Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Characterization of Cinnamon Extract—Polyphenol-Enriched Fractions
2.2. Cytotoxic Effect of Cinnamon Extracts on Colorectal Cancer Cell Lines
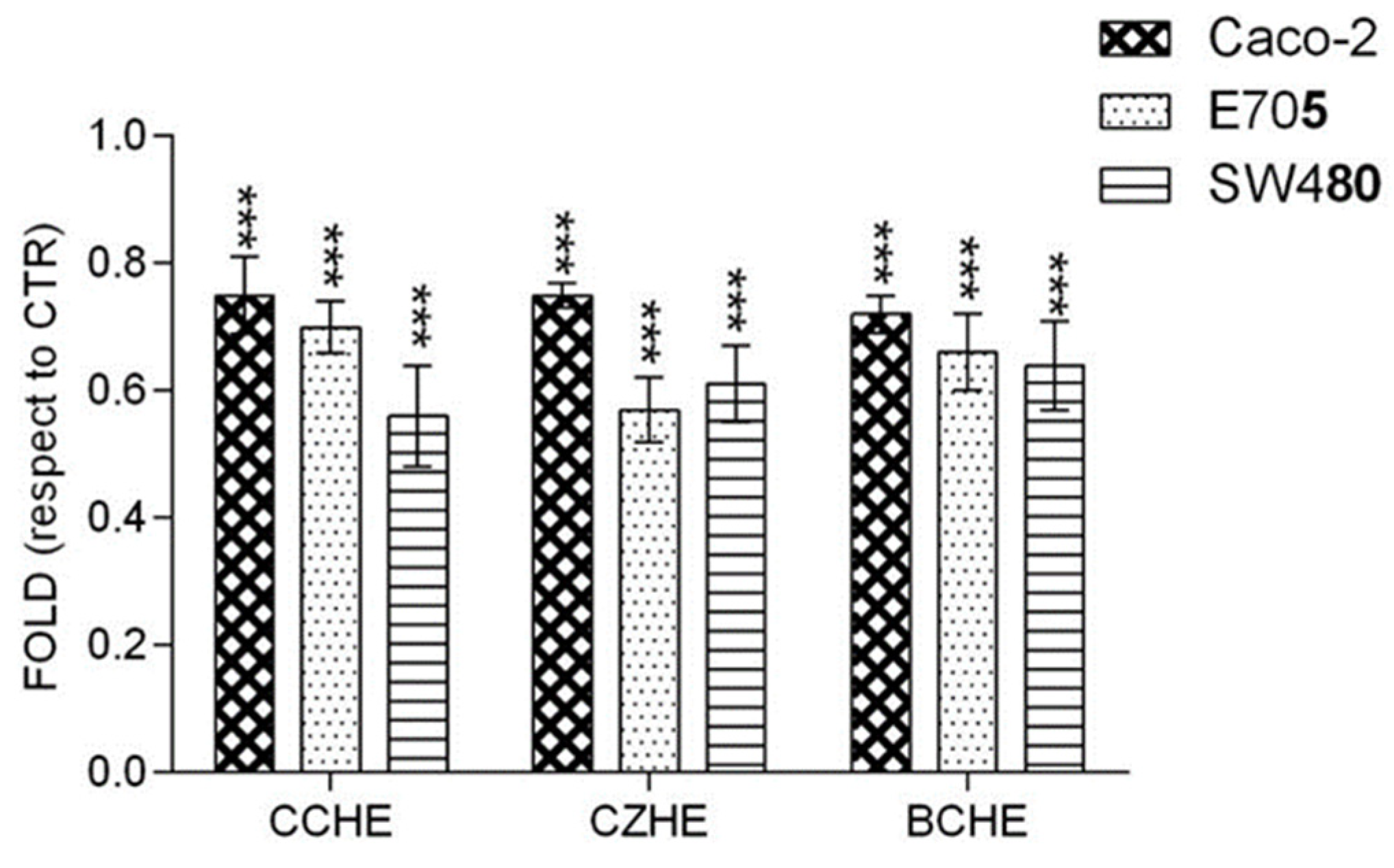
2.3. Additive Effect of Cinnamon Fractions Enriched in Polyphenols
2.4. Fractions Enriched in Polyphenols Induce Apoptosis in Colorectal Cancer Cell Lines
2.5. Fractions Enriched in Polyphenols Induce Apoptosis through ERK Activation and Reduce Mitochondrial Membrane Potential
3. Discussion
4. Materials and Methods
4.1. Cinnamon Extracts and Polyphenols-Enriched Fractions Preparation
4.2. UPLC–HRMS Characterization
4.3. Cell Cultures
4.4. Viability Assay
4.5. Annexin V-FITC Assay for Apoptosis
4.6. SDS-PAGE and Western Blotting
4.7. Mitochondrial Transmembrane Potential (MTP) Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Mendelsohn, J. The Epidermal Growth Factor Receptor as a Target for Cancer Therapy. Endocr. Relat. Cancer 2001, 8, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Voigt, M.; Braig, F.; Göthel, M.; Schulte, A.; Lamszus, K.; Bokemeyer, C.; Binder, M. Functional Dissection of the Epidermal Growth Factor Receptor Epitopes Targeted by Panitumumab and Cetuximab. Neoplasia 2012, 14, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Lungulescu, C.; Ianoşi, S.; Cherciu, I.; Schenker, M.; Săftoiu, A. Molecular Profiling of EGFR Status to Identify Skin Toxicity in Colorectal Cancer: A Clinicopathological Review. Curr. Health Sci. J. 2019, 45, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Foerster, C.G.; Cursiefen, C.; Kruse, F.E. Persisting Corneal Erosion Under Cetuximab (Erbitux) Treatment (Epidermal Growth Factor Receptor Antibody). Cornea 2008, 27, 612. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Frauenfelder, T.; Obrist, S.; Zaugg, K.; Corti, N.; Günthard, H.F. A Rare but Severe Pulmonary Side Effect of Cetuximab in Two Patients. Case Rep. 2012, 2012, bcr0320125973. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Martini, M.; Molinari, F.; Sartore-Bianchi, A.; Arena, S.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; Frattini, M.; Siena, S.; et al. Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer. JCO 2008, 26, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Siena, S. Molecular Mechanisms of Resistance to Cetuximab and Panitumumab in Colorectal Cancer. JCO 2010, 28, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.A.; Soundharrajan, I.; Govindan, N. Nutraceuticals as Potential Therapeutic Agents for Colon Cancer: A Review. Acta Pharm. Sin. B 2014, 4, 173–181. [Google Scholar] [CrossRef]
- Park, G.H.; Song, H.M.; Park, S.B.; Son, H.-J.; Um, Y.; Kim, H.-S.; Jeong, J.B. Cytotoxic Activity of the Twigs of Cinnamomum Cassia through the Suppression of Cell Proliferation and the Induction of Apoptosis in Human Colorectal Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Goto, H.; Kogure, T.; Kohta, K.; Shintani, T.; Itoh, T.; Terasawa, K. Extract Prepared from the Bark of Cinnamomum Cassia Blume Prevents Glutamate-Induced Neuronal Death in Cultured Cerebellar Granule Cells. Phytother. Res. 2000, 14, 466–468. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, B.-S.; Kim, M.-K. Suppression Effect of Cinnamomum Cassia Bark-Derived Component on Nitric Oxide Synthase. J. Agric. Food Chem. 2002, 50, 7700–7703. [Google Scholar] [CrossRef]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous Cinnamon Extract (ACE-c) from the Bark of Cinnamomum Cassiacauses Apoptosis in Human Cervical Cancer Cell Line (SiHa) through Loss of Mitochondrial Membrane Potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef]
- Ciaramelli, C.; Palmioli, A.; Angotti, I.; Colombo, L.; De Luigi, A.; Sala, G.; Salmona, M.; Airoldi, C. NMR-Driven Identification of Cinnamon Bud and Bark Components With Anti-Aβ Activity. Front. Chem. 2022, 10, 896253. [Google Scholar] [CrossRef]
- Lu, S.; Obianom, O.N.; Ai, Y. Novel Cinnamaldehyde-Based Aspirin Derivatives for the Treatment of Colorectal Cancer. Bioorganic Med. Chem. Lett. 2018, 28, 2869–2874. [Google Scholar] [CrossRef]
- Lee, Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, S. Anticancer Potential and Molecular Mechanisms of Cinnamaldehyde and Its Congeners Present in the Cinnamon Plant. Physiologia 2023, 3, 173–207. [Google Scholar] [CrossRef]
- Bovio, F.; Epistolio, S.; Mozzi, A.; Monti, E.; Fusi, P.; Forcella, M.; Frattini, M. Role of NEU3 Overexpression in the Prediction of Efficacy of EGFR-Targeted Therapies in Colon Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 8805. [Google Scholar] [CrossRef]
- Mazzoni, L.; Giampieri, F.; Suarez, J.M.A.; Gasparrini, M.; Mezzetti, B.; Hernandez, T.Y.F.; Battino, M.A. Isolation of Strawberry Anthocyanin-Rich Fractions and Their Mechanisms of Action against Murine Breast Cancer Cell Lines. Food Funct. 2019, 10, 7103–7120. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological Stress Response Terminology: Integrating the Concepts of Adaptive Response and Preconditioning Stress within a Hormetic Dose–Response Framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Duessel, S.; Heuertz, R.M.; Ezekiel, U.R. Growth Inhibition of Human Colon Cancer Cells by Plant Compounds. Clin. Lab. Sci. 2008, 21, 151–157. [Google Scholar] [PubMed]
- Roth-Walter, F.; Moskovskich, A.; Gomez-Casado, C.; Diaz-Perales, A.; Oida, K.; Singer, J.; Kinaciyan, T.; Fuchs, H.C.; Jensen-Jarolim, E. Immune Suppressive Effect of Cinnamaldehyde Due to Inhibition of Proliferation and Induction of Apoptosis in Immune Cells: Implications in Cancer. PLoS ONE 2014, 9, e108402. [Google Scholar] [CrossRef]
- Hoang, T.; Sohn, D.K.; Kim, B.C.; Cha, Y.; Kim, J. Efficacy and Safety of Systemic Treatments Among Colorectal Cancer Patients: A Network Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2022, 11, 756214. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Yuan, P.; Yang, Y.; Chen, K.; Jia, Q.; Li, Y. Immunosuppressive Effects of A-Type Procyanidin Oligomers from Cinnamomum tamala. Evid. Based Complement. Altern. Med. 2014, 2014, e365258. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, B.; Liu, Y.; Yu, X.; Cheng, G. Dual Effects of Active ERK in Cancer: A Potential Target for Enhancing Radiosensitivity (Review). Oncol. Lett. 2020, 20, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Harrington, P.D.B.; Chen, P. Metabolomic Profiling and Comparison of Major Cinnamon Species Using UHPLC–HRMS. Anal. Bioanal. Chem. 2020, 412, 7669–7681. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSn Profiling Method To Identify and Quantify Oligomeric Proanthocyanidins in Plant Products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]






| # | RT (Min) | ID | Molecular Formula | Monoisotopic Mass | Experimental m/z | Adduct Type | Abs. Error (ppm) | Source |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.85 | Cinnacassoside C | C19H28O13 | 464.1530 | 463.1458 | [M-H]− | 0.21 | CZ |
| 2 | 4.20 | A-type ProCy tetramer | C60H48O24 | 1152.2536 | 1151.2458 | [M-H]− | 0.37 | CZ |
| 3 | 4.25 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1354 | [M-H]− | 0.50 | CC |
| 4 | 4.27 | A-type ProCy tetramer | C60H48O24 | 1152.2540 | 1151.245 | [M-H]− | 1.11 | CC |
| 5 | 4.41 | Phenolic glycoside (NCGC00180160-01) | C19H28O12 | 448.1581 | 493.1564 | [M+FA-H]− | 0.31 | CC |
| 6 | 4.49 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1348 | [M-H]− | 0.56 | CC |
| 7 | 4.65 | A-type ProCy pentamer | C75H62O30 | 1440.3169 | 719.1531 | [M-2H]2− | 2.7 | CZ |
| 8 | 4.67 | Epicatechin | C15H14O6 | 290.0790 | 289.0716 | [M-H]− | 0.47 | CC |
| 11 | 4.83 | B-type ProCy trimer | C45H38O18 | 866.2058 | 865.1968 | [M-H]− | 1.79 | BC, CC |
| 12 | 4.85 | A-type ProCy pentamer | C75H62O30 | 1440.3169 | 719.1543 | [M-2H]− | 4.31 | CZ |
| 13 | 4.88 | 3,4,5-Trimethoxyphenyl 6-O-apiofuranosylglucopyranoside | C20H30O13 | 478.1686 | 477.1619 | [M-H]− | 1.05 | CZ |
| 14 | 4.89 | A-type ProCy pentamer | C75H60O30 | 1440.3170 | 719.1532 | [M-2H]2− | 2.79 | CC |
| 15 | 4.92 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1345 | [M-H]− | 1.09 | BC |
| 16 | 4.98 | A-type ProCy tetramer | C60H48O24 | 1152.2540 | 1151.246 | [M-H]− | 0.26 | CC |
| 17 | 5.01 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1352 | [M-H]− | 0.09 | BC, CC, CZ |
| 18 | 5.16 | B-type ProCy tetramer | C60H50O24 | 1154.2690 | 1153.2598 | [M-H]− | 1.87 | BC |
| 19 | 5.18 | Benzyl β-primeveroside | C18H26O10 | 402.1526 | 447.1505 | [M+FA-H]− | 0.54 | CZ, CC |
| 20 | 5.24 | A-type ProCy pentamer | C75H60O30 | 1440.3170 | 719.1524 | [M-2H]2− | 1.68 | CC |
| 21 | 5.33 | A-type ProCy trimer | C45H36O18 | 864.1902 | 863.1835 | [M-H]− | 0.75 | CC |
| 22 | 5.37 | Phenolic glycosides | C18H24O11 | 416.1319 | 415.1244 | [M-H]− | 0.47 | CZ |
| 23 | 5.37 | Catechin | C15H14O6 | 290.0790 | 289.0718 | [M-H]− | 0.05 | CC, BC |
| 24 | 5.46 | A-type ProCy tetramer | C60H48O24 | 1152.254 | 1151.245 | [M-H]− | 1.11 | CC |
| 25 | 5.48 | A-type ProCy trimer | C45H36O18 | 864.1902 | 863.1821 | [M-H]− | 0.95 | CZ |
| 26 | 5.53 | B-type ProCy trimer | C45H38O18 | 866.2058 | 865.1997 | [M-H]− | 1.35 | BC |
| 27 | 5.54 | A-type ProCy trimer | C45H36O18 | 864.1902 | 863.1946 | [M-H]− | 2.02 | CC |
| 28 | 5.63 | A-type ProCy tetramer | C60H48O24 | 1152.2536 | 1151.2454 | [M-H]− | 0.79 | CZ |
| 29 | 5.68 | B-type ProCy tetramer | C60H50O24 | 1154.2690 | 1153.264 | [M-H]− | 1.83 | BC |
| 30 | 5.68 | A-type ProCy tetramer | C60H48O24 | 1152.2540 | 1151.2469 | [M-H]− | 0.95 | CC |
| 31 | 5.73 | B-type ProCy pentamer | C75H62O30 | 1442.3330 | 720.1598 | [M-2H]2− | 0.69 | BC |
| 32 | 5.77 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1357 | [M-H]− | 1.02 | CC |
| 33 | 5.78 | B-type ProCy trimer | C45H38O18 | 866.2058 | 865.1985 | [M-H]− | 0.01 | BC |
| 34 | 5.83 | B-type ProCy pentamer | C75H62O30 | 1442.3330 | 720.1614 | [M-2H]2− | 2.89 | BC |
| 35 | 5.88 | A-type ProCy trimer | C45H36O18 | 864.1902 | 863.1832 | [M-H]− | 0.39 | CC |
| 36 | 5.90 | B-type ProCy hexamer | C90H75O36 | 1731.4040 | 864.1923 | [M-2H]2− | 1.54 | BC |
| 37 | 5.96 | B-type ProCy hexamer | C90H75O36 | 1731.4040 | 864.1924 | [M-2H]2− | 1.68 | BC |
| 38 | 6.04 | B-type ProCy heptamer | C105H86O42 | 2018.4590 | 1008.2208 | [M-2H]2− | 1.9 | BC |
| 39 | 6.08 | A-type ProCy trimer | C45H36O18 | 864.1902 | 863.1827 | [M-H]− | 0.17 | CC, CZ |
| 40 | 6.10 | Phenylethyl primeveroside | C19H28O10 | 416.1682 | 461.1678 | [M+FA-H]− | 1.12 | BC, CC |
| 41 | 6.15 | Quercetin 3-vicianoside | C26H28O16 | 596.1377 | 595.1301 | [M-H]− | 0.66 | BC |
| 42 | 6.16 | 4-Hydroxyacetophenone 4-O-(6′-O-beta-D-apiofuranosyl)-beta-D-glucopyranoside | C19H26O11 | 430.1475 | 429.1399 | [M-H]− | 0.73 | CZ |
| 43 | 6.29 | Lignan glycoside | C32H44O17 | 700.2579 | 699.2487 | [M-H]− | 2.74 | CZ |
| 44 | 6.36 | Lusitanicoside | C21H30O10 | 442.1839 | 441.1765 | [M-H]− | 0.24 | CZ |
| 45 | 6.37 | B-type ProCy trimer | C45H38O18 | 866.2058 | 865.1979 | [M-H]− | 0.79 | BC |
| 46 | 6.44 | B-type ProCy dimer | C30H26O12 | 578.1424 | 577.1352 | [M-H]− | 0.07 | BC, CC |
| 47 | 6.50 | Isoquercitrin | C21H20O12 | 464.0955 | 463.0887 | [M-H]− | 0.98 | BC |
| 48 | 6.54 | Cichorioside L | C25H38O11 | 514.2414 | 559.2401 | [M+FA-H]− | 1.55 | BC |
| 49 | 6.58 | Ptelatoside B | C20H28O10 | 428.1682 | 473.1667 | [M+FA-H]− | 0.63 | BC, CC, CZ |
| 50 | 6.61 | A-type ProCy trimer | C45H34O18 | 862.1745 | 861.1676 | [M-H]− | 0.46 | CC |
| 51 | 6.66 | Quercetin 3-xylosyl-(1-2)-alpha-L-arabinofuranoside | C25H26O15 | 566.1272 | 565.1201 | [M-H]− | 0.29 | BC |
| 52 | 6.69 | Phenolic glycosides | C20H28O10 | 428.1683 | 427.1608 | [M-H]− | 0.41 | CZ |
| 53 | 6.72 | A-type ProCy dimer | C30H24O12 | 576.1268 | 575.1193 | [M-H]− | 0.31 | CC |
| 54 | 6.76 | Phenolic glycoside | C20H28O10 | 428.1682 | 473.1666 | [M+FA-H]− | 0.37 | CC |
| 55 | 6.76 | Flavonoid glycoside | C39H34O13 | 710.1999 | 709.1921 | [M-H]− | 0.74 | BC |
| 56 | 6.82 | Rosavin | C20H28O10 | 428.1682 | 427.1614 | [M-H]− | 1.02 | BC |
| 57 | 6.92 | Phenethyl rutinoside | C20H30O10 | 430.1839 | 475.1819 | [M+FA-H]− | 1.45 | CC |
| 58 | 6.92 | Avicularin | C20H18O11 | 434.0849 | 433.0781 | [M-H]− | 0.99 | BC |
| 59 | 6.93 | Astragalin | C21H20O11 | 448.1006 | 447.0932 | [M-H]− | 0.19 | BC |
| 60 | 7.02 | Poncirin chalcone | C28H34O14 | 594.1949 | 593.1870 | [M-H]− | 1.06 | CC |
| 61 | 7.03 | Quercetin 3-(2-xylosylrhamnoside) | C26H28O15 | 580.1428 | 579.1353 | [M-H]− | 0.5 | BC |
| 62 | 7.11 | A-type procyanidin trimer | C45H34O18 | 862.1745 | 861.1685 | [M-H]− | 1.41 | CZ |
| 63 | 7.11 | Leeaoside | C24H40O11 | 504.2571 | 549.2559 | [M+FA-H]− | 1.19 | CZ |
| 64 | 7.18 | Quercitrin | C21H20O11 | 448.1006 | 447.0929 | [M-H]− | 0.87 | BC |
| 65 | 7.33 | Juglalin | C20H18O10 | 418.0900 | 417.0820 | [M-H]− | 1.72 | BC |
| 66 | 7.45 | Phenolic glycoside | C20H28O10 | 428.1682 | 427.1605 | [M-H]− | 1.19 | BC |
| 67 | 7.59 | Kaempferol-O-glycoside | C26H28O14 | 564.1479 | 563.1399 | [M-H]− | 1.31 | BC |
| 68 | 7.70 | Kaempferin | C21H20O10 | 431.0983 | 431.0983 | [M-H]− | 0.07 | BC |
| 69 | 7.74 | Flavonoid glycoside | C39H34O13 | 710.1999 | 709.1918 | [M-H]− | 1.17 | BC |
| 70 | 7.85 | Secoisolariciresinol | C20H26O6 | 362.1729 | 407.1704 | [M+FA-H]− | 1.87 | BC, CZ |
| 71 | 8.04 | Flavanone glycoside | C24H22O7 | 422.1366 | 421.1289 | [M-H]− | 0.88 | BC |
| 72 | 8.09 | Cinnammic acid | C9H8O2 | 148.0524 | 147.0448 | [M-H]− | 2.28 | BC |
| 73 | 8.55 | Piperic acid | C12H10O4 | 218.0579 | 217.0507 | [M-H]− | 0.33 | CZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmioli, A.; Forcella, M.; Oldani, M.; Angotti, I.; Sacco, G.; Fusi, P.; Airoldi, C. Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 16117. https://doi.org/10.3390/ijms242216117
Palmioli A, Forcella M, Oldani M, Angotti I, Sacco G, Fusi P, Airoldi C. Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines. International Journal of Molecular Sciences. 2023; 24(22):16117. https://doi.org/10.3390/ijms242216117
Chicago/Turabian StylePalmioli, Alessandro, Matilde Forcella, Monica Oldani, Irene Angotti, Grazia Sacco, Paola Fusi, and Cristina Airoldi. 2023. "Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines" International Journal of Molecular Sciences 24, no. 22: 16117. https://doi.org/10.3390/ijms242216117
APA StylePalmioli, A., Forcella, M., Oldani, M., Angotti, I., Sacco, G., Fusi, P., & Airoldi, C. (2023). Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines. International Journal of Molecular Sciences, 24(22), 16117. https://doi.org/10.3390/ijms242216117