Abstract
Salt stress negatively impacts crop production worldwide. Genetic diversity among barley (Hordeum vulgare) landraces adapted to adverse conditions should provide a valuable reservoir of tolerance genes for breeding programs. To identify molecular and biochemical differences between barley genotypes, transcriptomic and antioxidant enzyme profiles along with several morpho-physiological features were compared between salt-tolerant (Boulifa) and salt-sensitive (Testour) genotypes subjected to salt stress. Decreases in biomass, photosynthetic parameters, and relative water content were low in Boulifa compared to Testour. Boulifa had better antioxidant protection against salt stress than Testour, with greater antioxidant enzymes activities including catalase, superoxide dismutase, and guaiacol peroxidase. Transcriptome assembly for both genotypes revealed greater accumulation of differentially expressed transcripts in Testour compared to Boulifa, emphasizing the elevated transcriptional response in Testour following salt exposure. Various salt-responsive genes, including the antioxidant catalase 3, the osmoprotectant betaine aldehyde dehydrogenase 2, and the transcription factors MYB20 and MYB41, were induced only in Boulifa. By contrast, several genes associated with photosystems I and II, and light receptor chlorophylls A and B, were more repressed in Testour. Co-expression network analysis identified specific gene modules correlating with differences in genotypes and morpho-physiological traits. Overall, salinity-induced differential transcript accumulation underlies the differential morpho-physiological response in both genotypes and could be important for breeding salt tolerance in barley.
1. Introduction
Barley (Hordeum vulgare L.) is the fourth most economically important cereal crop worldwide in terms of both quantity and area under cultivation [1]. A versatile crop, barley is mainly used for feed and industry but also has great potential as healthy food source due to an abundance of dietary fiber and functional food constituents [2]. Domesticated thousands of years ago, barley is grown in a wide range of geographic and climatic conditions [3], reflecting high adaptability facilitated by genetic diversity [4]. Like most crops, barley development, productivity, and yield are impaired by environmental stresses such salinity. Soil salinity is recognized as a major environmental stress [5], and ever-changing global climatic factors amplify its effects. Salinity-induced plant responses include hyper-osmotic stress, ion toxicity due to imbalance of cellular ion homeostasis, nutritional imbalance, and oxidative damage due to the excessive generation of reactive oxygen species (ROS) [5,6,7].
Among cereals, barley is considered salt-tolerant [8,9], characterized by great variation in tolerance among cultivars [10]. Salinity tolerance in barley is a very complex process that involves the interaction of diverse regulatory pathways including water uptake and osmotic tolerance via osmoprotectant biosynthesis, photosynthesis regulation, hormone signaling, ion homeostasis adjustment, and antioxidant metabolism. All of these pathways are activated across complex salinity-sensing and signaling networks along with members of several stress-related gene expression regulator families [5].
Evolving in regions with marginal conditions, barley landraces adapted to harsh environments could provide a reservoir of tolerance alleles [11]. Therefore, exploration of landrace cultivars that exhibit contrasting responses toward salinity is of great interest for elucidating candidate genes and tolerance mechanisms. Such information could guide breeding programs that aim to enhance yields under stressful conditions.
In recent years, next-generation nucleic acid sequencing, including RNA sequencing (RNA-seq), has been widely employed in many plant species. This technology has been used to identify polymorphisms, characterize transcript populations, and detect differential gene expression networks between varieties/genotypes that demonstrate variable levels of stress tolerance [4,12]. These studies have allowed an improved understanding of the regulatory networks involved in plant stress responses and suggest avenues to increase tolerance and plant productivity [13].
The transcriptomic approach has been used to develop markers associated with salt tolerance. Several studies have focused on barley [14,15,16,17]. Other groups examined wheat (Triticum aestivum) [18,19] and maize [20] (Zea mays) RNA-seq data. Various studies have underscored the importance of analyzing transcriptomes from genotypes differing in stress tolerance in order to better understand stress tolerance processes in barley. Variation in root-specific transcriptional responses to salt stress were observed between barley genotypes that displayed contrasting tolerance phenotypes [21] and differential responses to drought and heat stress were identified between barley cultivars [22]. Moreover, sequencing of two wild barley accessions with contrasting drought tolerance revealed genotype-specific transcripts [23,24,25]. Several research studies have explored the physiological responses of barley to salt stress [26,27,28,29,30,31,32]. Studies have combined both physiological and transcriptomic approaches to examine drought [22,25] and heat stress responses in barley [22]; salt tolerance in rice [33] and drought stress response in maize [34]. Nevertheless, there is a lack of information about associations between physiological and transcriptomic responses to salt stress in barley. Therefore, it is of great interest to connect RNA-seq data to observed physiological changes induced by salt stress in contrasting barley genotypes.
Barley cultivars Boulifa (B; salt-tolerant) and Testour (T; salt-sensitive) were identified in a screening of 21 accessions that represent the genetic diversity among barley landraces in Tunisia [35]. Herein, these two barley genotypes, which demonstrate contrasting salinity tolerance, were subjected to severe salt stress (200 mM) and evaluated at different durations of exposure. Stress-response properties were investigated at both transcriptional and physiological levels. Transcriptomes of both tolerant and sensitive genotypes were sequenced, and gene expression changes were evaluated. Molecular functions, biological processes, and co-expression networks of salt-modulated genes are reported. Overall, the RNA-seq data demonstrated that B and T transcription profiles responded differentially to salt stress. These differences underlie the distinctive phenotypic plasticity of barley genotypes in response to salinity.
2. Results
2.1. Morphological and Physiological Responses of Barley Genotypes under Salt Stress
Two barley genotypes with differences in tolerance to salt stress were subjected to 200 mM NaCl for 24 h in a hydroponic culture system to assess morphological and physiological differences. At the end of treatment, both cultivars B and T exhibited a decrease in growth relative to their respective controls (Figure 1). Decreases of all measured parameters, fresh weight (FW), dry weight (DW), and shoot length (L), were more pronounced in the salt-sensitive genotype T relative to the salt-tolerant B (Table 1). Compared to within genotype controls DW decreased by 35.5% in T, whereas a 12.8% decline was measured in B (Table 1).
In response to salt stress, the relative water content (RWC) was significantly reduced in both genotypes compared to their corresponding controls and a smaller reduction in RWC was recorded for the tolerant B (4.5%) than the sensitive T (7.2%). With respect to osmotic potential (OP), the average decrease relative to controls was similar in both B and T with an average value of 17% (Table 1).
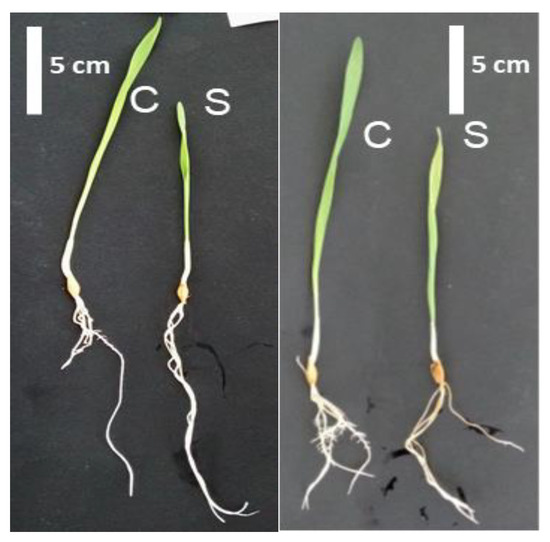


Figure 1.
Phenotype of Boulifa (B, right) and Testour (T, left) grown under control conditions (C) and salt (200 mM NaCl) treatment (S) for 24 h.

Table 1.
Growth parameters in leaves of control and salt-stressed seedlings.
Table 1.
Growth parameters in leaves of control and salt-stressed seedlings.
| Genotype | Treatment | FW | DW | L | RWC | OP |
|---|---|---|---|---|---|---|
| Boulifa | Control | 201.76 ± 1.1 b | 15.80 ± 0.2 b | 17.06 ± 0.75 b | 0.97 ± 0.04 a | −1.03 ± 0.05 b |
| Salt stress | 184.60 ± 1.6 c | 13.76 ± 0.7 c | 16.26 ± 0.40 b,c | 0.92 ± 0.02 b | −1.21 ± 0.12 a | |
| Testour | Control | 252.80 ± 0.9 a | 21.23 ± 0.8 a | 19.66 ± 1.10 a | 0.96 ± 0.08 a | −1.03 ± 0.03 b |
| Salt stress | 198.36 ± 1.1 b | 13.70 ± 1.2 c | 14.50 ± 0.81 c | 0.89 ± 0.02 c | −1.20 ± 0.05 a |
Data are mean ± SE of three replicates. Different lower-case letters (a, b, c) indicate that means in the same column with different superscripts are significantly different (Tukey’s HSD, p ≤ 0.05). Fresh weight (FW; mg), Dry weight (DW; mg), shoot length (L; cm), relative water content (RWC; %), osmotic potential (OP; Mpa).
Photosynthetic characteristics (net CO2 assimilation rate (Pn), stomatal conductance (Gs), transpiration rate (E), internal concentration of CO2 (Ci), and water-use efficiency (WUE)) were significantly decreased in both genotypes following 24 h of salt treatment (Table 2). Nevertheless, the tolerant genotype B had consistently higher Pn, Gs, E, Ci, and WUE than T. Declines in Pn were 45% and 30% of the control in T and B, respectively. A similar trend was observed for Gs (50% and 20% of the control in T and B, respectively). For E and Ci the effect of salt stress was less pronounced. In the salt-treated B, parameters E and Ci decreased by an average of 12% of control whereas in T this reduction was 31% and 20%, respectively. Relative to the respective controls, WUE decline was similar in both B and T genotype (~20%).
The photosynthetic pigment chlorophyll a (Chl a) was reduced by approximately 25% in both genotypes under 200 mM NaCl compared to their respective controls (Table 2). While chlorophyll b (Chl b) and Chl a were similarly reduced in genotype B, i.e., 25%, genotype T experienced a 42% reduction for Chl b, significantly different than Chl a in the T genotype (25%).


Table 2.
Effect of salt stress on photosynthetic parameters in leaves of control and salt-stressed seedlings of Boulifa and Testour.
Table 2.
Effect of salt stress on photosynthetic parameters in leaves of control and salt-stressed seedlings of Boulifa and Testour.
| Genotype | Treatment | Pn | Gs | E | Ci | WUE | Chl a | Chl b |
|---|---|---|---|---|---|---|---|---|
| Boulifa | Control | 7.67 ± 0.27 b | 0.06 ± 0.00 b | 0.55 ± 0.03 c | 236.33 ± 1.5 a | 14.00 ± 1.33 a | 4.64 ± 0.04 b | 1.99 ± 0.02 b |
| Salt stress | 5.39 ± 0.24 c | 0.05 ± 0.01 c | 0.47 ± 0.01 d | 210.66 ± 1.5 b | 11.32 ± 0.81 b | 3.62 ± 0.75 c | 1.45 ± 0.28 c | |
| Testour | Control | 9.35 ± 0.52 a | 0.08 ± 0.02 a | 0.91 ± 0.06 a | 171.33 ± 1.2 c | 10.24 ± 0.97 b | 6.43 ± 1.21 a | 2.69 ± 0.72 a |
| Salt stress | 5.15 ± 0.10 c | 0.04 ± 0.00 c | 0.63 ± 0.05 b | 135.33 ± 0.9 d | 8.17 ± 0.69 c | 4.85 ± 0.77 c | 1.56 ± 0.36 c |
Data are mean ± SE of three replicates. Different lower-case letters (a, b, c, d) indicate that means in the same column with different superscripts are significantly different (Tukey’s HSD, p ≤ 0.05). Net CO2 assimilation rate (Pn; μmol CO2 m−2 s−1), stomatal conductance (Gs; mol H2O m−2 s−1), transpiration rate (E; mmol H2O m−2 s−1), internal concentration of CO2 (Ci; μmol CO2 mol−1), and water use efficiency (WUE: nmol CO2 mol−1 H2O) and Chlorophyll a/b content (Chl and Chl b; mg/g MF).
2.2. Antioxidant Enzyme Responses to Salt Stress in Leaves and Roots
Differential activity of antioxidant enzymes between the barley genotypes was assessed. Changes in the major antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and glutathione reductase (GR) were analyzed in salt-stressed B and T early in development, 12 days after emergence. Salt exposure (200 mM, 24 h) induced oxidative stress in both genotypes as indicated by increased activity of antioxidant enzymes relative to respective controls, although overall the increases were greater in B than T (Table 3). When exposed to salt, SOD activity was elevated to a greater extent in B (50%) than in T (20%) compared to respective controls (Table 3). Similar trends were observed for CAT and GPX enzymes. Regarding APX and GR, no significant changes were detected for either genotype compared to their respective controls, even though a slight increase was noted in APX activity in T genotype (Table 3).

Table 3.
Antioxidant enzyme activities in leaves of control and salt-stressed seedlings.
2.3. RNA-seq Analysis of Salt Response in Barley Genotypes
RNA sequencing of three replicates for each salt stress treatment (0, 2, 8, and 24 h) resulted in an average of 23.9 million raw reads per sample and 62.5%, on average, of the reads from each sample aligned to the barley transcriptome and led to the identification of 32,943 genes (Table S1).
Principal Component Analysis (PCA) and hierarchical clustering were performed using 500 genes with the largest variance in expression to visualize the underlying structure of the RNA-seq data and assess the largest source of variation among the samples.
Samples separated into two major clusters of control (0 h) and salt-treated samples (2, 8, and 24 h) (Figure 2). The salt-stressed samples also separated according to the duration of exposure, though this signal was less distinct between 8 and 24 h salt treatments. Clustering of the genotypes was less prominent but replicates of both controls and salt-treated samples of B and T for the most part remained distinct (Figure 2 and Figure 3). In the PCA, the largest source of variation (31%) represented by the x-axis corresponds to controls (0 h) vs. salt stress (2, 8, and 24 h salt stress). The second largest source of variation (8%) represented by the y-axis may correspond to differences in genotype. According to PCA (Figure 3) and clustering (Figure 2), 24 h salt stress clustered the farthest from control samples (0 h), indicating that the duration of salt treatment was likely the largest source of variation in the data. Therefore, further bioinformatic analyses and correlation with physiological data were conducted on controls (0 h) and 24 h salt-treated samples of both genotypes.
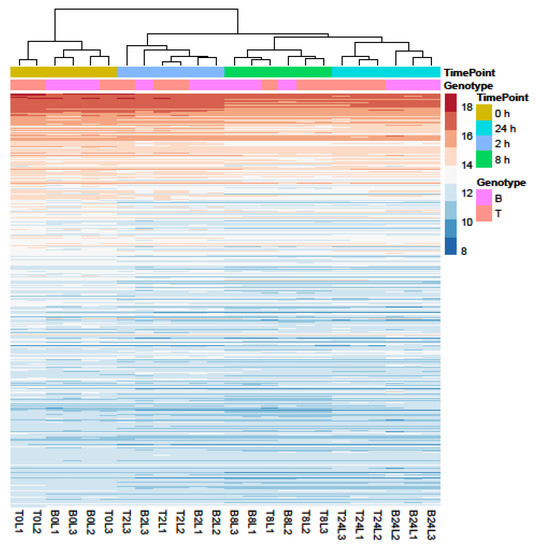
Figure 2.
Profiles of the 500 genes with the greatest variance in expression. Data were collected over four durations (0, 2, 8, and 24 h) of salt treatment in Boulifa (B, pink) and Testour (T, orange) seedlings. The colors indicate expression level of genes (see key on right). Sampling time points and genotypes (L1, L2, and L3) are shown above cluster plot. Biological replicates are labeled on the x-axis. The dendrogram (top) indicates sample clustering.
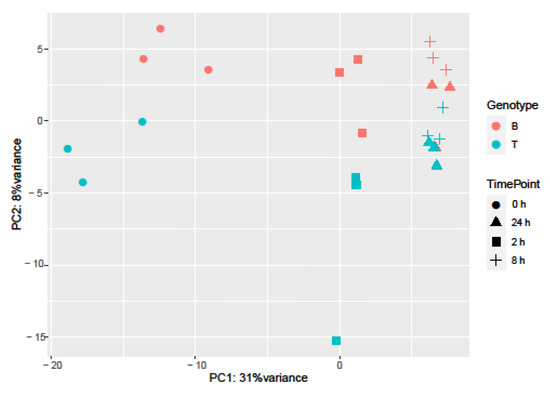
Figure 3.
Sources of variation in barley gene expression under salt stress. Principal component analysis was employed to explore influences on variation. Genotype is represented by color (Boulifa—orange; Testour—blue) and treatment is represented by shape (0, 2, 8, and 24 h salt stress, see key at right).
2.4. Differentially Expressed Genes in Boulifa and Testour in Response to Salt Stress
To explore the variation in transcriptional response between B and T genotypes in relation to salt stress and between control (0 h) and salt stress (24 h) treatments in each genotype, differential gene expression analysis was conducted for four contrasting groups (T 0 vs. B 0, T 24 vs. B 24, B 24 vs. B 0, and T 24 vs. T 0). Based on relative transcript abundance, differentially expressed genes (DEGs) were characterized as upregulated (↑) or downregulated (↓). The greatest number of DEGs was found between T 24 vs. T 0 (10,681:8362 ↑ and 3126 ↓; Figure 4a red) followed by B 24 vs. B 0 (4617:2528 ↑ and 2170 ↓; Figure 4a blue). These two contrast groups shared a high number of DEGs (3917; Figure 4a); only 700 DEGs were specific to B 24 vs. B 0. The elevated number of specific DEGs of T 24 vs. T 0 (6764), indicated that T activated a more robust transcriptional response than B at 24 h of salt stress treatment.
The lowest number of total DEGs, just 35 (11 ↑ and 24 ↓), was detected in the contrast between both analyzed genotypes under control conditions (T 0 vs. B 0; Figure 4b). Given that B and T arose in the same geographical location and likely share some genetic similarity, this was expected. However, after 24 h salt treatment, 530 DEGs were identified between T and B genotypes (362 ↑ and 168 ↓), indicating that the salt-induced transcriptomic responses were genotype specific. Furthermore, the number of T specific DEGs (520) was 20 times higher than B specific DEGs (25), which likely contributed to the differential responses to salt stress between these two genotypes and supported the morphological and physiological variation detected (Figure 4).
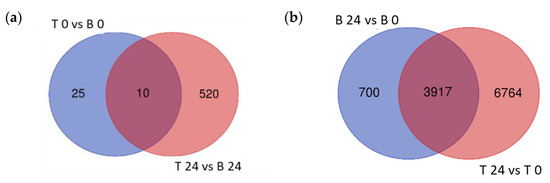

Figure 4.
Venn diagrams of the differentially expressed genes. Differentially expressed genes (DEGs) of the different contrast groups ((a) B 24 vs. B 0 and T 24 vs. T 0) and ((b) T 0 vs. B 0 and T 24 vs. B 24) were enumerated to illustrate the specific DEGs for each comparison and their overlap.
2.5. Gene Ontology Enrichment Analysis of DEGs
To further elucidate the regulatory variation in biological processes and molecular functions between both salt-stressed genotypes, DEGs identified from the four contrast groups (B 24 vs. B 0, T 24 vs. T 0, T 0 vs. B 0, and T 24 vs. B 24) were subjected to gene ontology (GO) enrichment analysis.
When comparing each genotype against its respective control, ‘catalytic activity’ was the most significantly enriched molecular function category in both genotypes (Figure S1a,b). In B, enriched DEGs were mainly involved in lyase, transferase, glycosyltransferase, and sucrose synthase activities. However, in T, catalytic activity was mainly represented by GO terms for ligase and aminoacyl−tRNA ligase activities. GO enrichment analysis of genotype-dependent DEGs under control conditions (T 0 vs. B 0) also revealed ‘catalytic activity’ as the main enriched category (Figure S1c). However, after 24 h salt treatment (T 24 vs. B 24), many more GO categories were enriched with the major identified subcategories of ‘binding’ and ‘catalytic activity’. For binding activity, several types of ion-, anion-, carbohydrate-, small-molecule-, and ATP-binding proteins were found. As for ‘catalytic activity’, transferase, kinase, and phosphotransferase activities were the most prominent GO terms in both genotypes following salt treatment (Figure 5).
Regarding biological processes, when comparing each salt-stressed genotype to its respective control, the major annotated categories were metabolic and cellular processes. For cellular process, protein folding was the most prominent in both B and T after 24 h salt treatment. However, for metabolic process the dominant annotated subcategories were different between B 24 vs. B 0 and T 24 vs. T 0. For B 24 vs. B 0, metabolic process subcategories were the predominant including carbohydrate metabolic, specifically, sucrose metabolism. Additionally, processes vital to photosynthesis were indicated. Dominant were organic substance metabolic processes such as tetrapyrrole and porphyrin−containing biosynthesis. Among the DEGs indicated, those ↑ respond to abiotic stress including oxygen-containing compounds, to water, and organonitrogen compounds.
For T 24 vs. T 0, the biological process pattern was much more complicated than in the B 24 vs. B 0 comparison. Over-represented categories included small molecule, organic substance, and cellular metabolic processes leading to tRNA aminoacylation, and porphyrin-containing processes. The ↑ DEGs in the sensitive genotype T following 24 h salt stress and compared to its respective control were highly representative of regulation processes such as protein phosphorylation, cellular protein modification, and establishment of proteins. The ↓ DEGs were carbohydrate metabolic, tetrapyrrole biosynthesis, and porphyrin−containing processes.
Comparing both analyzed genotypes under control conditions (T 0 vs. B 0) revealed that many more biological processes were enriched, including, response to stress, defense response and arginine catabolic process. Examination after 24 h salt stress (T 24 vs. B 24) processes specific to stress response such as to water and other abiotic stress and oxygen-containing compounds were allocated to the ↓ DEGs. The ↑ DEGs were enriched for protein phosphorylation and reproductive processes.
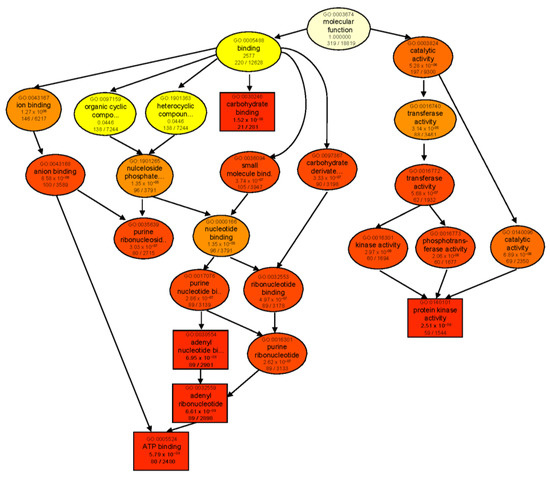

Figure 5.
GO enrichment analysis of DEGs of the contrast group T 24 vs. B 24. Rectangles/circles contain the GO term number and category name. The numerals at the bottom in each box are the p-value followed by the number of genes in the input list that overlapped with a given GO term over the total number of genes in the GO term. The colors indicate levels of statistical significance, with darker colors indicating a more significant level of enrichment. The rectangles indicate top five enriched terms.
2.6. Expression of Salt-Stress Responsive Genes
Annotation of the identified DEGs in barley genotypes B and T after 24 h salt treatment revealed the involvement of several stress responsive genes. These included genes encoding signaling activities and proteins involved in osmoprotection and photosynthesis. Genes encoding functions such as reactive oxygen species (ROS) production and scavenging, and transcription factors were also indicated (Table S2).
Several signaling and regulation genes were differentially expressed in both barley genotypes after 24 h salt treatment. These genes included hormone-related genes, kinases, calcium sensors and phospholipases. Compared with the B genotype, more differentially expressed signaling genes were detected in T (Table S2).
Several components of diverse hormone-related pathways exhibited varying expression patterns under salt stress conditions. Among them, ABA signaling pathway ABC transporter C family members and the 2C-type protein phosphatases (PP2C) were the most abundant. Additionally, expression of several auxin-responsive proteins and SAUR-like auxin-responsive proteins was largely ↓ under salt stress. Furthermore, several ethylene and Giberellin signaling pathways were differentially regulated in the barley genotypes (Table S2).
Regarding protein kinases, the receptor-like kinase (RLK) gene and the Leucine-rich receptor-like kinase (LRR-RLK) gene family were mainly ↑ in T. However, most of the other kinase groups including serine-threonine kinases and protein kinases were ↓ particularly in T. In addition, Ca2+ signaling and phospholipase pathways were differentially regulated in response to salt stress (Table S2).
Following salt treatment, various components of photosynthesis were repressed in both barley genotypes, whereas their expression levels decreased more strongly in T than in B. The expression level of many photosynthetic genes was ↓ only in T, such as the those encoding photosystem II (PSII) reaction center PsbP family protein PPD4, the photosystem I reaction center subunit II and the PSII subunit X. Furthermore, plastocyanin and Rubisco genes were repressed only in T. Likewise, several chlorophyll a-b binding proteins and photoreceptors exhibited downregulation mainly in T compared to B (Table S2).
In addition, genes involved in cell wall structure including several cellulose synthase family proteins, xyloglucan endotransglucosylase-hydrolase, and genes involved in cell wall extension and degradation were repressed under salt stress mainly in the T genotype (Table S2).
In response to salt stress, several genes involved in major compatible solutes biosynthesis were differentially expressed in both barley genotypes, including proline, sugars and glycine betaine. Among sugars, activities related to glucose and trehalose metabolism were ↓ mainly in T. In contrast, sucrose synthases were ↑ particularly in B. Different types of sugar transporters were also differentially regulated (Table S2).
Genes involved in the generation of ROS, i.e., respiratory burst oxidase homologs (RBOH), were ↑ in both barley genotypes under salt stress. Simultaneously, enzymatic and non-enzymatic ROS scavenging systems were differentially expressed under salt treatment. Several APX and glutathione S–transferase (GST) genes were differentially expressed in both barley genotypes. Only one and two catalase (CAT) genes were ↑ in T and B, respectively, and only one superoxide dismutase (SOD) was ↓ in T. Likewise, several non-enzymatic thioredoxin and chalcone synthase families were ↓ in both genotypes, while only one tocopherol cyclase and one ferritin were ↓ particularly in T. The oxidative stress 3 (OXS3) gene was ↑ in B and ↓ in T (Table S2).
Components of various ion channel families and membrane transporters were differentially regulated in both the T and B genotypes whereas, salt overly sensitive Na+/H+ exchanger (SOS) was ↑ only in B (Table S2).
Expression of various transcription factor (TF) family members were differentially expressed in both genotypes. These included ethylene-responsive TF, a member of the APETALA2/ERF family, and basic helix-loop-helix DNA-binding (bHLH) superfamily members. Members of the Homeobox-leucine zipper, NAC, MADS-box, and myb-domain protein families were represented among DEGs along with ABA-responsive, protein-related and heat shock TFs. Some TF families such as WRKY and bZIP were differentially regulated only in T. Moreover, one member of the growth-regulating factors family, GRF4, was repressed only in T (Table S2).
2.7. Co-Expressed DEGs in Response to Salt Stress
Weighted gene co-expression network analysis (WGCNA) was used to identify modules (clusters) of co-regulated genes that correlated with the morpho-physiological traits and genotypes. A total of 38 modules were identified and are numbered and color-coded on the y-axis of Figure 6.
Modules where expression levels were correlated with genotypes and morpho-physiological traits, namely modules 38, 36, 26, 22, 18, and 15, were further characterized. GO enrichment analysis indicated that several pathways were enriched for these modules (Supplementary Figure S1). Module 38 was positively correlated with the B genotype as well as all photosynthetic traits (Pn, Gs, Tr, Ci, and WUE). Module 36 correlated positively with B genotype and antioxidant enzymes (SOD, CAT, APX, GPX, and GR).
Module 26 was positively correlated with the T genotype and control conditions as well as growth traits (FW, DW), Chl b, and all photosynthetic traits but was negatively correlated with all antioxidant enzyme activities. Module 22 was positively correlated with the T genotype, growth traits (DW, L, and RWC), Chl a, and antioxidant enzymes (SOD, CAT, APX, GPX, and GR) but was highly negatively correlated with all photosynthetic traits (Pn, Gs, Tr, Ci, and WUE). Module 18 was positively correlated with T genotype and salt treatment as well as growth traits, Chl a, and antioxidant enzymes and negatively correlated with all photosynthetic traits. Module 15 was positively correlated with B genotype, salt treatment and growth traits (DW, L), Chl a, and all antioxidant enzymes but negatively correlated with all photosynthetic traits.
With respect to molecular function, GO analysis indicated that the most enriched categories in all modules were protein binding, transferase, kinase, and oxidoreductase activities. In module 38, transporter activity (ion/cation channel) was also enriched (Figure S2).
Regarding biological process, the most enriched categories were highly similar in all modules (metabolic, cellular and biological regulation processes, and localization), and the predominant annotated sub-categories varied depending on modules (Table 4; Figure S2). For instance, module 38 positively correlated with the tolerant genotype B, 0 h salt stress as well as all photosynthetic activities, and was also enriched in lipid biosynthetic process and transporter activity. In addition, module 36, which positively correlated with the tolerant genotype B, 24 h salt stress as well as all antioxidant enzyme activities, was also enriched for protein acetylation. Modules 18 and 26, positively correlated with the sensitive genotype T, were enriched in primary metabolic process and protein phosphorylation.
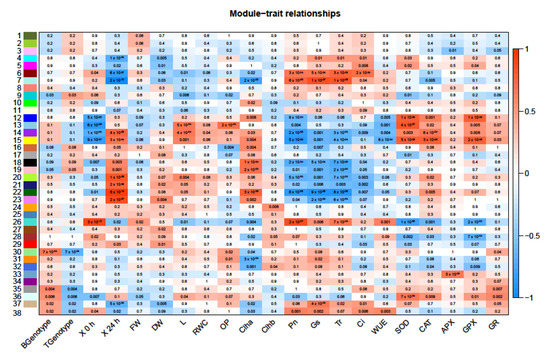


Figure 6.
Correlation of WGCNA co-expression modules to each genotype. The analysis considered several parameters including genotype (Boulifa, B and Testour, T), stress duration (0 h, 24 h) and morpho-physiological traits (fresh weight (FW), dry weight (DW), shoot length (L), relative water content (RWC), osmotic potential (OP), chlorophyll a/b content (Chl and Chl b), net CO2 assimilation rate (Pn), stomatal conductance (Gs), transpiration rate (E), internal concentration of CO2 (Ci), water use efficiency (WUE), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and glutathione reductase (GR)). Each numbered row corresponds to a module, each column to a trait (labelled along x-axis). Each cell contains the corresponding p-value and is color-coded by the strength of correlation according to the legend on the right (red and blue shading indicates level of correlation and ranges from +1 to −1).

Table 4.
The predominant annotated biological process categories and subcategories in the six selected modules.
Table 4.
The predominant annotated biological process categories and subcategories in the six selected modules.
| Module | GO Category | GO Subcategory |
|---|---|---|
| 15 | metabolic process | RNA metabolic process |
| cellular process | organelle organization | |
| localization | intracellular transport | |
| 38 | metabolic process | lipid biosynthetic process |
| cellular process | microtubule−based process | |
| 36 | metabolic process | primary metabolic process |
| metabolic process | protein acetylation | |
| metabolic process | histone acetylation | |
| cellular process | transcription by RNA | |
| cellular process | regulation of transcription | |
| biological regulation | organelle organization | |
| biological regulation | chromosome organization | |
| 22 | metabolic process | RNA metabolic process |
| metabolic process | transcription by RNA | |
| cellular process | organic cyclic compo… | |
| cellular process | nucleobase−containing | |
| biological regulation | regulation of protein | |
| biological regulation | regulation of catabolism | |
| biological regulation | ion homeostasis | |
| biological regulation | regulation of pH | |
| 26 | metabolic process | primary metabolic process |
| metabolic process | macromolecule modification | |
| cellular process | phosphate−containing | |
| cellular process | protein phosphorylation | |
| 18 | metabolic process | primary metabolic process |
| metabolic process | macromolecule modification | |
| cellular process | phosphate−containing | |
| cellular process | protein phosphorylation |
2.8. RNA-seq Data Validation by RT-qPCR
Eight randomly selected genes including four ↑ and four ↓ genes in B and T genotypes were analyzed by qRT-PCR in order to confirm RNA-seq data. As illustrated in Figure 7, the expression profiles of all selected genes at 24 h salt treatment compared to control conditions were in accordance with RNA-seq data.
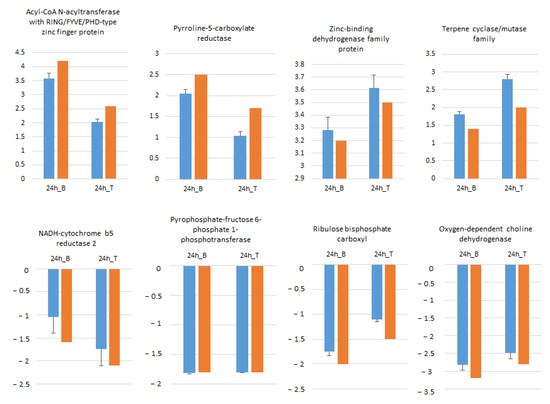
Figure 7.
Expression pattern validation of eight randomly selected genes in Boulifa and Testour leaves by qRT-PCR. The blue bar indicates the relative expression level determined by qRT-PCR using the 2−ΔΔCT method mean with associated standard error bar (n = 3) and alpha tubulin (TUB2) was used as reference gene for data normalization. The orange bar represents transcript abundance changes of RNA-seq data calculated by the Log2 fold change method.
3. Discussion
In this study, an integrative approach was employed to explore the role of gene expression in the differences in salt stress response between barley cultivars Boulifa (salt-tolerant) and Testour (salt-sensitive). We exploited the differential salt-tolerance response of these two genotypes to assign DEGs to various functional and biological processes. Additionally, WGCNA was used to elucidate specific gene modules differing between the two barley genotypes and subsequent correlation of modules with morpho-physiological traits.
When compared to their respective controls, the T phenotype was affected more by salt stress than the B phenotype. Growth characteristics were impacted including shoot length and fresh and dry leaf biomass. Compared to control plants the dry weight decrease was three times more pronounced in T than B. Similar repercussions of salt stress have been reported previously for barley [28] and maize [20]. Growth repression likely resulted from the effect of osmotic salt stress reducing water uptake [36,37]. Following 24 h salt treatment a greater decrease in RWC was also detected in T compared to B relative to their respective control plants, suggesting better water retention in B [37]. In addition to reduced water uptake and retention, decreases in several parameters associated with photosynthesis [28,29] and induced oxidative stress [38,39] may have contributed to the observed repression in plant growth. Consistent with previous reports [28,40], the sensitive genotype T exhibited stronger inhibition of all photosynthetic parameters (Pn, Gs, E, Ci and WUE) including photosynthetic pigment synthesis (Chl a and Chl b).
Stomatal conductance (Gs) was used to screen for salt tolerance in durum wheat by Rahnama et al. [41]. Here, Gs was reduced more than 2.5-fold in T compared to B relative to their respective controls. Tolerant barley plants maintained Gs rates under saline conditions, preventing water loss and promoting better yield [28]. Degradation of Chl b in the sensitive genotype T was greater than B in response to salt stress relative to their respective controls, corroborating previous studies [29,40], and could be due to higher induced ROS damage [29]. More greatly increased activity of antioxidant enzymes in B, mainly SOD, CAT and GPX, emphasized the role of induced ROS protection in improved salt tolerance [32,40]. These results suggest that the more robust maintenance of physiological salt tolerance mechanisms in B improved its capacity to maintain a relatively higher growth rate under salt stress.
The phenotypic differences in salt tolerance between B and T genotypes were supported by the RNA-seq analysis that revealed a much greater number of DEGs in T than B after 24 h salt treatment when compared to their respective controls. Approximately 2.6 times more salt-responsive genes were differentially regulated in T than in B. This suggests that the transcriptional response of the sensitive genotype T was strongly altered by the salt stress exposure. Low DEG abundance in tolerant genotypes relative to sensitive genotypes exposed to salt stress has been demonstrated for several plant species [42,43]. Ruiz et al. [43], reported fewer DEGs in glycophytes compared to halophytes under salt stress, suggesting that increases in DEGs under salt stress could be considered an indicator of plant sensitivity [44].
In the sensitive genotype T, 78.2% of DEGs were overexpressed and 21.8% were repressed under salt stress. Although there were fewer DEGs detected in the tolerant B genotype, similar ↑ and ↓ DEG proportions were detected. Several previous studies reported similar trends of DEG distribution between salt-sensitive and –tolerant genotypes of different species including barley [21], alfalfa [42], and quinoa [43]. The current results emphasize that the differential regulation of gene expression in T and B barley genotypes likely underlie the differential morpho-physiological response to salinity at an early stage of development.
3.1. Gene Expression Related to Photosynthesis, Osmoregulation, Oxidative Stress Response and Ion Homeostasis
Photosynthesis is the main source of energy needed for plant metabolism. It has been widely demonstrated that salt stress reduces photosynthetic efficiency, thus inhibiting plant growth via reduction of available resources and decreased cell division and expansion [28,29,45,46]. RNA-seq analyses revealed downregulation of several genes encoding major components of the photosynthetic reaction centers PSI and PSII. The core reactions of photosynthesis include NADP+ reduction and water splitting at PSI and light absorption at PSII. Both reaction centers participate in responses to environmental stress conditions [47,48]. In the sensitive T genotype 10 PSII components were repressed however only four components were ↓ in the tolerant B. Likewise, five PSI subunits were repressed in T compared to only one in B. Similar results were noted for barley leaves subjected to drought stress where repression of components in both photosystems was low in the drought-tolerant genotype compared to the drought-sensitive genotype [25]. Notably, plastocyanin, an electron transporter associated with photosynthesis, and Rubisco (ribulose-1,5-bisphophate carboxylase/oxygenase) accumulation factor 1, were ↓ only in T. Furthermore, the light receptors chlorophylls a and b and the chlorophyll a-b binding protein that capture and deliver excitation energy to PSI and PSII were highly ↓ in T compared to B. The differential regulation of photosynthesis-related genes in both genotypes promoted a more protective stomatal and non-stomatal response in B as exhibited by the smaller decline of all photosynthetic parameters (Pn, Gs, E, Ci and WUE). The greater accumulation of chlorophyll a and b in B relative to T suggests that stomatal and non-stomatal inhibition of chlorophyll synthesis could contribute to salinity tolerance in barley plants [29]. Marginal reduction in RWC in B relative to T may also point to stomatal response permitting growth even under saline conditions [28].
The higher photosynthetic efficiency of B could be related to cell wall organization in response to salinity. Downregulation of several genes involved in the biosynthesis of the cell wall such as the cellulose synthase protein, xyloglucan endotransglucosylase /hydrolase (XTH), was greater in T compared to B. Recognized as a cell-wall-modifying enzyme, XTH is involved in diverse physiological processes and its overexpression in Arabidopsis transgenic plants improves salt tolerance [49]. This finding is in agreement with previous RNA-seq studies on barley, maize, and plum genotypes with contrasting drought tolerance characteristics [25,34,50]. Notably, the glycine-rich cell wall structural protein was ↑ only in B. This protein is involved in cell wall plasticity and its overexpression confers enhanced tolerance to diverse abiotic stresses including salinity [51]. In addition, the hydroxyproline-rich glycoproteins (HRGP), with major roles in cell wall signal transduction cascades, plant development and stress tolerance [52] were more repressed in T compared to B. Furthermore, the arabinogalactan protein, a member of the HRGP family, was shown to be ↑ in response to salt stress in salt-adapted tobacco [52].
Wound-induced cell wall protein 1 (WIN1) transcripts rapidly increased in response to mechanical wounding and may correlate with cell death as it accumulates in senescing tissues [53]. Expression of win1 was ↑ in T and ↓ in B. This may be responsible for the more robust growth of B shoots under salt treatment. According to Savatin et al. [54], the increased accumulation of win1 transcripts was inhibited by adding osmoprotectants, emphasizing the importance of osmotic adjustment to sustain plant growth under adverse conditions [32].
Osmotic adjustment is positively correlated with stress tolerance. To cope with osmotic stress, plants activate the biosynthesis of diverse compatible solutes [28]. The major compatible solutes, including proline, sugars, and glycine betaine, accumulate under salt stress and tend to maintain low intracellular osmotic pressure by adjusting cytoplasmic water content, preventing harmful effects of salt stress [55]. The current RNA-seq data revealed that several key genes involved in the biosynthesis of these osmoprotectants were differentially regulated by salt stress in both genotypes.
Pyrroline-5-carboxylate reductase, which catalyzes the terminal step in proline biosynthesis from glutamate [56], was induced in both genotypes under salt stress. However, its expression was elevated more in B compared to T. Osmoprotectant glycine betaine is involved in the plant salt stress response [57]. Oxygen-dependent choline dehydrogenase, an enzyme involved in glycine betaine synthesis, was similarly repressed in both genotypes. Betaine aldehyde dehydrogenase 2, which catalyzes the conversion of betaine aldehyde from betaine, was induced only in B [58].
The expression of sugar biosynthetic genes, e. g. sucrose synthases, was differentially regulated in both genotypes. Sucrose synthase 1 and 6 were ↓ and ↑ in both genotypes, respectively. However, sucrose synthase 4 was ↑ only in B. Similarly, Cui et al. [59] detected elevated sucrose synthase activity and relatively high amounts of sucrose in low-temperature-treated Medicago. The GO enrichment analysis revealed overrepresentation of the subcategories of sucrose metabolic and organic substance metabolic processes. This highlights the involvement of osmotic and photosynthetic homeostasis in preventing salt stress damage and sustaining an improved growth rate under stressful conditions in the tolerant genotype B.
Overall, the relatively higher upregulation of some genes encoding osmoprotectants in B genotype could explain its better photosynthetic activity and higher leaf water content. Osmolytes can preserve cell membrane stability, prevent oxidative damage, and even act as salt-stress signaling molecules that influence stress-related gene expression [57]. Several studies have reported increased activity of enzymatic and non-enzymatic antioxidant components in salt tolerant plants following the elevation of ROS after salt treatment [46,60].
Genotype-specific transcriptional changes were detected for several peroxidases including ascorbate peroxidase. Glutathione S–transferase, catalase, and superoxide dismutase also followed genotype-specific patterns. For instance, catalase 3 was ↑ only in B and Fe superoxide dismutase 2 was ↓ only in T. Differential expression regulation of antioxidant genes under salt stress may explain the enhanced activities of their encoded enzymes mainly in B genotype. These results corroborate the reports of Yousefirad et al. [16] on mutant and wild barley subjected to salt stress and Harb et al. [25] on drought stress response of contrasting barley genotypes. The overexpression of APX and GST genes enhances salt tolerance in transgenic Arabidopsis plants [61,62]. Moreover, the upregulation of the oxidative stress 3 (OXS3) gene, involved in tolerance to oxidative stress, in B and its downregulation in T could emphasize the better oxidative stress tolerance in the B genotype. Indeed, the Arabidopsis mutant and overexpression lines of OXS3 exhibited oxidative stress sensitivity and tolerance, respectively [63].
Non-enzymatic ROS scavenging genes, including the thioredoxin family, protect cell components from damage under adverse conditions [60]. Differential regulation of this gene category was more dramatic in T compared to B. In addition, expression of the tocopherol cyclase, an antioxidant metabolite related gene, involved in protecting lipids from oxidation under environmental stresses [60,64], was exclusively ↓ in T. This highlights the greater need to protect against oxidative stress in the T genotype.
Several ion transporters were also differentially expressed in barley leaves in response to salt stress. Under salt stress, a high cytosolic K+/Na+ ratio is crucial for plants to survive [5]. The sodium transporter HKT is essential in maintenance of ion homeostasis under salinity conditions, a crucial role in plant salt tolerance [65,66]. Expression of this transporter was similarly enhanced in both barley genotypes following 24 h salt exposure. Potassium transporters, which influence salt tolerance through involvement in regulation of K+ absorption in leaves [65], were likewise upregulated in both genotypes. Upregulation of these ion transporters in response to saline conditions concurs with previous transcriptomic reports on mutant barley [16] and maize [20]. In contrast, expression of SOS1, involved in Na+ transport [67], was enhanced only in B. Overexpression of SOS1 significantly enhanced salt tolerance in Arabidopsis transgenic lines [68] suggesting that its upregulation in B influenced the salt tolerance of this genotype.
3.2. Signaling and Regulatory Proteins
Effective plant response to harmful conditions requires stress perception and signal transduction to activate expression of target genes. Several kinase signaling gene families were differentially expressed in both barley genotypes following salt stress treatment. Several MAPK kinases were overrepresented among the downregulated genes, especially in T. Members of the leucine-rich receptor-like protein kinase family (LRR-RLK), including the LRR receptor-like serine/threonine-protein kinase EFR (elongation factor Tu receptor) was downregulated. These family members are involved in both biotic and abiotic stress responses [69] and overexpression of LRR-RLK in Arabidopsis enhanced WUE [70]. The downregulation of several kinases in T compared to B could be one of the factors associated with salt sensitivity. These results concur with previous studies on barley and plum cultivars with contrasting drought stress responses [25,50].
3.3. Transcription Factors
Transcription factors, regulators of stress-related genes, are distributed in several gene families such as MYB, bZIP, NAC, CBF/DREB, HSF, WRKY, and ABF/ABRE [5]. According to our RNA-seq data, salt stress highly influenced expression of diverse TF gene families, especially in T.
The WRKY gene family is one of the largest plant TF families with members involved in plant development and stress responses [71]. One of the most important functions of WRKY genes is regulation of the salt stress response. Overexpression of several WRKY genes from maize and wild cotton (Gossypium aridum) enhanced the salt tolerance of transgenic Arabidopsis plants compared to wild type [72,73]. WRKY genes were differentially regulated exclusively in salt-stressed T. Three genes were ↑ and one ↓, emphasizing the complexity of transcriptional regulation and the involvement of several factors to overcome salinity [74].
The MYB domain-containing genes encode TFs widely involved in abiotic stress response, growth and development in plants [75]. These TFs have been associated with ROS signaling and induction of oxidative stress responses [74] and their overexpression in rice transgenic plants improved tolerance to freezing, dehydration and salt stress [76]. Several MYB domain genes were differentially regulated in both barley genotypes under salt treatment. In B, transcription of three MYB genes was enhanced, while transcription of three others was repressed. In T, expression of one MYB was enhanced while expression of four was repressed. The three induced MYB genes in B, MYB63, MYB20, and MYB41, are activators of lignin and wax biosynthesis in Arabidopsis and their disruption resulted in developmental defects [77,78]. Furthermore, MYB20 and MYB41, positively regulate ABA signaling in response to salt stress, and transgenic Arabidopsis lines overexpressing these two MYB genes showed enhanced salt tolerance [79]. Expression of MYB63 was highly ↑ in B relative to T; however MYB20 and MYB41 were induced only in B. The higher induction of these three MYB genes in B may be important for salt tolerance in this genotype.
Another group of TFs belong to the bHLH gene family and are efficient regulators of biosynthesis of several secondary metabolites, inducing flavonoids and anthocyanins involved in stress responses [74,80]. Overexpression of several bHLH genes increased the tolerance of different transgenic plants against drought and salinity by increasing flavonoids and anthocyanin accumulation [81,82]. In this study, three bHLH TFs were similarly regulated in both barley genotypes, two were enhanced and one was repressed.
It is worth noting that growth-regulating factor 4 (GRF4), a plant-specific TF with roles in stem and leaf cell expansion, proliferation, and development, and associated with growth maintenance under adverse environmental conditions was ↓ only in T. Overexpression of various GRF genes in Arabidopsis leads to larger leaf size compared to wild type [83]. Moreover, Huang et al. [84] found elevated GRF4 gene expression in wheat leaves treated with 96 h NaCl compared to control plants. This gene may play an active role in plant response to salt stress.
Overall, more regulatory processes were detected in the sensitive genotype T compared to B, implying that a more complicated physiological process occurred in T than in B when exposed to salt stress.
3.4. Identification of Genetic Modules Corresponding to Salt Stress
Weighted correlation network analysis (WGCNA) is an efficient tool for data exploration allowing gene screening related to traits and classification of co-expressed clusters with high biological significance [85]. We identified 38 gene modules, among them, six modules highly correlate transcript levels with genotypes and morpho-physiological traits (Figure 6).
Annotation of these modules confirmed that salt stress response mechanisms were genotype specific. Indeed, the modules (15, 36, 38), which correlated significantly with B genotype, also showed positive significant correlations with either photosynthetic or oxidative traits, which was not the case for the modules that correlated with T genotype. These data corroborate our findings from the physiological assays and highlight the possible use of physiological and/or antioxidant enzymes as salt stress tolerance markers.
Furthermore, GO enrichment analysis of the modules that significantly correlated with the tolerant genotype B revealed that lipid biosynthetic, protein acetylation (affecting diverse aspects of protein function such as enzymatic stability and activity [86]), and microtubule (function in the plant salt stress response [87]) processes were associated with these modules. Elevation of expression for genes involved in these processes could explain the higher growth maintenance in B genotype after 24 h salt treatment. The white module (#38) enriched for the GO annotation terms lipid biosynthesis and microtubule process, was significantly correlated with B at 0 h salt stress (absence of salt). In addition, several biological processes were enriched under control conditions when comparing T 0 vs. B 0, including response to stress and defense response. This could explain the fewer number of DEGs in B under 24 h salt stress compared to T and suggests that the salt tolerance of B is genotype-specific emphasizing the importance of the genotype itself to overcome stress [88,89].
The modules (18, 22, 26) that significantly correlate with the sensitive genotype T were enriched in several regulation processes, including that of protein, catabolism, primary metabolic processes, and protein phosphorylation, key steps in almost all cellular activity [90]. This is in accordance with more complex signaling and transcriptional regulation detected in the T genotype since more energy is needed for the sensitive genotype to overcome stress.
4. Conclusions
In this study, two barley genotypes differing in their tolerance to salinity (Boulifa and Testour) were evaluated for differential gene expression, antioxidant enzyme activity, and physiological responses following 200 mM NaCl treatment. Relative to Testour, the tolerant genotype Boulifa had better photosynthetic capacity, higher expression of antioxidant enzymes and activated expression of relevant biosynthetic pathways more strongly. This allowed better osmotic protection and antioxidant response, conferring differential growth performance between tested genotypes that was supported by higher, and possibly maladaptive, levels of transcriptional regulation in Testour compared to the Boulifa. In addition, comparison of leaf transcriptomes between salt-tolerant and sensitive genotypes following salt exposure allowed the identification of key candidate genes enhanced exclusively in the tolerant B including sucrose synthase 4, catalase 3, OXS3, and SOS1 as well as several TFs such as MYB20 and MYB41 and GRF4. Future studies should consider delving more deeply into the relationship between these factors and salt-stress responses to inform barley-breeding programs aimed at increasing salinity tolerance.
5. Materials and Methods
5.1. Plant Material, Growth Conditions, Salt Stress Treatment and Physiological Measurements
Seeds of two genetically distinct Tunisian barley landraces Boulifa (B, salt-tolerant) [91] and Testour (T, salt-sensitive) [35,92] were germinated in Petri dishes. Five-day-old seedlings were transferred to an aerated hydroponic system under controlled conditions (photoperiod: 16 h light/8 h dark, temperature: 25/19 °C, and relative humidity: 65%), acclimated for 3 days, and subjected to gradual salt stress application up to 200 mM following established methods [91]. Severe salt stress (200 mM) was adopted to allow clear discrimination between sensitive and tolerant genotypes without being drastic [35]. Leaves were harvested at 0 h (before adding NaCl), then again at 2, 8, and 24 h after exposure to 200 mM NaCl. At each time point, three pools of five plants of both genotypes were considered for antioxidant enzyme assays and RNA-seq analysis. Pooled samples were frozen in liquid nitrogen and stored at −80 °C. Growth assessment was conducted at 24 h after salt treatment on control and stressed leaves by measuring length (L) and fresh and dry weights (FW and DW, respectively). Relative water content (RWC) was calculated as RWC (%) = [(Fresh Weight − Dry Weight)/(Turgid Weight − Dry Weight)] × 100 [93] and osmotic potential (OP) was measured on leaf extracts using an osmometer (osmomat 3000 Type D-10553, Berlin, Germany) [26].
Photosynthetic parameters, namely net CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E), and internal concentration of CO2 (Ci) were also measured on control and stressed plants after 24 h of 200 mM NaCl treatment. Measurements were done under atmospheric CO2 using a portable photosynthetic system (LCpro+, Inc., Hoddesdon, UK) on the last fully expanded leaf. The data were collected at 10:00 am at an ambient CO2 concentration of 360 μmol mol−1 and photosynthetic active radiation in the leaf chamber of 980 µmol m−2 s−1. At the same time, point, chlorophyll a (Chl a) and chlorophyll b (Chl b) contents were spectrophotometrically determined according to Lichtenthaler [94].
5.2. Antioxidant Enzymes Assays
The extraction of antioxidant enzymes was performed from 500 mg of frozen leaf samples using 50 mM phosphate buffer solution (pH 7.8) added with 10% polyvinylpyrrolidone (PVP), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM EDTA, 10 mM DTT, and 0.1% triton X-100. For APX activity measurement, 5 mM ascorbate was added to the extraction buffer. The homogenates were centrifuged at 13,800 rpm for 15 min at 4 °C and supernatants were collected [95]. Protein contents (μg μL−1) were determined at 595 nm using a spectrophotometer following the method of Bradford [96]. SOD activity was evaluated by measuring the photoreduction inhibition of nitroblue tetrazolium at 560 nm [97]. CAT activity was detected by monitoring the degradation rate of H2O2 at 240 nm according to Cakmak and Marschner [98]. APX activity was assayed by following the rate of H2O2-dependent ascorbate peroxidation at 290 nm [99]. GPX activity was assessed by recording the increase in absorbance at 470 nm due to the guaiacol oxidation [100]. GR activity was measured following the GSSG (oxidized glutathione)-dependent oxidation of NADPH by the decrease in absorbance at 340 nm [101].
5.3. RNA Isolation, DNase Treatment and Sequencing
Three pools of leaves, each of which contained five control or stressed plants of the two studied genotypes, were used for total RNA isolation. The sampling was done at 0, 2, 8, and 24 h after salt stress application [91]. RNA extraction was performed using the ZR Plant RNA MiniPrep™ Kit (Zymo Research, Irvine, CA, USA). DNA was eliminated from RNA samples by TURBO DNA-free™ Kit (Promega, Madison, WI, USA). The quality and quantity of the isolated RNAs were checked by agarose gel electrophoresis (1%) and spectrophotometrically using BioPhotometer (Eppendorf BioPhotometer plus, Hamburg, Germany).
The three replicates of each treatment for both genotypes were sequenced at the Beijing Genomics Institute (BGI, Shenzhen, China) using the Illumina NextSeq 500 platform as described in our previous publication [91].
The clean sequencing reads were submitted to SRA database at NCBI (accession number: PRJNA821484).
5.4. Pseudoalignment and Differential Expression Analyses
The reads were pseudo-aligned to the barley transcriptome (from pigs barley genome database 2017) using Kallisto and transcript-level abundances were obtained. These were aggregated to gene-level abundances using tximport and normalized using DESeq2. Principal component analysis and hierarchical clustering was performed using 500 genes with the highest variance in expression in order to explore the underlying structure of the data in an unsupervised manner. Differential expression analysis was performed separately for the four contrasts: T 0 vs. B 0, T 24 vs. B 24, B 24 vs. B 0, and T 24 vs. T 0 in order to understand the relationship between salt stress and the two genotypes. DeSeq2 was used to normalize the gene abundances, estimate dispersion, model the abundances as a negative binomial distribution and perform a Wald test to identify significantly differentially expressed genes. Genes with adjusted p-value (after Benjamini–Hochberg correction) ≤0.01 and absolute log 2-fold change ≥1 were reported as significantly differentially expressed in each contrast.
5.5. Functional Enrichment Analysis of DEGs
Gene ontology terms enriched among each set of differentially expressed genes were identified using topGO, an R package for GO enrichment analysis. The differentially expressed genes for each contrast were separated into upregulated genes (adjusted p-value ≤ 0.01 and log 2-fold change ≥1) and downregulated genes (adjusted p-value ≤ 0.01 and log 2-fold change ≤ −1). A classic fisher enrichment test was performed using each list of differentially expressed genes to identify GO terms that were significantly overrepresented among that list. GO terms in the molecular function and biological process domains were identified in order to identify the biological functions likely dysregulated based on the differentially expressed genes.
5.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
The WGCNA R package was used to construct a scale-free co-expression network using all the genes in the dataset. WGCNA, rather than relying on p-value and fold change cutoffs to choose genes of interest, uses all the genes in the dataset to identify clusters of co-expressed genes called modules. Using correlation as a measure for co-expression, genes were clustered based on similarity in expression and a dynamic tree-cutting algorithm was used to identify modules (minimum size of 30 genes). The eigengene (most representative gene) of each module was correlated with metadata such as genotype, time point and physiological traits to identify modules of interest. Genes belonging to these modules were extracted and topGO was used to associate functional terms with each module of interest.
5.7. Quantitative Real-Time PCR Analysis
DNase treated RNAs were used for cDNA synthesis according to the manufacturer’s instructions of the GoScript™ Reverse Transcription System Kit (Promega). The qRT-PCR reactions were performed using the 7300 Real-Time PCR Detection System (Applied Biosystems, Foster City, CA, USA) and the Applied Biosystems Power SYBR Green qPCR Master Mix (Life technologies, Carlsbad, CA, USA). The 2−∆∆CT method of Schmittgen and Livak [102] was used to calculate the relative expression levels of the target genes and alpha tubulin (TUB2) was used as reference gene for data normalization. Fold change was calculated for the salt-treated plants relative to the controls. The primers used were designed by the Primer3 Input (version 0.4.0) software (created by Steve Lincoln, Mark Daly, and Eric S. Lander; http://bioinfo.ut.ee/primer3-0.4.0/) accessed on 22 November 2021 [103] and are listed in the Supplementary Table S3.
5.8. Statistical Analysis
The morpho-physiological data were analyzed by one-way analysis of variance (ANOVA). All experiments were repeated three times independently with six replications per treatment and the values are presented as mean ± standard error (SE). Means were separated by Tukey’s post hoc test (p ≤ 0.05) using SPSS program (SPSS software, version 11/PC SPSS 11.0, Inc., Chicago, IL, USA, 2001) developed by (Norman H. Nie, C. Hadlai (Tex) Hull and Dale H. Bent).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23095006/s1.
Author Contributions
Conceptualization, R.N.O., R.K.J., D.A. and T.A.R.; methodology, R.N.O., R.K.J., D.A. and G.A.; software, D.A.; validation, R.K.J., G.A. and A.G.; formal analysis, M.B.C., G.A. and S.M.; investigation, M.B.C. and S.M.; resources, M.B.C., S.M. and D.A.; data curation, M.B.C., G.A. and D.A.; writing—original draft preparation, R.N.O.; writing—review and editing, T.A.R., R.K.J. and D.A.; visualization, D.A.; supervision, R.K.J. and A.G.; project administration, R.N.O. and R.K.J.; funding acquisition, R.N.O. and R.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “International Center for Biosaline Agriculture (ICBA)”, grant number JRCAFS-23396, and the “CRDF Global”, grant number #62795.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study were submitted to the NCBI Sequence Read Archive (accession number: PRJNA821484).
Acknowledgments
The authors are thankful to Aida Bouajila and Badra Bouamama for prospecting barley genotypes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Tricase, C.; Amicarelli, V.; Lamonaca, E.; Leonardo, R.R. Economic analysis of the barley market and related uses. In Grasses as Food and Feed; IntechOpen: London, UK, 2018; Chapter 2. [Google Scholar]
- Zhou, M. Chapter 1. Barley production and consumption. Genetics and improvement of barley malt quality. In Genetics and Improvement of Barley Malt Quality; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.; Waugh, R. Barley: A translational model for adaptation to climate change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Schulte, D.; Close, T.J.; Graner, A.; Langridge, P.; Matsumoto, T.; Muehlbauer, G.; Sato, K.; Schulman, A.H.; Waugh, R.; Wise, R.P. The international barley sequencing consortium–At the threshold of efficient access to the barley genome. Plant Physiol. 2009, 149, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Jogaiah, S.; Govind, S.R.; Tran, L.S.P. Systems biology-based approaches toward understanding drought tolerance in food crops. Crit. Rev. Biotechnol. 2013, 33, 23–39. [Google Scholar] [CrossRef]
- Dai, F.; Nevo, E.; Wu, D.Z.; Comadran, J.; Zhou, M.X.; Qiu, L.; Chen, Z.H.; Beiles, A.; Chen, G.X.; Zhang, G.P. Tibet is one of the centers of domestication of cultivated barley. Proc. Natl. Acad. Sci. USA 2012, 109, 16969–16973. [Google Scholar] [CrossRef] [Green Version]
- Yahiaoui, S.; Cuesta-Marcos, A.; Gracia, M.P.; Medina, B.; Lasa, J.M.; Casas, A.M.; Ciudad, F.J.; Montoya, J.L.; Moralejo, M.; Molina-Cano, J.L.; et al. Spanish barley landraces outperform modern cultivars at low-productivity sites. Plant Breed. 2014, 133, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.K.; Terauchi, R.; McCouch, S.R. Harvesting the promising fruits of genomics: Applying genome sequencing technologies to crop breeding. PLoS Biol. 2014, 12, e1001883. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Gao, S.; Muegge, K.; Zhang, W.; Zhou, B. Advanced applications of RNA sequencing and challenges. Bioinform. Biol. Insights 2015, 9, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, M.; Kamboj, A.; Hove, R.M.; Loveridge, S.; El-Osta, A.; Bhave, M. Analysis of the barley leaf transcriptome under salinity stress using mRNA-Seq. Acta Physiol. Plant 2013, 35, 1915–1924. [Google Scholar] [CrossRef]
- Bahieldin, A.; Atef, A.; Sabir, J.S.; Gadalla, N.O.; Edris, S.; Alzohairy, A.M.; Radhwan, N.A.; Baeshen, M.N.; Ramadan, A.M.; Eissa, H.F.; et al. RNA-Seq analysis of the wild barley (H. spontaneum) leaf transcriptome under salt stress. Comptes Rendus. Biol. 2015, 338, 285–297. [Google Scholar] [CrossRef]
- Yousefirad, S.; Soltanloo, H.; Ramezanpour, S.S.; Nezhad, K.Z.; Shariati, V. The RNA-seq transcriptomic analysis reveals genes mediating salt tolerance through rapid triggering of ion transporters in a mutant barley. PLoS ONE 2020, 15, e0229513. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Fan, Y.; Shabala, S.; Li, C.; Lv, C.; Guo, B.; Xu, R.; Zhou, M. Understanding mechanisms of salinity tolerance in barley by proteomic and biochemical analysis of near-isogenic lines. Int. J. Mol. Sci. 2020, 21, 1516. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Teng, W.; Fang, S.; Li, H.; Li, B.; Chu, J.; Lia, Z.; Zhenga, Q. Transcriptome analysis of salt-stress response in three seedling tissues of common wheat. Crop J. 2019, 7, 378–392. [Google Scholar] [CrossRef]
- Duarte-Delgado, D.; Dadshani, S.; Schoof, H.; Oyiga, B.C.; Schneider, M.; Mathew, B.; Léon, J.; Ballvora, A. Transcriptome profiling at osmotic andionic phases of salt stress response in bread wheat uncovers trait-specific candidate genes. BMC Plant Biol. 2020, 20, 428. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Zeng, W.; Ding, Y.; Zhuang, Z.; Peng, Y. Comparing transcriptome expression profiles to reveal the mechanisms of salt tolerance and exogenous glycine betaine mitigation in maize seedlings. PLoS ONE 2020, 15, e0233616. [Google Scholar] [CrossRef]
- Hill, B.; Cassin, A.; Keeble-Gagnère, G.; Doblin, M.S.; Bacic, A.; Roessner, U. De novo transcriptome assembly and analysis of differentially expressed genes of two barley genotypes reveal root-zone-specific responses to salt exposure Camilla. Sci. Rep. 2016, 6, 31558–31572. [Google Scholar] [CrossRef] [Green Version]
- Cantalapiedra, C.P.; García-Pereira, M.J.; Gracia, M.P.; Igartua, E.; Casas, A.M.; Contreras-Moreira, B. Large differences in gene expression responses to drought and heat stress between elite barley cultivar scarlett and a spanish landrace. Front. Plant Sci. 2017, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Bedada, G.; Westerbergh, A.; Müller, T.; Galkin, E.; Bdolach, E.; Moshelion, M.; Fridman, E.; Schmid, K.J. Transcriptome sequencing of two wild barley (Hordeum spontaneum L.) ecotypes differentially adapted to drought stress reveals ecotype-specific transcripts. BMC Genom. 2014, 15, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübner, S.; Korol, A.B.; Schmid, K.J. RNA-Seq analysis identifies genes associated with differential reproductive success under drought-stress in accessions of wild barley Hordeum spontaneum. BMC Plant Biol. 2015, 15, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harb, A.; Simpson, C.; Guo, W.; Govindan, G.; Kakani, V.G.; Sunkar, R. The effect of drought on transcriptome and hormonal profiles in barley genotypes with contrasting drought tolerance. Front. Plant Sci. 2020, 11, 618491. [Google Scholar] [CrossRef] [PubMed]
- Imrul Mosaddek, A.; Huaxin, D.; Weite, Z.; Fangbin, C.; Guoping, Z.; Dongfa, S.; Feibo, W. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2012, 63, 49–60. [Google Scholar]
- Bornare, S.S.; Prasad, L.C.; Kumar, S. Comparative study of biochemical indicators of salinity tolerance of barley (Hordeum vulgare L.) with other crops: A review. Can. J. Plant Breed. 2013, 1, 97–102. [Google Scholar]
- Adem, G.D.; Roy, S.J.; Zhou, M.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014, 14, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.D.; Shabala, L.; Zhou, M.; Brodribb, T.; Corkrey, R.; Shabala, S. Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ. Exp. Bot. 2018, 153, 10–20. [Google Scholar] [CrossRef]
- Jabeen, Z.; Hussain, N.; Irshad, F.; Zeng, J.; Tahir, A.; Zhang, G. Physiological and antioxidant responses of cultivated and wild barley under salt stress. Plant Soil Environ. 2020, 66, 334–344. [Google Scholar] [CrossRef]
- Uçarlı, C.; Gürel, F. Diferential physiological and molecular responses of three-leaf stage barley (Hordeum vulgare L.) under salt stress within hours. Plant Biotechnol. Rep. 2020, 14, 89–97. [Google Scholar] [CrossRef]
- Ouertani, R.N.; Abid, G.; Karmous, C.; Ben Chikha, M.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, R.K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Li, M.; Huang, L.; Xu, J.; Zhang, F.; Cui, Y.; Fu, B.; Li, Z. Complex molecular mechanisms underlying seedling salt tolerance in rice revealed by comparative transcriptome and metabolomic profiling. J. Exp. Bot. 2016, 67, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lei, L.; Jinsheng, L.; Haiming, Z.; Weibin, S. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Ben Chikha, M.; Hessini, K.; Ourteni, R.N.; Ghorbel, A.; Zoghlami, N. Identification of barley landrace genotypes with contrasting salinity tolerance at vegetative growth stage. Plant Biotechnol. 2016, 33, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Negrao, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Weng, M.; Cui, L.; Liu, F.; Zhang, M.; Shan, L.; Yang, S.; Geng, X. Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- Nefissi Ouertani, R.; Jardak, R.; Ben Chikha, M.; Ben Yaala, W.; Abid, G.; Karmous, C.; Hamdi, Z.; Mejri, S.; Jansen, R.K.; Ghorbel, A. Genotype-specific patterns of physiological and antioxidative responses in barley under salinity stress. Cereal Res. Commun. 2022, 50, 1–13. [Google Scholar]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerancein durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Y.; Hettenhausen, C.; Lu, C.; Shen, G.; Zhang, C.; Li, J.; Song, J.; Lin, H.; Wu, J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, K.B.; Maldonado, J.; Biondi, S.; Silva, H. RNA-seq analysis of salt-stressed versus non salt-stressed transcriptomes of Chenopodium quinoa landrace R49. Genes 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osthoff, A.; Rose, P.D.D.; Baldauf, J.A.; Piepho, H.P.; Hochholdinger, F. Transcriptomic reprogramming of barley seminal roots by combined water deficit and salt stress. BMC Genom. 2019, 20, 325. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Shi, L.X.; Hall, M.; Funk, C.; Schröderab, W.P. Photosystem II, a growing complex: Updates on newly discovered components and low molecular mass proteins. Biochim. Biophys. Acta 2012, 1817, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Wang, H.; Yuan, Q.; Feng, Y. Structure and function of the photosystem supercomplexes. Front. Plant Sci. 2018, 9, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Choa, S.K.; Kima, J.E.; Parka, J.A.; Eomb, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [Green Version]
- Ksouri, N.; Jiménez, S.; Wells, C.E.; Contreras-Moreira, B.; Gogorcena, Y. Transcriptional responses in root and leaf of Prunus persica under drought stress using RNA sequencing. Front. Plant Sci. 2016, 7, 1715–1734. [Google Scholar] [CrossRef]
- Czolpinska, M.; Rurek, M. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant Sci. 2018, 9, 302. [Google Scholar] [CrossRef]
- Olmos, E.; Garcia De La Garma, J.; Gomez-Jimenez, M.C.; Fernandez-Garcia, N. Arabinogalactan proteins are involved in salt-adaptation and vesicle trafficking in tobacco by-2 cell cultures. Front. Plant Sci. 2017, 8, 1092. [Google Scholar] [CrossRef] [Green Version]
- Takabatake, R.; Seo, S.; Ito, N.; Gotoh, Y.; Mitsuhara, I.; Ohashi, Y. Involvement of wound-induced receptor-like protein kinase in wound signal transduction in tobacco plants. Plant J. 2006, 47, 249–257. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 16, 5–470. [Google Scholar] [CrossRef] [Green Version]
- Deinlein, U.; Aaron, B.S.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Qamar, A.; Mysore, K.S.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Ahmad, D.; Khan, M.A. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron. J. Biotechnol. 2015, 18, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Nomura, M.; Mori, H.; Jagendorf, A.T.; Ueda, A.; Takabe, T. An isozyme of betaine aldehyde dehydrogenase in barley. Plant Cell Physiol. 2001, 42, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Chai, H.; Yin, H.; Yang, M.; Hu, G.; Guo, M.; Yi, R.; Zhang, P. Full-length transcriptome sequencing reveals the low-temperature-tolerance mechanism of Medicago falcata roots. BMC Plant Biol. 2019, 19, 575. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Liu, D.; Liu, S. Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2007, 26, 1909–1917. [Google Scholar] [CrossRef]
- Sharma, R.; Sahoo, A.; Devendran, R.; Jain, M. Over-expression of a rice tau class glutathione S-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS ONE 2014, 9, e92900. [Google Scholar] [CrossRef] [Green Version]
- Blanvillain, R.; Kim, J.H.; Wu, S.; Lima, A.; Ow, D.W. OXIDATIVE STRESS 3 is a chromatin-associated factor involved in tolerance to heavy metals and oxidative stress. Plant J. 2009, 57, 654–665. [Google Scholar] [CrossRef]
- Fritsche, S.; Wang, X.; Jung, C. Recent advances in our understanding of tocopherol biosynthesis in plants: An overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketehouli, T.; Carther, K.F.I.; Noman, M.; Wang, F.W.; Li, X.W.; Li, H.Y. Adaptation of Plants to Salt Stress: Characterization of Na+ and K+ Transporters and Role of CBL Gene Family in Regulating Salt Stress Response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, H.T.; Guo, P.; Xia, X.L.; Yin, W.L. PdERECTA, a leucine-rich repeat receptor-like kinase of poplar, confers enhanced water use efficiency in Arabidopsis. Planta 2011, 234, 229–241. [Google Scholar] [CrossRef]
- Nan, H.; Li, W.; Lin, Y.; Gao, L. Genome-wide analysis of WRKY genes and their response to salt stress in the wild progenitor of Asian cultivated rice, Oryza rufipogon. Front. Genet. 2020, 11, 359. [Google Scholar] [CrossRef]
- Fan, X.; Guo, Q.; Xu, P.; Gong, Y.; Shu, H.; Yang, Y.; Ni, W.; Zhang, X.; Shen, X. Transcriptome-wide identification of salt-responsive members of the WRKY gene family in Gossypium aridum. PLoS ONE 2015, 10, e0126148. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Xu, H.; Dai, Y.; Deng, D.; Chen, J. ZmWRKY33, a WRKY maize transcription factor conferring enhanced salt stress tolerances in Arabidopsis. Plant Growth Regul. 2013, 70, 207–216. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Xu, Y.; Ma, Q.; Xu, W.; Wang, T.; Xue, Y.; Chong, K. Overexpression of an R1R2R3 MYB gene OsMYB3R-2, increases tolerance to freezing, drought, salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- An, J.P.; Li, H.H.; Song, L.Q.; Su, L.; Liu, X.; You, C.X.; Wang, X.F.; Hao, Y.J. The molecular cloning and functional characterization of MdMYC2, a bHLH transcription factor in apple. Plant Physiol. Biochem. 2016, 108, 24–31. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q.A. Grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Outchkourov, N.S.; Carollo, C.A.; Gomez-Roldan, V.; de Vos, R.C.; Bosch, D.; Hall, R.D.; Beekwilder, J. Control of anthocyanin and non-flavonoid compounds by anthocyanin-regulating MYB and bHLH transcription factors in Nicotiana benthamiana leaves. Front. Plant Sci. 2014, 5, 519. [Google Scholar] [CrossRef] [Green Version]
- Debernardi, J.M.; Mecchia, M.A.; Vercruyssen, L.; Smaczniak, C.; Kaufmann, K.; Inze, D.; Rodriguez, R.E.; Palatnik, J.F. Post-transcriptional control of GRF transcription factors by microRNA miR396 and GIF co-activator affects leaf size and longevity. Plant J. 2014, 79, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sircar, S.; Ramirez-Prado, J.S.; Manza-Mianza, D.; Antunez-Sanchez, J.; Brik-Chaouche, R.; Rodriguez-Granados, N.Y.; An, J.; Bergounioux, C.; Mahfouz, M.M.; et al. Polycomb-dependent differential chromatin compartmentalization determines gene coregulation in Arabidopsis. Genome Res. 2021, 31, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Langfelder, P.; Fuller, T.; Dong, J.; Li, A.; Hovarth, S. Weighted gene coexpression network analysis: State of the art. J. Biopharm. Stat. 2010, 20, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.G.; Baumgartner, J.T.; Xie, X.; Jew, K.M.; Basisty, N.; Schilling, B.; Kuhn, M.L.; Wolfe, A.J. Mechanisms, detection, and relevance of protein acetylation in prokaryotes. mBio 2019, 10, e02708-18. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Hammami, Z.; Sbei, H.; Kadri, K.; Jemel, Z.; Sahli, A.; Fraj, M.B.; Nasr, H.; Teixeira da Silva, J.A.; Trifa, Y. Evaluation of performance of different barley genotypes irrigated with saline water in South Tunisian Saharan conditions. Environ. Exp. Biol. 2016, 14, 15–21. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Nefissi Ouertani, R.; Arasappan, D.; Abid, G.; Ben Chikha, M.; Jardak, R.; Mahmoudi, H.; Mejri, S.; Ghorbel, A.; Ruhlman, T.A.; Jansen, R.K. Transcriptomic analysis of salt-stress-responsive genes in barley roots and leaves. Int. J. Mol. Sci. 2021, 22, 8155. [Google Scholar] [CrossRef]
- Ben Romdhane, M.; Riahi, L.; Selmi, A.; Jardak, R.; Bouajila, A.; Ghorbel, A.; Zoghlami, N. Low genetic differentiation and evidence of gene flow among barley landrace populations in Tunisia. Crop Sci. 2017, 57, 1585–1593. [Google Scholar] [CrossRef]
- Sade, N.; Galkin, E.; Moshelion, M. Measuring Arabidopsis, tomato and barley leaf relative water content (RWC). Bio-Protocol 2015, 5, 1451. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Rubio, M.C.; Gonzalez, E.M.; Minchin, F.R.; Webb, K.J.; Arrese-Igor, C.; Ramos, J.; Becana, M. Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiol. Plant 2002, 115, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principal of protein–dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Del Longo, O.T.; Gonzalez, C.A.; Pastori, G.M.; Trippi, V.S. Antioxidant defenses under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol. 1993, 34, 1023–1028. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and highlight intensity enhance activities of superoxide dismutase ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Tatiana, Z.; Yamashita, K.; Matsumoto, H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol. 1999, 40, 273–280. [Google Scholar]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, 115–127. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).