | Fibrosis 4 |
| FAI | Free androgen index |
| FSH | Follicle-stimulating hormone |
| GGT | Gamma-glutamyl transferase |
| GnRH | Gonadotropin-releasing hormone |
| GPER | G protein-coupled estrogen receptor |
| HA | Hyperandrogenemia |
| HH | Hereditary hemochromastosis |
| HOMA-IR | Homeostasis model of insulin resistance |
| IR | Insulin resistance |
| LH | Luteinizing hormone |
| MAPK | Mitogen-activated protein kinase |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MetS | Meabolic syndrome |
| mTOR | Mammalian target of rapamycin |
| PI3K | Phosphoinositide 3-kinase |
| PCOS | Polycystic ovary syndrome |
| ROS | Reactive oxygen species |
| SHBG | Sex hormone-binding globulin |
| T2DM | Type 2 diabetes mellitus |
| TS | Turner syndrome |
| WC | Waist circumference |
References
- Gustafsson, J.A.; Mode, A.; Norstedt, G.; Skett, P. Sex steroid induced changes in hepatic enzymes. Annu. Rev. Physiol. 1983, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Dolce, A.; Della Torre, S. Metabolic dysfunction-associated steatotic liver disease across women’s reproductive lifespan and issues. Clin. Mol. Hepatol. 2025, 31, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.B.; Seo, S.K.; Yun, B.H.; Cho, S.; Choi, Y.S.; Lee, B.S. Non-alcoholic fatty liver disease in polycystic ovary syndrome women. Sci. Rep. 2021, 11, 7085. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Sung, Y.A.; Hong, Y.S.; Song, D.K.; Jung, H.; Jeong, K.; Chung, H.; Lee, H. Non-alcoholic fatty liver disease is associated with hyperandrogenism in women with polycystic ovary syndrome. Sci. Rep. 2023, 13, 13397. [Google Scholar] [CrossRef]
- Alhermi, A.; Perks, H.; Nigi, V.; Altahoo, N.; Atkin, S.L.; Butler, A.E. The role of the liver in the pathophysiology of PCOS: A literature review. Biomolecules 2025, 15, 51. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Gui, Z.; Shi, W.; Zhou, F.; Yan, Y.; Li, Y.; Xu, Y. The role of estrogen receptors in intracellular estrogen signaling pathways, an overview. J. Steroid Biochem. Mol. Biol. 2025, 245, 106632. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of estrogens in the regulation of liver lipid metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [CrossRef]
- Bushi, A.; Ma, Y.; Adu-Amankwaah, J.; Wang, R.; Cui, F.; Xiao, R.; Zhao, J.; Yuan, J.; Tan, R. G protein-coupled estrogen receptor biased signaling in health and disease. Pharmacol. Ther. 2025, 269, 108822. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The influence of sex hormones in liver function and disease. Cells 2023, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, R.; Wang, Y.; Xiao, H.; Lin, W.; Diao, M.; He, S.; Mei, P.; Liao, Y. G protein-coupled estrogen receptor activates PI3K/AKT/mTOR signaling to suppress ferroptosis via SREBP1/SCD1-mediated lipogenesis. Mol. Med. 2024, 30, 28. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, X.; Xu, Y.; Cheng, D.; Xia, X.; Lv, X.; Yuan, G.; Peng, C. Estrogen mediates an atherosclerotic-protective action via estrogen receptor alpha/SREBP-1 signaling. Front. Cardiovasc. Med. 2022, 9, 895916. [Google Scholar] [CrossRef]
- Huang, W.Y.; Sun, P.M. Estrogen receptor-associated receptor α and peroxisome proliferator-activated receptor γ in metabolism and disease (Review). Mol. Med. Rep. 2021, 23, 156. [Google Scholar] [CrossRef]
- Antwi, M.B.; Lefere, S.; Clarisse, D.; Koorneef, L.; Heldens, A.; Onghena, L.; Decroix, K.; Fijalkowska, D.; Thommis, J.; Hellemans, M.; et al. PPARα-ERRα crosstalk mitigates metabolic dysfunction-associated steatotic liver disease progression. Metabolism 2025, 164, 156128. [Google Scholar] [CrossRef]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A.; et al. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, X.; Wang, G. Targeting SREBP-1-mediated lipogenesis as potential strategies for cancer. Front. Oncol. 2022, 12, 952371. [Google Scholar] [CrossRef]
- Yoon, M. PPARα in obesity: Sex difference and estrogen involvement. PPAR Res. 2010, 2010, 584296. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; Alviggi, C.; Lispi, M.; Conforti, A.; Hanyaloglu, A.C.; Chuderland, D.; Simoni, M.; Raine-Fenning, N.; Crépieux, P.; Kol, S.; et al. Reduced FSH and LH action: Implications for medically assisted reproduction. Hum. Reprod. 2021, 36, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The role of estrogen in insulin resistance: A review of clinical and preclinical data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Goulis, D.G. Menopause and metabolic dysfunction-associated steatotic liver disease. Maturitas 2024, 186, 108024. [Google Scholar] [CrossRef]
- Ko, S.H.; Jung, Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Emanuel, R.H.K.; Roberts, J.; Docherty, P.D.; Lunt, H.; Campbell, R.E.; Möller, K. A review of the hormones involved in the endocrine dysfunctions of polycystic ovary syndrome and their interactions. Front. Endocrinol. 2022, 13, 1017468. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of insulin and insulin resistance in androgen excess disorders. World J. Diabetes 2021, 12, 616–629. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Chatzikalil, E.; Kalopitas, G.; Patoulias, D.; Popovic, D.S.; Metallidis, S.; Kotsa, K.; Germanidis, G.; Koufakis, T. Metabolic dysfunction-associated steatotic liver disease and polycystic ovary syndrome: A complex interplay. J. Clin. Med. 2024, 13, 4243. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Lu, Y.; Wu, L. Association of metabolic-dysfunction associated steatotic liver disease with polycystic ovary syndrome. iScience 2024, 27, 108783. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse changes in body composition during the menopausal transition and relation to cardiovascular risk: A contemporary review. Womens Health Rep. 2022, 3, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ramezani-Binabaj, M.; Motalebi, M.; Karimi-Sari, H.; Rezaee-Zavareh, M.S.; Alavian, S.M. Are women with polycystic ovarian syndrome at a high risk of non-alcoholic Fatty liver disease; a meta-analysis. Hepat. Mon. 2014, 14, e23235. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M.; Moreira, G.V.; Cândido, A.L.; Couto, C.A.; Reis, F.M. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: Systematic review and meta-analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef]
- Wu, J.; Yao, X.Y.; Shi, R.X.; Liu, S.F.; Wang, X.Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: An update meta-analysis. Reprod. Health 2018, 15, 77. [Google Scholar] [CrossRef]
- Shengir, M.; Chen, T.; Guadagno, E.; Ramanakumar, A.V.; Ghali, P.; Deschenes, M.; Wong, P.; Krishnamurthy, S.; Sebastiani, G. Non-alcoholic fatty liver disease in premenopausal women with polycystic ovary syndrome: A systematic review and meta-analysis. JGH Open 2021, 5, 434–445. [Google Scholar] [CrossRef]
- Manzano-Nunez, R.; Santana-Dominguez, M.; Rivera-Esteban, J.; Sabiote, C.; Sena, E.; Bañares, J.; Tacke, F.; Pericàs, J.M. Non-alcoholic fatty liver disease in patients with polycystic ovary syndrome: A systematic review, meta-analysis, and meta-regression. J. Clin. Med. 2023, 12, 856. [Google Scholar] [CrossRef]
- Liu, D.; Gao, X.; Pan, X.F.; Zhou, T.; Zhu, C.; Li, F.; Fan, J.G.; Targher, G.; Zhao, J. The hepato-ovarian axis: Genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023, 21, 62. [Google Scholar] [CrossRef]
- Yao, K.; Zheng, H.; Peng, H. Association between polycystic ovary syndrome and risk of non-alcoholic fatty liver disease: A meta-analysis. Endokrynol. Pol. 2023, 74, 520–527. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, G.; Zhang, Y.; Li, R. Risk factors for metabolic dysfunction-associated steatotic liver disease in patients with polycystic ovary syndrome in East Asia: A review and meta-analysis. Endocr. Pract. 2025, 31, 668–676. [Google Scholar] [CrossRef]
- Loria, P.; Adinolfi, L.E.; Bellentani, S.; Bugianesi, E.; Grieco, A.; Fargion, S.; Gasbarrini, A.; Loguercio, C.; Lonardo, A.; Marchesini, G.; et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert. Committee. Dig. Liver Dis. 2010, 42, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Cordero, P.; Li, J.; Nguyen, V.; Oben, J.A. A guide to non-alcoholic fatty liver disease in childhood and adolescence. Int. J. Mol. Sci. 2016, 17, 947. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Rasquin, L.I.; Anastasopoulou, C. Polycystic Ovarian Syndrome. [Updated 2025 May 4]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459251/ (accessed on 30 May 2025).
- Lonardo, A.; Suzuki, A. Sexual dimorphism of NAFLD in adults. Focus on clinical aspects and implications for practice and translational research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Sarkar, M.; Terrault, N.; Chan, W.; Cedars, M.I.; Huddleston, H.G.; Duwaerts, C.C.; Balitzer, D.; Gill, R.M. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int. 2020, 40, 355–359. [Google Scholar] [CrossRef]
- Stefan, N.; Lonardo, A.; Targher, G. Role of steatotic liver disease in prediction and prevention of cardiometabolic diseases. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 136–137. [Google Scholar] [CrossRef]
- Lonardo, A.; Stefan, N.; Mantovani, A. Widening research horizons on metabolic dysfunction-associated steatotic liver disease and cancer. Trends Endocrinol. Metab. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Kumarendran, B.; O’Reilly, M.W.; Manolopoulos, K.N.; Toulis, K.A.; Gokhale, K.M.; Sitch, A.J.; Wijeyaratne, C.N.; Coomarasamy, A.; Arlt, W.; Nirantharakumar, K. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018, 15, e1002542. [Google Scholar] [CrossRef]
- Ballestri, S.; Mantovani, A.; Byrne, C.D.; Lonardo, A.; Targher, G. Diagnostic accuracy of ultrasonography for the detection of hepatic steatosis: An updated meta-analysis of observational studies. Metab. Target Organ Damage 2021, 1, 7. [Google Scholar] [CrossRef]
- Diamond, T.; Stiel, D.; Lunzer, M.; Wilkinson, M.; Roche, J.; Posen, S. Osteoporosis and skeletal fractures in chronic liver disease. Gut 1990, 31, 82–87. [Google Scholar] [CrossRef]
- Calanchini, M.; Moolla, A.; Tomlinson, J.W.; Cobbold, J.F.; Grossman, A.; Fabbri, A.; Turner, H.E. Liver biochemical abnormalities in Turner syndrome: A comprehensive characterization of an adult population. Clin. Endocrinol. 2018, 89, 667–676. [Google Scholar] [CrossRef]
- Han, J.C.; Reyes-Capo, D.P.; Liu, C.Y.; Reynolds, J.C.; Turkbey, E.; Turkbey, I.B.; Bryant, J.; Marshall, J.D.; Naggert, J.K.; Gahl, W.A.; et al. Comprehensive endocrine-metabolic evaluation of patients with Alström syndrome compared with BMI-matched controls. J. Clin. Endocrinol. Metab. 2018, 103, 2707–2719. [Google Scholar] [CrossRef] [PubMed]
- Viuff, M.H.; Stochholm, K.; Grønbaek, H.; Berglund, A.; Juul, S.; Gravholt, C.H. Increased occurrence of liver and gastrointestinal diseases and anaemia in women with Turner syndrome-a nationwide cohort study. Aliment. Pharmacol. Ther. 2021, 53, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Bourcigaux, N.; Dubost, E.; Buzzi, J.C.; Donadille, B.; Corpechot, C.; Poujol-Robert, A.; Christin-Maitre, S. Focus on liver function abnormalities in patients with Turner syndrome: Risk factors and evaluation of fibrosis risk. J. Clin. Endocrinol. Metab. 2023, 108, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.A.; Lee, H.W.; Ahn, S.H.; Lee, E.J.; Ku, C.R.; Kim, S.U. Positive association between nonalcoholic fatty liver disease and growth hormone deficiency in patients with nonfunctioning pituitary adenoma. Front. Endocrinol. 2023, 13, 1057769. [Google Scholar] [CrossRef]
- Lam, J.; Stoppa-Vaucher, S.; Antoniou, M.C.; Bouthors, T.; Ruiz, I.; Sekarski, N.; Rutz, T.; Fries, S.; Binz, P.A.; Bütschi, F.N.; et al. Turner syndrome: Skin, liver, eyes, dental and ENT evaluation should be improved. Front. Endocrinol. 2023, 14, 1190670. [Google Scholar] [CrossRef]
- Twohig, P.; Li, L.; Danford, D.; Craft, M.; Yetman, A.T. Prevalence of hepatic steatosis and fibrosis in Turner syndrome: A prospective case-control study. Liver Int. 2024, 44, 1309–1315. [Google Scholar] [CrossRef]
- Zaegel, N.; Brahimaj, R.; Battaglia-Hsu, S.; Lamiral, Z.; Feigerlova, E. Systemic inflammatory indices and liver dysfunction in Turner syndrome patients: A retrospective case-control study. J. Endocr. Soc. 2024, 8, bvae099. [Google Scholar] [CrossRef]
- Robeva, R.; Elenkova, A.; Kirilov, G.; Zacharieva, S. Metabolic risk in patients with a diminished ovarian reserve and premature ovarian insufficiency. J. Clin. Med. 2024, 13, 5105. [Google Scholar] [CrossRef]
- Ridder, L.O.R.; Just, J.; Hvas, C.L.; Nielsen, M.M.; Møller, H.J.; Grønbæk, H.; Gravholt, C.H. Elevated liver enzymes in Turner syndrome: The role of low-grade inflammation and hormonal imbalances. J. Endocr. Soc. 2025, 9, bvaf059. [Google Scholar] [CrossRef]
- Shankar Kikkeri, N.; Nagalli, S. Turner Syndrome. [Updated 2023 Aug 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554621/ (accessed on 30 May 2025).
- Khan, N.; Farooqui, A.; Ishrat, R. Turner Syndrome where are we? Orphanet J. Rare Dis. 2024, 19, 314. [Google Scholar] [CrossRef]
- Lonardo, A.; Weiskirchen, R. From hypothalamic obesity to metabolic dysfunction-associated steatotic liver disease: Physiology meets the clinics via metabolomics. Metabolites 2024, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.H.; Walsh, C.H. Hypogonadism in hereditary hemochromatosis. J. Clin. Endocrinol. Metab. 2005, 90, 2451–2455. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.L.; Rawla, P. Hemochromatosis. [Updated 2024 Oct 6]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430862/ (accessed on 30 May 2025).
- World Health Organization. Menopause. Available online: https://www.who.int/news-room/fact-sheets/detail/menopause (accessed on 30 May 2025).
- Jersild, M. Infectious hepatitis with subacute atrophy of the liver; an epidemic in women after the menopause. N. Engl. J. Med. 1947, 237, 8–11. [Google Scholar] [CrossRef]
- Codes, L.; Asselah, T.; Cazals-Hatem, D.; Tubach, F.; Vidaud, D.; Paraná, R.; Bedossa, P.; Valla, D.; Marcellin, P. Liver fibrosis in women with chronic hepatitis C: Evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut 2007, 56, 390–395. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Matsuo, K.; Gualtieri, M.R.; Cahoon, S.S.; Jung, C.E.; Paulson, R.J.; Shoupe, D.; Muderspach, L.I.; Wakatsuki, A.; Wright, J.D.; Roman, L.D. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause 2016, 23, 189–196. [Google Scholar] [CrossRef]
- Veronese, N.; Notarnicola, M.; Osella, A.R.; Cisternino, A.M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Rotolo, O.; Zinzi, I.; Chiloiro, M.; et al. Menopause does not affect fatty liver severity in women: A population study in a Mediterranean area. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 513–521. [Google Scholar] [CrossRef]
- Park, S.H.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; Kim, H.K.; Jang, H.J.; et al. Lack of association between early menopause and non-alcoholic fatty liver disease in postmenopausal women. Climacteric 2020, 23, 173–177. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Charoenngam, N.; Ponvilawan, B.; Mariano, M.; Thongpiya, J.; Yingchoncharoen, P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Menopause 2023, 30, 48–354. [Google Scholar] [CrossRef]
- Raverdy, V.; Chatelain, E.; Lasailly, G.; Caiazzo, R.; Vandel, J.; Verkindt, H. Combining diabetes, sex, and menopause as meaningful clinical features associated with NASH and liver fibrosis in individuals with class II and III obesity: A retrospective cohort study. Obesity 2023, 31, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, Y.J.; Kwon, Y.J.; Lee, J.W. Age at menopause and risk of metabolic dysfunction-associated fatty liver disease: A 14-year cohort study. Dig. Liver Dis. 2024, 56, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, S.; Feng, B.; Lu, Y.; Wang, Y.; Liao, W.; Wu, S.; Wang, L. Association between menopause, body composition, and nonalcoholic fatty liver disease: A prospective cohort in northern China. Maturitas 2025, 192, 108148. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Lankarani, K.; Jamalinia, M.; Zare, F.; Heydari, S.T.; Ardekani, A.; Lonardo, A. Liver-kidney-metabolic health, sex, and menopause impact total scores and monovessel vs. multivessel coronary artery calcification. Adv. Ther. 2025, 42, 1729–1744. [Google Scholar] [CrossRef]
- Bruno, S.; Maisonneuve, P.; Castellana, P.; Rotmensz, N.; Rossi, S.; Maggioni, M.; Persico, M.; Colombo, A.; Monasterolo, F.; Casadei-Giunchi, D.; et al. Incidence and risk factors for non-alcoholic steatohepatitis: Prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 2005, 330, 932. [Google Scholar] [CrossRef]
- Maldonado, S.S.; Cedars, M.I.; Yates, K.P.; Wilson, L.A.; Gill, R.; Terrault, N.A.; Suzuki, A.; Sarkar, M.A. Antimullerian hormone, a marker of ovarian reserve, is protective against presence and severity of NASH in premenopausal women. Clin. Gastroenterol. Hepatol. 2024, 22, 339–346.e5. [Google Scholar] [CrossRef]
- Hense, J.D.; Isola, J.V.V.; Garcia, D.N.; Magalhães, L.S.; Masternak, M.M.; Stout, M.B.; Schneider, A. The role of cellular senescence in ovarian aging. NPJ Aging 2024, 10, 35. [Google Scholar] [CrossRef]
- Verdiesen, R.M.G.; von Berg, J.; Said, M.A.; van der Harst, P.; Mahajan, A.; van Gils, C.H.; van der Schouw, Y.T.; Onland-Moret, N.C. Anti-Müllerian hormone and cardiometabolic disease in women: A two-sample Mendelian randomization study. Rev. Cardiovasc. Med. 2022, 23, 269. [Google Scholar] [CrossRef]
- Kim, S.E.; Min, J.S.; Lee, S.; Lee, D.Y.; Choi, D. Different effects of menopausal hormone therapy on non-alcoholic fatty liver disease based on the route of estrogen administration. Sci. Rep. 2023, 13, 15461. [Google Scholar] [CrossRef]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen biosynthesis and signal transduction in ovarian disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, X.; Han, J.; Cheng, Y.; Zhao, J.; Fang, W.; Gao, L. Critical illness and sex hormones: Response and impact of the hypothalamic-pituitary-gonadal axis. Ther. Adv. Endocrinol. Metab. 2025, 16, 20420188251328192. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Campbell, R.E. Abnormal GnRH pulsatility in polycystic ovary syndrome: Recent insights. Curr. Opin. Endocr. Metab. Res. 2020, 12, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Szybiak-Skora, W.; Cyna, W.; Lacka, K. New insights in the diagnostic potential of sex hormone-binding globulin (SHBG)-Clinical approach. Biomedicines 2025, 13, 1207. [Google Scholar] [CrossRef]
- Wallace, I.R.; McKinley, M.C.; Bell, P.M.; Hunter, S.J. Sex hormone binding globulin and insulin resistance. Clin. Endocrinol. 2013, 78, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Li, M.; Pan, F.; Xiao, Y.; Cui, W.; Hu, Y. Non-alcoholic fatty liver disease is an influencing factor for the association of SHBG with metabolic syndrome in diabetes patients. Sci. Rep. 2017, 7, 14532. [Google Scholar] [CrossRef]
- Wang, X.; Xie, J.; Pang, J.; Zhang, H.; Chen, X.; Lin, J.; Li, Q.; Chen, Q.; Ma, J.; Xu, X.; et al. Serum SHBG is associated with the development and regression of nonalcoholic fatty liver disease: A prospective study. J. Clin. Endocrinol. Metab. 2020, 105, dgz244. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hellberg, N.; Parini, P.; Vidal, O.; Bohlooly, M.; Rudling, M.; Lindberg, M.K.; Warner, M.; Angelin, B.; Gustafsson, J.A. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 2000, 278, 640–645, Erratum in: Biochem. Biophys. Res. Commun. 2006, 348, 326. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Geng, Y.; Li, F.; Hu, R.; Song, Y.; Zhang, M.; Song, K. The risk factors, pathogenesis and treatment of premature ovarian insufficiency. J. Ovarian Res. 2025, 18, 134. [Google Scholar] [CrossRef]
- Khan, M.S.; Kim, H.S.; Kim, R.; Yoon, S.H.; Kim, S.G. Dysregulated liver metabolism and polycystic ovarian syndrome. Int. J. Mol. Sci. 2023, 24, 7454. [Google Scholar] [CrossRef]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 2006, 12, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The signaling pathways in obesity-related complications. J. Cell Commun. Signal. 2024, 18, e12039. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, A.; Ar, S.; Rajagambeeram, R.; Nanda, S.K.; Daniel, M. SHBG and Insulin resistance—Nexus revisited. Bioinformation 2024, 20, 816–821. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, J.; Zhao, H.; He, B. Effect of sex hormone-binding globulin on polycystic ovary syndrome: Mechanisms, manifestations, genetics, and treatment. Int. J. Womens Health 2022, 14, 91–105. [Google Scholar] [CrossRef]
- Boots, C.E.; Jungheim, E.S. Inflammation and human ovarian follicular dynamics. Semin. Reprod. Med. 2015, 33, 270–275. [Google Scholar] [CrossRef]
- Stanciu, S.M.; Jinga, M.; Miricescu, D.; Stefani, C.; Nica, R.I.; Stanescu-Spinu, I.I.; Vacaroiu, I.A.; Greabu, M.; Nica, S. mTOR dysregulation, insulin resistance, and hypertension. Biomedicines 2024, 12, 1802. [Google Scholar] [CrossRef]
- Parazzini, F.; Gerli, S.; Favilli, A.; Vignali, M.; Ricci, E.; Cipriani, S.; Chiaffarino, F.; Dell’acqua, A.; Harari, S.; Bianchi, S. mTOR inhibitors and risk of ovarian cysts: A systematic review and meta-analysis. BMJ Open 2021, 11, e048190. [Google Scholar] [CrossRef]
- Ghafari, A.; Maftoohi, M.; Eslami Samrin, M.; Barani, S.; Banimohammad, M.; Samie, R. The last update on polycystic ovary syndrome (PCOS), diagnosis criteria, and novel treatment. Endocr. Metab. Sci. 2025, 17, 100228. [Google Scholar] [CrossRef]
- Iqbal, J.; Zaidi, M. Understanding estrogen action during menopause. Endocrinology 2009, 150, 3443–3445. [Google Scholar] [CrossRef]
- Kumariya, S.; Ubba, V.; Jha, R.K.; Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021, 17, 2706–2733. [Google Scholar] [CrossRef]
- Nash, Z.; Al-Wattar, B.H.; Davies, M. Bone and heart health in menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Devine, K.; Barber, K.; Comninos, A.N.; Conway, G.S.; Crown, A.; Davies, M.C.; Ewart, A.; Seal, L.J.; Smyth, A.; et al. Society for endocrinology guideline for understanding, diagnosing and treating female hypogonadism. Clin. Endocrinol. 2024, 101, 409–442. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, L.; Sun, D.; Wu, Y.; Yu, C.; Huang, Y.; Chan, S.O.; Ling, W.; Lv, J.; Li, L.; et al. Associations of alcohol consumption and genetic predisposition to hepatic steatosis with liver-related events: Results from large population-based cohort studies. Gastroenterology 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Perrett, R.M.; McArdle, C.A. Molecular mechanisms of gonadotropin-releasing hormone signaling: Integrating cyclic nucleotides into the network. Front. Endocrinol. 2013, 4, 180. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. [Updated 2023 Jul 30]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 30 May 2025).
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef]
- Mu, L.; Wang, G.; Yang, X.; Liang, J.; Tong, H.; Li, L.; Geng, K.; Bo, Y.; Hu, X.; Yang, R.; et al. Physiological premature aging of ovarian blood vessels leads to decline in fertility in middle-aged mice. Nat. Commun. 2025, 16, 72. [Google Scholar] [CrossRef]
- Gonçalves, C.R.; Vasconcellos, A.S.; Rodrigues, T.R.; Comin, F.V.; Reis, F.M. Hormone therapy in women with premature ovarian insufficiency: A systematic review and meta-analysis. Reprod. Biomed. Online 2022, 44, 1143–1157. [Google Scholar] [CrossRef]
- Lan, Y.; Jin, B.; Fan, Y.; Huang, Y.; Zhou, J. The circadian rhythm regulates the hepato-ovarian axis linking polycystic ovary syndrome and non-alcoholic fatty liver disease. Biochem. Genet. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Vassilatou, E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J. Gastroenterol. 2014, 20, 8351–8363. [Google Scholar] [CrossRef]
- Riemann, A.; Blaschke, M.; Jauho-Ghadimi, A.; Siggelkow, H.; Gollisch, K.S.C. Metformin improves the hepatic steatosis index in non-obese patients with polycystic ovary syndrome. J. Clin. Med. 2022, 11, 4294. [Google Scholar] [CrossRef]
- Wang, D.; He, B. Current perspectives on nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab. Syndr. Obes. 2022, 15, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- El Sobky, S.A.; Aboud, N.K.; El Assaly, N.M.; Fawzy, I.O.; El-Ekiaby, N.; Abdelaziz, A.I. Regulation of lipid droplet (LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs. Front. Med. 2022, 9, 903856. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Brady, C.W.; Fleckenstein, J.; Forde, K.A.; Khungar, V.; Molleston, J.P.; Afshar, Y.; Terrault, N.A. Reproductive health and liver disease: Practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 318–335. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).