Role Assessment of Water-Soluble Pharmaceutical Form of Phosphatidylcholine on the Catalytic Activity of Cytochrome P450 2C9 and 2D6
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the CYP2C9 Electrochemical System
- As pharmacologically active drugs that restore the structural integrity and functional activity of biological membranes and high-density lipoproteins;
- As a transport system for hydrophobic drugs, converting them from water-insoluble to water-soluble compounds and ensuring their delivery in increased quantities to target organs by improving pharmacokinetics.
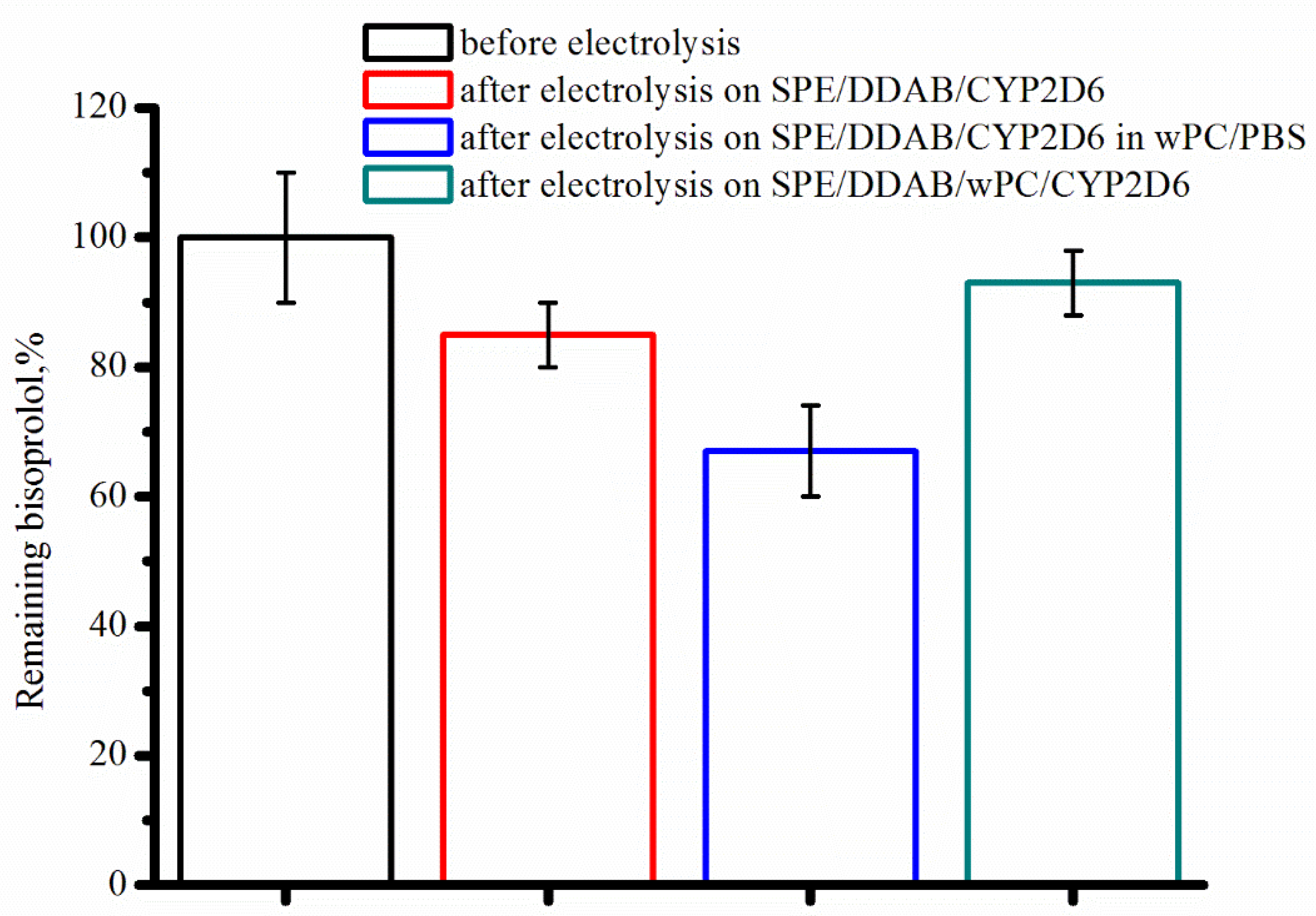
2.2. Characterization of the CYP2D6 Electrochemical System
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Zein, M.; Khazzeka, A.; El Khoury, A.; Al Zein, J.; Zoghaib, D.; Eid, A.H. Revisiting high-density lipoprotein cholesterol in cardiovascular disease: Is too much of a good thing always a good thing? Prog. Cardiovasc. Dis. 2024, 87, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bea, A.M.; González-Guerrero, A.; Cenarro, A.; Lamiquiz-Moneo, I.; Climent, E.; Jarauta, E.; Gracia-Rubio, I.; Benaiges, D.; Laclaustra, M.; Tejedor, T.; et al. Association of HDL cholesterol with all-cause and cardiovascular mortality in primary hypercholesterolemia. Atherosclerosis 2024, 400, 118617. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.; Kukharchuk, V.; Lisitsa, A.; Ponomarenko, E.; Romashova, Y.; Pleshakova, T.; Yarovaya, E.; Kutsenko, V.; Guseva, M.; Beregovykh, V.; et al. Ultra-small phospholipid nanoparticles in the treatment of combined hyperlipidemia: A randomized placebo-controlled clinical trial. Res. Pharm. Sci. (RPS) J. 2024, 19, 656–668. [Google Scholar] [CrossRef]
- Kudinov, V.A.; Torkhovskaya, T.I.; Zakharova, T.S.; Morozevich, G.E.; Artyushev, R.I.; Zubareva MYu Markin, S.S. High-density lipoprotein remodeling by phospholipid nanoparticles improves cholesterol efflux capacity and protects from atherosclerosis. Biomed. Pharmacother. 2021, 141, 111900. [Google Scholar] [CrossRef] [PubMed]
- Zubareva, M.; Kudinov, V.; Malyshev, P.; Rozhkova, T.; Markin, S.; Kukharchuk, V. Usage of a new pharmacological agent of ultra-small phospholipid nanoparticles (micelles) in the treatment of combined hyperlipidemia. Eur. Heart J. 2020, 41, 3007. [Google Scholar] [CrossRef]
- Tikhonova, E.G.; Sanzhakov, M.A.; Tereshkina, Y.A.; Kostryukova, L.V.; Khudoklinova, Y.Y.; Orlova, N.A.; Bobrova, D.V.; Ipatova, O.M. Drug Transport System Based on Phospholipid Nanoparticles: Production Technology and Characteristics. Pharmaceutics 2022, 14, 2522. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F. Human Cytochrome P450 Enzymes. In Cytochrome P450, 4th ed.; Ortiz de Montellano, P., Ed.; Springer: Cham, Switzerland, 2015; pp. 523–785. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human family 1–4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: An update. Arch. Toxicol. 2021, 95, 395–472. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Wang, Z.; Yang, W.; Huang, C.; Zhou, B.; Hu, Y.; Liu, S. Cytochromes P450 in biosensing and biosynthesis applications: Recent progress and future perspectives. Trends Anal. Chem. 2023, 158, 11679. [Google Scholar] [CrossRef]
- Mokhosoev, I.M.; Astakhov, D.V.; Terentiev, A.A.; Moldogazieva, N.T. Cytochrome P450 monooxygenase systems: Diversity and plasticity for adaptive stress response. Prog. Biophys. Mol. Biol. 2024, 193, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, V.; Paloncýová, M.; Berka, K.; Otyepka, M. Effect of lipid charge on membrane immersion of cytochrome P450 3A4. J. Phys. Chem. B 2016, 120, 11205–11213. [Google Scholar] [CrossRef] [PubMed]
- Brignac-Huber, L.M.; Reed, J.R.; Eyer, M.K.; Backes, W.L. Relationship between CYP1A2 localization and lipid microdomain formation as a function of lipid composition. Drug Metab. Dispos. 2013, 41, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhskiy, L.; Yablokov, E.; Gnedenko, O.; Burkatovskii, D.; Maslov, I.; Bogorodskiy, A.; Ershov, P.; Tsybruk, T.; Zelepuga, E.; Rutckova, T.; et al. The effect of membrane composition on the interaction between human CYP51 and its flavonoid inhibitor—Luteolin 7,3′-disulfate. BBA—Biomembranes 2024, 1866, 184286. [Google Scholar] [CrossRef]
- Huff, H.C.; Maroutsos, D.; Das, A. Lipid composition and macromolecular crowding effects on CYP2J2-mediated drug metabolism in nanodiscs. Protein Sci. 2019, 28, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Davies, M.J. Effect of crowding, compartmentalization and nanodomains on protein modification and redox signaling—Current state and future challenges. Free Radical. Biol. Med. 2023, 196, 81–92. [Google Scholar] [CrossRef]
- DasGupta, S.; Zhang, S.; Szostak, J.W. Molecular Crowding Facilitates Ribozyme-Catalyzed RNA Assembly. ACS Cent. Sci. 2023, 9, 1670–1678. [Google Scholar] [CrossRef]
- Küchler, A.; Yoshimoto, M.; Luginbühl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409–420. [Google Scholar] [CrossRef]
- Filippova, T.A.; Masamrekh, R.A.; Shumyantseva, V.V.; Khudoklinova, Y.Y.; Kuzikov, A.V. Voltammetric Analysis of (S)-O-Desmethylnaproxen for Determination of CYP2C9 Demethylase Activity. BioNanoScience 2023, 13, 1278–1288. [Google Scholar] [CrossRef]
- Horikiri, Y.; Suzuki, T.; Mizobe, M. Stereoselective metabolism of bisoprolol enantiomers in dogs and humans. Life Sci. 1998, 63, 1097–1108. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef]
- Sadeghi, S.J.; Ferrero, S.; Di Nardo, G.; Gilardi, G. Drug-drug interactions and cooperative effects detected in electrochemically driven human cytochrome P450 3A4. Bioelectrochem 2012, 86, 87–91. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Kuzikov, A.V.; Masamrekh, R.A.; Konyakhina, A.Y.; Romanenko, I.; Max, J.B.; Köhler, M.; Gilep, A.A.; Usanov, S.A.; et al. All-electrochemical nanocomposite two-electrode setup for quantification of drugs and study of their electrocatalytical conversion by cytochromes P450. Electrochim. Acta 2020, 336, 135579. [Google Scholar] [CrossRef]
- Panicco, P.; Castrignanò, S.; Sadeghi, S.J.; Nardo, G.D.; Gilardi, G. Engineered human CYP2C9 and its main polymorphic variants for bioelectrochemical measurements of catalytic response. Bioelectrochemistry 2021, 138, 107729. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Clark, D.S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Koroleva, P.I.; Shikh, E.V.; Makhova, A.A.; Kisel, M.S.; Haidukevich, I.V.; Gilep, A.A. Human Cytochrome P450 2C9 and Its Polymorphic Modifications: Electroanalysis, Catalytic Properties, and Approaches to the Regulation of Enzymatic Activity. Processes 2022, 10, 383. [Google Scholar] [CrossRef]
- Kuzikov, A.V.; Filippova, T.A.; Masamrekh, R.A.; Shumyantseva, V.V. Electroanalysis of 4′-Hydroxydiclofenac for CYP2C9 Enzymatic Assay. Electrocatalysis 2022, 13, 630–640. [Google Scholar] [CrossRef]
- Zuccarello, L.; Barbosa, C.; Todorovic, S.; Selivera, C.M. Electrocatalysis by heme enzymes—Applications in biosensing. Catalysts 2021, 11, 218. [Google Scholar] [CrossRef]
- Murray, R.W.; Bard, A.J. Electroanalytical Chemistry; Marcel Dekker: New York, NY, USA, 2001; p. 191. [Google Scholar]
- Rusling, J.F.; Wang, B.; Yun, S. Electrochemistry of redox enzymes. In Bioelectrochemistry: Fundametals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; JohnWiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 39–85. [Google Scholar]
- Kim, M.S.; Kim, J.E.; Lim, D.Y.; Huang, Z.; Chen, H.; Langfald, A.; Lubet, R.A.; Grubbs, C.J.; Dong, Z.; Bode, A.M. Naproxen induces cell-cycle arrest and apoptosis in human urinary bladder cancer cell lines and chemically induced cancers by targeting PI3K. Cancer Prev. Res. 2014, 7, 236–245. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Al-Hamad, S.S.; Lee, K.; Li, N.; Gary, B.D.; Keeton, B.; Piazza, G.A.; Abdel-Hamid, M.K. Novel non-cyclooxygenase inhibitory derivatives of naproxen for colorectal cancer chemoprevention. Med. Chem. Res. 2014, 23, 4177–4188. [Google Scholar] [CrossRef]
- Deb, J.; Majumder, J.; Bhattacharyya, S.; Jana, S.S. A novel naproxen derivative capable of displaying anti-cancer and anti-migratory properties against human breast cancer cells. BMC Cancer 2014, 14, 567. [Google Scholar] [CrossRef]
- Dong, A.N.; Ahemad, N.; Pan, Y.; Palanisamy, U.D.; Ong, C.E. Interactions of coumarin and amine ligands with six cytochrome P450 2D6 allelic variants: Molecular docking. Comp. Tox. 2023, 27, 100284. [Google Scholar] [CrossRef]
- Deodhar, M.; Turgeon, J.; Michaud, V. Contribution of CYP2D6 Functional Activity to Oxycodone Efficacy in Pain Management: Genetic Polymorphisms, Phenoconversion, and Tissue-Selective Metabolism. Pharmaceutics 2021, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Pechurskaya, T.A.; Lukashevich, O.P.; Gilep, A.A.; Usanov, S.A. Engineering, expression, and purification of “soluble” human cytochrome P45017alpha and its functional characterization. Biochemistry 2008, 73, 806–811. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The Carbon Monoxide-binding Pigment of Liver Microsomes: II. Solubilization, purification, and properties. J. Biol. Chem. 1964, 239, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 Enzymes as Drug Targets in Human Disease. Drug Metab. Dispos. 2024, 52, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, R.; Sheng, Y.; Gray, H.B.; Winkler, J.R. Tryptophan extends the life of cytochrome P450. Proc. Natl. Acad. Sci. USA 2023, 120, e2317372120. [Google Scholar] [CrossRef] [PubMed]
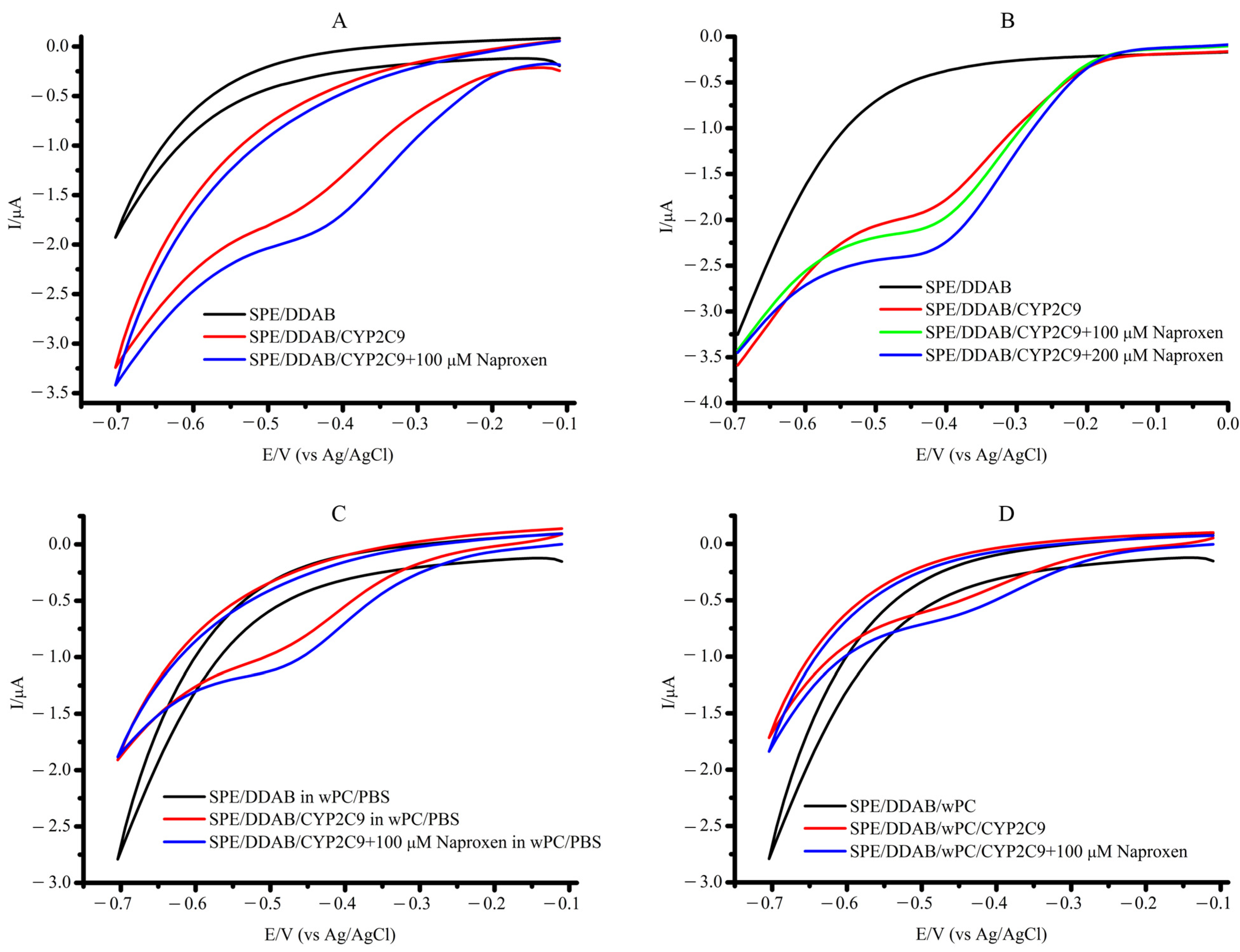
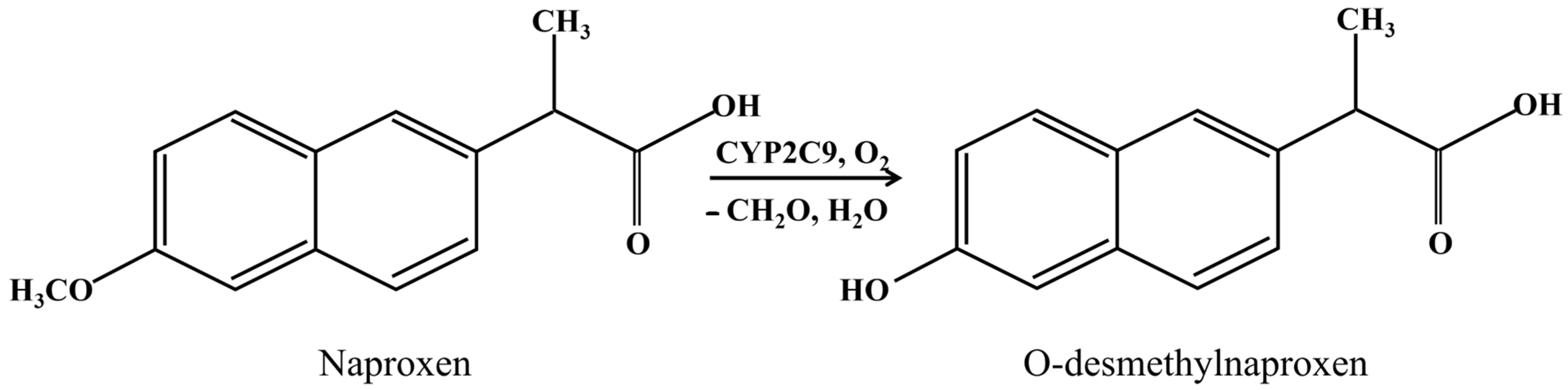
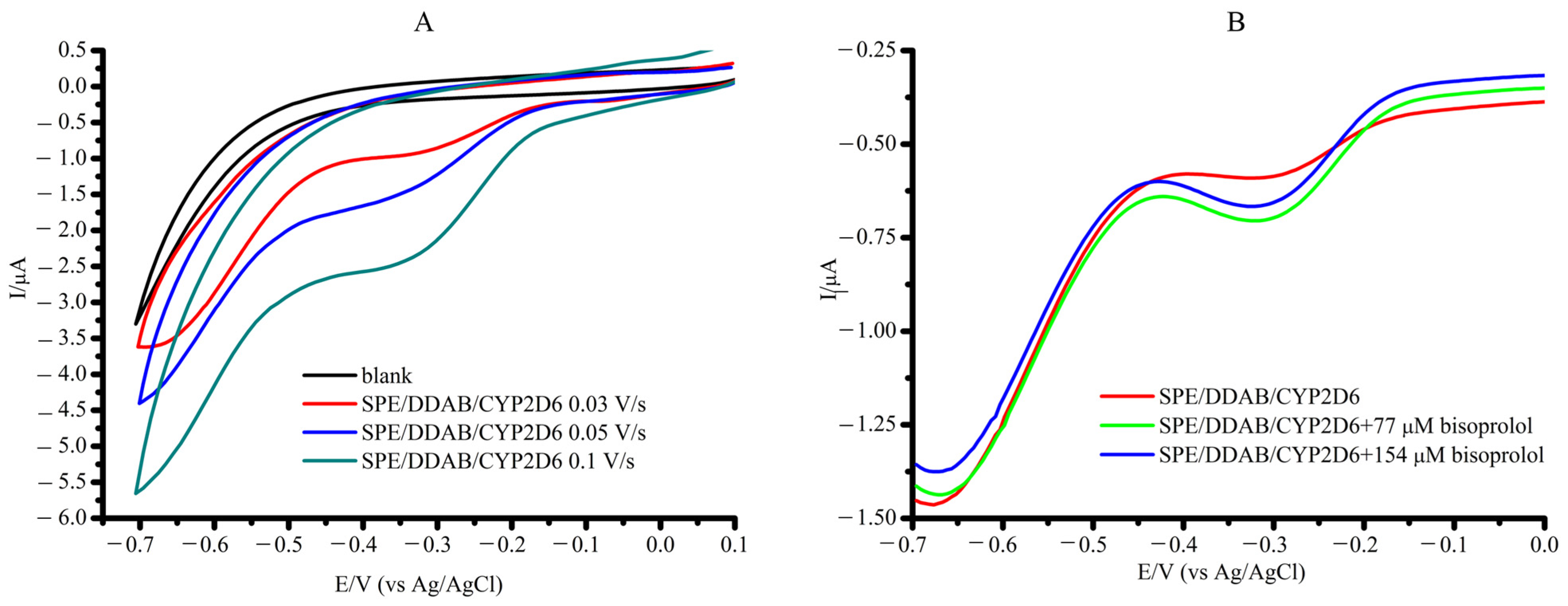
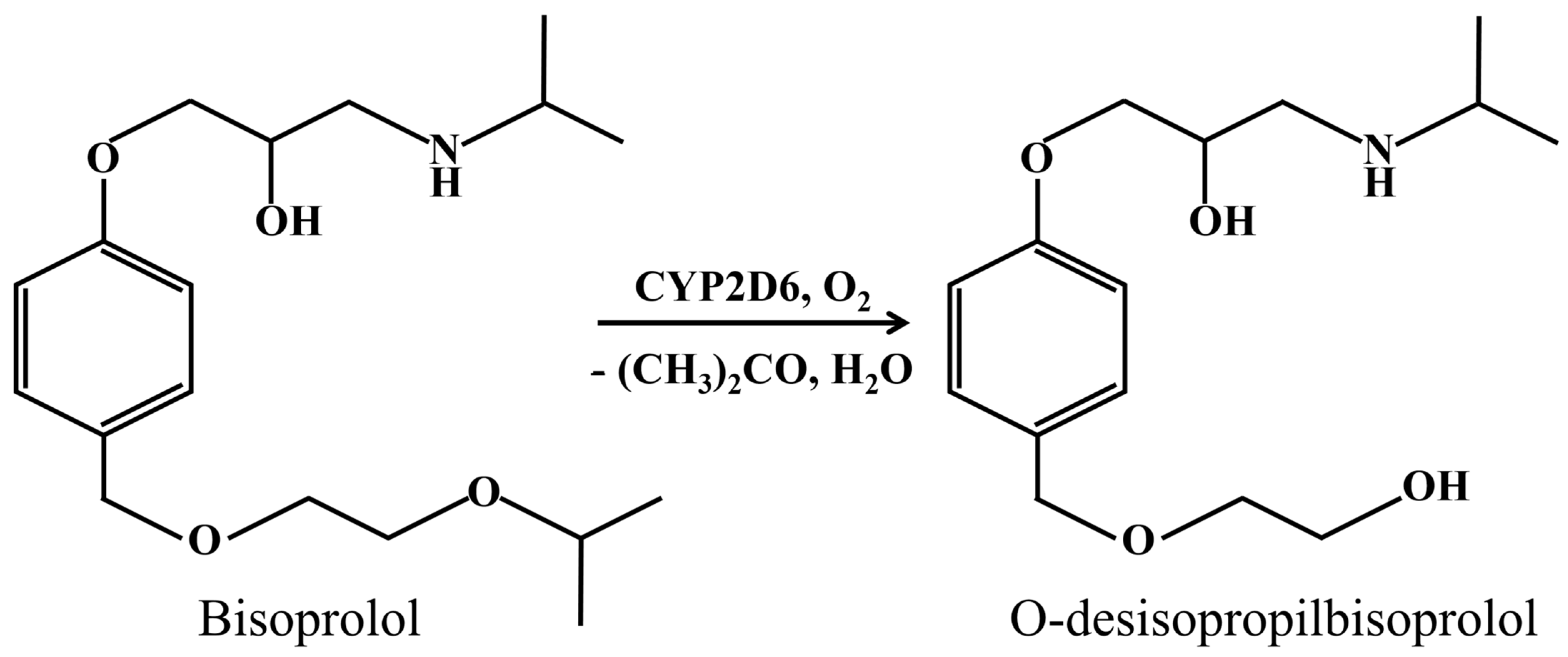
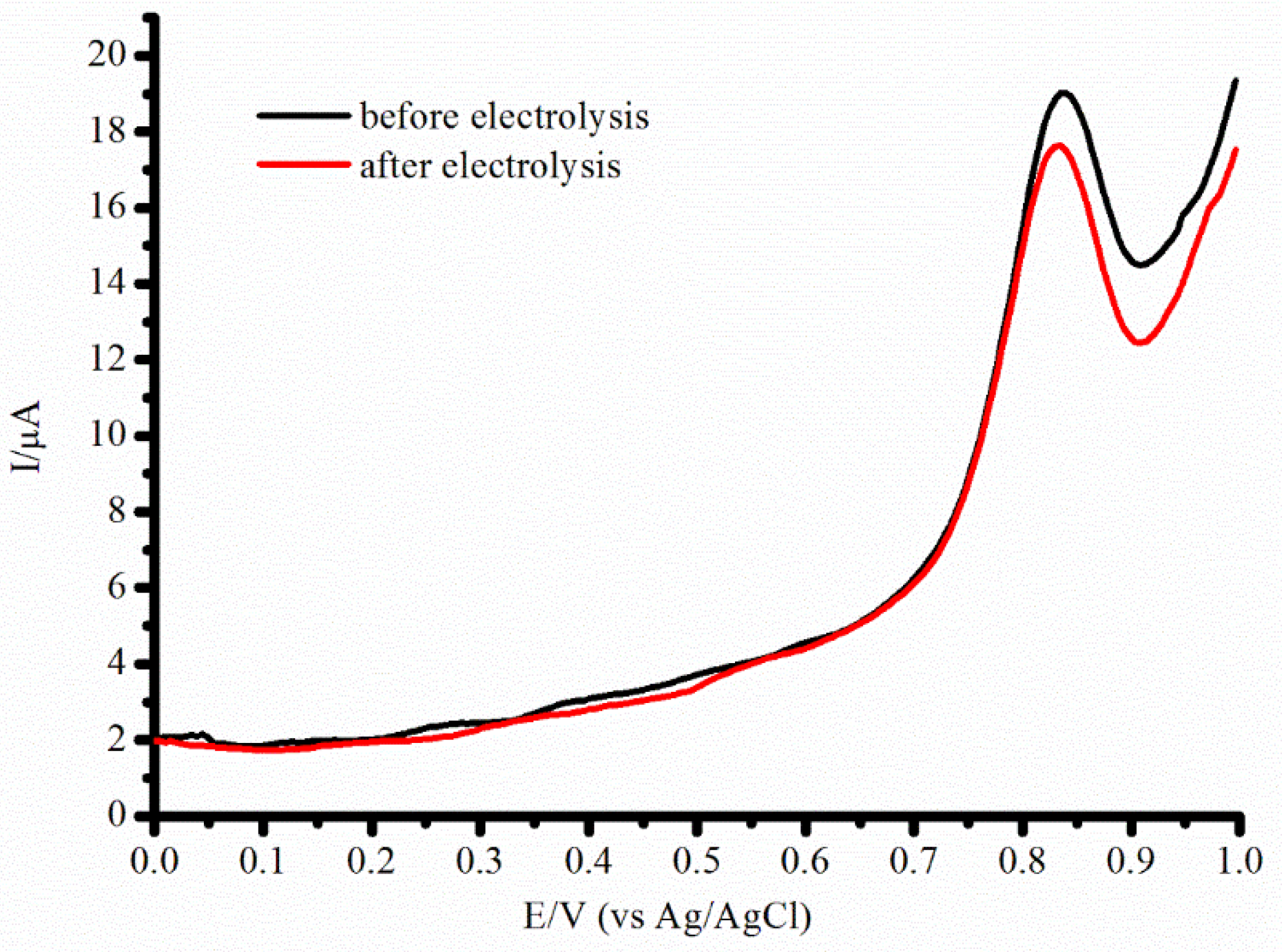
| System | Ered, V | Ecat, V | Eonset, V | Icat/Ired | Vmax, M/min | Relative Activity, % |
|---|---|---|---|---|---|---|
| SPE/DDAB/CYP2C9 | −0.495 ± 0.015 | In the presence of 100 µM naproxen | ||||
| −0.432 ± 0.024 | −0.266 ± 0.036 | 2.04 | 6.44 ± 1.17 × 10−11 | 100 ± 18 | ||
| SPE/DDAB/CYP2C9 in wPC/PBS | −0.465 ± 0.022 | −0.466 ± 0.021 | −0.288 ± 0.032 | 1.67 | 9.98 ± 0.69 × 10−11 | 155 ± 9 |
| SPE/DDAB/wPC/ CYP2C9 | −0.437 ± 0.017 | −0.422 ± 0.008 | −0.254 ± 0.024 | 1.91 | 1.04 ± 0.19 × 10−10 | 161 ± 18 |
| SPE/DDAB/ CYP2D6 | −0.373 ± 0.010 | In the presence of 77 µM Bisoprolol | ||||
| −0.383 ± 0.015 | −0.389 ± 0.015 | 1.31 | 0.40 ± 0.10 × 10−6 | 100 ± 15 | ||
| SPE/DDAB/CYP2D6 in wPC/PBS | −0.323 ± 0.009 | −0.353 ± 0.010 | −0.383 ± 0.010 | 2.61 | 0.57 ± 0.10 × 10−6 | 144 ± 8 |
| SPE/DDAB/wPC/ CYP2D6 | −0.313 ± 0.008 | −0.323 ± 0.009 | −0.365 ± 0.009 | 1.76 | 0.17 ± 0.09×10−6 | 47 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroleva, P.I.; Bulko, T.V.; Kuzikov, A.V.; Gilep, A.A.; Romashova, Y.A.; Tichonova, E.G.; Kostrukova, L.V.; Archakov, A.I.; Shumyantseva, V.V. Role Assessment of Water-Soluble Pharmaceutical Form of Phosphatidylcholine on the Catalytic Activity of Cytochrome P450 2C9 and 2D6. Int. J. Mol. Sci. 2025, 26, 4. https://doi.org/10.3390/ijms26010004
Koroleva PI, Bulko TV, Kuzikov AV, Gilep AA, Romashova YA, Tichonova EG, Kostrukova LV, Archakov AI, Shumyantseva VV. Role Assessment of Water-Soluble Pharmaceutical Form of Phosphatidylcholine on the Catalytic Activity of Cytochrome P450 2C9 and 2D6. International Journal of Molecular Sciences. 2025; 26(1):4. https://doi.org/10.3390/ijms26010004
Chicago/Turabian StyleKoroleva, Polina I., Tatiana V. Bulko, Alexey V. Kuzikov, Andrei A. Gilep, Yulia A. Romashova, Elena G. Tichonova, Lyubov V. Kostrukova, Alexander I. Archakov, and Victoria V. Shumyantseva. 2025. "Role Assessment of Water-Soluble Pharmaceutical Form of Phosphatidylcholine on the Catalytic Activity of Cytochrome P450 2C9 and 2D6" International Journal of Molecular Sciences 26, no. 1: 4. https://doi.org/10.3390/ijms26010004
APA StyleKoroleva, P. I., Bulko, T. V., Kuzikov, A. V., Gilep, A. A., Romashova, Y. A., Tichonova, E. G., Kostrukova, L. V., Archakov, A. I., & Shumyantseva, V. V. (2025). Role Assessment of Water-Soluble Pharmaceutical Form of Phosphatidylcholine on the Catalytic Activity of Cytochrome P450 2C9 and 2D6. International Journal of Molecular Sciences, 26(1), 4. https://doi.org/10.3390/ijms26010004






