St. John’s Wort Extract Ze 117 and Escitalopram Alter Plasma and Hippocampal Lipidome in a Rat Model of Chronic-Stress-Induced Depression
Abstract
1. Introduction
2. Results
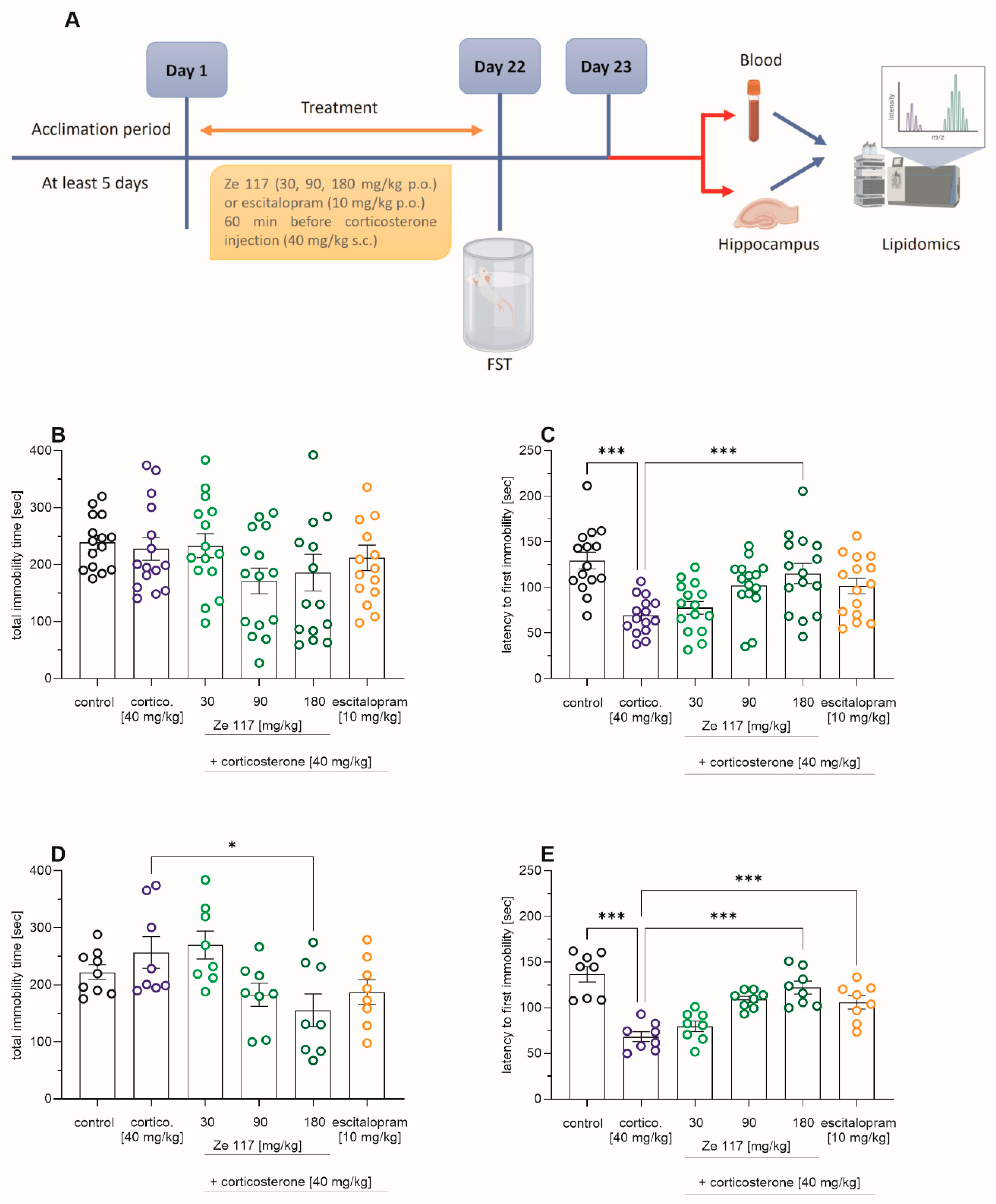
2.1. Effects of Ze 117 and Escitalopram Treatment on Corticosterone-Induced Depression-Like Behavior
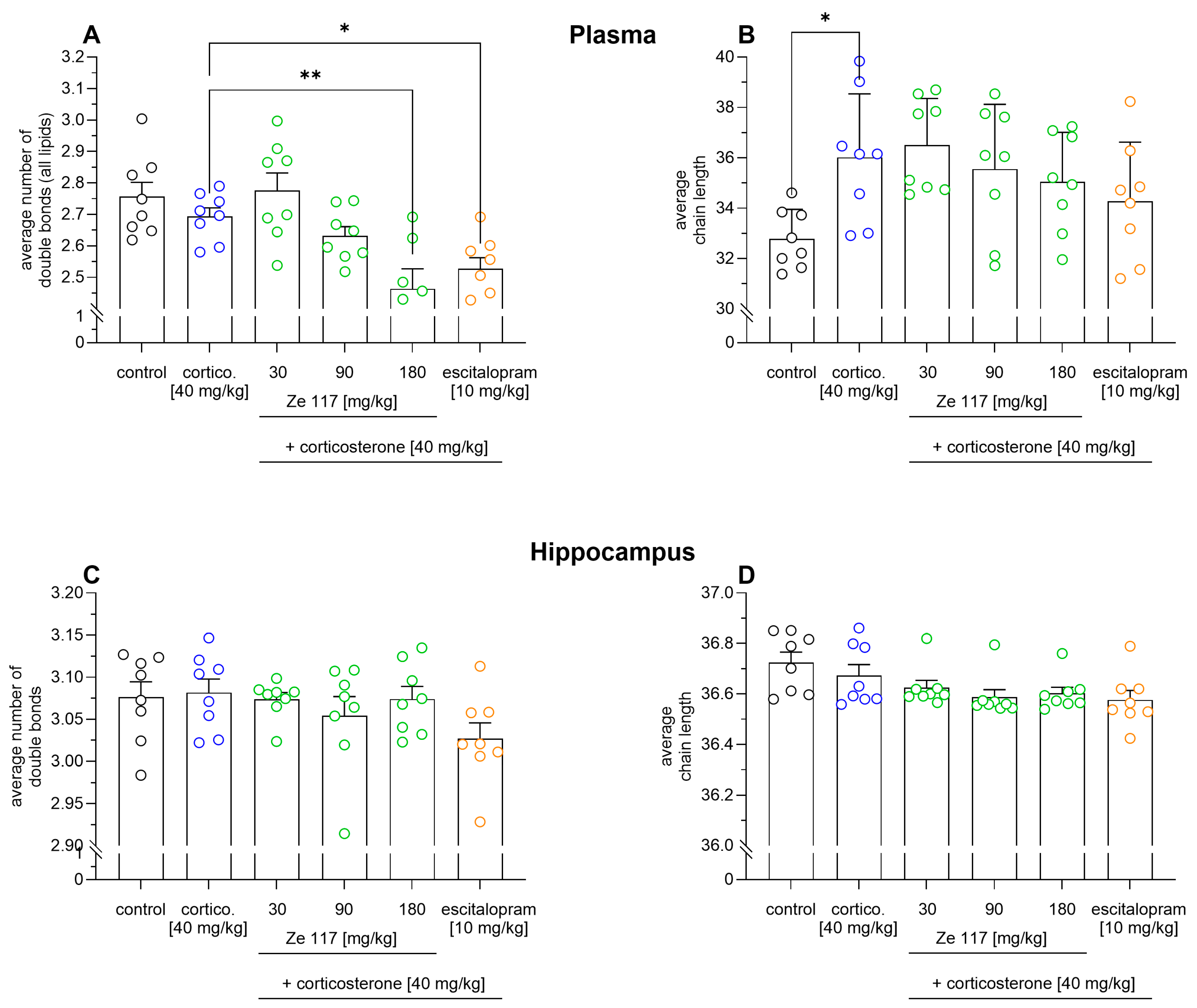
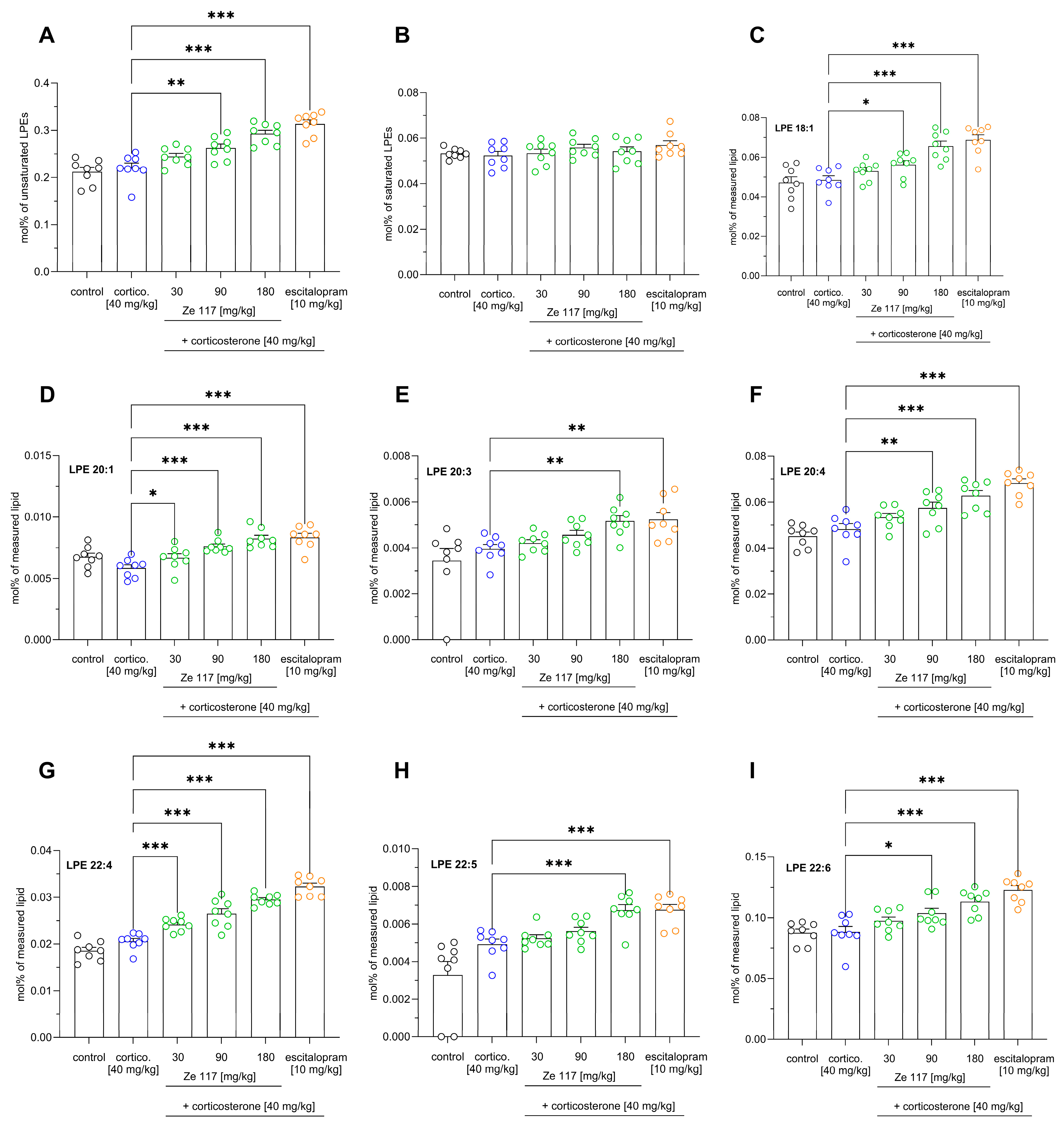
2.2. Ze 117 and Escitalopram Treatment Induces Changes in the Number of Double Bonds and Chain Lengths
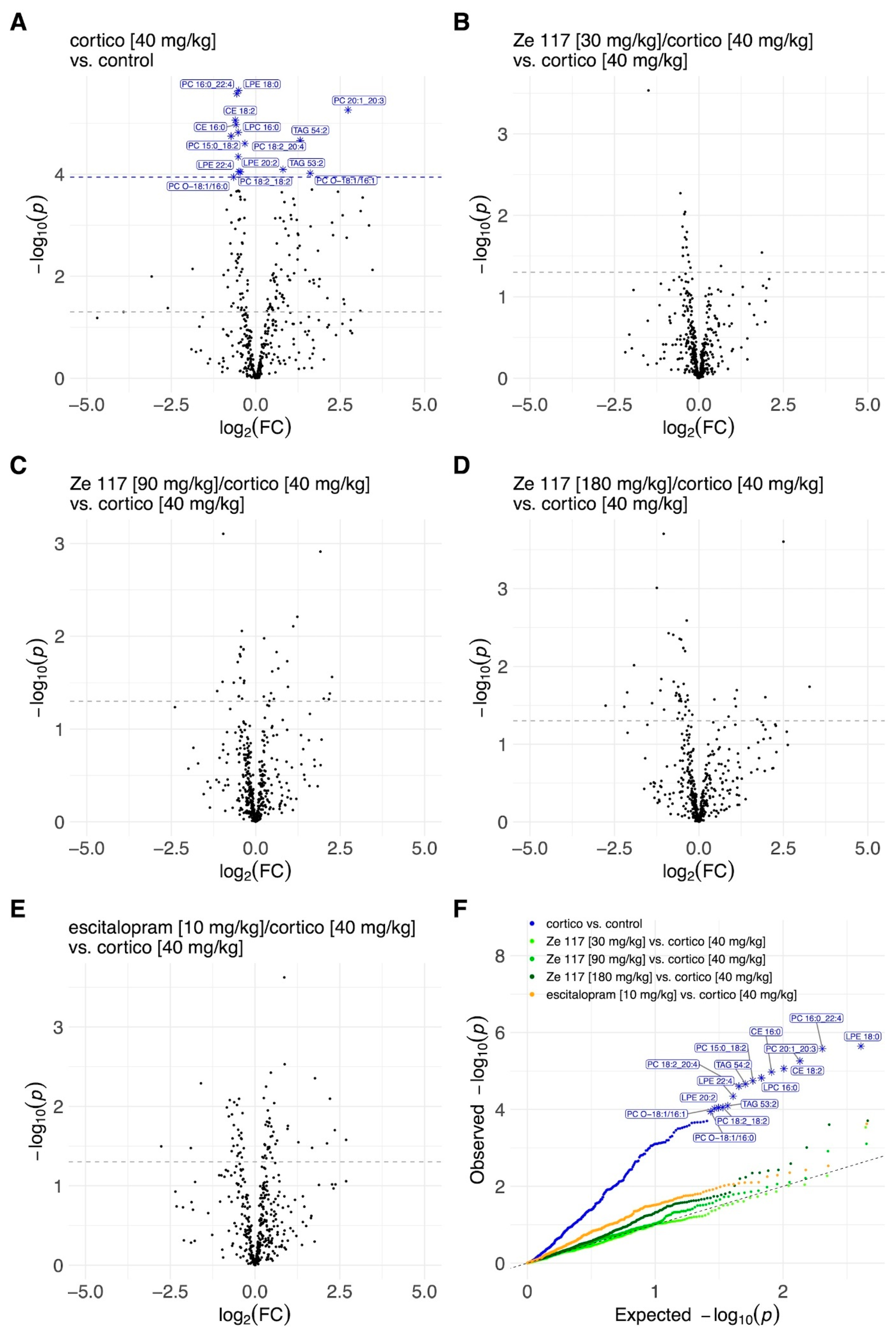
2.3. Effects of Ze 117 and Escitalopram Treatment on Key Lipid Metabolites in Plasma and Hippocampus
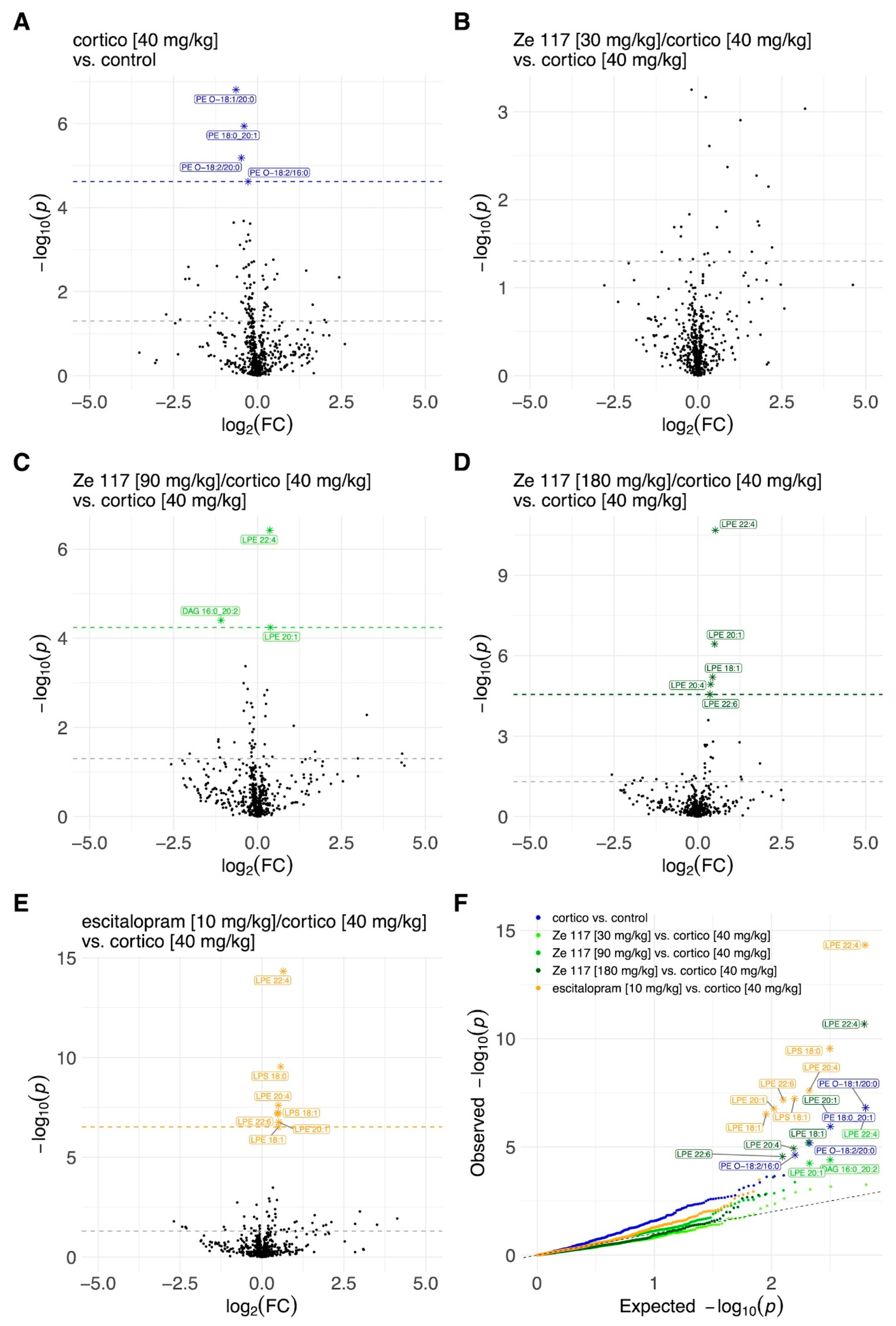
2.4. Effect of Ze 117 and Escitalopram Treatment on Specific Lipid Metabolites in Hippocampal Tissue
2.5. Correlation Analysis Between FST Behavior and Specific LPE Species in the Hippocampus of Corticosterone-Stressed Rats
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Ethical Statement
4.3. Treatments and Treatment Schedule
4.4. Modified Behavioral Despair Test
4.5. Biochemical Analysis
4.6. Sample Preparation for Lipidomics
4.7. MS Data Acquisition
4.8. Data Analysis and Post-Processing
4.9. Average Number of Double Bond and Average Chain Length of Fatty Acid Moieties
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.; Gianaros, P.J.; Manuck, S.B. A stage model of stress and disease. Perspect. Psychol. Sci. 2016, 11, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-B.; Lee, J.-H.; Park, S.-C. Park. The relationship between stress, inflammation, and depression. Biomedicines 2022, 10, 1929. [Google Scholar] [CrossRef]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hanna, J.; Ishak, W.W. Psychiatric symptoms in cushing’s syndrome: A systematic review. Innov. Clin. Neurosci. 2020, 17, 30–35. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32547845 (accessed on 12 December 2023). [PubMed]
- Santos, A.; Webb, S.M.; Resmini, E. Psychological complications of cushing’s syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 325–329. [Google Scholar] [CrossRef]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front. Psychiatry 2019, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and hpa axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Demuyser, T.; Bentea, E.; Deneyer, L.; Albertini, G.; Massie, A.; Smolders, I. Smolders. Disruption of the hpa-axis through corticosterone-release pellets induces robust depressive-like behavior and reduced bdnf levels in mice. Neurosci. Lett. 2016, 626, 119–125. [Google Scholar] [CrossRef]
- Sousa, N.; Almeida, O.F. Corticosteroids: Sculptors of the hippocampal formation. Rev. Neurosci. 2002, 13, 59–84. [Google Scholar] [CrossRef]
- Schaaf, M.J.; de Jong, J.; de Kloet, E.; Vreugdenhil, E. Downregulation of bdnf mrna and protein in the rat hippocampus by corticosterone. Brain Res. 1998, 813, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Santana, M.; Aveleira, C.; Simões, C.; Maciel, E.; Melo, T.; Santinha, D.; Oliveira, M.; Peixoto, F.; Domingues, P.; et al. Alterations in phospholipidomic profile in the brain of mouse model of depression induced by chronic unpredictable stress. Neuroscience 2014, 273, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 2005, 30, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Di Paolo, G.; et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry 2016, 21, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Bravo, F.V.; Chan, R.B.; Sousa, N.; Di Paolo, G.; Gil Oliveira, T. Differential lipid composition and regulation along the hippocampal longitudinal axis. Transl. Psychiatry 2019, 9, 144. [Google Scholar] [CrossRef]
- Pinto, B.; Conde, T.; Domingues, I.; Domingues, M.R. Adaptation of lipid profiling in depression disease and treatment: A critical review. Int. J. Mol. Sci. 2022, 23, 2032. [Google Scholar] [CrossRef]
- Walther, A.; Cannistraci, C.V.; Simons, K.; Durán, C.; Gerl, M.J.; Wehrli, S.; Kirschbaum, C. Lipidomics in major depressive disorder. Front. Psychiatry 2018, 9, 459. [Google Scholar] [CrossRef]
- Miranda, A.M.; Gil Oliveira, T. Lipids under stress–a lipidomic approach for the study of mood disorders. Bioessays 2015, 37, 1226–1235. [Google Scholar] [CrossRef]
- Skowronska-Krawczyk, D.; Budin, I. Aging membranes: Unexplored functions for lipids in the lifespan of the central nervous system. Exp. Gerontol. 2020, 131, 110817. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.; A Surma, M.; Simons, K. Organellar lipidomics—Background and perspectives. Curr. Opin. Cell Biol. 2013, 25, 406–413. [Google Scholar] [CrossRef]
- Muller, C.P.; Reichel, M.; Muhle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, H.; Bremer, S.; Häberlein, H.; Boonen, G.; Drewe, J.; Butterweck, V.; Franken, S. Impact of st. John’s wort extract ze 117 on stress induced changes in the lipidome of pbmc. Mol. Med. 2023, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, H.; Häberlein, H.; Boonen, G.; Drewe, J.; Butterweck, V.; Franken, S. Effect of st. John’s wort extract ze 117 on the lateral mobility of beta(1)-adrenergic receptors in c6 cells. Biomed. Pharmacother. 2023, 157, 114006. [Google Scholar] [CrossRef]
- Landy, K.; Rosani, A.; Estevez, R. Escitalopram. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Müller, W.; Singer, A.; Wonnemann, M.; Hafner, U.; Rolli, M.; Schäfer, C. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry 1998, 31 (Suppl. S1), 16–21. [Google Scholar] [CrossRef]
- Schmidt, M.; Butterweck, V. The mechanisms of action of st. John’s wort: An update. Wien. Med. Wochenschr. 2015, 165, 229–235. [Google Scholar] [CrossRef]
- Murray, F.; Smith, D.W.; Hutson, P.H. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008, 583, 115–127. [Google Scholar] [CrossRef]
- Xie, X.; Shen, Q.; Ma, L.; Chen, Y.; Zhao, B.; Fu, Z. Chronic corticosterone-induced depression mediates premature aging in rats. J. Affect. Disord. 2018, 229, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Castagné, V.; Porsolt, R.D.; Moser, P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur. J. Pharmacol. 2009, 616, 128–133. [Google Scholar] [CrossRef]
- Koek, W.; Sandoval, T.L.; Daws, L.C. Effects of the antidepressants desipramine and fluvoxamine on latency to immobility and duration of immobility in the forced swim test in adult male c57bl/6j mice. Behav. Pharmacol. 2018, 29, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Keksel, N.; Bussmann, H.; Unger, M.; Drewe, J.; Boonen, G.; Häberlein, H.; Franken, S. St john’s wort extract influences membrane fluidity and composition of phosphatidylcholine and phosphatidylethanolamine in rat c6 glioblastoma cells. Phytomedicine 2019, 54, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Hegger, M.; Winterhoff, H. Flavonoids of st. John’s wort reduce hpa axis function in the rat. Planta Med. 2004, 70, 1008–1011. [Google Scholar] [CrossRef]
- Butterweck, V.; Korte, B.; Winterhoff, H. Pharmacological and endocrine effects of hypericum perforatum and hypericin after repeated treatment. Pharmacopsychiatry 2001, 34 (Suppl. S1), 2–7. [Google Scholar] [CrossRef]
- Carta, M.; Lanore, F.; Rebola, N.; Szabo, Z.; Da Silva, S.V.; Lourenço, J.; Verraes, A.; Nadler, A.; Schultz, C.; Blanchet, C.; et al. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron 2014, 81, 787–799. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, P.; Zhao, X.; Zhang, Y.; Hu, C.; Li, J.; Zhao, J.; Zhou, J.; Xie, P.; Xu, G. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J. Proteome Res. 2015, 14, 2322–2330. [Google Scholar] [CrossRef]
- Hachem, M.; Nacir, H. Emerging role of phospholipids and lysophospholipids for improving brain docosahexaenoic acid as potential preventive and therapeutic strategies for neurological diseases. Int. J. Mol. Sci. 2022, 23, 3969. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Hisano, K.; Kawase, S.; Mimura, T.; Yoshida, H.; Yamada, H.; Haniu, H.; Tsukahara, T.; Kurihara, T.; Matsuda, Y.; Saito, N.; et al. Structurally different lysophosphatidylethanolamine species stimulate neurite outgrowth in cultured cortical neurons via distinct g-protein-coupled receptors and signaling cascades. Biochem. Biophys. Res. Commun. 2021, 534, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hisano, K.; Yoshida, H.; Kawase, S.; Mimura, T.; Haniu, H.; Tsukahara, T.; Kurihara, T.; Matsuda, Y.; Saito, N.; Uemura, T. Abundant oleoyl-lysophosphatidylethanolamine in brain stimulates neurite outgrowth and protects against glutamate toxicity in cultured cortical neurons. J. Biochem. 2021, 170, 327–336. [Google Scholar] [CrossRef]
- Tsukahara, T.; Hara, H.; Haniu, H.; Matsuda, Y. The combined effects of lysophospholipids against lipopolysaccharide-induced inflammation and oxidative stress in microglial cells. J. Oleo Sci. 2021, 70, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Duan, X.; Tian, X.; Shen, H.; Sun, Q.; Chen, G. Nadph oxidase: A potential target for treatment of stroke. Oxid. Med. Cell. Longev. 2016, 2016, 5026984. [Google Scholar] [CrossRef]
- Boleti, A.P.d.A.; Flores, T.M.d.O.; Moreno, S.E.; dos Anjos, L.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An overview of neurodegenerative and metabolic diseases and of biotechnological studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Schwendler, A.; Hüther, J.; Schwarzbach, H.; Schwarz, A.; Kolb, C.; Abdel-Aziz, H.; Kinscherf, R. Neurotrophic, cytoprotective, and anti-inflammatory effects of st. John’s wort extract on differentiated mouse hippocampal ht-22 neurons. Front. Pharmacol. 2017, 8, 955. [Google Scholar] [CrossRef]
- Butterweck, V. Mechanism of action of st john’s wort in depression: What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef]
- Butterweck, V.; Winterhoff, H.; Herkenham, M. St john’s wort, hypericin, and imipramine: A comparative analysis of mrna levels in brain areas involved in hpa axis control following short-term and long-term administration in normal and stressed rats. Mol. Psychiatry 2001, 6, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Ruhé, H.G.; Assies, J.; Lok, A.; Koeter, M.W.; Visser, I.; Bockting, C.L.; Schene, A.H. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology 2013, 38, 1607–1617. [Google Scholar] [CrossRef]
- Benatti, C.; Alboni, S.; Blom, J.M.; Mendlewicz, J.; Tascedda, F.; Brunello, N. Molecular changes associated with escitalopram response in a stress-based model of depression. Psychoneuroendocrinology 2018, 87, 74–82. [Google Scholar] [CrossRef]
- Makide, K.; Kitamura, H.; Sato, Y.; Okutani, M.; Aoki, J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009, 89, 135–139. [Google Scholar] [CrossRef]
- HMPC. Assessment Report on Hypericum perforatum L., Herba; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.A.; Herzog, R.; Vasilj, A.; Klose, C.; Christinat, N.; Morin-Rivron, D.; Simons, K.; Masoodi, M.; Sampaio, J.L. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Schuhmann, K.; Schwudke, D.; Sampaio, J.L.; Bornstein, S.R.; Schroeder, M.; Shevchenko, A. Lipidxplorer: A software for consensual cross-platform lipidomics. PLoS ONE 2012, 7, e29851. [Google Scholar] [CrossRef]
- Herzog, R.; Schwudke, D.; Schuhmann, K.; Sampaio, J.L.; Bornstein, S.R.; Schroeder, M.; Shevchenko, A. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011, 12, R8. [Google Scholar] [CrossRef]
- Lenth, R.V.B.; Buerkner, P.; Gine-Vazquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Estimated Marginal Means, Aka Least-Squares Means; 2023; Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 12 November 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussmann, H.; Bremer, S.; Hernier, A.M.; Drewe, J.; Häberlein, H.; Franken, S.; Freytag, V.; Boonen, G.; Butterweck, V. St. John’s Wort Extract Ze 117 and Escitalopram Alter Plasma and Hippocampal Lipidome in a Rat Model of Chronic-Stress-Induced Depression. Int. J. Mol. Sci. 2024, 25, 12667. https://doi.org/10.3390/ijms252312667
Bussmann H, Bremer S, Hernier AM, Drewe J, Häberlein H, Franken S, Freytag V, Boonen G, Butterweck V. St. John’s Wort Extract Ze 117 and Escitalopram Alter Plasma and Hippocampal Lipidome in a Rat Model of Chronic-Stress-Induced Depression. International Journal of Molecular Sciences. 2024; 25(23):12667. https://doi.org/10.3390/ijms252312667
Chicago/Turabian StyleBussmann, Hendrik, Swen Bremer, Anne Marie Hernier, Jürgen Drewe, Hanns Häberlein, Sebastian Franken, Virginie Freytag, Georg Boonen, and Veronika Butterweck. 2024. "St. John’s Wort Extract Ze 117 and Escitalopram Alter Plasma and Hippocampal Lipidome in a Rat Model of Chronic-Stress-Induced Depression" International Journal of Molecular Sciences 25, no. 23: 12667. https://doi.org/10.3390/ijms252312667
APA StyleBussmann, H., Bremer, S., Hernier, A. M., Drewe, J., Häberlein, H., Franken, S., Freytag, V., Boonen, G., & Butterweck, V. (2024). St. John’s Wort Extract Ze 117 and Escitalopram Alter Plasma and Hippocampal Lipidome in a Rat Model of Chronic-Stress-Induced Depression. International Journal of Molecular Sciences, 25(23), 12667. https://doi.org/10.3390/ijms252312667






