Is Physical Activity an Efficient Strategy to Control the Adverse Effects of Persistent Organic Pollutants in the Context of Obesity? A Narrative Review
Abstract
1. Introduction
2. Persistent Organic Pollutants (POPs)
2.1. What Are Persistent Organic Pollutants (POPs)?
2.2. Dose-Response Relationship and Synergistic Effects of POPs
2.3. POPs Nowadays
2.4. POPs as Obesogens
3. PA and POPs in the Context of Obesity
3.1. PA and Obesity
3.2. The Link between POPs, PA, Adipogenesis, and Lipid Accumulation
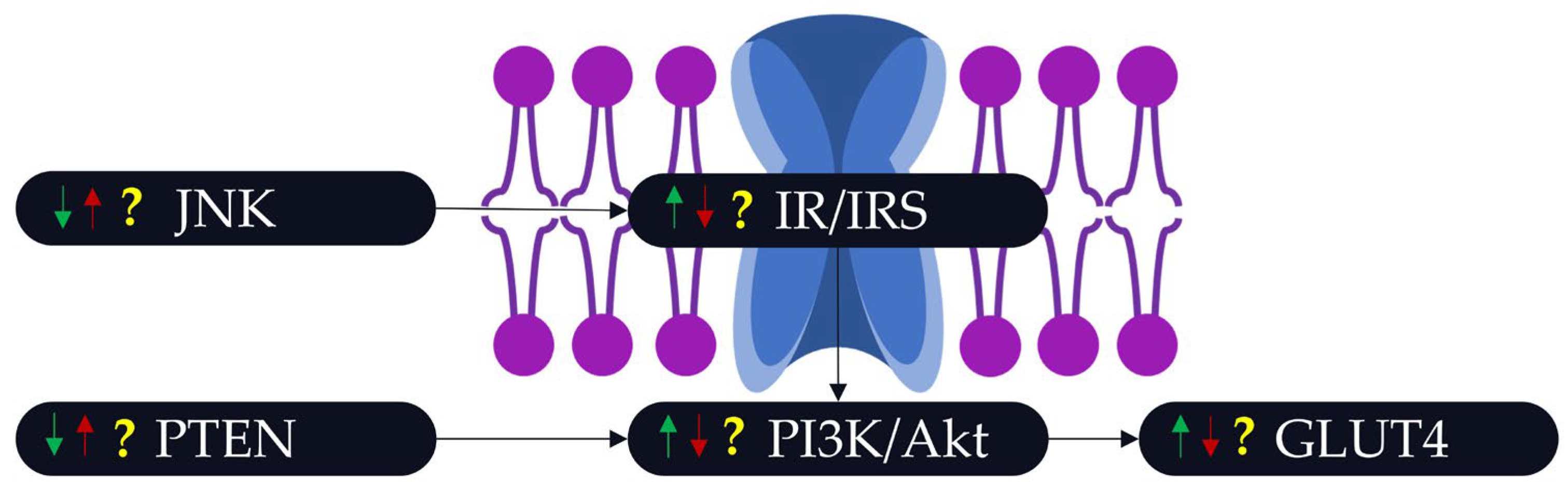
3.3. The Link between POPs, PA, and Insulin Resistance/Insulin Sensitivity
3.4. The Link between POPs, PA, and Inflammatory Function
3.5. The Link between POPs, PA, and Gut Microbiota
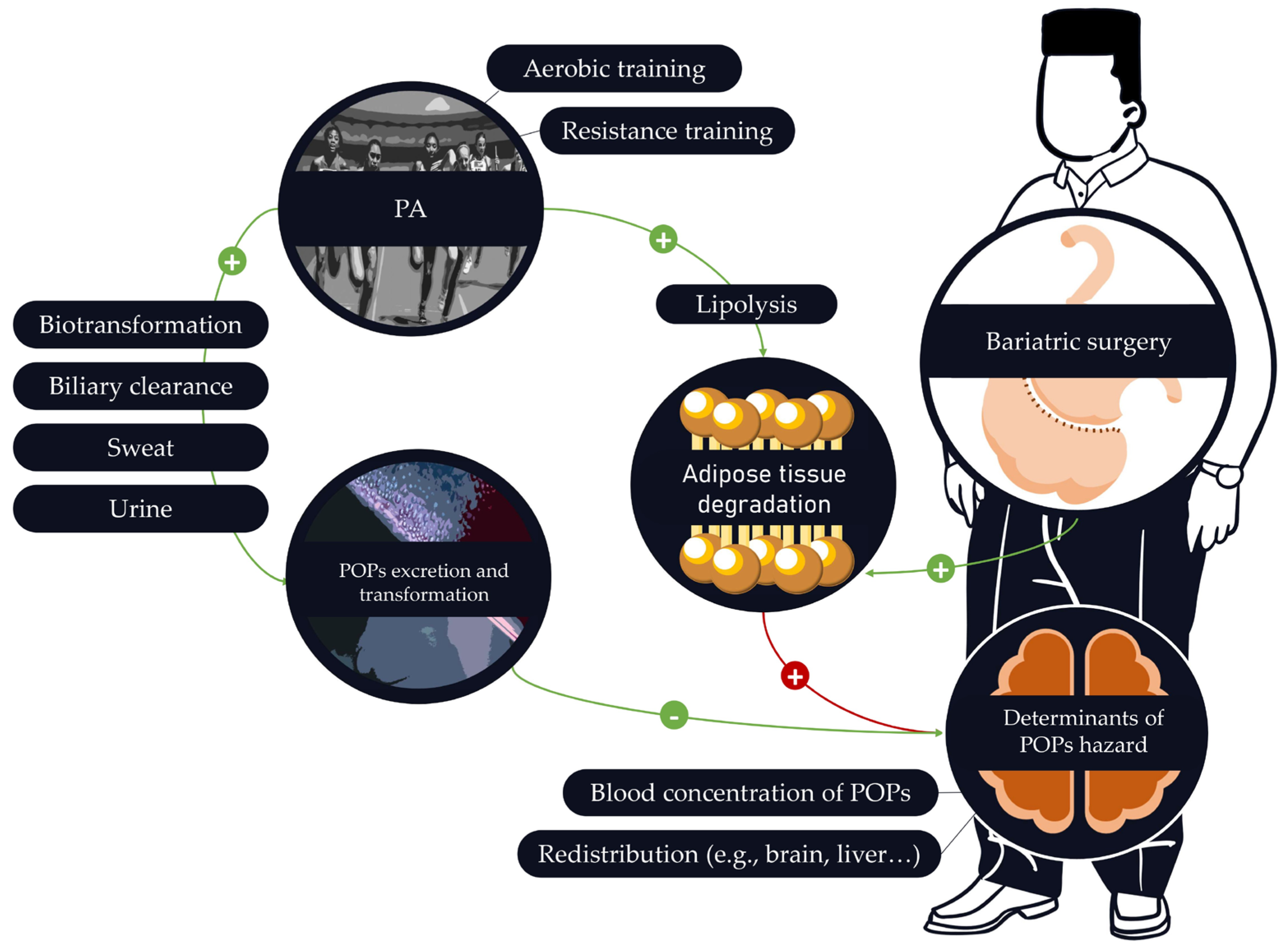
3.6. Effects of PA on the Mobilization of POPs
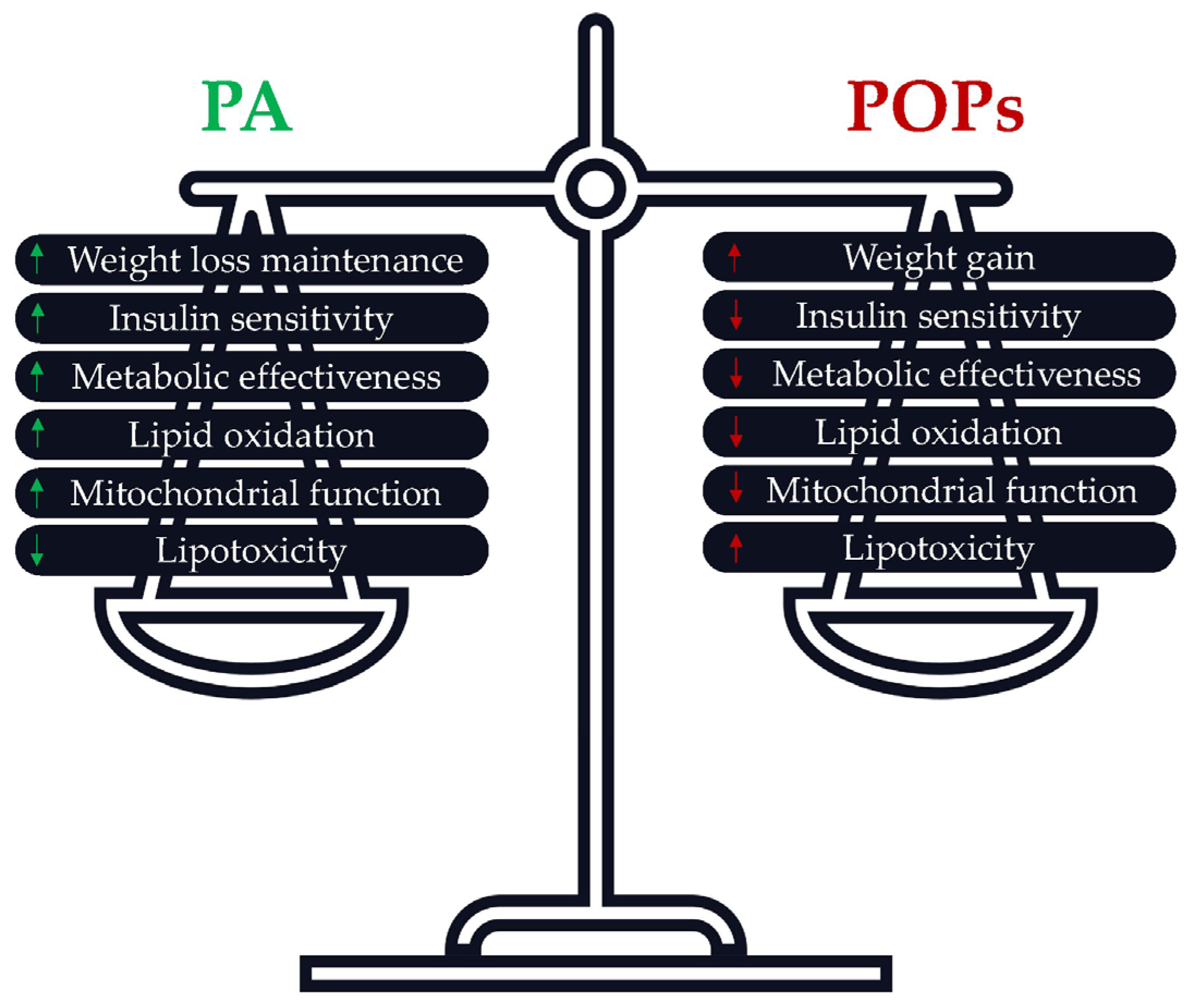
3.7. Is PA an Accurate Solution to Prevent POPs’ Adverse Effects?
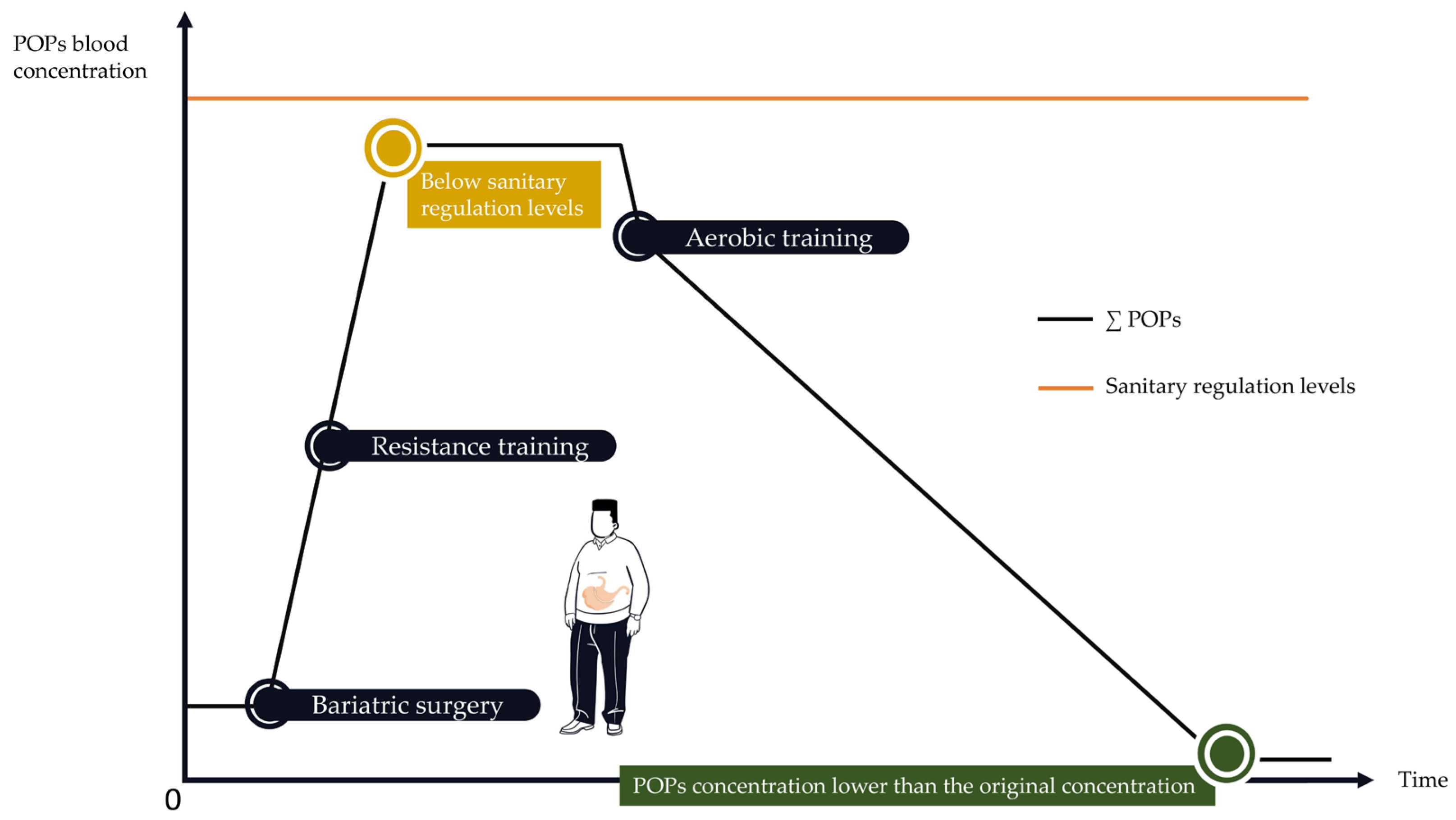
4. When Physical Activity Is Highly Recommended but Potentially Harmful: The Case of Bariatric Surgery
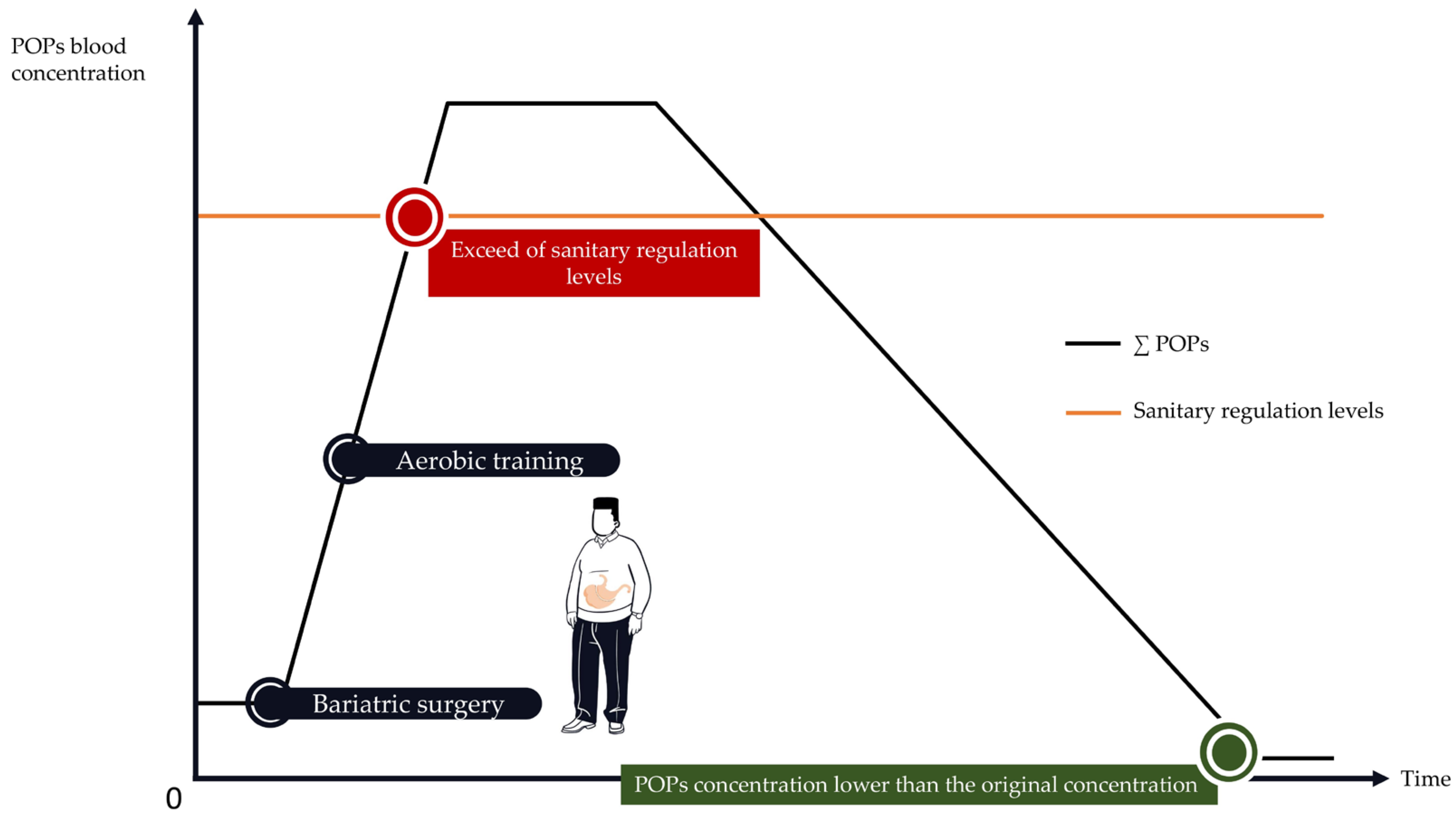
4.1. Bariatric Surgery Is Associated with an Important Increase in POP Blood Concentrations
4.2. How to Implement PA Programs to Protect against POPs’ Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSM | American College of Sports Medicine |
| AhR | Aryl hydrocarbon Receptor |
| Akt | or Protein Kinase B (PKB) |
| AMPK | AMP-Activated Protein Kinase |
| APA | Adapted Physical Activity |
| ATM/NEMO pathway | Ataxia Telangiectasia Mutated/NF-κB Essential Modifier pathway |
| BaP | Benzo(a)Pyrene |
| C/EBPα | CCAAT Enhancer-Binding Protein alpha |
| C/EBPβ | CCAAT Enhancer-Binding Protein beta |
| C/EBPδ | CCAAT Enhancer-Binding Protein delta |
| CAT | Chloramphenicol AcetylTransferase |
| CCAAT | Cytosin-Cytosin-Adenosin-Adenosin-Thymidin |
| CCL2 | Macrophage Chemoattractant Protein-1 (MCP-1) |
| CCL3 | Macrophage Inflammatory Protein-1 alpha (MIP-1α) |
| CCL4 | Macrophage Inflammatory Protein-1 beta (MIP-1β) |
| CRP | C-Reactive Protein |
| DDT | DichloroDiphenylTrichloroethane |
| EASO | European Asylum Support Office |
| eNOS | Endothelial Nitric Oxide Synthase |
| FABP | Fatty Acid-Binding Protein |
| FAS | Fatty Acid Synthase |
| FFAs | Free Fatty Acids |
| GLUT4 | Glucose Transporter type 4 |
| GR | Glucocorticoid Receptor |
| GSH-Px | Glutathion Peroxydase |
| IFNγ | Interferon-gamma |
| IκB | Inhibitor κB |
| IL-12p70 | Interleukin 12p70 |
| IL-17A | Interleukin 17A |
| IL-1Ra | Interleukin-1Ra |
| IL-1β | Interleukin 1beta |
| IL-2 | Interleukin 2 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| INSERM | Institut National de la Santé et de la Recherche Médicale |
| IRS | Insulin Receptor Substrate |
| IVM | Integrated Vector Management |
| JNK | Jun N-terminal Kinase |
| LPL | Lipoprotein Lipase |
| MDA | Malondialdehyde |
| NADPH oxidase | Nicotinamide Adenine Dinucleotide Phosphate oxidase |
| NF-κB | Nuclear Factor-Kappa B |
| OCPs | OrganoChlorine Pesticides |
| PA | Physical Activity |
| PBDEs | PolyBromoDiphénylEthers |
| PCBs | PolyChlorinated Biphenyls |
| PGC1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| PI3K | Phosphoinositide 3-Kinase |
| POPs | Persistent Organic Pollutants |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| PTEN | Phosphatase and TENsin homolog |
| ROS | Reactive Oxygen Species |
| SHBG | Sex Hormone Binding Globulin |
| SOCS3 | Supressor Of Cytokine Signaling 3 |
| SOD | SuperOxyde Dismutase |
| SREBP-1 | Sterol Regulatory Element-Binding Protein-1 |
| TNFα | Tumor Necrosis Factor alpha |
| TYK-2/STAT-3 | Tyrosine Kinase-2/Signal Transducer and Activator of Transcription 3 |
| VAT | Visceral Adipose Tissue |
References
- Cercato, C.; Fonseca, F.A. Cardiovascular Risk and Obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Ciangura, C.; Touizer, E.; Basdevant, A. Who Is Considered Obese? Why? Clinical and Therapeutic Implications. J. Visc. Surg. 2010, 147, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Hepatology. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing Causal Links between Chemical Exposures and Obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Aaseth, J.; Javorac, D.; Djordjevic, A.; Bulat, Z.; Skalny, A.; Zaitseva, I.; Aschner, M.; Tinkov, A. The Role of Persistent Organic Pollutants in Obesity: A Review of Laboratory and Epidemiological Studies. Toxics 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; Vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Bartrons, M.; Catalan, J.; Penuelas, J. Spatial and Temporal Trends of Organic Pollutants in Vegetation from Remote and Rural Areas. Sci. Rep. 2016, 6, 25446. [Google Scholar] [CrossRef]
- White, K.B.; Kalina, J.; Scheringer, M.; Přibylová, P.; Kukučka, P.; Kohoutek, J.; Prokeš, R.; Klánová, J. Temporal Trends of Persistent Organic Pollutants across Africa after a Decade of MONET Passive Air Sampling. Environ. Sci. Technol. 2021, 55, 9413–9424. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Jansen, A.; Lyche, J.L.; Polder, A.; Aaseth, J.; Skaug, M.A. Increased Blood Levels of Persistent Organic Pollutants (POP) in Obese Individuals after Weight Loss—A Review. J. Toxicol. Environ. Health Part B 2017, 20, 22–37. [Google Scholar] [CrossRef]
- Lee, D.-H.; Steffes, M.W.; Sjödin, A.; Jones, R.S.; Needham, L.L.; Jacobs, D.R. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS ONE 2011, 6, e15977. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Porta, M.; Jacobs, D.R.; Vandenberg, L.N. Chlorinated Persistent Organic Pollutants, Obesity, and Type 2 Diabetes. Endocr. Rev. 2014, 35, 557–601. [Google Scholar] [CrossRef]
- Brulport, A.; Le Corre, L.; Chagnon, M.-C. Chronic Exposure of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) Induces an Obesogenic Effect in C57BL/6J Mice Fed a High Fat Diet. Toxicology 2017, 390, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fleury, S.; Rivière, G.; Allès, B.; Kesse-Guyot, E.; Méjean, C.; Hercberg, S.; Touvier, M.; Bemrah, N. Exposure to Contaminants and Nutritional Intakes in a French Vegetarian Population. Food Chem. Toxicol. 2017, 109, 218–229. [Google Scholar] [CrossRef]
- Kahleova, H.; Tonstad, S.; Rosmus, J.; Fisar, P.; Mari, A.; Hill, M.; Pelikanova, T. The Effect of a Vegetarian versus Conventional Hypocaloric Diet on Serum Concentrations of Persistent Organic Pollutants in Patients with Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Le Garf, S.; Anty, R. Place de l’Activité Physique Adaptée dans le parcours de soins: Cas du patient présentant une stéatose hépatique non-alcoolique (NAFLD). Nutr. Clin. Métabolisme 2022, 36, 247–255. [Google Scholar] [CrossRef]
- Müller, M.J. Reports of the EASO Physical Activity Working Group: Diverse Insights, Evidence-based Recommendations, and Future Perspectives. Obes. Rev. 2021, 22, e13254. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Dias, S.; Strasser, B.; Hoffmann, G. Impact of Different Training Modalities on Anthropometric and Metabolic Characteristics in Overweight/Obese Subjects: A Systematic Review and Network Meta-Analysis. PLoS ONE 2013, 8, e82853. [Google Scholar] [CrossRef]
- Vissers, D.; Hens, W.; Taeymans, J.; Baeyens, J.-P.; Poortmans, J.; Van Gaal, L. The Effect of Exercise on Visceral Adipose Tissue in Overweight Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e56415. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Van Baak, M.A.; Pramono, A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of Different Types of Regular Exercise on Physical Fitness in Adults with Overweight or Obesity: Systematic Review and Meta-analyses. Obes. Rev. 2021, 22, e13239. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Cai, L.; Wang, Y. Effects of Physical Activity Interventions on Cognitive Performance of Overweight or Obese Children and Adolescents: A Systematic Review and Meta-Analysis. Pediatr. Res. 2021, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child. Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Wu, Z.; Bucher, N.L.R.; Farmer, S.R. Induction of Peroxisome Proliferator-Activated Receptor gamma during the Conversion of 3T3 Fibroblasts into Adipocytes Is Mediated by C/EBPbeta, C/EBPdelta, and Glucocorticoids. Mol. Cell. Biol. 1996, 16, 4128–4136. [Google Scholar] [CrossRef]
- Stewart, W.C.; Pearcy, L.A.; Floyd, Z.E.; Stephens, J.M. STAT5A Expression in Swiss 3T3 Cells Promotes Adipogenesis In Vivo in an Athymic Mice Model System. Obesity 2011, 19, 1731–1734. [Google Scholar] [CrossRef]
- González-Casanova, J.E.; Pertuz-Cruz, S.L.; Caicedo-Ortega, N.H.; Rojas-Gomez, D.M. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. BioMed Res. Int. 2020, 2020, 7453786. [Google Scholar] [CrossRef]
- Liu, W.; Qin, H.; Pan, Y.; Luo, F.; Zhang, Z. Low Concentrations of Perfluorooctane Sulfonate Repress Osteogenic and Enhance Adipogenic Differentiation of Human Mesenchymal Stem Cells. Toxicol. Appl. Pharmacol. 2019, 367, 82–91. [Google Scholar] [CrossRef]
- Wen, Q.; Xie, X.; Zhao, C.; Ren, Q.; Zhang, X.; Wei, D.; Emanuelli, B.; Du, Y. The Brominated Flame Retardant PBDE 99 Promotes Adipogenesis via Regulating Mitotic Clonal Expansion and PPARγ Expression. Sci. Total Environ. 2019, 670, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mangum, L.H.; Howell, G.E.; Chambers, J.E. Exposure to p,P′-DDE Enhances Differentiation of 3T3-L1 Preadipocytes in a Model of Sub-Optimal Differentiation. Toxicol. Lett. 2015, 238, 65–71. [Google Scholar] [CrossRef]
- Zheng, F.; Cai, Y. Concurrent Exercise Improves Insulin Resistance and Nonalcoholic Fatty Liver Disease by Upregulating PPAR-γ and Genes Involved in the Beta-Oxidation of Fatty Acids in ApoE-KO Mice Fed a High-Fat Diet. Lipids Health Dis. 2019, 18, 6. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Mo, L.; Wang, Q. The Role of Exercise Intensity on Fatty Liver in Rats. Chin. J. Physiol. 2022, 65, 301. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Mohebbi, H.; Symonds, M.E.; Karimi, P.; Akbari, A.; Tabari, E.; Faridnia, M.; Moghaddami, K. The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients 2020, 12, 925. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, H.; Jin, W.; Lee, H.J. Acute Exercise Regulates Adipogenic Gene Expression in White Adipose Tissue. Biol. Sport. 2016, 33, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Y.; Ma, Y.; Wen, D. High-Intensity Interval versus Moderate-Intensity Continuous Training: Superior Metabolic Benefits in Diet-Induced Obesity Mice. Life Sci. 2017, 191, 122–131. [Google Scholar] [CrossRef]
- Asada, M.; Rauch, A.; Shimizu, H.; Maruyama, H.; Miyaki, S.; Shibamori, M.; Kawasome, H.; Ishiyama, H.; Tuckermann, J.; Asahara, H. DNA Binding-Dependent Glucocorticoid Receptor Activity Promotes Adipogenesis via Krüppel-like Factor 15 Gene Expression. Lab. Investig. 2011, 91, 203–215. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Masse, L.; Kim, S.; Schlezinger, J.J.; Webster, T.F.; Stapleton, H.M. Characterization of Adipogenic Chemicals in Three Different Cell Culture Systems: Implications for Reproducibility Based on Cell Source and Handling. Sci. Rep. 2017, 7, 42104. [Google Scholar] [CrossRef]
- Wilson, J.; Berntsen, H.F.; Zimmer, K.E.; Verhaegen, S.; Frizzell, C.; Ropstad, E.; Connolly, L. Do Persistent Organic Pollutants Interact with the Stress Response? Individual Compounds, and Their Mixtures, Interaction with the Glucocorticoid Receptor. Toxicol. Lett. 2016, 241, 121–132. [Google Scholar] [CrossRef]
- Dassonvalle, J.; Díaz-Castro, F.; Donoso-Barraza, C.; Sepúlveda, C.; Pino-de La Fuente, F.; Pino, P.; Espinosa, A.; Chiong, M.; Llanos, M.; Troncoso, R. Moderate Aerobic Exercise Training Prevents the Augmented Hepatic Glucocorticoid Response Induced by High-Fat Diet in Mice. Int. J. Mol. Sci. 2020, 21, 7582. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Johnson, D.N.; Choudhury, R.A.; Brady, M.J. Environmental Endocrine Disruptors Promote Adipogenesis in the 3T3-L1 Cell Line through Glucocorticoid Receptor Activation. Obesity 2010, 18, 1283–1288. [Google Scholar] [CrossRef]
- Bi, P.; Shan, T.; Liu, W.; Yue, F.; Yang, X.; Liang, X.-R.; Wang, J.; Li, J.; Carlesso, N.; Liu, X.; et al. Inhibition of Notch Signaling Promotes Browning of White Adipose Tissue and Ameliorates Obesity. Nat. Med. 2014, 20, 911–918. [Google Scholar] [CrossRef]
- Derecka, M.; Gornicka, A.; Koralov, S.B.; Szczepanek, K.; Morgan, M.; Raje, V.; Sisler, J.; Zhang, Q.; Otero, D.; Cichy, J.; et al. Tyk2 and Stat3 Regulate Brown Adipose Tissue Differentiation and Obesity. Cell Metab. 2012, 16, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Duan, Y.; Zhang, X.; Yu, Q.; Di, Q.; Song, Y.; Li, P.; Gong, Y. Aryl Hydrocarbon Receptor (AhR) Regulates Adipocyte Differentiation by Assembling CRL4B Ubiquitin Ligase to Target PPARγ for Proteasomal Degradation. J. Biol. Chem. 2019, 294, 18504–18515. [Google Scholar] [CrossRef]
- Moyer, B.J.; Rojas, I.Y.; Kerley-Hamilton, J.S.; Nemani, K.V.; Trask, H.W.; Ringelberg, C.S.; Gimi, B.; Demidenko, E.; Tomlinson, C.R. Obesity and Fatty Liver Are Prevented by Inhibition of the Aryl Hydrocarbon Receptor in Both Female and Male Mice. Nutr. Res. 2017, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Rojas, I.Y.; Moyer, B.J.; Ringelberg, C.S.; Tomlinson, C.R. Reversal of Obesity and Liver Steatosis in Mice via Inhibition of Aryl Hydrocarbon Receptor and Altered Gene Expression of CYP1B1, PPARα, SCD1, and Osteopontin. Int. J. Obes. 2020, 44, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Dieudonne, M.N.; Pecquery, R.; Leneveu, M.C.; Giudicelli, Y. Opposite Effects of Androgens and Estrogens on Adipogenesis in Rat Preadipocytes: Evidence for Sex and Site-Related Specificities and Possible Involvement of Insulin-Like Growth Factor 1 Receptor and Peroxisome Proliferator-Activated Receptorγ 21. Endocrinology 2000, 141, 649–656. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, L.; Kang, Q.; Lee, H.K.; Li, D.; Chung, A.C.K.; Cai, Z. Chronic Exposure to Tetrabromodiphenyl Ether (BDE-47) Aggravates Hepatic Steatosis and Liver Fibrosis in Diet-Induced Obese Mice. J. Hazard. Mater. 2019, 378, 120766. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, L.; Zhao, T.; Ding, S.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Zhang, X.; et al. Effect of the TYK-2/STAT-3 Pathway on Lipid Accumulation Induced by Mono-2-Ethylhexyl Phthalate. Mol. Cell. Endocrinol. 2019, 484, 52–58. [Google Scholar] [CrossRef]
- Qi, W.; Xu, Q.; Xu, Y.; Wang, Z.; Yang, L.; Guo, S.; Shi, Y.; Zhao, T.; Zhou, L.; Ye, L. Effect of Notch Pathway on Lipid Accumulation Induced by Mono-2-Ethylhexyl Phthalate on 3T3-L1 Cells. Ecotoxicol. Environ. Saf. 2021, 208, 111472. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Ogier, V.; Jacquenet, S.; Notet, V.; Sibille, P.; Mejean, L.; Bihain, B.E.; Yen, F.T. Benzo[a]Pyrene Impairs Beta-Adrenergic Stimulation of Adipose Tissue Lipolysis and Causes Weight Gain in Mice. A Novel Molecular Mechanism of Toxicity for a Common Food Pollutant. FEBS J. 2006, 273, 1362–1372. [Google Scholar] [CrossRef]
- Chehade, L.; Khouri, H.; Malatier--Ségard, J.; Caron, A.; Mauger, J.-F.; Chapados, N.A.; Aguer, C. Acute Exposure to Environmentally Relevant Levels of DDT Alters Muscle Mitochondrial Function in Vivo in Rats but Not in Vitro in L6 Myotubes: A Pilot Study. Toxicol. Rep. 2022, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Gourronc, F.A.; Perdew, G.H.; Robertson, L.W.; Klingelhutz, A.J. PCB126 Blocks the Thermogenic Beiging Response of Adipocytes. Env. Sci. Pollut. Res. 2020, 27, 8897–8904. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.Q.; Berntsen, H.F.; Verhaegen, S.; Ropstad, E.; Connolly, L.; Igout, A.; Muller, M.; Scippo, M.L. A Mixture of Persistent Organic Pollutants Relevant for Human Exposure Inhibits the Transactivation Activity of the Aryl Hydrocarbon Receptor in Vitro. Environ. Pollut. 2019, 254, 113098. [Google Scholar] [CrossRef]
- Shanaki, M.; Khosravi, M.; Khoshdooni-Farahani, A.; Dadashi, A.; Heydari, M.F.; Delfan, M.; Jafary, H.; Gorgani-Firuzjaee, S. High-Intensity Interval Training Reversed High-Fat Diet–Induced M1-Macrophage Polarization in Rat Adipose Tissue via Inhibition of NOTCH Signaling. JIR 2020, 13, 165–174. [Google Scholar] [CrossRef]
- Trenerry, M.K.; Carey, K.A.; Ward, A.C.; Farnfield, M.M.; Cameron-Smith, D. Exercise-Induced Activation of STAT3 Signaling Is Increased with Age. Rejuven. Res. 2008, 11, 717–724. [Google Scholar] [CrossRef]
- Lázaro, I.; Ferré, R.; Plana, N.; Aragonès, G.; Girona, J.; Merino, J.; Heras, M.; Cabré, A.; Masana, L. Cambios de estilo de vida disminuyen las concentraciones plasmáticas de FABP4 en pacientes con riesgo cardiovascular. Rev. Española De. Cardiol. 2012, 65, 152–157. [Google Scholar] [CrossRef]
- Fiebig, R.G.; Hollander, J.M.; Ney, D.; Boileau, R.; Jeffery, E.; Li Ji, L. Training Down-Regulates Fatty Acid Synthase and Body Fat in Obese Zucker Rats. Med. Sci. Sports Exerc. 2002, 34, 1106–1114. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-de Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise Training Improves Mitochondrial Respiration and Is Associated with an Altered Intramuscular Phospholipid Signature in Women with Obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Hey-Mogensen, M.; Højlund, K.; Vind, B.F.; Wang, L.; Dela, F.; Beck-Nielsen, H.; Fernström, M.; Sahlin, K. Effect of Physical Training on Mitochondrial Respiration and Reactive Oxygen Species Release in Skeletal Muscle in Patients with Obesity and Type 2 Diabetes. Diabetologia 2010, 53, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Gashi, A.; Gontarev, S.; Zivkovic, V.; Gjorgovski, I.; Azemi, A. The Effect of Aerobic Physical Activity in Adrenaline Level in White Laboratory Rats. Med. Arch. 2020, 74, 84. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Control of Energy Expenditure in Humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef]
- Bonfante, I.L.P.; Duft, R.G.; Mateus, K.C.D.S.; Trombeta, J.C.D.S.; Finardi, E.A.R.; Ramkrapes, A.P.B.; Brunelli, D.T.; Mori, M.A.D.S.; Chacon-Mikahil, M.P.T.; Velloso, L.A.; et al. Acute/Chronic Responses of Combined Training on Serum Pro-Thermogenic/Anti-Inflammatory Inducers and Its Relation With Fed and Fasting State in Overweight Type 2 Diabetic Individuals. Front. Physiol. 2022, 12, 736244. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.; Joisten, N.; Walzik, D.; Koliamitra, C.; Schoser, D.; Bloch, W.; Zimmer, P. Acute Exercise Impacts AhR and PD-1 Levels of CD8+ T-Cells—Exploratory Results from a Randomized Cross-over Trial Comparing Endurance versus Resistance Exercise. Eur. J. Appl. Physiol. 2021, 121, 637–644. [Google Scholar] [CrossRef]
- Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.-F.; Fleisch, A.F.; Lin, P.-I.D.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; et al. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Adiposity. JAMA Netw. Open 2018, 1, e181493. [Google Scholar] [CrossRef]
- McComb, J.; Mills, I.G.; Muller, M.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Human Blood-Based Exposure Levels of Persistent Organic Pollutant (POP) Mixtures Antagonise Androgen Receptor Transactivation and Translocation. Environ. Int. 2019, 132, 105083. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kim, C.-Y.; Lee, H.-K.; Kang, I.-H.; Kim, M.-G.; Jung, K.-K.; Kwon, Y.-K.; Nam, H.-S.; Hong, S.-K.; Kim, H.-S.; et al. Estrogenic Activity of Persistent Organic Pollutants and Parabens Based on the Stably Transfected Human Estrogen Receptor-α Transcriptional Activation Assay (OECD TG 455). Toxicol. Res. 2011, 27, 181–184. [Google Scholar] [CrossRef]
- Horii, N.; Sato, K.; Mesaki, N.; Iemitsu, M. Increased Muscular 5α-Dihydrotestosterone in Response to Resistance Training Relates to Skeletal Muscle Mass and Glucose Metabolism in Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0165689. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of Physical Activity on Sex Hormones in Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef]
- Malone, J.I.; Hansen, B.C. Does Obesity Cause Type 2 Diabetes Mellitus (T2DM)? Or Is It the Opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. JNK at the Crossroad of Obesity, Insulin Resistance, and Cell Stress Response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Jaiswal, R.; Paliwal, S.; Dwivedi, J.; Sharma, S. An Insight into PI3k/Akt Pathway and Associated Protein–Protein Interactions in Metabolic Syndrome: A Recent Update. J. Cell. Biochem. 2023, 124, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, M.K.; Kwak, M.K.; Kim, D.; Hong, E.-G. Association between Thyroid Hormones and Insulin Resistance Indices Based on the Korean National Health and Nutrition Examination Survey. Sci. Rep. 2021, 11, 21738. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Lee, E.; Moon, T.C.; Jung, H.; Lin, C.X.; Nam, K.-S.; Baek, S.H.; Min, H.-K.; Chang, H.W. Expression of Cyclooxygenase-2 and Pro-Inflammatory Cytokines Induced by 2,2′,4,4′,5,5′-Hexachlorobiphenyl (PCB 153) in Human Mast Cells Requires NF-kB Activation. Biol. Pharm. Bull. 2002, 25, 1165–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, W.; Zhu, X.; Wang, L.; Ding, G.; Wang, X.; Sheng, Y.; Lv, S.; Yu, J.; Liu, J.; Duan, Y. 2,3’,4,4’,5-Pentachlorobiphenyl Induced Thyroid Dysfunction by Increasing Mitochondrial Oxidative Stress. J. Toxicol. Sci. 2022, 47, 555–565. [Google Scholar] [CrossRef]
- Du, G.; Sun, J.; Zhang, Y. Perfluorooctanoic Acid Impaired Glucose Homeostasis through Affecting Adipose AKT Pathway. Cytotechnology 2018, 70, 479–487. [Google Scholar] [CrossRef]
- Rajesh, P.; Sathish, S.; Srinivasan, C.; Selvaraj, J.; Balasubramanian, K. Diethyl Hexyl Phthalate (DEHP) Is Associated with Insulin Resistance in Adipose Tissue of Male Rat: Protective Role of Antioxidant Vitamins (C & E). J. Cell. Biochem. 2013, 114, 558–569. [Google Scholar] [CrossRef]
- Boosani, C.S.; Agrawal, D.K. PTEN Modulators: A Patent Review. Expert Opin. Ther. Pat. 2013, 23, 569–580. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, K.-S.; Jacobs, D.R.; Lee, D.-H. Persistent Organic Pollutants in Adipose Tissue Should Be Considered in Obesity Research: Obesity and Persistent Organic Pollutants. Obes. Rev. 2017, 18, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Marinho, R.; Moura, L.P.D.; Rodrigues, B.D.A.; Pauli, L.S.S.; Silva, A.S.R.D.; Ropelle, E.C.C.; Souza, C.T.D.; Cintra, D.E.C.; Ropelle, E.R.; Pauli, J.R. Effects of Different Intensities of Physical Exercise on Insulin Sensitivity and Protein Kinase B/Akt Activity in Skeletal Muscle of Obese Mice. Einstein 2014, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Heled, Y.; Shapiro, Y.; Shani, Y.; Moran, D.S.; Langzam, L.; Braiman, L.; Sampson, S.R.; Meyerovitch, J. Physical Exercise Enhances Protein Kinase C δ Activity and Insulin Receptor Tyrosine Phosphorylation in Diabetes-Prone Psammomys Obesus. Metabolism 2003, 52, 1028–1033. [Google Scholar] [CrossRef]
- Luciano, E.; Carneiro, E.; Carvalho, C.; Carvalheira, J.; Peres, S.; Reis, M.; Saad, M.; Boschero, A.; Velloso, L. Endurance Training Improves Responsiveness to Insulin and Modulates Insulin Signal Transduction through the Phosphatidylinositol 3-Kinase/Akt-1 Pathway. Eur. J. Endocrinol. 2002, 147, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chibalin, A.V.; Yu, M.; Ryder, J.W.; Song, X.M.; Galuska, D.; Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Exercise-Induced Changes in Expression and Activity of Proteins Involved in Insulin Signal Transduction in Skeletal Muscle: Differential Effects on Insulin-Receptor Substrates 1 and 2. Proc. Natl. Acad. Sci. USA 2000, 97, 38–43. [Google Scholar] [CrossRef]
- Muñoz, V.R.; Gaspar, R.C.; Severino, M.B.; Macêdo, A.P.A.; Simabuco, F.M.; Ropelle, E.R.; Cintra, D.E.; da Silva, A.S.R.; Kim, Y.-B.; Pauli, J.R. Exercise Counterbalances Rho/ROCK2 Signaling Impairment in the Skeletal Muscle and Ameliorates Insulin Sensitivity in Obese Mice. Front. Immunol. 2021, 12, 702025. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhu, Y.; Yan, H.; Lu, Y. Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. Int. J. Environ. Res. Public Health 2021, 18, 13141. [Google Scholar] [CrossRef]
- Braun, J.M.; Papandonatos, G.D.; Li, N.; Sears, C.G.; Buckley, J.P.; Cecil, K.M.; Chen, A.; Eaton, C.B.; Kalkwarf, H.J.; Kelsey, K.T.; et al. Physical Activity Modifies the Relation between Gestational Perfluorooctanoic Acid Exposure and Adolescent Cardiometabolic Risk. Environ. Res. 2022, 214, 114021. [Google Scholar] [CrossRef]
- Berdanier, C.D.; De Dennis, S.K. Effect of Exercise on the Responses of Rats to DDT. J. Toxicol. Environ. Health 1977, 2, 651–656. [Google Scholar] [CrossRef]
- Cardenas, A.; Hivert, M.-F.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Lin, P.-I.D.; Fleisch, A.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; et al. Associations of Perfluoroalkyl and Polyfluoroalkyl Substances With Incident Diabetes and Microvascular Disease. Diabetes Care 2019, 42, 1824–1832. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Phillips, M.C.; Dheer, R.; Santaolalla, R.; Davies, J.M.; Burgueño, J.; Lang, J.K.; Toborek, M.; Abreu, M.T. Intestinal Exposure to PCB 153 Induces Inflammation via the ATM/NEMO Pathway. Toxicol. Appl. Pharmacol. 2018, 339, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Petriello, M.C.; Zhu, B.; Hennig, B. PCB 126 Induces Monocyte/Macrophage Polarization and Inflammation through AhR and NF-κB Pathways. Toxicol. Appl. Pharmacol. 2019, 367, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, P.; Findlay, C.S.; Robidoux, M.A.; Haman, F.; Blais, J.M.; Tremblay, A.; Springthorpe, S.; Pal, S.; Seabert, T.; Krümmel, E.M.; et al. Dysregulation of Cytokine Response in Canadian First Nations Communities: Is There an Association with Persistent Organic Pollutant Levels? PLoS ONE 2012, 7, e39931. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Y.; Wang, J.; Zhao, Y.; Li, K.; Jing, Y.; Zhang, X.; Liu, Q.; Geng, X.; Li, G.; et al. Long-Term Persistent Organic Pollutants Exposure Induced Telomere Dysfunction and Senescence-Associated Secretary Phenotype. J. Gerontol. Ser. A 2018, 73, 1027–1035. [Google Scholar] [CrossRef]
- Kim, K.-S.; Hong, N.-S.; Jacobs, D.R.; Lee, D.-H. Interaction Between Persistent Organic Pollutants and C-Reactive Protein in Estimating Insulin Resistance Among Non-Diabetic Adults. J. Prev. Med. Public. Health 2012, 45, 62–69. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Amero, P.; Santoro, A.; Monnolo, A.; Simeoli, R.; Di Guida, F.; Mattace Raso, G.; Meli, R. Polychlorinated Biphenyls (PCB 101, PCB 153 and PCB 180) Alter Leptin Signaling and Lipid Metabolism in Differentiated 3T3-L1 Adipocytes. Toxicol. Appl. Pharmacol. 2014, 279, 401–408. [Google Scholar] [CrossRef]
- Lim, J.; Jee, S.H. Association between Serum Levels of Adiponectin and Polychlorinated Biphenyls in Korean Men and Women. Endocrine 2015, 48, 211–217. [Google Scholar] [CrossRef]
- Amine, Z.E.; Mauger, J.-F.; Imbeault, P. CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-Exposed to PCB126 and Hypoxia. Cells 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, H.F.; Fonnum, F.; Walaas, S.I.; Bogen, I.L. Low-Chlorinated Non-Dioxin-like Polychlorinated Biphenyls Present in Blood and Breast Milk Induce Higher Levels of Reactive Oxygen Species in Neutrophil Granulocytes than High-Chlorinated Congeners. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 588–597. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive Oxygen Species (ROS) in Macrophage Activation and Function in Diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Ye, L. The Effect of Di-2-Ethylhexyl Phthalate on Inflammation and Lipid Metabolic Disorder in Rats. Ecotoxicol. Environ. Saf. 2019, 170, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nichols, R.G.; Correll, J.; Murray, I.A.; Tanaka, N.; Smith, P.B.; Hubbard, T.D.; Sebastian, A.; Albert, I.; Hatzakis, E.; et al. Persistent Organic Pollutants Modify Gut Microbiota–Host Metabolic Homeostasis in Mice Through Aryl Hydrocarbon Receptor Activation. Environ. Health Perspect. 2015, 123, 679–688. [Google Scholar] [CrossRef]
- Sriwijitkamol, A.; Christ-Roberts, C.; Berria, R.; Eagan, P.; Pratipanawatr, T.; DeFronzo, R.A.; Mandarino, L.J.; Musi, N. Reversal by Exercise Training. Diabetes 2006, 55, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, X. Anti-Inflammatory Effect of Exercise Training through Reducing Inflammasome Activation-Related Inflammatory Cytokine Levels in Overweight/Obese Populations: A Systematic Review and Meta-Analysis. Complement. Ther. Clin. Pract. 2022, 49, 101656. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, F.; Lebaka, V.R.; Mohammed, A.; Ji, L.; Zhang, Y.; Korivi, M. Calorie Restriction With Exercise Intervention Improves Inflammatory Response in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 754731. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef]
- Peng, J.; Yin, L.; Wang, X. Central and Peripheral Leptin Resistance in Obesity and Improvements of Exercise. Horm. Behav. 2021, 133, 105006. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of Leptin and Adiponectin in Insulin Resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Maillard, F.; Vazeille, E.; Barnich, N.; Sirvent, P.; Otero, Y.F.; Combaret, L.; Madeuf, E.; Sourdrille, A.; Delcros, G.; et al. Tissue-Specific Oxidative Stress Modulation by Exercise: A Comparison between MICT and HIIT in an Obese Rat Model. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Li, K.; Zhu, X.; Wang, Y.; Zheng, S.; Dong, G. Effect of Aerobic Exercise Intervention on DDT Degradation and Oxidative Stress in Rats. Saudi J. Biol. Sci. 2017, 24, 664–671. [Google Scholar] [CrossRef]
- Murphy, M.O.; Petriello, M.C.; Han, S.G.; Sunkara, M.; Morris, A.J.; Esser, K.; Hennig, B. Exercise Protects against PCB-Induced Inflammation and Associated Cardiovascular Risk Factors. Environ. Sci. Pollut. Res. 2016, 23, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Domazet, S.L.; Jensen, T.K.; Wedderkopp, N.; Nielsen, F.; Andersen, L.B.; Grøntved, A. Exposure to Perfluoroalkylated Substances (PFAS) in Relation to Fitness, Physical Activity, and Adipokine Levels in Childhood: The European Youth Heart Study. Environ. Res. 2020, 191, 110110. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.R.; Keylock, K.T.; Cromwell, H.C.; Meserve, L.A. Exercise Influences the Impact of Polychlorinated Biphenyl Exposure on Immune Function. PLoS ONE 2020, 15, e0237705. [Google Scholar] [CrossRef]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise Attenuates PCB-Induced Changes in the Mouse Gut Microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota in Obesity. WJG 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Snedeker, S.M.; Hay, A.G. Do Interactions Between Gut Ecology and Environmental Chemicals Contribute to Obesity and Diabetes? Environ. Health Perspect. 2012, 120, 332–339. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Agarwal, M.; Hoffman, J.; Ngo Tenlep, S.Y.; Santarossa, S.; Pearson, K.J.; Sitarik, A.R.; Cassidy-Bushrow, A.E.; Petriello, M.C. Maternal Polychlorinated Biphenyl 126 (PCB 126) Exposure Modulates Offspring Gut Microbiota Irrespective of Diet and Exercise. Reprod. Toxicol. 2023, 118, 108384. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Shin, J.-Y.; Kim, S.-A.; Jacobs, D.R.; Lee, D.-H. Can Habitual Exercise Help Reduce Serum Concentrations of Lipophilic Chemical Mixtures? Association between Physical Activity and Persistent Organic Pollutants. Diabetes Metab. J. 2020, 44, 764–774. [Google Scholar] [CrossRef]
- Minh, T.B.; Watanabe, M.; Tanabe, S.; Yamada, T.; Hata, J.; Watanabe, S. Specific Accumulation and Elimination Kinetics of Tris(4-Chlorophenyl)Methane, Tris(4-Chlorophenyl)Methanol, and Other Persistent Organochlorines in Humans from Japan. Environ. Health Perspect. 2001, 109, 927–935. [Google Scholar] [CrossRef]
- Yiamouyiannis, C.A.; Sanders, R.A.; Watkins, J.B.; Martin, B.J. Chronic Physical Activity: Hepatic Hypertrophy and Increased Total Biotransformation Enzyme Activity. Biochem. Pharmacol. 1992, 44, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yiamouyiannis, C.A.; Martin, B.J.; Watkins, J.B. Chronic Physical Activity Alters Hepatobiliary Excretory Function in Rats. J. Pharmacol. Exp. Ther. 1993, 265, 321–327. [Google Scholar]
- Watkins, J.B.; Crawford, S.T.; Sanders, R.A. Chronic Voluntary Exercise May Alter Hepatobiliary Clearance of Endogenous and Exogenous Chemicals in Rats. Drug Metab. Dispos. 1994, 22, 537–543. [Google Scholar]
- Xu, Y.; Gao, H.; Du, Z.; Liu, H.; Cheng, Q.; Zhang, F.; Ye, J.; Wang, A.; Dou, Y.; Ma, B.; et al. A New Approach for Reducing Pollutants Level: A Longitudinal Cohort Study of Physical Exercises in Young People. BMC Public Health 2022, 22, 223. [Google Scholar] [CrossRef]
- Genuis, S.J.; Beesoon, S.; Birkholz, D. Biomonitoring and Elimination of Perfluorinated Compounds and Polychlorinated Biphenyls through Perspiration: Blood, Urine, and Sweat Study. ISRN Toxicol. 2013, 2013, 483832. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Lane, K.; Birkholz, D. Human Elimination of Organochlorine Pesticides: Blood, Urine, and Sweat Study. BioMed Res. Int. 2016, 2016, 1624643. [Google Scholar] [CrossRef]
- Genuis, S.K.; Birkholz, D.; Genuis, S.J. Human Excretion of Polybrominated Diphenyl Ether Flame Retardants: Blood, Urine, and Sweat Study. BioMed Res. Int. 2017, 2017, 3676089. [Google Scholar] [CrossRef] [PubMed]
- Deger, M.; Kapila, V.; Denys, M.A.; Aridogan, I.A.; Everaert, K.; Herve, F. The Impact of Movement, Physical Activity and Position on Urine Production: A Pilot Study. Int. J. Clin. Pract. 2021, 75, e14743. [Google Scholar] [CrossRef]
- Lin, P.-I.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.-F.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; Oken, E. Per- and Polyfluoroalkyl Substances and Kidney Function: Follow-up Results from the Diabetes Prevention Program Trial. Environ. Int. 2021, 148, 106375. [Google Scholar] [CrossRef]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R.J. Optimizing the Restoration and Maintenance of Fluid Balance after Exercise-Induced Dehydration. J. Appl. Physiol. 2017, 122, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, P.; Ravanelli, N.; Chevrier, J. Can POPs Be Substantially Popped out through Sweat? Environ. Int. 2018, 111, 131–132. [Google Scholar] [CrossRef]
- Louis, C.; Tinant, G.; Mignolet, E.; Thomé, J.-P.; Debier, C. PCB-153 Shows Different Dynamics of Mobilisation from Differentiated Rat Adipocytes during Lipolysis in Comparison with PCB-28 and PCB-118. PLoS ONE 2014, 9, e106495. [Google Scholar] [CrossRef]
- Domazet, S.L.; Grøntved, A.; Jensen, T.K.; Wedderkopp, N.; Andersen, L.B. Higher Circulating Plasma Polychlorinated Biphenyls (PCBs) in Fit and Lean Children: The European Youth Heart Study. Environ. Int. 2020, 136, 105481. [Google Scholar] [CrossRef]
- Harmouche-Karaki, M.; Mahfouz, Y.; Salameh, P.; Matta, J.; Helou, K.; Narbonne, J.-F. Patterns of PCBs and OCPs Exposure in a Sample of Lebanese Adults: The Role of Diet and Physical Activity. Environ. Res. 2019, 179, 108789. [Google Scholar] [CrossRef]
- Domazet, S.L.; Jensen, T.K.; Grøntved, A. Response to Correspondence ENVINT_2020_552 “Can Habitual Exercise Really Increase Serum Concentrations of Persistent Organic Pollutants? Environ. Int. 2020, 140, 105616. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Lee, D.-H. Can Habitual Exercise Really Increase Serum Concentrations of Persistent Organic Pollutants? Environ. Int. 2020, 140, 105615. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, C.; Després, J.-P.; Tremblay, A. Plasma Organochlorine Concentrations in Endurance Athletes and Obese Individuals. Med. Sci. Sports Exerc. 2002, 34, 1971–1975. [Google Scholar] [CrossRef]
- Carlisle, A.J.; Sharp, N.C.C. Exercise and Outdoor Ambient Air Pollution. Br. J. Sports Med. 2001, 35, 214–222. [Google Scholar] [CrossRef]
- Martinez, B.; Robey, N.M.; Da Silva, B.F.; Ditz, H.; Sobczak, W.J.; Deliz Quiñones, K.Y.; Bowden, J.A. Swimming with PFAS in Public and Private Pools. Chemosphere 2023, 310, 136765. [Google Scholar] [CrossRef] [PubMed]
- Falcó, G.; Bocio, A.; Llobet, J.M.; Domingo, J.L. Health Risks of Dietary Intake of Environmental Pollutants by Elite Sportsmen and Sportswomen. Food Chem. Toxicol. 2005, 43, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Vu Tran, Z. Aerobic Exercise, Lipids and Lipoproteins in Overweight and Obese Adults: A Meta-Analysis of Randomized Controlled Trials. Int. J. Obes. 2005, 29, 881–893. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Borghese, M.M.; Liang, C.L.; Owen, J.; Fisher, M. Individual and Mixture Associations of Perfluoroalkyl Substances on Liver Function Biomarkers in the Canadian Health Measures Survey. Environ. Health 2022, 21, 85. [Google Scholar] [CrossRef]
- Lignell, S.; Winkvist, A.; Bertz, F.; Rasmussen, K.M.; Glynn, A.; Aune, M.; Brekke, H.K. Environmental Organic Pollutants in Human Milk before and after Weight Loss. Chemosphere 2016, 159, 96–102. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Diamond, M.L.; Peaslee, G.F.; Peng, H.; Blum, A.; Wang, Z.; Shalin, A.; Whitehead, H.D.; Green, M.; Schwartz-Narbonne, H.; et al. Per- and Polyfluoroalkyl Substances in North American School Uniforms. Environ. Sci. Technol. 2022, 56, 13845–13857. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.L.; Tupper, S. Ski Wax Use Contributes to Environmental Contamination by Per- and Polyfluoroalkyl Substances. Chemosphere 2020, 261, 128078. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Plassmann, M.M.; Cousins, I.T. Levels of Per- and Polyfluoroalkyl Substances (PFAS) in Ski Wax Products on the Market in 2019 Indicate No Changes in Formulation. Environ. Sci Process Impacts 2020, 22, 2142–2146. [Google Scholar] [CrossRef] [PubMed]
- Grønnestad, R.; Vázquez, B.P.; Arukwe, A.; Jaspers, V.L.B.; Jenssen, B.M.; Karimi, M.; Lyche, J.L.; Krøkje, Å. Levels, Patterns, and Biomagnification Potential of Perfluoroalkyl Substances in a Terrestrial Food Chain in a Nordic Skiing Area. Environ. Sci. Technol. 2019, 53, 13390–13397. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.A.; Doherty, B.T.; Gilbert-Diamond, D.; Romano, M.E.; Claus Henn, B. Waxing Activity as a Potential Source of Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Other Environmental Contaminants among the US Ski and Snowboard Community. Environ. Res. 2022, 215, 114335. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Coquillard, P.; Brucker-Davis, F.; Marchand, P.; Cano-Sancho, G.; Boda, M.; Antignac, J.-P.; Iannelli, A.; Gugenheim, J.; Le Bizec, B.; et al. Sustained Bloodstream Release of Persistent Organic Pollutants Induced by Extensive Weight Loss after Bariatric Surgery: Implications for Women of Childbearing Age. Environ. Int. 2021, 151, 106400. [Google Scholar] [CrossRef]
- Jansen, A.; Polder, A.; Müller, M.H.B.; Skjerve, E.; Aaseth, J.; Lyche, J.L. Increased Levels of Persistent Organic Pollutants in Serum One Year after a Great Weight Loss in Humans: Are the Levels Exceeding Health Based Guideline Values? Sci. Total Environ. 2018, 622–623, 1317–1326. [Google Scholar] [CrossRef]
- Jansen, A.; Aaseth, J.O.; Lyche, J.L.; Berg, J.P.; Müller, M.H.B.; Lydersen, S.; Farup, P.G. Do Changes in Persistent Organic Pollutants after Bariatric Surgery Cause Endocrine Disruption? Chemosphere 2023, 313, 137461. [Google Scholar] [CrossRef]
- Brown, R.H.; Ng, D.K.; Steele, K.; Schweitzer, M.; Groopman, J.D. Mobilization of Environmental Toxicants Following Bariatric Surgery. Obesity 2019, 27, 1865–1873. [Google Scholar] [CrossRef]
- Bellicha, A.; Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of Exercise Training before and after Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Rev. 2021, 22, e13296. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Goodpaster, B.H. A Role for Exercise after Bariatric Surgery? Diabetes Obes. Metab. 2016, 18, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on Cardiovascular Risk Factors in Adults with Overweight and/or Obesity: A Meta-Analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef]
- Ismail, I.; Keating, S.E.; Baker, M.K.; Johnson, N.A. A Systematic Review and Meta-Analysis of the Effect of Aerobic vs. Resistance Exercise Training on Visceral Fat: Exercise for Visceral Fat. Obes. Rev. 2012, 13, 68–91. [Google Scholar] [CrossRef]
- In, G.; Taskin, H.E.; Al, M.; Alptekin, H.K.; Zengin, K.; Yumuk, V.; Ikitimur, B. Comparison of 12-Week Fitness Protocols Following Bariatric Surgery: Aerobic Exercise Versus Aerobic Exercise and Progressive Resistance. Obes. Surg. 2021, 31, 1475–1484. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, Q.A.; Le Garf, S.; Martin, V.; Colson, S.S.; Chevalier, N. Is Physical Activity an Efficient Strategy to Control the Adverse Effects of Persistent Organic Pollutants in the Context of Obesity? A Narrative Review. Int. J. Mol. Sci. 2024, 25, 883. https://doi.org/10.3390/ijms25020883
Serrano QA, Le Garf S, Martin V, Colson SS, Chevalier N. Is Physical Activity an Efficient Strategy to Control the Adverse Effects of Persistent Organic Pollutants in the Context of Obesity? A Narrative Review. International Journal of Molecular Sciences. 2024; 25(2):883. https://doi.org/10.3390/ijms25020883
Chicago/Turabian StyleSerrano, Quentin A., Sébastien Le Garf, Vincent Martin, Serge S. Colson, and Nicolas Chevalier. 2024. "Is Physical Activity an Efficient Strategy to Control the Adverse Effects of Persistent Organic Pollutants in the Context of Obesity? A Narrative Review" International Journal of Molecular Sciences 25, no. 2: 883. https://doi.org/10.3390/ijms25020883
APA StyleSerrano, Q. A., Le Garf, S., Martin, V., Colson, S. S., & Chevalier, N. (2024). Is Physical Activity an Efficient Strategy to Control the Adverse Effects of Persistent Organic Pollutants in the Context of Obesity? A Narrative Review. International Journal of Molecular Sciences, 25(2), 883. https://doi.org/10.3390/ijms25020883






