Abstract
Extracellular vesicles (EVs) are nano-scaled vesicles released from all cell types into extracellular fluids and specifically contain signature molecules of the original cells and tissues, including the placenta. Placenta-derived EVs can be detected in maternal circulation at as early as six weeks of gestation, and their release can be triggered by the oxygen level and glucose concentration. Placental-associated complications such as preeclampsia, fetal growth restriction, and gestational diabetes have alterations in placenta-derived EVs in maternal plasma, and this can be used as a liquid biopsy for the diagnosis, prediction, and monitoring of such pregnancy complications. Alpha-thalassemia major (“homozygous alpha-thalassemia-1”) or hemoglobin Bart’s disease is the most severe form of thalassemia disease, and this condition is lethal for the fetus. Women with Bart’s hydrops fetalis demonstrate signs of placental hypoxia and placentomegaly, thereby placenta-derived EVs provide an opportunity for a non-invasive liquid biopsy of this lethal condition. In this article, we introduced clinical features and current diagnostic markers of Bart’s hydrops fetalis, extensively summarize the characteristics and biology of placenta-derived EVs, and discuss the challenges and opportunities of placenta-derived EVs as part of diagnostic tests for placental complications focusing on Bart’s hydrop fetalis.
1. Introduction
Thalassemia is the most common hematologic genetic disease in Southeast Asia. Alpha-thalassemia major (homozygous alpha-thalassemia-1) or hemoglobin (Hb) Bart’s disease is the most severe form of thalassemia disease [1,2,3,4,5]. The prevalence of Hb Bart’s disease is approximately 0.23% [6], with a deletion frequency in Southeast Asia as high as 4.5–5% [7]. The term Hb Bart’s hydrops fetalis was first described in 1960 [8] and the fetuses with this condition are characterized by severe anemia, hypoxia, heart failure, and hydrops fetalis (accumulation of body fluid) [9]. Unfortunately, these fetuses usually die in utero or in the early neonatal period, although the survivors after gene therapy or bone marrow transplantation have been reported [10,11,12]. These survivors remain transfusion dependent.
Molecular diagnosis of Hb Bart’s hydrops fetalis is characterized by homozygous α-thalassemia-1 (-/-); therefore, the entire delta and globin gene clusters are lacking but present only Hb Bart’s (γ4) [1]. Such Hb has a high oxygen affinity, creating widespread tissue hypoxia and fetal anemia. Hemodynamic changes in fetal anemia are cardiomyopathy, increased intravascular volume, hepatic extramedullary hematopoiesis, and widespread endothelial cell damage resulting in cardiomegaly, hepatosplenomegaly, and placentomegaly [13,14]. Currently, prenatal screening of this condition is possibly performed by the determination of the mean corpuscular volume, dichlorophenolindophenol, osmotic fragility, or Hb typing [15,16]. Any couple at risk of Hb Bart’s hydrops fetalis is counseled to perform an invasive diagnostic test (chorionic villus sampling, amniocentesis, or cordocentesis) or to follow up with a non-invasive approach by performing ultrasonography to evaluate the cardiac diameter/thoracic ratio (CTR), middle cerebral artery peak systolic velocity (MCA PSV), or placental thickness [17,18,19,20,21]. Maternal blood biomarkers including non-invasive prenatal diagnosis tests such as prenatal cell-free fetal DNA for the identification of Hb Bart’s hydrops fetalis are poor predictors, thus, they are not routinely used in a clinical setting [22,23,24,25,26,27].
With a better understanding of intercellular communication, the role of extracellular vesicles (EVs) has emerged [28,29,30,31,32]. Accumulating evidence has shown that placenta-derived EVs can be identified in maternal plasma at as early as six weeks of gestation and their concentrations have increased as a function of gestation in normal pregnancy [33]. Maternal plasma concentrations of placenta-derived EVs released by the syncytiotrophoblast are further increased in pregnancy complications associated with placental hypoxia [34,35,36,37]. In this article, we will summarize the current evidence of biomarkers for the identification of Hb Bart’s hydrops fetalis, a condition associated with placental hypoxia [38], and discuss the challenges and opportunities of clinical implications of placenta-derived EVs for this lethal disease.
2. Bart’s Hydrops Fetalis: Clinical Perspectives and Current Diagnostic Procedures
In Hb Bart’s disease, the placenta can range from near normal in appearance to enlarged, friable, pale, and edematous (hydropic) [39,40] (Figure 1a). Their weights are often excessive, as much as 2 kg [21]. These changes reflect the adaptive placental response to severe and prolonged intrauterine hypoxia, beginning as early as the first trimester of pregnancy. The placental changes in Hb Bart’s disease involve both villous maturation and vascularity [41,42,43].
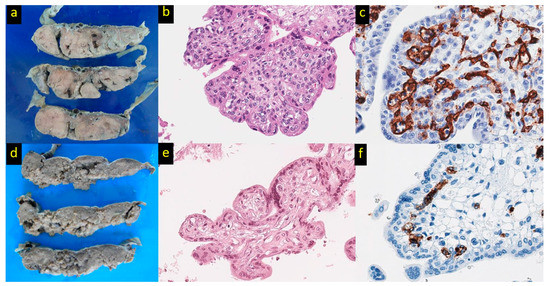
Figure 1.
Placental pathology in Hb Bart’s disease. (a) The placenta belonging to Hb Bart’s disease shows enlarged, pale, and edematous cut surfaces in comparison to the placenta of non-Hb Bart’s disease (d). (b) Immature intermediate villi with a bulbous contour possessing conspicuous myofibroblasts at the periphery beneath the trophoblastic layer referred to as peripheral villous stromal hypercellularity in comparison to the control (e) (hematoxylin and eosin, original magnification ×400). (c) A branching vascular pattern with increased vessel number and endothelial thickness in comparison to the control (f) (CD34 immunostaining, original magnification ×600).
In the normal placenta, mesenchymal villi first develop at 5 weeks’ of gestation, serving as the precursors to more mature villous types. Around 8 weeks of gestation, mesenchymal villi develop into immature intermediate villi, which become the predominant type by 14–20 weeks of gestation. As the pregnancy proceeds, immature intermediate villi transform into stem villi. Throughout the third trimester, mesenchymal villi differentiate into mature intermediate villi, which then later produce terminal villi growing in grape-like clusters as the final stage of villous development [44]. The placenta in Hb Bart’s hydrops increases the number of immature intermediate villi, which persist despite advancing in gestational age, and this finding is referred to as “generalized delayed villous maturation” [38,40]. Although Hb Bart’s fetuses suffer from severe anemia, the survival in the embryonic period is uneventful because of the production of the embryonic Hbs Portland I (ζ2γ2) and Gower I (ζ2ε2), which maintain the Fetal-placental circulation [45]. The switching from embryonic to abnormal fetal Hb (Hb Bart’s) that occurs around 6–8 weeks of gestation is concurrent with the transition of mesenchymal villi to immature intermediate villi [19,21,40]. This switch to an abnormal Hb may initiate or aggravate the increase in the number of immature intermediate villi, resulting in the persistence of such villi throughout gestation.
Other villous changes in the Hb Bart’s placenta involve the villous stroma and the cytotrophoblastic cells that cover the villous stroma. Cytotrophoblastic cells tend to be numerous and conspicuous in the Hb Bart’s placenta throughout gestation, in contrast to placentas in uncomplicated pregnancies where these cells become scarce by the third trimester [40]. Chronic hypoxia stimulates the proliferation of such cells but inhibits their fusion and differentiation into syncytiotrophoblast [42]. Within the stroma of the immature and mature intermediate villi of the Hb Bart’s placenta, there is often a characteristic increase in the number of stromal cells at the periphery beneath the trophoblast layer, a finding referred to as “peripheral villous stromal hypercellularity (PVSH)” [40] (Figure 1b). This change is seen in >80% of Hb Bart’s cases and is usually multifocal. PVSH is hypothesized to be a placental adaptation to chronic hypoxia, in utero. The immature intermediate villi have a large diameter, and their predominance in the Hb Bart’s placenta leads to the narrowing of the intervillous space, impeding fetoplacental oxygen and nutrition transfer. Contraction of the myofibroblastic cells in PVSH would serve to reduce the villous size, thereby widening the intervillous space for an increase in the maternal blood flow.
Vascular formation is a crucial step in placental development, and this is influenced by numerous factors, one of which is chronic hypoxia [21,41,42]. The pattern of the placental vascular adaptive response is influenced by the source of chronic hypoxia and can be classified into pre-placental (maternal), placental, and post-placental (fetal) sources. Pre-placental causes of hypoxia include maternal anemia, maternal diabetes, or maternal smoking, whereas placental causes result from a restricted supply of normoxic maternal blood to the placenta, as seen with preeclampsia at term and fetal growth restriction with preserved end-diastolic flow in umbilical arteries. In these situations, the oxygen level of the intervillous space is low and this stimulates a predominantly branching pattern of villous vascularization. On the other hand, in post-placental hypoxia, there is a failure of the fetoplacental extraction of oxygen from the intervillous space. Post-placental hypoxia can be observed in stillbirth or fetal growth restriction with an absent or reverse end-diastolic umbilical blood flow. The relatively high oxygen level in the intervillous space stimulates a predominantly non-branching pattern of villous vascularization. An increased number of villous vessels can be seen in pre-placental, placental, and post-placental hypoxia (Figure 1c).
Patterns of placental vasculature can be accurately assessed with the aid of computer-assisted measurements on placental histologic sections [38]. In Hb Bart’s, the number of villous vessels in the placenta is markedly increased. There is also a significant increase in the vascular perimeter and endothelial thickness. The etiology of the hypoxia-induced placental vascularization is multifactorial in Hb Bart’s disease. Morphometric studies have identified a branching vascular pattern, a change that is associated with pre-placental and placental hypoxia. This change is thought to have its basis for the marked placental enlargement, which compromised the blood flow, from the uterine distention and the generally diminished intervillous space due to the numerous intermediate types of villi. However, post-placental hypoxia is also likely to be in effect, given the greatly reduced capacity of Hb Bart’s to extract oxygen from the intervillous space.
Current Biomarkers for Hemoglobin Bart’s Disease
The non-invasive prenatal diagnosis test (NIPD) for fetal Hb Bart’s disease utilizes sonographic and maternal serum biomarkers as screening tools. Nonetheless, in actual practice, only sonographic markers are available, whereas serum biomarkers are rarely used in practice. Sonographic markers can accurately predict fetal Hb Bart’s disease as early as the late first trimester (12–15 weeks of gestation), with a detection rate of 90–95 percent [46,47,48,49]. Almost all cases of fetal Hb Bart’s disease are detected at gestational age (GA) 18–22 weeks. Leng et al. showed that the overall sensitivity and specificity of sonographic markers throughout the gestational period are 100 percent and 95.6 percent, respectively [50]. The potential sonographic markers for predicting fetal Hb Bart’s disease [48,49,51,52,53] include the following:
- a cardiac diameter/thoracic diameter ratio (CTR) with a cut-off value of 0.5 at mid-pregnancy;
- a high peak systolic velocity of the middle cerebral artery (MCA-PSV) with a cut-off value of 1.5 multiple of median (MoM), and;
- an increase in the placental thickness with a cut off value of 1.8 cm in the late first trimester and 3 cm at mid-pregnancy.
Additionally, several other sonographic markers can also support the diagnosis of fetal Hb Bart’s disease, including nuchal translucency, heart circumference, liver length, splenic circumference, and splenic artery peak systolic velocity [49,54,55,56,57]. The performance of sonographic markers is shown in Table 1. Serial ultrasound screening for Hb Bart’s disease during pregnancy, beginning in the first trimester and continuing every 2–4 weeks until 24 weeks, has a sensitivity of 100 percent in detecting pre-hydropic signs and a false positive rate of 10.9 percent. It also reduces the rate of invasive procedures by 70 percent. Hence, in normal practice, the sonographic marker is recommended for the routine screening of fetal Hb Bart’s disease [58]. The main limitations of serial ultrasound are that it requires specific equipment and it is operator-dependent.

Table 1.
Performance of Sonographic Markers in Predicting Fetal Hb Bart’s Disease.
The studies of maternal serum biomarkers, as a part of the second trimester Down syndrome screening [gestational age (GA) 18–22 weeks], demonstrated that serum biomarkers could also predict Hb Bart’s disease. Several maternal serum biomarkers used in routine fetal Down syndrome screening, such as free beta-human chorionic gonadotropin (β-hCG), inhibin-A, pregnancy-associated plasma protein-A (PAPP-A), alpha-fetoprotein (MAFP), and unconjugated estriol (uE3), are probably useful in predicting fetal Hb Bart’s disease. Increased β-hCG, inhibin-A, and PAPP-A levels have been noted from the increased placental hormone synthesis due to placentomegaly in fetal Hb Bart’s disease [22]. Additionally, elevated MAFP and decreased uE3 levels are observed because of hepatomegaly caused by extramedullary hematopoiesis secondary to fetal anemia [64]. According to the study conducted by Wanapirak et al. in 2018 [27], the most significant single predictor is MAFP with a cut-off greater than 1.5 MoM. (The sensitivity of 87.2 percent and the specificity of 74.5 percent). The combination prediction model including AFP and uE3 provided the best diagnostic performance. With the probability cut-off point greater than 0.5, the model gave an acceptable sensitivity of 61.5 percent and a high specificity of 98.1 percent [23]. However, using serum markers as a single tool in screening for fetal Hb Bart’s is not satisfactory. These markers are primarily used to screen for Down syndrome and neural tube defects in fetuses. Moreover, fetal anemia secondary to any causes other than Hb Bart’s disease, such as alloimmunization or Parvovirus B19, can result in an unexpected increase in the MAFP levels. However, the clinicians should take advantage of the second-trimester maternal serum screening to simultaneously screen for fetal Hb Bart’s disease, especially in geographical areas of high prevalence. Combining serum markers (MAFP) with sonographic markers such as CTR, placental thickness, and MCA-PSV may be beneficial with a sensitivity of approximately 48–69 percent and a specificity of 88.9–100 percent [64], as shown in Table 2. Similarly, the placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) commonly used in predicting preeclampsia were also demonstrated to be useful in screening for fetal Hb Bart’s disease. Thus, in fetal Hb Bart’s disease, a significant increase in PlGF and a decrease in the sFlt-1/PlGF ratio are observed, although sFlt-1 is marginally enhanced (p values of 0.008, 0.139, and 0.001 respectively) [24].

Table 2.
Performance of Maternal Biomarkers in Predicting Fetal Hb Bart’s Disease.
The novel technology of NIPD using maternal plasma cell-free fetal DNA (cffDNA), has been developed and is now utilized to screen common fetal aneuploidies, microdeletion/ microduplication syndromes, and monogenic disorders after 10 weeks of gestation. In thalassemia, significant progress has been achieved in developing NIPD as an effective screening method. The challenge for development is separating cffDNA from maternal DNA. Several techniques such as reverse transcription-quantitative PCR (RT-qPCR) using the gap-PCR technique, droplet digital PCR (ddPCR), and next-generation sequencing (NGS) have been developed for NIPD [6,65,66,67,68,69,70,71]. The NGS techniques in particular, are highly sensitive and enable the detection of small amounts of cffDNA. According to a recent study involving 878 cases at risk of fetal Hb Bart’s disease, the sensitivity and specificity in detecting fetal Hb Bart’s disease as early as 11–13 weeks of gestation were 98.98 percent and 96.06 percent, respectively [6]. However, the high costs, complex techniques, and unavailability in clinical laboratories make them inappropriate to use in routine clinical practices. In addition, this technique requires further validation. Thus, the search for other biomarkers is continuing. Table 3 demonstrates the advantages and disadvantages of different biomarkers for the identification of Hb Bart’s hydrops fetalis.

Table 3.
Advantages and disadvantages of different biomarkers for the identification of Hb Bart’s hydrops fetalis.
3. Extracellular Vesicles (EVs) as a Source of Non-Invasive Liquid Biopsy
EVs are membrane-bound vesicles containing cytosol from secreting cells enclosed in lipid bilayer structures, released into the extracellular environment from all cell types including trophoblasts [72], erythrocytes [73], and endothelial cells [74]. They carry specific cargos containing lipids, proteins, and nucleic acids (i.e., mRNAs, microRNAs, long non-coding RNA, and DNA fragments). EVs play a crucial role in cell-to-cell communication including fetal–maternal communication by the regulation of several biological processes, mediated by their surface receptors and their contents [29,36,75,76,77,78,79,80,81,82]. The concept of EVs was first introduced in 1860s by Charles Darwin [83] who proposed the theory of Pangenesis to understand the mechanisms of inheritance and the cause of natural variation [84]. Other than cell division for transferring genetic information, every cell in the body can produce particles containing diverse molecules called “gemmules” traversing to other cell types [83,84]. The first evidence of cell trafficking between the mother and the fetus was reported by Georg Schmorl in 1893 who demonstrated the fetal cells in the form of thrombin containing multinucleated syncytial giant cells in the lungs of pregnant women with eclampsia [85,86]. In the context of EV, placenta-derived EVs enter target cells in the uterus by endocytosis and are trafficked to early and late endosomes [87]. This pathway might allow for the trafficking of EVs across the syncytiotrophoblast and their release on the opposite surface from multivesicular bodies. Both the feto-maternal and maternal−fetal trafficking of exosomes during pregnancy can be demonstrated by the use of fetal cell-derived fluorescently labeled exosomes or genetically engineered mice, in which fetal and maternal exosomes could be distinguished [88,89]. The detection of fetal exosomes in maternal plasma discloses their potential as biomarkers for pregnancy monitoring using minimally invasive liquid biopsy.
3.1. Extracellular Vesicle Subpopulations
EVs are classified into three subpopulations, i.e., exosomes, microvesicles, and apoptotic bodies, based on their sizes, molecular markers, and contents [28]. Figure 2 shows EV biogenesis and molecular compositions. Exosomes are originated by the inward budding of the plasma membrane (endosomal route) forming multivesicular bodies which internalize to generate intraluminal vesicles subsequently released into the extracellular environment as exosomes [90,91,92]. The term intraluminal vesicles is used when the vesicles are inside the multivesicular bodies. In contrast, exosomes are described when the contents of the multivesicular bodies are released into the extracellular environment by cell membrane exocytosis [93]. Typically, exosomes can be distinguished from microvesicles and apoptotic bodies by their size, origination, formation, isolation methods, and protein enrichment as demonstrated in Table 4. To avoid confusion of the EV nomenclature in previous publications, the general term of EV is applied for exosomes and microvesicles (unless specified) throughout this review.
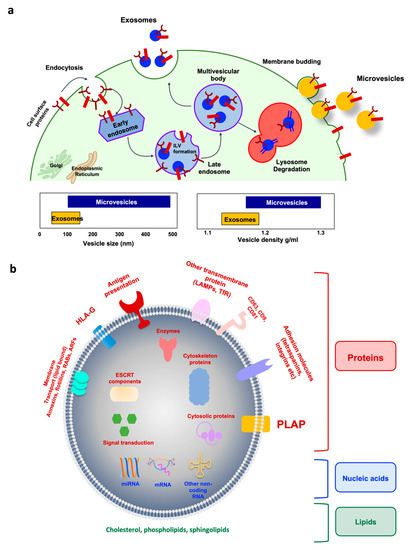
Figure 2.
Extracellular vesicle biogenesis and compositions. (a) Exosome biogenesis and intracellular life are depicted on the left. Cell surface proteins are endocytosed and trafficked to early endosomes. Once sorted to late endosomes, the endosomal sorting complex required for transport of (ESCRT)-0 complex recruits ubiquitinated proteins, while ESCRT-I and -II mediate the budding of intraluminal vesicles (ILVs). The multivesicular body (MVB) can either follow a degradation pathway fusing with lysosomes or proceed to release the ILV contents (as exosomes) to the extracellular space by an exocytic step. Microvesicle biogenesis via plasma membrane budding is illustrated on the right. Transmembrane proteins are clustered in discrete membrane domains that promote outward membrane budding. Tetraspanins and other proteins abundant at the domain may have a role by promoting the sorting of other components. Lipid-anchored (myristoylation, palmitoylation) proteins accumulate proteins in the lumen as well as contribute to membrane curvature. Additional mechanisms of microvesicle formation include the calcium-activated scramblases, which randomize the distribution of lipids between the two faces of the plasma membrane. The cytoskeleton becomes looser, while cytosolic proteins and RNA molecules are sorted into microvesicles. The specific ATPase VPS4 mediates the disassembly of the spiral by pulling its end. (b) Representative structure of exosomes with cargos. Note that placenta alkaline phosphatase (PLAP) is a specific marker of placenta-derived EVs. ARFs, ADP ribosylation factors; CD, cluster of differentiation; ESCRT, endosomal sorting complex required for transport; LAMPs, lysosome-associated membrane glycoproteins; mRNA, messenger RNA; miRNA, microRNA; RABs, member of RAS superfamily of small G proteins; TfR, transferrin receptor.

Table 4.
EV classification and their biophysical characteristics.
It is now well-established that EVs contain a variety of proteins, lipids, various RNA species (mRNA, microRNA, or other non-coding RNAs), and small amount of DNA as well as putative surface proteins or ligands that bind to receptors on target cells. The cargo from EVs has a distinct molecular signature reflecting the originating cells which facilitate the selective incorporation and triggering of biochemical and/or phenotypic changes in the recipient cells [28,94,95,96]. The cargo contents can be absorbed when EVs circulate, and this leads to the modification of the target gene expression, signal, and biological function of the recipient cells [97]. The expression of exosomal cargos is altered according to the microenvironment [98,99,100].
3.2. EV Isolation and Characterization
Several approaches have been applied to isolate EVs, even though there is no universal method that is suitable to all projects [101]. EV isolation based on a single step of polymer/precipitation agents (e.g., polyethylene glycol), high-speed ultracentrifugation, or low-molecular weight centrifugal filters provides the highest amount of EV recovered from the biofluids. However, they come with a trade-off in the specificity due to the significant contamination of vesicular (i.e., microvesicles, apoptotic bodies) and non-vesicular (e.g., free and precipitated proteins, lipoprotein micelles) components [101]. The single step EV isolation by high-molecular weight centrifugal filters, size-exclusion chromatography, tangential flow filtration, or membrane affinity columns, as well as the differential ultracentrifugation with the low/intermediate centrifuge prior to the high-speed, shows an improved specificity while maintaining the intermediate recovery yield [101,102,103,104]. The main advantage of these single-step protocols is the turnaround time and the compatibility with large-scale, exosome-molecular studies, in which many samples have to be processed for EV isolation within a relatively short period of time.
Combinatorial EV isolation based on two methods (e.g., differential ultracentrifugation coupled with ethylene glycol precipitation, size-exclusion chromatography) [105,106], the immunoaffinity isolation based on the specific EV molecules [107,108], or three combined methods, i.e., step-wise centrifugation, ultrafiltration, and size exclusion chromatography [109,110], usually provide better EV specificity and thus are suitable when the specific goals involve the EV functions or subpopulations. The combined isolation methods are very useful when the known contaminants are expected to significantly interfere with the downstream analysis, i.e., the measuring biomarkers could be presented as free soluble proteins and EV-containing proteins. Note that the multi-step combinatorial protocols and immunoaffinity procedures provide a low recovery yield with as few contaminates as possible [101], so that the downstream analysis and data interpretation can be straightforward with high confidence. However, the reproducibility and the upscaling of the isolated EVs for the whole project should be addressed at the initial phase of the studies. Therefore, one can choose the method by considering the degrees of recovery and specificity of the isolated EVs and their compatibility with the downstream applications [101].
Once isolated, EV-enriched specimens are required to be validated for the EV presence following the International Society for Extracellular Vesicles (ISEV) guideline [101]. In general, EV characterization must be performed to demonstrate both the particle and protein evidence. Particle evidence usually requires two different but complementary methods to be satisfied [101]. These include one technique for high-resolution images of the EV morphology (e.g., transmission electron microscopy or atomic force microscopy) and another technique for the EV size distribution (i.e., nanoparticle tracking analysis, high-resolution flow cytometry, or asymmetric-flow field-flow fractionation) [111,112,113,114]. Protein evidence could be satisfied by demonstrating three positive (enriched) and one negative (depleted) EV marker as follows: (i) the enrichment of plasma membrane-associated EV markers (e.g., CD9, CD63, CD81, MHC molecules, integrins, LAMP1/2, syndecans); (ii) the enrichment of cytosolic proteins involved in EV biogenesis or with a lipid/membrane protein-binding ability (e.g., TSG101, ALIX, flotillin-1 and -2, caveolins, annexins); (iii) the depletion of proteins commonly found as EV contaminants (i.e., free soluble proteins such as lipoproteins, serum albumin, and cytokines) or subcellular compartment proteins (e.g., histones, cytochrome C, calnexin, and HSP90B1) depending on the matrix [101]. Immunodetection methods such as Western blotting and ELISA are often used to show the EV protein evidence and in some circumstances, quantitative proteomics can be applied to identify and quantify multiple EV protein markers to support the claim of EV presence in the isolates [115,116]. Figure 3 demonstrates the commonly used methods for the detection of EVs as well as their cargos.
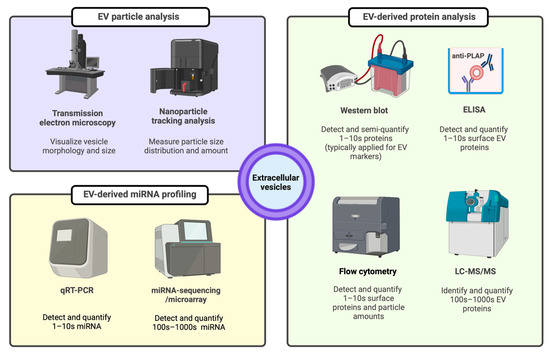
Figure 3.
EV characterization methods for particle, protein, and miRNA evidence. ELISA: enzyme linked immunosorbent assay, EV: extracellular vesicle, LC-MS/MS: liquid chromatography-tandem mass spectrometry, miRNA: microRNA, PLAP: placental alkaline phosphatase protein, qRT-PCR: quantitative real-time polymerase chain reaction.
3.3. Placenta-Derived EVs
In pregnancy, the discovery of circulating fetal genetic material in the maternal plasma has enhanced the non-invasive prenatal diagnosis [117,118,119,120,121,122,123,124,125,126]. The placenta secretes a large number of EVs into maternal circulation since they are shed from the syncytiotrophoblast into the intervillous space and then flushed via the uterine veins into the maternal circulation [127]. The placental EVs were then confirmed that they are of placental origin by using the placental alkaline phosphatase (PLAP) marker. In addition, such fragments are detected in all pregnant women, but not in the non-pregnant population, suggesting a pregnancy-specific marker. The placenta-derived exosome can induce apoptosis and the down regulation of T cell responses contributing to fetal immune tolerance [128]. Subsequently, other investigators have also isolated placenta-derived EVs using human placenta explant cultures from the first trimester of normal pregnancies and reported that PLAP is a marker of placental exosomes [129,130,131,132]. Although 87% homology is found with the intestinal alkaline phosphatase gene, there are differences at their carboxyl terminal end [133,134], which is unique to the placenta.
Accumulating evidence shows that placenta-derived EVs exhibit multiple functions, including the following.
3.3.1. Regulating Trophoblast Migration and Angiogenesis
Placenta-derived EVs can regulate trophoblast migration and angiogenesis [72,98,99,135]. In in vitro studies, placenta-derived EVs isolated from pregnant women are biologically active since they can induce endothelial cell migration [132]. In addition, the release of EVs from cytotrophoblast culture depends on the oxygen tension whereby the low oxygen tension environment promotes extravillous trophoblast (EVT) cell migration, endovascular invasion, and proliferation. Thus, it is possible that placenta-derived EVs are involved in the process of placentation [98,136].
3.3.2. Promoting Maternal–Fetal Immune Tolerance
Placenta-derived EVs from in vitro-cultured placental explants down-regulated the NK cell receptor NKG2D and impaired the cytotoxic function of T cells from the peripheral blood mononuclear cells of pregnant women [130,137,138]. Furthermore, placenta-derived EVs suppressed T cell signaling components and induced lymphocyte apoptosis leading to immune tolerance [139]. Despite the immunosuppressive activity of placenta-derived EVs, the risk of infection caused by the EVs remains unremarkable.
3.3.3. Mediating Maternal–Fetal Intercommunication
Placenta-derived EVs play a crucial role in the crosstalk between the feto–placental unit or maternal–fetal communication [36,82,140]. Studies demonstrated that placental-specific microRNAs within C19MC-derived miRNAs can be detected in the maternal circulation via EVs where they function in placental–maternal signaling [141,142]. For example, placenta-derived EVs can be taken up by endothelial cells, immune cells, or platelets and they can modulate their functions such as generating pro-inflammatory signals, inducing death ligands, mediating nitric oxide signaling, or producing damage-associated molecular pattern (DAMP) molecules [143,144,145,146]. In addition, non-placental cells incubated with placenta-derived EVs harboring C19MC miRNA clusters attenuate the viral replication in recipient cells through the induction of the autophagy pathway [142]. Therefore, EVs regulate the maternal immune response to maintain a normal pregnancy and protect against viral infections such as Cytomegalovirus infection [143,147,148].
3.3.4. Regulating Maternal Metabolic Homeostasis
Placenta-derived EVs regulate maternal metabolic homeostasis by transferring the gene information to target cells [29]. For example, miRNAs derived from the placenta-derived EVs of women with gestational diabetes have been shown to reduce insulin sensitivity and glucose uptake by striated muscle [100,149,150,151,152].
Collectively, placenta-derived EVs are specifically packaged with signaling molecules including protein, mRNA, microRNA, and non-coding RNA. They play a role in the translational activities of angiogenesis, immunomodulation, cell signaling, and metabolic homeostasis. As a result, placental exosome signaling represents a fundamental pathway mediating the maternal–fetal intercellular communication [153,154].
3.4. Pathophysiological Roles of Placenta-Derived EVs in Pregnancy Complications
Placenta-derived EVs have a unique feature due to the presence of specific placental alkaline phosphatase (PLAP) [129,132,139], HLA-G [155], and miRNAs such as those within the chromosome 19 miRNA cluster [75,142,156]. They can be identified by size, buoyant density, and the presence of CD63 as well as PLAP antigens. Placenta-derived EVs are identified in maternal plasma as early as sixth weeks of gestation [33] and the concentration of placenta-derived EVs in maternal plasma increases progressively as a function of gestation [33,132]. Furthermore, the concentration of maternal plasma placenta-derived EVs is approximately 20-fold higher than that observed in non-pregnant women, indicating a pregnancy-specific protein [132,139]. It has been hypothesized that placental and fetal communication as well as the presence of fetal cells in maternal circulation occur beginning from the fourth week of gestation, although intervillous circulation has not been completely established [157].
Several factors such as hypoxia, high glucose concentrations, and inflammatory signals trigger the release of placenta-derived EVs [98,100,158]. A high glucose concentration (25 mM) significantly increased the number of EVs from the first trimester primary trophoblast cells and such EVs triggered the release of pro-inflammatory cytokines from human umbilical vein endothelial cells [100]. A similar observation has been demonstrated in pregnant women with gestational diabetes mellitus [149,150,151]. Specifically, women with gestational diabetes have a higher maternal plasma-derived exosome concentration than those with a normal pregnancy from the 11th week of gestation onwards [150]. EVs isolated from the plasma of women with gestational diabetes induce the release of pro-inflammatory cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4, IL-6, IL-8, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) [150]. Thus, it is possible that the dysregulation of EVs may be involved in the pro-inflammatory status in gestational diabetes.
In in vitro studies, the number of EVs released from extravillous trophoblast (EVT) cells is higher than that from EVT cultured under a hypoxic condition (1% oxygen) compared to normal oxygenation (8% oxygen) [158]. Interestingly, EVs isolated from EVT cultured at 1% oxygen reduce the endothelial cell migration but increase the production of TNF-α. Hypoxic conditions also influence exosome contents as well as their biological activities, such as protein and miRNA, against target cells [98,99,158]. These findings have important clinical implications since the determination of the placenta-derived exosome concentration and their cargo contents in maternal plasma can be used as a non-invasive method for the early detection and diagnosis as well as monitoring of placental-associated complications [159]. In modern clinical obstetrics, the term “liquid biopsy” refers to the molecular diagnosis or biomarker determination in biological fluids, especially blood, for the evaluation of pregnancy-related complications or prenatal screenings. Compelling evidence demonstrated that the aberration exosome number and their contents are observed in women with preeclampsia [29,34,37,160], diabetes [35,150,161,162,163], fetal growth restriction [164], and preterm birth [116,165,166,167,168]. Figure 4 shows the role of placental debris and EVs in placental-associated complications.
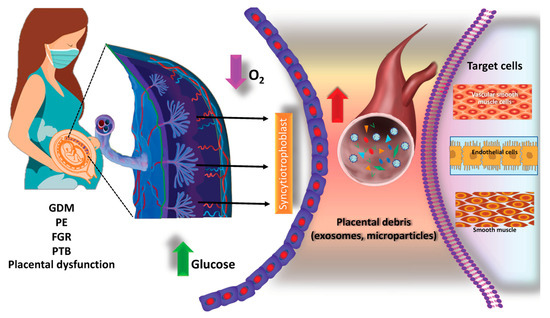
Figure 4.
Potential roles of placental debris and exosome changes during pregnancy-related complications. Placental debris and EVs (including exosomes and microvesicles) released from the placenta under different micro-environment conditions (such as hypoxia or high glucose) and their targeting of neighboring cells in the placenta and distant organs such as skeletal muscles. EVs are released by placental cells (such as syncytiotrophoblasts, cytotrophoblasts (CTs), and extravillous trophoblasts (EVTs)) and other cells in the placenta such as the placental mesenchymal stem cells. EVs from the placenta can enter maternal circulation and target distant cells such as skeletal muscles, endothelial cells, or vascular smooth muscle cells. Placenta-derived EVs’ concentration, content, and bioactivity changes in pregnancy and pregnancy-related disorders including GDM, PE, PTB, and FGR. GDM: gestational diabetes, PE: preeclampsia; FGR: fetal growth restriction, PTB: preterm birth.
Several studies have demonstrated that placental-specific EVs are implicated in the pathogenesis of preeclampsia since they generate inflammatory responses, since they generate inflammatory responses, increase anti-angiogenesis, activate platelets, and induce endothelial cell activation. In an experimental study, syncytiotrophoblast EVs from explant cultures of a preeclamptic placenta induced the release of proinflammatory cytokines such as IL-1β, IL-6, IL-18, macrophage inhibitory protein-1-α and -β, or TNF-α in peripheral mononuclear cells [169]. In addition, the administration of EVs to pregnant mice induces embryonic death or a small size of the embryo and placenta, as well as preeclampsia-like features including elevated blood pressure, proteinuria, enlarged glomeruli, thickened glomerular basement membrane, and increased maternal plasma sFlt-1 concentrations [170]. These changes were not observed in non-pregnant mice. The mechanism responsible for the development of preeclampsia is attributed to the activation of maternal platelets in the placental vascular bed, induced via an adenosine triphosphate (ATP) release and inflammasome activation [170]. In humans, the evidence has demonstrated an alteration in the EVs and their cargos in the placenta and maternal plasma of women with preeclampsia [29,34,37,160]. However, an alteration in EVs in the placenta or maternal circulation can be found in other great obstetrical syndromes such as fetal growth restriction [164] and gestational diabetes [163]. Figure 5 illustrates a possible role of placenta-derived exosomes in Bart’s hydrops fetalis and in the pathogenesis of mirror syndrome.
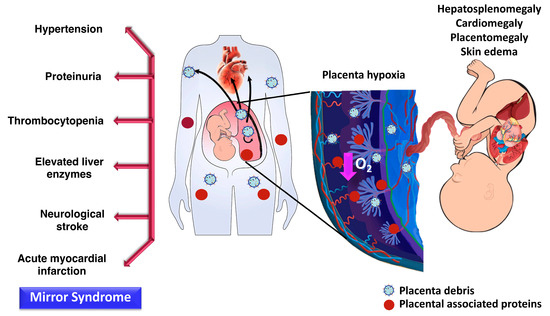
Figure 5.
The possible roles of placenta-derived exosomes in the pathogenesis of Bart’s hydrops fetalis. Features of fetus with Bart’s hydrops are hepatosplenomegaly, cardiomegaly, ascites, pleural effusion, skin edema, and/or placentomegaly. Women with Bart’s hydropic fetuses can be presented with preeclampsia features called “mirror syndrome”. Placental hypoxia in Bart’s hydrops fetus is hypothesized to trigger the release of placental debris or placenta-derived exosomes which enter maternal circulation.
4. Placenta-Derived EVs as Hb Bart’s Liquid Biopsy: Challenges and Future Perspectives
While placenta-derived EVs are promising as the source of candidate biomarkers with clinical relevance, there are several challenges when conducting EV biomarker studies. As aforementioned, one needs to ensure the performance and compatibility of the EV isolation method chosen to support the specific goals/downstream applications [101]. It should be emphasized that different isolation methods could impact the downstream EV molecular analysis [171], while it is common that the isolated EV samples fail the quality control experiments. Nonetheless, this challenge can be simply addressed by the practice of reproducible EV isolation and some preliminary experiments to choose the suitable isolation method for achieving the qualified EV samples for downstream analyses. In addition, EVs presented in biofluids are heterogeneous in nature [172]. For example, plasma EVs are contributed from the local vascular endothelial cells and distant organ systems (e.g., brain, heart, lung, liver, intestine, and kidneys) and they can change in both quantity and quality during the healthy and disease states. The key to address this issue relies on the study design that focuses on the inclusion and exclusion criteria, the clinical context of using the candidate EV biomarkers, and a sufficient study population to support the statistical power. In addition, the validation phase of biomarker candidates should be performed with independent larger cohorts or multi-center studies [173]. Alternatively, the application of tissue-specific markers, for example, targeting PLAP for the placenta-derived EV subtype [29], is a potential approach to minimize the EV heterogeneity caused by the unrelated organ systems. The multiplex detection of PLAP-positive EVs in relation to other EV molecular profiles (i.e., proteins and miRNAs) should be pursued in future studies involving biomarker discovery and validation for placental-associated complications.
Only a few studies have described exosomes or microvesicles from hemoglobinopathy adult patients [174,175,176,177,178,179]. In addition, most of these studies examined the role of microparticles in circulation from adults with Beta-thalassemia/Hb E disease in relation to the hypercoagulable state. The authors demonstrated that microparticles obtained from Beta-thalassemia/Hb E patients can induce an inflammatory response, endothelial dysfunction [174], and stimulate platelet activation as well as aggregation leading to thrombus formation [175]. Thus far, there is no study determining placenta-derived EVs in pregnant women with Hb Bart’s hydrops fetalis. Since placental hypoxia is a main feature of Bart’s hydropic fetus, it can trigger the release of placental debris or placental-derived exosomes which subsequently enter maternal circulation. In addition, in some cases of Bart’s hydrops fetalis, Ballantyne or mirror syndrome characterized by a simultaneous edematous state of the mother, fetus, and placenta (also called triple edema) can be observed. In this syndrome, the mother may develop proteinuria, hypertension, and even severe preeclampsia [180,181,182].
Altogether, we hypothesize that the placenta-derived exosome concentration might have a role in the identification of women with Hb Bart’s fetuses beginning in early gestation and this might be implicated in the genesis of mirror syndrome in some cases. Ultimately, we propose that the placenta-derived exosome might be used as a liquid biopsy tool for the identification of fetuses at risk for this condition and this might reduce the rate of unnecessary invasive prenatal diagnosis determinations. In this direction, an integrative strategy has been proposed to combine current biomarkers with placenta-derived EVs for the identification of Bart’s hydrops fetalis throughout gestation (Figure 6). Importantly, prior to clinical use, it is necessary to standardize the protocols of exosome isolation and characterization, while the diagnostic performance of placenta-derived EVs should be evaluated as a solitary biomarker and as part of the combined biomarkers in the large-scale prospective cohorts or multicenter studies.
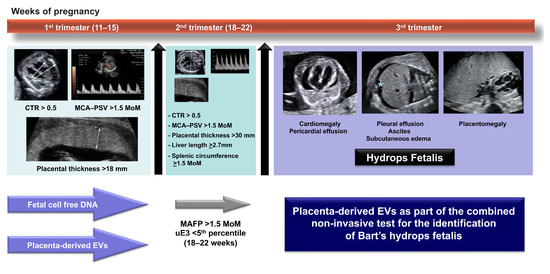
Figure 6.
An integrative diagnostic model combining current biomarkers and placenta-derived EVs for the identification of Bart’s hydrops fetalis throughout gestation. CTR: cardiothoracic diameter ratio, MCA-PSV: middle cerebral artery peak systolic velocity, MoM: multiple of median, MAFP: maternal alpha fetoprotein, uE3: unconjugated estriol.
Last but not least, several drugs are known to modulate the EV release and cargo contents, and thus are actively investigated to harness EV-modulated therapeutic effects against non-communicable and infectious diseases [183,184,185]. Among these medications, antiplatelets are commonly used during pregnancy. Low-dose aspirin prophylaxis is indicated in patients at a high-risk of developing preeclampsia [186]. Dual antiplatelet therapy (aspirin and P2Y12 receptor blocker) has been used to manage pregnancy-associated myocardial infarction following a percutaneous coronary intervention (PCI) with intracoronary stenting [187,188,189]. Interestingly, aspirin suppressed the cargo levels of proinflammatory cytokines and mediators in platelet-derived exosomes [190]. Ticagrelor, a potent P2Y12 receptor blocker, induced the growth of cardiac-derived mesenchymal stem cells and enhanced the release of anti-hypoxic exosomes with cardioprotective effects [191]. While future investigations of placenta-derived EVs as the liquid biopsy should take into account the prescribed medications during pregnancy, one can foresee a translational potential of EV modulatory agents, especially antiplatelets, to mitigate the pathophysiological consequences of placental hypoxia and pregnancy complications through the modulations of placenta-derived EV biogenesis and molecular cargos.
5. Conclusions
Pregnancy is associated with a significant increase in the number of total EVs and placenta-derived EVs in maternal circulation across gestation. Hypoxia and hyperglycemia are the main triggers for the release of placenta-derived EVs. Compelling evidence supports placental-associated complications such as preeclampsia and fetal growth restriction as having some alterations in the number and the contents of EVs. Therefore, changes in the profile of placenta-derived EVs are a very attractive tool for the identification of placental-associated complications in asymptomatic pregnant women. Placental hypoxia in women with Bart’s hydrops fetuses may exhibit changes in the numbers and/or functions of placental-derived EVs, and thus could serve as a liquid biopsy tool for an early non-invasive prenatal diagnosis. Nonetheless, standardization of the method for placenta-derived EV detection is needed in order to open a new avenue for an early identification, monitoring, and therapeutic intervention of women at risk for Bart’s hydrops fetalis and other placental-associated conditions.
Author Contributions
Conceptualization, P.C. and S.C.; data curation, P.C., S.L., M.T., W.C., P.S.T. and P.P.; writing—original draft preparation, P.C.; writing—review and editing, P.C., S.L., M.T., W.C., P.P., P.S.T., T.T. and S.C.; visualization, P.C., S.L., M.T., W.C., P.P., P.S.T. and S.C.; supervision, T.T. and S.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Royal Thai College of Obstetricians and Gynaecologists and Faculty of Medicine Ramathibodi Hospital (RF_65027 to P.C.). The APC was funded by the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data have been presented in the manuscript.
Acknowledgments
We thank Panachai Nimitpanya and Jitpisuth Tantasiri for their comments on the manuscript. This work was supported by the Faculty of Medicine at Ramathibodi Hospital, Mahidol University. Figure 3 was created with Biorender.com (accessed on 23 December 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Flint, J.; Hill, A.V.; Bowden, D.K.; Oppenheimer, S.J.; Sill, P.R.; Serjeantson, S.W.; Bana-Koiri, J.; Bhatia, K.; Alpers, M.P.; Boyce, A.J.; et al. High frequencies of alpha-thalassaemia are the result of natural selection by malaria. Nature 1986, 321, 744–750. [Google Scholar] [CrossRef]
- Wanapirak, C.; Muninthorn, W.; Sanguansermsri, T.; Dhananjayanonda, P.; Tongsong, T. Prevalence of thalassemia in pregnant women at Maharaj Nakorn Chiang Mai Hospital. J. Med. Assoc. Thail. 2004, 87, 1415–1418. [Google Scholar]
- Chui, D.H. Alpha-thalassemia: Hb H disease and Hb Barts hydrops fetalis. Ann. N. Y. Acad. Sci. 2005, 1054, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Jatavan, P.; Chattipakorn, N.; Tongsong, T. Fetal hemoglobin Bart’s hydrops fetalis: Pathophysiology, prenatal diagnosis and possibility of intrauterine treatment. J. Matern. Fetal. Neonatal Med. 2018, 31, 946–957. [Google Scholar] [CrossRef]
- Yang, J.; Peng, C.F.; Qi, Y.; Rao, X.Q.; Guo, F.; Hou, Y.; He, W.; Wu, J.; Chen, Y.Y.; Zhao, X.; et al. Noninvasive prenatal detection of hemoglobin Bart hydrops fetalis via maternal plasma dispensed with parental haplotyping using the semiconductor sequencing platform. Am. J. Obstet. Gynecol. 2020, 222, 185.e1–185.e17. [Google Scholar] [CrossRef]
- Xiong, F.; Sun, M.; Zhang, X.; Cai, R.; Zhou, Y.; Lou, J.; Zeng, L.; Sun, Q.; Xiao, Q.; Shang, X.; et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin. Genet. 2010, 78, 139–148. [Google Scholar] [CrossRef]
- Lie-Injo, L.E. Alpha-chain thalassemia and hydrops fetalis in Malaya: Report of five cases. Blood 1962, 20, 581–590. [Google Scholar]
- King, A.J.; Higgs, D.R. Potential new approaches to the management of the Hb Bart’s hydrops fetalis syndrome: The most severe form of α-thalassemia. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 353–360. [Google Scholar] [CrossRef]
- Chik, K.W.; Shing, M.M.; Li, C.K.; Leung, T.F.; Tsang, K.S.; Yuen, H.L.; Cheng, S.B.; Yuen, P.M. Treatment of hemoglobin Bart’s hydrops with bone marrow transplantation. J. Pediatr. 1998, 132, 1039–1042. [Google Scholar] [CrossRef]
- Jiang, F.; Li, D.Z. Outcome of survivors with hemoglobin Bart’s hydrops fetalis syndrome: The most severe form of α-thalassemia. Pediatr. Transplant. 2021, 25, e14090. [Google Scholar] [CrossRef]
- Chan, W.Y.K.; Lee, P.P.W.; Lee, V.; Chan, G.C.F.; Leung, W.; Ha, S.Y.; Cheuk, D.K.L. Outcomes of allogeneic transplantation for hemoglobin Bart’s hydrops fetalis syndrome in Hong Kong. Pediatr. Transplant. 2021, 25, e14037. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.T.; Wong, V.C.; So, W.W.; Ma, H.K.; Chan, V.; Todd, D. Homozygous alpha-thalassaemia: Clinical presentation, diagnosis and management. A review of 46 cases. Br. J. Obstet. Gynaecol. 1985, 92, 680–684. [Google Scholar] [CrossRef]
- Thammavong, K.; Luewan, S.; Jatavan, P.; Tongsong, T. Foetal haemodynamic response to anaemia. ESC Heart Fail. 2020, 7, 3473–3482. [Google Scholar] [CrossRef]
- Tongsong, T.; Wanapirak, C.; Sirivatanapa, P.; Sanguansermsri, T.; Sirichotiyakul, S.; Piyamongkol, W.; Chanprapaph, P. Prenatal control of severe thalassaemia: Chiang Mai strategy. Prenat. Diagn. 2000, 20, 229–234. [Google Scholar] [CrossRef]
- Tongsong, T.; Wanapirak, C.; Sirivatanapa, P.; Sa-nguansermsri, T.; Sirichotiyakul, S.; Piyamongkol, W.; Chanprapaph, P.; Steger, H.F.; Sekararithi, R.; Tuggapichitti, A. Prenatal eradication of Hb Bart’s hydrops fetalis. J. Reprod. Med. 2001, 46, 18–22. [Google Scholar]
- Tongsong, T.; Boonyanurak, P. Placental thickness in the first half of pregnancy. J. Clin. Ultrasound JCU 2004, 32, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Tongsong, T.; Tatiyapornkul, T. Cardiothoracic ratio in the first half of pregnancy. J. Clin. Ultrasound 2004, 32, 186–189. [Google Scholar] [CrossRef]
- Tongsong, T.; Wanapirak, C.; Sirichotiyakul, S.; Chanprapaph, P. Sonographic markers of hemoglobin Bart disease at midpregnancy. J. Ultrasound Med. 2004, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Thammavong, K.; Luewan, S.; Tongsong, T. Performance of Fetal Cardiac Volume Derived from VOCAL (Virtual Organ Computer-Aided AnaLysis) in Predicting Hemoglobin (Hb) Bart’s Disease. J. Clin. Med. 2021, 10, 4651. [Google Scholar] [CrossRef]
- Thammavong, K.; Luewan, S.; Wanapirak, C.; Tongsong, T. Ultrasound Features of Fetal Anemia Lessons From Hemoglobin Bart Disease. J. Ultrasound Med. 2021, 40, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Tongprasert, F.; Wanapirak, C.; Tongsong, T. Maternal serum hCG, PAPP-A and AFP as predictors of hemoglobin Bart disease at mid-pregnancy. Prenat. Diagn. 2011, 31, 430–433. [Google Scholar] [CrossRef]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Second trimester maternal serum markers and a predictive model for predicting fetal hemoglobin Bart’s disease. J. Matern. Fetal. Neonatal Med. 2013, 26, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Comparison of maternal serum PlGF and sFlt-1 between pregnancies with and without fetal hemoglobin Bart’s disease. Prenat. Diagn. 2013, 33, 1272–1275. [Google Scholar] [CrossRef]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Second trimester maternal serum inhibin-A in fetal anemia secondary to hemoglobin Bart’s disease. J. Matern. Fetal. Neonatal Med. 2014, 27, 1005–1009. [Google Scholar] [CrossRef]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Comparison of cardiac troponin T and N-terminal pro-B-type natriuretic peptide between fetuses with hemoglobin Bart’s disease and nonanemic fetuses. Prenat. Diagn. 2014, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Wanapirak, C.; Piyamomgkol, W.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Jatavan, P.; Tongsong, T. Second-trimester maternal serum screening for fetal Down syndrome: As a screening test for hemoglobin Bart’s disease: A prospective population-based study. Prenat. Diagn. 2018, 38, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. CCS 2021, 19, 47. [Google Scholar] [CrossRef]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- Nair, S.; Salomon, C. Extracellular vesicles as critical mediators of maternal-fetal communication during pregnancy and their potential role in maternal metabolism. Placenta 2020, 98, 60–68. [Google Scholar] [CrossRef]
- Sadovsky, Y.; Ouyang, Y.; Powell, J.S.; Li, H.; Mouillet, J.F.; Morelli, A.E.; Sorkin, A.; Margolis, L. Placental small extracellular vesicles: Current questions and investigative opportunities. Placenta 2020, 102, 34–38. [Google Scholar] [CrossRef]
- Palma, C.; Jellins, J.; Lai, A.; Salas, A.; Campos, A.; Sharma, S.; Duncombe, G.; Hyett, J.; Salomon, C. Extracellular Vesicles and Preeclampsia: Current Knowledge and Future Research Directions. Subcell Biochem. 2021, 97, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Taweevisit, M.; Thorner, P.S. Hemoglobin Bart hydrops fetalis: A model for studying vascular changes in placental hypoxia. Placenta 2016, 44, 98–103. [Google Scholar] [CrossRef]
- Taweevisit, M.; Thorner, P.S. Hydrops fetalis in the stillborn: A series from the central region of Thailand. Pediatr. Dev. Pathol. 2010, 13, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Taweevisit, M.; Thorner, P.S. Peripheral villous stromal hyperplasia: A distinctive placental lesion in hemoglobin bart hydrops fetalis. Pediatr. Dev. Pathol. 2012, 15, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J. Utility of diagnosing various histological patterns of diffuse chronic hypoxic placental injury. Pediatr. Dev. Pathol. 2012, 15, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J. Hypoxic patterns of placental injury: A review. Arch. Pathol. Lab. Med. 2013, 137, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J. Placental hypoxic overlap lesions: A clinicoplacental correlation. J. Obstet. Gynaecol. Res. 2015, 41, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Turowski, G.; Vogel, M. Re-view and view on maturation disorders in the placenta. Apmis 2018, 126, 602–612. [Google Scholar] [CrossRef]
- Songdej, D.; Babbs, C.; Higgs, D.R. An international registry of survivors with Hb Bart’s hydrops fetalis syndrome. Blood 2017, 129, 1251–1259. [Google Scholar] [CrossRef]
- Lam, Y.H.; Ghosh, A.; Tang, M.H.; Lee, C.P.; Sin, S.Y. Early ultrasound prediction of pregnancies affected by homozygous alpha-thalassaemia-1. Prenat. Diagn 1997, 17, 327–332. [Google Scholar] [CrossRef]
- Lam, Y.H.; Tang, M.H.; Lee, C.P.; Tse, H.Y. Prenatal ultrasonographic prediction of homozygous type 1 alpha-thalassemia at 12 to 13 weeks of gestation. Am. J. Obstet. Gynecol 1999, 180, 148–150. [Google Scholar] [CrossRef]
- Leung, K.Y.; Cheong, K.B.; Lee, C.P.; Chan, V.; Lam, Y.H.; Tang, M. Ultrasonographic prediction of homozygous alpha0-thalassemia using placental thickness, fetal cardiothoracic ratio and middle cerebral artery Doppler: Alone or in combination? Ultrasound Obstet. Gynecol. 2010, 35, 149–154. [Google Scholar] [CrossRef]
- Sirichotiyakul, S.; Luewan, S.; Srisupundit, K.; Tongprasert, F.; Tongsong, T. Prenatal ultrasound evaluation of fetal Hb Bart’s disease among pregnancies at risk at 11 to 14 weeks of gestation. Prenat. Diagn. 2014, 34, 230–234. [Google Scholar] [CrossRef]
- Leung, K.Y.; Liao, C.; Li, Q.M.; Ma, S.Y.; Tang, M.H.; Lee, C.P.; Chan, V.; Lam, Y.H. A new strategy for prenatal diagnosis of homozygous alpha(0)-thalassemia. Ultrasound Obstet. Gynecol. 2006, 28, 173–177. [Google Scholar] [CrossRef]
- Leung, W.C.; Oepkes, D.; Seaward, G.; Ryan, G. Serial sonographic findings of four fetuses with homozygous alpha-thalassemia-1 from 21 weeks onwards. Ultrasound Obstet. Gynecol. 2002, 19, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Raungrongmorakot, K.; Chaemsaithong, P. Middle cerebral artery peak systolic velocity in fetuses with homozygous alpha-thalassemia-1: Case series. J. Med. Assoc. Thai. 2010, 93, S114–S117. [Google Scholar] [PubMed]
- Srisupundit, K.; Piyamongkol, W.; Tongsong, T. Identification of fetuses with hemoglobin Bart’s disease using middle cerebral artery peak systolic velocity. Ultrasound Obstet. Gynecol. 2009, 33, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Luewan, S.; Tongprasert, F.; Piyamongkol, W.; Wanapirak, C.; Tongsong, T. Fetal liver length measurement at mid-pregnancy among fetuses at risk as a predictor of hemoglobin Bart’s disease. J. Perinatol. 2010, 31, 157–160. [Google Scholar] [CrossRef]
- Tongsong, T.; Piyamongkol, W.; Tongprasert, F.; Srisupundit, K.; Luewan, S. Fetal Splenic Artery Peak Velocity (SPA-PSV) at Mid-Pregnancy as a Predictor of Hb Bart’s Disease. Ultraschall Med. 2011, 32, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Srisupundit, K.; Tongprasert, F.; Luewan, S.; Sirichotiyakul, S.; Tongsong, T. Splenic circumference at midpregnancy as a predictor of hemoglobin Bart’s disease among fetuses at risk. Gynecol. Obstet. Investig. 2011, 72, 63–67. [Google Scholar] [CrossRef]
- Siwawong, W.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Fetal cardiac circumference derived by spatiotemporal image correlation as a predictor of fetal hemoglobin Bart disease at midpregnancy. J. Ultrasound Med. 2013, 32, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Harn-a-morn, P.; Wanapirak, C.; Sirichotiyakul, S.; Srisupundit, K.; Tongprasert, F.; Luewan, S.; Tongsong, T. Effectiveness of ultrasound algorithm in prenatal diagnosis of Hemoglobin Bart’s disease among pregnancies at risk. Int. J. Gynecol. Obstet. 2022; in press. [Google Scholar] [CrossRef]
- Li, X.; Qiu, X.; Huang, H.; Zhao, Y.; Li, X.; Li, M.; Tian, X. Fetal heart size measurements as new predictors of homozygous alpha-thalassemia-1 in mid-pregnancy. Congenit. Heart Dis. 2018, 13, 282–287. [Google Scholar] [CrossRef]
- Traisrisilp, K.; Sirilert, S.; Tongsong, T. The performance of cardio-biparietal ratio measured by 2D ultrasound in predicting fetal hemoglobin Bart disease during midpregnancy: A pilot study. Prenat. Diagn. 2019, 39, 647–651. [Google Scholar] [CrossRef]
- Thathan, N.; Traisrisilp, K.; Luewan, S.; Srisupundit, K.; Tongprasert, F.; Tongsong, T. Screening for hemoglobin Bart’s disease among fetuses at risk at mid-pregnancy using the fetal cardiac diameter to biparietal diameter ratio. BMC Pregnancy Childbirth 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Tongsong, T.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Jatavan, P. Fetal Cardiac Remodeling in Response to Anemia: Using Hemoglobin Bart’s Disease as a Study Model. Ultraschall Med. 2018, 41, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Srisupundit, K.; Piyamongkol, W.; Tongprasert, F.; Luewan, S.; Tongsong, T. Reference range of fetal splenic circumference from 14 to 40 weeks of gestation. Arch. Gynecol. Obstet. 2010, 283, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Second trimester maternal serum alpha-fetoprotein (MSAFP) as predictor of fetal hemoglobin Bart’s disease. Prenat. Diagn. 2014, 34, 1277–1282. [Google Scholar] [CrossRef]
- Tungwiwat, W.; Fucharoen, S.; Fucharoen, G.; Ratanasiri, T.; Sanchaisuriya, K. Development and application of a real-time quantitative PCR for prenatal detection of fetal alpha(0)-thalassemia from maternal plasma. Ann. N. Y. Acad. Sci. 2006, 1075, 103–107. [Google Scholar] [CrossRef]
- Long, X.J.; Long, G.F.; Lin, W.X. [Noninvasive prenatal diagnosis of Hb Bart’s hydrops fetus using cell-free fetal DNA in maternal plasma]. Zhonghua Xue Ye Xue Za Zhi 2009, 30, 175–178. [Google Scholar]
- Pornprasert, S.; Sukunthamala, K.; Kunyanone, N.; Sittiprasert, S.; Thungkham, K.; Junorse, S.; Pongsawatkul, K.; Pattanaporn, W.; Jitwong, C.; Sanguansermsri, T. Analysis of real-time PCR cycle threshold of alpha-thalassemia-1 Southeast Asian type deletion using fetal cell-free DNA in maternal plasma for noninvasive prenatal diagnosis of Bart’s hydrops fetalis. J. Med. Assoc. Thail. 2010, 93, 1243–1248. [Google Scholar]
- Ho, S.S.; Chong, S.S.; Koay, E.S.; Ponnusamy, S.; Chiu, L.; Chan, Y.H.; Rauff, M.; Baig, S.; Chan, J.; Su, L.L.; et al. Noninvasive prenatal exclusion of haemoglobin Bart’s using foetal DNA from maternal plasma. Prenat. Diagn. 2010, 30, 65–73. [Google Scholar] [CrossRef]
- Sirichotiyakul, S.; Charoenkwan, P.; Sanguansermsri, T. Prenatal diagnosis of homozygous alpha-thalassemia-1 by cell-free fetal DNA in maternal plasma. Prenat. Diagn. 2012, 32, 45–49. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, C.S.; Jin, L.; Lou, H.; Song, Y. Prenatal detection of thalassemia by cell-free fetal DNA (cffDNA) in maternal plasma using surface enhanced Raman spectroscopy combined with PCR. Biomed. Opt. Express 2018, 9, 3167–3176. [Google Scholar] [CrossRef]
- Sawakwongpra, K.; Tangmansakulchai, K.; Ngonsawan, W.; Promwan, S.; Chanchamroen, S.; Quangkananurug, W.; Sriswasdi, S.; Jantarasaengaram, S.; Ponnikorn, S. Droplet-based digital PCR for non-invasive prenatal genetic diagnosis of α and β-thalassemia. Biomed. Rep. 2021, 15, 82. [Google Scholar] [CrossRef]
- Salomon, C.; Yee, S.; Scholz-Romero, K.; Kobayashi, M.; Vaswani, K.; Kvaskoff, D.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front. Pharmacol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Southcombe, J.H.; Tannetta, D.S.; Redman, C.W.; Sargent, I.L. Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol. Reprod. 2013, 89, 151. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef]
- Ouyang, Y.; Mouillet, J.F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific microRNAs in exosomes—Good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Clemmens, H.; Lambert, D.W. Extracellular vesicles: Translational challenges and opportunities. Biochem. Soc. Trans. 2018, 46, 1073–1082. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- De Wever, O.; Hendrix, A. A supporting ecosystem to mature extracellular vesicles into clinical application. Embo J. 2019, 38, e101412. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2021, 12, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Mouillet, J.F.; Sorkin, A.; Sadovsky, Y. Trophoblastic extracellular vesicles and viruses: Friends or foes? Am. J. Reprod. Immunol. 2021, 85, e13345. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.R. The Variation of Animals and Plants under Domestication; J. Murray: London, UK, 1868; Volume 1. [Google Scholar]
- Liu, Y.; Chen, Q. 150 years of Darwin’s theory of intercellular flow of hereditary information. Nat. Rev. Mol. Cell Biol. 2018, 19, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Schmorl, G. Pathologisch-Anatomische Unteruchungen Uber Puerperal-Eklampsie; Verlag FCW: Leipzig, Germany, 1893. [Google Scholar]
- Lapaire, O.; Holzgreve, W.; Oosterwijk, J.C.; Brinkhaus, R.; Bianchi, D.W. Georg Schmorl on trophoblasts in the maternal circulation. Placenta 2007, 28, 1–5. [Google Scholar] [CrossRef]
- Li, H.; Pinilla-Macua, I.; Ouyang, Y.; Sadovsky, E.; Kajiwara, K.; Sorkin, A.; Sadovsky, Y. Internalization of trophoblastic small extracellular vesicles and detection of their miRNA cargo in P-bodies. J. Extracell. Vesicles 2020, 9, 1812261. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Choi, K.; Choi, C.; Menon, R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am. J. Obstet. Gynecol. 2019, 221, 502.e1–502.e12. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Lei, J.; Saade, G.; Salomon, C.; Burd, I.; Menon, R. Feto-Maternal Trafficking of Exosomes in Murine Pregnancy Models. Front. Pharmacol. 2016, 7, 432. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Aliotta, J.M. Cellular phenotype.e switching and microvesicles. Adv. Drug Deliv. Rev. 2010, 62, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.D.; Lodha, S.; Sahoo, S. EV Cargo Sorting in Therapeutic Development for Cardiovascular Disease. Cells 2021, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Salomon, C.; Kobayashi, M.; Ashman, K.; Sobrevia, L.; Mitchell, M.D.; Rice, G.E. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS ONE 2013, 8, e79636. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.D.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Welton, J.L.; Webber, J.P.; Botos, L.A.; Jones, M.; Clayton, A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J. Extracell. Vesicles 2015, 4, 27269. [Google Scholar] [CrossRef] [PubMed]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef]
- Koh, Y.Q.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N.; Mitchell, M.D. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front. Biosci. (Landmark Ed.) 2018, 23, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Whiteside, T.L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol. Biol. 2021, 2265, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Yousif, G.; Qadri, S.; Parray, A.; Akhthar, N.; Shuaib, A.; Haik, Y. Exosomes Derived Neuronal Markers: Immunoaffinity Isolation and Characterization. NeuroMol. Med. 2021, 24, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Kongsomros, S.; Pongsakul, N.; Panachan, J.; Khowawisetsut, L.; Pattanapanyasat, K.; Hongeng, S.; Thitithanyanont, A. Anti-SARS-CoV-2 effect of extracellular vesicles released from mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12201. [Google Scholar] [CrossRef] [PubMed]
- Panachan, J.; Rojsirikulchai, N.; Pongsakul, N.; Khowawisetsut, L.; Pongphitcha, P.; Siriboonpiputtana, T.; Chareonsirisuthigul, T.; Phornsarayuth, P.; Klinkulab, N.; Jinawath, N.; et al. Extracellular Vesicle-Based Method for Detecting MYCN Amplification Status of Pediatric Neuroblastoma. Cancers 2022, 14, 2627. [Google Scholar] [CrossRef]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Arkesteijn, G.J.; Nauwelaers, F.A.; van den Engh, G.; Nolte-’t Hoen, E.N.; Wauben, M.H. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A 2016, 89, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Greis, K.D. Multiplex Biomarker Screening Assay for Urinary Extracellular Vesicles Study: A Targeted Label-Free Proteomic Approach. Sci. Rep. 2018, 8, 15039. [Google Scholar] [CrossRef]
- Menon, R.; Dixon, C.L.; Sheller-Miller, S.; Fortunato, S.J.; Saade, G.R.; Palma, C.; Lai, A.; Guanzon, D.; Salomon, C. Quantitative Proteomics by SWATH-MS of Maternal Plasma Exosomes Determine Pathways Associated With Term and Preterm Birth. Endocrinology 2019, 160, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Lo, Y.M. Fetomaternal cellular and plasma DNA trafficking: The Yin and the Yang. Ann. N. Y. Acad. Sci. 2001, 945, 119–131. [Google Scholar] [CrossRef]
- Chiu, R.W.; Lo, Y.M. Application of fetal DNA in maternal plasma for noninvasive prenatal diagnosis. Expert Rev. Mol. Diagn. 2002, 2, 32–40. [Google Scholar] [CrossRef]
- Chiu, R.W.; Lo, Y.M. Non-invasive prenatal diagnosis: On the horizon? Pharmacogenomics 2003, 4, 191–200. [Google Scholar] [CrossRef]
- Bianchi, D.W. Circulating fetal DNA: Its origin and diagnostic potential-a review. Placenta 2004, 25 (Suppl. A), S93–S101. [Google Scholar] [CrossRef]
- Wataganara, T.; Bianchi, D.W. Fetal cell-free nucleic acids in the maternal circulation: New clinical applications. Ann. N. Y. Acad. Sci. 2004, 1022, 90–99. [Google Scholar] [CrossRef]
- Lo, Y.M.; Chiu, R.W. Prenatal diagnosis: Progress through plasma nucleic acids. Nat. Rev. Genet. 2007, 8, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Bianchi, D.W. Fetal fraction and noninvasive prenatal testing: What clinicians need to know. Prenat. Diagn. 2020, 40, 155–163. [Google Scholar] [CrossRef]
- Chiu, R.W.K.; Lo, Y.M.D. Cell-free fetal DNA coming in all sizes and shapes. Prenat. Diagn. 2021, 41, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D. Noninvasive prenatal testing: Advancing through a virtuous circle of science, technology and clinical applications. Prenat. Diagn. 2021, 41, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000, 21, 597–602. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; O’Connor, S.M.; Lam, G.K.; Taylor, D.D. Shed membrane fragment modulation of CD3-zeta during pregnancy: Link with induction of apoptosis. J. Reprod. Immunol. 2002, 56, 29–44. [Google Scholar] [CrossRef]
- Kam, W.; Clauser, E.; Kim, Y.S.; Kan, Y.W.; Rutter, W.J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc. Natl. Acad. Sci. USA 1985, 82, 8715–8719. [Google Scholar] [CrossRef]
- Hedlund, M.; Stenqvist, A.C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: Evidence for immunosuppressive function. J. Immunol. 2009, 183, 340–351. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef]
- Henthorn, P.S.; Raducha, M.; Kadesch, T.; Weiss, M.J.; Harris, H. Sequence and characterization of the human intestinal alkaline phosphatase gene. J. Biol. Chem. 1988, 263, 12011–12019. [Google Scholar] [CrossRef]
- Kniss, D.A.; Xie, Y.; Li, Y.; Kumar, S.; Linton, E.A.; Cohen, P.; Fan-Havard, P.; Redman, C.W.; Sargent, I.L. ED(27) trophoblast-like cells isolated from first-trimester chorionic villi are genetically identical to HeLa cells yet exhibit a distinct phenotype. Placenta 2002, 23, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Yee, S.W.; Mitchell, M.D.; Rice, G.E. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed. Res. Int. 2014, 2014, 693157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Liu, X.B.; Huang, S.; Bi, X.Y.; Wang, H.X.; Xie, L.X.; Wang, Y.Q.; Cao, X.F.; Lv, J.; Xiao, F.J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Nagaeva, O.; Chen, T.; Stendahl, U.; Antsiferova, J.; Mogren, I.; Hernestål, J.; Baranov, V. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: A possible novel immune escape mechanism for fetal survival. J. Immunol. 2006, 176, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Salomon, C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin. Immunopathol. 2018, 40, 425–437. [Google Scholar] [CrossRef]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef]
- Chang, G.; Mouillet, J.F.; Mishima, T.; Chu, T.; Sadovsky, E.; Coyne, C.B.; Parks, W.T.; Surti, U.; Sadovsky, Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017, 31, 2760–2770. [Google Scholar] [CrossRef]
- Luo, S.S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A.; et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef]
- Donker, R.B.; Mouillet, J.F.; Chu, T.; Hubel, C.A.; Stolz, D.B.; Morelli, A.E.; Sadovsky, Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef]
- Atay, S.; Gercel-Taylor, C.; Suttles, J.; Mor, G.; Taylor, D.D. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am. J. Reprod. Immunol. 2011, 65, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Kambe, S.; Yoshitake, H.; Yuge, K.; Ishida, Y.; Ali, M.M.; Takizawa, T.; Kuwata, T.; Ohkuchi, A.; Matsubara, S.; Suzuki, M.; et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol. Reprod. 2014, 91, 129. [Google Scholar] [CrossRef] [PubMed]
- Collett, G.P.; Redman, C.W.; Sargent, I.L.; Vatish, M. Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget 2018, 9, 6707–6717. [Google Scholar] [CrossRef] [PubMed]
- Czernek, L.; Düchler, M. Exosomes as Messengers Between Mother and Fetus in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4264. [Google Scholar] [CrossRef]
- Atay, S.; Gercel-Taylor, C.; Taylor, D.D. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. Am. J. Reprod. Immunol. 2011, 66, 259–269. [Google Scholar] [CrossRef]
- Hamilton, S.T.; Hahn, F.; Sonntag, E.; Marschall, M.; Rawlinson, W.D. A placental specific miRNA miR-517a-3p exerts anti-human cytomegalovirus activity. Placenta 2021, 112, 62–65. [Google Scholar] [CrossRef]
- Patil, R.; Ghosh, K.; Shetty, S. Comment on Salomon et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, e24–e25. [Google Scholar] [CrossRef]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef]
- Salomon, C.; Rice, G.E. Reply. Am. J. Obstet. Gynecol. 2016, 214, 766–767. [Google Scholar] [CrossRef]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5157–5169. [Google Scholar] [CrossRef]
- Record, M. Intercellular communication by exosomes in placenta: A possible role in cell fusion? Placenta 2014, 35, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Elfeky, O.; Kinhal, V.; Dutta, S.; Lai, A.; Jayabalan, N.; Nuzhat, Z.; Palma, C.; Rice, G.E.; Salomon, C. Review: Fetal-maternal communication via extracellular vesicles—Implications for complications of pregnancies. Placenta 2017, 54, 83–88. [Google Scholar] [CrossRef]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.; Tsui, N.B.; Lau, T.K.; Leung, T.N.; Chiu, R.W.; Panesar, N.S.; Lit, L.C.; Chan, K.W.; Lo, Y.M. mRNA of placental origin is readily detectable in maternal plasma. Proc. Natl. Acad. Sci. USA 2003, 100, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Jurkovic, D.; Campbell, S. Current topic: In vivo investigation of the placental circulations by Doppler echography. Placenta 1995, 16, 323–331. [Google Scholar] [CrossRef]
- Truong, G.; Guanzon, D.; Kinhal, V.; Elfeky, O.; Lai, A.; Longo, S.; Nuzhat, Z.; Palma, C.; Scholz-Romero, K.; Menon, R.; et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells—Liquid biopsies for monitoring complications of pregnancy. PLoS ONE 2017, 12, e0174514. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Escudero, C.A.; Herlitz, K.; Troncoso, F.; Acurio, J.; Aguayo, C.; Roberts, J.M.; Truong, G.; Duncombe, G.; Rice, G.; Salomon, C. Role of Extracellular Vesicles and microRNAs on Dysfunctional Angiogenesis during Preeclamptic Pregnancies. Front. Physiol. 2016, 7, 98. [Google Scholar] [CrossRef]
- Jayabalan, N.; Nair, S.; Nuzhat, Z.; Rice, G.E.; Zuñiga, F.A.; Sobrevia, L.; Leiva, A.; Sanhueza, C.; Gutiérrez, J.A.; Lappas, M.; et al. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front. Endocrinol. (Lausanne) 2017, 8, 239. [Google Scholar] [CrossRef]
- Nair, S.; Guanzon, D.; Jayabalan, N.; Lai, A.; Scholz-Romero, K.; Kalita de Croft, P.; Ormazabal, V.; Palma, C.; Diaz, E.; McCarthy, E.A.; et al. Extracellular vesicle-associated miRNAs are an adaptive response to gestational diabetes mellitus. J. Transl. Med. 2021, 19, 360. [Google Scholar] [CrossRef]
- Nair, S.; Ormazabal, V.; Lappas, M.; McIntyre, H.D.; Salomon, C. Extracellular vesicles and their potential role inducing changes in maternal insulin sensitivity during gestational diabetes mellitus. Am. J. Reprod. Immunol. 2021, 85, e13361. [Google Scholar] [CrossRef]
- Miranda, J.; Paules, C.; Nair, S.; Lai, A.; Palma, C.; Scholz-Romero, K.; Rice, G.E.; Gratacos, E.; Crispi, F.; Salomon, C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction—Liquid biopsies to monitoring fetal growth. Placenta 2018, 64, 34–43. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.; Sheller-Miller, S.; Salomon, C. Protein Profile Changes in Circulating Placental Extracellular Vesicles in Term and Preterm Births: A Longitudinal Study. Endocrinology 2020, 161, bqaa009. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Shahin, H. Extracellular vesicles in spontaneous preterm birth. Am. J. Reprod. Immunol. 2021, 85, e13353. [Google Scholar] [CrossRef] [PubMed]
- Radnaa, E.; Richardson, L.S.; Sheller-Miller, S.; Baljinnyam, T.; de Castro Silva, M.; Kumar Kammala, A.; Urrabaz-Garza, R.; Kechichian, T.; Kim, S.; Han, A.; et al. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab. Chip 2021, 21, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Dixon, C.L.; Cayne, S.; Radnaa, E.; Salomon, C.; Sheller-Miller, S. Differences in cord blood extracellular vesicle cargo in preterm and term births. Am. J. Reprod. Immunol. 2022, 87, e13521. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Jones, C.J.; Aplin, J.D.; Abrahams, V.M. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol. Reprod. 2012, 86, 103. [Google Scholar] [CrossRef]
- Tobin, B.W.; Marchello, M.J. Islet transplantation reverses carcass protein loss in diabetic rats without inducing disproportionate fat accumulation. Diabetologia 1995, 38, 881–888. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Chatchen, S.; Svasti, J. Plasma prefractionation methods for proteomic analysis and perspectives in clinical applications. Proteom. Clin. Appl. 2017, 11, 1600135. [Google Scholar] [CrossRef]
- Kheansaard, W.; Phongpao, K.; Paiboonsukwong, K.; Pattanapanyasat, K.; Chaichompoo, P.; Svasti, S. Microparticles from β-thalassaemia/HbE patients induce endothelial cell dysfunction. Sci. Rep. 2018, 8, 13033. [Google Scholar] [CrossRef] [PubMed]
- Klaihmon, P.; Lertthammakiat, S.; Anurathapan, U.; Pakakasama, S.; Sirachainan, N.; Hongeng, S.; Pattanapanyasat, K. Activated platelets and leukocyte activations in young patients with β-thalassemia/HbE following bone marrow transplantation. Thromb. Res. 2018, 169, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Siriworadetkun, S.; Thubthed, R.; Thiengtavor, C.; Paiboonsukwong, K.; Khuhapinant, A.; Fucharoen, S.; Pattanapanyasat, K.; Vadolas, J.; Svasti, S.; Chaichompoo, P. Elevated levels of circulating monocytic myeloid derived suppressor cells in splenectomised β-thalassaemia/HbE patients. Br. J. Haematol. 2020, 191, e72–e76. [Google Scholar] [CrossRef]
- Atipimonpat, A.; Siwaponanan, P.; Khuhapinant, A.; Svasti, S.; Sukapirom, K.; Khowawisetsut, L.; Pattanapanyasat, K. Extracellular vesicles from thalassemia patients carry iron-containing ferritin and hemichrome that promote cardiac cell proliferation. Ann. Hematol. 2021, 100, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Klaihmon, P.; Khuhapinant, A.; Kheansaard, W.; Pattanapanyasat, K. Internalization of cell-derived microparticles triggers endothelial pro-inflammatory responses. Asian Pac. J. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Levin, C.; Koren, A.; Rebibo-Sabbah, A.; Levin, M.; Koifman, N.; Brenner, B.; Aharon, A. Extracellular Vesicle MicroRNA That Are Involved in β-Thalassemia Complications. Int. J. Mol. Sci. 2021, 22, 9760. [Google Scholar] [CrossRef] [PubMed]
- John, A.H.; Duncan, A.S. The maternal syndrome associated with hydrops foetalis. J. Obstet. Gynaecol. Br. Commonw. 1964, 71, 61–65. [Google Scholar] [CrossRef]
- Kaiser, I.H. Ballantyne and triple edema. Am. J. Obstet. Gynecol. 1971, 110, 115–120. [Google Scholar] [CrossRef]
- Gedikbasi, A.; Oztarhan, K.; Gunenc, Z.; Yildirim, G.; Arslan, O.; Yildirim, D.; Ceylan, Y. Preeclampsia due to fetal non-immune hydrops: Mirror syndrome and review of literature. Hypertens. Pregnancy 2011, 30, 322–330. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef] [PubMed]
- Kongsomros, S.; Suksatu, A.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Prasongtanakij, S.; Jearawuttanakul, K.; Borwornpinyo, S.; Hongeng, S.; Thitithanyanont, A.; Chutipongtanate, S. Anti-SARS-CoV-2 Activity of Extracellular Vesicle Inhibitors: Screening, Validation, and Combination with Remdesivir. Biomedicines 2021, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S. EV-out or EV-in: Tackling cell-to-cell communication within the tumor microenvironment to enhance anti-tumor efficacy using extracellular vesicle-based therapeutic strategies. OpenNano 2022, 8, 100085. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Tweet, M.S.; Lewey, J.; Smilowitz, N.R.; Rose, C.H.; Best, P.J.M. Pregnancy-Associated Myocardial Infarction: Prevalence, Causes, and Interventional Management. Circ. Cardiovasc. Interv. 2020, 13, e20008687. [Google Scholar] [CrossRef]
- Verbruggen, M.; Mannaerts, D.; Muys, J.; Jacquemyn, Y. Use of ticagrelor in human pregnancy, the first experience. BMJ Case Rep. 2015, bcr2015212217. [Google Scholar] [CrossRef]
- Nana, M.; Morgan, H.; Moore, S.; Lee, Z.X.; Ang, E.; Nelson-Piercy, C. Antiplatelet therapy in pregnancy: A systematic review. Pharmacol. Res. 2021, 168, 105547. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Goetzl, L.; Karliner, J.S.; Tang, N.; Pulliam, L. Human plasma platelet-derived exosomes: Effects of aspirin. FASEB J. 2016, 30, 2058–2063. [Google Scholar] [CrossRef]
- Casieri, V.; Matteucci, M.; Pasanisi, E.M.; Papa, A.; Barile, L.; Fritsche-Danielson, R.; Lionetti, V. Ticagrelor Enhances Release of Anti-Hypoxic Cardiac Progenitor Cell-Derived Exosomes Through Increasing Cell Proliferation In Vitro. Sci. Rep. 2020, 10, 2494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).