Abstract
Parthenocarpy and stenospermocarpy are the two mechanisms underlying the seedless fruit set program. Seedless fruit occurs naturally and can be produced using hormone application, crossbreeding, or ploidy breeding. However, the two types of breeding are time-consuming and sometimes ineffective due to interspecies hybridization barriers or the absence of appropriate parental genotypes to use in the breeding process. The genetic engineering approach provides a better prospect, which can be explored based on an understanding of the genetic causes underlying the seedlessness trait. For instance, CRISPR/Cas is a comprehensive and precise technology. The prerequisite for using the strategy to induce seedlessness is identifying the crucial master gene or transcription factor liable for seed formation/development. In this review, we primarily explored the seedlessness mechanisms and identified the potential candidate genes underlying seed development. We also discussed the CRISPR/Cas-mediated genome editing approaches and their improvements.
1. Introduction
Seedlessness is one of the most valuable agricultural traits in fruit crops that consumers appreciate for fresh consumption and value-added processed products [1,2]. It enriches the eating quality of the fruits due to their expanded edible pulp and the absence of hard seeds with an awful taste. Further, seedlessness could prevent browning and bitterness caused by seeds [3]. Moreover, it improves many other fruit biometric characteristics regarding acid/sugar levels, dry matter, firmness, and overall shelf-life qualities of climacteric fruit due to reduced ethylene generated by seeds [4]. Seedlessness can also mitigate fruit yield losses caused by environmental stresses that affect pollination and fertilization processes [5]. Finally, it occurred independently of pollination and fertilization, which increases fruit production, particularly in dioecious species, due to the uselessness of the pollen source staminate trees. Studies on fruit seedlessness suggest that the trait is coordinated by intricate systems involving hormonal, genetic, and environmental factors [6,7]. Therefore, there are many causes underlying the seedless fruit set program [8]. The most classical reasons include male sterility, degradation of mother pollen cells, embryonic abortion, and chromosomal irregularities during meiosis leading to triploidy.
In typical seeded fruit, the ovary proliferates after fertilization through a coordinated program of molecular, biochemical, and structural changes that stimulate fruit size enlargement due to the interplay of cell division, differentiation, and expansion of sporophytic and gametophytic tissues [9]. Research on mechanisms underlying fruit seedlessness has highlighted the potential involvement of two distinct strategies, parthenocarpy and stenospermocarpy [10]. In parthenocarpy, true seedlessness occurs, and the ovary develops into fruit independent of pollination and fertilization [11]. However, two different procedures were identified for the parthenocarpic fruit set program. The obligatory-parthenocarpy, where a plant always produces seedless fruits (i.e., pineapple), and the facultative-parthenocarpy, where seedless fruits only develop if pollination is prevented (i.e., watermelon) [12]. Parthenocarpic fruit development is triggered by the deregulation of the hormone balance in ovary tissues, mainly auxin, gibberellins (GAs), and/or cytokinins (CKs). Applying these hormones to unpollinated ovaries at anthesis can stimulate pollination-independent ovary growth and produce parthenocarpic seedless fruit, strongly supporting their individual and overlapped roles during early fruit development [13]. An earlier study reported that the growth of tomato fruit is coordinated by a delicate balance between auxin and GA, whereby auxin is needed to mediate cell division and GA is required to organize cell expansion [7]. The parthenocarpy trait stability in fruit crops primarily occurs through elective pressure for seedlessness during domestication and breeding [8]. However, parthenocarpic genotypes were also identified in wild species and non-fruit crops [14].
In stenospermocarpy, pollination and fertilization typically occur; however, the seed growth is prematurely aborted due to the cessation of seed coat and endosperm development, resulting in expanded fruit size with seminal rudiments or seed traces [15]. The fact that different degrees of seedlessness were observed in progeny grapevines resulting from crossing seeded and stenospermocarpy seedless parents adds more complexity to the integrative regulatory network and signaling pathways underlying the stenospermocarpy seedless fruit set machinery [16]. Despite recent advances in grape biology, the molecular basis that triggers stenospermocarpy fruit development is largely unknown [10]. The efforts to unravel the molecular basis for stenospermocarpy in grapes were able to identify and functionally characterize several genes that can be potentially involved in the procedure [17,18,19]. Although the results did not show an ultimate gene network, they at least shed light on potential molecular mechanisms that synchronize stenospermocarpy machinery.
Fruit size and weight are positive commercial attributes, through which the number of developed seeds per fruit is positively correlated with the two characters [20]. Parthenocarpy fruit set results in considerably smaller fruit size than seeded fruit due to the absence of seed initiation, leading to reduced hormone levels necessary to sustain fruit growth [7]. However, stenospermocarpy does not compromise or, in the worst-case scenario, slightly reduce the fruit size because the ovary-growth event occurs after pollination and fertilization, making stenospermocarpy seedlessness a more attractive trait for breeding (Figure 1). CKs are essential to determining ovary size before fertilization. However, the slightly compromised size of the stenospermocarpy fruit is due to the availability of CKs post-fertilization, which negatively regulate cell expansion during fruit development [21].
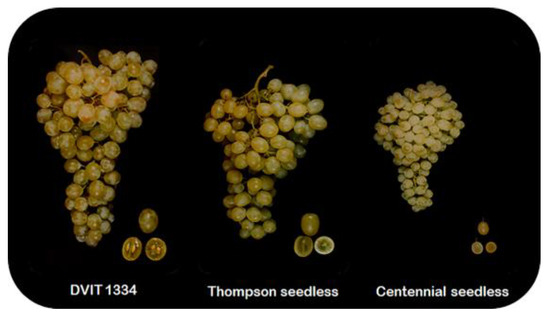
Figure 1.
Close-up cluster views of Thompson seeded mutant (DVIT 1334), Thompson seedless, and Centennial seedless grape genotypes exhibiting seeded, stenospermocarpy, and parthenocarpy fruit set programs, respectively.
Parthenocarpy seedlessness can be induced by applying hormones to unpollinated inflorescences at anthesis, via fostering self-incompatibility, or through generating triploid plants using conventional breeding practices [4,22]. Nevertheless, all the strategies are laborious, time-consuming, and sometimes not possible to use due to the absence of proper parental genetic resources. In the meantime, no treatment or application that can induce stenospermocarpy seedlessness has been identified yet. Accordingly, both seedlessness mechanisms are important, depending on growth conditions and commercial value. This has opened up the opportunity for the genetic engineering approaches that have given encouraging results, both in the quality and quantity of seedless fruit production.
Genetic engineering technology is a promising approach that has contributed considerably to crop improvement. Over 20 genetically modified (GM) crop entities had been commercialized by 2018—including soybeans, cotton, maize, and canola—with a share of world production ranging between 29–78% [23,24]. Some other GM crops are produced depending on the country, such as potato, apple, alfalfa (North America), papaya (Hawaii), eggplant, squash, safflower, pineapple, and sugar cane (different countries) [25]. The leading countries for GM crop cultivation are the USA, Brazil, Argentina, Canada, and India, with total productive land spaces ranging between 11.6–75 M ha, in addition to Paraguay, China, Pakistan, South Africa, and Australia, with entire land spaces ranging between 0.8–5 M ha [23,26]. Before commercialization, these crops had to pass through a prolonged and extensive regulatory process. The US Department of Agriculture (USDA) does not impose any GMO regulations on plants with targeted mutagenesis by self-repair mechanisms if they are free from Agrobacterium, any transgene, or foreign genetic materials. Accordingly, there is a high probability that CRISPR/Cas RNPs could be exempt from current GMO regulation [27,28]. CRISPR/Cas technology has been implemented to gain desired traits in many crops [29,30]. For instance, the disease resistance trait was developed in rice [31], tomato [32,33], cucumber [34], wheat [35,36], citrus [37], and Arabidopsis [38] by engineering disease-susceptible genes. However, the technology needs a comprehensive understanding of the gene(s) coordinating the desired trait. Accordingly, several other factors are essential, such as appropriate gRNA selection, promoter choice, and a suitable Cas protein. In this review article, we investigate and scrutinize genes that function as positive regulators of seed formation. Further, we discussed diverse CRISPR/Cas genome editing approaches to introduce a seedless character. The knowledge and information generated from this review will improve our critical thinking toward introducing innovative, high-value quality attributes to fruit crops.
2. Genes Coordinating the Seedlessness Trait
2.1. Auxin-Related Genes
Applying synthetic auxins to unpollinated flowers induces parthenocarpic fruit growth by modulating early cell division, resulting in an increase in the pericarp volume [4]. Several molecular studies have demonstrated the role of auxin in triggering and coordinating the transition from flower to fruit [39,40,41,42]. The auxin-mediated parthenocarpy seedless induction occurs by either altering auxin synthesis or signaling.
In the auxin pathway, seven gene candidates acting as positive regulators of the seeded fruit set program were identified (Figure 2; Table 1). The SlIAA9 and SlARF7/AtARF8 TFs belong to the AUX/IAA and auxin response factor (ARF) gene families, respectively.
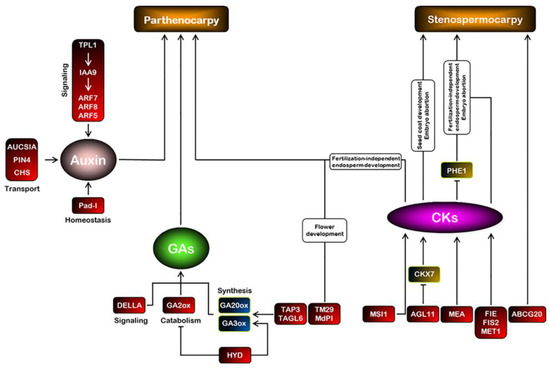
Figure 2.
Schematic model of hormonal regulation of seedless fruit set. Parthenocarpy is obtained either by exogenous treatments or by genetic manipulations of phytohormones. Gene names in red boxes represent the gene loss-of-function mutation or downregulation that causes parthenocarpy or stenospermocarpy seedlessness. The gene name in the red boxes could be identified in Table 1. GA20ox (GA 20 oxidase) and GA3ox (GA 3 oxidase), GA biosynthetic genes; GA2ox (GA 2 oxidase), a GA catabolic enzyme; CKX7 (cytokinin oxidases/dehydrogenases), a CK-degrading enzyme; and PHE1 (PHERES1), a type I MADS-box gene.
Aux/IAAs and ARF-transcriptional regulators interact in homo- and heterodimers, forming complexes that repress auxin-dependent changes in gene expression and, therefore, auxin action. Auxin binding to an F-box receptor promotes SCFTIR1/AFB complex formation, leading to the ubiquitin-dependent proteolysis of Aux/IAA [43,44]. Loss of Aux/IAA repressors allows ARF-mediated auxin-responsive changes in gene transcription. Transgenic tomato and Arabidopsis plants with suppressed SlIAA9, SlARF7, or AtARF8 mRNA displayed fruit/silique development before fertilization, giving rise to parthenocarpy seedless fruit development [45,46]. Similarly, the amiRNA SlARF5 lines exhibited ovary growth and formed seedless tomato fruits following emasculation. These parthenocarpic fruits developed fewer locular tissues, and the fruit size and weight declined in transgenic lines compared to wild-type fruits [47].
The transcriptional co-repressor (TPL) is an upstream central regulatory hub to control phytohormone pathways. SlTPLs participate in the auxin-signaling pathway by interacting with Aux/IAA proteins in tomatoes, particularly IAA9. There is no interaction between SlTPL1 and the ARF activators ARF7, ARF8, or ARF5. Accordingly, IAA9 is the connection link between SlTPL1 and ARFs. The down-regulation of SlTPL1 in tomato plants produced facultative parthenocarpy fruit associated with a significant decline in the expression of ARF-related genes. Transgenic SlTPL1-RNAi plants produced WT-like fruits having no pleiotropic effect under normal growth conditions [48].
Another gene, PARENTAL ADVICE-1 (Pad-1), encodes an aminotransferase, which is involved in auxin homeostasis. The role of Pad-1 in unpollinated ovaries is to prevent the excessive accumulation of IAA, resulting in a precocious fruit set. A loss-of-function mutant, pad-1 caused high accumulation of IAA in the tomato and pepper ovaries, suggesting that Pad-1 protein is involved in auxin homeostasis during ovary development [49].
Similarly, several genes involved in auxin transport were identified as master genes that enhance parthenocarpy fruit set. The AUxin Cum Silencing Action (AUCSIA) is a green plant gene family encoding a mini-protein involved in several aspects of auxin biology, including polar auxin transport [50,51]. Silencing of the AUCSIA in tomato and Arabidopsis caused fruit set independent of pollination and fertilization that produced facultative and obligatory parthenocarpic fruits [51]. Further, auxin efflux transport is conducted by the PIN-FORMED (PIN) protein family. In tomato, the application of auxin efflux transport inhibitors produced parthenocarpic fruit development. Silencing of the SlPIN4 gene resulted in parthenocarpic fruits due to precocious fruit development [52]. Finally, chalcone synthase (CHS) encodes a key enzyme that catalyzes the first committed step in the flavonoid biosynthetic pathway [53]. Flavonoids act as negative regulators of auxin transport that affect auxin sensitivity [54]. Down-regulation of CHS mRNA produced parthenocarpic seedless tomato fruits probably by enhancing polar auxin transport [55].
2.2. Gibberellin-Related Genes
Applying active gibberellins (GA1, GA3, or GA4) to unpollinated flowers induces parthenocarpy fruit set in several plant species [39,41]. The role of GAs in the fruit set was also supported by the analysis of the natural tomato mutants (pat, pat2, and pat-K) that produce parthenocarpic fruit [56,57]. In higher plants, it is essential to maintain optimal levels of phytohormones to ensure typical growth and development (Figure 2). Hence, plants have to retain a mechanism to remove any excess active compounds or their biosynthetic precursors to ensure the proper function of phytohormones. Such a strategy can prevent the progressive accumulation of hormones. The flux of active GAs is regulated by the balance between their rates of biosynthesis and deactivation. The GA20ox and GA3ox genes encode key enzymes of bioactive GA synthesis, whereas GA2ox is the major GA inactivation enzyme [58]. Modifying the regulation of genes by adjusting GA flux can subsequently alter the processes regulated by GA [59]. GA2oxs-silenced tomato plants displayed parthenocarpic fruit growth. However, the mutant plants exhibited branching inhibition due to the high accumulation of active GA4 in axillary buds [60].
Seedless fruits have also been produced by modifying the GA-signaling pathway. According to the relief of restraint model, DELLA proteins operate as growth repressors, and GA-mediated DELLA degradation is a critical step to overcome this restraint [61]. At low GA levels, DELLA proteins impair the activity of basic helix-loop-helix (bHLH) transcription factors by interacting with their DNA binding domain [62]. The binding of GA to its GID1 receptor results in a conformational change that promotes the interaction of GID1 with DELLA [63]. The GA–GID1–DELLA complex is subsequently recognized by the SCFSLY1/GID2 E3 ubiquitin-ligase complex, which mediates the ubiquitination of DELLA proteins. This ubiquitin mark destines the DELLA proteins for degradation via the 26S proteasome, thereby allowing growth by releasing their inhibitory interaction with GA-dependent gene partners. In agreement with their function as growth repressors, lacking one or more DELLA proteins within the plant elicited constitutive activation of the GA-signaling pathway independent of GA presence, in which the mutant plants exhibited a GA-overdose phenotype, including parthenocarpic fruit development [64]. Antisense DELLA tomato and Arabidopsis plants produced seedless fruits [65]. However, the resultant fruits were smaller and displayed elongated shapes compared with typical fruits.
2.3. Cytokinin-Related Genes
CKs influence seed development and play other roles in plant growth [39]. They are involved in regulating ovary size and ovule development, which affect seed size and number [21]. Polycomb group (PcG) proteins regulate the expression of the signature CKs genes [66]. In Arabidopsis, the genes of MEDEA (MEA), FERTILIZATION INDEPENDENT ENDOSPERM (FIE), and FERTILIZATION INDEPENDENT SEED 2 (FIS2) encode the PcG protein that controls seed development via synchronizing embryo and endosperm proliferation (Figure 2). The MEA protein holds a characteristic SET domain that confers histone methyltransferase activity. The mea mutation caused seed abortion in Arabidopsis, primarily mediated by epigenetically deregulating the expression of the type I MADS-box gene PHERES1 (PHE1) [67].
The FIE gene directly contributes to female reproductive development. The mutant fie by the female gametophyte caused embryo abortion by influencing the central cell’s development. Mutants of fie can replicate the central cell nucleus and stimulate endosperm development independent of fertilization procedures [68]. Similarly, fis1 and fis2 mutants generated barely-formed pro-embryos that did not develop beyond the globular stage, causing seed abortion. The arrested embryos’ emerged due to continued endosperm growth until the cellularization stage [69]. Further, MULTICOPY SUPPRESSOR OF IRA 1 (MSI1) is a WD-40 domain protein that forms a complex with the MEA and FIE proteins. It is another member of the conserved FIS polycomb group complex that belongs to the PRC2 type. Mutant plants heterozygous for msi1 were able to produce parthenocarpic siliques in Arabidopsis [70]. The msi1 mutant gametophytes initiated endosperm development in the absence of fertilization at high penetrance. It showed a seed abortion ratio of 50%, with seeds aborting when the mutant allele is maternally inherited, irrespective of a paternal WT or mutant MSI1 allele.
DNA methyltransferase 1 (MET1) is a central regulator of parentally imprinted genes that affect seed growth. The met1 loss-of-function mutant caused a reduction in seed size, presumably linked to the silencing of the paternal allele of growth enhancers in the endosperm, which nurtures the embryo [71]. MET1 and MEDEA exhibited overlapping expression patterns in reproductive tissues pre- and post-fertilization. Apparently, there is a mechanistic association between two major epigenetic pathways involved in histone and DNA methylation in plants through the physical interaction of MET1 with FIS-PRC2, the core component of MEDEA. This concerted action is relevant for the repression of seed development in the absence of fertilization [72].
2.4. MADS-Box Genes
The MADS-box gene family of transcription factors (TFs) are crucial regulatory networks underlying multiple developmental pathways in plants, animals, and fungi [73,74]. MADS-box proteins interact with members of the same family or with diverse other proteins to orchestrate different developmental programs that respond to external and internal stimuli signals, such as growth-, hormone-, and defense-signaling [75]. Based on structural characteristics, the MADS-box TFs are classified into two major groups: type I and type II [76]. Type I MADS-box TFs hold an SRF-like domain, whereas type II comprises the Myocyte Enhancer Factor 2-like (MEF2-like) domain, known as the MIKC genes in plants [77]. MIKC genes can be further divided into MIKCC and MIKC* subfamilies. MIKCC genes have been widely reported due to their involvement in diverse biological functions in plants, particularly floral organ specification, flowering time regulation, and fruit development and ripening [78,79]. The approach used to elucidate MADS-box gene function was through the analysis of plant phenotypes resulting from their downregulation or overexpression.
Several lines of evidence have associated seed initiation/development and the seedless fruit set program with the alteration of several MADS-box members belonging to type II lineage MICKC subfamilies (Figure 2). The silencing of different tomato MADS-box gene members of class B, including TAP3, TM6, SlGLO1, and SlGLO2, produced mutant plants that developed fruits with no or few seeds [80]. For instance, the downregulation of the tomato floral homeotic gene APETALA3 (TAP3) in the ovary results in male sterility and parthenocarpic fruit development [81]. Emasculation and manual pollination assays using WT pollen suggested a liable pollen impairment phenotype involved in the facultative parthenocarpy fruit set of the TAP3-silenced plants [82]. The parthenocarpic fruit development in TAP3-downregulated ovaries was associated with increased GA levels, suggesting that stamen development negatively regulates fruit set by repressing GA biosynthesis. A different floral homeotic gene that belongs to the same family, designated as PISTILLATA (MdPI), has been identified as the determinant underlying parthenocarpy fruit set in apples [83]. In addition to the altered fruit set program, the apple mutant trees produce a distinct flower phenotype, where petals are converted to sepals and stamens to carpels. The apple mutants exhibited retrotransposon insertion events in intron 4 or intron 6, which abolished the typical MdPI gene expression.
Another distinct tomato gene, AGAMOUS-Like 6 (TAGL6), encodes a MADS-box protein of the subfamily AGL6 [78]. Transcriptome analysis followed by marker-assisted mapping established that a mutation in TAGL6 is responsible for an interesting EMS-induced tomato mutant [84]. The mutant plants exhibited a facultative parthenocarpy fruit phenotype under heat stress conditions without pleiotropic effects on vegetative and reproductive development. The Tagl6 mutation showed typical characteristics of WT plants, excluding the parthenocarpic fruit set, in terms of pollen viability, sexual reproduction capacity, and fruit biometrics, making TAGL6 an attractive target gene for facultative parthenocarpy. Gene expression analysis and CRISPR/Cas9 gene knockout confirmed the role of TAGL6 as a critical regulator coordinating the transition from the state of ‘ovary arrest’ to fertilization-triggered fruit set resumption. Once the down-regulation of TAGL6 is alleviated, the ovary/fruit development resumes and continues to reach its full potential.
The Tomato MADS-box 29 (TM29) gene is the ortholog of the Arabidopsis SEPALLATA (AtSEP) genes that belong to the E class [78]. Based on transcript abundance and evaluation of silenced mutants, it was suggested that TM29 behaves like AtSEP1 via coordinating floral organ development and identity. The tm29 plants produced aberrant flowers with phenotypic alterations in the organs of the inner three whorls [85]. The yellow petals and stamens have been converted into green color. The reproductive organs of stamens and ovaries were sterile; however, the ovaries continued growing into parthenocarpic fruit. The fruits were malformed, as they emerged from ectopic shoots with partially developed leaves and secondary flowers. These shoots resembled the primary transgenic flowers and continued to produce parthenocarpic fruit and ectopic shoots.
Finally, an interesting homeotic MADS-box gene classified as a D member AGAMOUS-Like 11 (AGL11) was demonstrated to coordinate seed development. Several phenomic, genetic, biochemical, and transcriptomic approaches allowed the identification of the Arabidopsis AGL11 gene, called SEEDSTICK (STK), as a master regulator coordinating ovule identity and the flavonoid pathway, particularly proanthocyanidins synthesis linked to seed coat development [86,87]. Afterward, an AGL11 ortholog named SHELL was identified in oil palm as a crucial regulator for the thickness of the coconut-like shell surrounding the kernel [88]. Through a homozygosity mapping by sequencing approach, it was demonstrated that the thin kernel shell phenotype was associated with two independent mutations within the DNA-binding domain. Later, VviAGL11 was identified as a substantial gene controlling seed morphogenesis in cultivated grapevine [16,18]. A missense mutation detected within the C-terminal region of the gene was associated with a reduced VviAGL11 transcription level and subsequently considered the direct cause of triggering the seedless stenospermocarpy fruit set program. Interestingly, the genetic characterization of the mutant highlighted the dominant inheritance of the seedless trait [16]. Subsequently, two AGL11 homologs were identified in tomato, SlAGL11 and SlMBP3 [89]. Genetic analysis of numerous tomato genotypes, along with the functional analysis of the two genes via CRISPR/Cas9 and silencing approaches, suggested the critical role played by SlMBP3 in regulating the structure of locular tissue in tomatoes. Individual knockout mutations did not influence seed development; however, the dual SlMBP3/SlAGL11 mutant lines displayed smaller plants with a dramatic reduction in fruit size/weight and under-developed seeds showing a complete inability to germinate. Apparently, SlMBP3 and SlAGL11 have overlapping functions in seed development, through which the absence of an ortholog can be covered by the presence of the other.
2.5. Other Genes
2.5.1. HYDRA (HYD) Gene
The SPOROCYTELESS/NOZZLE (SPL/NZZ) gene is a floral organ-building gene that encodes a protein related to MADS-box transcription factors. The SPL/NZZ plays a central role in controlling early anther cell differentiation and stamen identity [90,91,92]. The direct activation of SPL/NZZ by the MADS-box AG is instructed for early anther development [93]. The tomato HYDRA gene (HYD) encodes a putative SPL/NZZ transcription factor. The SlHYD is essential for preventing precocious ovary growth, flower maturation, and an appropriate fruit set program [94]. The tomato hyd mutant produces seedless fruit due to the impaired formation of male and female germlines, triggering parthenocarpic fruit set development. Interestingly, the precocious growth of the ovary in the hyd mutant was associated with changes in the expression of genes involved in gibberellin (GA) metabolism, particularly the accumulation of SlGA3ox and the suppression of SlGA2ox (Figure 2).
2.5.2. Binding Cassette G Transporter
The ATP-binding cassette (ABC) transporter is one of the largest and oldest protein families. The tonoplast-localized ATP-binding cassette pumps various secondary metabolite substrates across the vacuolar membrane into the vacuole using the energy generated by ATP [95]. The VviABCG20 encodes a putative ATP-binding cassette G transporter in grape. The gene was identified as a differentially expressed gene during seed development or seed abortion of the seeded and stenospermocarpy seedless grapes, respectively (Figure 2) [19]. Silencing of the VviABCG20 ortholog in tomato (SlABCG20) resulted in plants that set fruit with no or few seeds, suggesting its potential involvement in seed development. Interestingly, the VviDof14 gene, which acts as a negative regulator of VviABCG20, showed a higher expression level in Thompson seedless grape [96].

Table 1.
Transcription factor genes involved in seed formation could be utilized to induce seedlessness.
Table 1.
Transcription factor genes involved in seed formation could be utilized to induce seedlessness.
| Gene Name | Species | Protein | References |
|---|---|---|---|
| Auxin-related genes | |||
| IAA9 | S. lycopersicum | Auxin repressor Aux/IAA 9 | [40] |
| ARF7/8 | A. thaliana; S. lycopersicum | Auxin-response factor 7/8 | [45,46] |
| ARF5 | S. lycopersicum | Auxin-response factor 5 | [47] |
| TPL1 | S. lycopersicum | Transcriptional co-repressor TOPLESS 1 | [48] |
| Pad-1 | S. lycopersicum | Proteasome subunit alpha type-7 | [49] |
| AUCSIA | S. lycopersicum | AUxin Cum Silencing Action | [51] |
| PIN4 | S. lycopersicum | Auxin efflux carrier component 4 | [52] |
| CHS | S. lycopersicum | Chalcone synthase | [55] |
| Gibberellin-related genes | |||
| GA2ox | S. lycopersicum | Gibberellin 2-oxidase | [60] |
| DELLA | A. thaliana; S. lycopersicum | DELLA protein GAI | [64,65] |
| Cytokinin-related genes | |||
| MEA | A. thaliana | SET domain-containing protein | [67] |
| FIE | A. thaliana | Transducin/WD40 repeat-like superfamily protein | [68] |
| FIS2 | A. thaliana | VEFS-Box of polycomb protein | [69] |
| MSI | A. thaliana | Transducin/WD40 repeat-like superfamily protein | [70] |
| MET1 | A. thaliana | DNA (cytosine-5)-methyltransferase 1 | [71,72] |
| MADS-box genes | |||
| TAP3 | S. lycopersicum | Tomato APETALA 3 | [81] |
| PI | Malus domestica | PISTILLATA | [83] |
| TAGL6 | S. lycopersicum | AGAMOUS-Like 6 | [84] |
| TM29 | S. lycopersicum | Tomato MADS-box 29 | [85] |
| AGL11 | V. vinifera; E. guineensis | AGAMOUS-Like 11 | [16,88] |
| Other genes | |||
| HYDRA | S. lycopersicum | SPOROCYTELESS/NOOZLE-like protein | [94] |
| ABCG20 | A. thaliana | ABC-2 type transporter family protein | [96] |
3. Genome Editing Technology—CRISPR-Cas
Targeted genes or genome editing technologies have been explored for the last three decades. The zinc finger nucleases (ZFNs), transcription activator-like effector (TALE) nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR) are the three primary strategies that have been developed and utilized for genome editing. However, the CRISPR-associated protein 9 (CRISPR-Cas9) technology has emerged as the most potent tool [97]. In general, CRISPR-mediated genome editing mandates several requirements, including a guide RNA (gRNA) composed of 20 synthetic nucleotide sequences that binds to target DNA and a nuclease enzyme (Cas9) that breaks the DNA near the protospacer adjacent motif (PAM) sequence [98]. Cas9 is not active under natural conditions, as the active Cas9-sgRNA complex can only be assembled when the Cas9 encloses the sgRNA. Then, the active complex scans the double-strand DNA to identify and bind to the complementary sequences. Afterward, the enzyme’s HNH domain cleaves the DNA closely before the PAM sequence, while the RuvC domain breaks another strand, causing a double-strand break (DSB). The DSBs are eventually repaired by endogenous DNA repair mechanisms, such as non-homologous end joining (NHEJ) and homology-directed repair (HDR), causing nucleotide insertions and/or deletions (indels) at the desired sites.
4. Improved CRISPR-Cas9 Technology
A notable deficiency of the early experiments with the CRISPR/Cas9 system was the high rate of off-target cleavages caused by the formation of a mismatched complex between gRNA and DNA. Several strategies were reported to improve target site specificity and efficiency, such as modifying the Cas9 enzyme [99,100], increasing the length of the PAM sequence [101,102,103], generating new Cas proteins (i.e., CRISPR-Cas12a) [104], and modifying the CRISPR technology itself.
Several changes were applied to the Cas9 enzyme via modifying the cleavage domain of Cas9-D10A or Cas9-H840A, which enriched the specificity of cleaving target DNA [99,100]. The VQR variants of SpCas9 recognize NGA PAMs, and the VRER variants recognize NGCG PAMs, greatly expanding the genome editing range [101]. Moreover, Cas9 can be deactivated (dCas9 or CRISPRi) by a point mutation in the RuvC and HNH nuclease domains. Co-expression of dCas9 and a sgRNA prevents transcription elongation and subsequently averts protein function, which diminishes gene expression [105]. Gene expression can also be regulated by fusing dCas9 with a repressor or an activator. For instance, dCas9-VP64 and dCas9-p65AD can efficiently trigger gene expression [106]. Cas9 combined with histone-modified/DNA-methylated enzymes can modulate the epigenetic modification of genes [107]. A light-controlled endogenous gene expression circuit was reported. This circuit was developed by fusing the light-inducible proteins (CRY2 and CIB1) to a transactivation domain and dCas9 [108]. Furthermore, the fusion of Cas9 with a fluorescent protein was declared to label the DNA in a particular compartment, facilitating the study of complex chromosomal architecture and nuclear organization [109].
The discovery of different nucleases that recognize different PAMs has enriched the CRISPR/Cas strategy in terms of specificity and efficiency. For instance, the Nmecas9 enzyme, derived from Neisseria meningitidis, recognizes an 8-mer (50-NNNNGATT) PAM. This longer PAM sequence can reduce off-target cleavage and increase target specificity [110]. Likewise, the Sacas9 enzyme, derived from Staphylococcus aureus, recognizes a 6-mer (50-NNGRRT) PAM sequence [99]. The St1cas9 and St3cas9 derived from Streptococcus thermophilus recognize a 7-mer (5′-NNAGAAW) and a 5-mer (5′-NGGNG) PAM, respectively. The St1cas9 and St3cas9 minimized off-target rates while editing the human PRKDC and CARD11 loci, compared to the enzyme SpCas9 derived from Streptococcus pyogenes [102]. Furthermore, CRISPR-CpfI, developed from Prevotella and Francisella, is a class II type V endonuclease that recognizes the 5′-TTTN-3′ PAM sequence [111]. It can be used effectively in plants and animals with reduced or no off-target effects [112]. Unlike Cas9, another endonuclease, NgAgo derives from Natronobacterium gregoryi and operates on 24 nucleotides of ssDNA with 5′-phosphorylation as a guide. It can bind 5′-phosphorylated single-stranded guide DNA (gDNA) of ~24 nucleotides and efficiently generate gDNA sequence-specific DNA double-strand breaks. This editing process does not require the presence of PAM [113]. Finally, the C2c2, derived from Leptotrichia shahii, showed two nuclease functions that cleave single-stranded RNA [114].
In addition to the previous efforts to improve Cas9 engineering, several studies were performed to enhance the CRISPR technology. Highly efficient multiplex genome editing showed the new dimensions of plant biology and crop breeding. Sophisticated genetic engineering objectives became feasible, including multigene knockouts, gene or promoter knock-ins, gene activation and repression, chromosomal deletion and translocation, epigenome modifications, and many others. Multiplex editing was executed by expressing Cas9 (or Cas9-derived effectors) together with multiple gRNAs targeting multiple sites. The multiple gRNA toolbox system can be constructed by either expressing the gRNAs individually or simultaneously. The two strategies have advantages and disadvantages regarding cloning readiness, the number of expressed gRNAs, and editing efficiency.
Moreover, the recently developed CRISPR-TSKO technology can evaluate gene function based on a tissue-specific knockout [115]. CRISPR-TSKO is able to modify the genome of specific cells, tissues, and organs of different allelic backgrounds for plant disease-resistant capacity engineering. This tissue-specific knockdown can be a better option for comprehensively understanding signaling and tolerance mechanisms. Cas9 expression under the control of the egg cell-specific promoter EC1.2 and the germline-specific promoter SPL produced a heritable mutant in Arabidopsis [116,117]. Recently, Cas9 was expressed under the control of the fiber-specific -NST3/SND1 promoter to target the essential Arabidopsis gene HCL (encoding a hydroxycinnamoyl transferase) [118].
Base editing (BE) is another newly developed strategy for precise genome editing that enables irreversible base conversion at a specific site. The BE machinery is a complex of a catalytically impaired Cas protein, guide RNA (gRNA), and nucleobase deaminase domain that can convert specific base pairs [119]. All four transition mutations, C → T, G → A, A → G, and T → C, can be introduced in the genome with the available CRISPR/Cas base editors. The cytosine base editor (CBE) can establish a G–C to A–T mutation, while the adenine base editor (ABE) can alter an A–T base pair into a G–C. In RNA, the conversion of adenine (A) to inosine (I) is also possible with the RNA base editor [120].
The latest addition, “Prime Editing” is based on “search-and-replace”. The technology can force targeted insertions/deletions within the gene. Interestingly, it does not require double-strand breaks (DSBs) or donor DNA templates. The earlier version of prime editors (PE1) uses RNA-programmable nickase and a prime editing guide RNA (pegRNA) fused with reverse transcriptase (RT). The updated PE2 version exhibited higher editing efficiency because it used an engineered RT. While the latest PE3 version utilizes two guide RNAs and further increases the editing efficiency by producing nick at specific locations on the non-edited strands to induce its replacement. Prime editing offers much lower off-target activity than Cas9, far fewer byproducts with similar/higher efficiency than Cas9-initiated HDR, and complementary strengths over base editor technology [121].
5. Genetic Engineering Strategies for Seedlessness Breeding
Parthenocarpy seedless fruits can be accomplished either by exogenous application of plant growth regulators, conventional breeding, interspecies hybridization, or polyploidy breeding. However, none of these strategies is feasible to induce stenospermocarpy seedless. For instance, muscadine seedless grape breeding is not viable due to the absence of the trait within the species. The only available seedless muscadine genotype, “Fry Seedless”, is parthenocarpic with limited commercial value and cannot be used as a crossing parent in the breeding program due to male sterility (Figure 3) [122]. Muscadine and bunch grape are classified under the Euvitis genera; however, the pronounced differences in their phenomic, metabolomic, and genomic characteristics represented by the dissimilarities in stress responses, horticultural and reproductive growth characteristics, and genome structure enabled us to classify them into two different genera, Muscadinia and Vitis [123]. Accordingly, introducing the stenospermocarpy seedless trait to muscadine grapes via generating Vitis x Muscadinia interspecific hybrids is challenging due to the differences in chromosome number and genetic incompatibility [124]. Alternatively, developing triploid seedless muscadine grapes might be an option that avoids the genetic barrier between species. However, the attempt to establish a triploid seedless muscadine grape did not produce satisfactory genotypes that can be promoted into new cultivars due to limited reproductive growth qualities [125]. Hence, genetic engineering could be a promising strategy for introducing a seedless trait.

Figure 3.
Close-up cluster view of muscadine cultivars Majesty (female flower, seeded), Noble (perfect flower, seeded), and Fry seedless (perfect flower, parthenocarpy seedless).
Advanced genome-editing tools, such as CRISPR-TSKO, precise base editing, or prime editing approaches, were efficiently applied in different crops for precise genome editing that prevented or minimized the pleotropic effects [115,126]. However, this approach is considered GMO and may affect consumer acceptance. Interestingly, the Cas9-free lines can be selected by crossing out the transgenes from the segregating populations or through RNP-mediated protoplast transformation and regeneration [38,127]. Moreover, the CRISPR reagent (RNP) can be delivered to the germline cells using viral vectors like the Tobacco rattle virus (TRV). Thus, an inherited mutation could be achieved using the seeds from the germline-edited plant [128].
The model presented in Figure 4 illustrates our suggested strategy for introducing a seedless trait using genome editing technology.
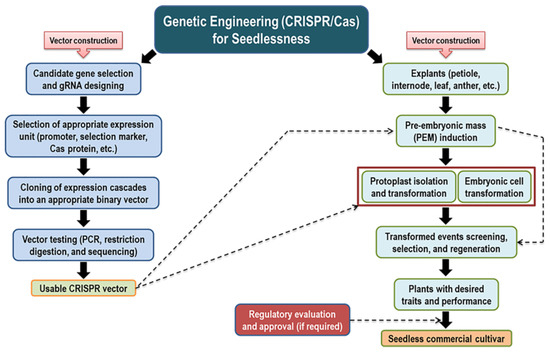
Figure 4.
Visual scheme of gaining the seedlessness trait using genetic engineering (CRISPR/Cas) strategy.
(I) Establishing a plant regeneration system based on embryonic suspension cell culture. Genetic transformation using embryogenic cell suspension cultures is a good opportunity because of their higher organogenetic potential [129,130,131,132]. Regeneration of putatively transformed cells and subsequent grafting of transgenic micro-shoots on rootstocks may shorten the juvenile period for flowering and fruiting [133].
(II) Selecting appropriate candidate gene(s) and regulatory elements for targeted genome editing. The AGL11 is considered the only identified upstream regulatory gene that controls the ovule/seed development, and its Arabidopsis mutant phenotype (stk) displayed compromised seed characteristics. [87]. In the case of V. vinifera, stenospermocarpy seedlessness is associated with a SNP mutation in VviAGL11 (R197L) [16]. The availability of whole genome sequence (i.e., muscadine whole genome sequence) [134] facilitates target gene selection. In the current review, we highlighted many other genes associated with seed development/abortion (Table 1). These genes could be target candidates for genome editing. Organ-specific promoter-driven Cas protein expression has been reported on the CRISPR platform [116,135,136,137]. Using a seed-specific promoter could achieve the goal more effectively and efficiently because the desired expression will occur only in seeds. Seed development-specific promoters have been characterized using various genes and different plant species [96,138,139,140].
(III) Cloning and assembly of a binary vector. Guide RNA (gRNA) design for the particular gene and cloning the gRNAs into appropriate vector backbones are the primary tasks for genome editing vector construction. Different online tools for gRNA design (i.e., CRISPOR [141], CRISPR-P [142], CCTop [143], CHOPCHOP [144], and GuideMaker [145]) with customized features are openly accessible. High-efficiency cloning technology (MoClo) [146] and readymade cloning materials of different expression modules are readily available from addgene (https://www.addgene.org/) and other sources. Adopting the MoClo cloning technology would accelerate vector construction efficiency. Recently, it has been shown that gRNA possessing the same restriction site as the type II restriction enzyme used for the GG reaction does not affect MoClo cloning and subsequent genome editing efficiency, which expanded the gRNA selection options [147].
(IV) Transformation of embryogenic cells or protoplasts and regeneration of putatively transformed cells into a complete plant. Using the appropriate transformation system increases the chances of getting transformed events. Among the different types of transformation processes, Agrobacterium-mediated or direct protoplast transformation could be adopted. Interestingly, RNP (ribonucleoprotein) mediated genome editing of protoplasts could avoid current GMO regulations, as the USDA does not consider the plant a GMO if the engineering involves a plant self-repair mechanism.
(V) Screening for potential transgenic events and securing approval from regulatory agencies. The procedure is associated with both molecular and phenotypic evaluations. Molecular screening means genomic and proteomic studies of the desired genome engineering plant(s) to confirm that desired change(s) in the genome, and phenotypic screening means the study of visual changes (either positive or negative) in the plants. If satisfactory performance is achieved, the new plant genotype needs approval from regulatory authorities before release for commercialization.
6. Bio-Engineered Food Regulation and Consumer Acceptance
Conventional breeding has limited application for developing innovative value-added cultivars because of extreme heterozygosity, which is fostered by inbreeding depression. Perennial crops are characterized by long juvenility, extended breeding cycles, large plant size, poor fecundity, and high heterozygosity due to outcrossing fertilization. Accordingly, the development and introduction of improved cultivars is challenging, and the breeders lack the capacity to generate new cultivars quickly in response to evolving consumer/industry preferences and crisis circumstances (i.e., climate change). New cost-effective breeding technologies with obvious potential for enhanced improvement of economically viable crops have emerged from advances in genomic research and the refinement of cell culture tools. The technologies are well adapted to enrich precision breeding efficiency by enabling accurate, targeted, and reliable changes to the genome. This caused rapid changes in the landscape of life sciences, providing many novel biological applications by targeting several economically important traits. CRISPR/Cas technology has been developed to disrupt specific genomic loci with a very limited number of off-target alterations, resulting in plants with edited alleles. This method of delivery is effective in producing non-GMO plants due to its ability to avoid issues that arise from the stable insertion of T-DNAs into the genome.
Safety subject is a concern for bioengineered food. Accordingly, different regulatory organizations work in cooperation. Three U.S. organizations, including the food and drug administration (FDA), the U.S. department of agriculture (USDA), and the environmental protection agency (EPA) co-regulate the pre- and post-release of bio-engineered food products. In European Union (EU) countries, European food safety authority (EFSA) looks after the bioengineered products. The factor of evaluation between the U.S. and EU is also different. The U.S. approach focuses on the end product. Bop-engineered foods fall under the FDA classification of “generally recognized as safe”. They do not have to be approved before entering the market, and they typically do not require special labeling. However, the FDA recommends that companies go through a voluntary consultation process to determine whether their new GM foods would require premarket approval. Approval is necessary if the GM food contains high levels of toxic substances, allergens, or reduced levels of key nutrients. Interestingly, bioengineered food is not considered GM if the product is free from Agrobacterium, transgenes, and foreign genetic materials. Hence, there is a high probability that current GMO regulations could be avoided by CRISPR/Cas RNP-mediated precise genetic engineering [27,28]. However, the EU imposed more stringent regulations on GM foods than the US. The EU’s regulatory approach focuses more on the process than the product. As all GM foods are made with different processes than natural (conventional) foods, they are supposed to be regulated. All GM food products must require premarket approval and proper labeling.
Despite all of these, a good number of bioengineered crops have been commercialized [23,24]. Bioengineered papaya, sweet corn, squash, potato, apple, and eggplant have been released for fresh consumption [25]. Despite that, there have not been any safety issues noticed so far, but there is still concern about consumer acceptance of bioengineered products. The primary concern is somehow justified due to the non-directional changes resulting from genetic transformation in some cases [148]. Interestingly, there should be a different approach to mitigate or eliminate these off-target effects [115,120,121].
7. Conclusions
Tissue and developmental stage-specific mutagenesis of candidate gene(s) by CRISPR-TSKO and CRISPR-based precise nucleotide editing are favorable options for seedlessness breeding since these strategies overcome the interspecies hybridization barrier and prevent/minimize the pleiotropic effects of genetic engineering. Moreover, it could develop transgene-free if RNP-mediated transformation is subjected. Appropriate gene targeting, gRNA designing, appropriate promoter and Cas protein selection, cloning of all modules into a proper binary vector, transforming plant cells, and subsequent regeneration are the steps for genetic engineering-mediated seedlessness trait gaining. Finding or establishing a good transformation and plant regeneration system could largely improve the desired plant recovery rate. This article summarizes the genes that could be targeted with the CRISPR/Cas platform. It also proposed a model and provided sources of related, useful information for executing genome-editing projects for gaining seedlessness. This approach could be applied to other crops as well. However, in the case of working with plants of different ploidy, hybrids, and plants with different degrees of sex expression, additional technologies will be required for a preliminary assessment of the genotype and a special protocol for evaluating the results since it is not enough to guarantee them simply by the presence or absence of an edited genome region.
Author Contributions
Conceptualization, writing—original draft preparation, visualization (M.M.); writing—review and editing (A.G.D., A.I., A.E.-k. and V.T.); conceptualization, funding acquisition, project administration, supervision, writing—review and editing, visualization (I.E.-S.); All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 1890 Institution Teaching, Research, and Extension Capacity Building Grants (CBG) Program (grant no. 2019-38821-29150; project accession no. 1018161) from the USDA National Institute of Food and Agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We want to express our deep and sincere gratitude to the Viticulture Advisory Council (VAC) and the Florida Grape Growers Association (FGGA) for their continued support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wijesinghe, S.A.E.C.; Evans, L.J.; Kirkland, L.; Rader, R. A global review of watermelon pollination biology and ecology: The increasing importance of seedless cultivars. Sci. Hortic. 2020, 271, 109493. [Google Scholar] [CrossRef]
- Vignati, E.; Lipska, M.; Dunwell, J.M.; Caccamo, M.; Simkin, A.J. Options for the generation of seedless cherry, the ultimate snacking product. Planta 2022, 256, 90. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, A.; Scalzo, R.L.; Rotino, G.L.; Acciarri, N.; Spena, A.R.; Vitelli, G.; Bertolo, G. Freezing effect on some quality parameters of transgenic parthenocarpic eggplants. J. Food Eng. 2003, 56, 285–287. [Google Scholar] [CrossRef]
- Pandolfini, T. Seedless fruit production by hormonal regulation of fruit set. Nutrients 2009, 1, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Acciarri, N.; Restaino, F.; Vitelli, G.; Perrone, D.; Zottini, M.; Pandolfini, T.; Spena, A.; Rotino, G. Genetically modified parthenocarpic eggplants: Improved fruit productivity under both greenhouse and open field cultivation. BMC Biotechnol. 2002, 2, 4. [Google Scholar] [CrossRef]
- Bouquet, A.; Danglot, Y. Inheritance of seedlessness in grapevine (Vitis vinifera L.). Vitis 1996, 35, 35–42. [Google Scholar]
- de Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Sherif, S.; El Kayal, W.; Mahboob, A.; Abubaker, K.; Ravindran, P.; Jyothi-Prakash, P.A.; Kumar, P.P.; Jayasankar, S. Characterization of gibberellin-signalling elements during plum fruit ontogeny defines the essentiality of gibberellin in fruit development. Plant Mol. Biol. 2014, 84, 399–413. [Google Scholar] [CrossRef]
- Ledbetter, C.A.; Ramming, D.W. Seedlessness in grapes. Hortic. Rev. 1989, 11, 159–184. [Google Scholar]
- Ingrosso, I.; Bonsegna, S.; De Domenico, S.; Laddomada, B.; Blando, F.; Santino, A.; Giovinazzo, G. Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol. Biochem. 2011, 49, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Gorguet, B.; van Heusden, A.W.; Lindhout, P. Parthenocarpic fruit development in tomato. Plant Biol. 2005, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, S.; Frova, C.; Torti, G.; Soressi, G.P. Relationship between set, development, and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978, 19, 1281–1288. [Google Scholar] [CrossRef]
- Picarella, M.E.; Mazzucato, A. The occurrence of seedlessness in higher plants; Insights on roles and mechanisms of parthenocarpy. Front. Plant Sci. 2019, 9, 2018. [Google Scholar] [CrossRef]
- Doligez, A.; Bouquet, A.; Danglot, Y.; Lahogue, F.; Riaz, S.; Meredith, P.; Edwards, J.; This, P. Genetic mapping of grapevine (Vitis vinifera L.) applied to the detection of QTLs for seedlessness and berry weight. Theor. Appl. Genet. 2002, 105, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Torres-Pérez, R.; Mauri, N.; Diestro, N.; Cabezas, J.A.; Marchal, C.; Lacombe, T.; Ibáñez, J.; Tornel, M.; Carreño, J.; et al. The major origin of seedless grapes is associated with a missense mutation in the MADS-box gene VviAGL11. Plant Physiol. 2018, 177, 1234–1253. [Google Scholar] [CrossRef] [PubMed]
- Hanania, U.; Velcheva, M.; Or, E.; Flaishman, M.; Sahar, N.; Perl, A. Silencing of chaperonin 21 that was differentially expressed in inflorescence of seedless and seeded grapes, promoted seed abortion in tobacco and tomato fruits. Transgenic Res. 2007, 16, 515–525. [Google Scholar] [CrossRef]
- Malabarba, J.; Buffon, V.; Mariath, J.E.A.; Gaeta, M.L.; Dornelas, M.C.; Margis-Pinheiro, M.; Pasquali, G.; Revers, L.F. The MADS-box gene Agamous-like 11 is essential for seed morphogenesis in grapevine. J. Exp. Bot. 2017, 68, 1493–1506. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, B.; Li, Y.; Van Nocker, S.; Wang, Y.; Zhang, C. Differential expression of the seed-specific gene ABCG20 between seedless and seeded grapes and its roles in tomato seed development. S. Afr. J. Bot. 2020, 131, 428–436. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef]
- Di Marzo, M.; Herrera-Ubaldo, H.; Caporali, E.; Novák, O.; Strnad, M.; Balanzà, V.; Ezquer, I.; Mendes, M.A.; de Folter, S.; Colombo, L. SEEDSTICK controls Arabidopsis fruit size by regulating cytokinin levels and FRUITFULL. Cell Rep. 2020, 30, 2846–2857. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Ollitrault, P.; Navarro, L. Production of tetraploid plants of non apomictic citrus genotypes. Plant Cell Rep. 2009, 28, 1837–1846. [Google Scholar] [CrossRef]
- ISAAA. Global Status of Commercialized Biotech/GM Crops; ISAAA Brief No. 54; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Redden, R. Genetic modification for agriculture—Proposed revision of GMO regulation in Australia. Plants 2021, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G. Genetically engineered foods and their regulation: The way forward after twenty years of adoption. In Regulatory Focus; Regulatory Affairs Professionals Society: Rockville, MD, USA, 2016. [Google Scholar]
- Brookes, G. Crop Biotechnology Continues to Provide Higher Farmer Income and Significant Environmental Benefits; PG Economics: Dorchester, UK, 2020. [Google Scholar]
- Jones, H.D. Regulatory uncertainty over genome editing. Nat. Plants 2015, 1, 14011. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. US regulation misses some GM crops. Nature 2013, 500, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Thomazella, D.P.T.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schauer, N.; Usadel, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latché, A.; Pech, J.C.; Fernie, A.R.; Bouzayen, M. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 2009, 21, 1428–1452. [Google Scholar] [CrossRef]
- Dorcey, E.; Urbez, C.; Blázquez, M.A.; Carbonell, J.; Perez-Amador, M.A. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009, 58, 318–332. [Google Scholar] [CrossRef]
- Vriezen, W.H.; Feron, R.; Maretto, F.; Keijman, J.; Mariani, C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 2008, 177, 60–76. [Google Scholar] [CrossRef]
- Dharmasiri, N.; Estelle, M. Auxin signaling and regulated protein degradation. Trends Plant Sci. 2004, 9, 302–308. [Google Scholar] [CrossRef]
- Leyser, O. Dynamic integration of auxin transport and signalling. Curr. Biol. 2006, 16, R424–R433. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Hooper, L.C.; Johnson, S.D.; Rodrigues, J.C.; Vivian-Smith, A.; Koltunow, A.M. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007, 145, 351–366. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Wolters-Arts, M.; Feron, R.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009, 57, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Feng, Q.; Qin, L.; Pan, C.; Lamin-Samu, A.T.; Lu, G. Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci. Rep. 2018, 8, 2971. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Song, S.; Zhu, X.; Lin, Y.; Pan, Z.; Chen, L.; Chen, D.; Hu, G.; Huang, B.; Chen, M.; et al. SlTPL1 silencing induces facultative parthenocarpy in tomato. Front. Plant Sci. 2021, 12, 672232. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Miyatake, K.; Endo, M.; Urashimo, S.; Kawanishi, T. Loss of function of the Pad-1 aminotransferase gene, which is involved in auxin homeostasis, induces parthenocarpy in Solanaceae plants. Proc. Natl. Acad. Sci. USA 2020, 117, 12784–12790. [Google Scholar] [CrossRef] [PubMed]
- Pandolfini, T.; Molesini, B.; Spena, A. AUCSIA: An ancestral green plant miniprotein and the emergence of auxin transport. Plant Signal. Behav. 2013, 8, e22928. [Google Scholar] [CrossRef]
- Molesini, B.; Pandolfini, T.; Pii, Y.; Korte, A.; Spena, A. Arabidopsis thaliana AUCSIA-1 regulates auxin biology and physically interacts with a kinesin-related protein. PLoS ONE 2012, 7, e41327. [Google Scholar] [CrossRef]
- Mounet, F.; Moing, A.; Kowalczyk, M.; Rohrmann, J.; Petit, J.; Garcia, V.; Maucourt, M.; Yano, K.; Deborde, C.; Aoki, K.; et al. Down-regulation of a single auxin efflux transport protein in tomato induces precocious fruit development. J. Exp. Bot. 2012, 63, 4901–4917. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Q.; Shen, W.; El Mohtar, C.A.; Zhao, X.; Gmitter, F.G. Functional study of CHS gene family members in citrus revealed a novel CHS gene affecting the production of flavonoids. BMC Plant Biol. 2018, 18, 189. [Google Scholar] [CrossRef]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Schijlen, E.G.; de Vos, C.H.; Martens, S.; Jonker, H.H.; Rosin, F.M.; Molthoff, J.W.; Tikunov, Y.M.; Angenent, G.C.; van Tunen, A.J.; Bovy, A.G. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007, 144, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Fos, M.; Nuez, F.; García-Martínez, J.L. The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol. 2000, 122, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Olimpieri, I.; Siligato, F.; Caccia, R.; Mariotti, L.; Ceccarelli, N.; Soressi, G.P.; Mazzucato, A. Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 2007, 226, 877–888. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Martínez-Bello, L.; Moritz, T.; López-Díaz, I. Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J. Exp. Bot. 2015, 66, 5897–5910. [Google Scholar] [CrossRef]
- Harberd, N.P. Relieving DELLA restraint. Science 2003, 299, 1853–1854. [Google Scholar] [CrossRef]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Lglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef]
- Murase, K.; Hirano, Y.; Sun, T.P.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef]
- Fuentes, S.; Ljung, K.; Sorefan, K.; Alvey, E.; Harberd, N.P.; Østergaard, L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 2012, 24, 3982–3996. [Google Scholar] [CrossRef] [PubMed]
- Martí, C.; Orzáez, D.; Ellul, P.; Moreno, V.; Carbonell, J.; Granell, A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007, 52, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Jacob, E.; Hod-Dvorai, R.; Ben-Mordechai, O.L.; Boyko, Y.; Avni, O. Dual function of polycomb group proteins in differentiated murine T helper (CD4+) cells. J. Mol. Signal. 2011, 6, 5. [Google Scholar] [CrossRef]
- Köhler, C.; Hennig, L.; Spillane, C.; Pien, S.; Gruissem, W.; Grossniklaus, U. The polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 2003, 17, 1540–1553. [Google Scholar] [CrossRef]
- Ohad, N.; Margossian, L.; Hsu, Y.C.; Williams, C.; Repetti, P.; Fischer, R.L. A mutation that allows endosperm development without fertilization. Proc. Natl. Acad. Sci. USA 1996, 93, 5319–5324. [Google Scholar] [CrossRef]
- Chaudhury, A.M.; Ming, L.; Miller, C.; Craig, S.; Dennis, E.S.; Peacock, W.J. Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 4223–4228. [Google Scholar] [CrossRef]
- Guitton, A.E.; Berger, F. Loss of function of MULTICOPY SUPPRESSOR OF IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Curr. Biol. 2005, 15, 750–754. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.; Luo, M.; Chaudhury, A.; Berger, F. DNA methylation causes predominant maternal controls of plant embryo growth. PLoS ONE 2008, 3, e2298. [Google Scholar] [CrossRef]
- Schmidt, A.; Wöhrmann, H.J.; Raissig, M.T.; Arand, J.; Gheyselinck, J.; Gagliardini, V.; Heichinger, C.; Walter, J.; Grossniklaus, U. The polycomb group protein MEDEA and the DNA methyltransferase MET1 interact to repress autonomous endosperm development in Arabidopsis. Plant J. 2013, 73, 776–787. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Pelaz, S.; Liljegren, S.J.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; de Pouplana, L.R.; Martínez-Castilla, L.; Yanofsky, M.F. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 2000, 97, 5328–5333. [Google Scholar] [CrossRef]
- Saedler, H.; Becker, A.; Winter, K.U.; Kirchner, C.; Theißen, G. MADS-box genes are involved in floral development and evolution. Acta Biochim. Pol. 2001, 48, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Castelán-Muñoz, N.; Herrera, J.; Cajero-Sánchez, W.; Arrizubieta, M.; Trejo, C.; García-Ponce, B.; Sánchez, M.d.l.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. MADS-box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Front. Plant Sci. 2019, 10, 853. [Google Scholar] [CrossRef]
- De Bodt, S.; Raes, J.; Van de Peer, Y.; Theißen, G. And then there were many: MADS goes genomic. Trends Plant Sci. 2003, 8, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.; Melzer, R.; Theißen, G. MIKC-type MADS-domain proteins: Structural modularity, protein interactions and network evolution in land plants. Gene 2005, 347, 183–198. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef] [PubMed]
- Daminato, M.; Masiero, S.; Resentini, F.; Lovisetto, A.; Casadoro, G. Characterization of TM8, a MADS-box gene expressed in tomato flowers. BMC Plant Biol. 2014, 14, 319. [Google Scholar] [CrossRef]
- Geuten, K.; Irish, V. Hidden variability of floral homeotic B genes in solanaceae provides a molecular basis for the evolution of novel functions. Plant Cell 2010, 22, 2562–2578. [Google Scholar] [CrossRef]
- Okabe, Y.; Yamaoka, T.; Ariizumi, T.; Ushijima, K.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Kusano, M.; Shinozaki, Y.; Pulungan, S.I.; et al. Aberrant stamen development is associated with parthenocarpic fruit set through up-regulation of gibberellin biosynthesis in tomato. Plant Cell Physiol. 2019, 60, 38–51. [Google Scholar] [CrossRef]
- Medina, M.; Roque, E.; Pineda, B.; Cañas, L.; Rodriguez-Concepción, M.; Beltrán, J.P.; Gómez-Mena, C. Early anther ablation triggers parthenocarpic fruit development in tomato. Plant Biotechnol. J. 2013, 11, 770–779. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Y.; Morris, B.A. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc. Natl. Acad. Sci. USA 2001, 98, 1306–1311. [Google Scholar] [CrossRef]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Morris, B.A.; Sutherland, P.; Veit, B.; Yao, J.L. Down-regulation of TM29, a tomato SEPALLATA homolog, causes parthenocarpic fruit development and floral reversion. Plant Physiol. 2002, 130, 605–617. [Google Scholar] [CrossRef]
- Favaro, R.; Pinyopich, A.; Battaglia, R.; Kooiker, M.; Borghi, L.; Ditta, G.; Yanofsky, M.F.; Kater, M.M.; Colombo, L. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell 2003, 15, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Mizzotti, C.; Ezquer, I.; Paolo, D.; Rueda-Romero, P.; Guerra, R.F.; Battaglia, R.; Rogachev, I.; Aharoni, A.; Kater, M.M.; Caporali, E.; et al. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014, 10, e1004856. [Google Scholar] [CrossRef]
- Singh, R.; Low, E.T.; Ooi, L.C.; Ong-Abdullah, M.; Ting, N.C.; Nagappan, J.; Nookiah, R.; Amiruddin, M.D.; Rosli, R.; Manaf, M.A.; et al. The oil palm SHELL gene controls oil yield and encodes a homologue of SEEDSTICK. Nature 2013, 500, 340–344. [Google Scholar] [CrossRef]
- Huang, B.; Hu, G.; Wang, K.; Frasse, P.; Maza, E.; Djari, A.; Deng, W.; Pirrello, J.; Burlat, V.; Pons, C.; et al. Interaction of two MADS-box genes leads to growth phenotype divergence of all-flesh type of tomatoes. Nat. Commun. 2021, 12, 6892. [Google Scholar] [CrossRef] [PubMed]
- Schiefthaler, U.; Balasubramanian, S.; Sieber, P.; Chevalier, D.; Wisman, E.; Schneitz, K. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1999, 96, 11664–11669. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Ye, D.; Xu, J.; Sundaresan, V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 1999, 13, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.; Parameswaran, S.; Ito, T.; Seubert, B.; Auer, M.; Rymaszewski, A.; Jia, G.; Owen, H.A.; Zhao, D. The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Physiol. 2009, 151, 1401–1411. [Google Scholar] [CrossRef]
- Ito, T.; Wellmer, F.; Yu, H.; Das, P.; Ito, N.; Alves-Ferreira, M.; Riechmann, J.L.; Meyerowitz, E.M. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 2004, 430, 356–360. [Google Scholar] [CrossRef]
- Rojas-Gracia, P.; Roque, E.; Medina, M.; Rochina, M.; Hamza, R.; Angarita-Díaz, M.P.; Moreno, V.; Pérez-Martín, F.; Lozano, R.; Cañas, L.; et al. The parthenocarpic hydra mutant reveals a new function for a SPOROCYTELESS-like gene in the control of fruit set in tomato. New Phytol. 2017, 214, 1198–1212. [Google Scholar] [CrossRef]
- Locher, K.P. Review. Structure and mechanism of ATP-binding cassette transporters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, W.; Shi, Y.; Wang, Y.; Zhang, C. Cloning and activity analysis of the highly expressed gene VviABCG20 promoter in seed and its activity is negatively regulated by the transcription factor VviDof14. Plant Sci. 2022, 315, 111152. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mahfouz, M.M.; Mansoor, S. CRISPR-TSKO: A tool for tissue-specific genome editing in plants. Trends Plant Sci. 2020, 25, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR-Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, W.; Zhang, J.; Zhou, J.; Wang, J.; Chen, L.; Wang, L.; Hodgkins, A.; Iyer, V.; Huang, X.; et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 2014, 11, 399–402. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Liu, Q.; Li, J.; Wang, K. Increasing the efficiency of CRISPR-Cas9-VQR precise genome editing in rice. Plant Biotechnol. J. 2018, 16, 292–297. [Google Scholar] [CrossRef]
- Müller, M.; Lee, C.M.; Gasiunas, G.; Davis, T.H.; Cradick, T.J.; Siksnys, V.; Bao, G.; Cathomen, T.; Mussolino, C. Streptococcus thermophilus CRISPR-Cas9 systems enable specific editing of the human genome. Mol. Ther. 2016, 24, 636–644. [Google Scholar] [CrossRef]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Paul, B.; Montoya, G. CRISPR-Cas12a: Functional overview and applications. Biomed. J. 2020, 43, 8–17. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Rusk, N. CRISPRs and epigenome editing. Nat. Methods 2013, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Polstein, L.R.; Gersbach, C.A. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat. Chem. Biol. 2015, 11, 198–200. [Google Scholar] [CrossRef]
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.-W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 2013, 155, 1479–1491. [Google Scholar] [CrossRef]
- Lee, C.M.; Cradick, T.J.; Bao, G. The Neisseria meningitidis CRISPR-Cas9 system enables specific genome editing in mammalian cells. Mol. Ther. 2016, 24, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 2017, 35, 31. [Google Scholar] [CrossRef]
- Song, G.; Jia, M.; Chen, K.; Kong, X.; Khattak, B.; Xie, C.; Li, A.; Mao, L. CRISPR/Cas9: A powerful tool for crop genome editing. Crop J. 2016, 4, 75–82. [Google Scholar] [CrossRef]
- Wei, Q.; Liao, J.; Yu, X.; Wang, E.J.; Wang, C.; Luu, H.H.; Haydon, R.C.; Lee, M.J.; He, T.C. An NgAgo tool for genome editing: Did CRISPR/Cas9 just find a competitor? Genes Dis. 2016, 3, 169–170. [Google Scholar] [CrossRef]
- Zaidi, S.S.-e.-A.; Mahfouz, M.M.; Mansoor, S. CRISPR-Cpf1: A new tool for plant genome editing. Trends Plant Sci. 2017, 22, 550–553. [Google Scholar] [CrossRef]
- Decaestecker, W.; Buono, R.A.; Pfeiffer, M.L.; Vangheluwe, N.; Jourquin, J.; Karimi, M.; Van Isterdael, G.; Beeckman, T.; Nowack, M.K.; Jacobs, T.B. CRISPR-TSKO: A technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell 2019, 31, 2868–2887. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, Z.; Feng, Z.; Wei, P.; Zhang, H.; Botella, J.R.; Zhu, J.K. Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol. J. 2016, 14, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P.; Xing, H.-L.; Dong, L.; Zhang, H.-Y.; Han, C.-Y.; Wang, X.-C.; Chen, Q.-J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015, 16, 144. [Google Scholar] [CrossRef]
- Liang, Y.; Eudes, A.; Yogiswara, S.; Jing, B.; Benites, V.T.; Yamanaka, R.; Cheng-Yue, C.; Baidoo, E.E.; Mortimer, J.C.; Scheller, H.V.; et al. A screening method to identify efficient sgRNAs in Arabidopsis, used in conjunction with cell-specific lignin reduction. Biotechnol. Biofuels 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Monsur, M.B.; Shao, G.; Lv, Y.; Ahmad, S. Base editing: The ever expanding clustered regularly interspaced short palindromic repeats (CRISPR) tool kit for precise genome editing in plants. Genes 2020, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Yang, Y. CRISPR/Cas-mediated base editing: Technical considerations and practical applications. Trends Biotechnol. 2019, 37, 1121–1142. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Baisouny, F.M.; Himelrick, D.G. Muscadine grapes. J. Am. Pomol. Soc. 2002, 56, 207. [Google Scholar] [CrossRef]
- Park, M.; Sarkhosh, A.; Tsolova, V.; El-Sharkawy, I. Horizontal transfer of LTR retrotransposons contributes to the genome diversity of Vitis. Int. J. Mol. Sci. 2021, 22, 10446. [Google Scholar] [CrossRef]
- Lu, J.; Schell, L.; Ramming, D. Interspecific hybridization between Vitis rotundifolia and Vitis vinifera and evaluation of the hybrids. Acta Hortic. 1998, 528, 481–486. [Google Scholar] [CrossRef]
- Xu, X.; Lu, J.; Bradley, F. Applications of polyploids in muscadine grape (Vitis rotundifolia Michx.) breeding. Acta Hortic. 2014, 1046, 411–417. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Zhong, Y.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Exploration of susceptible genes with clustered regularly interspaced short palindromic repeats–tissue-specific knockout (CRISPR-TSKO) to enhance host resistance. CRC Crit. Rev. Plant Sci. 2020, 39, 387–417. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Dai, X.; Zhang, D.; Zhao, Y. An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol. 2016, 171, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Mansoor, S. Viral vectors for plant genome engineering. Front. Plant Sci. 2017, 8, 539. [Google Scholar] [CrossRef]
- Omar, A.A.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. Somatic embryogenesis: Still a relevant technique in citrus improvement. Methods Mol. Biol. 2016, 1359, 289–327. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Citrus cell suspension culture establishment, maintenance, efficient transformation and regeneration to complete transgenic plant. Plants 2021, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Yaakob, Z.; Anuar, N. Factors affecting in vitro regeneration of Ficus carica L. and genetic fidelity studies using molecular marker. J. Plant Biochem. Biotechnol. 2021, 30, 304–316. [Google Scholar] [CrossRef]
- Li, Z.T.; Kim, K.-H.; Dhekney, S.A.; Jasinski, J.R.; Creech, M.R.; Gray, D.J. An optimized procedure for plant recovery from somatic embryos significantly facilitates the genetic improvement of Vitis. Hortic. Res. 2014, 1, 14027. [Google Scholar] [CrossRef]
- Dutt, M.; Grosser, J.W. An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Rep. 2010, 29, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Vera, D.; Kambiranda, D.; Gajjar, P.; Cadle-Davidson, L.; Tsolova, V.; El-Sharkawy, I. Chromosome-level genome sequence assembly and genome wide association study of Muscadinia rotundifolia reveal the genetics of 12 berry-related traits. Hortic. Res. 2021, 9, uhab011. [Google Scholar] [CrossRef]
- Ordon, J.; Bressan, M.; Kretschmer, C.; Dall’Osto, L.; Marillonnet, S.; Bassi, R.; Stuttmann, J. Optimized Cas9 expression systems for highly efficient Arabidopsis genome editing facilitate isolation of complex alleles in a single generation. Funct. Integr. Genom. 2020, 20, 151–162. [Google Scholar] [CrossRef]
- Ren, Q.; Zhong, Z.; Wang, Y.; You, Q.; Li, Q.; Yuan, M.; He, Y.; Qi, C.; Tang, X.; Zheng, X.; et al. Bidirectional promoter-based CRISPR-Cas9 systems for plant genome editing. Front. Plant Sci. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Zheng, N.; Li, T.; Dittman, J.D.; Su, J.; Li, R.; Gassmann, W.; Peng, D.; Whitham, S.A.; Liu, S.; Yang, B. CRISPR/Cas9-based gene editing using egg cell-specific promoters in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 800. [Google Scholar] [CrossRef]
- Gong, P.; Wei, R.; Li, Y.; Wang, R.; Tang, Y.; Wang, L.; Zhu, H.; Wang, Y.; Zhang, C. Molecular cloning and functional characterization of a seed-specific VvβVPE gene promoter from Vitis vinifera. Planta 2019, 250, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Choi, J.Y.; Shin, H.Y.; Bae, J.M.; Shin, J.S. Seed-specific expression of seven Arabidopsis promoters. Gene 2014, 553, 17–23. [Google Scholar] [CrossRef]
- Tang, G.; Xu, P.; Li, P.; Zhu, J.; Chen, G.; Shan, L.; Wan, S. Cloning and functional characterization of seed-specific LEC1A promoter from peanut (Arachis hypogaea L.). PLoS ONE 2021, 16, e0242949. [Google Scholar] [CrossRef]
- Haeussler, M.; Schönig, K.; Eckert, H.; Eschstruth, A.; Mianné, J.; Renaud, J.-B.; Schneider-Maunoury, S.; Shkumatava, A.; Teboul, L.; Kent, J.; et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016, 17, 148. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, M.; Thumberger, T.; Del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. CCTop: An Intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 2015, 10, e0124633. [Google Scholar] [CrossRef] [PubMed]
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef]
- Poudel, R.; Rodriguez, L.T.; Reisch, C.R.; Rivers, A.R. GuideMaker: Software to design CRISPR-Cas guide RNA pools in non-model genomes. GigaScience 2022, 11, giac007. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Zhong, G. Having a same type IIS enzyme’s restriction site on guide RNA sequence does not affect golden gate (GG) cloning and subsequent CRISPR/Cas mutagenesis. Int. J. Mol. Sci. 2022, 23, 4889. [Google Scholar] [CrossRef] [PubMed]
- Chaban, I.; Khaliluev, M.; Baranova, E.; Kononenko, N.; Dolgov, S.; Smirnova, E. Abnormal development of floral meristem triggers defective morphogenesis of generative system in transgenic tomatoes. Protoplasma 2018, 255, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).