The Plasmodium falciparum RING Finger Protein PfRNF1 Forms an Interaction Network with Regulators of Sexual Development
Abstract
1. Introduction
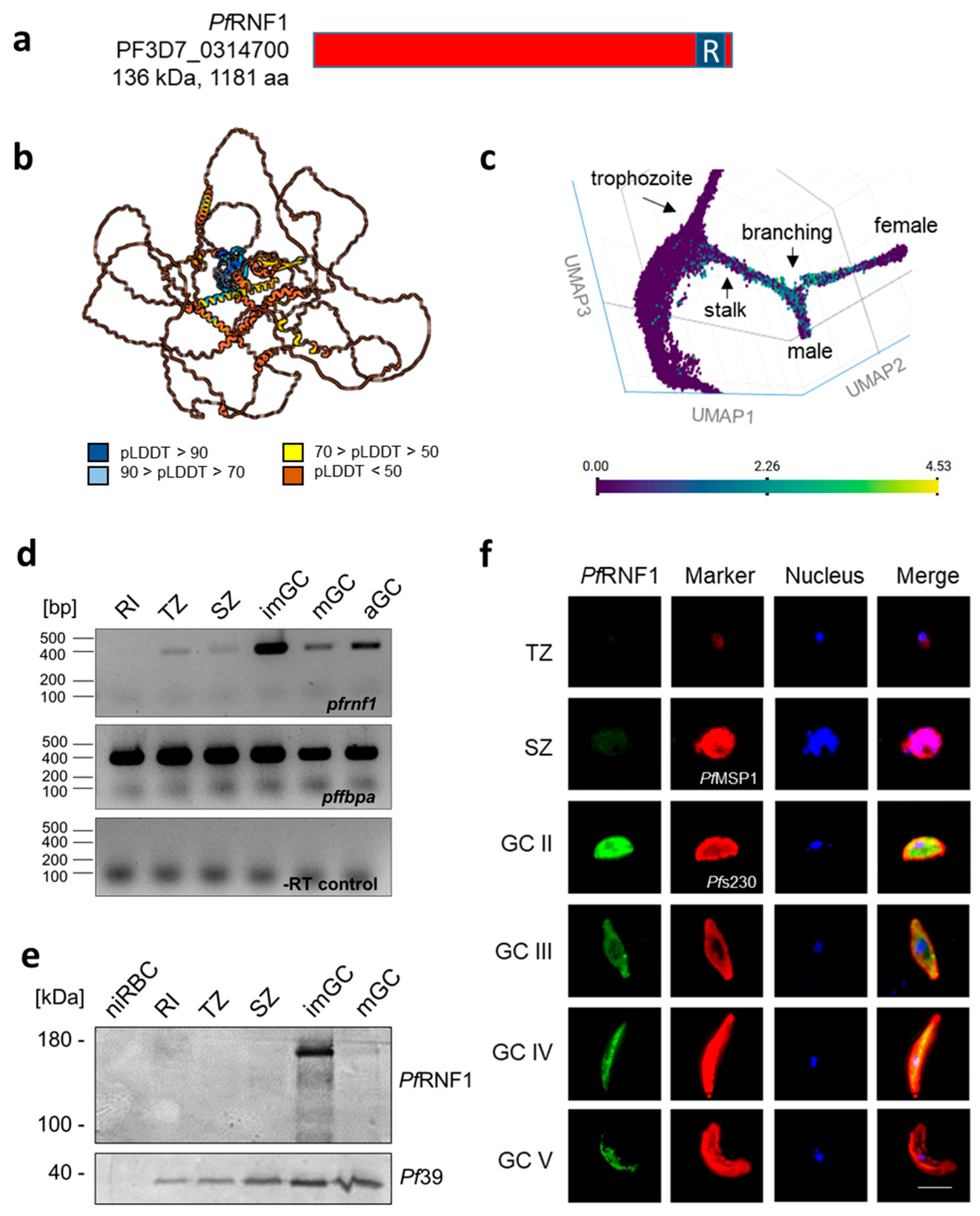
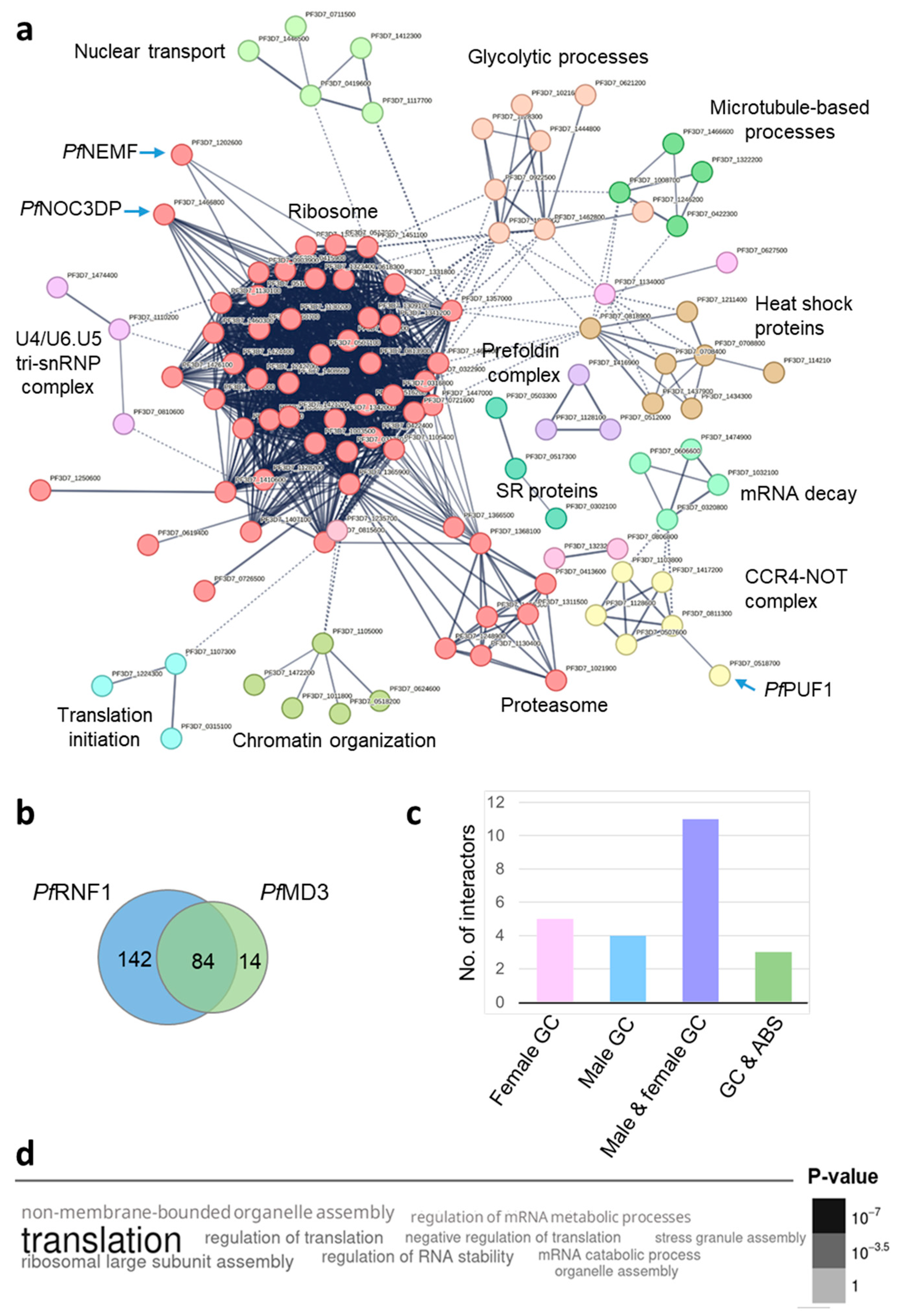
2. Results
3. Discussion
4. Materials and Methods
4.1. Gene Identifiers
4.2. Antibodies
4.3. Parasite Culture
4.4. Generation of Line PfRNF1-pffnpa-GFP-BirA
4.5. Semi-Quantitative RT-PCR
4.6. Western Blotting
4.7. Indirect Immunofluorescence Assay
4.8. BioID-MS Analysis
4.9. Bioinformatics
4.10. Data Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aGC | Activated gametocyte |
| AMA1 | Apical membrane antigen 1 |
| Ap2 | Apetala 2 |
| BioID-MS | Proximity-dependent biotin identification with mass spectrometry |
| CCP2 | LCCL domain-containing protein 2 |
| FBPA | Fructose bisphosphate aldolase |
| FD | Female development |
| GD | Gametocyte development |
| GDV1 | Gametocyte development protein 1 |
| GFP | Green fluorescent protein |
| HP1 | Heterochromatin protein 1 |
| IFA | Indirect immunofluorescence assay |
| imGC | Immature gametocyte |
| MD | Male development |
| mGC | Mature gametocyte |
| niRBC | Non-infected red blood cell |
| PCR | Polymerase chain reaction |
| RBP | RNA-binding protein |
| RBUL | RNA-binding E3 ubiquitin ligase |
| RI | Ring stage |
| RNF1 | RING finger protein 1 |
| RT-PCR | Reverse transcriptase PCR |
| SZ | Schizont |
| TSA | Trichostatin A |
| TZ | Trophozoite |
| UPS | Ubiquitin proteasome system |
| WT NF54 | Wildtype strain NF54 |
| ZFP | Zinc finger protein |
| ZNF4 | Zinc finger protein 4 |
References
- WHO. World Malaria Report 2024. Available online: https://www.who.int/publications/i/item/9789240104440 (accessed on 23 January 2025).
- Beri, D.; Balan, B.; Tatu, U. Commit, hide and escape: The story of Plasmodium gametocytes. Parasitology 2018, 145, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Josling, G.A.; Williamson, K.C.; Llinás, M. Regulation of Sexual Commitment and Gametocytogenesis in Malaria Parasites. Annu. Rev. Microbiol. 2018, 72, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.S.; Brancucci, N.M. Regulation of sexual commitment in malaria parasites—A complex affair. Curr. Opin. Microbiol. 2024, 79, 102469. [Google Scholar] [CrossRef]
- Yuda, M.; Kaneko, I.; Iwanaga, S.; Murata, Y.; Kato, T. Female-specific gene regulation in malaria parasites by an AP2-family transcription factor. Mol. Microbiol. 2020, 113, 40–51. [Google Scholar] [CrossRef]
- Li, Z.; Cui, H.; Guan, J.; Liu, C.; Yang, Z.; Yuan, J. Plasmodium transcription repressor AP2-O3 regulates sex-specific identity of gene expression in female gametocytes. EMBO Rep. 2021, 22, e51660. [Google Scholar] [CrossRef]
- van Biljon, R.; van Wyk, R.; Painter, H.J.; Orchard, L.; Reader, J.; Niemand, J.; Llinás, M.; Birkholtz, L.M. Hierarchical transcriptional control regulates Plasmodium falciparum sexual differentiation. BMC Genom. 2019, 20, 920. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Dziedziech, A.; Macedo, D.; Huppertz, F.; Veith, Y.; Postel, Z.; Christ, E.; Scheytt, R.; Slotte, T.; Henriksson, J.; et al. Single-cell transcriptomics reveal transcriptional programs underlying male and female cell fate during Plasmodium falciparum gametocytogenesis. Nat. Commun. 2024, 15, 7177. [Google Scholar] [CrossRef]
- Mair, G.R.; Braks, J.A.M.; Garver, L.S.; Wiegant, J.C.A.G.; Hall, N.; Dirks, R.W.; Khan, S.M.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Regulation of sexual development of Plasmodium by translational repression. Science 2006, 313, 667–669. [Google Scholar] [CrossRef]
- Mair, G.R.; Lasonder, E.; Garver, L.S.; Franke-Fayard, B.M.D.; Carret, C.K.; Wiegant, J.C.A.G.; Dirks, R.W.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010, 6, e1000767. [Google Scholar] [CrossRef]
- Miao, J.; Li, J.; Fan, Q.; Li, X.; Li, X.; Cui, L. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell Sci. 2010, 123, 1039–1049. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Q.; Parker, D.; Li, X.; Li, J.; Cui, L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 2013, 9, e1003268. [Google Scholar] [CrossRef] [PubMed]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell. Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef]
- Bennink, S.; Pradel, G. The molecular machinery of translational control in malaria parasites. Mol. Microbiol. 2019, 112, 1658–1673. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Farrukh, A.; Pradel, G. Zinc finger proteins of Plasmodium falciparum. Cell. Microbiol. 2021, 23, e13387. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Kamaliyan, Z.; Clarke, T.L. Zinc finger proteins: Guardians of genome stability. Front. Cell Dev. Biol. 2024, 12, 1448789. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Kiesow, M.J.; Papst, O.; Orchard, L.M.; Filarsky, M.; Rosinski, A.N.; Voss, T.S.; Llinás, M.; Pradel, G. Transcriptional Profiling Defines Histone Acetylation as a Regulator of Gene Expression during Human-to-Mosquito Transmission of the Malaria Parasite Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2017, 7, 320. [Google Scholar] [CrossRef]
- Hanhsen, B.; Farrukh, A.; Pradel, G.; Ngwa, C.J. The Plasmodium falciparum CCCH Zinc Finger Protein ZNF4 Plays an Important Role in Gametocyte Exflagellation through the Regulation of Male Enriched Transcripts. Cells 2022, 11, 1666. [Google Scholar] [CrossRef]
- Farrukh, A.; Musabyimana, J.P.; Distler, U.; Mahlich, V.J.; Mueller, J.; Bick, F.; Tenzer, S.; Pradel, G.; Ngwa, C.J. The Plasmodium falciparum CCCH zinc finger protein MD3 regulates male gametocytogenesis through its interaction with RNA-binding proteins. Mol. Microbiol. 2024, 121, 543–564. [Google Scholar] [CrossRef]
- Russell, A.J.C.; Sanderson, T.; Bushell, E.; Talman, A.M.; Anar, B.; Girling, G.; Hunziker, M.; Kent, R.S.; Martin, J.S.; Metcalf, T.; et al. Regulators of male and female sexual development are critical for the transmission of a malaria parasite. Cell Host Microbe 2023, 31, 305–319.e10. [Google Scholar] [CrossRef]
- Thapa, P.; Shanmugam, N.; Pokrzywa, W. Ubiquitin Signaling Regulates RNA Biogenesis, Processing, and Metabolism. Bioessays 2020, 42, e1900171. [Google Scholar] [CrossRef] [PubMed]
- Guseva, E.A.; Emelianova, M.A.; Sidorova, V.N.; Tyulpakov, A.N.; Dontsova, O.A.; Sergiev, P.V. Diversity of Molecular Functions of RNA-Binding Ubiquitin Ligases from the MKRN Protein Family. Biochemistry 2024, 89, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Musabyimana, J.P.; Distler, U.; Sassmannshausen, J.; Berks, C.; Manti, J.; Bennink, S.; Blaschke, L.; Burda, P.-C.; Flammersfeld, A.; Tenzer, S.; et al. Plasmodium falciparum S-Adenosylmethionine Synthetase Is Essential for Parasite Survival through a Complex Interaction Network with Cytoplasmic and Nuclear Proteins. Microorganisms 2022, 10, 1419. [Google Scholar] [CrossRef]
- Aurrecoechea, C.; Brestelli, J.; Brunk, B.P.; Dommer, J.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; Harb, O.S.; et al. PlasmoDB: A functional genomic database for malaria parasites. Nucleic Acids Res. 2009, 37, D539–D543. [Google Scholar] [CrossRef]
- Vembar, S.S.; Macpherson, C.R.; Sismeiro, O.; Coppée, J.-Y.; Scherf, A. The PfAlba1 RNA-binding protein is an important regulator of translational timing in Plasmodium falciparum blood stages. Genome Biol. 2015, 16, 212. [Google Scholar] [CrossRef]
- Muñoz, E.E.; Hart, K.J.; Walker, M.P.; Kennedy, M.F.; Shipley, M.M.; Lindner, S.E. ALBA4 modulates its stage-specific interactions and specific mRNA fates during Plasmodium yoelii growth and transmission. Mol. Microbiol. 2017, 106, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Nag, S.; Goyal, M.; Saha, D.; Siddiqui, A.A.; Mazumder, S.; Debsharma, S.; Pramanik, S.; Bandyopadhyay, U. Nuclease activity of Plasmodium falciparum Alba family protein PfAlba3. Cell Rep. 2023, 42, 112292. [Google Scholar] [CrossRef]
- Chalabi Hagkarim, N.; Grand, R.J. The Regulatory Properties of the Ccr4-Not Complex. Cells 2020, 9, 2379. [Google Scholar] [CrossRef]
- Hart, K.J.; Oberstaller, J.; Walker, M.P.; Minns, A.M.; Kennedy, M.F.; Padykula, I.; Adams, J.H.; Lindner, S.E. Plasmodium male gametocyte development and transmission are critically regulated by the two putative deadenylases of the CAF1/CCR4/NOT complex. PLoS Pathog. 2019, 15, e1007164. [Google Scholar] [CrossRef]
- Hart, K.J.; Power, B.J.; Rios, K.T.; Sebastian, A.; Lindner, S.E. The Plasmodium NOT1-G paralogue is an essential regulator of sexual stage maturation and parasite transmission. PLoS Biol. 2021, 19, e3001434. [Google Scholar] [CrossRef]
- Shrestha, S.; Li, X.; Ning, G.; Miao, J.; Cui, L. The RNA-binding protein Puf1 functions in the maintenance of gametocytes in Plasmodium falciparum. J. Cell Sci. 2016, 129, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Tadauchi, T.; Inada, T.; Matsumoto, K.; Irie, K. Posttranscriptional regulation of HO expression by the Mkt1-Pbp1 complex. Mol. Cell Biol. 2004, 24, 3670–3681. [Google Scholar] [CrossRef]
- Gomes, A.R.; Marin-Menendez, A.; Adjalley, S.H.; Bardy, C.; Cassan, C.; Lee, M.C.S.; Talman, A.M. A transcriptional switch controls sex determination in Plasmodium falciparum. Nature 2022, 612, 528–533. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Masamba, P.; Iwalokun, B.A.; Kappo, A.P. Zooming into the structure-function of RING finger proteins for anti-cancer therapeutic applications. Am. J. Cancer Res. 2023, 13, 2773–2789. [Google Scholar]
- Musabyimana, J.P.; Musa, S.; Manti, J.; Distler, U.; Tenzer, S.; Ngwa, C.J.; Pradel, G. The Plasmodium falciparum histone methyltransferase SET10 participates in a chromatin modulation network crucial for intraerythrocytic development. mSphere 2024, 9, e0049524. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Howick, V.M.; Russell, A.J.C.; Andrews, T.; Heaton, H.; Reid, A.J.; Natarajan, K.; Butungi, H.; Metcalf, T.; Verzier, L.H.; Rayner, J.C.; et al. The Malaria Cell Atlas: Single parasite transcriptomes across the complete Plasmodium life cycle. Science 2019, 365, eaaw2619. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrukh, A.; Musa, S.; Distler, U.; Tenzer, S.; Pradel, G.; Ngwa, C.J. The Plasmodium falciparum RING Finger Protein PfRNF1 Forms an Interaction Network with Regulators of Sexual Development. Int. J. Mol. Sci. 2025, 26, 5470. https://doi.org/10.3390/ijms26125470
Farrukh A, Musa S, Distler U, Tenzer S, Pradel G, Ngwa CJ. The Plasmodium falciparum RING Finger Protein PfRNF1 Forms an Interaction Network with Regulators of Sexual Development. International Journal of Molecular Sciences. 2025; 26(12):5470. https://doi.org/10.3390/ijms26125470
Chicago/Turabian StyleFarrukh, Afia, Sherihan Musa, Ute Distler, Stefan Tenzer, Gabriele Pradel, and Che Julius Ngwa. 2025. "The Plasmodium falciparum RING Finger Protein PfRNF1 Forms an Interaction Network with Regulators of Sexual Development" International Journal of Molecular Sciences 26, no. 12: 5470. https://doi.org/10.3390/ijms26125470
APA StyleFarrukh, A., Musa, S., Distler, U., Tenzer, S., Pradel, G., & Ngwa, C. J. (2025). The Plasmodium falciparum RING Finger Protein PfRNF1 Forms an Interaction Network with Regulators of Sexual Development. International Journal of Molecular Sciences, 26(12), 5470. https://doi.org/10.3390/ijms26125470







