Abstract
A large number of studies have reported the relationships between leptin levels and diabetes or obesity. However, the results are still controversial, and no consensus has been reached. Therefore, the purpose of the study was to collect data from various databases to perform a meta-analysis and address the inconsistencies in these studies. A systematic literature search was conducted on PubMed, Web of Science, and EBSCO for relevant available articles. The pooled standard mean difference (SMD) with 95% confidence interval (CI) was used to estimate the association by a meta-analysis. Fifteen reports with 1,388 cases and 3,536 controls were chosen for the meta-analysis. First, an increase in leptin levels in serum (SMD 0.69; 95% CI 0.36–1.02 ng/mL) and plasma (SMD 0.46; 95% CI 0.18–0.74 ng/mL) was observed in individuals with diabetes compared to controls. This increased level was also observed by gender and population. Second, statistical analysis showed that leptin levels in serum were significantly increased in individuals with obesity (SMD 1.03; 95% CI 0.72–1.34 ng/mL). This meta-analysis analyzed leptin in individuals with diabetes or obesity and emphasized the importance of monitoring serum/plasma leptin levels in patients with these diseases. However, more comprehensive studies are necessary in order to draw firm conclusions.
1. Introduction
Obesity and diabetes are global health problems and risk factors for premature death. Patients with obesity are more likely to develop type 2 diabetes mellitus (T2DM) which has become one of the most prevalent chronic diseases worldwide, affecting more than 250 million people [1]. Both obesity and diabetes have been related to several cardiovascular, neurological, and other clinical complications. In relation to this, the physiopathology of obesity and T2DM is frequently associated with alterations in lipids and glucose metabolism, insulin signaling, adipose tissue development and inflammation, among others [2].
Leptin is a peptide hormone secreted by adipocytes, with a crucial role in the regulation of body mass through a negative feedback mechanism between adipose tissue and the hypothalamus [3]. In this sense, leptin acts as an appetite-regulating factor that induces a decrease in food intake and an increase in energy consumption by inducing anorexigenic factors and suppressing neuropeptides [4]. Thus, alterations in leptin levels are highly associated with metabolic comorbidities such as obesity and diabetes.
Several studies have been carried out to determine the association between leptin levels and obesity or diabetes. For example, Huang et al. [5] conducted a study and observed elevated serum leptin levels in patients with T2DM. On the contrary, the data provided by Yin et al. [6] did not confirm this association, since they observed reduced leptin levels in patients with diabetes compared to healthy controls. Other studies suggest that leptin resistance is a key factor in the development of obesity. However, the leptin levels have been reported responding to changes in feeding and adiposity in the population. Therefore, leptin expression and circulating levels increase and reflect the degree of adiposity [7].
In view of that, understanding the role of leptin in metabolic disorders is indeed complex, and due to the discrepancies in the findings of previous studies that suggest that leptin levels could be a consequence of the pathologies and not a prognostic marker, we performed a meta-analysis to investigate the role of leptin in individuals with diabetes or obesity. We will also explore sources of heterogeneity between studies using subgroup meta-analysis and meta-regression.
2. Materials and Methods
2.1. Design
The systematic review and meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (protocol ID: CRD42023389891).
Search Strategy
A systematic search of the PubMed and EBSCO databases up to February 2023 was conducted using medical subject headings (MeSH) or free-text words. The search keywords were “leptin” and “diabetes” or “obesity”. The references cited in the studies and in review articles were also examined to identify additional reports. These studies were screened by reading their titles, abstracts, and contents (Figure 1). Only published studies with full-text reports were included.
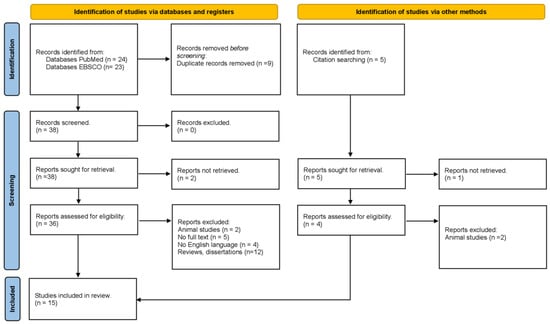
Figure 1.
PRISMA flowchart of the inclusion process.
2.2. Inclusion/Exclusion Criteria
Articles were included when they met the following criteria: (1) Patients with clinical diagnoses of obesity or with diagnoses of diabetes; (2) A control group; (3) Leptin levels of cases and controls (mean ± SD); (4) English language; (5) Articles published in peer-reviewed journals; (6) Whole information available in the article. Articles were excluded if: (1) The data were not fully available after contacting the authors by email; (2) Reviews or meta-analyses; (3) Duplicated studies.
2.3. Data Extraction
All data were extracted independently by two reviewers (YHD and MAOA) according to the inclusion and exclusion criteria. The following data were extracted from the articles: first author’s name, year of publication, country, type of biological sample, diagnostic, total leptin levels, and population size of cases and controls. Disagreements were initially discussed and then resolved by a third independent author (CATZ).
2.4. Quality Appraisal
The Newcastle–Ottawa Scale (NOS) was used to assess study quality and adapted for cross-sectional studies. The NOS scale assesses the methodological quality of three categories of studies: study selection, group comparability, and exposure. A total of nine stars can be rated according to certain criteria: for the selection category, nine stars can be awarded, a maximum of two stars for the comparability category, and three stars for the result category. The scores obtained on the NOS scale were then used to assign study quality as good (>6 stars) or poor (<6 stars).
2.5. Statistical Analyses
Comprehensive Meta-analysis version 2 software (Biostat, Englewood, NJ, USA) was used to treat quantitative data. Standardized mean differences (SMD) and their 95% confidence intervals (CIs) were calculated and represented the differences in mean leptin levels between individuals with obesity or diabetes, and healthy individuals as controls. Heterogeneity was assessed using the Q test (p < 0.05) and the I2 test (>50%).
Subgroup and meta-regression analyses were performed to address the reasons for heterogeneity. The subgroup analysis was conducted based on gender, ethnicity, and biological sample. Meta-regression was performed to investigate whether covariates (sample size and mean age) accounted for the observed effect. Sensitivity analyses were conducted to assess the stability of the pooled results. Possible publication bias was explored using Egger’s linear regression test (Egger’s test). The value of p < 0.05 was considered statistically significant.
3. Results
3.1. Literature Screening Process and Results
Figure 1 indicates the overall flow of the study selection, literature search, and number of the included studies. At the end of the search, a total of 52 articles were obtained and filtered; for example, duplicate articles were eliminated (n = 9). Reviews and dissertations were then eliminated (n = 12). Articles were then reviewed to ensure that they covered all three aspects of the inclusion criteria (diabetic patients, obese patients, and leptin levels). After a meticulous review of each article, 15 studies met the inclusion criteria [5,6,8,9,10,11,12,13,14,15,16,17,18,19,20].
3.2. Methodological Characteristics of the Studies
The characteristics of the included studies are shown in Table 1. The sample size of the 15 included studies ranged from 3536 for controls and 1388 cases (people with diabetes/obesity), with a total of 4924 participants. Of the 15 studies analyzed, only two showed data from individuals with obesity and diabetes [14,19]. The selected studies were divided into groups and subgroups; for example, in the analysis, the diabetic population was grouped into diabetics, healthy subjects, according to their ethnicity, and biological sample. On the other hand, studies with obese populations were grouped into obese, healthy subjects, and according to the biological sample used in the analysis.

Table 1.
Main characteristics of eligible studies.
3.3. Meta-Analysis of Leptin Levels in Individuals with Diabetes vs. Controls
A total of 12 studies [6,8,9,10,12,13,14,15,16,17,18,19] contained analyzable data and were included in the meta-analysis. The analysis indicated that total leptin in blood was significantly increased in individuals with diabetes, compared to controls (SMD 0.55; 95% CI 0.34–0.77; p < 0.0001; Figure 2) (Table 2). According to subgroup results, increased SMD of total leptin levels was significantly associated with sex (Male, SMD 0.34; 95% CI 0.17–0.50; p < 0.0001; Female, SMD 0.34; 95% CI 0.16–0.53; p = 0.00) and biological sample (Plasma, SMD 0.46; 95% CI 0.18–0.74; p < 0.0001; Serum, SMD 0.69; 95% CI 0.36–1.02; p = 0.00). Regarding the ethnicity, diabetic patients (Middle East) showed increased leptin levels compared to controls (SMD 0.47; 95% CI 0.21–0.74; p < 0.0001). However, the results were inversely associated with SMD difference of leptin (SMD −0.76; 95% CI −0.95–−0.57; p < 0.0001) in the Caucasian population. The heterogeneity test indicated that homogeneity was ideal for all analysis (I2 = < 50%, p > 0.05) (Table 2).
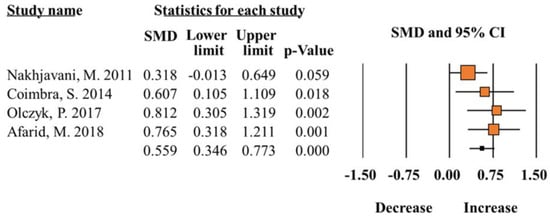
Figure 2.
Meta-analysis of leptin levels in patients with diabetes compared to healthy controls (I2 = 21.72) [12,15,16,17].

Table 2.
Analysis of leptin levels in patients with diabetes or with obesity.
3.4. Meta-Analysis of Total Leptin Levels in Individual with Obesity vs. Controls
A total of five studies [5,11,12,19,20] reported the comparison of total leptin levels of obese patients and controls. The results showed that the difference between total leptin levels of obese patients compared with controls was statistically significant (SMD 1.09; 95% CI 0.82–1.35; p = 0.00; Figure 3) (Table 2). The increase in total leptin levels was further confirmed with serum concentrations between obese patients and controls (SMD 1.03; 95% CI 0.72–1.34; p = 0.00). The heterogeneity test showed good homogeneity (I2 =< 50%, p > 0.05).
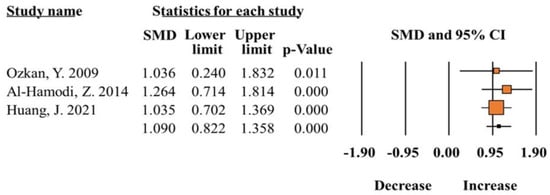
Figure 3.
Meta-analysis of leptin levels in patients with obesity compared to healthy controls (I2 = 00.00) [5,11,14].
3.5. Evaluation of Publication Bias
Publication bias was analyzed by using the Begg’s test and Egger’s test. The results showed that there was no publication bias in the results (Figure 4). The statistical results of publication bias are shown in Table 2.
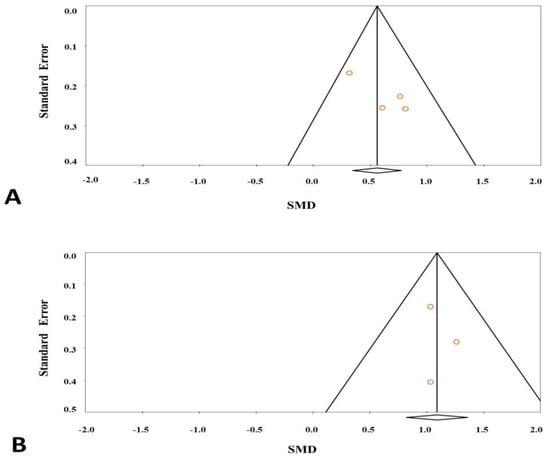
Figure 4.
Funnel plot for studies in leptin levels for subjects with diabetes (A) or obesity (B) versus control subjects.
3.6. Meta-Regression and Sensitivity Analysis
Meta-regression analysis was conducted to examine the effect of the covariates on effect size. The covariates, namely sample size (β = 0.40, p = 0.64) and mean age (β = 0.85, p = 0.77), did not have any effect on leptin levels. Sensitivity analysis was performed to examine the influence set by the individual study on the pooled SMD, by sequentially excluding each case-control study. Consistently, our data were stable and reliable in all meta-analyses.
3.7. Quality Appraisal
Our quality assessment using the NOS scale for studies is presented in Table 3. The mean total score of NOS was 7.1. Therefore, all studies were identified as of good methodological quality due to the low risk of bias (scores > 6).

Table 3.
Quality assessment of included studies according to the modified Newcastle–Ottawa Scale (NOS).
4. Discussion
Metabolic syndrome comprises a cluster of cardiometabolic risk factors that include obesity, hyperglycemia, hypertension, and dyslipidemias. The growing prevalence of metabolic syndrome is becoming a serious health problem and economic burden; moreover, the difficulty in the management of metabolic syndrome is linked to its multifactorial nature. Obesity and diabetes are strongly associated with the prognosis of other diseases such as atherosclerosis. Adipose tissue secretes adipokines that affect whole-body metabolism. Evidence suggests that adipokines such as adiponectin, leptin, and interleukin-6 can play important roles in atherosclerosis development, progression, as well as regression [7,21]. Therefore, the monitoring of blood adipokines levels in patients with these diseases is important. Here, we explore the correlation between leptin levels and diabetes or obesity through a meta-analysis. Our findings demonstrate that increased leptin levels are associated with increased risk of diabetes or obesity, compared with population control subjects.
Leptin plays an important role in the pathophysiology of metabolic syndrome. Leptin is primarily produced by white adipose tissue; it decreases insulin sensitivity and, therefore, could lead to decreased glucose tolerance. The insulin-producing β cell may be negatively affected by chronically elevated leptin levels, leading to decreased responsiveness of the receptor system in β cells, resulting in failure to suppress insulin secretion. The resulting hyperinsulinemia could, in turn, exacerbate obesity and further increase leptin levels and gene expression in white adipose tissue. Therefore, elevated leptin levels may contribute to obesity and insulin resistance as a consequence of this, in a positive feedback loop, may promote the development of metabolic disorders [22]. Kim et al. demonstrated that obesity and the chronic consumption of high-fat diets produce significant changes in the blood–brain barrier (BBB) [23], and also in brain regions that contain neurons with high metabolic demands, such as those of the arcuate core of the hypothalamus and hippocampus. The arcuate nucleus (ARH) is an important leptin-sensing site because it converts peripheral signals into neuronal responses [24].
Additionally, in the present study, when leptin levels were compared in individuals with obesity, they showed higher leptin levels (point estimate 1.09, lower-upper range: 0.82–1.35) than individuals with diabetes (point estimate 0.55, lower-upper intervals: 0.34–0.77). Although plasma leptin levels are high, impaired leptin transport to the brain among obese individuals results in leptin resistance [25]. In addition to the above, it is known that the main cause of low leptin levels is related to the deficiency of its transport through the blood–brain barrier; that is, the leptin transport mechanism is saturated or defective in people with obesity [26]. In fact, this hormone plays an important role in the negative feedback loop between adipose tissue and the brain. Therefore, the combination of high levels of leptin and serum triglycerides may be a marker of obesity “at risk” [27]. Adipose tissue dysfunction in obesity causes hypertriglyceridemia; this is due to increased hepatic production of very-low-density lipoproteins (VLDL), as well as decreased triglyceride hydrolysis [28]. Consequently, triglycerides inhibit the transport of leptin through the blood–brain barrier, so that its levels rise in the brain and redirect the use of calories toward food-seeking activities.
Racial and ethnic differences in obesity and diabetes require a thorough understanding of mediator factors, including lipid, hormone, and lipoprotein metabolism. Lipid levels are positively associated in people with metabolic disorders; for example, Pan et al. [29] and Colin et al. [30] specifically compared obesity-related risk factors. Their results suggest that increases in BMI correspond to higher odds ratios (ORs) in Chinese compared with Caucasians for hypercholesterolemia, hypertriglyceridemia, and diabetes. In fact, Gaillard et al. [31] confirmed that serum triglycerides, measured by traditional enzymatic methods, are significantly lower in African-American women than in American women with prediabetes. The above findings are consistent with our report and those of other investigators because we observed a notable difference in the analysis by populations. For example, in studies with the Caucasian population, it was observed that this significant association is related to a decrease in levels of leptin. Therefore, we can conclude that lipids play a fundamental role in metabolic disorders. This trend among the aforementioned populations can be attributed to a strong genetic–environmental interaction, as it has been intensified by rapid lifestyle changes in a growing economy.
Although there have been several studies on the relationship between leptin levels and diabetes or obesity, the conclusions have not been consistent. In recent years, attention has focused on the role of visceral adipose tissue due to the synthesis and release of a number of adipokines (leptin and adiponectin) from adipocytes. There are a number of physiological and metabolic changes associated with obesity that may contribute to increased leptin levels [7]. Due to the above, our results should be taken with caution, since the positive association observed could be a consequence of the pathologies, and not a prognostic marker. It will be necessary to verify the results and clarify the mechanism through which this may occur.
Some limitations of the present meta-analysis need to be addressed. First, although the present analysis includes 15 studies, it is relatively small compared to other meta-analyses on such conditions. We consider that the number of eligible studies included in our meta-analysis is small, so to validate our results we suggest including a larger number of studies in future research. On the other hand, the analysis of subgroups by ethnicity revealed an important association in Caucasian populations; however, there are few studies based on this population, so we could not make a comparison to see if there is any change in leptin levels in people with obesity or diabetes related to ethnicity. We suggest further studies investigating this association in Caucasian and other populations.
5. Conclusions
In conclusion, this current meta-analysis demonstrated that leptin levels were significantly upregulated in cases, compared with those in the controls. However, we suggest conducting further studies with a larger number of patients and in diverse populations to verify the results and clarify the mechanisms involved.
Author Contributions
Conceptualization, C.A.T.-Z. and T.B.G.-C.; methodology, M.d.l.Á.O.-A. and Y.H.-D.; software, A.D.G.-M.; validation, I.E.J.-R. and A.F.; formal analysis, T.B.G.-C.; investigation, M.L.L.-N.; resources, A.F.; data curation, A.F.; writing—original draft preparation, M.d.l.Á.O.-A. and Y.H.-D.; writing—review and editing, M.L.L.-N.; visualization, H.N.; supervision, C.A.T.-Z.; project administration, M.L.L.-N.; funding acquisition, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liao, Y.C.; Lim, Y.S.; Chu, P.W.; Chen, S.K. Inflammatory Milieu Induces Mitochondrial Alterations and Neuronal Activations in Hypothalamic POMC Neurons in a Time-Dependent Manner. Mol. Neurobiol. 2022, 60, 1164–1178. [Google Scholar] [CrossRef]
- Ayo-Martin, O.; García-García, J.; Hernández-Fernández, F.; Gómez-Hontanilla, M.; Gómez-Fernández, I.; Andrés-Fernández, C.; Lamas, C.; Alfaro-Martínez, J.J.; Botella, F.; Segura, T. Cerebral hemodynamics in obesity: Relationship with sex, age, and adipokines in a cohort-based study. GeroScience 2021, 43, 1465–1479. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. Leptin: A pivotal regulator of human energy homeostasis. Am. J. Clin. Nutr. 2009, 89, 980S–984S. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Huang, J.; Peng, X.; Dong, K.; Tao, J.; Yang, Y. The Association Between Insulin Resistance, Leptin, and Resistin and Diabetic Nephropathy in Type 2 Diabetes Mellitus Patients with Different Body Mass Indexes. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2357–2365. [Google Scholar] [CrossRef]
- Yin, H.; Tian, S.; Huang, R.; Cai, R.; Guo, D.; Lin, H.; Wang, J.; Wang, S. Low Plasma Leptin and High Soluble Leptin Receptor Levels Are Associated With Mild Cognitive Impairment in Type 2 Diabetic Patients. Front. Aging Neurosci. 2018, 10, 132. [Google Scholar] [CrossRef]
- Flier, J.S.; Ahima, R.S. Leptin physiology and pathophysiology: Knowns and unknowns 30 years after its discovery. J. Clin. Investig. 2024, 134, 1. [Google Scholar] [CrossRef]
- Hanaki, K.; Becker, D.J.; Arslanian, S.A. Leptin before and after insulin therapy in children with new-onset type 1 diabetes. J. Clin. Endocrinol. Metab. 1999, 84, 1524–1526. [Google Scholar] [CrossRef]
- Tatti, P.; Masselli, L.; Buonanno, A.; Di Mauro, P.; Strollo, F. Leptin levels in diabetic and nondiabetic subjects. Endocrine 2001, 15, 305–308. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Al-Rubeaan, K.; Mohieldin, M.; Al-Katari, M.; Jones, A.F.; Kumar, S. Serum leptin and its relation to anthropometric measures of obesity in pre-diabetic Saudis. Cardiovasc. Diabetol. 2007, 6, 18. [Google Scholar] [CrossRef]
- Ozkan, Y.; Aydin, S.; Donder, E.; Koca, S.S.; Aydin, S.; Ozkan, B.; Sahin, I. Effect of orlistat on the total ghrelin and leptin levels in obese patients. J. Physiol. Biochem. 2009, 65, 215–223. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Esteghamati, A.; Tarafdari, A.M.; Nikzamir, A.; Ashraf, H.; Abbasi, M. Association of plasma leptin levels and insulin resistance in diabetic women: A cross-sectional analysis in an Iranian population with different results in men and women. Gynecol. Endocrinol. 2011, 27, 14–19. [Google Scholar] [CrossRef]
- Morteza, A.; Nakhjavani, M.; Asgarani, F.; Ghaneei, A.; Esteghamati, A.; Mirmiranpour, H. The lost correlation between leptin and CRP in type 2 diabetes. Eur. Cytokine Netw. 2013, 24, 53–59. [Google Scholar] [CrossRef]
- Al-Hamodi, Z.; Al-Habori, M.; Al-Meeri, A.; Saif-Ali, R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2014, 6, 99. [Google Scholar] [CrossRef]
- Coimbra, S.; Brandão Proença, J.; Santos-Silva, A.; Neuparth, M.J. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: A close linkage with obesity and length of the disease. BioMed Res. Int. 2014, 2014, 701915. [Google Scholar] [CrossRef]
- Olczyk, P.; Koprowski, R.; Komosinska-Vassev, K.; Jura-Półtorak, A.; Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Mencner, Ł.; Telega, A.; Ivanova, D.; Olczyk, K. Adiponectin, Leptin, and Leptin Receptor in Obese Patients with Type 2 Diabetes Treated with Insulin Detemir. Molecules 2017, 22, 1274. [Google Scholar] [CrossRef]
- Afarid, M.; Attarzadeh, A.; Farvardin, M.; Ashraf, H. The Association of Serum Leptin Level and Anthropometric Measures With the Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2018, 7, 156–162. [Google Scholar]
- Bidulescu, A.; Dinh, P.C., Jr.; Sarwary, S.; Forsyth, E.; Luetke, M.C.; King, D.B.; Liu, J.; Davis, S.K.; Correa, A. Associations of leptin and adiponectin with incident type 2 diabetes and interactions among African Americans: The Jackson heart study. BMC Endocr. Disord. 2020, 20, 31. [Google Scholar] [CrossRef]
- Katsogiannos, P.; Kamble, P.G.; Pereira, M.J.; Sundbom, M.; Carlsson, P.O.; Eriksson, J.W.; Espes, D. Changes in Circulating Cytokines and Adipokines After RYGB in Patients with and without Type 2 Diabetes. Obesity 2021, 29, 535–542. [Google Scholar] [CrossRef]
- Sitar-Tǎut, A.V.; Cozma, A.; Fodor, A.; Coste, S.C.; Orasan, O.H.; Negrean, V.; Pop, D.; Sitar-Tǎut, D.A. New Insights on the Relationship between Leptin, Ghrelin, and Leptin/Ghrelin Ratio Enforced by Body Mass Index in Obesity and Diabetes. Biomedicines 2021, 9, 1657. [Google Scholar] [CrossRef]
- Grasso, P. Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline. Front. Aging Neurosci. 2022, 14, 861350. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Gong, X.; Yao, J.; He, D.; Du, W. Effect of Gender on Serum Leptin in Type 2 Diabetes Mellitus: A System Review and Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 4875799. [Google Scholar] [CrossRef]
- Kim, D.W.; Glendining, K.A.; Grattan, D.R.; Jasoni, C.L. Maternal Obesity in the Mouse Compromises the Blood-Brain Barrier in the Arcuate Nucleus of Offspring. Endocrinology 2016, 157, 2229–2242. [Google Scholar] [CrossRef]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic control of energy expenditure and thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef]
- Amorim, M.R.; Aung, O.; Mokhlesi, B.; Polotsky, V.Y. Leptin-mediated neural targets in obesity hypoventilation syndrome. Sleep 2022, 45, zsac153. [Google Scholar] [CrossRef]
- Tacad, D.K.M.; Tovar, A.P.; Richardson, C.E.; Horn, W.F.; Krishnan, G.P.; Keim, N.L.; Krishnan, S. Satiety Associated with Calorie Restriction and Time-Restricted Feeding: Peripheral Hormones. Adv. Nutr. 2022, 13, 792–820. [Google Scholar] [CrossRef]
- Milek, M.; Moulla, Y.; Kern, M.; Stroh, C.; Dietrich, A.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; Kovacs, P.; et al. Adipsin Serum Concentrations and Adipose Tissue Expression in People with Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2222. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; Hontecillas-Prieto, L.; García-Domínguez, D.J.; Zapata, F.; Palazón-Carrión, N.; Sánchez-León, M.L.; Tami, M.; Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; et al. Obesity and Risk for Lymphoma: Possible Role of Leptin. Int. J. Mol. Sci. 2022, 23, 15530. [Google Scholar] [CrossRef]
- Pan, W.H.; Flegal, K.M.; Chang, H.Y.; Yeh, W.T.; Yeh, C.J.; Lee, W.C. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am. J. Clin. Nutr. 2004, 79, 31–39. [Google Scholar] [CrossRef]
- Colin Bell, A.; Adair, L.S.; Popkin, B.M. Ethnic differences in the association between body mass index and hypertension. Am. J. Epidemiol. 2002, 155, 346–353. [Google Scholar] [CrossRef]
- Gaillard, T.; Osei, K. Ethnic differences in serum lipids and lipoproteins in overweight/obese African-American and white American women with pre-diabetes: Significance of NMR-derived lipoprotein particle concentrations and sizes. BMJ Open Diabetes Res. Care 2016, 4, e000246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).