Effect of Nanosecond Pulsed Currents on Directions of Cell Elongation and Migration through Time-Lapse Analysis
Abstract
1. Introduction
2. Results and Discussion
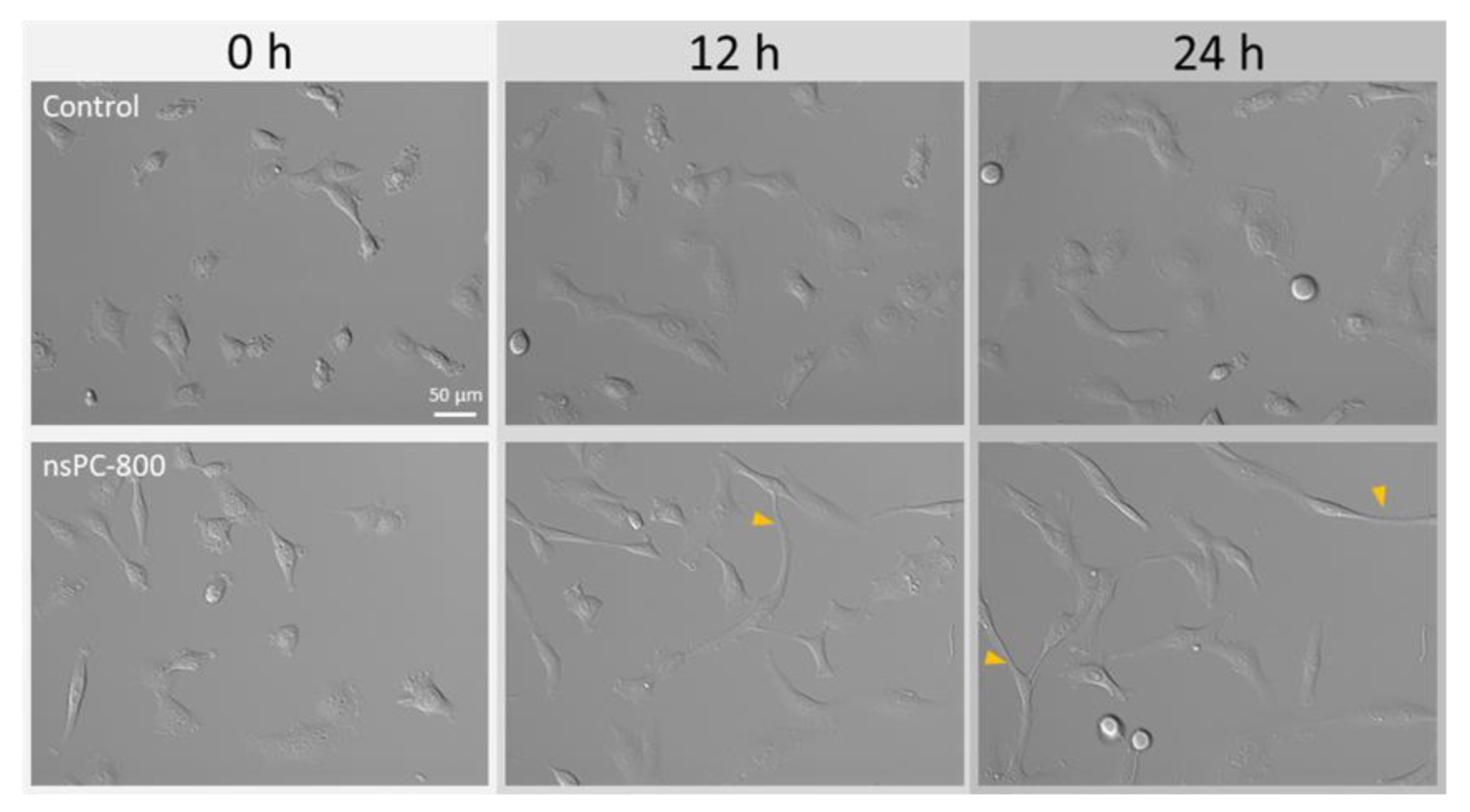
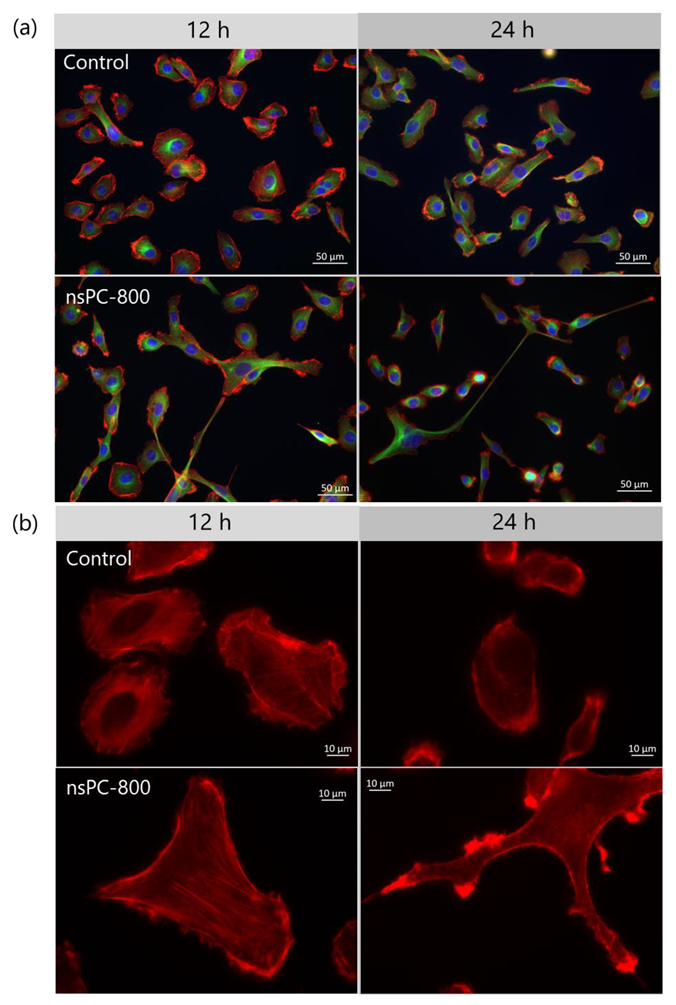
2.1. nsPC Stimulation Change HT-1080 Morphology with Stress Fiber Formation
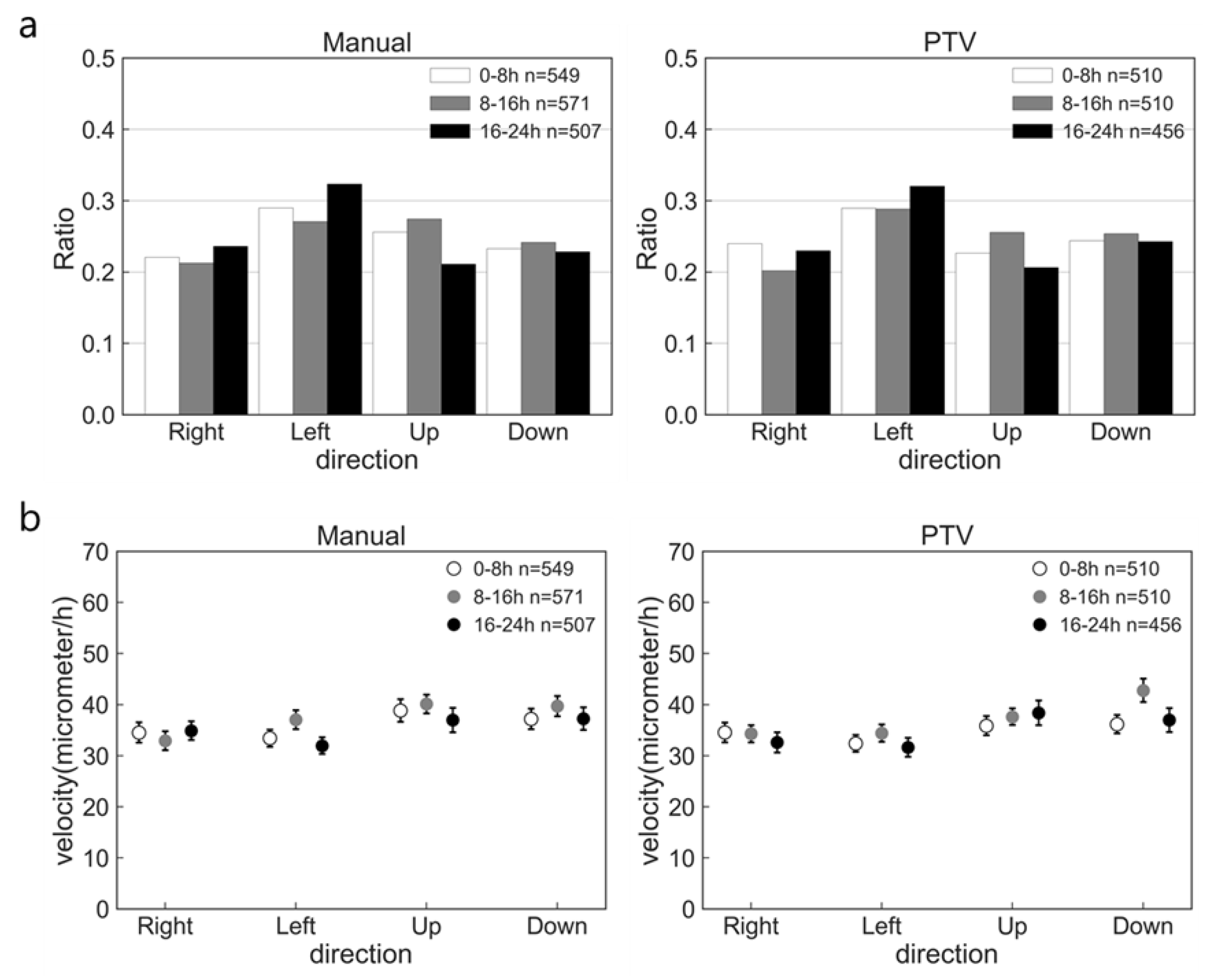
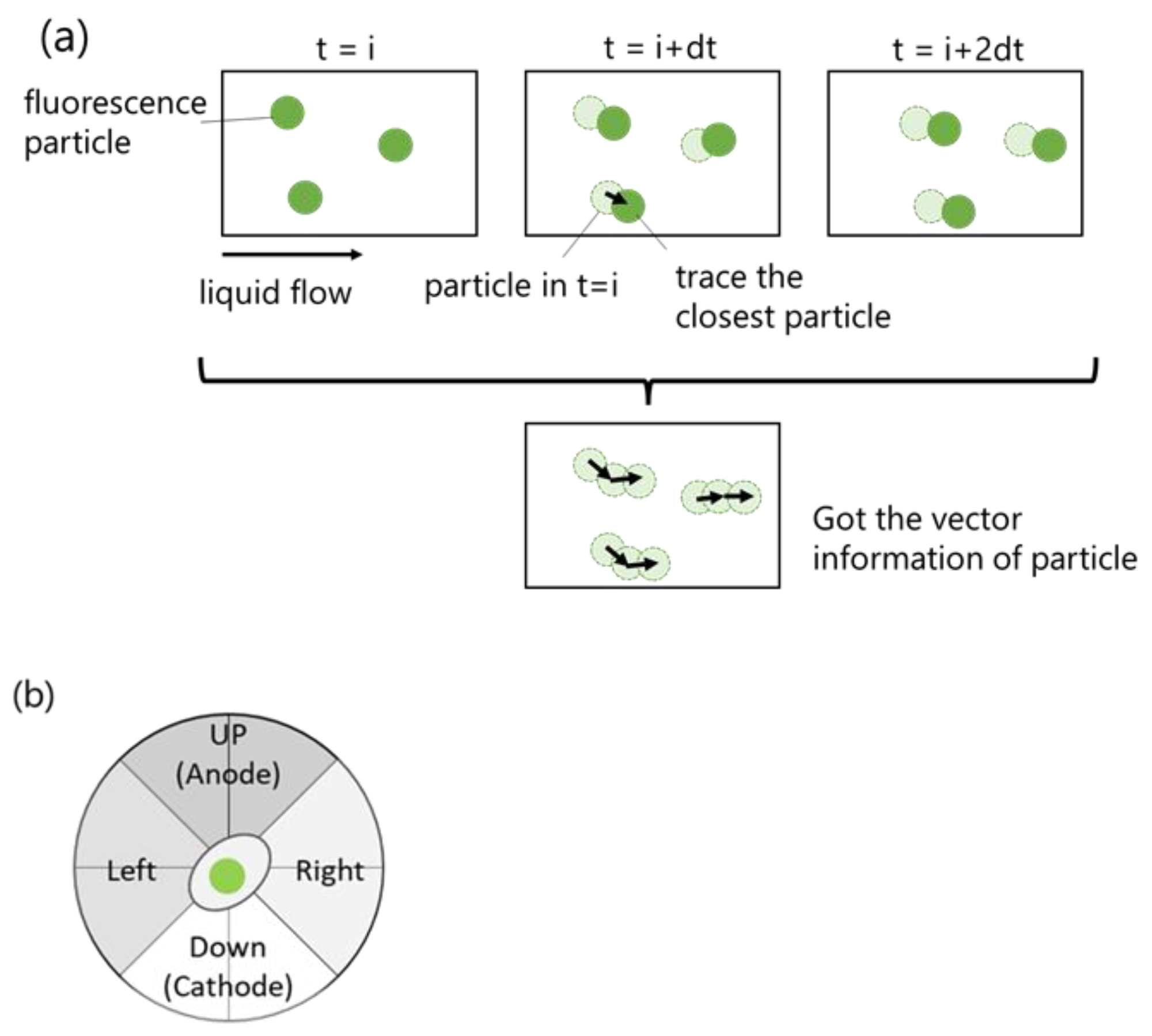
2.2. Accuracy of PTV Method Compared with Manual Method
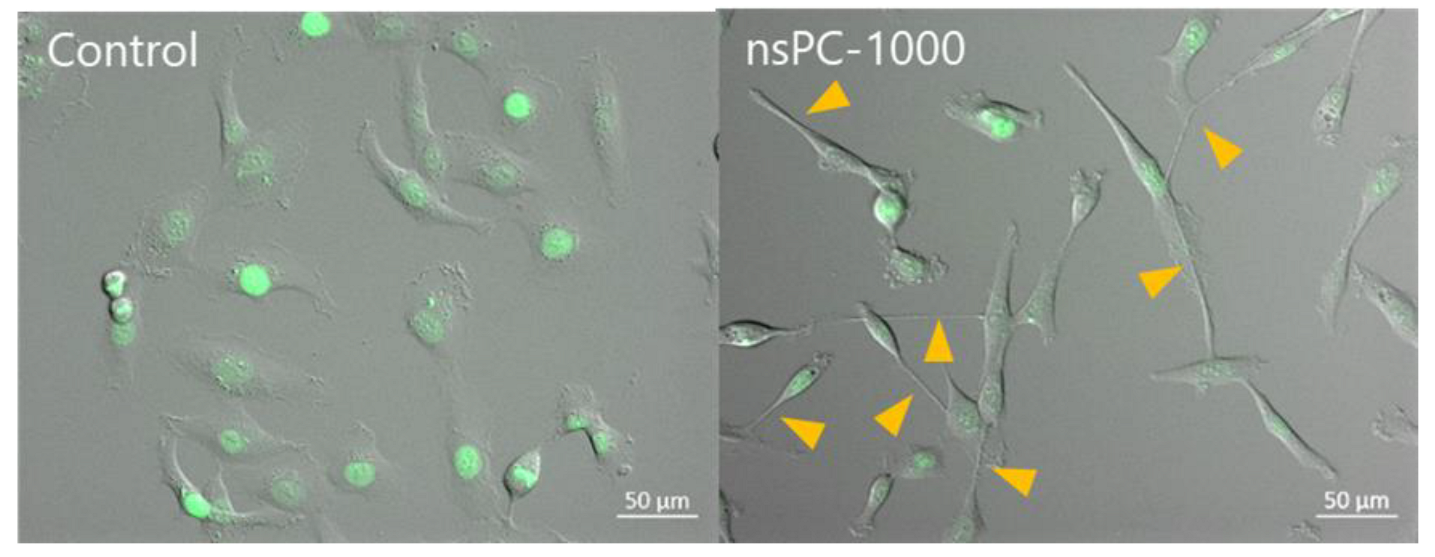
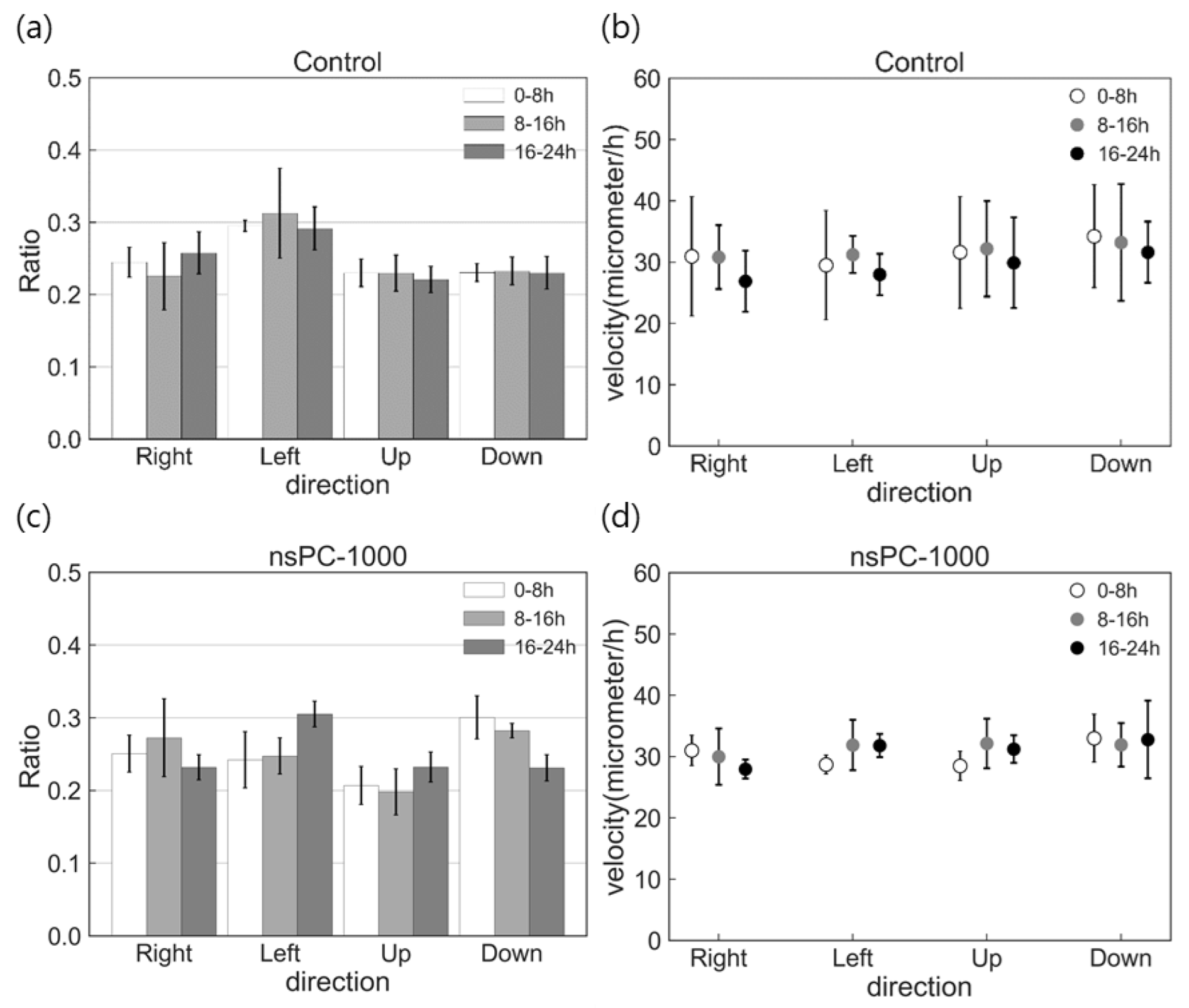
2.3. Effect of nsPC on Cell Migration
3. Materials and Methods
3.1. Cell Culture
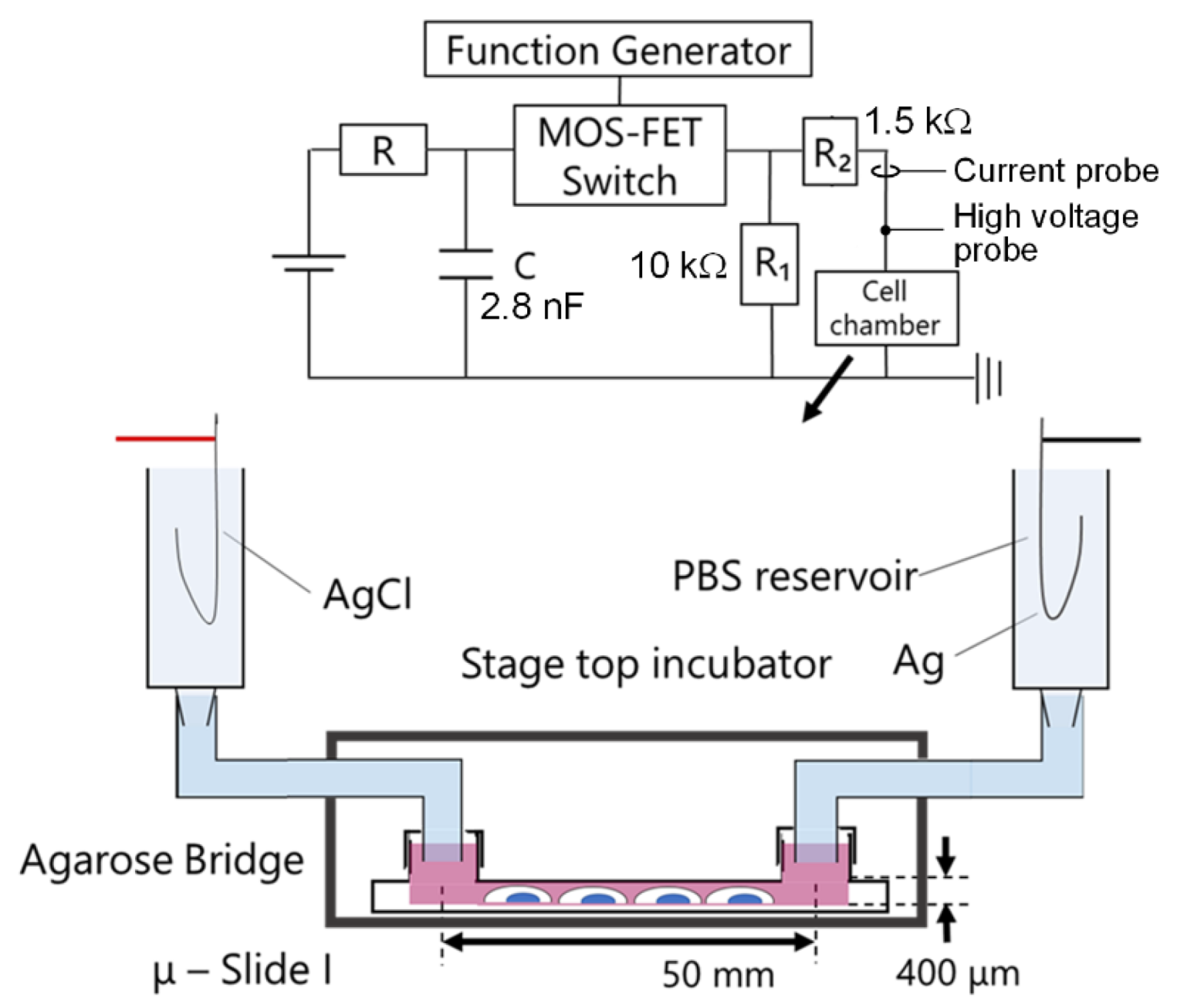
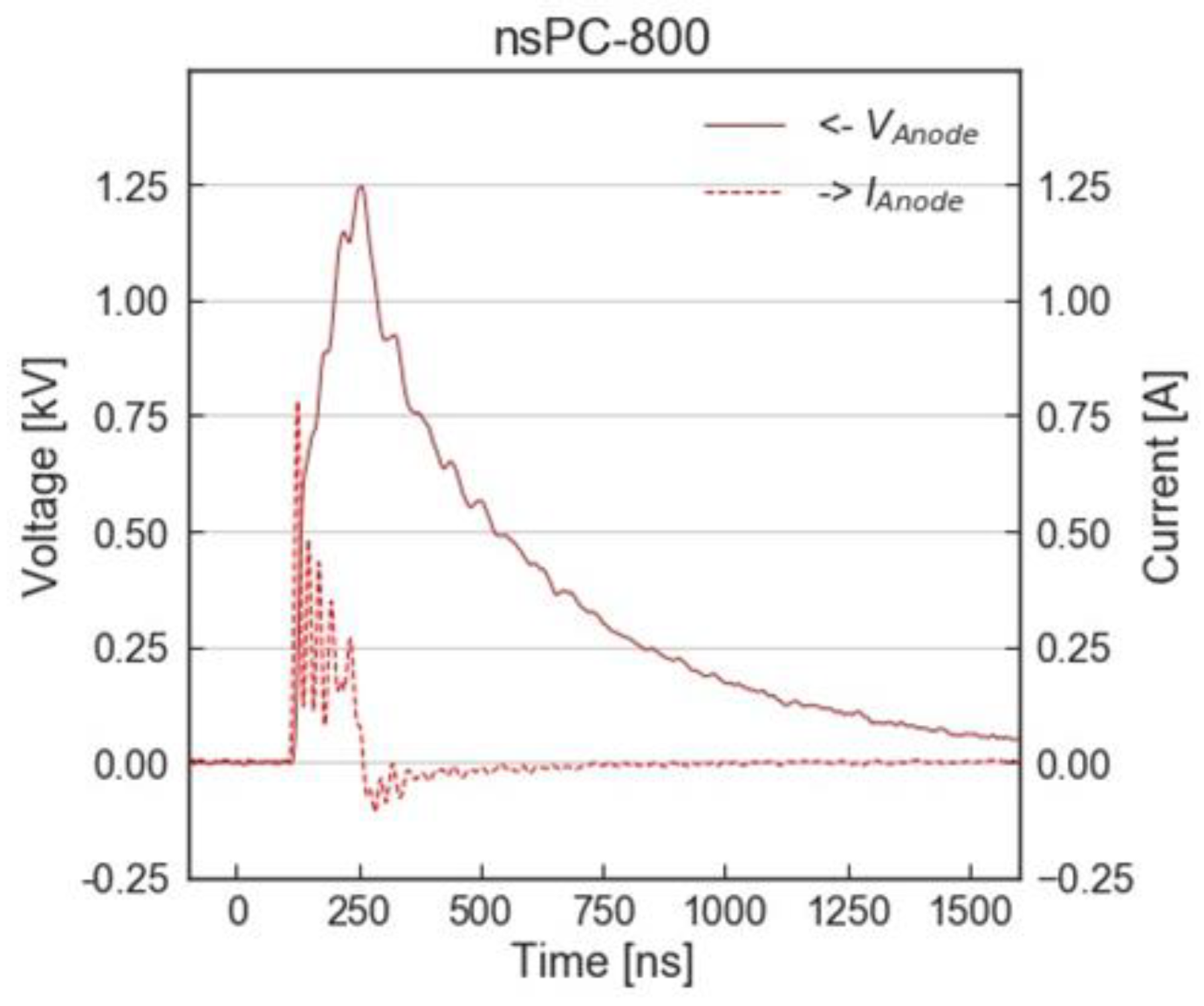
3.2. Experimental Setup with nsPC
3.3. Cell Staining
3.4. Microscopy and Time-Lapse Analysis
3.5. Automatic Cell Migration Analysis by Algorithm
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine–current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Reuter, S.; Von Woedtke, T.; Weltmann, K.D. The kINPen–A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Muramatsu, K.; Sato, T.; Nakajima, T.; Nagasawa, T.; Nakatani, T.; Fujimura, S. Sterilization in liquids by air plasma under intermittent discharge. Mech. Eng. J. 2020, 7, 19-00431. [Google Scholar] [CrossRef]
- Sato, T.; Miyahara, T.; Doi, A.; Ochiai, S.; Urayama, T.; Nakatani, T. Sterilization mechanism for Escherichia coli by plasma flow at atmospheric pressure. Appl. Phys. Lett. 2006, 89, 073902. [Google Scholar] [CrossRef]
- Xu, Z.; Lan, Y.; Ma, J.; Shen, J.; Han, W.; Hu, S.; Ye, C.; Xi, W.; Zhang, Y.; Yang, C. Applications of atmospheric pressure plasma in microbial inactivation and cancer therapy: A brief review. Plasma Sci. Technol. 2020, 22, 103001. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Katsumata, Y.; Ishikawa, K.; Kondo, H.; Hashizume, H.; Okazaki, Y.; Toyokuni, S.; Nakamura, K.; Yoshikawa, N.; et al. Oxidative stress-dependent and -independent death of glioblastoma cells induced by non-thermal plasma-exposed solutions. Sci. Rep. 2019, 9, 13657. [Google Scholar] [CrossRef]
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 372001. [Google Scholar] [CrossRef]
- Yokoyama, M.; Johkura, K.; Sato, T. Gene expression responses of HeLa cells to chemical species generated by an atmospheric plasma flow. Biochem. Biophys. Res. Commun. 2014, 450, 1266–1271. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornin, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Chang, C.H.; Yano, K.; Sato, T. Distinct biological actions of electrical and chemical factors of cold atmospheric pressure plasma and their synergistic cytotoxic effects. Int. J. Plasma Environ. Sci. Technol. 2020, 14, e03004. [Google Scholar]
- Cortese, B.; Palamà, I.E.; D’Amone, S.; Gigli, G. Influence of electrotaxis on cell behaviour. Integr. Biol. 2014, 6, 817–830. [Google Scholar] [CrossRef]
- Weaver, J.C.; Smith, K.C.; Esser, A.T.; Son, R.S.; Gowrishankar, T.R. A brief overview of electroporation pulse strength–duration space: A region where additional intracellular effects are expected. Bioelectrochemistry 2012, 87, 236–243. [Google Scholar] [CrossRef]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans. Biomed. Eng. 2015, 62, 4–20. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 435, 580–585. [Google Scholar] [CrossRef]
- Burke, R.C.; Bardet, S.M.; Carr, L.; Romanenko, S.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Nanosecond pulsed electric fields depolarize transmembrane potential via voltage-gated K+, Ca2+ and TRPM8 channels in U87 glioblastoma cells. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 2040–2050. [Google Scholar] [CrossRef]
- Guo, S.; Burcus, N.I.; Hornef, J.; Jing, Y.; Jiang, C.; Heller, R.; Beebe, S.J. Nano-Pulse Stimulation for the Treatment of Pancreatic Cancer and the Changes in Immune Profile. Cancers 2018, 10, 217. [Google Scholar] [CrossRef]
- Chang, C.H.; Yano, K.; Okumura, T.; Sato, T. Effect of plasma-generator-supplied nanosecond pulsed current on cell response. J. Phys. D Appl. Phys. 2019, 52, 185402. [Google Scholar] [CrossRef]
- Chang, C.H.; Yano, K.; Sato, T. Nanosecond pulsed current under plasma-producing conditions induces morphological alterations and stress fiber formation in human fibrosarcoma HT-1080 cells. Arch. Biochem. Biophys. 2020, 681, 108252. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, K.; Nakabayashi, T.; Li, L.; Ohta, N. Effects of Nanosecond Pulsed Electric Field on Intracellular NADH Autofluorescence: A Comparison between Normal and Cancer Cells. ACS Omega 2017, 2, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Awasthi, K.; Hsu, H.Y.; Hsieh, P.Y. Differentiation between normal and cancer cells with autofluorescence lifetime microscopy and nanosecond pulsed electric field effects. SPIE BiOS 2019, 10890, 51. [Google Scholar]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef]
- Wolf, K.; Mazo, I.; Leung, H.; Engelke, K.; von Andrian, U.H.; Deryugina, E.I.; Strongin, A.Y.; Brocker, E.B.; Friedl, P. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003, 160, 267–277. [Google Scholar] [CrossRef]
- Sannino, G.; Marchetto, A.; Kirchner, T.; Grunewald, T.G.P. Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transition in Mesenchymal Tumors: A Paradox in Sarcomas? Cancer Res. 2017, 77, 4556–4561. [Google Scholar] [CrossRef]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef]
- Shankar, J.; Nabi, I.R. Actin Cytoskeleton Regulation of Epithelial Mesenchymal Transition in Metastatic Cancer Cells. PLoS ONE 2015, 10, e0132759. [Google Scholar]
- Song, B.; Gu, Y.; Pu, J.; Reid, B.; Zhao, Z.; Zhao, M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nature 2007, 2, 1479–1489. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Li, L.; Huang, C.; Lin, J.; Zhu, G.; Chen, Z.; Wu, N.; Feng, H. Superoxide plays critical roles in electrotaxis of fibrosarcoma cells via activation of ERK and reorganization of the cytoskeleton. Free Radic. Biol. Med. 2012, 52, 1888–1896. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Wei, C.; Chow, J.K.; Nguy, L.; Nguyen, H.K.; Schmidt, C.E. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J. Neural Eng. 2013, 10, 046011. [Google Scholar] [CrossRef]
- Hammerick, K.E.; Longaker, M.T.; Prinz, F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010, 397, 12–17. [Google Scholar] [CrossRef]
| 0–8 h | 8–16 h | 16–24 h | |
|---|---|---|---|
| Capture ratio in PTV (vs. manual) [%] | 93.0 ± 0.7 | 90.1 ± 5.0 | 96.2 ± 5.2 |
| Temperature (°C) | Start | 24 h Later |
|---|---|---|
| Control | 35.0 ± 0.9 | 34.2 ± 0.5 |
| nsPC-800 | 34.4 ± 0.7 | |
| nsPC-1000 | 34.8 ± 0.3 | |
| pH | Start | 24 h Later |
| Control | 7.7 ± 0.1 | 7.3 ± 0.1 |
| nsPC-800 | 7.4 ± 0.3 | |
| nsPC-1000 | 7.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, H.; Uehara, S.; Chang, C.-H.; Yano, K.-i.; Sato, T. Effect of Nanosecond Pulsed Currents on Directions of Cell Elongation and Migration through Time-Lapse Analysis. Int. J. Mol. Sci. 2023, 24, 3826. https://doi.org/10.3390/ijms24043826
Tada H, Uehara S, Chang C-H, Yano K-i, Sato T. Effect of Nanosecond Pulsed Currents on Directions of Cell Elongation and Migration through Time-Lapse Analysis. International Journal of Molecular Sciences. 2023; 24(4):3826. https://doi.org/10.3390/ijms24043826
Chicago/Turabian StyleTada, Hayato, Satoshi Uehara, Chia-Hsing Chang, Ken-ichi Yano, and Takehiko Sato. 2023. "Effect of Nanosecond Pulsed Currents on Directions of Cell Elongation and Migration through Time-Lapse Analysis" International Journal of Molecular Sciences 24, no. 4: 3826. https://doi.org/10.3390/ijms24043826
APA StyleTada, H., Uehara, S., Chang, C.-H., Yano, K.-i., & Sato, T. (2023). Effect of Nanosecond Pulsed Currents on Directions of Cell Elongation and Migration through Time-Lapse Analysis. International Journal of Molecular Sciences, 24(4), 3826. https://doi.org/10.3390/ijms24043826






