The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming
Abstract
1. Introduction
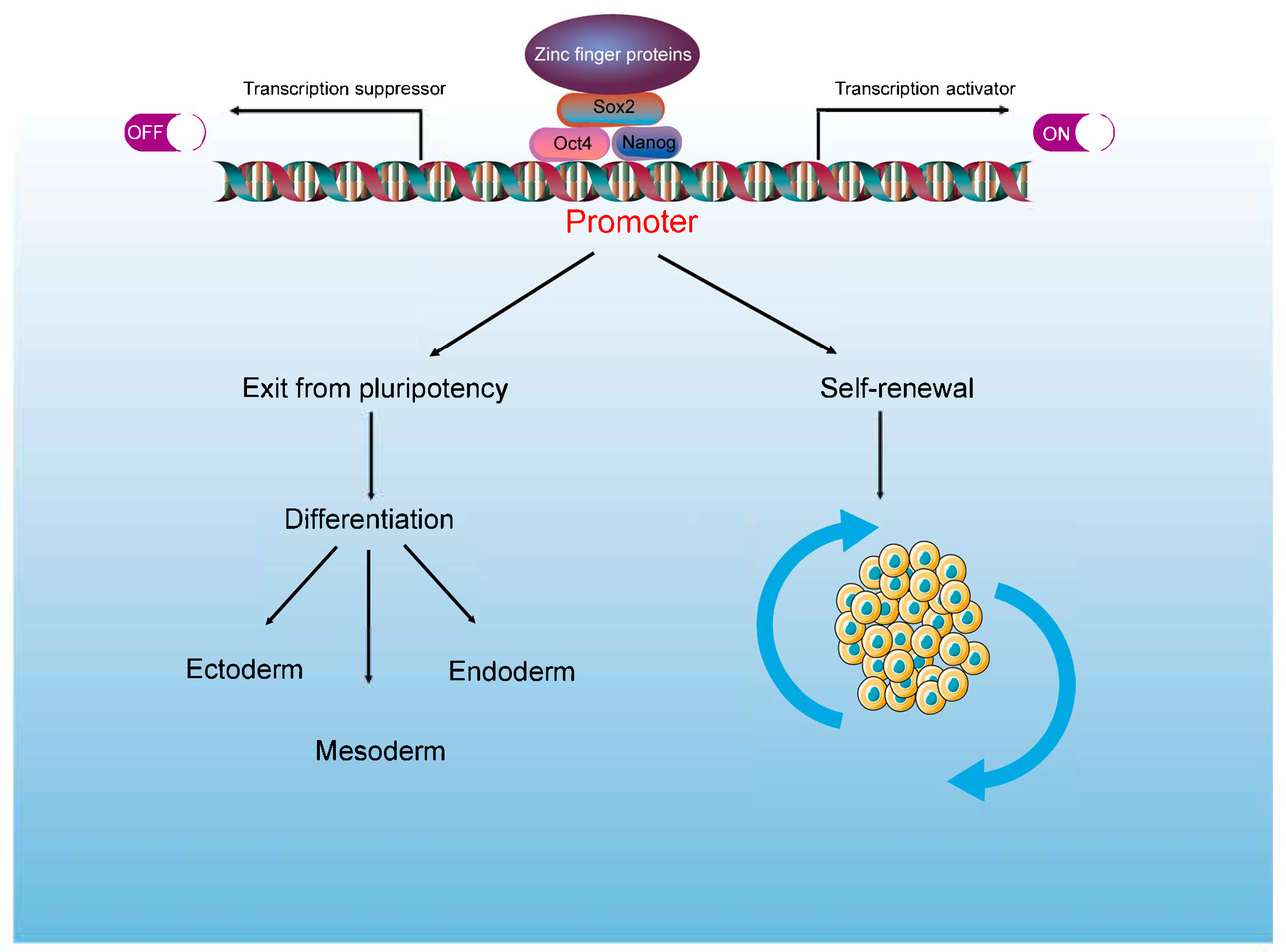
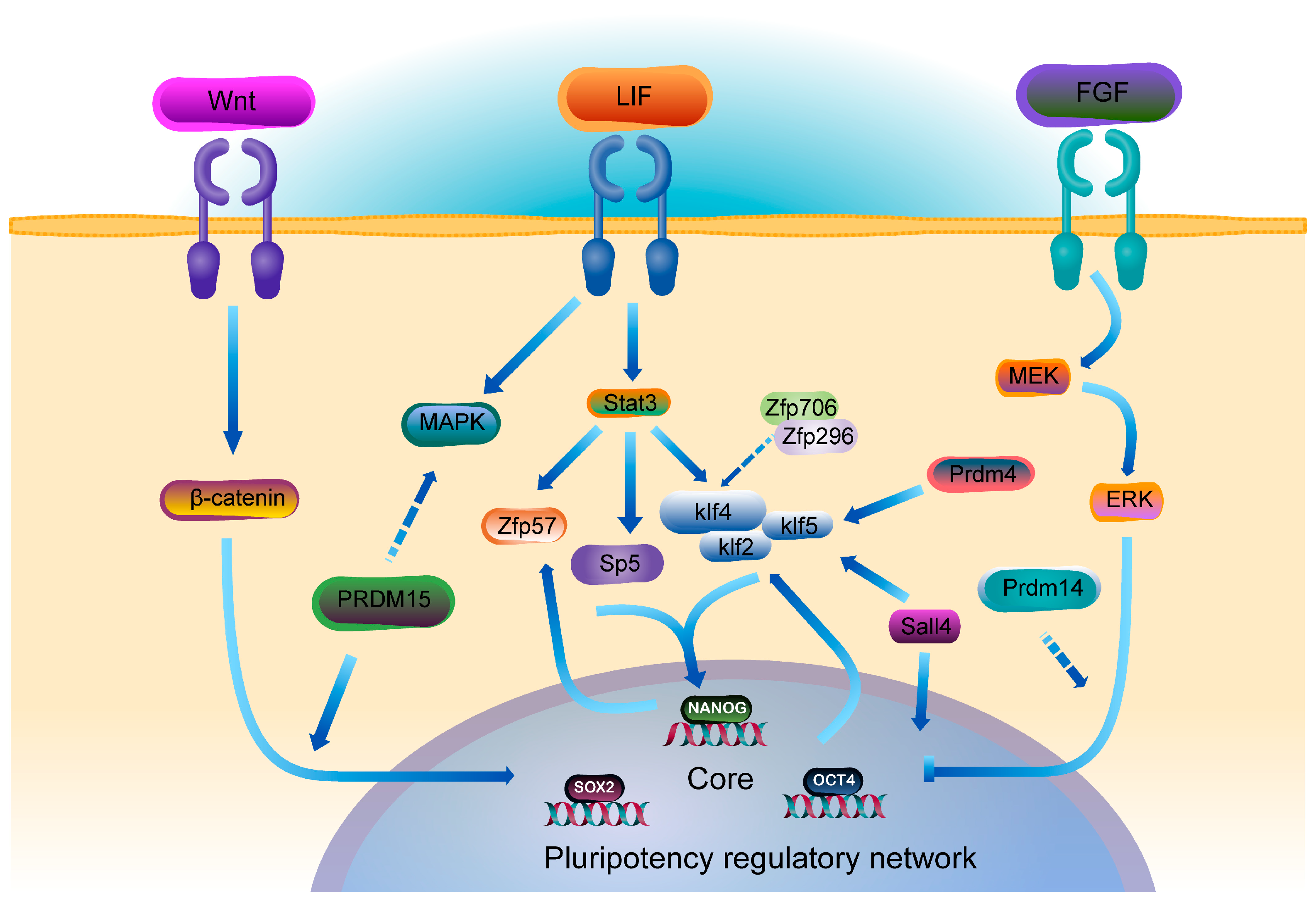
2. ZFPs and the Transcriptional Regulatory Networks of PSCs
3. ZFPs and Epigenetic State of PSCs
3.1. ZFPs and DNA Methylation
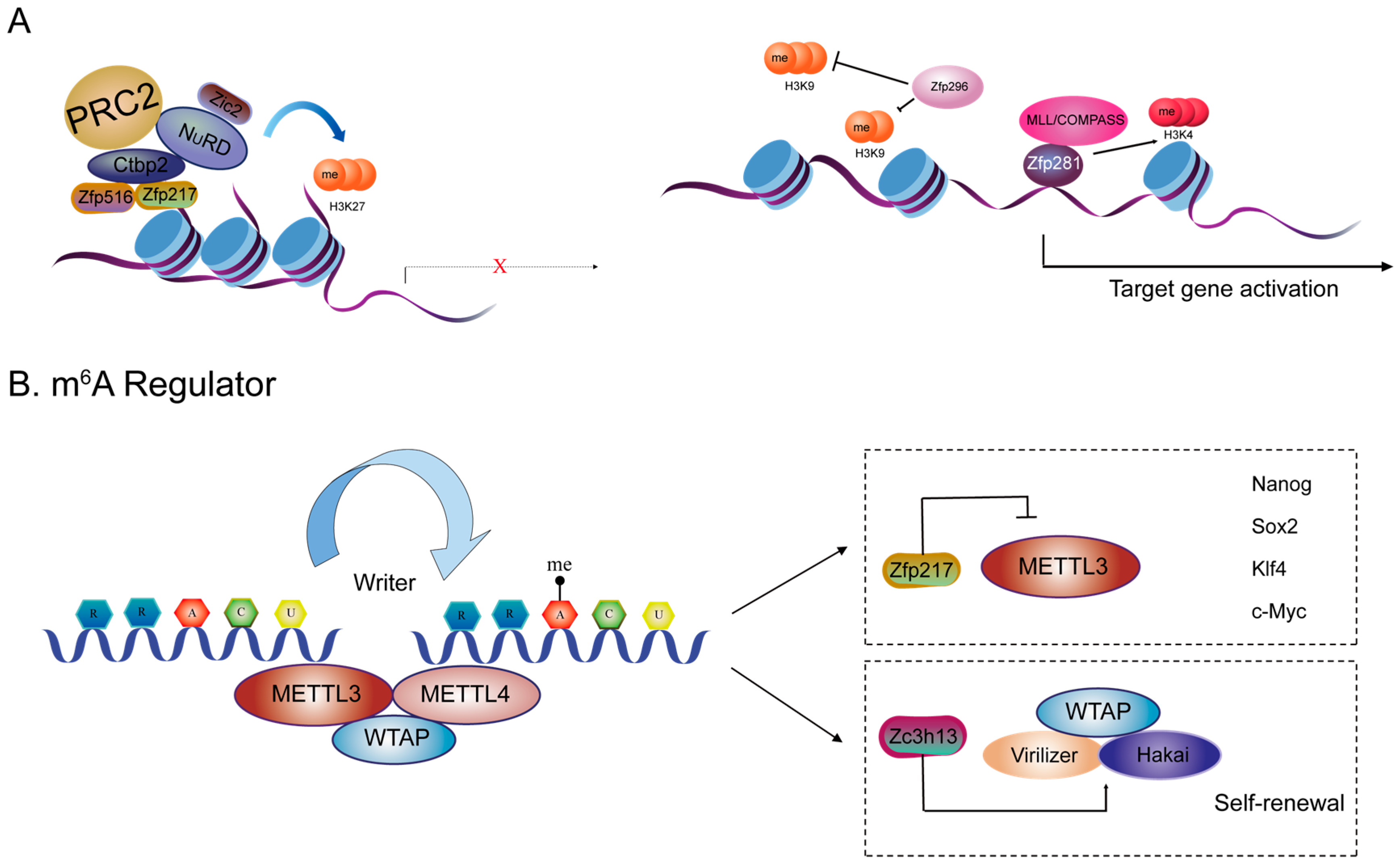
3.2. ZFPs and Histone Modifications
3.3. ZFPs and N6-Methyladenosine (m6A) Methylation
4. ZFPs and ERV Regulatory Network in PSCs
5. ZFPs in Embryonic Development
6. ZFPs Act as Regulator of PSCs
6.1. ZFPs Act as Regulator of Diverse Pluripotent States
6.2. ZFPs Act as Regulators of ES Cell Identity
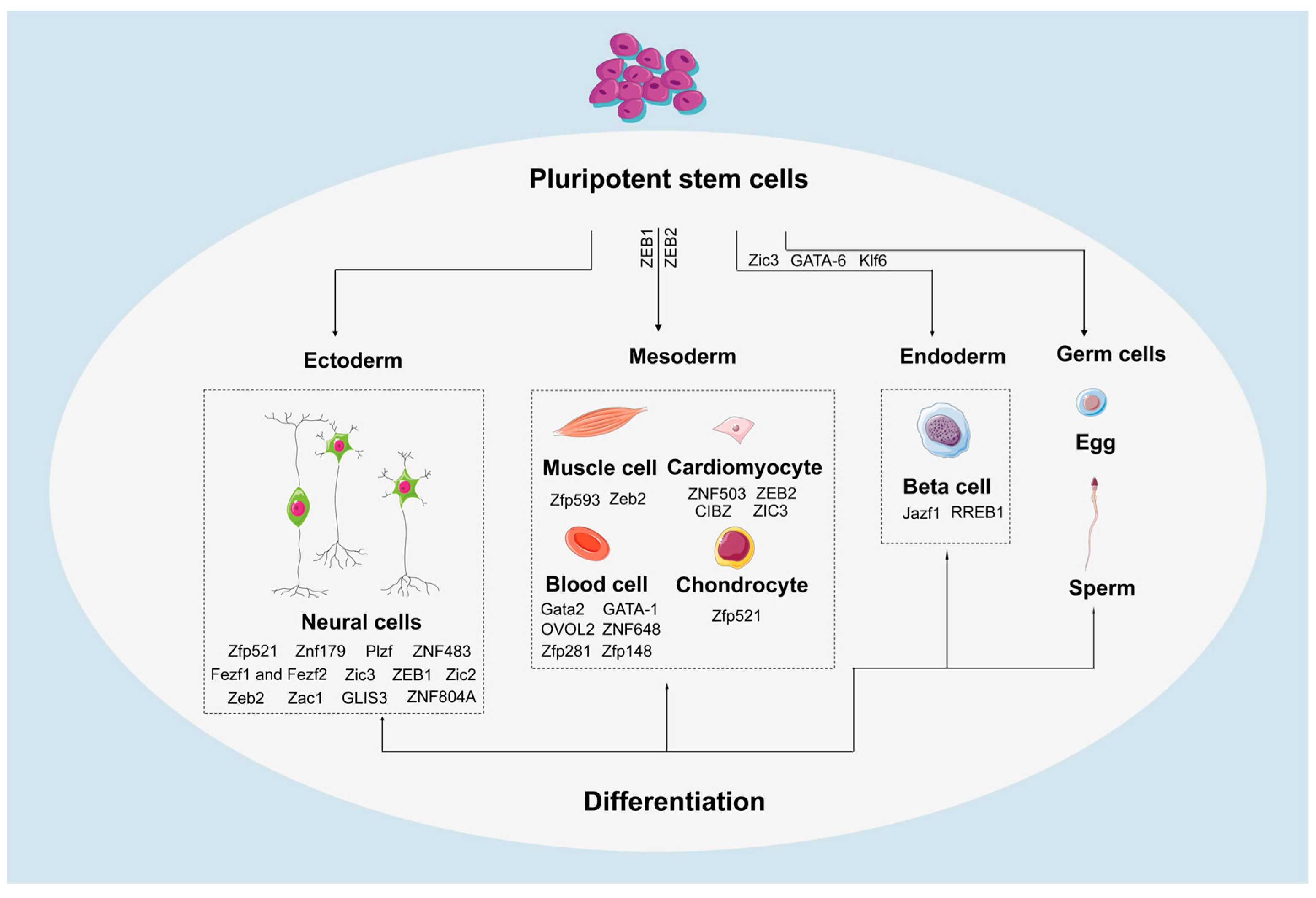
7. The Role of ZFPs in Differentiation of PSCs
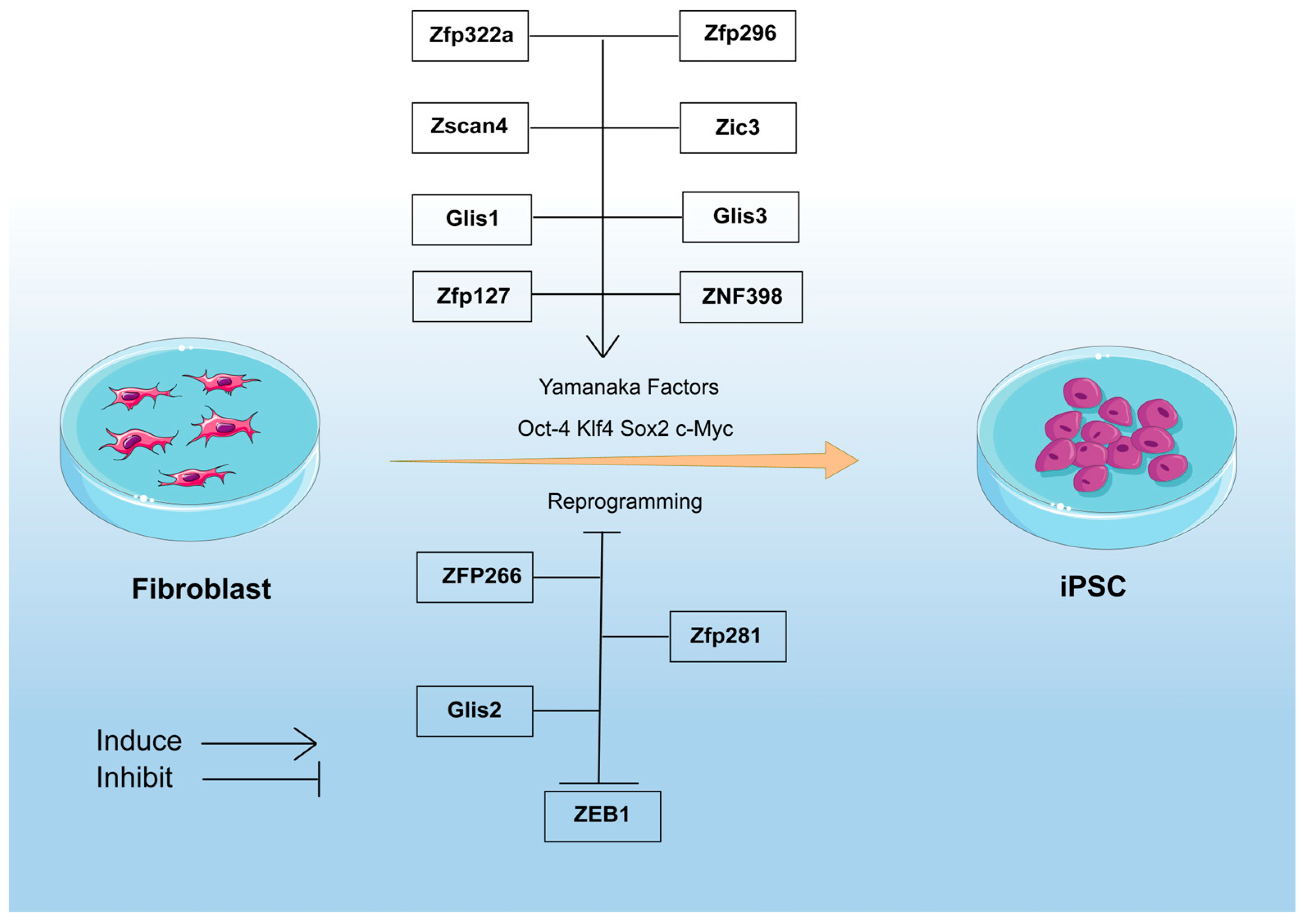
8. ZFPs in Regulation of Somatic Cell Reprogramming
8.1. ZFPs in Promoting Somatic Cell Reprogramming
8.2. ZFPs in Blocking Somatic Cell Reprogramming
9. The Role of ZFPs in Human Health
10. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Fingering too many proteins. Cell 1988, 53, 675. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Krishna, S.S.; Majumdar, I.; Grishin, N.V. Structural classification of zinc fingers: Survey and summary. Nucleic Acids Res. 2003, 31, 532–550. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Lee, S.J.; Michel, S.L.J. Structural metal sites in nonclassical zinc finger proteins involved in transcriptional and translational regulation. Acc. Chem. Res. 2014, 47, 2643–2650. [Google Scholar] [CrossRef]
- Wang, Z.X.; Teh, C.H.; Chan, C.M.; Chu, C.; Rossbach, M.; Kunarso, G.; Allapitchay, T.B.; Wong, K.Y.; Stanton, L.W. The transcription factor Zfp281 controls embryonic stem cell pluripotency by direct activation and repression of target genes. Stem Cells 2008, 26, 2791–2799. [Google Scholar] [CrossRef]
- Fidalgo, M.; Shekar, P.C.; Ang, Y.S.; Fujiwara, Y.; Orkin, S.H.; Wang, J. Zfp281 functions as a transcriptional repressor for pluripotency of mouse embryonic stem cells. Stem Cells 2011, 29, 1705–1716. [Google Scholar] [CrossRef]
- Ma, Z.; Swigut, T.; Valouev, A.; Rada-Iglesias, A.; Wysocka, J. Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat. Struct. Mol. Biol. 2011, 18, 120–127. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yang, H.; Wang, S.; Guo, B.; Yu, L.; Wang, Z.; Fu, J. The zinc finger transcription factor 191 is required for early embryonic development and cell proliferation. Exp. Cell Res. 2006, 312, 3990–3998. [Google Scholar] [CrossRef]
- Su, J.; Miao, X.; Archambault, D.; Mager, J.; Cui, W. ZC3H4-a novel Cys-Cys-Cys-His-type zinc finger protein-is essential for early embryogenesis in mice. Biol. Reprod. 2021, 104, 325–335. [Google Scholar] [CrossRef]
- Ow, J.R.; Ma, H.; Jean, A.; Goh, Z.; Lee, Y.H.; Chong, Y.M.; Soong, R.; Fu, X.Y.; Yang, H.; Wu, Q. Patz1 regulates embryonic stem cell identity. Stem Cells Dev. 2014, 23, 1062–1073. [Google Scholar] [CrossRef]
- Yu, S.; Ma, H.; Ow, J.R.; Goh, Z.; Chiang, R.; Yang, H.; Loh, Y.H.; Wu, Q. Zfp553 Is Essential for Maintenance and Acquisition of Pluripotency. Stem Cells Dev. 2016, 25, 55–67. [Google Scholar] [CrossRef]
- Chen, X.; Fang, F.; Liou, Y.C.; Ng, H.H. Zfp143 regulates Nanog through modulation of Oct4 binding. Stem Cells (Dayt. Ohio) 2008, 26, 2759–2767. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, X.; Lin, C.; Smith, E.R.; Marshall, S.A.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Shilatifard, A. Zic2 is an enhancer-binding factor required for embryonic stem cell specification. Mol. Cell 2015, 57, 685–694. [Google Scholar] [CrossRef]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef]
- Yu, J.; Thomson, J.A. Pluripotent stem cell lines. Genes. Dev. 2008, 22, 1987–1997. [Google Scholar] [CrossRef]
- Harrison, S.E.; Sozen, B.; Christodoulou, N.; Kyprianou, C.; Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 2017, 356, eaal1810. [Google Scholar] [CrossRef]
- Sozen, B.; Amadei, G.; Cox, A.; Wang, R.; Na, E.; Czukiewska, S.; Chappell, L.; Voet, T.; Michel, G.; Jing, N.; et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 2018, 20, 979–989. [Google Scholar] [CrossRef]
- Kim, J.; Chu, J.; Shen, X.; Wang, J.; Orkin, S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008, 132, 1049–1061. [Google Scholar] [CrossRef]
- Yeo, J.-C.; Ng, H.-H. The transcriptional regulation of pluripotency. Cell Res. 2013, 23, 20–32. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Dai, Q.; Yang, Q.; Zhang, Y.; Wang, X.; Xie, W.; Luo, Z.; Lin, C. A permissive chromatin state regulated by ZFP281-AFF3 in controlling the imprinted Meg3 polycistron. Nucleic Acids Res. 2017, 45, 1177–1185. [Google Scholar] [CrossRef]
- Sasai, N.; Matsuda, E.; Sarashina, E.; Ishida, Y.; Kawaichi, M. Identification of a novel BTB-zinc finger transcriptional repressor, CIBZ, that interacts with CtBP corepressor. Genes. Cells Devoted Mol. Cell. Mech. 2005, 10, 871–885. [Google Scholar] [CrossRef]
- Nishii, T.; Oikawa, Y.; Ishida, Y.; Kawaichi, M.; Matsuda, E. CtBP-interacting BTB zinc finger protein (CIBZ) promotes proliferation and G1/S transition in embryonic stem cells via Nanog. J. Biol. Chem. 2012, 287, 12417–12424. [Google Scholar] [CrossRef]
- Ru, W.; Koga, T.; Wang, X.; Guo, Q.; Gearhart, M.D.; Zhao, S.; Murphy, M.; Kawakami, H.; Corcoran, D.; Zhang, J.; et al. Structural studies of SALL family protein zinc finger cluster domains in complex with DNA reveal preferential binding to an AATA tetranucleotide motif. J. Biol. Chem. 2022, 298, 102607. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.R.; Bassal, M.A.; Tan, H.K.; Kurland, J.V.; Yong, K.J.; Young, J.J.; Yang, Y.; Li, F.; Lee, J.D.; Liu, Y.; et al. Zinc Finger Protein SALL4 Functions through an AT-Rich Motif to Regulate Gene Expression. Cell Rep. 2021, 34, 108574. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jeong, H.-W.; Kong, N.; Yang, Y.; Carroll, J.; Luo, H.R.; Silberstein, L.E.; Yupoma; Chai, L. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS ONE 2009, 4, e5577. [Google Scholar] [CrossRef]
- Yang, J.; Chai, L.; Fowles, T.C.; Alipio, Z.; Xu, D.; Fink, L.M.; Ward, D.C.; Ma, Y. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 19756–19761. [Google Scholar] [CrossRef]
- Tanimura, N.; Saito, M.; Ebisuya, M.; Nishida, E.; Ishikawa, F. Stemness-related factor Sall4 interacts with transcription factors Oct-3/4 and Sox2 and occupies Oct-Sox elements in mouse embryonic stem cells. J. Biol. Chem. 2013, 288, 5027–5038. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, X.; Zhang, J.; Loh, Y.-H.; Low, T.-Y.; Zhang, W.; Zhang, W.; Sze, S.-K.; Lim, B.; Ng, H.-H. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J. Biol. Chem. 2006, 281, 24090–24094. [Google Scholar] [CrossRef]
- Baglivo, I.; Esposito, S.; De Cesare, L.; Sparago, A.; Anvar, Z.; Riso, V.; Cammisa, M.; Fattorusso, R.; Grimaldi, G.; Riccio, A.; et al. Genetic and epigenetic mutations affect the DNA binding capability of human ZFP57 in transient neonatal diabetes type 1. FEBS Lett. 2013, 587, 1474–1481. [Google Scholar] [CrossRef]
- Zuo, X.; Sheng, J.; Lau, H.T.; McDonald, C.M.; Andrade, M.; Cullen, D.E.; Bell, F.T.; Iacovino, M.; Kyba, M.; Xu, G.; et al. Zinc finger protein ZFP57 requires its co-factor to recruit DNA methyltransferases and maintains DNA methylation imprint in embryonic stem cells via its transcriptional repression domain. J. Biol. Chem. 2012, 287, 2107–2118. [Google Scholar] [CrossRef]
- Shi, H.; Strogantsev, R.; Takahashi, N.; Kazachenka, A.; Lorincz, M.C.; Hemberger, M.; Ferguson-Smith, A.C. ZFP57 regulation of transposable elements and gene expression within and beyond imprinted domains. Epigenet. Chromatin 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, X.; Gao, Y.; Gao, X.; Li, X.; Zhong, Y.; Wang, X.; Liu, C.; Shi, T.; Lv, J.; et al. Zbtb34 promotes embryonic stem cell proliferation by elongating telomere length. Aging 2022, 14, 7126–7136. [Google Scholar] [CrossRef]
- Karemaker, I.D.; Vermeulen, M. ZBTB2 reads unmethylated CpG island promoters and regulates embryonic stem cell differentiation. EMBO Rep. 2018, 19, e44993. [Google Scholar] [CrossRef]
- Gowher, H.; Stuhlmann, H.; Felsenfeld, G. Vezf1 regulates genomic DNA methylation through its effects on expression of DNA methyltransferase Dnmt3b. Genes. Dev. 2008, 22, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, S.; Bozek, G.; Martin, T.; Gallagher, S.J.; Payne, C.J.; Storb, U. Ssm1b expression and function in germ cells of adult mice and in early embryos. Mol. Reprod. Dev. 2017, 84, 596–613. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Sugito, K.; Yoshizawa, S.; Uekusa, S.; Furuya, T.; Ikeda, T.; Koshinaga, T.; Shinojima, Y.; Hasegawa, R.; Mishra, R.; et al. DNA hypomethylation at the ZNF206-exon 5 CpG island associated with neuronal differentiation in mice and development of neuroblastoma in humans. Int. J. Oncol. 2012, 40, 31–39. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, T.W.; Kang, B.H.; Kim, J.H.; Lee, J.S.; Lee, H.T.; Hwang, I.Y.; Shin, J.; Lee, J.H.; Cho, E.J.; et al. Zinc finger proteins orchestrate active gene silencing during embryonic stem cell differentiation. Nucleic Acids Res. 2018, 46, 6592–6607. [Google Scholar] [CrossRef]
- Fidalgo, M.; Huang, X.; Guallar, D.; Sanchez-Priego, C.; Valdes, V.J.; Saunders, A.; Ding, J.; Wu, W.-S.; Clavel, C.; Wang, J. Zfp281 Coordinates Opposing Functions of Tet1 and Tet2 in Pluripotent States. Cell Stem Cell 2016, 19, 355–369. [Google Scholar] [CrossRef]
- Russo, R.; Russo, V.; Cecere, F.; Valletta, M.; Gentile, M.T.; Colucci-D’Amato, L.; Angelini, C.; Riccio, A.; Pedone, P.V.; Chambery, A.; et al. ZBTB2 protein is a new partner of the Nucleosome Remodeling and Deacetylase (NuRD) complex. Int. J. Biol. Macromol. 2021, 168, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.; Paramanathan, S.; Bardet, A.F.; Hess, D.; Smallwood, S.A.; Elling, U.; Betschinger, J. The BTB-domain transcription factor ZBTB2 recruits chromatin remodelers and a histone chaperone during the exit from pluripotency. J. Biol. Chem. 2021, 297, 100947. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, M.; Faiola, F.; Pereira, C.-F.; Ding, J.; Saunders, A.; Gingold, J.; Schaniel, C.; Lemischka, I.R.; Silva, J.C.R.; Wang, J. Zfp281 mediates Nanog autorepression through recruitment of the NuRD complex and inhibits somatic cell reprogramming. Proc. Natl. Acad. Sci. USA 2012, 109, 16202–16207. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Han, X.; Chew, J.L.; Liu, J.; Chiu, K.P.; Choo, A.; Orlov, Y.L.; Sung, W.-K.; Shahab, A.; Kuznetsov, V.A.; et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell 2007, 1, 286–298. [Google Scholar] [CrossRef]
- Vignaux, P.A.; Bregio, C.; Hathaway, N.A. Contribution of promoter DNA sequence to heterochromatin formation velocity and memory of gene repression in mouse embryo fibroblasts. PLoS ONE 2019, 14, e0217699. [Google Scholar] [CrossRef]
- Matsuura, T.; Miyazaki, S.; Miyazaki, T.; Tashiro, F.; Miyazaki, J.I. Zfp296 negatively regulates H3K9 methylation in embryonic development as a component of heterochromatin. Sci. Rep. 2017, 7, 12462. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.; Zheng, X.; Wang, F.; Deng, Y.; Zhang, Q.; Wang, G.; Zhang, Y.; Liu, X. The Novel Role of Zfp296 in Mammalian Embryonic Genome Activation as an H3K9me3 Modulator. Int. J. Mol. Sci. 2023, 24, 11377. [Google Scholar] [CrossRef]
- Miyazaki, S.; Yamano, H.; Motooka, D.; Tashiro, F.; Matsuura, T.; Miyazaki, T.; Miyazaki, J.I. Zfp296 knockout enhances chromatin accessibility and induces a unique state of pluripotency in embryonic stem cells. Commun. Biol. 2023, 6, 771. [Google Scholar] [CrossRef]
- Ishiuchi, T.; Ohishi, H.; Sato, T.; Kamimura, S.; Yorino, M.; Abe, S.; Suzuki, A.; Wakayama, T.; Suyama, M.; Sasaki, H. Zfp281 Shapes the Transcriptome of Trophoblast Stem Cells and Is Essential for Placental Development. Cell Rep. 2019, 27, 1742–1754.e6. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Walsh, M.J.; Aguiló, F. ZNF217/ZFP217 Meets Chromatin and RNA. Trends Biochem. Sci. 2016, 41, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Song, L.; Su, G.; Di, A.; Bai, C.; Wei, Z.; Li, G. Melatonin restores the pluripotency of long-term-cultured embryonic stem cells through melatonin receptor-dependent m6A RNA regulation. J. Pineal Res. 2020, 69, e12669. [Google Scholar] [CrossRef]
- Aguilo, F.; Zhang, F.; Sancho, A.; Fidalgo, M.; Di Cecilia, S.; Vashisht, A.; Lee, D.F.; Chen, C.H.; Rengasamy, M.; Andino, B.; et al. Coordination of m(6)A mRNA Methylation and Gene Transcription by ZFP217 Regulates Pluripotency and Reprogramming. Cell Stem Cell 2015, 17, 689–704. [Google Scholar] [CrossRef]
- Wen, J.; Lv, R.; Ma, H.; Shen, H.; He, C.; Wang, J.; Jiao, F.; Liu, H.; Yang, P.; Tan, L.; et al. Zc3h13 Regulates Nuclear RNA m6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell 2018, 69, 1028–1038.e6. [Google Scholar] [CrossRef]
- Gautam, P.; Yu, T.; Loh, Y.-H. Regulation of ERVs in pluripotent stem cells and reprogramming. Curr. Opin. Genet. Dev. 2017, 46, 194–201. [Google Scholar] [CrossRef]
- Rowe, H.M.; Friedli, M.; Offner, S.; Verp, S.; Mesnard, D.; Marquis, J.; Aktas, T.; Trono, D. De novo DNA methylation of endogenous retroviruses is shaped by KRAB-ZFPs/KAP1 and ESET. Development 2013, 140, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Ecco, G.; Cassano, M.; Kauzlaric, A.; Duc, J.; Coluccio, A.; Offner, S.; Imbeault, M.; Rowe, H.M.; Turelli, P.; Trono, D. Transposable Elements and Their KRAB-ZFP Controllers Regulate Gene Expression in Adult Tissues. Dev. Cell 2016, 36, 611–623. [Google Scholar] [CrossRef]
- Tan, X.; Xu, X.; Elkenani, M.; Smorag, L.; Zechner, U.; Nolte, J.; Engel, W.; Pantakani, D.V. Zfp819, a novel KRAB-zinc finger protein, interacts with KAP1 and functions in genomic integrity maintenance of mouse embryonic stem cells. Stem Cell Res. 2013, 11, 1045–1059. [Google Scholar] [CrossRef]
- Oleksiewicz, U.; Gładych, M.; Raman, A.T.; Heyn, H.; Mereu, E.; Chlebanowska, P.; Andrzejewska, A.; Sozańska, B.; Samant, N.; Fąk, K.; et al. TRIM28 and Interacting KRAB-ZNFs Control Self-Renewal of Human Pluripotent Stem Cells through Epigenetic Repression of Pro-differentiation Genes. Stem Cell Rep. 2017, 9, 2065–2080. [Google Scholar] [CrossRef]
- Briers, S.; Crawford, C.; Bickmore, W.A.; Sutherland, H.G. KRAB zinc-finger proteins localise to novel KAP1-containing foci that are adjacent to PML nuclear bodies. J. Cell Sci. 2009, 122, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Oleksiewicz, U.; Czerwińska, P.; Wróblewska, J.; Klimczak, M.; Wiznerowicz, M. Disruption of RING and PHD Domains of TRIM28 Evokes Differentiation in Human iPSCs. Cells 2021, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, A.; Ecco, G.; Duc, J.; Offner, S.; Turelli, P.; Trono, D. Individual retrotransposon integrants are differentially controlled by KZFP/KAP1-dependent histone methylation, DNA methylation and TET-mediated hydroxymethylation in naive embryonic stem cells. Epigenetics Chromatin 2018, 11, 7. [Google Scholar] [CrossRef]
- Jacobs, F.M.; Greenberg, D.; Nguyen, N.; Haeussler, M.; Ewing, A.D.; Katzman, S.; Paten, B.; Salama, S.R.; Haussler, D. An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 2014, 516, 242–245. [Google Scholar] [CrossRef]
- Wolf, D.; Goff, S.P. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 2009, 458, 1201–1204. [Google Scholar] [CrossRef]
- Wang, C.; Goff, S.P. Differential control of retrovirus silencing in embryonic cells by proteasomal regulation of the ZFP809 retroviral repressor. Proc. Natl. Acad. Sci. USA 2017, 114, E922–E930. [Google Scholar] [CrossRef]
- Seah, M.K.Y.; Wang, Y.; Goy, P.A.; Loh, H.M.; Peh, W.J.; Low, D.H.P.; Han, B.Y.; Wong, E.; Leong, E.L.; Wolf, G.; et al. The KRAB-zinc-finger protein ZFP708 mediates epigenetic repression at RMER19B retrotransposons. Development 2019, 146, dev170266. [Google Scholar] [CrossRef]
- Dai, Q.; Shen, Y.; Wang, Y.; Wang, X.; Francisco, J.C.; Luo, Z.; Lin, C. Striking a balance: Regulation of transposable elements by Zfp281 and Mll2 in mouse embryonic stem cells. Nucleic Acids Res. 2017, 45, 12301–12310. [Google Scholar] [CrossRef] [PubMed]
- Osipovich, A.B.; Dudek, K.D.; Trinh, L.T.; Kim, L.H.; Shrestha, S.; Cartailler, J.-P.; Magnuson, M.A. ZFP92, a KRAB domain zinc finger protein enriched in pancreatic islets, binds to B1/Alu SINE transposable elements and regulates retroelements and genes. PLoS Genet. 2023, 19, e1010729. [Google Scholar] [CrossRef]
- Turelli, P.; Castro-Diaz, N.; Marzetta, F.; Kapopoulou, A.; Raclot, C.; Duc, J.; Tieng, V.; Quenneville, S.; Trono, D. Interplay of TRIM28 and DNA methylation in controlling human endogenous retroelements. Genome Res. 2014, 24, 1260–1270. [Google Scholar] [CrossRef]
- Schlesinger, S.; Lee, A.H.; Wang, G.Z.; Green, L.; Goff, S.P. Proviral silencing in embryonic cells is regulated by Yin Yang 1. Cell Rep. 2013, 4, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Haring, N.L.; van Bree, E.J.; Jordaan, W.S.; Roels, J.R.E.; Sotomayor, G.C.; Hey, T.M.; White, F.T.G.; Galland, M.D.; Smidt, M.P.; Jacobs, F.M.J. ZNF91 deletion in human embryonic stem cells leads to ectopic activation of SVA retrotransposons and up-regulation of KRAB zinc finger gene clusters. Genome Res. 2021, 31, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P. Mammalian zygotic genome activation. Semin. Cell Dev. Biol. 2018, 84, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, W.; Hu, S.; Wang, X.; Zhang, Y. Inherited DNA methylation primes the establishment of accessible chromatin during genome activation. Genome Res. 2018, 28, 998–1007. [Google Scholar] [CrossRef]
- Xia, W.; Xu, J.; Yu, G.; Yao, G.; Xu, K.; Ma, X.; Zhang, N.; Liu, B.; Li, T.; Lin, Z.; et al. Resetting histone modifications during human parental-to-zygotic transition. Science 2019, 365, 353–360. [Google Scholar] [CrossRef]
- Li, X.; Ito, M.; Zhou, F.; Youngson, N.; Zuo, X.; Leder, P.; Ferguson-Smith, A.C. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell 2008, 15, 547–557. [Google Scholar] [CrossRef]
- Ogawa, S.; Yamada, M.; Nakamura, A.; Sugawara, T.; Nakamura, A.; Miyajima, S.; Harada, Y.; Ooka, R.; Okawa, R.; Miyauchi, J.; et al. Zscan5b Deficiency Impairs DNA Damage Response and Causes Chromosomal Aberrations during Mitosis. Stem Cell Rep. 2019, 12, 1366–1379. [Google Scholar] [CrossRef]
- Srinivasan, R.; Nady, N.; Arora, N.; Hsieh, L.J.; Swigut, T.; Narlikar, G.J.; Wossidlo, M.; Wysocka, J. Zscan4 binds nucleosomal microsatellite DNA and protects mouse two-cell embryos from DNA damage. Sci. Adv. 2020, 6, eaaz9115. [Google Scholar] [CrossRef]
- Sakamoto, M.; Abe, S.; Miki, Y.; Miyanari, Y.; Sasaki, H.; Ishiuchi, T. Dynamic nucleosome remodeling mediated by YY1 underlies early mouse development. Genes. Dev. 2023, 37, 590–604. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Hoang, D.; Tinkham, M.; Patel, A.; Sun, M.-A.; Wolf, G.; Baker, M.; Chien, H.-C.; Lai, K.-Y.N.; et al. A placental growth factor is silenced in mouse embryos by the zinc finger protein ZFP568. Science 2017, 356, 757–759. [Google Scholar] [CrossRef]
- Ishiguro, K.I.; Nakatake, Y.; Chikazawa-Nohtomi, N.; Kimura, H.; Akiyama, T.; Oda, M.; Ko, S.B.; Ko, M.S. Expression analysis of the endogenous Zscan4 locus and its coding proteins in mouse ES cells and preimplantation embryos. Vitr. Cell Dev. Biol. Anim. 2017, 53, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Y.; Seah, M.K.Y.; Brooks, I.R.; Quek, D.H.P.; Huxley, D.R.; Foo, C.S.; Lee, L.T.; Wollmann, H.; Guo, H.; Messerschmidt, D.M.; et al. Global translation during early development depends on the essential transcription factor PRDM10. Nat. Commun. 2020, 11, 3603. [Google Scholar] [CrossRef]
- Nishio, M.; Matsuura, T.; Hibi, S.; Ohta, S.; Oka, C.; Sasai, N.; Ishida, Y.; Matsuda, E. Heterozygous loss of Zbtb38 leads to early embryonic lethality via the suppression of Nanog and Sox2 expression. Cell Prolif. 2022, 55, e13215. [Google Scholar] [CrossRef]
- Falco, G.; Lee, S.L.; Stanghellini, I.; Bassey, U.C.; Hamatani, T.; Ko, M.S. Zscan4: A novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev. Biol. 2007, 307, 539–550. [Google Scholar] [CrossRef]
- Dang, L.T.; Wong, L.; Tropepe, V. Zfhx1b induces a definitive neural stem cell fate in mouse embryonic stem cells. Stem Cells Dev. 2012, 21, 2838–2851. [Google Scholar] [CrossRef]
- Dang, L.T.H.; Tropepe, V. FGF dependent regulation of Zfhx1b gene expression promotes the formation of definitive neural stem cells in the mouse anterior neurectoderm. Neural Dev. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.P.; Breyak, E.; Nishinakamura, R.; Nakagawa, Y. The zinc finger transcription factor Sall1 is required for the early developmental transition of microglia in mouse embryos. Glia 2022, 70, 1720–1733. [Google Scholar] [CrossRef]
- Ebrahim, N.; Kondratyev, N.; Artyuhov, A.; Timofeev, A.; Gurskaya, N.; Andrianov, A.; Izrailov, R.; Volchkov, E.; Dyuzheva, T.; Kopantseva, E.; et al. Human pancreatic islet-derived stromal cells reveal combined features of mesenchymal stromal cells and pancreatic stellate cells. Stem Cell Res. Ther. 2024, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mojica-Perez, S.P.; Azaria, R.D.; Schultz, M.; Parent, J.M.; Niu, W. Loss of POGZ alters neural differentiation of human embryonic stem cells. Mol. Cell. Neurosci. 2022, 120, 103727. [Google Scholar] [CrossRef]
- Horisawa-Takada, Y.; Kodera, C.; Takemoto, K.; Sakashita, A.; Horisawa, K.; Maeda, R.; Shimada, R.; Usuki, S.; Fujimura, S.; Tani, N.; et al. Meiosis-specific ZFP541 repressor complex promotes developmental progression of meiotic prophase towards completion during mouse spermatogenesis. Nat. Commun. 2021, 12, 3184. [Google Scholar] [CrossRef]
- Rogers, M.B.; Hosler, B.A.; Gudas, L.J. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development 1991, 113, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Osipovich, A.B.; Dudek, K.D.; Greenfest-Allen, E.; Cartailler, J.-P.; Manduchi, E.; Potter Case, L.; Choi, E.; Chapman, A.G.; Clayton, H.W.; Gu, G.; et al. A developmental lineage-based gene co-expression network for mouse pancreatic β-cells reveals a role for Zfp800 in pancreas development. Development 2021, 148, dev196964. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kobayashi, S.; Hiratani, I. Epigenetic differences between naïve and primed pluripotent stem cells. Cell. Mol. Life Sci. CMLS 2018, 75, 1191–1203. [Google Scholar] [CrossRef]
- Romayor, I.; Herrera, L.; Burón, M.; Martin-Inaraja, M.; Prieto, L.; Etxaniz, J.; Inglés-Ferrándiz, M.; Pineda, J.R.; Eguizabal, C. A Comparative Study of Cell Culture Conditions during Conversion from Primed to Naive Human Pluripotent Stem Cells. Biomedicines 2022, 10, 1358. [Google Scholar] [CrossRef]
- Ng, H.-H.; Surani, M.A. The transcriptional and signalling networks of pluripotency. Nat. Cell Biol. 2011, 13, 490–496. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, L.; Xue, Y.; Idris, M.O.; Liu, J.; Pei, D.; Shi, Y.; Liao, B.; Li, F. ZBTB7A regulates primed-to-naïve transition of pluripotent stem cells via recognition of the PNT-associated sequence by zinc fingers 1-2 and recognition of γ-globin -200 gene element by zinc fingers 1–4. FEBS J. 2023, 290, 3896–3909. [Google Scholar] [CrossRef]
- Lea, R.A.; McCarthy, A.; Boeing, S.; Fallesen, T.; Elder, K.; Snell, P.; Christie, L.; Adkins, S.; Shaikly, V.; Taranissi, M.; et al. KLF17 promotes human naive pluripotency but is not required for its establishment. Development 2021, 148, dev199378. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Stadler, M.B.; Rittirsch, M.; Hess, D.; Lukonin, I.; Winzi, M.; Smith, A.; Buchholz, F.; Betschinger, J. Zfp281 orchestrates interconversion of pluripotent states by engaging Ehmt1 and Zic2. EMBO J. 2020, 39, e102591. [Google Scholar] [CrossRef]
- Wen, X.; Lin, Z.; Wu, H.; Cao, L.; Fu, X. Zfp281 Inhibits the Pluripotent-to-Totipotent State Transition in Mouse Embryonic Stem Cells. Front. Cell Dev. Biol. 2022, 10, 879428. [Google Scholar] [CrossRef]
- Huang, X.; Bashkenova, N.; Yang, J.; Li, D.; Wang, J. ZFP281 recruits polycomb repressive complex 2 to restrict extraembryonic endoderm potential in safeguarding embryonic stem cell pluripotency. Protein Cell 2021, 12, 213–219. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Enriquez-Gasca, R.; Gould, P.A.; Holt, J.H.; Conde, L.; Ecco, G.; Herrero, J.; Gifford, R.; Trono, D.; Kassiotis, G.; et al. A satellite DNA array barcodes chromosome 7 and regulates totipotency via ZFP819. Sci. Adv. 2022, 8, eabp8085. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Liu, G.; Oh, Y.; Oh, S.; Yang, S.; Mandjikian, L.; Rani, N.; Almeida, M.C.; Kosik, K.S.; Jang, J. ZBTB12 is a molecular barrier to dedifferentiation in human pluripotent stem cells. Nat. Commun. 2023, 14, 632. [Google Scholar] [CrossRef]
- Wang, Z.X.; Kueh, J.L.; Teh, C.H.; Rossbach, M.; Lim, L.; Li, P.; Wong, K.Y.; Lufkin, T.; Robson, P.; Stanton, L.W. Zfp206 is a transcription factor that controls pluripotency of embryonic stem cells. Stem Cells 2007, 25, 2173–2182. [Google Scholar] [CrossRef]
- Yu, H.B.; Kunarso, G.; Hong, F.H.; Stanton, L.W. Zfp206, Oct4, and Sox2 are integrated components of a transcriptional regulatory network in embryonic stem cells. J. Biol. Chem. 2009, 284, 31327–31335. [Google Scholar] [CrossRef]
- Zhang, W.; Walker, E.; Tamplin, O.J.; Rossant, J.; Stanford, W.L.; Hughes, T.R. Zfp206 regulates ES cell gene expression and differentiation. Nucleic Acids Res. 2006, 34, 4780–4790. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chan, Y.-S.; Loh, Y.-H.; Cai, J.; Tong, G.-Q.; Lim, C.-A.; Robson, P.; Zhong, S.; Ng, H.-H. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 2008, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Zhang, J.; Chia, N.Y.; Chan, Y.S.; Sim, H.S.; Tan, K.S.; Oh, S.K.; Ng, H.H.; Choo, A.B. KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells. Stem Cells 2009, 27, 2114–2125. [Google Scholar] [CrossRef]
- Ema, M.; Mori, D.; Niwa, H.; Hasegawa, Y.; Yamanaka, Y.; Hitoshi, S.; Mimura, J.; Kawabe, Y.-i.; Hosoya, T.; Morita, M.; et al. Krüppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell 2008, 3, 555–567. [Google Scholar] [CrossRef]
- Parisi, S.; Passaro, F.; Aloia, L.; Manabe, I.; Nagai, R.; Pastore, L.; Russo, T. Klf5 is involved in self-renewal of mouse embryonic stem cells. J. Cell Sci. 2008, 121, 2629–2634. [Google Scholar] [CrossRef]
- Tang, L.; Wang, M.; Liu, D.; Gong, M.; Ying, Q.L.; Ye, S. Sp5 induces the expression of Nanog to maintain mouse embryonic stem cell self-renewal. PLoS ONE 2017, 12, e0185714. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takamura, H.; Tada, Y.; Akagi, T.; Oyama, K.; Miyashita, T.; Tajima, H.; Kitagawa, H.; Fushida, S.; Yokota, T.; et al. Nanog positively regulates Zfp57 expression in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2014, 453, 817–820. [Google Scholar] [CrossRef]
- Fang, F.; Xia, N.; Angulo, B.; Carey, J.; Cady, Z.; Durruthy-Durruthy, J.; Bennett, T.; Sebastiano, V.; Reijo Pera, R.A. A distinct isoform of ZNF207 controls self-renewal and pluripotency of human embryonic stem cells. Nat. Commun. 2018, 9, 4384. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Loh, Y.H.; Zhang, W.W.; Li, Y.X.; Chen, X.; Wang, Y.N.; Bakre, M.; Ng, H.H.; Stanton, L.W. Zic3 is required for maintenance of pluripotency in embryonic stem cells. Mol. Biol. Cell 2007, 18, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Hong, F.H.; Kunarso, G.; Stanton, L.W. The pluripotency regulator Zic3 is a direct activator of the Nanog promoter in ESCs. Stem Cells (Dayt. Ohio) 2010, 28, 1961–1969. [Google Scholar] [CrossRef]
- Karantzali, E.; Lekakis, V.; Ioannou, M.; Hadjimichael, C.; Papamatheakis, J.; Kretsovali, A. Sall1 Regulates Embryonic Stem Cell Differentiation in Association with Nanog. J. Biol. Chem. 2011, 286, 1037–1045. [Google Scholar] [CrossRef]
- Massé, J.; Piquet-Pellorce, C.; Viet, J.; Guerrier, D.; Pellerin, I.; Deschamps, S. ZFPIP/Zfp462 is involved in P19 cell pluripotency and in their neuronal fate. Exp. Cell Res. 2011, 317, 1922–1934. [Google Scholar] [CrossRef]
- Massé, J.; Laurent, A.; Nicol, B.; Guerrier, D.; Pellerin, I.; Deschamps, S. Involvement of ZFPIP/Zfp462 in chromatin integrity and survival of P19 pluripotent cells. Exp. Cell Res. 2010, 316, 1190–1201. [Google Scholar] [CrossRef]
- Storm, M.P.; Kumpfmueller, B.; Thompson, B.; Kolde, R.; Vilo, J.; Hummel, O.; Schulz, H.; Welham, M.J. Characterization of the Phosphoinositide 3-Kinase-Dependent Transcriptome in Murine Embryonic Stem Cells: Identification of Novel Regulators of Pluripotency. Stem Cells (Dayt. Ohio) 2009, 27, 764–775. [Google Scholar] [CrossRef]
- Pérez-Palacios, R.; Climent, M.; Santiago-Arcos, J.; Macías-Redondo, S.; Klar, M.; Muniesa, P.; Schoorlemmer, J. YY2 in Mouse Preimplantation Embryos and in Embryonic Stem Cells. Cells 2021, 10, 1123. [Google Scholar] [CrossRef]
- Galan-Caridad, J.M.; Harel, S.; Arenzana, T.L.; Hou, Z.E.; Doetsch, F.K.; Mirny, L.A.; Reizis, B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 2007, 129, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Tu, E.Y.; Weisberg, S.; Esquilin, M.; Chambers, S.M.; Liu, B.; Carson, C.T.; Studer, L.; Reizis, B.; Tomishima, M.J. ZFX Controls the Self-Renewal of Human Embryonic Stem Cells. PLoS ONE 2012, 7, e0042302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mongan, N.P.; Martin, K.M.; Gudas, L.J. The putative human stem cell marker, Rex-1 (Zfp42): Structural classification and expression in normal human epithelial and carcinoma cell cultures. Mol. Carcinog. 2006, 45, 887–900. [Google Scholar] [CrossRef]
- Masui, S.; Ohtsuka, S.; Yagi, R.; Takahashi, K.; Ko, M.S.H.; Niwa, H. Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev. Biol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Yamane, M.; Fujii, S.; Ohtsuka, S.; Niwa, H. Zscan10 is dispensable for maintenance of pluripotency in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2015, 468, 826–831. [Google Scholar] [CrossRef]
- Chan, E.M.; Ratanasirintrawoot, S.; Park, I.-H.; Manos, P.D.; Loh, Y.-H.; Huo, H.; Miller, J.D.; Hartung, O.; Rho, J.; Ince, T.A.; et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 2009, 27, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, Y.; Shimosato, D.; Murakami, K.; Takahashi, K.; Niwa, H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 2008, 135, 909–918. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Gao, W.; Yang, H.-B.; Zhang, B.; Zhu, Z.-Y.; Xue, Y.-F. Screening for genes essential for mouse embryonic stem cell self-renewal using a subtractive RNA interference library. Stem Cells 2006, 24, 2661–2668. [Google Scholar] [CrossRef]
- Guallar, D.; Pérez-Palacios, R.; Climent, M.; Martínez-Abadía, I.; Larraga, A.; Fernández-Juan, M.; Vallejo, C.; Muniesa, P.; Schoorlemmer, J. Expression of endogenous retroviruses is negatively regulated by the pluripotency marker Rex1/Zfp42. Nucleic Acids Res. 2012, 40, 8993–9007. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Jafarnejad, S.M.; Tam, I.S.; Gonatopoulos-Pournatzis, T.; Matta-Camacho, E.; Tsukumo, Y.; Yanagiya, A.; Li, W.; Atlasi, Y.; Caron, M.; et al. Control of embryonic stem cell self-renewal and differentiation via coordinated alternative splicing and translation of YY2. Proc. Natl. Acad. Sci. USA 2016, 113, 12360–12367. [Google Scholar] [CrossRef]
- Akagi, T.; Usuda, M.; Matsuda, T.; Ko, M.S.H.; Niwa, H.; Asano, M.; Koide, H.; Yokota, T. Identification of Zfp-57 as a downstream molecule of STAT3 and Oct-3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 2005, 331, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Guo, G.; Wray, J.; Eyres, I.; Nichols, J.; Grotewold, L.; Morfopoulou, S.; Humphreys, P.; Mansfield, W.; Walker, R.; et al. Oct4 and LIF/Stat3 Additively Induce Kruppel Factors to Sustain Embryonic Stem Cell Self-Renewal. Cell Stem Cell 2009, 5, 597–609. [Google Scholar] [CrossRef]
- Leeb, M.; Dietmann, S.; Paramor, M.; Niwa, H.; Smith, A. Genetic exploration of the exit from self-renewal using haploid embryonic stem cells. Cell Stem Cell 2014, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kakegawa, M.; Koide, H.; Akagi, T.; Yokota, T. Zfp296 is a novel Klf4-interacting protein and functions as a negative regulator. Biochem. Biophys. Res. Commun. 2013, 441, 411–417. [Google Scholar] [CrossRef]
- Bogani, D.; Morgan, M.A.; Nelson, A.C.; Costello, I.; McGouran, J.F.; Kessler, B.M.; Robertson, E.J.; Bikoff, E.K. The PR/SET domain zinc finger protein Prdm4 regulates gene expression in embryonic stem cells but plays a nonessential role in the developing mouse embryo. Mol. Cell. Biol. 2013, 33, 3936–3950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaji, M.; Ueda, J.; Hayashi, K.; Ohta, H.; Yabuta, Y.; Kurimoto, K.; Nakato, R.; Yamada, Y.; Shirahige, K.; Saitou, M. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 2013, 12, 368–382. [Google Scholar] [CrossRef]
- Leitch, H.G.; McEwen, K.R.; Turp, A.; Encheva, V.; Carroll, T.; Grabole, N.; Mansfield, W.; Nashun, B.; Knezovich, J.G.; Smith, A.; et al. Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 2013, 20, 311–316. [Google Scholar] [CrossRef]
- Grabole, N.; Tischler, J.; Hackett, J.A.; Kim, S.; Tang, F.; Leitch, H.G.; Magnúsdóttir, E.; Surani, M.A. Prdm14 promotes germline fate and naive pluripotency by repressing FGF signalling and DNA methylation. EMBO Rep. 2013, 14, 629–637. [Google Scholar] [CrossRef]
- Mzoughi, S.; Zhang, J.; Hequet, D.; Teo, S.X.; Fang, H.; Xing, Q.R.; Bezzi, M.; Seah, M.K.Y.; Ong, S.L.M.; Shin, E.M.; et al. PRDM15 safeguards naive pluripotency by transcriptionally regulating WNT and MAPK-ERK signaling. Nat. Genet. 2017, 49, 1354–1363. [Google Scholar] [CrossRef]
- Winata, C.L.; Kondrychyn, I.; Korzh, V. Changing Faces of Transcriptional Regulation Reflected by Zic3. Curr. Genom. 2015, 16, 117–127. [Google Scholar] [CrossRef]
- Zhao, X.; Monson, C.; Gao, C.; Gouon-Evans, V.; Matsumoto, N.; Sadler, K.C.; Friedman, S.L. Klf6/copeb is required for hepatic outgrowth in zebrafish and for hepatocyte specification in mouse ES cells. Dev. Biol. 2010, 344, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Liu, J.; Qi, Y.; Graham, A.M.; Parmacek, M.S.; Li, S. GATA-6 promotes cell survival by up-regulating BMP-2 expression during embryonic stem cell differentiation. Mol. Biol. Cell 2012, 23, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.H.; Nichols, J.; Watson, C.J. The KRAB domain zinc finger protein, Zfp157, is expressed in multiple tissues during mouse embryogenesis and in specific cells in adult mammary gland and skin. Genesis 2013, 51, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.; Jouneau, A.; Larsson, M.; Oudin, J.-F.; Adenot, P.; Omar, J.; Brochard, V.; Andersson, L. Ablation of ZC3H11A causes early embryonic lethality and dysregulation of metabolic processes. Proc. Natl. Acad. Sci. USA 2023, 120, e2216799120. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, C.; Spallotta, F.; Savoia, M.; Kuenne, C.; Guenther, S.; Re, A.; Wingert, S.; Rehage, M.; Sürün, D.; Siragusa, M.; et al. Zeb1-Hdac2-eNOS circuitry identifies early cardiovascular precursors in naive mouse embryonic stem cells. Nat. Commun. 2018, 9, 1281. [Google Scholar] [CrossRef]
- Ninfali, C.; Siles, L.; Esteve-Codina, A.; Postigo, A. The mesodermal and myogenic specification of hESCs depend on ZEB1 and are inhibited by ZEB2. Cell Rep. 2023, 42, 113222. [Google Scholar] [CrossRef]
- Stryjewska, A.; Dries, R.; Pieters, T.; Verstappen, G.; Conidi, A.; Coddens, K.; Francis, A.; Umans, L.; van Ijcken, W.F.J.; Berx, G.; et al. Zeb2 Regulates Cell Fate at the Exit from Epiblast State in Mouse Embryonic Stem Cells. Stem Cells 2017, 35, 611–625. [Google Scholar] [CrossRef]
- Zhu, L.; Harutyunyan, K.G.; Peng, J.L.; Wang, J.; Schwartz, R.J.; Belmont, J.W. Identification of a novel role of ZIC3 in regulating cardiac development. Hum. Mol. Genet. 2007, 16, 1649–1660. [Google Scholar] [CrossRef][Green Version]
- Busser, B.W.; Lin, Y.; Yang, Y.; Zhu, J.; Chen, G.; Michelson, A.M. An Orthologous Epigenetic Gene Expression Signature Derived from Differentiating Embryonic Stem Cells Identifies Regulators of Cardiogenesis. PLoS ONE 2015, 10, e0141066. [Google Scholar] [CrossRef]
- Kotoku, T.; Kosaka, K.; Nishio, M.; Ishida, Y.; Kawaichi, M.; Matsuda, E. CIBZ Regulates Mesodermal and Cardiac Differentiation of by Suppressing T and Mesp1 Expression in Mouse Embryonic Stem Cells. Sci. Rep. 2016, 6, 34188. [Google Scholar] [CrossRef]
- Di Filippo, E.S.; Costamagna, D.; Giacomazzi, G.; Cortés-Calabuig, Á.; Stryjewska, A.; Huylebroeck, D.; Fulle, S.; Sampaolesi, M. Zeb2 Regulates Myogenic Differentiation in Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 2525. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; McLeod, M.A.; Orsech, H.C.; Cirelli, A.M.; Waddell, D.S. Zinc finger protein 593 is upregulated during skeletal muscle atrophy and modulates muscle cell differentiation. Exp. Cell Res. 2019, 383, 111563. [Google Scholar] [CrossRef]
- Seo, K.W.; Roh, K.H.; Bhandari, D.R.; Park, S.B.; Lee, S.K.; Kang, K.S. ZNF281 Knockdown Induced Osteogenic Differentiation of Human Multipotent Stem Cells In Vivo and In Vitro. Cell Transpl. 2013, 22, 29–40. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, R.H.; Kim, T.M.; Kim, D.W.; Jeon, Y.J.; Huh, S.H.; Oh, S.Y.; Kyba, M.; Kataoka, H.; Choi, K.; et al. OVOL2 is a critical regulator of ER71/ETV2 in generating FLK1+, hematopoietic, and endothelial cells from embryonic stem cells. Blood 2014, 124, 2948–2952. [Google Scholar] [CrossRef]
- Kitajima, K.; Kanokoda, M.; Nakajima, M.; Hara, T. Domain-specific biological functions of the transcription factor Gata2 on hematopoietic differentiation of mouse embryonic stem cells. Genes. Cells Devoted Mol. Cell. Mech. 2018, 23, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, D.; Tanaka, M.; Kitajima, K.; Zheng, J.; Yen, H.; Murotani, T.; Yamatodani, A.; Nakano, T. Differential context-dependent effects of friend of GATA-1 (FOG-1) on mast-cell development and differentiation. Blood 2008, 111, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.J.; Patry, C.A.A.; Ghamari, A.; Pregernig, G.; Yuan, D.; Zheng, K.N.; Piers, T.; Hibbs, M.; Li, J.; Fidalgo, M.; et al. Zfp281 (ZBP-99) plays a functionally redundant role with Zfp148 (ZBP-89) during erythroid development. Blood Adv. 2019, 3, 2499–2511. [Google Scholar] [CrossRef]
- Woo, A.J.; Moran, T.B.; Schindler, Y.L.; Choe, S.K.; Langer, N.B.; Sullivan, M.R.; Fujiwara, Y.; Paw, B.H.; Cantor, A.B. Identification of ZBP-89 as a novel GATA-1-associated transcription factor involved in megakaryocytic and erythroid development. Mol. Cell. Biol. 2008, 28, 2675–2689. [Google Scholar] [CrossRef]
- Ferguson, D.C.J.; Mokim, J.H.; Meinders, M.; Moody, E.R.R.; Williams, T.A.; Cooke, S.; Trakarnsanga, K.; Daniels, D.E.; Ferrer-Vicens, I.; Shoemark, D.; et al. Characterization and evolutionary origin of novel C2H2 zinc finger protein (ZNF648) required for both erythroid and megakaryocyte differentiation in humans. Haematologica 2021, 106, 2859–2873. [Google Scholar] [CrossRef]
- Brown, L.; Brown, S. Zic2 is expressed in pluripotent cells in the blastocyst and adult brain expression overlaps with makers of neurogenesis. Gene Expr. Patterns 2009, 9, 43–49. [Google Scholar] [CrossRef]
- Birkhoff, J.C.; Brouwer, R.W.W.; Kolovos, P.; Korporaal, A.L.; Bermejo-Santos, A.; Boltsis, I.; Nowosad, K.; van den Hout, M.; Grosveld, F.G.; van IJcken, W.F.; et al. Targeted chromatin conformation analysis identifies novel distal neural enhancers of ZEB2 in pluripotent stem cell differentiation. Hum. Mol. Genet. 2020, 29, 2535–2550. [Google Scholar] [CrossRef] [PubMed]
- Pao, P.C.; Huang, N.K.; Liu, Y.W.; Yeh, S.H.; Lin, S.T.; Hsieh, C.P.; Huang, A.M.; Huang, H.S.; Tseng, J.T.; Chang, W.C.; et al. A novel RING finger protein, Znf179, modulates cell cycle exit and neuronal differentiation of P19 embryonal carcinoma cells. Cell Death Differ. 2011, 18, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.J.; Lee, K.H.; Huang, C.C.; Wang, I.F.; Hsieh, C.C.; Lin, H.C.; Lee, Y.C. Purα regulates the induction of Znf179 transcription during neuronal differentiation. Biochem. Biophys. Res. Commun. 2020, 533, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Tsou, J.-H.; Yang, Y.-C.; Pao, P.-C.; Lin, H.-C.; Huang, N.-K.; Lin, S.-T.; Hsu, K.-S.; Yeh, C.-M.; Lee, K.-H.; Kuo, C.-J.; et al. Important Roles of Ring Finger Protein 112 in Embryonic Vascular Development and Brain Functions. Mol. Neurobiol. 2017, 54, 2286–2300. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Ko, C.Y.; Lee, K.H.; Chen, I.H.; Kao, T.J.; Chang, W.C.; Hsu, T.I.; Lee, Y.C. E2f1 regulates the induction of promyelocytic leukemia zinc finger transcription in neuronal differentiation of pluripotent P19 embryonal carcinoma cells. Biochem. Biophys. Res. Commun. 2019, 512, 629–634. [Google Scholar] [CrossRef]
- Rraklli, V.; Södersten, E.; Nyman, U.; Hagey, D.W.; Holmberg, J. Elevated levels of ZAC1 disrupt neurogenesis and promote rapid in vivo reprogramming. Stem Cell Res. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, A.; Spengler, D. A new coactivator function for Zac1’s C2H2 zinc finger DNA-binding domain in selectively controlling PCAF activity. Mol. Cell. Biol. 2008, 28, 6078–6093. [Google Scholar] [CrossRef]
- Yasui, G.; Katayama, S.; Kubota, Y.; Takatsuka, H.; Ito, M.; Inazu, T. Zinc finger protein 483 (ZNF483) regulates neuronal differentiation and methyl-CpG-binding protein 2 (MeCP2) intracellular localization. Biochem. Biophys. Res. Commun. 2021, 568, 68–75. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, L.; Xia, L.; Lu, X.; Zhu, W.; Ding, D.; Du, M.; Zhang, D.; Wang, H.; Hu, B. Zinc finger E-box-binding homeobox 1 (ZEB1) is required for neural differentiation of human embryonic stem cells. J. Biol. Chem. 2018, 293, 19317–19329. [Google Scholar] [CrossRef]
- Winata, C.L.; Kondrychyn, I.; Kumar, V.; Srinivasan, K.G.; Orlov, Y.; Ravishankar, A.; Prabhakar, S.; Stanton, L.W.; Korzh, V.; Mathavan, S. Genome wide analysis reveals Zic3 interaction with distal regulatory elements of stage specific developmental genes in zebrafish. PLoS Genet. 2013, 9, e1003852. [Google Scholar] [CrossRef]
- Kamiya, D.; Banno, S.; Sasai, N.; Ohgushi, M.; Inomata, H.; Watanabe, K.; Kawada, M.; Yakura, R.; Kiyonari, H.; Nakao, K.; et al. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 2011, 470, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Birkhoff, J.C.; Korporaal, A.L.; Brouwer, R.W.W.; Nowosad, K.; Milazzo, C.; Mouratidou, L.; van den Hout, M.; van Ijcken, W.F.J.; Huylebroeck, D.; Conidi, A. Zeb2 DNA-Binding Sites in Neuroprogenitor Cells Reveal Autoregulation and Affirm Neurodevelopmental Defects, Including in Mowat-Wilson Syndrome. Genes 2023, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Kumar, D.; Conway, A.E.; Park, K.; Jothi, R.; Jetten, A.M. GLIS3 Transcriptionally Activates WNT Genes to Promote Differentiation of Human Embryonic Stem Cells into Posterior Neural Progenitors. Stem Cells 2019, 37, 202–215. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakazawa, M.; Kani, S.; Bae, Y.K.; Shimizu, T.; Kageyama, R.; Hibi, M. Zinc finger genes Fezf1 and Fezf2 control neuronal differentiation by repressing Hes5 expression in the forebrain. Development 2010, 137, 1875–1885. [Google Scholar] [CrossRef]
- Bai, M.; Li, G.; Jiapaer, Z.; Guo, X.; Xi, J.; Wang, G.; Ye, D.; Chen, W.; Duan, B.; Kang, J. Linc1548 Promotes the Transition of Epiblast Stem Cells Into Neural Progenitors by Engaging OCT6 and SOX2. Stem Cells 2022, 40, 22–34. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Esfahani, A.K.; Peymani, M.; Nejati, A.S.; Ghaedi, K.; Nasr-Esfahani, M.H.; Baharvand, H. Zinc finger protein 521 overexpression increased transcript levels of Fndc5 in mouse embryonic stem cells. J. Biosci. 2016, 41, 69–76. [Google Scholar] [CrossRef]
- Kumar, A.; Declercq, J.; Eggermont, K.; Agirre, X.; Prosper, F.; Verfaillie, C.M. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J. Mol. Cell Biol. 2012, 4, 252–255. [Google Scholar] [CrossRef][Green Version]
- Shen, S.; Pu, J.; Lang, B.; McCaig, C.D. A zinc finger protein Zfp521 directs neural differentiation and beyond. Stem Cell Res. Ther. 2011, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.; Hesse, E.; Seriwatanachai, D.; Kiviranta, R.; Saito, H.; Yamana, K.; Neff, L.; Atfi, A.; Coillard, L.; Sitara, D.; et al. Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev. Cell 2010, 19, 533–546. [Google Scholar] [CrossRef]
- Wu, M.; Hesse, E.; Morvan, F.; Zhang, J.-P.; Correa, D.; Rowe, G.C.; Kiviranta, R.; Neff, L.; Philbrick, W.M.; Horne, W.C.; et al. Zfp521 antagonizes Runx2, delays osteoblast differentiation in vitro, and promotes bone formation in vivo. Bone 2009, 44, 528–536. [Google Scholar] [CrossRef]
- Pedrosa, E.; Sandler, V.; Shah, A.; Carroll, R.; Chang, C.; Rockowitz, S.; Guo, X.; Zheng, D.; Lachman, H.M. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J. Neurogenet. 2011, 25, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Deans, P.J.M.; Raval, P.; Sellers, K.J.; Gatford, N.J.F.; Halai, S.; Duarte, R.R.R.; Shum, C.; Warre-Cornish, K.; Kaplun, V.E.; Cocks, G.; et al. Psychosis Risk Candidate ZNF804A Localizes to Synapses and Regulates Neurite Formation and Dendritic Spine Structure. Biol. Psychiatry 2017, 82, 49–61. [Google Scholar] [CrossRef]
- Chen, J.; Lin, M.; Hrabovsky, A.; Pedrosa, E.; Dean, J.; Jain, S.; Zheng, D.; Lachman, H.M. ZNF804A Transcriptional Networks in Differentiating Neurons Derived from Induced Pluripotent Stem Cells of Human Origin. PLoS ONE 2015, 10, e0124597. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, W.; Park, S.; Jeong, J.; Kim, D.; Jang, S.; Kim, S.Y.; Sung, Y.; Kim, M.O.; Choi, S.K.; et al. Jazf1 acts as a regulator of insulin-producing β-cell differentiation in induced pluripotent stem cells and glucose homeostasis in mice. FEBS J. 2021, 288, 4412–4427. [Google Scholar] [CrossRef] [PubMed]
- Mattis, K.K.; Krentz, N.A.J.; Metzendorf, C.; Abaitua, F.; Spigelman, A.F.; Sun, H.; Ikle, J.M.; Thaman, S.; Rottner, A.K.; Bautista, A.; et al. Loss of RREB1 in pancreatic beta cells reduces cellular insulin content and affects endocrine cell gene expression. Diabetologia 2023, 66, 674–694. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Buganim, Y.; Faddah, D.A.; Jaenisch, R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 2013, 14, 427–439. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Jaenisch, R. Molecular control of induced pluripotency. Cell Stem Cell 2014, 14, 720–734. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lv, W.; Ye, X.; Wang, L.; Zhang, M.; Yang, H.; Okuka, M.; Zhou, C.; Zhang, X.; Liu, L.; et al. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res. 2013, 23, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-W.; Paek, J.-S.; Cho, H.-J.; Lee, C.-S.; Lee, H.-J.; Park, I.-H.; Roh, T.-Y.; Kang, C.-M.; Yang, H.-M.; Park, Y.-B.; et al. Role of Zscan4 in secondary murine iPSC derivation mediated by protein extracts of ESC or iPSC. Biomaterials 2015, 59, 102–115. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Zhang, M.-L.; Lin, H.-P.; Gao, C.; Song, J.-B.; Zheng, Z.; Li, L.; Zhang, Y.; Shen, X.; Zhang, H.; et al. The Zscan4-Tet2 Transcription Nexus Regulates Metabolic Rewiring and Enhances Proteostasis to Promote Reprogramming. Cell Rep. 2020, 32, 107877. [Google Scholar] [CrossRef] [PubMed]
- Declercq, J.; Sheshadri, P.; Verfaillie, C.M.; Kumar, A. Zic3 enhances the generation of mouse induced pluripotent stem cells. Stem Cells Dev. 2013, 22, 2017–2025. [Google Scholar] [CrossRef]
- Ma, H.; Ng, H.M.; Teh, X.; Li, H.; Lee, Y.H.; Chong, Y.M.; Loh, Y.H.; Collins, J.J.; Feng, B.; Yang, H.; et al. Zfp322a Regulates mouse ES cell pluripotency and enhances reprogramming efficiency. PLoS Genet. 2014, 10, e1004038. [Google Scholar] [CrossRef]
- Fischedick, G.; Klein, D.C.; Wu, G.M.; Esch, D.; Höing, S.; Han, D.W.; Reinhardt, P.; Hergarten, K.; Tapia, N.; Schöler, H.R.; et al. Zfp296 Is a Novel, Pluripotent-Specific Reprogramming Factor. PLoS ONE 2012, 7, e0034645. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Noh, H.B.; Kim, H.-T.; Lee, K.-I.; Hwang, D.-Y. Glis family proteins are differentially implicated in the cellular reprogramming of human somatic cells. Oncotarget 2017, 8, 77041–77049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasuoka, Y.; Matsumoto, M.; Yagi, K.; Okazaki, Y. Evolutionary History of GLIS Genes Illuminates Their Roles in Cell Reprograming and Ciliogenesis. Mol. Biol. Evol. 2020, 37, 100–109. [Google Scholar] [CrossRef]
- Yoshioka, N.; Gros, E.; Li, H.-R.; Kumar, S.; Deacon, D.C.; Maron, C.; Muotri, A.R.; Chi, N.C.; Fu, X.-D.; Yu, B.D.; et al. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell 2013, 13, 246–254. [Google Scholar] [CrossRef]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, K.; Wang, T.; Wu, Y.; Xing, G.; Chen, M.; Hao, Z.; Zhang, C.; Zhang, J.; Ma, B.; et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat. Metab. 2020, 2, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Yamanaka, S. Glis1, a unique pro-reprogramming factor, may facilitate clinical applications of iPSC technology. Cell Cycle (Georget. Tex.) 2011, 10, 3613–3614. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Li, D.; Liu, Y.; Guo, J.; Li, C.; Yao, Y.; Wang, Y.; Zhao, G.; Wang, X.; et al. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Jdp2-Jhdm1b-Mkk6-Glis1-Nanog-Essrb-Sall4. Cell Rep. 2019, 27, 3473–3485. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Ahn, H.S.; Park, J.Y.; Yang, H.M.; Cho, H.J.; Kim, H.S. Imprinted gene Zinc finger protein 127 is a novel regulator of master pluripotency transcription factor, Oct4. BMB Rep. 2018, 51, 242–248. [Google Scholar] [CrossRef]
- Wei, J.; Antony, J.; Meng, F.; MacLean, P.; Rhind, R.; Laible, G.; Oback, B. KDM4B-mediated reduction of H3K9me3 and H3K36me3 levels improves somatic cell reprogramming into pluripotency. Sci. Rep. 2017, 7, 7514. [Google Scholar] [CrossRef] [PubMed]
- Zorzan, I.; Pellegrini, M.; Arboit, M.; Incarnato, D.; Maldotti, M.; Forcato, M.; Tagliazucchi, G.M.; Carbognin, E.; Montagner, M.; Oliviero, S.; et al. The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs. Nat. Commun. 2020, 11, 2364. [Google Scholar] [CrossRef]
- Kaemena, D.F.; Yoshihara, M.; Beniazza, M.; Ashmore, J.; Zhao, S.; Bertenstam, M.; Olariu, V.; Katayama, S.; Okita, K.; Tomlinson, S.R.; et al. B1 SINE-binding ZFP266 impedes mouse iPSC generation through suppression of chromatin opening mediated by reprogramming factors. Nat. Commun. 2023, 14, 488. [Google Scholar] [CrossRef]
- Faiola, F.; Yin, N.; Fidalgo, M.; Huang, X.; Saunders, A.; Ding, J.; Guallar, D.; Dang, B.; Wang, J. NAC1 Regulates Somatic Cell Reprogramming by Controlling Zeb1 and E-cadherin Expression. Stem Cell Rep. 2017, 9, 913–926. [Google Scholar] [CrossRef]
- An, J.; Zheng, Y.; Dann, C.T. Mesenchymal to Epithelial Transition Mediated by CDH1 Promotes Spontaneous Reprogramming of Male Germline Stem Cells to Pluripotency. Stem Cell Rep. 2017, 8, 446–459. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Wu, P.; Hu, S.; Zhang, Y.; Chen, C. miR-200c-141 Enhances Sheep Kidney Cell Reprogramming into Pluripotent Cells by Targeting ZEB1. Int. J. Stem Cells 2021, 14, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Barington, M.; Bak, M.; Kjartansdóttir, K.R.; Hansen, T.v.O.; Birkedal, U.; Østergaard, E.; Hove, H.B. Novel Alu insertion in the ZEB2 gene causing Mowat-Wilson syndrome. Am. J. Med. genetics. Part A 2024, 194, e63581. [Google Scholar] [CrossRef]
- Treccarichi, S.; Vinci, M.; Musumeci, A.; Rando, R.G.; Papa, C.; Saccone, S.; Federico, C.; Failla, P.; Ruggieri, M.; Calì, F.; et al. Investigating the Role of the Zinc Finger Protein ZC2HC1C on Autism Spectrum Disorder Susceptibility. Medicina 2025, 61, 574. [Google Scholar] [CrossRef]
- Laugwitz, L.; Cheng, F.; Collins, S.C.; Hustinx, A.; Navarro, N.; Welsch, S.; Cox, H.; Hsieh, T.-C.; Vijayananth, A.; Buchert, R.; et al. ZSCAN10 deficiency causes a neurodevelopmental disorder with characteristic oto-facial malformations. Brain A J. Neurol. 2024, 147, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Gu, S.; Han, X.; Ding, H.; Jiang, Y.; Zhang, X.; Yao, C.; Hong, S.; Zhang, J.; Shen, Y.; et al. The transcription factor ZEB2 drives the formation of age-associated B cells. Science 2024, 383, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fattah, S.A.; Abdel Fattah, M.A.; Mesbah, N.M.; Saleh, S.M.; Abo-Elmatty, D.M.; Mehanna, E.T. The expression of zinc finger 804a (ZNF804a) and cyclin-dependent kinase 1 (CDK1) genes is related to the pathogenesis of rheumatoid arthritis. Arch. Physiol. Biochem. 2022, 128, 688–693. [Google Scholar] [CrossRef]
- Zhao, G.-F.; Huang, L.-B. Zinc finger E-box binding homebox 2 alleviated experimental osteoarthritis in rats. Connect. Tissue Res. 2023, 64, 323–336. [Google Scholar] [CrossRef]
- Hu, J.-Q.; Zheng, D.-C.; Huang, L.; Yang, X.; Ning, C.-Q.; Zhou, J.; Yu, L.-L.; Zhou, H.; Xie, Y. Suppression of ZEB1 by Ethyl caffeate attenuates renal fibrosis via switching glycolytic reprogramming. Pharmacol. Res. 2024, 209, 107407. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, X.; Wang, C.; Wang, M.; Li, M.; Li, X.; Yao, L.; Xu, Y. Zinc finger protein 593 promotes breast cancer development by ensuring DNA damage repair and cell-cycle progression. iScience 2024, 27, 111513. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, X.; Wu, J.; Chen, W.; Ren, D.; Zhong, C.; Meng, Z.; Shi, Y.; Zhu, J. ZNF652 exerts a tumor suppressor role in lung cancer by transcriptionally downregulating cyclin D3. Cell Death Dis. 2024, 15, 792. [Google Scholar] [CrossRef]
- Wei, X.; Liu, J.; Cheng, J.; Cai, W.; Xie, W.; Wang, K.; Lin, L.; Hou, J.; Cai, J.; Zhuo, H. Super-enhancer-driven ZFP36L1 promotes PD-L1 expression in infiltrative gastric cancer. eLife 2024, 13, RP96445. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Ke, Y.; Pan, J.; Zhou, M.; Zeng, H.; Song, G.; Yu, Z.; Fu, B.; Liu, Y.; Huang, D.; et al. ZNF320 is a hypomethylated prognostic biomarker involved in immune infiltration of hepatocellular carcinoma and associated with cell cycle. Aging 2022, 14, 8411–8436. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Zhang, X.; Gao, Y.; Tian, H.; Fan, L.; Yang, P. SOX4-BMI1 axis promotes non-small cell lung cancer progression and facilitates angiogenesis by suppressing ZNF24. Cell Death Dis. 2024, 15, 698. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Geng, Q.; Li, H.; Wu, M.; Chan, B.; Wang, S.; Sheng, W. ZNF263 cooperates with ZNF31 to promote the drug resistance and EMT of pancreatic cancer through transactivating RNF126. J. Cell. Physiol. 2024, 239, e31259. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, Y.; Hu, H.; Li, F. ZFP64 Promotes Gallbladder Cancer Progression through Recruiting HDAC1 to Activate NOTCH1 Signaling Pathway. Cancers 2023, 15, 4508. [Google Scholar] [CrossRef]
- Zhang, H.; Han, B.; Tian, S.; Gong, Y.; Liu, L. ZNF740 facilitates the malignant progression of hepatocellular carcinoma via the METTL3/HIF-1A signaling axis. Int. J. Oncol. 2024, 65, 105. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Gui, F.; Peng, F.; Deng, W.; Zhu, Q. METTL3-mediated m6A modification of ZNF384 promotes hepatocellular carcinoma progression by transcriptionally activating ACSM1. Clin. Transl. Oncol. 2025, 27, 2256–2268. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Q.; Ma, X.; Jiang, Y.; Wang, Y.; Jia, S.; Nie, Y.; Deng, R.; Zhou, P.; Zhang, S.; et al. ZNF131-BACH1 transcriptionally accelerates RAD51-dependent homologous recombination repair and therapy-resistance of non-small-lung cancer cells by preventing their degradation from CUL3. Theranostics 2024, 14, 7241–7264. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Zhao, H.; Qiu, Y.; Liu, Z.; Zhang, X.; Fan, M.; Zhang, Y.; Zhang, Y. ZNF480 Accelerates Chemotherapy Resistance in Breast Cancer by Competing With TRIM28 and Stabilizing LSD1 to Upregulate the AKT-GSK3β-Snail Pathway. Mol. Carcinog. 2025, 64, 192–208. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L.; Li, X.; Hu, Y.; Luo, N. Cancer-associated fibroblasts promote doxorubicin resistance in triple-negative breast cancer through enhancing ZFP64 histone lactylation to regulate ferroptosis. J. Transl. Med. 2025, 23, 247. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Zhou, L.; Zhao, X.; Ge, C.; Zhao, F.; Xie, H.; Chen, T.; Tian, H.; Li, H.; et al. FBXO9 Mediates the Cancer-Promoting Effects of ZNF143 by Degrading FBXW7 and Facilitates Drug Resistance in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 930220. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, Y.; Yan, Z.; Yang, J.; Da, M. Zinc finger protein 263 promotes colorectal cancer cell progression by activating STAT3 and enhancing chemoradiotherapy resistance. Sci. Rep. 2024, 14, 21827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, P.; Yu, S.; Tang, C.; Wang, Y.; Shen, Z.; Chen, W.; Liu, T.; Cui, Y. Targeting ZFP64/GAL-1 axis promotes therapeutic effect of nab-paclitaxel and reverses immunosuppressive microenvironment in gastric cancer. J. Exp. Clin. Cancer Res. CR 2022, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Sun, J.; Jing, F.; Xing, Y.; Luan, M.; Feng, Z.; Ma, X.; Wang, Y.; Jia, Y. GLI2 inhibits cisplatin sensitivity in gastric cancer through DEC1/ZEB1 mediated EMT. Cell Death Dis. 2025, 16, 204. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Moreno, A.M.; Alemán, F.; Catroli, G.F.; Hunt, M.; Hu, M.; Dailamy, A.; Pla, A.; Woller, S.A.; Palmer, N.; Parekh, U.; et al. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci. Transl. Med. 2021, 13, eaay9056. [Google Scholar] [CrossRef]
| Name | Aliases | Role | Species | Target Genes | Mechanism | References |
|---|---|---|---|---|---|---|
| Zfp281 | Znf281(mouse) ZBP-99 (human) | mESC | Mouse | Nanog Nanog | Regulate pluripotency by activation and repression of target genes | [7,8] |
| Patz1 | 8430401L15Rik, Mazr, Patz, Zfp278 (mouse) MAZR, PATZ, RIAZ, ZBTB19, ZNF278, ZSG, dJ400N23 (human) | mESC | Mouse | Oct4, Nanog | Maintain pluripotency | [12] |
| Zfp553 | 2600009K23Rik, C330013F15Rik, Znf48 (mouse) | mESC | Mouse | Oct4, Nanog | Maintain pluripotency | [13] |
| Zfp143 | D7Ertd805e, KRAB14, SBF, Staf, Zfp79, Zfp80-rs1, Znf143, pHZ-1 (mouse) | mESC | Mouse | Oct4, Nanog | Maintain pluripotency | [14] |
| Sall4 | 5730441M18Rik, C330011P20Rik, Tex20 (mouse) DRRS, HSAL4, IVIC, ZNF797 (human) | mESC | Mouse | Oct4, Nanog, Sox2 | Maintain pluripotency | [28,29,30] |
| Zfp819 | 4930427I11Rik, 4933405K07Rik (mouse) | mESC | Mouse | Oct4, Nanog, Sox2 | Downregulation of pluripotency marker genes | [59] |
| Zscan10 | Zfp206, Zkscan10, Znf206 (mouse) OFNS, ZFP206, ZNF206 (human) | mESC | Mouse | Oct4, Nanog, Sox2 | 1, Pluripotency factor 2, No impact on self-renewal | [104,105,106,125] |
| klf2 | Lklf (mouse) LKLF (human) | mESC | Mouse | Nanog | Sustain self-renewal | [107] |
| klf4 | EZF, Gklf, Zie (mouse) EZF, GKLF (human) | mESC hESC | Mouse Human | Nanog | Sustain self-renewal | [107,108] |
| Klf5 | 4930520J07Rik, Bteb2, CKLF, IKLF (mouse) BTEB2, CKLF, IKLF (human) | mESC | Mouse | Nanog | Sustain self-renewal | [109,110] |
| Sp5 | - | mESC | Mouse | Nanog | Sustain self-renewal | [111] |
| Zfp57 | G19, Zfp-57 (mouse) C6orf40, TNDM1, ZNF698, bA145L22, bA145L22.2 (human) | mESC | Mouse | Nanog | Downstream target of Nanog | [112] |
| ZNF207 | 8430401D15Rik, BuGZ, Zep, Znf207 (mouse) BuGZ, hBuGZ (human) | hESC | Human | OCT4 | Required for self-renewal and pluripotency | [113] |
| Zic3 | Bn, Ka (mouse) HTX, HTX1, VACTERLX, ZNF203 (human) | mESC | Mouse | Oct4, Nanog, Sox2 | Maintain pluripotency | [114,115] |
| Sall1 | Msal-3 (mouse) HEL-S-89, HSAL1, Sal-1, TBS, ZNF794 (human) | mESC | Mouse | Nanog, Sox2 | Regulate pluripotency | [116] |
| Zfp462 | 6030417H05, 9430078C22Rik, Gt4-2, Zfpip, Znf462 (mouse) WSKA, ZFPIP, Zfp462 (human) | P19 | Mouse | Oct4, Nanog, Sox2 | Maintain pluripotency | [117,118] |
| Zscan4c | Gm397, XM_142517, Zscan4d (mouse) | mESC | Mouse | - | Regulator of pluripotency | [119] |
| YY2 | ZNF631 (human) | mESC | Mouse | Zscan4 Oct4 | Required for self-renewal | [120,130] |
| ZFX | Zfx55,6, Zfx6, Zfx (mouse) MRXS37, ZNF926 (human) | mESC hESC | Mouse Human | Tbx3, Tcl1 | Promote self-renewal | [121,122] |
| Zfp42 | Rex-1, Rex1, Zfp-42 (mouse) REX-1, REX1, ZNF754, zfp-42 (human) | mESC hESCF9 | Mouse Human | - | Pluripotency marker | [123,124,127,128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Wu, Q. The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming. Int. J. Mol. Sci. 2025, 26, 5106. https://doi.org/10.3390/ijms26115106
Qian Y, Wu Q. The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming. International Journal of Molecular Sciences. 2025; 26(11):5106. https://doi.org/10.3390/ijms26115106
Chicago/Turabian StyleQian, Yiwei, and Qiang Wu. 2025. "The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming" International Journal of Molecular Sciences 26, no. 11: 5106. https://doi.org/10.3390/ijms26115106
APA StyleQian, Y., & Wu, Q. (2025). The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming. International Journal of Molecular Sciences, 26(11), 5106. https://doi.org/10.3390/ijms26115106







