Abstract
Fruits are excellent sources of substrate for various fermented products, including fruit vinegars, which are typically produced by submerged fermentation. Some evidence suggests that fruit vinegar consumption can alleviate certain disorders, including hyperlipidemia, inflammation, and hyperglycemia. Fruit vinegars also have bacteriostatic and antihypertensive actions. Recent studies also suggest that apple vinegar may offer benefits in treating insulin resistance, osteoporosis, and certain neurological diseases such as Alzheimer’s disease; it may also support weight loss. Recent studies in animal and human models have considerably broadened our understanding of the biological properties of not only fruit vinegars but also oxymels, i.e., mixtures of vinegar and honey or sugar. This paper reviews the current state of knowledge regarding vinegars and oxymels, with a special emphasis on their chemical composition and the mechanisms behind their biological activity and pro-health potential. The multidirectional effects of fruit vinegars and oxymels result from the synergy of different chemical compounds, including organic acids (mainly acetic acid), phenolic compounds, vitamins, minerals, and fermentation products. However, more studies are needed to understand the interactions between all the different components, not only the phenolic compounds and organic acids. In addition, more research is needed on their mechanisms of action. Although no serious side effects have been noted to date, further studies with large sample sizes are needed to understand the possible side effects of long-term fruit vinegar and oxymel use.
1. Introduction
Health-promoting foods have recently enjoyed increasing interest from consumers. Fruits, for example, are valuable sources of vitamins, minerals, and fiber and contain a range of phytochemicals, such as phenolic compounds, known to benefit health by exerting anti-inflammatory, hypoglycemic, antioxidant, and anti-platelet activity. A range of apples are popular choices for producing a range of alcoholic beverages and vinegars. Most fruit vinegar production is based on aromatic and juicy apple species, such as McIntosch, Cox Orange, Boskop, and Alkmene [1,2,3,4,5]. However, fruit vinegars can also be made from many other fruits, including grapes, blackberries, strawberries, oranges, pineapples, and bananas, as well as fruit juices. They can be prepared by a two-stage process based on alcoholic and acetic acid fermentation.
Food fermentation is one of the oldest forms of biotechnology and was initially used as a means of preservation. Currently, there are about 5000 varieties of fermented food products [3,6,7], and they can have unique characteristics, tastes, and flavors depending on the ingredients, the fermentation process, and the participating microorganisms.
A key determinant of vinegar quality is its proportions of specific compounds, particularly phenolic compounds. However, the most important criterion for assessing the quality of vinegar, along with the aroma, is its acetic acid content. In addition, an important part is played by the sugar content in the fruit as this determines the amount of alcohol produced and thus the acetic acid content. The acetic acid content should not be less than 5–7% in fruit vinegars, while the maximum ethanol content should not exceed 1%. There are several ways to obtain vinegars, for example, the Orleans method (one of the oldest methods, i.e., the surface method), the generator drip method, or the depth method [2,8,9,10,11,12,13,14].
According to historical records, fruit vinegars were first prepared and utilized by the ancient Egyptians, Babylonians, and Sumerians. In addition to their use as taste enhancers, they have also been employed as medicines: the ancient Egyptians used apple vinegar to treat mushroom poisoning and a lack of appetite, while Hippocrates recommended vinegar as a disinfectant for wounds and apple vinegar for treating colds, coughs, and digestive ailments. They began to be used medicinally by Europeans in the 17th century; however, currently, in Africa, America, and Europe, fruit vinegars are mainly added to food for their flavor [15]. They have also been included in syrups and antiseptics as an antimicrobial agent [8,12].
Today, fruit vinegars are popular natural products with multiple uses and are often used as dressings, particularly in salads. Apple vinegar is becoming particularly popular, mainly because of its organoleptic profile and its phenolic compound profile, which is believed to confer various biological properties [2,4,5].
Chemically speaking, fruit vinegars are aqueous solutions of acetic acid, with a mixture of inter alia organic acids, mineral salts, dyes, esters, aldehydes, and ketones [2,13]. They are manufactured using various production techniques, the choice of which determines their taste, color, consistency, chemical composition, smell, or biological properties. Their production process is carried out in two stages. In the first stage, anaerobic digestion takes place, in which sugars are converted into ethanol with the participation of yeast. In the next step, ethanol is converted to acetic acid with the participation of acetic acid bacteria (AAB) from the Acetobacteriaceae family, including Acetobacter, Komagataeibacter, Gluconacetobacter, and Gluconobacter [2,13,16]. More details about the microorganisms used for fruit fermentation are described by Yuan et al. [7].
The consumption of fruit vinegars has been positively associated with good health. Fruit vinegar consumption is associated with inter alia better control of diabetes, blood pressure, and obesity, associated with better lipid metabolism [2,8,17,18,19,20,21,22,23,24,25,26]. In addition to fruit vinegars, recent studies have considerably broadened our understanding of the biological properties of oxymels, i.e., mixtures of vinegar and honey or sugar. They can be used in a simple form or enriched with various medicinal plant roots, seeds, leaves, fruits, and vegetables extracts. One widely used oxymel is squill oxymel, which has gained recognition in the West and continues to be used today [27].
The term oxymel is derived from the Greek words oxymeli, meaning acid and honey. It has been used for centuries as a therapeutic drink with a unique flavor and is still a commercially produced traditional drink in various countries. The use of oxymel dates back to the time of Hippocrates [8,27]. In ancient Persia, oxymel was known as serkangabin, derived from the combination of serkeh (vinegar) and angabin (honey). Persian medicine books describe various types of oxymel and their preparation methods, side effects, benefits, and indications; they include over 1200 types of oxymels, including one containing apple vinegar and honey, often named powerdrink [27].
Recently, few papers have described the chemical characteristics and biological properties of various vinegars [12,14,26]. However, Ousaaid et al. [2] only demonstrated the chemical composition of fruit vinegars and their therapeutic application. In addition, only one systematic review on oxymel has been published [27]. The present work reviews the up-to-date literature concerning the pro-health potential of fruit vinegars, especially filtered and pasteurized clear vinegars, and oxymels; it also examines their chemical composition and the mechanisms behind their biological properties. Of course, artisanal vinegars, i.e., live vinegars, are also available, but no comprehensive literary data on their health-promoting potential currently exist.
2. Methodology for Literature Search
A literature search was performed of PubMed, Science Direct, Scopus, Springer, Web of Knowledge, Web of Science, and Google Scholar, using various combinations of the keywords “fruit vinegar”, “oxymel”, “biological activity”, and “pro-health potential”. No time criteria were applied to the search, but recent papers were evaluated first. The review included book chapters, review papers, and research papers. The abstracts of any identified articles were initially analyzed to confirm whether they met the inclusion criteria. Any relevant identified articles were summarized. After obtaining the full texts of the included studies, the reference sections were also manually examined to identify any additional new articles.
3. Phytochemical Characteristics of Fruit Vinegar and Oxymel
3.1. Fruit Vinegar
Fruit vinegars contain a cocktail of chemical compounds. The main components are organic acids, particularly acetic acid, which makes up about 30–50% of the total organic acid content. These are accompanied by melanoidins formed between sugars and nitrogen compounds such as amino acids, peptides and proteins, and tetramethylpeyrazine, also known as ligustrazine. In addition, vinegars contain a range of phenolic compounds, minerals (e.g., sodium, potassium, calcium, zinc, iron, copper, phosphorus, and magnesium), vitamins (A, C, and others), amino acids, monosaccharides (e.g., glucose, fructose, xylose, mannitose, and arabinose), disaccharides (such as sucrose, maltose, and mycose), and pectin (Figure 1).
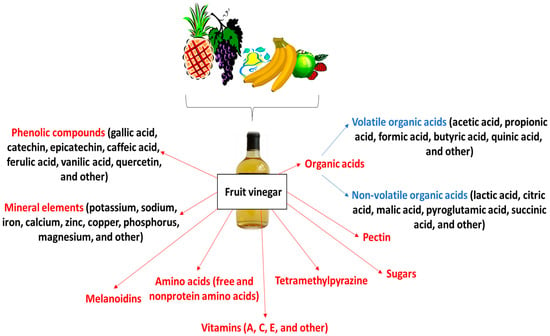
Figure 1.
Chemical characteristic of fruit vinegars.
They contain free and nonprotein amino acids, mainly derived from raw materials and proteins obtained by microbial decomposition, and their total concentration increases with aging time. Glucose and fructose may be present, and they provide a sweet taste to fruit vinegars. A comparison of the chemical compositions of different fruit vinegars is shown in Table 1. For example, apple vinegar has 24–226 mg/L Br and Ca, 19.4 mg/L Fe, and 7–195 mg/L Mg [2,13,15].
Phenolic Compounds and Organic Acids
In addition to phenolic compounds, organic acids are also believed to play an important part in the beneficial effects of fruit vinegars [28].
The phenolic compound composition of a fruit vinegar is influenced by its raw material [29,30,31,32,33,34]. For example, chlorogenic acid predominates in apple vinegar [29], and “Rauch”-made vinegar has the highest total phenolic compound content (281 mg gallic acid equivalent (GAE)/L) among commercial apple vinegars (33–57 mg GAE/L) [35]. The content of phenolic compounds obtained from dark fruits ranges from 367.2 mg GAE/L in raspberry vinegar to 1443.6 mg GAE/L in cherry and elderberry vinegar [36]. In grape vinegars, the content ranges from 71.01 to 2228.79 mg GAE/L [11].
A recent study by Abdali et al. [37] found that the chemical composition of apple vinegar, including acetic acid and phenolic compound content, depended on the choice of apple cultivar. A study of fruit vinegars available on the Polish Food Market (apple, rhubarb, lemon, and pear vinegar) by Melkis and Jakubczyk [38] found lemon vinegar to have the highest vitamin C content (15.95 mg/100 mL) but apple vinegar to have the highest flavonoid content (70.22 mg RE/L). Ozdemir et al. [39] note that sea buckthorn fruit vinegar is also a good source of phenolic compounds such as gallic acid (763.9 mg GAE/L) and that the major phenolic compound in Rosa canina L. vinegar is catechin (5.66 mg/L). It also appears that the phenolic compound content of a vinegar may be related to its antioxidant activity [36].
Suksamran et al. [40] report that mangosteen rind vinegar, mangosteen flesh vinegar, and mangosteen rind plus flesh vinegar have higher levels of phenolic compounds and greater antioxidant activity than apple vinegar. Also, a recent study by Uram-Dudek et al. [36] found dark fruit vinegars to have higher antioxidant activity, i.e., 21.3 to 77.5% tested by the 2,2-diphenyl-1-picrlhydrazyl (DPPH) method, compared with other vinegars [36].
Budak et al. [41,42] report the total phenolic content to be 1483.66 mg GAE/L in grape juice and 2690 and 2461 mg/L in vinegar obtained by traditional and industrial methods, respectively; they also indicate that fruit vinegars have higher antioxidant activity than wines and fruit juices. In addition, their bioaccessibility may be decreased or increased by fermentation. For example, the gallic acid and p-hydroxybenzoic acid in commercial “Turkish” apple vinegar were found to be less bioaccessible than those in apple fruits [43]. However, no other studies have examined the bioavailability of the other phenolic compounds present in fruit vinegars.
Nevertheless, a range of antioxidant compounds are present in fruit vinegars, and these can derive from the fruits or arise during the fermentation process [43,44]. The antioxidant 1,4-lactone of D-saccharic acid is also formed during fermentation [45], demonstrating that the method of production can also influence the final antioxidant potential [41,42,46]. Also, Antoniewicz et al. [47] monitored the antioxidant activity and phenolic compounds content of grape vinegars during the two-month fermentation process and the subsequent six-month storage under various conditions. It was found that storage conditions and time both affected the phenolic compound content and thus the antioxidant potential of the vinegar; the content was also influenced by the grape variety and the preparation method.
While the characteristic aroma of vinegar is attributed to the presence of acetic acid, it may also be influenced by a variety of chemical compounds formed during acetic acid fermentation [39]. For example, Budak et al. [41,42] report that peach vinegar has high concentrations of acids (68%), alcohols (10%), and esters (8%), which contribute to its odor. More details about chemical characteristic of fruit vinegars are given in reviews by Xia et al. [25] and Ousaaid et al. [2].

Table 1.
Comparison of selected chemical ingredients in the different fruit vinegars.
Table 1.
Comparison of selected chemical ingredients in the different fruit vinegars.
| Selected Chemical Ingredients | Fruit Vinegar | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pomagranate | Blackberry | Blueberry | Mulberry | Cherry | Apple | Plum | Kiwi | Grape | Persimmon | Pineapple | |
| Amino acids | Aspartic acid, glutamic acid, serine, alanine, glycine, arginine, threonine, lysine, valine, isoleucine, methionine, leucine, phenylalanine, and GABA [48] | Aspartic acid, glutamic acid, serine, alanine, glycine, arginine, threonine, lysine, valine, isoleucine, methionine, leucine, phenylalanine, and GABA [48] | Aspartic acid, glutamic acid, serine, alanine, glycine, arginine, threonine, lysine, valine, isoleucine, methionine, leucine, phenylalanine, and GABA [48] | Aspartic acid, glutamic acid, serine, alanine, glycine, arginine, threonine, lysine, valine, isoleucine, methionine, leucine, phenylalanine, and GABA [48] | Aspartic acid, glutamic acid, serine, alanine, glycine, arginine, threonine, lysine, valine, isoleucine, methionine, leucine, phenylalanine, and GABA [48] | - | - | - | - | - | - |
| Sugars | Fructose and glucose [48] | Fructose and glucose [48] | Fructose and glucose [48] | Fructose and glucose [48] | Fructose and glucose [48] | - | - | - | - | - | - |
| Organic acid | Barbituric acid, shikimic acid, citric acid, succinic acid, and acetic acid [4] | Maleic acid, barbituric acid, shikimic acid, adipic acid, citric acid, succinic acid, lactic acid, acetic acid, and propionic acid [4] | Malonic acid, barbituric acid, quinic acid, shikimic acid, citric acid, tartaric acid, malic acid, succinic acid, lactic acid, and acetic acid [4] | - | Isobutyric acid, isovaleric acid, hexanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodacanoic acid, tetradecanoic acid, methyl acetate, ethyl acetate, ethyl propanoate, isobutyl acetate, isoamyl acetate, ethyl caproate, ethyl caprylate, ethyl decanoate, benzyl acetate, phenethyl acetate, ethanol, isobutyl alcohol, hexanol, nonanol, and benzyl alcohol [4,49] | Acetic acid, lactic acid, quinic acid, malonic acid, barbituric acid, oxalic acid-dihydrate, shikimic acid, adipic acid, oxalic acid, tartaric acid, propanedioic acid, malic acid, succinic acid, propionic acid, isobutyric acid, butryric acid, isovaleric acid, and citric acid [4,29,30,50] | Acetic acid, tartaric acid, and lactic acid [51] | Acetic acid, lactic acid, quinic acid, tartaric acid, propanedioic acid, malic acid, succinic acid, and citric acid [29] | Malonic acid, barbituric acid, shikimic acid, adipic acid, citric acid, tartaric acid, succinic acid, lactic acid, acetic acid, fumaric acid, and propionic acid [4] | - | Methyl ester, ethyl acetate, isobutyl acetate, iobutanol, acetoin, benzaldehyde, propanoic acid, butanoic acid, isobutyric acid, methylbutanoic acid, naphthalene, and phenylrthyl alcohol [52] |
| Phenolic compounds | Gallic acid, galloylglucoside, protocatechuic acid, punicalagin, catechin, vanillic acid, syringic acid, ethyl acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, ferulic acid hexoside, tyrosol, and trans-p-coumaric derivate [53,54] | - | - | - | Gallic acid, chlorogenic acid, p-coumaric acid, caffeic acid, ferulic acid, catechin, protocatechuic acid, caftaric acid, furoic acid, protocatechualdehyde, tyrosol, catequin, vanillic acid, syringic acid, vanillin, syringaldehyde, coniferyl aldehyde, sinapaldehyde, and epicatechin [49,55] | Chlorogenic acid, 4-coumaroylqunic acid, isomer of chlorogenic acid, isomer of 4-coumaroylqunic acid, p-hydroxybenzoic acid, protocatechuic acid, gallic acid, vanillic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, catechin, syringic acid, epicatechin, gallate, procyanidin B2, luteolin-3-O-rutinose; isorhamnetin-3-O-rutionse, isorhamnetin-3-O-glucoside, kaempferol-3-O-glucoside, quercetin-3-orhamnoside, quercetin, rutin, luteolin, apigenin, phloretin, phloridzin, and phloridzin [29,30,53,56,57,58] | Ellagic acid, caffeoylquainic acid derivatives, p-coumaric acid derivatives, cyjandidn 3-o-galactoside, cyjanidin 3-orobinobioside and pelargonidine 3-o-galactoside, pelargonidine 3-o-robinoside, aromadendrin 7-o-glucoside, quercetin 3-o-galactoside, quercetin 3-o-glucuronide, and kaempferol 3-o-galactoside [59] | Gallic acid, vanillic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, epicatechin, gallate, chlorogenic acid, trans-ferulic cid, and phloridzin [29] | Gallic acid, p-hydroxybenzoic acid, catechin, epicatechin, caffeic acid, chlorogenic acid, syringic acid, p-coumaric acid, tyrosol, protocatechiuc acid, caftaric acid, coutaric acid, fertaric acid, vanilic acid, syringing acid, procyanidin B2, quercetin-3-O-galactoside, kaempferol-3-O-rutinoside, rutin isorhamnetib-3-O-glucoside, ferulic acid, and quercetin [41,42,57] | Gallic acid, catechin hydrate, chlorogenic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, epicatechin gallate, and phloridzin [29] | Catechol, peonidin, catechin 3-O-gallate, m-coumaric acid, ferulic acid, mullein, genistein, 4-ethylcatechol, 6-prenylnaringenin, gallic acid, spinacetin, and malvidin 3-O-arabinoside [60] |
| Mineral composition | - | - | - | - | K, Na, Ca, Zn, Mg, Fe, P, Ni, and Mg [61] | K, Na, Ca, Mg, Fe, P, Ni, Mn, and Zn [61,62] | - | - | - | - | - |
3.2. Oxymel
However, relatively little information exists about the chemical components of oxymels. An oxymel is not always only a mixture of vinegar and honey or sugar: it can be enriched with various medical plants and fruit and vegetable extracts. For example, Abolghasemi et al. [63] found that Zataria multiflora Boiss. oxymel contained various chemical compounds. The authors created an oxymel by boiling a mixture of three units of sugar to one unit of vinegar and one unit of water. Gas chromatography/mass spectrometry (GC/MS) demonstrated that the tested oxymel contained terpenes, including carvacrol and thymol, as well as inter alia β-cymene and terpinene. HPLC testing confirmed the presence of caffeic acid (10.9 mg/L), quercetin (652.7 mg/L), p-coumaric acid (13.1 mg/L), eugenol (91 mg/L), and rosmarinic acid (116.2 mg/L).
4. Biological Activity of Fruit Vinegars
The consumption of fruit vinegars has been found to have pro-health properties in animal and human models.
4.1. Antihyperglycemic Effect
Mitrou et al. [64] noted that apple vinegar (30 mL/day) decreased the concentration of blood glucose in humans with type 2 diabetes (n = 11); the authors attributed this to a combination of stimulating glucose uptake and enhancing insulin activity in skeletal muscle. Also, this beneficial action may be due to the presence of acetic acid, which acts via mitogen-activated protein kinase (MAPK). Other studies indicate that other organic acids also counteract the activity of hydrolyzing enzymes such as lactase, maltase, trehalase, and sucrase. Moreover, Ousaaid et al. [61] observed that 2 mL of apple vinegar daily for five weeks decreased the risk of developing hyperglycemia stimulated by a hypercaloric diet in rats.
4.2. Antihyperlipidemic Effect
A few papers propose that fruit vinegars may be used to treat dyslipidemia. For example, Bahesheti et al. [65] indicate that supplementation with apple vinegar (30 mL twice a day, for eight weeks) improves lipid profiles, including triglyceride, low-density lipoprotein (LDL), and total cholesterol levels, in patients with hyperlipidemia (n = 19). Also, the consumption of fruit vinegars, including pomegranate vinegar, appears to have similar effects in obese mice and overweight females [17,66,67]. It is possible that this influence on lipid metabolism may take place though the activation of 5′AMP-activated protein kinase (AMPK) by acetic acid in adipose tissue [66].
4.3. Antioxidative Effect
Fruit vinegars contain antioxidant phenolic compounds, which may protect against the oxidative stress associated with various diseases. Interestingly, fruit vinegars have been found to demonstrate these properties both in vitro and in vivo. For example, Bouazza et al. [68] reported that vinegars from pomegranate (Punica granatum L), prickly pear (Opuntia ficus-indica (L.) Mill.), and apple (Malus domestica Borkh.) stimulated the activity of various antioxidant enzymes, including glutathione reductase, glutathione peroxidase, and superoxide dismutase, and increased total antioxidant status. However, they also decreased lipid peroxidation, measured by TBARS level, by about 44% in the liver and about 61% in plasma in rats fed a high-fat diet (80 cal/day). In addition, the pomegranate vinegar yielded a significant reduction in lipid profile levels: total cholesterol, 65%; triglycerides, 68%; low-density lipoprotein (LDL), 76%; and atherogenic index, 80%. In this experiment, fifty male Wistar rats were orally dosed with fruit vinegars (7 mL/kg) once daily for 28 weeks. Halima et al. [69] also noted that the consumption of apple vinegar attenuated oxidative stress and reduced the risk of obesity in male Wistar rats receiving a high-fat diet.
4.4. Anti-Inflammatory Effect
The results of Wakuda et al. [70] demonstrated that pear vinegar reduced the levels of inflammatory cytokines, including serum interleukin-6 (Il-6) and IL-8, in mice with acute colitis induced by a sodium sulfate (n = 54). Other authors indicate that consumption of apple vinegar inhibits cyclooxygenase-2 (COX-2) in patients with type 2 diabetes [71]. In addition, Cudrania tricuspidata fruit vinegar suppressed inflammatory marker expression, decreasing IL-6, TNF-α, MCP-1, iNOS, and nitric oxide (NO), in 3T3-L1 adipocytes and Raw264.7 macrophages in vitro [31,32]. A more detailed review of the anti-inflammatory properties of fruit vinegars is given in a review by Ousaaid et al. [72], which discusses their use in the prevention and treatment of various inflammatory diseases including colitis, arthritis, atopic dermatitis, and asthma; the authors note that artisanal fruit vinegars are transformed into anti-inflammatory compounds in the digestive system more effectively than commercial varieties, and their properties may promote anti-inflammatory activity by influencing pro-inflammatory cytokine production and altering the intestinal microbiota. In addition, Abdali et al. [37] reported that supplementation of apple vinegar (10 mL/kg) reduced carrageenan-stimulated inflammation by about 37% in Wistar rats. The dosing also appeared to exert antidepressant properties, reducing immobility time by about 30% among the rats.
4.5. Other Biological Properties
Recently, the results of Seyidoglu et al. [73] demonstrated that all used hawthorn vinegars, especially ultrasound-treated vinegar, had positive actions on intestinal health and boosted immunity in Wistar albino rats (n = 56). In this study, the authors used traditional production of vinegar (0.5 mL/kg bw), thermal pasteurization of vinegar (0.5 and 1 mL/kg bw), and ultrasound treatment of vinegar (0.5 and 1 mL/kg bw). They were administered by oral gavage daily (for 45 days). Various other authors [74,75] suggest that a possible mechanism of hawthorn vinegar could be related to phenolic compounds.
Na et al. [76] noted that fruit vinegar decreased blood pressure in spontaneously hypertensive rats, which they attributed to the downregulation of angiotensin II receptor type 1 (AT1R) expression via the AMPK/PGC-1α-PPARγ pathway. Similar changes in protein expression were also found in SVAREC cells treated with 200 or 400 μmol/L acetate; the authors therefore suggested that the antihypertensive effects of the used vinegar may be due to its acetic acid content. Recently, Tang et al. [23] found that Fardh vinegar, made from the fruit of the date palm (Pheonix dactylifera L.), inhibited angiotensin-converting enzyme 2 (ACE2) activity in vitro and displayed good antioxidant activity by radical scavenging. Ali et al. [18,19] and Li et al. [77] also found that vinegars from red and black date fruits demonstrated antioxidant potential in vitro.
Tripathi and Mazumder [78] found that apple vinegar inhibited the activity of monoamine oxidase (MAO). This may have a protective effect against Alzheimer’s disease as MAO catalyzes the oxidative deamination of amines in the brain and peripheral tissues, whose buildup plays a part in AD. It is also involved in the metabolism of monoamines and is vital for cognition.
Apple vinegar and other fruit vinegars have been found to have in vitro antimicrobial properties against bacteria, such as Bacillus subtilis, Escherichia coli, Salmonella, and Staphylococcus aureus [37,79,80,81], and against certain fungi, including Candida albicans spp. [37,79,82]. Some papers indicate that these vinegars may also improve skin barrier integrity in atopic dermatitis [83,84,85].
A recent study by Yim et al. [86] found that mulberry vinegar intake (1 mg/kg bw) preserved bone mineral density in ovariectomized rats, mainly by inhibiting osteoclastic activity. They suggested that this vinegar may develop as a functional food for anti-osteoporosis in menopausal females. An in vitro study by Bang et al. [87] found that mulberry vinegar (10–100 µg/mL) prevented neuroinflammation in C6 glial cells stimulated by lipopolysaccharide (LPS)/interferon-γ (IFN-γ) by regulating the NF-κB signaling pathway; treatment inhibited COX-2 and iNOS production and increased glial activation, through the downregulation of ionized calcium-binding adapter molecule-1 (Iba-1) and glial fibrillary acidic protein (GFAP).
It has also been found that apple vinegar supplementation (1 mL/kg/day, for five weeks) ameliorated changes in blood platelets, blood cell count, mean corpuscular volume, hemoglobin concentration, and mean capsulated hemoglobin induced by phenylhydrazine in Wistar rats [2]. Shams et al. [88] examined the effect of apple vinegar on fallicuogenesis and ovarian kisspeptin in rats with non-alcoholic fatty liver disease caused by a high-fat diet (n = 28). Supplementation of apple vinegar was found to increase ovarian kisspeptin expression and raise primordial, estradiol, and small primary follicles. In addition, the used vinegars demonstrated anti-lipidemic and anti-glycemic properties.
Recent data indicate that orange vinegar and its main phenolic compounds (coumaric acid, epicatechin, and catechin) reduce the levels of pro-inflammatory factors, reactive oxygen species, and NADPH in Caco-2 cells. They have also been found to protect against the accumulation of advanced glycation end products, which play roles in the development of various degenerative disorders [24].
The biological activities of various fruit vinegars, including their anti-inflammatory, antioxidant, and neuroprotective properties, identified in animal and human models are presented in more detail in Table 2. These vinegars may also counteract the development of various disorders, including diabetes and cardiovascular diseases. Recent studies also suggest that apple vinegar may offer benefits in treating insulin resistance, osteoporosis, and certain neurological diseases such as Alzheimer’s disease; it may also support weight loss. The biological properties of fruit vinegars and their potential molecular mechanisms are also presented in Figure 2. In addition, this figure demonstrates modes of action of vinegar components, including phenolic compounds, vitamins, and acetic acid. For example, phenolic compounds and vitamins exhibit antioxidant properties. Acetic acid was found to suppress body fat accumulation, and it may determine the anti-obesity and cardioprotection properties of fruit vinegars.

Table 2.
The various biological activities of fruit vinegars and oxymels, based on animal and human models.
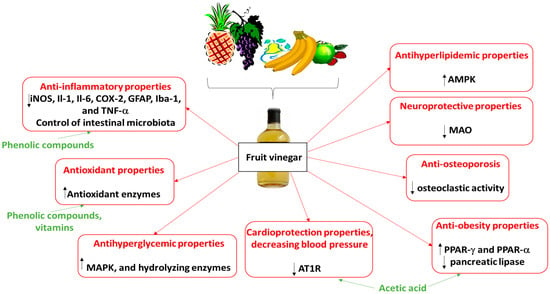
Figure 2.
Biological properties of fruit vinegars and their potential molecular mechanisms. AMPK, 5′AMP-activated protein kinase; AT1R, angiotensin II receptor type 1; COX, cyclooxygenase; GFAP, glial fibrillary acidic protein; Il, interleukin; Iba-1, ionized calcium-binding adapter molecule-1; iNOS, inducible nitric oxide synthase; MAO, monoamine oxidase; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferators-activated receptors; TNF-α, tumor necrosis factor-α.
5. Biological Activity of Oxymels
Oxymels also appear to possess pro-health properties and may also serve as effective and safe treatment options for managing various diseases, including obesity, metabolic syndrome, and asthma. Numerous preclinical and clinical studies indicate the beneficial action of oxymels [90,92,104]. For example, Abolmaali et al. [92] observed that oxymel containing vinegar, honey, and squill bulb, at doses of 100, 200, and 400 mg/kg, decreased the severity of seizure induced by penta-lenetetrazole in mice (n = 39) compared with controls; it also reduced the mortality rate of the animals in a dose-dependent manner.
It was also found that 0.1 mL of warm oxymel consisting of vinegar and sugar, once per day for 10 days, was also found to decrease IL-4 gene expression, perivascular and peribronchial inflammation, and hypersecretion in mice with asthma (n = 24) [90]. Squill oxymel containing honey, vinegar, and Drima martima (L.) stem, administered at 10 mL, twice a day, appeared to be a safe and effective treatment in patients with moderate to severe persistent asthma (n = 60) [102], as was squill oxymel, 10 mL twice a day for four weeks, in patients with chronic obstructive pulmonary disease (n = 42) [103].
Other results indicate that oxymel composed of vinegar, sugar, and thyme (300 or 500 mg/kg body weight (BW)/day, for 12 weeks) ameliorates obesity in Sprague–Dawley rats (n = 80) [93]. The authors suggest that it exerts its anti-obesity effects by improving lipid metabolism, inflammation, and oxidative stress, as indicated by serum and hepatic TBARS level and antioxidant enzyme activity. It also appears that the oxymel may also regulate the expression of sterol regulatory element-binding transcription factor (SREBP), carnitine palmitoyl transferase I (CPT-1), nuclear factor kappa B (NF-κB), and erythroid 2-related factor 2 (Nrf-2) on the genetic level.
Sarbaz Hoseini et al. [91] found oxymel (1 mL/day, for eight weeks) to have anti-diabetic effects in Wistar rats with type 2 diabetes and Zataria oxymel (0.75 mg Zataria multiflora Boiss. in 10 mL oxymel) to reduce insulin resistance in overweight patients (n = 200). Another study found oxymel (10 mL/kg BW, orally, for 14 days) to change the lipid profile in Wistar rats with hyperlipidemia (n = 30) [89].
Berberis vulgaris oxymel also appears effective in the treatment of patients with refractory primary sclerosing cholangitis and primary biliary cholangitis (n = 87). The tested oxymel did not have any apparent adverse effect on the kidney [99]. However, oxymel did not appear to demonstrate any significant effects on blood pressure in healthy volunteers [95,96].
More details about different biological activities of oxymels in animal and human models are given in Table 2. Interestingly, in most cases, oxymels have been studied on models of various diseases, including asthma, obesity, and migraine; comparatively few have been conducted on healthy people (Table 2). The most frequently tested type was simple oxymel, i.e., consisting of vinegar and a sweet component (honey or sugar), with squill oxymel being less common. Some other types also contain Z. multiflora, C. spinose, and B. vulgaris. Most importantly, like the fruit vinegars, none of the used oxymels had any adverse effects on animals or humans.
Recently, oxymel has been used successfully on wounds as a topical application against antibiotic-resistant bacteria. While both vinegar and honey have been used historically as antiseptics, the combination was found to be 1000 times more effective at killing bacteria than vinegar alone and 100,000 times more than honey alone [27,63].
Although the mechanism behind the antimicrobial action of oxymel remains unclear, it is likely that it acts on a number of different levels simultaneously. It is also possible that the different ingredients, e.g., vinegar and its main component, acetic acid, phenolic compounds, and sweet components, may demonstrate synergy when applied together. Furthermore, the biological effects of the oxymel may be further enhanced by the presence of plant components.
6. Conclusions
For the first time, this review paper demonstrates that not only fruit vinegars but also oxymels appear to be good candidates for dietary supplements with beneficial effects on chronic conditions, such as obesity and asthma. They are also easy to administer: oxymels can be taken by the spoonful or added to beverages, such as water or tea, and the combination of honey and vinegar has a pleasant taste. Fruit vinegars and oxymels are also less expensive than other supplements or functional foods and can even be prepared at home.
The multidirectional effects of fruit vinegars and oxymels result from the synergy of different chemical compounds, including organic acids (mainly acetic acid), phenolic compounds, vitamins, minerals, and fermentation products (esters, aldehydes, and ketones). Together, these give the preparation its characteristic taste and aroma [25,26,46,105]. However, more studies are needed to understand the interactions between all the different components, not only the phenolic compounds and organic acids. In addition, more research is needed on their mechanisms of action. Although no serious side effects have been noted to date, further studies with large sample sizes are needed to understand the possible side effects of long-term fruit vinegar and oxymel use. These studies should include healthy people and those with various diseases, in addition to animals, and should examine their potential interactions with functional foods, supplements, and drugs.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
AAB, acetic acid bacteria; AMPK, 5′AMP-activated protein kinase; AT1R, angiotensin II receptor type 1; BMI, body mass index; BW, body weight; COX, cyclooxygenase; CPT-1, carnitime palmitoyl transferase I; DPPH, 2,2-diphenyl-1-picrlhydrazyl; GAE, gallic acid equivalent; HDL, high-density lipoprotein; Il, interleukin; iNOS, inducible nitric oxide synthase; LDL, low-density lipoprotein; MAO, monoamine oxidase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor kappa B; NO, nitric oxide; Nrf-2, erytroid 2-related factor 2; PPAR, peroxisome proliferators-activated receptors; SREBP, sterol regulatory element-binding transcription factor; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor-α.
References
- Guine, R.P.F.; Brroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple fermented products: An overview of technology, properties and health effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Ousaaid, D.; Mechchate, H.; Laaroussi, H.; Hano, C.; Bakour, M.; El Ghouizi, A.; Cnte, R.; Lyoussi, B.; El Arabi, I. Fruits vinegar: Quality characteristics, phytochemistry, and functionality. Molecules 2022, 27, 222. [Google Scholar] [CrossRef]
- Da Silva Monteiro Wanderley, B.R.; Ferreira, A.L.A.; Nunes, I.L.; de Mello Castanho Amboni, R.D.; de Sena Aquino, A.C.M.; Fritzen-Freire, C.B. The role of fruit vinegar in food science: Perspectives among consumers, the scientific community and patent holders. Biotechnol. Res. Innov. 2023, 7, e2023015. [Google Scholar]
- Yildiz, E. Characterization of fruit vinegars via bioactive and organic acid profile using chemometrics. Foods 2023, 12, 3769. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoz, K.; Echave, J.; Garcia-Oliveira, P.; Seyyedi-Mansour, S.; Donn, P.; Xiao, J.; Trząskowska, M.; Prieto, M.A. Polyphenolic profile, processing impact, and bioaccessibility of apple fermented products. Crit. Rev. Food Sci. Nutr. 2023, 1, 1–15. [Google Scholar]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent advances in Kombucha tea: Microbial consortium, chemical parameters, health implications and biocellulose production. Inter. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent advances of fermented fruits: A review on strains, fermentation strategies, and functional activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Luzon-Quintana, L.M.; Castro, R.; Duran-Guerrero, E. Biotechnological process in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Cavadaroglu, C.; Ozen, B. Autentication of vinegars with targeted and non-targeted methods. Food Rev. Inter. 2021, 1, 41–58. [Google Scholar]
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT-Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Chen, G.L.; Zheng, F.J.; Lin, B.; Yang, Y.X.; Fang, X.C.; Verma, K.K.; Yang, L.F. Vinegar: A potential source of healthy and functional food with special reference to sugarcane vinegar. Front. Nutr. 2023, 1, 1145862. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, R.; Kowalska, M.; Kaczmarczyk, M.; Pietrasik, D.; Walasek-Janusz, M. Fruit vinegars—Obtaining and health-promoting properties. Ann. Horticult. 2023, 32, 1–32. [Google Scholar]
- Khalifa, S.A.M.; El-Shabasy, R.M.; Tahir, H.E.; Abo-Atya, D.M.; Saeed, A.; Abolibda, T.Z.; Guo, Z.; Zou, X.; Du, D.Z.M.; Kai, G.; et al. Vinegar—A beneficial food additivie: Production, safety, possibilities, and applications from ancient to modern times. Food Fun. 2024, 15, 10262–10282. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Koysomboon, C.; Zhang, H.; Lu, Z.; Zhang, X.; Chen, F. Vinegar volatile organic compounds: Analytical methods, constituents, and formation process. Front. Microbiol. 2022, 1, 907883. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy apple juice fermented by lactobacillus prevents obesity via modulating gut microbiota and protecting intestinal tract health. Nutients 2021, 13, 971. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.A.; Park, G.G.; Jang, J.K.; Park, Y.S. Semi-continuous fermentation of onion vinegar and its functional properties. Molecules 2017, 22, 1313. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ma, H.; Wali, A.; Ayim, I.; Rashid, M.T.; Younas, S. A double-blinded, randomized, placebo-controlled study evaluating the impact of dates vinegar consumption on blood biochemical and hematological parameters in patients with type 2 diabetes. Trop. J. Pharm. Res. 2019, 17, 2463–2469. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Wali, A.; Ayim, I.; Sharif, M.N. Daily data vinegar consumption improves hyperlipidemia, β-carotenoid and inflammatory biomarkers in mildly hypercholesterolemic adults. J. Herb. Med. 2019, 1, 17–18. [Google Scholar]
- Ali, Z.; Li, J.; Zhang, Y.; Naeem, N.; Younas, S.; Javeed, F. Dates (Pheonix dactyliflora) and date vinegar: Preventive role against various diseases and related in vivo mechanism. Food Rev. Inter. 2022, 38, 480–507. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Lan, Y.; Li, X.; Luo, L.; Duan, X.; Lei, M.; Liu, G.; Yang, Z.; Mai, X.; et al. Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. EBioMed 2019, 45, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Erdal, B.; Akalin, R.B.; Yilmaz, B.; Bozgeyik, E.; Yikmis, S. Application of ultrasound to the organic cornelian cherry (Cornus mas L.) vinegar: Changes in antibacterial, antidiabetic, antihypertensive, and anticancer activities. J. Food Proc. Prev. 2022, 46, e16952. [Google Scholar]
- Tang, M.; Wang, Z.; Luo, J.; Zhu, T.; Song, F.; Chen, H. Preparation, chemical profile, antioxidative activities, and angiotensin-converting enzyme 2 inhibitory effect of data fruit vinegar. J. Food Sci. 2024, 89, 684–700. [Google Scholar] [CrossRef]
- Wu, Q.; Kong, Y.; Liang, Y.; Niu, M.; Feng, N.; Zhang, C.; Qi, Y.; Guo, Z.; Xiao, J.; Zhou, M.; et al. Protective mechanism of fruit vinegar polyphenols against AGEs-induced Caco-2 cell damage. Food Chem. X 2023, 19, 100736. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Xia, T.; Kang, C.; Qiang, X.; Zhang, X.; Li, S.; Liang, K.; Wang, Y.; Wang, J.; Cao, H.; Wang, M. Beneficial effect of vinegar consumption associated with regulating gut microbiome and metabolome. Cur. Res. Food Sci. 2024, 8, 100566. [Google Scholar] [CrossRef]
- Darani, N.S.; Vaghasloo, M.A.; Kazemi, A.; Amri, H.; Rampp, T.; Hashempur, M.H. Oxymel: A systematic review of preclinical and clinical studies. Heliyon 2023, 9, e22649. [Google Scholar] [CrossRef] [PubMed]
- Ousaaid, D.; Ghouizi, A.E.; Laaroussi, H.; Bakour, M.; Mechchate, H.; Es-safi, I.; Al Kamaly, O.; Saleh, A.; Conte, R.; Lyoussi, B.; et al. Anti-anemic effect of antioxidant-rich apple vinegar against phenylhydrazine-induced hemolytic anemia in rats. Life 2022, 12, 239. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of organic acids and phenolic compounds of cereal vinegars and fruit vinegars in China. J. Food Proc. Preserv. 2017, 41, e12937. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid content of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, M.K.; Yeo, S.H.; Kim, S. Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci. Rep. 2020, 10, 21102. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.E.; Yeo, S.H.; Kim, S. Anti-inflammatory and cytotoxicity effects of Cudrania tricuspidata fruits vinegar in a co-culture system with RAW264.7 macrophages and 3T3-L1 adipocytes. Food 2020, 9, 1232. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavor using electronic nose and electronic tongue. Sci. Hortcult. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Orozco-Flores, L.A.; Salas, E.; Rocha-Gutierrez, B.; Peralta-Pérez, M.D.R.; Gonzalez-Sanchez, G.; Ballinas-Casarrubias, L. Determination of polyphenolic profile of apple pomace (Malus domestica golden delicious variety) by HPLC-MS. ACS Omega 2024, 9, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhu, Y.; Wang, X.; Zhang, J.; Tian, C.; Liu, L.; Meng, Y.; Guo, Y. Phenolic composition of apple products and by-products based on cold pressing technology. J. Food Sci. Technol. 2019, 56, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Uram-Dudek, A.; Wajs, I.; Paradowska, K. The analysis of antioxidant properties of fermented active vinegar from fruits. Herbalism 2023, 1, 111. [Google Scholar] [CrossRef]
- Abdali, Y.E.; Saghrouchni, H.; Kara, M.; Mssillou, I.; Allali, A.; Bin Jardan, Y.A.; Kafkas, N.E.; El-Assri, E.M.; Nafidi, H.A.; Bourhia, M.; et al. Exploring the bioactive compounds in some apple vinegar samples and their biological activities. Plants 2023, 12, 3850. [Google Scholar] [CrossRef]
- Melkis, K.; Jakubczyk, K. The chemical profiles and antioxidant properties of live fruit or vegetable vinegars available on the Polish food market. Foods 2024, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property of the vinegar produced from rosehip fruit (Rosa canina). LWT 2022, 154, 112716. [Google Scholar] [CrossRef]
- Suksamran, N.; Anantawat, V.; Wattanaarsakit, P.; Wei, C.; Rahman, A.; Majima, H.J.; Tangpong, J. Mangosteen vinegar from Garcinia mangostana: Quality improvement and antioxidant properties. Heliyon 2022, 8, e11943. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Budak, N.H.; Ozdemir, N.; Gokirmakl, C. The changes of physicochemical properties, antioxidants, organic, and key volatile compounds associated with the flavor of peach (Prunus cerasus L. Batsch) vinegar during the fermentation process. J. Food Biochem. 2022, 46, e13978. [Google Scholar] [CrossRef]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit antioxidants during vinegar processing: Changes in content and in vitro bio-accessibility. Int. J. Mol. Sci. 2016, 17, 1658. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Esteban-Diez, I.; Saez-Gonzalez, C.; Gonzalez-Saiz, J.M. Vinegar classification based on feture extraction and selection from headspace solid-phase microextraction/gas chromatography volatile analyses: A feasibility study. Anal. Chim. Acta 2008, 608, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Wójtowicz, M.; Dabrowski, M.; Jaworska, D. Acetic acid bacteria as potential next-generation probiotics. Żywność Nauk Technol. Jakość 2020, 27, 15–27. [Google Scholar]
- Antoniewicz, J.; Janda-Milczerek, K. Grape vinegars–characteristics, properties and safety of use. Med. Ogólna Nauk. Zdrowiu 2021, 27, 379–386. [Google Scholar] [CrossRef]
- Antoniewicz, J.; Kochman, J.; Jakubczyk, K.; Janda-Milczarek, K. The influence of time and storage conditions on the antioxidant potential and total content in homemade grape vinegars. Molecular 2021, 26, 7616. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, H.K.; Shin, H.S. Physicochemical properties and antioxidant activities of commercial vinegar drinks in Korea. Food Sci. Biotech. 2012, 21, 1729–1734. [Google Scholar] [CrossRef]
- Ozen, M.; Ozdemir, N.; Filiz, B.E.; Budak, N.H.; Kok-Tas, T. Souir cherry (Prunus cerasus L.) vinegars produced from Fresh fruit or juice concentrate: Bioactive compounds, volatile aroma compounds and antioxidant capacities. Food Chem. 2020, 309, 125664. [Google Scholar] [CrossRef]
- Mateos-Aparico, I.; de la Pena, R.J.; Perez-Cozar, M.L.; Ruperez, P.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioact. Carbohydr. Diet. Fibre 2020, 23, 1016. [Google Scholar]
- Liu, F.; He, Y. Application of successive projections algorithm for variable selection to determine organic acids of plum vinegar. Food Chem. 2009, 115, 1430–1436. [Google Scholar] [CrossRef]
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous vinegar fermentation from a pineapple by-product using the co-inoculation of yeast and thermotolerant acetic acid bacteria and their physiochemical properties. 3 Biotech 2020, 10, 115. [Google Scholar] [CrossRef]
- Aykin, E.; Budak, N.H.; Guzel-Seydim, Z.B. Bioactive components of mother vinegar. J. Am. Coll. Nutr. 2015, 34, 80–89. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Gomez, J.; Lasanta, C.; Castro, R.; Sainz, R.; Hamdi, M. Benchmarking laboratory-sacle pomegranate vinegar against commercial wine vinegars: Antioxidant activity and chemical composition. J. Sci. Food Agric. 2018, 98, 4749–4758. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Duran-Guerrero, E.; Rodriguez-Dodero, M.C.; Barroso, C.G.; Castro, R. Use of ultrasound at a pilot scale to accelerate the ageing of sherry vinegar. Ultrason. Sonochem. 2020, 69, 105244. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogasawara, Y.; Endou, K.; Fujimori, S.; Koyama, M.; Akano, H. Phenolic compounds responsible for the superoxide dismutase-like activity in high-brix apple vinegar. J. Agric. Food Chem. 2010, 58, 10124–10132. [Google Scholar] [CrossRef]
- Kelebek, H.; Kadiroglu, P.; Demircan, N.B.; Selli, S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. J. Inst. Brew. 2017, 123, 407–416. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A new approach to the use of apple pomace in cider making for the recovery of phenolic compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piorecki, N. Bioactive compounds in cornelian cherry vinegars. Molecules 2018, 379, 379. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Lucini, L.; Torchion, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; ElGhouzi, A.; Aboulghazi, A.; Lyoussi, B.; ElArabi, I. Beneficial effects of apple vinegar on hyperglycemia and hyperlipidemia in hypercaloric-fed rats. J. Diabetes 2020, 1, 9284987. [Google Scholar] [CrossRef] [PubMed]
- Akpinar-Bayzit, A.; Turan, M.A.; Yilmaz-Ersan, L.; Taban, N. Inductively coupled plasma optical-emission spectroscopy determination of major and minbor elements in vinegar. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 64–68. [Google Scholar]
- Abolghasemi, J.; Jahromi, M.A.F.; Sharifi, M.H.; Mazloom, Z.; Hosseini, L.; Zamani, N.; Nimrouzi, M. Effects of Zataria oxymel on obesity, insulin resistance and lipid profile: A randomized, controlled, triple-blind trial. J. Integr. Med. 2020, 18, 401–408. [Google Scholar] [CrossRef]
- Mitrou, P.; Petsiou, E.; Papakonstantinou, E.; Maratou, E.; Lamadiari, V.; Dimitriadis, P.; Spanoudi, F.; Rapis, S.A.; Dimitriadis, G. Vinegar consumption increases insulin-stimulated glucose uptake by the forearm muscle in human with type 2 diabetes. J. Diabetes Res. 2015, 1, 175204. [Google Scholar] [CrossRef]
- Bahesheti, Z.; Chan, Y.H.; Nia, H.S.; Hajihosseini, F.; Nazari, R.; Shaabani, M. Influence of apple cider vinegar on blood lipids. Life Sci. 2012, 9, 2431–2440. [Google Scholar]
- Park, J.E.; Kim, J.Y.; Kim, J.; Kim, Y.J.; Kim, M.J.; Kwon, S.W.; Kwon, O. Pomegranate vinegar beverage reduces visceral fat accumulation in association with AMPK activation in overweight women: A double-blind, randomized, and placebo-controlled trial. J. Funct. Foods 2014, 8, 274–281. [Google Scholar] [CrossRef]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y.; Chua, J.Y.H.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Long, K. Anti-obesity and anti-inflammation effects of synthetic acetic acid vinegar and nipa vinegar on high-fat-diet-induced obese mice. Sci. Rep. 2017, 7, 6664. [Google Scholar] [CrossRef]
- Bouazza, A.; Bitam, A.; Amiali, M.; Bounihi, A.; Yargui, L.; Koceir, E.A. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm. Biol. 2016, 54, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Halima, B.H.; Sonia, G.; Sarra, K.; Houda, B.J.; Fethi, B.S.; Abdallah, A. Apple cider vinegar attenuates oxidative stress and reduces the risk of obesity in high-fat-fed male Wistar rats. J. Med. Food 2018, 21, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, T.; Azuma, K.; Saimoto, H.; Ifuku, S.; Morimoto, M.; Arifuku, I.; Asaka, M.; Tsuka, T.; Imagawa, T.; Okamoto, Y. Protective effects of galacturonic acid-rich vinegar brewed from Japanese pear in a dextran sodium sulfate-induced acute colitis model. J. Funct. Foods 2013, 5, 516–523. [Google Scholar] [CrossRef]
- Golzarand, M.; Ebrahimi-Mamaghani, M.; Arefhosseini, S.R.; Asgarzadeh, A.A. Effect of processed Berberis vulgairs in apple vinegar on blood pressure and inflammatory markers in type 2 diabetic patients. Iran. J. Diabetes Metab. Disord. 2008, 7, 15–20. [Google Scholar]
- Ousaaid, D.; Bakour, M.; Laaroussi, H.; ElGhouizi, A.; Lysussi, B.; ElArabi, I. Fruit vinegar as a promising source of natural anti-inflammatory agents: An up-to-date review. DARU J. Pharm. Sci. 2023, 1, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Seyidoglu, N.; Karakci, D.; Bakir, B.; Yikmis, S. Hawthorn vinegar in health with a Focus on immune responses. Nutrients 2024, 16, 1868. [Google Scholar] [CrossRef] [PubMed]
- Elango, C.; Devaraj, S. Immunomodulatory effect of hawthorn extract in an experimental stroke model. J. Neuroinflamm. 2010, 7, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Yu, J.C.; Fu, M.F.; Wang, X.F.; Chang, X.D. Regulatory effects of hawthorn polyphenols on hyperglycemic, inflammatory, insulin resistance responses, and alleviation of aortic injury in type 2 diabetic rats. Food Res. Int. 2021, 142, 110239. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Chu, X.; Jiang, S.; Li, C.; Li, G.; He, Y.; Liu, Y.; Li, Y.; Sun, C. Vinegar decreases blood pressure by down-regulating AT1R expression via the AMPK/PGC-1α/PPARγ pathway in spontaneously hypertensive rats. Eur. J. Nutr. 2016, 55, 1245–1253. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Dong, L.; Huan, Y.; Yoshimoto, M.; Zhu, Y.; Tada, I.; Wang, X.; Zhao, S.; Zhang, F.; et al. Comprehensive metabolomics comparison of five cereal vinegars using non-targeted and chemical isotope labeling LC-MS analysis. Metabolites 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mazumder, P.M. Neuroprotective efficacy of apple cider vinegar on zinc-high fat diet-induced mono amino oxidase alteration in murine model of AD. J. Am. Coll. Nutr. 2021, 41, 658–667. [Google Scholar]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; Ennaji, H.; Lyoussi, B.; ElArabi, I. Antifungal and antibacterial activities of apple vinegar of different cultivars. Int. J. Microbiol. 2021, 1, 6087671. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, D.; Ward, M.; Skah, A.J. Antibacterial apple cider vinegar eradicates methicillin resistant Staphylococcus aureus and resistant Escherichia coli. Sci. Rep. 2021, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, N.K.; Abdelmotilib, N.M.; Salem-Bekhit, M.M.; Salem, M.M.; Singh, S.; Dandrawy, M.K. Antibacterial efficiency of apple vinegar margination on beef-borne Salmonella. Open Veter. J. 2024, 14, 274–283. [Google Scholar] [CrossRef]
- Mota, A.C.L.G.; de Castro, R.D.; de Araujo Oliveira, J.; de Oliveira Lima, E. Antifungal activity of apple cider vinegar on Candida species involved in denture stomatitis. J. Prosthodont. 2015, 24, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.R.; Treister, A.D.; Tesic, V.; Lee, K.C.; Lio, P.A. A split body trial comparing dilute bleach vs. dilute apple cider vinegar compresses for atopic dermatitis in Chicago: A pilot study. J. Dermatol. Cosmetol. 2019, 3, 22–24. [Google Scholar]
- Luu, L.A.; Flowers, R.H.; Kellams, A.L.; Zeichner, S.; Preston, D.C.; Zlotoff, B.J.; Wisniewski, J.A. Apple cider vinegar soaks [0.5%] as a treatment for atopic dermatitis do not improve skin barrier integrity. Pediatr. Dermatol. 2019, 36, 634–639. [Google Scholar] [CrossRef]
- Luu, L.A.; Flowers, R.H.; Gao, Y.; Wu, M.; Gaserino, S.; Kellams, A.L.; Preston, D.C.; Zlotoff, B.J.; Wisniewski, J.A.; Zeichner, S.L. Apple cider vinegar soaks do not alter the skin bacterial microbiome in atopic dermatitis. PLoS ONE 2021, 16, e0252272. [Google Scholar] [CrossRef]
- Yim, E.J.; Jo, S.W.; Kang, H.J.; Park, S.K.; Yu, K.Y.; Jeong, D.Y.; Park, S. Protection against osteoporosis by fermented mulberry vinegar supplementation via inhibiting osteoclastic activity in overiectomized rats and osteoclastic cells. Fermentation 2022, 8, 211. [Google Scholar] [CrossRef]
- Bang, S.I.; Kim, H.Y.; Seo, W.T.; Lee, A.Y.; Cho, E.J. Mulberry vinegar attenuates lipopolysaccharide and interferon gamma-induced inflammatory responses in C6 glial cells. J. Food Biochem. 2022, 46, e14197. [Google Scholar] [CrossRef]
- Shams, F.; Aghajani-Nasab, M.; Ramzezanpour, M.; Fatideh, R.H.; Mohammadghasemi, F. Effect of apple vinegar on folliculogenesis and ovarian kisspeptin in a high-fat diet-induced nonalcoholic fatty liver disease in rat. BMC Endocr. Disord. 2022, 22, 330. [Google Scholar] [CrossRef]
- Lucia, E.; Lidya, K.; Annisa, T. Efficacy of honey vinegar in hyperlipidemic rats (Rattus norvegicus). In Unity in Diversity and Standardization of Clinical Pharmacy Services; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 139–141. [Google Scholar]
- Faryabi, R.; Vaghaloo, M.A.; Athari, S.S.; Boskabady, M.H.; Zangii, B.M.; Kaveh, S.; Kabiri, M. Immunomodulatory effect of SINA 1.2 therapy protocol in asthmatic mice model: A combination of oxymel and sauna. Iran. J. Allergy Asthma Immunol. 2022, 1, 128–140. [Google Scholar] [CrossRef]
- Sarbaz Hoseini, Z.; Alizadeh Vaghasloo, M.; Ababzadeh, S.; Heidari, H.; Mohammadbeigi, A.; Khalaj, A.; Asghari, M. The effect of combination therapy (thermal therapy and oxymel) on insulin resistance and Langerhans islands in diabetic rats. IRCMJ 2019, 21, 90752. [Google Scholar] [CrossRef]
- Abolmaali, M.; Motevalian, M.; Mehrzadi, S.; Shojaii, A. Anticonvulsant effects of squill oxymel (a traditional formulation) in mice. Physiol. Pharm. 2022, 26, 1–6. [Google Scholar] [CrossRef]
- Nimrouzi, M.; Abolghasemi, J.; Sharifi, M.H.; Nasiri, K.; Akbari, A. Thyme oxymel by improving of inflammation, oxidative stress, dyslipidemia and homeostasis of some trace elements ameliorates obesity induced by high-fructose/fat diet in male rat. Biomed. Pharmacother. 2020, 126, 110079. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh Rishehri, S.M.; Heidaribeni, M.; Feizi, A.; Ramezani, H.; Jamali, Z.; Entezari, M.H. The effect of honey vinegar syrup on anthropometric indices and blood pressure in healthy subjects: A randomized clinical trial. Qom Univ. Med. Sci. J. 2015, 9, 21–31. [Google Scholar]
- Derakhshandeh Rishehri, S.M.; Heidari-Beni, M.; Feizi, A.; Askari, G.R.; Entezari, M.H. Effect of honey vinegar syrup on blood sugar and lipid profile in healthy subjects. Int. J. Prev. Med. 2014, 5, 1608–1614. [Google Scholar] [PubMed]
- Yikmis, S.; Ozpancar, N.; Bozkir, C.; Gol, B.G. Functional sirkencubin syrup with purple basil; bioactive properties, organoleptic acceptability, and possible effects on blood pressure. Food Sci. Technol. 2020, 40, 550–557. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Emaminejad, S.; Parvinroo, S.; Ashouri, A.; Alizadeh, I. The effect of oxymel syrup on some cardiovascular risk factors in overweight and obese people: A randomized controlled trial study. Trad. Integr. Med. 2021, 1, 204–215. [Google Scholar] [CrossRef]
- Firoozabadi, M.D.; Navabzadeh, M.; Roudsari, M.K.; Zahmatkash, M. Comparative efficacy trial of cupping and serkangabin versus conventional therapy of migraine headaches: A randomized, open-label, comparative efficacy trial. J. Res. Med. Sci. 2014, 1, 1134–1139. [Google Scholar]
- Naghibi, Z.; Rakhshandeh, H.; Jarahi, L.; Hosseini, S.M.; Yousefi, M. Evaluation of the effects of additional therapy with Berberies vulgaris oxymel in patients with refractory primary sclerosing cholangitis and primary biliary cholangitis: A quasi-experimental study. AJP 2021, 11, 154–164. [Google Scholar]
- Vahid, H.; Bonakdaran, S.; Khorasani, Z.M.; Jarahi, L.; Rakhshandeh, H.; Ghorbani, A.; Zarghi, N.; Yonsefi, H. Effect of Cappris spinose extract on metabolic parameters in patients with type-2 diabetes: Arandomized controlled trial. Endocr. Metab. Immune Disord.—Drug Targets 2019, 19, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Riah, S.M.; Meybodi, M.K.E.; Ahmadian-Attari, M.M.; Esmaeili, S.; Mokaberinejad, R. Effect of squill oxymel on knee osteoarthritis: A triple-blind, randomized, controlled clinical trial. Tradit. Integr. Med. 2023, 1, 119–129. [Google Scholar] [CrossRef]
- Nejatbaskhsh, F.; Karegar-Borzi, H.; Amin, G.; Eslaminejad, A.; Hosseini, M.; Bozorgi, M.; Gharabaghi, M.A. Squill oxymel, a traditional formulation from Drima maritima (L.) stearn, a s an add-on treatment in patients with moderate to severe persistant asthma: A pilot, triple-blind, randomized clinical trial. J. Ethnopharmacol. 2017, 196, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Araghi, M.; Eslaminejad, A.; Karegar-Bozi, H.; Mazloomzadeh, S.; Najatbakhsh, F. An add-on treatment for moderate COPD with squill-oxymel (a traditional formulation from Drimia martitima (L.) stearn): A pilot randomized triple-blinded placebo-controlled clinical trial. Evid.-Based Complement. Altern. Med. 2022, 1, 5024792. [Google Scholar]
- Prham, H.I.; Yilmaz, I.; Tekiner, I.H. Maulana and sekanjabin (oxymel): A ceremonial relationship with gastronomic and health perspectives. J. Ethn. Food 2022, 9, 12. [Google Scholar]
- Ozturk, M.; Yalcin, O.; Tekgunduz, C.; Tekgunduz, E. Origin of the effects of optical spectrum and flow behavior in determining the quality of dry fig, jujube, pomegranate, date palm and concentrated grape vinegars. Spectrochim. Acta 2022, 270, 120792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).