Abstract
Immune checkpoint inhibitors (ICIs) have a modest clinical activity when administered as monotherapy against breast cancer (BC), the most common malignancy in women. Novel combinatorial strategies are currently being investigated to overcome resistance to ICIs and promote antitumor immune responses in a greater proportion of BC patients. Recent studies have shown that the BC abnormal vasculature is associated with immune suppression in patients, and hampers both drug delivery and immune effector cell trafficking to tumor nests. Thus, strategies directed at normalizing (i.e., at remodeling and stabilizing) the immature, abnormal tumor vessels are receiving much attention. In particular, the combination of ICIs with tumor vessel normalizing agents is thought to hold great promise for the treatment of BC patients. Indeed, a compelling body of evidence indicates that the addition of low doses of antiangiogenic drugs to ICIs substantially improves antitumor immunity. In this review, we outline the impact that the reciprocal interactions occurring between tumor angiogenesis and immune cells have on the immune evasion and clinical progression of BC. In addition, we overview preclinical and clinical studies that are presently evaluating the therapeutic effectiveness of combining ICIs with antiangiogenic drugs in BC patients.
1. Introduction
The advent of immunotherapy has paved the way for treating highly aggressive, previously incurable cancers in a considerable percentage of patients [1]. In particular, immune checkpoint (IC) inhibitors (ICIs) that reactivate dysfunctional and/or exhausted T cells have shown remarkable efficacy against a wide range of solid and hematologic tumors [2]. However, the administration of ICIs as monotherapy has shown limited efficacy and high side effects in certain types of tumors [3]. Among the latter is breast cancer (BC) [4,5,6], the most common female malignancy worldwide [7], which, being poorly immunogenic, has for a long time been considered as generically resistant to immunotherapy [8]. In the last two decades, however, it has been understood that this concept does not apply indiscriminately to all BC patients [9]. In fact, the thorough characterization of BC heterogeneity has allowed the delineation of molecular subtypes with specific pathological features and clinical outcomes [9]. BC subtypes include luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-positive, and basal-like BC [10]. Luminal tumors express hormone receptors [11], while HER2-positive tumors are characterized by HER2 overexpression [11]. On their part, basal-like BCs express neither hormone receptors nor HER2, thereby being named triple-negative BCs (TNBCs) [11]. Amidst the BC subtypes, TNBC has emerged as an attractive candidate for the evaluation of novel immunotherapy approaches. This is because TNBC displays high genomic instability that leads to the generation of tumor-specific neoantigens, overexpression of the IC programmed death-ligand 1 (PD-L1), and a high density of tumor-infiltrating lymphocytes (TILs) [12,13,14]. Altogether, these features argue in favor of TNBC responsiveness to immunotherapy. Therefore, in an attempt to overcome the modest clinical activity of ICIs administered as monotherapy, treatments involving the combination of ICIs and immunogenic chemotherapy are being evaluated [4,6].
To date, two immunotherapy agents, atezolizumab (an antibody directed against PD-L1) and pembrolizumab (an anti-PD1 antibody), have been approved in combination with chemotherapy for PD-L1 positive, advanced TNBC [15,16]. Interestingly, patients who have received cisplatin or doxorubicin followed by the administration of nivolumab (another antibody against PD1) experience the upregulation of immune-related genes involved in PD-1/PD-L1 and cytotoxic T-cell pathways (NCT04159818 [17]). Several studies have also evaluated ICIs’ combination with chemotherapeutics during neoadjuvant treatment [18]. However, the addition of PD-1/PD-L1 inhibitors to chemotherapy has been reported to increase the rate of pathologic complete response (pCR) only in a small fraction of patients with early-stage BC [6]. Indeed, most patients respond initially and then develop resistance [6]. In addition, unsatisfactory activity has been observed in BC subtypes other than PD-L1-positive TNBCs [6].
To date, no solid data can accurately explain the pattern of response or resistance to ICIs in BC patients [19]. Thus, a deeper understanding of the mechanisms for BC resistance to immunotherapy is required to achieve pCR in a greater number of patients. In this regard, the analysis of the tumor microenvironment (TME) holds great promise [19].
The TME consists of stromal and immune cells lying on an extracellular matrix traversed by blood and lymphatic vessels [20]. Compared with its non-malignant counterpart, each component of the TME is abnormal in a fashion that fuels tumor progression and resistance to therapy [21]. In particular, the TME of BC is characterized by hypoxia, a low pH, and a high interstitial fluid pressure [22,23]: all these features not only reduce the efficacy of anticancer therapies, but also hamper immune cells entrance in the tumor nest [24]. This evidence suggests that normalizing the TME of BC could improve the efficacy of antitumor chemo/immunotherapy.
In this review, we focus on one important component of the TME, the tumor vessels. Specifically, we discuss the strategies currently under investigation to improve the effectiveness of immunotherapy via the normalization of BC vasculature.
2. The Abnormal Tumor Vasculature of Breast Cancer
In tumors, the formation of new blood vessels is mainly accomplished through angiogenesis, the multistep process in which endothelial cells lining pre-existing vessels degrade the basement membrane and migrate into the perivascular space to form capillary structures: the latter will eventually cavitate, permitting blood influx [25,26,27]. Tumor vessel formation is boosted when angiogenesis is accompanied by vasculogenesis, a process involving the recruitment of immature endothelial cell precursors from the bone marrow to the nascent vessels [28]. Additional events leading to the expansion of the tumor vasculature are the vasculogenic mimicry (in which cancer cells form channels allowing blood inflow), and the vascular co-option (in which cancer cells line the abluminal surface of pre-existing normal vessels) [29].
Whatever the mechanism is that is responsible for their development, the newly formed blood vessels display both protumor and antitumor properties: in fact, if on the one hand they supply oxygen and nutrients to the neoplastic mass and allow the spread of metastatic cells, on the other hand they favor the infiltration of the tumor by the immune cells [30].
Notably, unlike normal vessels, tumor vessels are tortuous and chaotically organized, with wide gaps between endothelial cells, detached pericytes, and basal membranes that are either too thick or too thin [21]. All these features are typical of BC [31]. Importantly, BC biopsies are routinely evaluated for vessel density, which reliably predicts the risk of BC recurrence/metastasis and, thereby, BC patients’ survival rate [32,33].
Of upmost interest, a previous in vivo study showed that inoculating BC cells at different anatomical sites (mammary gland, cranium, and dorsal skin) leads to the establishment of abnormal vascularization whose features significantly differ from site to site [34].
However, whatever the involved anatomic site is, tumor vessels are characterized by a loss of structural integrity and functional aberrations that promote inflammation and tissue fibrosis, and, at the cellular level, DNA hypermethylation, genomic instability, trans-differentiation, and resistance to apoptosis [35]. The proliferating tumor mass squeezes blood vessels leading to flow stasis and thereby limiting the access of both drugs and immune cells to the tumor [36]. In addition, flow stasis causes vessel permeability and blood concentration, thus lowering tissue pH and oxygen [36]. Notably, due to their abnormal vasculature, about 25–40% of invasive BC exhibits hypoxic regions [37,38]. There, the hypoxia-inducible transcription factor (HIF) is activated, leading to the expression of molecular players that further stimulate angiogenesis [39]. These reciprocal interactions establish a vicious cycle [39] that contributes to BC aggressiveness and/or its resistance to therapy [38]. Concerning this last aspect, it is well established that the abnormal structure of tumor vessels can hamper the homogeneous distribution of anticancer drugs within the tumor [40,41]. In addition, one should consider that to be fully effective, chemotherapeutics, such as those currently employed to treat BCs, require adequate oxygen levels [35,42]. Moreover, because of the prevalence of leaky vessels in the tumor, tumor cells enter the systemic circulation, eventually giving rise to metastases [43]. The latter are the major cause of morbidity and mortality among BC patients [44]. Approximately 20–30% of early-stage BC patients will develop metastases, most frequently at the liver, lung, bone, or brain [44]. Despite therapeutic advances in BC, prevention of metastasis is still a challenge, and aberrant angiogenesis is the essential early stage of this complex process [45].
Finally, the hypervascularization of BC regulates the dysfunctional homing of lymphocytes. These events lead to immunosuppression, reduced immune surveillance, and poor trafficking of immune effector cells to the TME [46,47]. It is therefore reasonable to speculate that the abnormal vasculature could favor BC resistance to immunotherapy by jeopardizing the adhesion of immune effector cells to the endothelium and their intrusion into TME.
The Molecular Players of Aberrant Vasculature in Breast Cancer
Dysregulated tumor-associated angiogenesis is orchestrated by a variety of molecular players, such as vascular endothelial growth factor (VEGF), interleukin 8 (IL-8), pleiotrophin, angiopoietin-1, angiopoietin-2, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF)-2, and transforming growth factor beta-1 (TGFβ-1). Clinical studies have shown that elevated levels of these cytokines are associated with a worse prognosis of several tumor types [48,49,50] and also play a critical role in BC progression [31,51].
The VEGF family (VEGF-A–F and their receptors, VEGFR-1–3 and neuropilin) are key to the angiogenesis associated to BC [52,53,54,55]. In addition, the binding of VEGF-A to VEGFR-1 or VEGFR-2 has a role in BC development [56,57], while VEGF-D is important to BC metastasization via lymphatic vessels [55]. Consistently, VEGF levels in tumor tissue or serum positively correlate with the severity of the prognosis of BC patients [53,58,59].
Others have shown that BCs expressing high levels of IL-8 are particularly aggressive and invasive [60]. In fact, IL-8 triggers the expression of the extracellular-matrix-degrading matrix metalloproteinases (MMPs) enzymes, which, in turn, promote both tumor cell invasion and angiogenesis [61]. Notably, IL-8-overexpressing BC cell lines also synthesize high levels of VEGF [60,62,63,64], whose pro-tumorigenic activities are potentiated by IL-8 [61,65]. As for VEGF, the FGFs also spark angiogenesis in BC. In this context, it is of note that the expression of FGF-2 is increased in patients treated with VEGF antagonists [66]. For its part, angiopoietin 2, which is predominantly found in hypoxic tumor tissues [67,68], regulates the maturation of BC blood vessels by acting in a complementary manner to the VEGF pathway [69,70].
Additional factors that are expressed in BC tissues in a fashion that positively correlates with both the intensity of angiogenesis and tumor aggressiveness include TGFβ-1, pleiotrophin, placental growth factor, and PDGF [71].
In addition, the vasculogenic mimicry has been associated with poor prognosis, tumor aggressiveness, metastasis, and drug resistance in BC as well as other types of tumor [72,73].
3. Impact of Abnormal Breast Tumor Vascular on Immune Cells
Antitumor immunity is exerted by both tissue-resident immune cells and those recruited intratumorally from the blood [74]. In this context, the abnormal tumor angiogenesis can be considered an important mechanism of immune evasion (Figure 1).

Figure 1.
Abnormal tumor angiogenesis promotes tumor immune evasion. (A) Wide gaps between endothelial cells, detached pericytes, and thick/thin basement membranes, together with high levels of VEGF and low expression of endothelial adhesion molecules, reduce adhesion, extravasation, and infiltration of leukocytes into the tumor bed and contribute to establish the immune-desert and immune-excluded BC phenotype. (B) Ability of TME to promote the release of VEGF, other angiogenic factors, and protumor cytokines capable of inhibiting the maturation and function of DCs (characterized by low expression of CD80, CD83, CD86, etc.). (C) The exhaustion state of T cells is directly induced by the binding between the VEGF-R receptor and the VEGF-A ligand, major source of the inhibition of their effector function. (D) Vascular microenvironment produces multiple cytokines that recall immunosuppressive cells such as TAM2, Treg, and MDSC and reduce tumor infiltration and activity of DC, NK, and T cells. The figure was created with BioRender.com.
Indeed, an effective antitumor immune response requires not only the activation of effector immune cells, but also their access to the tumor parenchyma, where the efficacy of immunotherapies must be deployed [75]. To infiltrate a tumor, immune cells must enter the tumor’s blood vessels, adhere to the endothelium, and transmigrate through the vessel wall [76]. All this may be prevented by the presence of an aberrant tumor vasculature, which could also explain the establishment of the immune-excluded tumor phenotype, commonly identified in BC, and associated with anti-PD1 resistance [77]. In this regard, it is noteworthy that human ductal BCs are characterized by high levels of VEGF-C/D leading to the decreased expression of endothelial adhesion molecules such as ICAM-1. This lowers the adhesion, extravasation, and infiltration of leukocytes into the tumor bed, thus establishing a physical barrier for their intratumor trafficking [78,79].
Moreover, angiogenic factors detectable at high levels in the TME can induce tumor-associated immune suppression through several mechanisms. First, VEGF inhibits dendritic cell (DC) maturation and antigen presentation, thereby hindering T-cell activation and consequently reducing the T-cell-mediated antitumor immune response [80]. Second, increased levels of angiogenic factors correspond to a direct inhibition of cytotoxic T lymphocytes’ (CTLs’) trafficking, proliferation, and effector function [81,82]. Third, high amounts of pro-angiogenic messengers promote the intratumor recruitment and proliferation of immunosuppressive cells [83,84]. All these processes can simultaneously occur in BCs.
Terminally differentiated DCs are key players in adaptive antitumor immunity [85] and secrete cytokines including IL-12 and IL-18 that inhibit endothelial cell proliferation [86,87]. However, tumor cells release other cytokines (e.g., VEGF, β-defensin, CXCL12, HGF, and CXCL8) that recruit immature DCs from the peripheral blood in the TME and, at the same time, hamper DCs’ maturation and function [88]. In this regard, in vitro experiments have shown that BC-derived cell lines secrete VEGF, which, in turn, inhibits the differentiation, maturation, and function of DCs from the healthy donor, and that VEGF gene silencing is followed by an increase in the expression of activation markers such as CD80, CD83, CD86, and HLA-DR on the DC’s surface [89]. The VEGF inhibitory effect on DC maturation has also been confirmed in animal models of BC [90].
Notably, when it is overexpressed, VEGF-A directly interferes with hematopoiesis [91] and impairs T-cell development in the thymus [84]: both effects are likely to be involved in the immune compromission observed in BCs. The binding of VEGF-A to the VEGFR expressed by T cells also contributes to their exhaustion status [81,92], a phenomenon characterized by the co-expression of several ICs, such as PD-1, T-cell immunoglobulin mucin-3 (Tim-3), cytotoxic T-lymphocyte-associated protein (CTLA-4), and lymphocyte activation gene 3 (Lag3), which results in a gradual loss of lymphocyte effector function [93]. VEGF-A has been reported to increase the expression of PD-1, CTLA-4, Tim-3, and Lag3 on CD8+ T cells in a variety of tumors [81]. In BC tissues, the expression of VEGF-A is positively correlated to that of PD-L1 [94]. Of interest, high levels of VEGF-A and PD-L1 parallel a low number of TILs in the BC-TME [94]. Consistently, low levels of VEGF-A are accompanied by an abundance of CD8+ T cells in the TME of BCs, and this predicts a long disease-free survival in BC patients [95,96,97].
Finally, the tumor vasculature stimulates the function of protumor immune cells in BC [98]. Among them, M2-like protumor macrophages (TAM), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) play an important role in BC progression [99,100].
Based on the type of stimuli they are subjected to, TAMs polarize into classically activated macrophages (M1, characterized by the expression of antitumoral cytokines) or into alternatively activated macrophages (M2, thought to be involved in cancer progression) [101]. In BC, TAM polarization is regulated by several TME-derived factors [102]. For example, upon VEGF binding to VEGFR2 expressed on their membrane, TAMs polarize to an M2-like phenotype [103,104] and secrete angiogenic and immunosuppressive cytokines (e.g., IL-10, TGFβ, and VEGF) that favor tumor progression [105,106,107]. Interestingly, macrophages of BC-bearing mice have been found to express both VEGFR1 and VEGFR2, while those from tumor-free mice express only VEGFR1 [90]. Analogously, VEGFR2+/CD45bright/CD14+ monocytes are present in the blood of BC patients but not healthy controls [108]. Not surprisingly, a high number of tumor-infiltrating macrophages is a marker of poor prognosis for BC patients [102].
On their part, Tregs secrete VEGFA in a fashion paralleling BC clinical progression [109]. In this regard, one should consider that the Treg-specific transcription factor FOXP3 cooperates with STAT3 to induce VEGF-A expression in Tregs, thus triggering angiogenesis [109]. Since they positively correlate not only with VEGF expression and BC vascularity but also with BC growth rate, invasiveness, and metastasis, FOXP3 levels have been thought to be capable of monitoring BC clinical progression [110].
The immunosuppressive Tregs are recruited in the TME by the MDSCs that populate BCs [111]. Recently, it has been shown that BC cells release both IL-34, that induces myeloid stem cells’ differentiation into monocytic MDSCs [111], and CXCL17, that promotes the accumulation of MDSCs within the lung where they favor the development of a metastatic niche [112]. Results from further animal studies indicate that BC metastases to the lung are facilitated by the loss of the Shb gene in endothelial cells, which is followed by the recruitment of monocytic MDSCs in the lung [113]. Altogether, these findings explain why the infiltration of BC by MDSCs or an increase in MDSCs in peripheral blood correlate with BC progression and metastatic burden in patients [114].
Notably, the hypoxia and acidosis present in TME because of the abnormal tumor vasculature can in turn promote local and systemic immunosuppression. In immune competent syngeneic BC mouse models, factors secreted by hypoxic tumor cells recruit CD11b+/Ly6Cmed/Ly6G+ myeloid cells and suppress natural killer (NK) cell functions [115]. In BC cells, hypoxia induces the expression of BIRC2, which counters the capability of CXCL9 to recruit CD8+ T cells and NK cells to the tumor, and hence increases tumor growth and resistance to anti-PD-1 therapy [116]. In hypoxic BC cells, the HIF-1 transcription factor is activated together with anaerobic metabolism and lactate dehydrogenase (LDHA) production [117]. The expression of both HIF1α and LDH5 defines “cold”, immunologically silent BCs and poor prognosis of patients [117].
4. Vessel Normalization Strategies in Breast Cancer
It has long been known that drugs inhibiting the formation of new blood vessels or damaging already formed tumor vessels can delay cancer progression [118]. However, antiangiogenic agents, often used at high doses, have shown some limitations in clinical applications since the destruction of blood vessels caused by these drugs promotes hypoxia, which, as we have already reported, accelerates tumor progression [119].
To date, growing evidence indicates that normalization rather than destruction of the tumor vasculature might be an effective antitumor strategy. Vascular normalization involves the judicious dosing of antiangiogenic agents to reverse the abnormal phenotype of the tumor vasculature [120]. To this end, the restoration of structurally and functionally fit blood vessels will be achieved through a series of normalizing events that include the fostering of a tighter connection between adjacent endothelial cells, a greater pericyte coverage, and the restoration of vascular basement membrane integrity to decrease vascular permeability and interstitial fluid pressure [121]. Although the structure and function of tumor vessels are unlikely to become completely normal (hence the term “normalized vessels”), this reversion can transiently render the distribution of blood flow more uniform and reduce the area of anoxia and acidosis within tumors [122]. Thus, the direct and anticipated consequences of vessel normalization are: (1) a strengthened immune response against cancer cells, through both vessel maturation and the relief of immunosuppression induced by hypoxia and/or angiogenic factors; (2) improved delivery of anticancer therapeutics and oxygen into the tumor bed; and (3) a decreased likelihood by the tumor to metastasize [100].
Vessel Normalization Improves Immunotherapy and Vice Versa: Preclinical Evidence in Breast Cancer
Recently, reciprocal interactions between the remodeling of tumor vessels and the reprogramming of the immune microenvironment have been demonstrated. On one side, in fact, vascular normalization enhances vascular perfusion and thereby increases intratumor infiltration of immune cells; on the other side, activated immune cells have a key role in normalizing the tumor vasculature [123,124,125]. For this reason, several preclinical studies have also been carried out in models of BC, showing that the inhibition of angiogenesis alone or in combination with various immunotherapies boosts antitumor immunity even in this aggressive neoplasm (Figure 2).
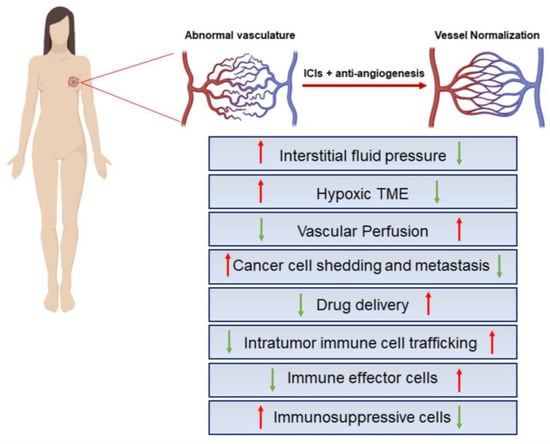
Figure 2.
Key effects of IC blockers combined with antiangiogenics. Structural and functional abnormalities of tumor blood vessels lead to impaired blood flow and perfusion, hypoxic TME, limited drug delivery to the tumor, increased invasiveness of tumor cells, enhanced tumor infiltration of immunosuppressive cells, and impaired antitumor immune responses. ICI administration in combination with antiangiogenic factors may subvert this scenario in support of an immunosupportive BC-TME. The red and green arrows represent the indicated hyper-activated or down-modulated cellular processes, respectively. The figure was created with BioRender.com.
For instance, the use of BC murine models has shown that targeting the tumor vasculature with low doses of anti-VEGFR2 antibodies not only results in a homogeneous distribution of functional tumor vessels, but also facilitates tumor infiltration by CD4+ and CD8+ T cells and reverts the TAM phenotype from the pro-tumorigenic M2-like one to the antitumor, M1-like one [126]. Moreover, administration of the VEGF inhibitor bevacizumab to BC-bearing mice reduces the intratumor infiltration of protumor TAMs and MDSCs, as well as diminishing tumor vessel density and BC growth [90]. Similarly, mice treatment with DC101 (a rat monoclonal antibody directed against mouse VEGFR2), in addition to suppressing BC growth, attenuates the MDSCs’ inhibitory effect on T cells and reduces the number of Tregs in both primary BCs and lung metastases of BC [127]. Likewise, VEGF165b, an antiangiogenic isoform of VEGF-A, inhibits the MDSCs’ and Tregs’ accumulation in the spleens and tumors of BC-bearing mice [128].
In recent years, the possibility of combining antiangiogenic drugs with immunotherapy has grown to achieve an even better clinical outcome than that provided by the individual approaches. Specifically, consistent with the fact that BCs overexpressing the Neu proto-oncogene display high VEGF levels, the combination of DC101 with Neu-specific vaccination accelerates tumor regression in murine models of BC by augmenting the cytotoxic activity of CD8+ T cells [129].
Similar effects have been obtained in BC-bearing mice treated with the immune-stimulator recombinant fusion protein B7.2-IgG in combination with SU6668an, an inhibitor of the tyrosine kinase activity of three angiogenic receptors, namely VEGFR2, PDGFR-beta, and FGFR1 [130].
Furthermore, other studies have supported the rationale of co-targeting angiogenesis and ICIs for BC therapy, positioning immune cells as key effectors of antiangiogenic agents [131,132,133,134,135,136,137,138,139].
The combination of DC101 with anti-PD-L1 antibodies promotes the formation of high endothelial venules in mouse BC, enabling intratumor infiltration of cytotoxic T cells and thereby sensitizing BCs to anti-PD-L1 therapy [131].
In addition, the dual blockade of angiopoietin-2 and VEGF-A by a bispecific antibody (A2V) causes the normalization or regression of tumor vessels, the extravasation and perivascular accumulation of activated CD8+ cytotoxic T lymphocytes, the necrosis of BC, and, consequently, the presentation of neoantigens by intratumor phagocytes [132]. The concomitant blockade of PD-1 further enhances tumor control by A2V [132]. Similarly, a combination of angiopoietin-2 blockers, VEGF inhibitors, and anti-PD-L1 antibodies can successfully treat human metastatic BC xenografts and syngeneic BCs in mice [133].
More recently, Li et al. tested the combination of anti-PD-1 antibodies and different doses of VEGFR2-targeting agents in syngeneic BC mouse models, demonstrating a dose-dependent synergism between antiangiogenic therapy and IC blockade. Specifically, mice treatment with low doses of anti-VEGFR2 antibodies not only normalizes tumor vessels but also results in more robust immune cell infiltration, thus providing important insights into the optimal strategies for combining immunotherapy with molecular-targeted agents [134]. In the BC EMT-6/CDDP model, the administration of anti-PD-L1 antibodies is effective as an adjuvant monotherapy, while the combination of anti-PD-L1 antibodies with paclitaxel and VEGF antagonists gives better efficiency results in a neoadjuvant setting [135].
Notably, the use of several mouse models deficient in vascular normalization or T lymphocytes has allowed the delineation of an unexpected role of CD4+ T cells as a major immune cell population associated with vascular reprogramming. Indeed, the depletion of CD4+ T lymphocytes impairs vascular normalization [136]. In addition, ICIs facilitate vessel normalization in BC through a mechanism mediated by CD4+ T cells in an IFN gamma-dependent manner [136].
On the other hand, Zheng et al. primarily ascribed to CD8+ T cells’ activation the mechanism by which to achieve the increased vessel perfusion and antitumor effects of IC blockade. The authors demonstrated on several clinically relevant BC models, including orthotopic BCs (EO771, 4T1, and MCaP0008) and spontaneous BCs (MMTV-PyVT, which mirrors BC progression in humans), that CTLA4 and PD-1 antagonists increase blood perfusion of the tumor while they exert antitumor activities [123].
Intriguingly, Kabir et al. identified Myct1 as a new critical factor for tumor angiogenesis, nearly exclusively expressed in endothelial cells. The authors found that Myct1 expression is crucial for cancer progression through the regulation of both tumor angiogenesis and tumor immunity. Indeed, Myct1 deficiency reduces angiogenesis, and facilitates the trans-endothelial migration of cytotoxic T lymphocytes and the polarization of macrophages toward the M1 phenotype. Moreover, when Myct1 targeting is combined with anti-PD-1 treatment, the tumor regression is complete, with a significant long-term survival of BC-bearing mice [137]. Moreover, TNBC-bearing mice treated with the TGF-β inhibitor TRANILAST combined with the nanomedicine DOXIL as a vessel normalizing strategy, show a marked reduction in extracellular matrix components and an increase in intratumor vessel diameter and pericyte coverage: this leads to the infiltration of T cells and M1 macrophages into the tumor and improves the efficacy of anti-PD-1/anti-CTLA-4 antibodies [138]. Recently, nanocomplexes were prepared that release sunitinib (a vascular normalizing drug) and BMS-202 (a PD-1/PD-L1 blocker) in tumor tissues. The administration of such nanocomplexes to BC-bearing mice resulted in the significant inhibition of tumor growth coupled with excellent efficacy of antitumor immunity, which supports a potential new approach for BC treatment [139].
Importantly, all these studies suggest that in the case of combined therapeutic regimens, beyond identifying the suboptimal dose of the angiogenesis inhibitor, it is equally crucial to administer the other drugs, such as ICIs, during the window of normalization induced by the angiogenesis inhibitors. This will yield better results in terms of drug delivery into the tumor core as well as of triggering antitumor immunity.
5. Effect of Antiangiogenic Agents Combined with Immune Checkpoint Inhibitors in Breast Cancer: Clinical Studies
Previous studies have evaluated the effect of bevacizumab added to neoadjuvant chemotherapy in TNBC patients. Despite an improvement in progression-free survival, this combination has resulted in an increased incidence of adverse events and has not augmented the overall survival of BC patients [140,141,142,143]. In general, the clinical benefit of antiangiogenic drugs as a monotherapy or in association with chemotherapy remains controversial in BC [144,145].
To date, based on the results from the previously illustrated preclinical studies providing evidence that angiogenesis-induced immunosuppression can be exploited to improve immunotherapy, the addition of antiangiogenic agents to ICIs is considered an attractive treatment approach. Hence, this novel combination therapy is currently being addressed in clinical trials for many malignances. Thus far, the Food and Drug Administration has approved combinations of ICIs and antiangiogenic agents for the treatment of renal cell carcinoma [146], non-small-cell lung cancer [147], hepatocellular carcinoma [148], and endometrial carcinoma [149]. Regarding BC, numerous phase I and II clinical trials are currently underway (Table 1).

Table 1.
Active, recruiting, and completed clinical studies of angiogenic inhibitors combined with ICIs for BC (data source: clinicalTrials.gov, December 2022).
An explorative analysis confirmed that (1) staining positivity for CD8 identifies TNBCs that are likely to benefit from immunotherapy, and (2) as angiogenesis discriminates patients with low CD8+ T-cell infiltration, angiogenesis inhibitors may facilitate IC blockade. These observations were preparatory for the launch of a phase II clinical trial (NCT04129996), which was conducted in 48 late-stage TNBC patients to assess the feasibility of combining FAMITINIB (an angiogenesis inhibitor), with CAMRELIZUMAB (a monoclonal antibody directed against PD-1) and conventional chemotherapeutics. Notably, patients’ response rate was above 80%, and no treatment-related deaths were reported [150].
Another phase II study (NCT04734262) evaluated the efficacy and safety of a chemotherapy-free regimen in which SITRAVATINIB (tyrosine kinase receptor inhibitor against the TYRO3, AXL, MERTK, and VEGF family) was given in combination with TISLELIZUMAB to patients with relapsed and/or metastatic TNBC, regardless of PD-L1 status. BC patients included in the study were divided into two cohorts: cohort A receiving 70 mg SITRAVATINIB plus 200 mg TISLELIZUMAB, and cohort B receiving 100 mg SITRAVATINIB plus 200 mg TISLELIZUMAB. Patients of cohort A demonstrated clinically significant antitumor activity and a manageable safety profile.
A recent phase II study (NCT04303741) employed CAMRELIZUMAB (an anti-PD-1 antibody) in combination with APATINIB (a tyrosine kinase inhibitor specifically directed against VEGFR2) and ERIBULIN (an inducer of tumor vessel normalization) [151]. This therapy resulted in the transformation of a “cold” tumor to an “inflamed” tumor and was demonstrated to be safe and effective in patients with heavily pretreated advanced TNBC, even in those negative for PD-L1, or in those who have progressed after several lines of treatment, including ICIs [151].
The phase II study NCT04914390 was initiated in August 2021 with the aim to evaluate the efficacy and safety of combining ANLOTINIB (a novel multitarget tyrosine kinase inhibitor that effectively inhibit VEGFR, FGFR, c-KIT, c-MET, and RET) with TISLELIZUMAB (a humanized immunoglobulin G4 anti-PD-1 monoclonal antibody engineered to minimize binding to FcγR on macrophages) and chemotherapy, as a neoadjuvant treatment in TNBC. The clinical trial will enroll a total of 32 patients, for which pCR rate, invasive disease-free survival, event-free survival, overall survival, and safety will be evaluated.
Additional studies are currently underway to evaluate the efficacy of this type of therapeutic combination in other BC subtypes. For instance, the pilot clinical trial NCT02802098 is seeking to explore the efficacy of combining the anti-PD-L1 durvalumab plus the antiangiogenic bevacizumab after bevacizumab monotherapy for advanced HER2-negative BC [152]. Peripheral blood mononuclear cells and fresh pre-durvalumab tumor biopsies have been analyzed to assess vascular normalization and to characterize the immune infiltrate. Preliminary results are encouraging and suggest that the antiangiogenic treatment exerts an immune-priming effect, with a systemic and intratumor reduction in Tregs [152].
In another recent phase Ib study (NCT02802098), patients with HER2-negative metastatic BC were treated with durvalumab plus bevacizumab. The results indicated that CD8+ memory effector T cells are increased in the peripheral blood from patients with stable BC but not from patients with progressed BC [153].
Recently, a patient affected by advanced metaplastic BC not responsive to standard adjuvant chemotherapy partially responded to the immunotherapeutic TORIPALIMAB combined with the angiogenesis inhibitor ANLOTINIB [154].
Finally, and most importantly, one should consider that as tumor vessel normalization ameliorates drug delivery into the tumor core, combination strategies require not only low doses of antiangiogenics but also even lower doses of ICIs: this improves antitumor immune responses, and simultaneously reduces the risk of clinical side effects that are associated with the administration of the individual therapeutic agents. Indeed, antiangiogenic therapy can suppress the reactive capillary hemangioma caused by anti-PD-1 antibodies [155,156] or reduce the risk of cerebral edema promoted by other ICIs [157], hence being effective in treating the brain metastases of BC [158].
6. Conclusions and Perspectives
Understanding the mechanisms of resistance to immunotherapy may enable the design of new therapeutic combinations that are hopefully more effective against BCs than the conventional ones.
The important role that tumor vessels play in evading the immune response is currently considered a major obstacle to overcome. Not surprisingly, clinical trials testing the combination of antiangiogenic agents with ICIs have increased since 2018 [75]. Nevertheless, efforts are needed to select the BC patients most likely to benefit from this therapeutic combination. Some studies have suggested that due to the transient window of antiangiogenic therapy and the low PD-L1 positivity rate in patients with advanced BC, an antiangiogenic therapy combined with immunotherapy may yield better clinical benefits in early-stage BC [159]. In contrast, results from other studies indicate that such a combined therapy is likely to be more promising in the neoadjuvant setting [159]. Therefore, to achieve more effective anti-BC therapies, the crosstalk between tumor vessels and immune cells has to be comprised, and many unsolved questions have to be answered. For instance, current combinatorial treatments focus on monoclonal antibodies as bevacizumab among antiangiogenics. However, the efficacy of this therapeutic approach is compromised over time by the induction of the expression of other angiogenic factors such as the FGFs [160]. Further studies will thus be needed to select additional antiangiogenic drugs in combination with the immunotherapy of choice. This could substantially improve the clinical outcomes of BC patients. In this regard, the effectiveness of combined antiangiogenics and immunotherapeutic against BC could be enhanced by the addition of chemotherapy and radiotherapy, which have been proven to increase the therapeutic efficacy of ICIs [161,162].
Further investigation should address the use of multimodal therapies directed against BC: timing, dosing, and toxicity will require careful consideration.
Finally, no reliable biomarkers predicting BC responsiveness to antiangiogenic agents or ICIs are available at the present time, and the selection of combination therapy is based on the positive results of monotherapies. Therefore, the identification of biomarkers foreseeing combination therapy outcomes would be of great importance.
Author Contributions
O.M. and G.B. wrote the paper; G.V., I.P., R.B., S.P., C.A.P., O.C.B., M.R., L.A. and A.M. revised it. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Italian Ministry of Health (grant no. OR/0906).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BC | breast cancer |
| CTL | cytotoxic T lymphocytes |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein |
| DC | dendritic cell |
| FGF | fibroblast growth factor |
| HER2 | human epidermal growth factor receptor 2 |
| HIF | hypoxia-inducible transcription factor |
| IC | immune checkpoint |
| ICIs | immune checkpoint inhibitors |
| IL-8 | interleukin 8 |
| Lag3 | lymphocyte activation gene 3 |
| LDHA | lactate dehydrogenase |
| MDSCs | myeloid-derived suppressor cells |
| MMPs | matrix metalloproteinases |
| pCR | pathologic complete response |
| PDGF | platelet-derived growth factor |
| PD-L1 | programmed death-ligand 1 |
| TAM | M2-like protumor macrophages |
| TGFβ-1 | transforming growth factor beta-1 |
| TILs | tumor-infiltrating lymphocytes |
| Tim-3 | T-cell immunoglobulin mucin-3 |
| TME | tumor microenvironment |
| TNBCs | triple-negative BCs |
| Tregs | regulatory T cells |
| VEGF | vascular endothelial growth factor |
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Giovannoni, R.; Fruci, D.; Gemignani, F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin. Cancer Biol. 2020, 79, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Mezni, E.; Behi, K.; Gonçalves, A. Immunotherapy and breast cancer: An overview. Curr. Opin. Oncol. 2022, 34, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M.; Villamar, D.; He, G.; Pearson, A.T.; Nanda, R. The emerging role of immune checkpoint inhibitors for the treatment of breast cancer. Expert Opin. Investig. Drugs 2021, 31, 531–548. [Google Scholar] [CrossRef]
- Mph, K.D.M.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L.; Keegan, M.T.H.; Dvm, A.J.; Mph, R.L.S. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas (TCGA) Research Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef]
- Dai, X.-L.; Han, Z.-B.; Yang, Y.-T.; Qiu, J.; Liu, Y.-F.; Feng, Y.-Z. Efficacy and prognosis of neoadjuvant chemotherapy is correlated with breast cancer molecular classification. Int. J. Clin. Pharmacol. Ther. 2015, 53, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef]
- Atezolizumab Combo Approved for PD-L1–Positive TNBC. Cancer Discov. 2019, 9, OF2. [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Ge, Y.; Domschke, C.; Stoiber, N.; Schott, S.; Heil, J.; Rom, J.; Blumenstein, M.; Thum, J.; Sohn, C.; Schneeweiss, A.; et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 2011, 61, 353–362. [Google Scholar] [CrossRef]
- Garufi, G.; Palazzo, A.; Paris, I.; Orlandi, A.; Cassano, A.; Tortora, G.; Scambia, G.; Bria, E.; Carbognin, L. Neoadjuvant therapy for triple-negative breast cancer: Potential predictive biomarkers of activity and efficacy of platinum chemotherapy, PARP- and immune-checkpoint-inhibitors. Expert Opin. Pharmacother. 2020, 21, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef]
- Ben-Baruch, A. Host microenvironment in breast cancer development: Inflammatory cells, cytokines and chemokines in breast cancer progression: Reciprocal tumor–microenvironment interactions. Breast Cancer Res. 2002, 5, 31–36. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef]
- De Heer, E.C.; Jalving, M.; Harris, A.L. HIFs, angiogenesis, and metabolism: Elusive enemies in breast cancer. J. Clin. Investig. 2020, 130, 5074–5087. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Huang, Z.; Zuo, T.; Lu, Q.; Wu, G.; Shen, Q. Reducing Interstitial Fluid Pressure and Inhibiting Pulmonary Metastasis of Breast Cancer by Gelatin Modified Cationic Lipid Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 29457–29468. [Google Scholar] [CrossRef]
- Palucka, A.K.; Coussens, L.M. The basis of oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Priya, S.K.; Nagare, R.; Sneha, V.; Sidhanth, C.; Bindhya, S.; Manasa, P.; Ganesan, T. Tumour angiogenesis-Origin of blood vessels. Int. J. Cancer 2016, 139, 729–735. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, M.; Mishima, Y.; Sahin, I.; Manier, S.; Glavey, S.; Vacca, A.; Roccaro, A.M.; Ghobrial, I.M. Role of endothelial progenitor cells in cancer progression. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Pezzella, F. Vascular Co-Option and Other Alternative Modalities of Growth of Tumor Vasculature in Glioblastoma. Front. Oncol. 2022, 12, 874554. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Badodekar, N.; Sharma, A.; Patil, V.; Telang, G.; Sharma, R.; Patil, S.; Vyas, N.; Somasundaram, I. Angiogenesis induction in breast cancer: A paracrine paradigm. Cell Biochem. Funct. 2021, 39, 860–873. [Google Scholar] [CrossRef]
- Linderholm, B.; Tavelin, B.; Grankvist, K.; Henriksson, R. Does vascular endothelial growth factor (VEGF) predict local relapse and survival in radiotherapy-treated node-negative breast cancer? Br. J. Cancer 1999, 81, 727–732. [Google Scholar] [CrossRef]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis—Correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Cho, H.-H.; Kim, H.; Nam, S.Y.; Lee, J.E.; Han, B.-K.; Ko, E.Y.; Choi, J.S.; Park, H.; Ko, E.S. Measurement of Perfusion Heterogeneity within Tumor Habitats on Magnetic Resonance Imaging and Its Association with Prognosis in Breast Cancer Patients. Cancers 2022, 14, 1858. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Holm, C.; Landberg, G. Common Molecular Mechanisms of Mammary Gland Development and Breast Cancer. Cell. Mol. Life Sci. 2007, 64, 3233–3247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.-K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in Breast Cancer—Scientific Translation to Therapeutic and Diagnostic Clinical Applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef]
- Potente, M.; Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y.R. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.K.; Veinot-Drebot, L.; Tjan, E.; Tannock, I.F. Effect of Transient Hypoxia on Sensitivity to Doxorubicin in Human and Murine Cell Lines. Gynecol. Oncol. 1990, 82, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. npj Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, J.; Kozłowska, A.; Kocki, J. Breast cancer metastasis–insight into selected molecular mechanisms of the phenomenon. Postep. Hig. I Med. Dosw. 2015, 69, 447–451. [Google Scholar] [CrossRef]
- Ribatti, D. Immunosuppressive effects of vascular endothelial growth factor (Review). Oncol. Lett. 2022, 24, 1–6. [Google Scholar] [CrossRef]
- Oshi, M.; Newman, S.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Endo, I.; Nagahashi, M.; Takabe, K. Intra-Tumoral Angiogenesis Is Associated with Inflammation, Immune Reaction and Metastatic Recurrence in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6708. [Google Scholar] [CrossRef]
- Martins, S.F.; Garcia, E.A.; Luz, M.A.M.; Pardal, F.; Rodrigues, M.; Filho, A.L. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom. Proteom. 2013, 10, 55–67. [Google Scholar]
- Cañadas, I.; Taus, Á.; Villanueva, X.; Arpí, O.; Pijuan, L.; Rodríguez, Y.; Menéndez, S.; Mojal, S.; Rojo, F.; Albanell, J.; et al. Angiopoietin-2 is a negative prognostic marker in small cell lung cancer. Lung Cancer 2015, 90, 302–306. [Google Scholar] [CrossRef]
- Melaiu, O.; Catalano, C.; De Santi, C.; Cipollini, M.; Figlioli, G.; Pellè, L.; Barone, E.; Evangelista, M.; Guazzelli, A.; Boldrini, L.; et al. Inhibition of the platelet-derived growth factor receptor beta (PDGFRB) using gene silencing, crenolanib besylate, or imatinib mesylate hampers the malignant phenotype of mesothelioma cell lines. Genes Cancer 2017, 8, 438–452. [Google Scholar] [CrossRef]
- Kern, F.G.; Lippman, M.E. The role of angiogenic growth factors in breast cancer progression. Cancer Metastasis Rev. 1996, 15, 213–219. [Google Scholar] [CrossRef]
- Niu, G. Vascular Endothelial Growth Factor as an Anti-Angiogenic Target for Cancer Therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Ruggieri, S.; Tamma, R.; Simone, G.; Mangia, A. Angiogenesis and Antiangiogenesis in Triple-Negative Breast cancer. Transl. Oncol. 2016, 9, 453–457. [Google Scholar] [CrossRef]
- Longatto-Filho, A.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and Breast Cancer. J. Oncol. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Eroğlu, A.; Ersöz, C.; Karasoy, D.; Sak, S.D. Vascular endothelial growth factor (VEGF)-C, VEGF-D, VEGFR-3 and D2-40 expressions in primary breast cancer: Association with lymph node metastasis. Adv. Clin. Exp. Med. 2017, 26, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Srabovic, N.; Mujagic, Z.; Mujanovic-Mustedanagic, J.; Softic, A.; Muminovic, Z.; Rifatbegovic, A.; Begic, L. Vascular Endothelial Growth Factor Receptor-1 Expression in Breast Cancer and Its Correlation to Vascular Endothelial Growth Factor A. Int. J. Breast Cancer 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 108–121. [Google Scholar] [CrossRef]
- Schneider, B.P.; Miller, K.D. Angiogenesis of Breast Cancer. J. Clin. Oncol. 2005, 23, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Sahana, K.R.; Akila, P.; Prashant, V.; Chandra, B.S.; Suma, M.N. Quantitation of Vascular Endothelial Growth Factor and Interleukin-6 in Different Stages of Breast Cancer. Rep. Biochem. Mol. Biol. 2017, 6, 33–39. [Google Scholar]
- Chelouche-Lev, D.; Miller, C.P.; Tellez, C.; Ruiz, M.; Bar-Eli, M.; Price, J.E. Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: Implications for therapy. Eur. J. Cancer 2004, 40, 2509–2518. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Razmkhah, M.; Jaberipour, M.; Hosseini, A.; Safaei, A.; Khalatbari, B.; Ghaderi, A. Expression profile of IL-8 and growth factors in breast cancer cells and adipose-derived stem cells (ASCs) isolated from breast carcinoma. Cell. Immunol. 2010, 265, 80–85. [Google Scholar] [CrossRef]
- Marjon, P.L.; Bobrovnikova-Marjon, E.V.; Abcouwer, S.F. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol. Cancer 2004, 3, 4. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Chen, L.; Li, S.; Shi, Q.; Jordan, C.; Huang, R.-P. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int. J. Cancer 2004, 109, 507–515. [Google Scholar] [CrossRef]
- Martin, D.; Galisteo, R.; Gutkind, J. CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFκB through the CBM (Carma3/Bcl10/Malt1) Complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Gyanchandani, R.; Alves, M.V.O.; Myers, J.N.; Kim, S. A Proangiogenic Signature Is Revealed in FGF-Mediated Bevacizumab-Resistant Head and Neck Squamous Cell Carcinoma. Mol. Cancer Res. 2013, 11, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, P.; Thomas, M.; Kruse, K.; Helfrich, I.; Wolter, V.; Deppermann, C.; Schadendorf, D.; Thurston, G.; Fiedler, U.; Augustin, H.G. Host-Derived Angiopoietin-2 Affects Early Stages of Tumor Development and Vessel Maturation but Is Dispensable for Later Stages of Tumor Growth. Cancer Res. 2009, 69, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Gerald, D.; Chintharlapalli, S.; Augustin, H.G.; Benjamin, L.E. Angiopoietin-2: An Attractive Target for Improved Antiangiogenic Tumor Therapy. Cancer Res. 2013, 73, 1649–1657. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Dhami, S.P.S.; Patmore, S.; Comerford, C.; Byrne, C.M.; Cavanagh, B.; Castle, J.; Kirwan, C.C.; Kenny, M.; Schoen, I.; O’Donnell, J.S.; et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. J. Thromb. Haemost. 2022, 20, 2350–2365. [Google Scholar] [CrossRef]
- Relf, M.; Lejeune, S.; Scott, P.A.; Fox, S.; Smith, K.; Leek, R.; Moghaddam, A.; Whitehouse, R.; Bicknell, R.; Harris, A.L. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. . Cancer Res. 1997, 57, 963–969. [Google Scholar]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Andonegui-Elguera, M.A.; Alfaro-Mora, Y.; Cáceres-Gutiérrez, R.; Caro-Sánchez, C.H.S.; Herrera, L.A.; Díaz-Chávez, J. An Overview of Vasculogenic Mimicry in Breast Cancer. Front. Oncol. 2020, 10, 220. [Google Scholar] [CrossRef]
- Horton, B.L.; Fessenden, T.B.; Spranger, S. Tissue Site and the Cancer Immunity Cycle. Trends Cancer 2019, 5, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Hammerl, D.; Martens, J.W.M.; Timmermans, M.; Smid, M.; Trapman-Jansen, A.M.; Foekens, R.; Isaeva, O.I.; Voorwerk, L.; Balcioglu, H.E.; Wijers, R.; et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat. Commun. 2021, 12, 5668. [Google Scholar] [CrossRef]
- Dirkx, A.E.M.; Egbrink, M.G.A.O.; Kuijpers, M.J.E.; Van Der Niet, S.T.; Heijnen, V.V.T.; Steege, J.C.A.B.-T.; Wagstaff, J.; Griffioen, A.W. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003, 63, 2322–2329. [Google Scholar] [PubMed]
- Steege, J.C.A.B.-T.; Baeten, C.I.M.; Thijssen, V.L.J.L.; Satijn, S.A.; Verhoeven, I.C.L.; Hillen, H.F.P.; Wagstaff, J.; Griffioen, A.W. Angiogenic Profile of Breast Carcinoma Determines Leukocyte Infiltration. Clin. Cancer Res. 2004, 10, 7171–7178. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages In Vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR Pathway Blockade Inhibits Tumor-Induced Regulatory T-cell Proliferation in Colorectal Cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ohm, J.E.; Gabrilovich, D.I.; Sempowski, G.D.; Kisseleva, E.; Parman, K.S.; Nadaf, S.; Carbone, D.P. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003, 101, 4878–4886. [Google Scholar] [CrossRef]
- Lucarini, V.; Melaiu, O.; Tempora, P.; D’Amico, S.; Locatelli, F.; Fruci, D. Dendritic Cells: Behind the Scenes of T-Cell Infiltration into the Tumor Microenvironment. Cancers 2021, 13, 433. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Asselin-Paturel, C.; Trinchieri, G. Production of type I interferons. J. Exp. Med. 2005, 202, 461–465. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Zhang, S.; Li, Y. Inhibition of vascular endothelial growth factor by small interfering RNA upregulates differentiation, maturation and function of dendritic cells. Exp. Ther. Med. 2014, 9, 120–124. [Google Scholar] [CrossRef]
- Roland, C.L.; Dineen, S.P.; Lynn, K.D.; Sullivan, L.A.; Dellinger, M.T.; Sadegh, L.; Sullivan, J.P.; Shames, D.S.; Brekken, R.A. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol. Cancer Ther. 2009, 8, 1761–1771. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Dikov, M.M.; Novitskiy, S.V.; Mosse, C.A.; Yang, L.; Carbone, D.P. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 2007, 110, 624–631. [Google Scholar] [CrossRef]
- Meder, L.; Schuldt, P.; Thelen, M.; Schmitt, A.; Dietlein, F.; Klein, S.; Borchmann, S.; Wennhold, K.; Vlasic, I.; Oberbeck, S.; et al. Combined VEGF and PD-L1 Blockade Displays Synergistic Treatment Effects in an Autochthonous Mouse Model of Small Cell Lung Cancer. Cancer Res. 2018, 78, 4270–4281. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hirakata, T.; Kurozumi, S.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; Oyama, T.; Shirabe, K. VEGF-A Is Associated with the Degree of TILs and PD-L1 Expression in Primary Breast Cancer. Vivo 2020, 34, 2641–2646. [Google Scholar] [CrossRef]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e5. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Linderholm, B.K.; Lindh, B.; Beckman, L.; Erlanson, M.; Edin, K.; Tavelin, B.; Bergh, J.; Grankvist, K.; Henriksson, R. Prognostic Correlation of Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor in 1307 Primary Breast Cancers. Clin. Breast Cancer 2003, 4, 340–347. [Google Scholar] [CrossRef]
- Missiaen, R.; Mazzone, M.; Bergers, G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin. Cancer Biol. 2018, 52, 107–116. [Google Scholar] [CrossRef]
- Paluskievicz, C.M.; Cao, X.; Abdi, R.; Zheng, P.; Liu, Y.; Bromberg, J.S. T Regulatory cells and priming the suppressive tumor microenvironment. Front. Immunol. 2019, 10, 2453. [Google Scholar] [CrossRef]
- Munn, L.L.; Jain, R.K. Vascular regulation of antitumor immunity. Science 2019, 365, 544–545. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Dong, F.; Ruan, S.; Wang, J.; Xia, Y.; Le, K.; Xiao, X.; Hu, T.; Wang, Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Sun, X.; Bernhardt, S.M.; Glynn, D.J.; Hodson, L.J.; Woolford, L.; Evdokiou, A.; Yan, C.; Du, H.; Robertson, S.A.; Ingman, W.V. Attenuated TGFB signalling in macrophages decreases susceptibility to DMBA-induced mammary cancer in mice. Breast Cancer Res. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Jia, X.-H.; Feng, G.-W.; Wang, Z.-L.; Du, Y.; Shen, C.; Hui, H.; Peng, D.; Li, Z.-J.; Kong, D.-L.; Tian, J. Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects. Oncotarget 2016, 7, 20934–20944. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef]
- Vroling, L.; Yuana, Y.; Schuurhuis, G.J.; van Hinsbergh, V.W.M.; Gundy, C.; de Haas, R.; van Cruijsen, H.; Boven, E.; Hoekman, K.; Broxterman, H.J. VEGFR2 expressing circulating (progenitor) cell populations in volunteers and cancer patients. Thromb. Haemost. 2007, 98, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Kajal, K.; Bose, S.; Panda, A.K.; Chakraborty, D.; Chakraborty, S.; Pati, S.; Sarkar, T.; Dhar, S.; Roy, D.; Saha, S.; et al. Transcriptional regulation of VEGFA expression in T-regulatory cells from breast cancer patients. Cancer Immunol. Immunother. 2021, 70, 1877–1891. [Google Scholar] [CrossRef]
- Gupta, S.; Joshi, K.; Wig, J.; Arora, S.K. Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 2007, 46, 792–797. [Google Scholar] [CrossRef]
- Kajihara, N.; Kobayashi, T.; Otsuka, R.; Nio-Kobayashi, J.; Oshino, T.; Takahashi, M.; Imanishi, S.; Hashimoto, A.; Wada, H.; Seino, K.-I. Tumor-derived interleukin-34 creates an immunosuppressive and chemoresistant tumor microenvironment by modulating myeloid-derived suppressor cells in triple-negative breast cancer. Cancer Immunol. Immunother. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Yen, M.-C.; Chang, W.-A.; Tsai, P.-H.; Pan, Y.-C.; Liao, S.-H.; Kuo, P.-L. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef]
- He, Q.; Jamalpour, M.; Bergquist, E.; Anderson, R.L.; Gustafsson, K.; Welsh, M. Mouse Breast Carcinoma Monocytic/Macrophagic Myeloid-Derived Suppressor Cell Infiltration as a Consequence of Endothelial Dysfunction in Shb-Deficient Endothelial Cells Increases Tumor Lung Metastasis. Int. J. Mol. Sci. 2021, 22, 11478. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, C.; Roxå, A.; Mehmeti, M.; Leandersson, K.; Larsson, A.-M. Clinical relevance of systemic monocytic-MDSCs in patients with metastatic breast cancer. Cancer Immunol. Immunother. 2020, 69, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Sceneay, J.; Parker, B.S.; Smyth, M.J.; Möller, A. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. Oncoimmunology 2013, 2, e22355. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Gkegka, A.G.; Pouliliou, S.; Biziota, E.; Kakolyris, S.; Koukourakis, M. Hypoxia and anaerobic metabolism relate with immunologically cold breast cancer and poor prognosis. Breast Cancer Res. Treat. 2022, 194, 13–23. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Comunanza, V.; Bussolino, F. Therapy for Cancer: Strategy of Combining Anti-Angiogenic and Target Therapies. Front. Cell Dev. Biol. 2017, 5, 101. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Duda, D.G.; Jain, R.K.; Willett, C.G. Antiangiogenics: The Potential Role of Integrating This Novel Treatment Modality with Chemoradiation for Solid Cancers. J. Clin. Oncol. 2007, 25, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Audiger, C.; Popovic, N.; Akla, N.; Lanthier, K.; Legault-Navarrete, I.; Melichar, H.; Costantino, S.; Lesage, S.; Larrivée, B. BMP9 signaling promotes the normalization of tumor blood vessels. Oncogene 2020, 39, 2996–3014. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fang, Z.; Liu, X.; Deng, S.; Zhou, P.; Wang, X.; Zhang, C.; Yin, R.; Hu, H.; Chen, X.; et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Investig. 2018, 128, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, R.; Zhou, P.; Liu, X.; Fan, P.; Qian, L.; Dong, L.; Zhang, C.; Zheng, X.; Deng, S.; et al. DLL1 orchestrates CD8+T cells to induce long-term vascular normalization and tumor regression. Proc. Natl. Acad. Sci. USA 2021, 118, e2020057118. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Secondini, C.; Coquoz, O.; Spagnuolo, L.; Spinetti, T.; Peyvandi, S.; Ciarloni, L.; Botta, F.; Bourquin, C.; Rüegg, C. Arginase inhibition suppresses lung metastasis in the 4T1 breast cancer model independently of the immunomodulatory and anti-metastatic effects of VEGFR-2 blockade. Oncoimmunology 2017, 6, e1316437. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, W.; Liang, C.; Wang, X.; Zhi, L.; Guo, C.; Niu, Z.; Zhu, W. VEGF165b and its mutant demonstrate immunomodulatory, not merely anti-angiogenic functions, in tumor-bearing mice. Mol. Immunol. 2020, 122, 132–140. [Google Scholar] [CrossRef]
- Manning, E.A.; Ullman, J.G.; Leatherman, J.M.; Asquith, J.M.; Hansen, T.R.; Armstrong, T.D.; Hicklin, D.J.; Jaffee, E.M.; Emens, L.A. A Vascular Endothelial Growth Factor Receptor-2 Inhibitor Enhances Antitumor Immunity through an Immune-Based Mechanism. Clin. Cancer Res. 2007, 13, 3951–3959. [Google Scholar] [CrossRef]
- Huang, X.; Wong, M.K.; Yi, H.; Watkins, S.; Laird, A.D.; Wolf, S.F.; Gorelik, E. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2-IgG fusion protein. Cancer Res. 2002, 62, 5727–5735. [Google Scholar]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9, eaak9679. [Google Scholar] [CrossRef] [PubMed]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Rmili, C.W.; Kiialainen, A.; Kienast, Y.; Mueller, H.-J.; Ooi, C.-H.; Laoui, D.; et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017, 9, eaak9670. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.T.; Man, S.; Xu, P.; Chow, A.; Paez-Ribes, M.; Lee, C.R.; Pirie-Shepherd, S.R.; Emmenegger, U.; Kerbel, R.S. Efficacy of Cotargeting Angiopoietin-2 and the VEGF Pathway in the Adjuvant Postsurgical Setting for Early Breast, Colorectal, and Renal Cancers. Cancer Res. 2016, 76, 6988–7000. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724. [Google Scholar] [CrossRef]
- Wu, F.; Xu, P.; Chow, A.; Man, S.; Krüger, J.; Khan, K.; Paez-Ribes, M.; Pham, E.; Kerbel, R.S. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br. J. Cancer 2018, 120, 196–206. [Google Scholar] [CrossRef]
- Tian, L.; Goldstein, A.; Wang, H.; Lo, H.C.; Kim, I.S.; Welte, T.; Sheng, K.; Dobrolecki, L.E.; Zhang, X.; Putluri, N.; et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017, 544, 250–254. [Google Scholar] [CrossRef]
- Kabir, A.U.; Subramanian, M.; Lee, D.H.; Wang, X.; Krchma, K.; Wu, J.; Naismith, T.; Halabi, C.M.; Kim, J.Y.; Pulous, F.E.; et al. Dual role of endothelial Myct1 in tumor angiogenesis and tumor immunity. Sci. Transl. Med. 2021, 13, eabb6731. [Google Scholar] [CrossRef]
- Panagi, M.; Voutouri, C.; Mpekris, F.; Papageorgis, P.; Martin, M.R.; Martin, J.D.; Demetriou, P.; Pierides, C.; Polydorou, C.; Stylianou, A.; et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 2020, 10, 1910–1922. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, T.; Zhang, S.; Song, G.; Meng, T.; Yuan, H.; Hu, F. Combination of tumor vessel normalization and immune checkpoint blockade for breast cancer treatment via multifunctional nanocomplexes. Biomater. Sci. 2022, 10, 4140–4155. [Google Scholar] [CrossRef]
- Thomssen, C.; Pierga, J.-Y.; Pritchard, K.I.; Biganzoli, L.; Cortes-Funes, H.; Petráková, K.; Kaufman, B.; Duenne, A.; Smith, I. First-Line Bevacizumab-Containing Therapy for Triple-Negative Breast Cancer: Analysis of 585 Patients Treated in the ATHENA Study. Oncology 2012, 82, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Robert, N.J.; Diéras, V.; Glaspy, J.; Brufsky, A.M.; Bondarenko, I.; Lipatov, O.N.; Perez, E.A.; Yardley, D.A.; Chan, S.Y.; Zhou, X.; et al. RIBBON-1: Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Chemotherapy With or Without Bevacizumab for First-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative, Locally Recurrent or Metastatic Breast Cancer. J. Clin. Oncol. 2011, 29, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Kimmick, G.; Hopkins, J.; Marcom, P.K.; Rocha, G.; Welch, R.; Broadwater, G.; Blackwell, K. Nab-Paclitaxel/Bevacizumab/Carboplatin Chemotherapy in First-Line Triple Negative Metastatic Breast Cancer. Clin. Breast Cancer 2013, 13, 416–420. [Google Scholar] [CrossRef]
- Brufsky, A.; Valero, V.; Tiangco, B.; Dakhil, S.; Brize, A.; Rugo, H.S.; Rivera, R.; Duenne, A.; Bousfoul, N.; Yardley, D.A. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: Subgroup analysis of the RIBBON-2 trial. Breast Cancer Res. Treat. 2012, 133, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Sasich, L.D.; Sukkari, S.R. The US FDAs withdrawal of the breast cancer indication for Avastin (bevacizumab). Saudi Pharm. J. 2011, 20, 381–385. [Google Scholar] [CrossRef]
- Cameron, D.; Brown, J.; Dent, R.; Jackisch, C.; Mackey, J.; Pivot, X.; Steger, G.G.; Suter, T.M.; Toi, M.; Parmar, M.; et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Xu, Y.; Chen, L.; Fan, L.; Ma, X.-Y.; Zhao, S.; Song, X.-Q.; Hu, X.; Yang, W.-T.; Chai, W.-J.; et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: Concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol. Cancer 2022, 21, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tian, Z.; Lin, Y.; Li, H.; Zhu, Z.; Liu, Q.; Su, S.; Zeng, Y.; Jia, W.; et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Fandino, M.; Holgado, E.; Manso, L.; Morales, S.; Bermejo, B.; Colomer, R.; Apala, J.V.; Blanco, R.; Muñoz, M.; Caleiras, E.; et al. Immuno-priming durvalumab with bevacizumab in HER2-negative advanced breast cancer: A pilot clinical trial. Breast Cancer Res. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Quintela-Fandino, M.; Sànchez, L.M.; Martín, E.H.; Moreno, M.; Murillo, S.M.; Heras, B.B.D.L.; Gimenez, D.M.; Bosch, R.C.; Cortijo, L.G.; Hornedo, J.; et al. Addition of durvalumab (Dur) upon progression to bevacizumab (Bev) maintenance in advanced HER2-negative (HERNEG) breast cancer (BC): Safety, efficacy and biomarkers. Ann. Oncol. 2018, 29, ix24. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Jiang, Y. Partial Response After Toripalimab Plus Anlotinib for Advanced Metaplastic Breast Carcinoma: A Case Report. Front. Endocrinol. 2022, 13, 810747. [Google Scholar] [CrossRef]
- Xuelian, C.; Lanying, M.; Xi, W.; Hongnan, M.; Dawei, W.; Bo, L.; Dong, Q.; Hongtu, Z.; Jing, H.; Binghe, X. Reactive capillary hemangiomas: A novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol. Med. 2019, 16, 173–181. [Google Scholar] [CrossRef]
- Fadlallah, A.; Chelala, E.; Dirani, A. Intravitreal anti-VEGF injection for the treatment of progressive juxtapapillary retinal capillary hemangioma: A case report and mini review of the literature. Clin. Ophthalmol. 2013, 7, 2143–2146. [Google Scholar] [CrossRef]
- Qin, L.; Li, X.; Stroiney, A.; Qu, J.; Helgager, J.; Reardon, D.A.; Young, G.S. Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 2017, 59, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, W.S.; Ley, C.D.; Farrar, C.; Duyverman, A.M.; Lahdenranta, J.; Lacorre, D.A.; Batchelor, T.T.; Di Tomaso, E.; Duda, D.G.; Munn, L.L.; et al. Edema Control by Cediranib, a Vascular Endothelial Growth Factor Receptor–Targeted Kinase Inhibitor, Prolongs Survival Despite Persistent Brain Tumor Growth in Mice. J. Clin. Oncol. 2009, 27, 2542–2552. [Google Scholar] [CrossRef]
- Chen, W.; Shen, L.; Jiang, J.; Zhang, L.; Zhang, Z.; Pan, J.; Ni, C.; Chen, Z. Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment. Biomark. Res. 2021, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Sainson, R.C.; Oon, C.E.; Turley, H.; Leek, R.; Sheldon, H.; Bridges, E.; Shi, W.; Snell, C.; Bowden, E.T.; et al. DLL4-Notch Signaling Mediates Tumor Resistance to Anti-VEGF Therapy In Vivo. Cancer Res. 2011, 71, 6073–6083. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; He, P.; Shao, B.; Liu, F.; Xiang, Z.; Yang, T.; Zeng, Y.; He, T.; Ma, J.; et al. Effective Combinations of Immunotherapy and Radiotherapy for Cancer Treatment. Front. Oncol. 2022, 12, 809304. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).