Abstract
Intracranial aneurysms (IAs) are abnormal dilations of the cerebral vessels, which pose a persistent threat of cerebral hemorrhage. Inflammation is known to contribute to IA development. The nuclear factor “kappa-light-chain-enhancer” of activated B-cells (NF-κB) is the major driver of inflammation. It increases the expression of inflammatory markers and matrix metalloproteinases (MMPs), which contribute heavily to the pathogenesis of IAs. NF-κB activation has been linked to IA rupture and resulting subarachnoid hemorrhage. Moreover, NF-κB activation can result in endothelial dysfunction, smooth muscle cell phenotypic switching, and infiltration of inflammatory cells in the arterial wall, which subsequently leads to the initiation and progression of IAs and consequently results in rupture. After a systematic search, abstract screening, and full-text screening, 30 research articles were included in the review. In this systematic review, we summarized the scientific literature reporting findings on NF-κB’s role in the pathogenesis of IAs. In conclusion, the activation of the NF-κB pathway was associated with IA formation, progression, and rupture.
1. Introduction
Intracranial aneurysms are asymptomatic abnormal dilations of the intracranial blood vessels with an increased diameter compared to the parent artery. IAs occur in 3-5% of the population with a gender ratio of 1:1 and a mean age of 50, but after the age of 50 years, the gender ratio changes significantly with the increasing number of female cases [,]. The rupture of IAs is a persistent risk for hemorrhage with an incidence rate of around 7.9 per 100,000 person-years []. About 15% of intracranial hemorrhage patients die before reaching a hospital. Even after state-of-the-art medical intervention, the mortality rate of SAH patients is very high (~40%) [,], and the rupture of IAs can lead to a lifelong disability.
The pathophysiology of IAs is complex. Although many factors play together in the formation, progression, and rupture of IAs, the research hitherto suggests that inflammation heavily contributes to IAs from formation to rupture [,]. The endothelial dysfunction, smooth muscle cells (SMCs) phenotypic switching, infiltration and accumulation of inflammatory cells in the arterial walls, and the expression and release of pro-inflammatory cytokines such as interleukin (IL) -1β, and tumor necrosis factor-alpha (TNF-α), chemokines such as monocyte chemoattractant protein-1 (MCP-1), and IL-8, cell adhesion molecules, namely, vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), and extracellular matrix remodeling proteinases such as MMPs including MMP-2 and MMP-9 have been implicated in IA formation and rupture [,]. The expression of these pro-inflammatory markers and MMPs is regulated by NF-κB transcription activity []. Experimental studies have shown that NF-κB activation increases the expression of these markers, and by blocking NF-κB activation, the expression of these inflammatory markers and MMPs could be reduced [,,]. Previously, deficiency of NF-κB subunit P50 in mice has been shown to decrease the incidence of IA formation and reduced macrophage infiltration []. Moreover, silencing the adenomatous polyposis coli (APC) gene in rats promoted IA formation with increased NF-κB protein expression and activation []. It is worth noting that potential acquired risk factors for IA formation and rupture including smoking, alcohol abuse, obesity, and oxidative stress activate NF-κB and increase the expression of pro-inflammatory markers and MMPs [,,,].
In this systematic review, we focused on the contribution of NF-κB in the formation, progression, and rupture of IAs.
2. Methods
2.1. Systematic Literature Search
The PRISMA guidelines were followed for the scientific literature search. The search for research articles reporting findings on NF-κB’s role in IA formation, progression, and rupture was conducted in June 2023 in PubMed, BASE, and Embase search engines. The detailed search strategy was “NF-κB” or “NF-kappaB” in the “intracranial aneurysm” and “cerebral aneurysms” and “ruptured cerebral aneurysm” and “intracranial aneurysm” and “Un-ruptured intracranial aneurysm” and “Unruptured intracranial aneurysm” and “Un-ruptured cerebral aneurysms” and “Unruptured cerebral aneurysms”. The search results were deduplicated, and the irrelevant research articles were screened out after reading the titles.
2.2. Literature Screening Criteria
The original peer-reviewed research article reporting the findings, which included expression of NF-κB in IA walls in animal or human studies, targeting gene manipulation to block NF-κB activation, and using pharmacological compounds or other strategies that directly or indirectly manipulated NF-κB expression and/or activation were included in this review. The studies reporting NF-κB findings in human IAs with only mutated genomes without comparing them to normal genomes were excluded.
3. Results
The systematic search on search engines PubMed, Embase, and BASE for scientific literature reporting NF-κB’s role in aneurysm biology delivered a total of 118 records. After deduplication and title screening, 92 unique research articles were left. The abstract screening delivered 38 research articles for full-text screening. The full-text screening delivered 30 scientific research articles. The flowchart for the systematic search is shown in Figure 1. Eight research articles reported findings on human samples, and 25 studies reported NF-κB findings using animal models. Three studies reported both experimental and clinical data.
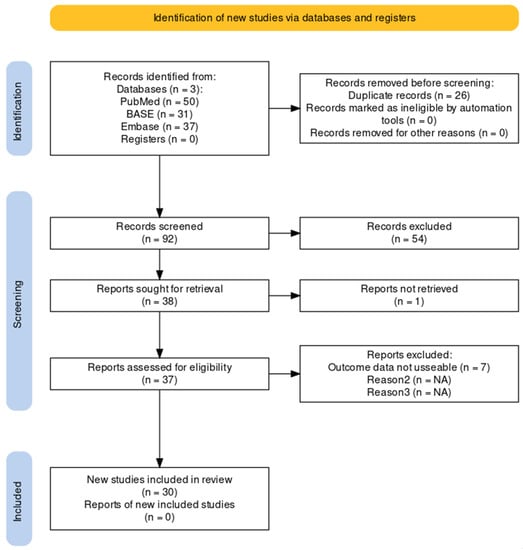
Figure 1.
Flowchart showing a systematic search of the literature []. A systematic search for scientific literature was performed in June 2023.
3.1. Clinical Studies
Seven clinical studies reported NF-κB expression and/or activation in IAs compared to normal cerebral or nonvascular diseased arteries. The findings of these studies are summarized in Table 1. One study provided findings on NF-κB genotype correlation to IA formation. According to this study, ATTG1/ATTG2 and ATTG2/ATTG2 genotypes compared with the ATTG1/ATTG1 genotype were linked to a significantly decreased risk of IAs, suggesting that the ATTG2 allele may be a protective factor against IA formation [].

Table 1.
Studies reporting NF-κB status in patients with IAs compared to control with nonvascular diseased arteries or normal cerebral arteries.
3.2. Animal Experimental Studies
Among the included studies, 16 studies reported findings on NF-κB’s role in IA formation, progression, and rupture. In addition to NF-κB, these studies reported findings on the regulation of inflammatory markers, MMPs, internal elastic lamina (IEL) loss, media thinning, vascular smooth muscle cells (VSMCs) loss and apoptosis, and macrophage infiltration. The findings of these studies are summarized in Table 2. Three studies used rabbits, 18 studies used rats, and 8 studies used mouse models of IAs. In rabbit models, the common carotid artery was ligated to induce IAs. In rats, all studies used left common carotid artery and bilateral posterior renal arteries ligation with increased salt intake with or without β-aminopropionitrile (BAPN) to induce IAs. In mice, IAs were induced either by using the same IA model as that of rats or by using deoxycorticosterone acetate-salt hypertension combined with a single elastase injection with or without BAPN.

Table 2.
Animal studies reporting NF-κB status in IA animals compared to control animals.
In total, 21 studies used different approaches to decrease the incidence of aneurysm formation and rupture and to ameliorate aneurysmal changes. Among these studies, 2 studies used the rabbit IA model, 6 studies used the rat IA model, and 7 studies used the mouse model of IAs. In all studies, the NF-κB expression and/or activation was directly or indirectly inhibited, except in one study, where the protein expression and phosphorylation of NF-κB P65 were enhanced. The findings of these studies are summarized in Table 3.

Table 3.
Studies reporting the effects of pharmacological/other treatments or genetic manipulations on NF-κB status and IA formation/rupture in animals.
4. Discussion
The clinical studies provided ample convincing evidence that the NF-κB P65 subunit is overexpressed at mRNA and protein levels with increased phosphorylation in IAs compared to normal arteries and nonvascular diseased tissue (Table 1) [,,,,,]. Elevated NF-κB activation was linked to the increased diameter of IAs []. In addition to that, polymorphism in the genotype of the NF-κB promoter was linked to a lower risk of IAs []. Similar to clinical studies, experimental studies using different animal models showed the elevated mRNA expression, protein levels, and/or activation of NF-κB in IA tissue compared to normal control arteries (Table 2) [,,,,,,,,,,,,,]. The animal experimental studies revealed that blocking NF-κB P50 expression and NF-κB activation reduced the incidence of IA formation (Table 3) [,], while enhanced NF-κB activation was linked to increased IA formation (Table 3) []. Moreover, the treatments, which reduced the formation, growth, and rupture of IAs, also mitigated the mRNA expression, protein levels, and/or phosphorylation of NF-κB P65 (Table 3) [,,,,,,,,,,,,,,,,], suggesting the heavy contribution of NF-κB activation in IA formation and rupture.
NF-κB activation increases the transcription and protein expression of inflammatory markers and MMPs []. Higher mRNA expression, protein levels, and concentrations of pro-inflammatory markers in serum and IA walls have been reported in clinical and experimental animal studies (Table 1 and Table 2) [,,,,,,,,,,,,]. The mRNA and protein expression of these inflammatory markers were lowered by pharmacological treatments and genetic manipulations, which also reduced the incidence of IA formation, growth, and rupture (Table 3) [,,,,,,,,,,,,]. Furthermore, blocking NF-κB expression and activation reduced the expression of pro-inflammatory markers including IL-1β, MCP-1, and VCAM-1 (Table 3) []. In addition to that, animal experimental studies showed that blocking the expression of these pro-inflammatory markers, including TNF-α, IL-1β, and MCP-1, or blocking their function via inhibiting their receptors or knocking out their receptors in mice could significantly reduce the formation, progression, and rupture of IAs [,,,]. It is interesting to note that APC gene silencing in rats, which promoted IA formation and rupture, resulted in enhanced mRNA expression, protein levels, and phosphorylation of NF-κB P65 with elevated mRNA and protein expression of TNF-α, IL-1β, IL-6, MCP-1, MMP2, and MMP-9 (Table 3) []. These findings demonstrate the causative role of NF-κB activation in IA pathology.
Furthermore, inhibition of NF-κB activation resulted in reduced expression of MMP-2 and MMP-9 in IA walls of experimental animals (Table 3) []. Higher mRNA expression, protein levels, serum concentration, and strong staining intensity of these MMPs in human and animal IA tissue have been reported (Table 1 and Table 2) [,,,,,,,,]. Experimental studies have shown that NF-κB activation results in increased transcription and protein levels of both MMPs [,,]. The strategies, which lowered the incidence of IA formation and rupture, also reduced the mRNA expression, protein levels, and/or activation of MMPs (Table 3) [,,,,,,,,,,,,,,]. Moreover, blocking the expression and activation of these MMPs reduced the formation and progression of IAs in experimental animal studies [,,]. The rupture and progression of IAs depend on the balance between the proteins causing degeneration and regeneration of the extracellular matrix. NF-κB plays an important role in regulating this balance, as, on one hand, NF-κB transcription activity increases the expression of MMPs [,,], and, on the other hand, it reduces the expression of procollagens and LOX [], which can consequently destabilize IA walls, leading to IA rupture.
NF-κB activation was detected in macrophages, endothelial cells, and SMCs in IA walls of experimental animals [,,]. Macrophages were predominantly infiltrated and accumulated in IA walls compared to normal arteries in human and arteries in SHAM-operated animals, and their number increased with the progression of IAs in experimental animal studies (Table 2) [,,,,]. In addition to macrophages, infiltration and accumulation of neutrophils, mast cells, and T cells were also detected in IA walls (Table 2) [,]. Blocking NF-κB expression and activation and the treatments reducing IA formation and rupture attenuated the infiltration and accumulation of these inflammatory cell types in IA tissue (Table 3) [,,,,,,,,,,,,]. Blocking or suppressing cell-specific NF-κB activation provided interesting results. It has been shown that macrophage-specific NF-κB inhibition and suppression could potentially reduce the incidence of IA formation, while blocking and suppressing NF-κB activation in endothelial cells did not reduce the incidence of IA formation []. The inhibition of NF-κB in macrophages reduced CCL2 expression and, consequently, ameliorated macrophage infiltration in IA walls []. Contrary to these findings, macrophages do not seem to be the only culprit, as clodronate liposomes lowered the number of circulating and infiltrated macrophages in IA tissue, but it did not reduce aneurysmal damage in the rabbit model of IAs []. The treatment with clodronate liposomes did not reduce the mRNA and protein levels of MMP-2 and MMP-9 in IA walls []. Furthermore, immunofluorescence staining showed that SMCs were the source of increased MMP-2 and MMP-9 with increased NF-κB P65 and MCP-1 levels [], suggesting the contribution of NF-κB expression and activation in SMCs to IA initiation. The vascular and inflammatory cells orchestrate the cellular and molecular events leading to IA initiation, progression, and rupture, thus development of IAs cannot be attributed to a single cell type. Moreover, different experimental animal models and time points of investigation after IA induction could affect the outcomes of the studies, which can explain the contradictory results discussed above.
Taken together, these studies suggest that the pharmacological drugs, which can block NF-κB activation and can suppress the consequent expression and release of cytokines, chemokines, cell adhesion molecules, and MMPs in macrophages and SMCs, can be suitable candidates that might reduce IA formation and rupture. Experimental animal studies will be needed to further explore the potential of these drug candidates.
5. Conclusions
Clinical and experimental studies have shown the activation of NF-κB in IA formation. NF-κB contributes to IA formation and rupture probably via increasing the transcription of inflammatory markers and MMPs. Blocking NF-κB expression and/or activation attenuated mRNA expression and protein levels of inflammatory markers and MMPs and reduced IA formation and rupture in different animal models. Limitations: For this systematic review, a meta-analysis was not performed. Moreover, the inclusion criteria were strictly limited to NF-κB regulation, and the studies reporting findings on NF-κB upstream/downstream signaling molecules without investigating NF-κB regulation were not included in the study.
Author Contributions
Conceptualization, D.K.; methodology, D.K.; investigation, D.K.; resources, S.M.; writing—original draft preparation, D.K.; writing—review and editing, S.M. and J.F.C.; supervision, S.M.; project administration, S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by University of Helsinki. This work was supported by grants from Peek and Cloppenburg Stiftung 2021, BMBF, and Forschungskommission HHU Düsseldorf 2020 and 2022 to S. Muhammad.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.-Y.; Ha, S.W.; Suh, S.H. Prevalence of Unruptured Intracranial Aneurysms: A Single Center Experience Using 3T Brain MR Angiography. Neurointervention 2021, 16, 117–121. [Google Scholar] [CrossRef]
- Etminan, N.; Chang, H.-S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- Hackenberg, K.A.; Hänggi, D.; Etminan, N. Unruptured Intracranial Aneurysms. Stroke 2018, 49, 2268–2275. [Google Scholar] [CrossRef]
- Rehman, S.; Phan, H.T.; Reeves, M.J.; Thrift, A.G.; Cadilhac, D.A.; Sturm, J.; Breslin, M.; Callisaya, M.L.; Vemmos, K.; Parmar, P.; et al. Case-Fatality and Functional Outcome after Subarachnoid Hemorrhage (SAH) in INternational STRoke oUtComes sTudy (INSTRUCT). J. Stroke Cerebrovasc. Dis. 2021, 31, 106201. [Google Scholar] [CrossRef]
- Muhammad, S.; Chaudhry, S.R.; Dobreva, G.; Lawton, M.T.; Niemelä, M.; Hänggi, D. Vascular Macrophages as Therapeutic Targets to Treat Intracranial Aneurysms. Front. Immunol. 2021, 12, 630381. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Wisniewski, K.; Tokarz, P.; Jaskólski, D.J.; Blasiak, J. NF-κB-Mediated Inflammation in the Pathogenesis of Intracranial Aneurysm and Subarachnoid Hemorrhage. Does Autophagy Play a Role? Int. J. Mol. Sci. 2018, 19, 1245. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Shimamura, M.; Nakagami, H.; Wakayama, K.; Moriwaki, T.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N.; et al. NF-κB Is a Key Mediator of Cerebral Aneurysm Formation. Circulation 2007, 116, 2830–2840. [Google Scholar] [CrossRef]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of transcription factor NF-κB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar] [CrossRef]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Lai, X.-L.; Deng, Z.-F.; Zhu, X.-G.; Chen, Z.-H. Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-κB signaling pathway mediated inflammatory response. Biosci. Rep. 2019, 39, BSR20181909. [Google Scholar] [CrossRef]
- Zhou, H.; Khan, D.; Gerdes, N.; Hagenbeck, C.; Rana, M.; Cornelius, J.F.; Muhammad, S. Colchicine Protects against Ethanol-Induced Senescence and Senescence-Associated Secretory Phenotype in Endothelial Cells. Antioxidants 2023, 12, 960. [Google Scholar] [CrossRef]
- Li, X.; Khan, D.; Rana, M.; Hänggi, D.; Muhammad, S. Doxycycline Attenuated Ethanol-Induced Inflammaging in Endothelial Cells: Implications in Alcohol-Mediated Vascular Diseases. Antioxidants 2022, 11, 2413. [Google Scholar] [CrossRef]
- Starke, R.M.; Thompson, J.W.; Ali, M.S.; Pascale, C.L.; Lege, A.M.; Ding, D.; Chalouhi, N.; Hasan, D.M.; Jabbour, P.; Owens, G.K.; et al. Cigarette Smoke Initiates Oxidative Stress-Induced Cellular Phenotypic Modulation Leading to Cerebral Aneurysm Pathogenesis. Arter. Thromb. Vasc. Biol. 2018, 38, 610–621. [Google Scholar] [CrossRef]
- Zhou, H.; Khan, D.; Hussain, S.M.; Gerdes, N.; Hagenbeck, C.; Rana, M.; Cornelius, J.F.; Muhammad, S. Colchicine inhibited oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: Implications in vascular diseases. bioRxiv 2023. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sima, X.; Xu, J.; Li, J.; You, C. Association Between NFKB1 −94 Insertion/Deletion ATTG Polymorphism and Risk of Intracranial Aneurysm. Genet. Test. Mol. Biomarkers 2013, 17, 620–624. [Google Scholar] [CrossRef]
- Kamińska, J.; Tylicka, M.; Dymicka-Piekarska, V.; Mariak, Z.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Canonical NF-κB signaling pathway and GRO-α/CXCR2 axis are activated in unruptured intracranial aneurysm patients. Sci. Rep. 2022, 12, 21375. [Google Scholar] [CrossRef]
- Cheng, W.-T.; Wang, N. Correlation between MMP-2 and NF-κ B expression of intracranial aneurysm. Asian Pac. J. Trop. Med. 2013, 6, 570–573. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, X.; Zhang, X.; Zhang, Y.; Luo, G. Exosomal microRNA-23b-3p from bone marrow mesenchymal stem cells maintains T helper/Treg balance by downregulating the PI3k/Akt/NF-κB signaling pathway in intracranial aneurysm. Brain Res. Bull. 2020, 165, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, Y.; Feng, J.; Li, M.; Jiang, Z. Involvement of TLR2/4-MyD88-NF-κB signaling pathway in the pathogenesis of intracranial aneurysm. Mol. Med. Rep. 2021, 23, 230. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Yu, G.; Zhong, X.; Yao, H.; Yang, Q. Role of inflammatory responses in the pathogenesis of human cerebral aneurysm. Genet. Mol. Res. 2015, 14, 9062–9070. [Google Scholar] [CrossRef]
- Wei, L.; Yang, C.; Li, K.Q.; Zhong, C.L.; Sun, Z.Y. 3-Aminobenzamide protects against cerebral artery injury and inflammation in rats with intracranial aneurysms. Die Pharm.-Int. J. Pharm. Sci. 2019, 74, 142–146. [Google Scholar] [CrossRef]
- Mandelbaum, M.; Kolega, J.; Dolan, J.M.; Siddiqui, A.H.; Meng, H. A Critical Role for Proinflammatory Behavior of Smooth Muscle Cells in Hemodynamic Initiation of Intracranial Aneurysm. PLoS ONE 2013, 8, e74357. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, D.; Tian, Y.; Wei, H.; Zhou, Z.; Liu, L.; Wang, D.; Dong, J.-F.; Jiang, R.; Zhang, J. Aspirin Inhibits Degenerative Changes of Aneurysmal Wall in a Rat Model. Neurochem. Res. 2015, 40, 1537–1545. [Google Scholar] [CrossRef]
- Hayashi, K.; Kataoka, H.; Minami, M.; Ikedo, T.; Miyata, T.; Shimizu, K.; Nagata, M.; Yang, T.; Yamamoto, Y.; Yokode, M.; et al. Association of zinc administration with growth suppression of intracranial aneurysms via induction of A20. J. Neurosurg. 2020; ahead-of-print. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, Y.; Dai, D.; Xu, Z. Expression of NF-κB, MCP-1 and MMP-9 in a Cerebral Aneurysm Rabbit Model. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2014, 41, 200–205. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Egashira, K.; Hashimoto, N. Impact of Monocyte Chemoattractant Protein-1 Deficiency on Cerebral Aneurysm Formation. Stroke 2009, 40, 942–951. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Huang, X.; Zhang, Y.; Wang, D.; Wei, H.; Dong, J.; Jiang, R.; Zhang, J. Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain Res. 2014, 1593, 65–75. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, J.; You, L.; Zhang, J.; Li, B. Pharmacological inhibition of STAT3 by BP-1-102 inhibits intracranial aneurysm formation and rupture in mice through modulating inflammatory response. Pharmacol. Res. Perspect. 2021, 9, e00704. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, M.; Peng, X.; Jiang, G. Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-κB-mediated inflammatory mechanisms in experimental rats. Open Life Sci. 2021, 16, 128–141. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Nishimura, M.; Ishibashi, R.; Morishita, R.; Miyamoto, S. Regression of Intracranial Aneurysms by Simultaneous Inhibition of Nuclear Factor-κB and Ets With Chimeric Decoy Oligodeoxynucleotide Treatment. Neurosurgery 2012, 70, 1534–1543. [Google Scholar] [CrossRef]
- Jin, T.; An, Q.; Qin, X.; Hu, Y.; Hu, J.; Zhou, B.; Leng, B. Resveratrol inhibits cerebral aneurysms in mice via downregulating the NF-κB pathway. Acta Biochim. Pol. 2022, 69, 613–618. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M.; Ishibashi, R.; Kataoka, H.; Takagi, Y.; Hashimoto, N.; Liu, L.; Zhang, Q.; Xiong, X.-Y.; Gong, Q.-W.; et al. Toll-like receptor 4 expression during cerebral aneurysm formation. J. Neurosurg. 2010, 113, 851–858. [Google Scholar] [CrossRef]
- Ishibashi, R.; Aoki, T.; Nishimura, M.; Hashimoto, N.; Miyamoto, S. Contribution of Mast Cells to Cerebral Aneurysm Formation. Curr. Neurovascular Res. 2010, 7, 113–124. [Google Scholar] [CrossRef]
- Aoki, T.; Fukuda, M.; Nishimura, M.; Nozaki, K.; Narumiya, S. Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation. Acta Neuropathol. Commun. 2014, 2, 34. [Google Scholar] [CrossRef]
- Ikedo, T.; Minami, M.; Kataoka, H.; Hayashi, K.; Nagata, M.; Fujikawa, R.; Higuchi, S.; Yasui, M.; Aoki, T.; Fukuda, M.; et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Prevents Intracranial Aneurysm Growth by Suppressing Macrophage Infiltration and Activation. J. Am. Heart Assoc. 2017, 6, e004777. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Hashimoto, N. Nifedipine Inhibits the Progression of An Experimentally Induced Cerebral Aneurysm in Rats with Associated Down-Regulation of NF-Kappa B Transcriptional Activity. Curr. Neurovascular Res. 2008, 5, 37–45. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M.; Matsuoka, T.; Yamamoto, K.; Furuyashiki, T.; Kataoka, H.; Kitaoka, S.; Ishibashi, R.; Ishibazawa, A.; Miyamoto, S.; et al. PGE2-EP2signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br. J. Pharmacol. 2011, 163, 1237–1249. [Google Scholar] [CrossRef]
- Aoki, T.; Frȍsen, J.; Fukuda, M.; Bando, K.; Shioi, G.; Tsuji, K.; Ollikainen, E.; Nozaki, K.; Laakkonen, J.; Narumiya, S. Prostaglandin E2–EP2–NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci. Signal. 2017, 10, eaah6037. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. Reduced Collagen Biosynthesis Is the Hallmark of Cerebral Aneurysm. Arter. Thromb. Vasc. Biol. 2009, 29, 1080–1086. [Google Scholar] [CrossRef]
- Ma, J.; Hou, D.; Wei, Z.; Zhu, J.; Lu, H.; Li, Z.; Wang, X.; Li, Y.; Qiao, G.; Liu, N. Tanshinone IIA attenuates cerebral aneurysm formation by inhibiting the NF-κB-mediated inflammatory response. Mol. Med. Rep. 2019, 20, 1621–1628. [Google Scholar] [CrossRef]
- Wang, D.; Lai, D.; Peng, C. TWIST1 silencing attenuates intracranial aneurysms by inhibiting NF-κB signaling. Trop. J. Pharm. Res. 2022, 21, 927–932. [Google Scholar] [CrossRef]
- Moriwaki, T.; Takagi, Y.; Sadamasa, N.; Aoki, T.; Nozaki, K.; Hashimoto, N. Impaired Progression of Cerebral Aneurysms in Interleukin-1β–Deficient Mice. Stroke 2006, 37, 900–905. [Google Scholar] [CrossRef]
- Starke, R.M.; Chalouhi, N.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Wada, K.; Shimada, K.; Hasan, D.M.; Greig, N.H.; et al. Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J. Neuroinflamm. 2014, 11, 77. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Morimoto, M.; Nozaki, K.; Hashimoto, N. Macrophage-Derived Matrix Metalloproteinase-2 and -9 Promote the Progression of Cerebral Aneurysms in Rats. Stroke 2007, 38, 162–169. [Google Scholar] [CrossRef]
- Morimoto, M.; Kume, N.; Miyamoto, S.; Mizoguchi, A.; Nozaki, K.; Sadamasa, N.; Kita, T.; Hashimoto, N. The Roles of MMPs for Cerebral Aneurysm Formation; Springer: Tokyo, Japan, 2002; pp. 223–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).