Abstract
Layered two-dimensional transition metal dichalcogenides and their heterostructures are of current interest, owing to the diversity of their applications in many areas of materials nanoscience and technologies. With this in mind, we have examined the three molecular dimers of the tungsten dichalcogenide series, (WCh2)2 (Ch = S, Se, Te), using density functional theory to provide insight into which interactions, and their specific characteristics, are responsible for the interfacial/interlayer region in the room temperature 2H phase of WCh2 crystals. Our calculations at various levels of theory suggested that the Te···Te chalcogen bonding in (WTe2)2 is weak, whereas the Se···Se and S···S bonding interactions in (WSe2)2 and (WS2)2, respectively, are of the van der Waals type. The presence and character of Ch···Ch chalcogen bonding interactions in the dimers of (WCh2)2 are examined with a number of theoretical approaches and discussed, including charge-density-based approaches, such as the quantum theory of atoms in molecules, interaction region indicator, independent gradient model, and reduced density gradient non-covalent index approaches. The charge-density-based topological features are shown to be concordant with the results that originate from the extrema of potential on the electrostatic surfaces of WCh2 monomers. A natural bond orbital analysis has enabled us to suggest a number of weak hyperconjugative charge transfer interactions between the interacting monomers that are responsible for the geometry of the (WCh2)2 dimers at equilibrium. In addition to other features, we demonstrate that there is no so-called van der Waals gap between the monolayers in two-dimensional layered transition metal tungsten dichalcogenides, which are gapless, and that the (WCh2)2 dimers may be prototypes for a basic understanding of the physical chemistry of the chemical bonding environments associated with the local interfacial/interlayer regions in layered 2H-WCh2 nanoscale systems.
1. Introduction
Group VI transition metal dichalcogenides (TMDs or TMDCs) are a class of layered materials discovered during the last century [1,2,3,4]. Due to the fact that they find application in a wide range of technological areas, they have been the focus of intense research more recently (for example, [5,6,7,8,9,10]). Stable TMDCs (approximately 60 in number) have the generic formula AB2, [11,12], where A is a transition metal and B is chalcogen (S, Se, Te). These materials are sometimes described with formulae such as ME2, MX2, and MCh2 (M = transition metal and X, Ch, E = chalcogen derivative) [3,13,14,15], among others. TMDCs that have been particularly intensively studied are the dichalcogenides of W and Mo [3,16,17].
In layered AB2 structures, each B–A–B monolayer comprises three atomic layers, with the transition metal (A) layer sandwiched between two chalcogen layers (Figure 1). The gap between two monolayers is referred to as the van der Waals gap [18,19], an interlayer gap within which the metal-bonded chalcogenides of the two monolayers interact non-covalently to hold the structure together [15,20,21,22]. The weak interactions between the layers allow for their mechanical exfoliation [18]. Simply referring to the interaction between the monolayers as being due to weak van der Waals forces may be simplistic and overlooks the actual nature of the interaction between the dichalcogenides of the neighboring monolayers.
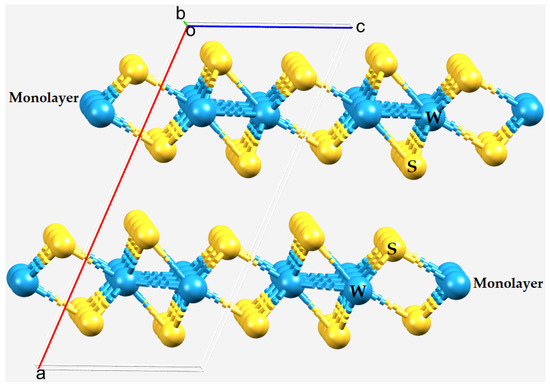
Figure 1.
The bilayer structure of 2H-WS2 (651384–ICSD [2]) viewed with the b-axis (slightly offset) perpendicular to the page. Atom type is shown.
Interest in group VI TMDCs stems from their potential to display marked anisotropic physical properties, including intercalation akin to other layered materials, such as graphene and silicate clay minerals, and an indirect-to-direct bandgap crossover when the monolayer is exposed to bright photoluminescence at room temperature [15,23], as well as for the valley degree of freedom associated with the non-centrosymmetric semiconducting phase [24]. TMDC monolayers behave differently to bulk materials that feature an indirect bandgap. For instance, the monolayer and bulk forms of MoS2 display a direct and an indirect bandgap of 1.80 and 1.29 eV, respectively [25]; both the character and the magnitude of the bandgap changes significantly when passing from the monolayer to the bulk form.
As the number of layers decreases, the material exhibits different optical characteristics. In particular, MoSe2 has a crossover from an indirect bandgap of 1.1 eV to a direct bandgap of 1.55 eV [26] on passing from a bilayer to a monolayer. Similarly, WS2 shows a crossover from an indirect bandgap of 1.4 eV to a direct bandgap transition of 2.1 eV [27]. Clearly, these are superior materials compared to metallic graphene [28], insofar as they exist in different phases depending on the temperature and pressure and feature some material and optical properties required for technological applications [3,29,30,31]. The most commonly observed crystalline phases of transition metal dichalcogenides are the 2H, 1T, 3R, and Td phases, featuring, respectively, a hexagonal, trigonal, rhombohedral, and distorted octahedral symmetry of the crystal lattice [5,15,20,32].
Although several experimental and theoretical studies have been reported on AB2 systems in the solid state (i.e., on the bulk crystals, as well as on the layered WCh2 systems) [33,34,35,36], the basic physical chemistry of these systems in the gas phase is not yet well understood [37]. In the calculations of these systems in the solid-state, periodic boundary conditions are often invoked, and the properties reported are often limited to the geometry, lattice constants, band structure, density of states spectra, electron–hole recombination effect, exciton binding, and an analysis of stability; for example, [5,7,22,38,39]. Another focus was the determination of the nature of the dependence of the band dispersion on the mono-, bi-, and multi-layered WCh2 systems [35,36,40]. These are important properties of a solid-state material, and they assist one in predicting whether the material is suitable for synthesis, whether it is environmentally stable (in air, water, light, and atmospheric ambient temperature and pressure, etc.), and for applications in various areas of optoelectronics.
The importance of transition metal dichalcogenide materials, and what appears to be an incomplete understanding of the nature of the chemical bonding in the interfacial region between the monolayers of the metal dichalcogenide bilayer and multi-layered systems, prompted this study. As in our recent report on MoCh2 systems [41], we focus here only on the dimers of the tungsten dichalcogenide series. We employ density functional theory (DFT) at the M06-2X level [42], with and without accounting for the effects of dispersion, in conjunction with three basis sets (LANL08, def2-TZVPPD, and Aug-cc-pVTZ(-PP) [43]).
The geometric, electronic, orbital, energetic, and charge-density based bond critical point and isosurface topologies of bonding interactions responsible for the formation of the three dimers, WCh2 (Ch = S, Se, Te), are discussed. (We use the term WCh2 rather than AB2 or ME2, in line with the IUPAC definition of chalcogen bonding (ChB) [44].) The orbital features are examined using natural bond orbital (NBO) analysis [45,46], whereas the charge-density-based features are examined using the quantum theory of atoms in molecules (QTAIM) [47], interaction region indicator (IRI) [48], independent gradient model (IGM) [49,50], and reduced density gradient (RDG) [51] approaches. We provide our views on whether or not the molecular electrostatic surface potential (MESP) method, often utilized to determine the active and inactive regions on molecular surfaces [52,53,54], is suitable for providing insight into the chemistry responsible for the attraction between Ch atoms of the isolated tungsten dichalcogenide monomer molecules leading to the formation of the dimers of tungsten dichalcogenides.
The intermolecular geometries that emerge from the gas phase calculations on (WCh2)2, and those known for the crystalline phase, are compared to address the titled concern: whether or not the chalcogen-centered non-covalent bonding interactions in (WCh2)2 dimers can serve as prototypes for a fundamental understanding of the local interfacial/interlayer chemical bonding environment in two-dimensional, layered tungsten dichalcogenides.
2. Results and Discussion
2.1. The Nature of the Interlayer/Interfacial Bonding Interactions in WCh2 Crystals
The inter-layer contacts (dotted lines in cyan) between the tungsten bonded Ch sites in the WS2, WSe2, and WTe2 crystals of the 2H phase, in which the 4 × 1 × 1 supercells were used, are shown in Figure 2. The contacts are illustrated within the framework of the respective unit cells.
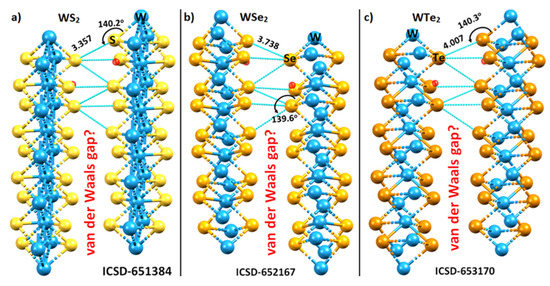
Figure 2.
The nature of the interfacial (or interlayer) Ch···Ch bonding interactions between the metal bonded Ch (Ch = S, Se, Te) sites of the two monolayers in the 2H-WCh2 crystals: (a) WS2; (b) WSe2; and (c) WTe2. Selected Ch···Ch bond distances (Å) and ∠W–Ch···Ch bond angles (degrees) are shown. All Ch···Ch interlayer distances are equivalent for a given WCh2 crystal. The ICSD reference code is shown for each case. The so-called van der Waals gap between the chalcogenide layers is depicted in each occasion. Crystallographic axes are not shown.
The S···S, Se···Se, and Te···Te inter-layer distances in the respective systems are either less than (for the former two) or slightly greater than twice the van der Waals radius of the respective chalcogen atom. For instance, the interlayer bond distances of 3.357 and 3.738 Å in WS2 and WSe2 are less than twice the vdW radius, 3.60 and 3.80 Å, of the S and Se atoms, respectively, whereas that of 4.007 Å in WTe2 is slightly longer than twice the vdW radius of Te, 4.00 Å (Bondi’s radius used: rvdW (S) = 1.80 Å; rvdW (S) = 1.90 Å; rvdW (S) = 2.00 Å) [55]). From these distances alone, and since the bond distance is a measure of the bond strength, one might conclude that the Te···Te non-covalent interactions in WTe2 are weaker than the S···S and Se···Se bonds in WS2 and WSe2, respectively, and are of van der Waals type. Whether or not this immediate conclusion is misleading can only be verified once the energy of these interactions is calculated. We discuss this in a following section that summarizes the binding energy of the WCh2 dimers.
The Ch···Ch non-covalent links between the monolayers in the WCh2 crystals are significantly bent, with ∠W–Ch···Ch = 140.2°, 139.6°, and 140.3° when Ch = S, Se, and Te, respectively. The non-linearity in these interactions could be a consequence of a relatively less negative Ch site in one monolayer interacting attractively with a relatively more negative junction region, delocalized over the triangular face formed by the three Ch sites, on the neighboring layer. This becomes evident if one examines the distance between the Ch site and the centroid of the triangular Ch···Ch···Ch face in the second monolayer. This distance is 2.804, 3.110, and 3.463 Å in WS2, WSe2, and WTe2, respectively (Figure 3), which is much smaller than the Ch···Ch inter-layer distances of the corresponding systems. Although these interactions are not linear and do not show up exactly along the W–Ch bond extensions, they could still be regarded as Ch···Ch bonded interactions. The validity of this view is put forward in the following section, where the nature of the electrostatic potential on the WCh2 surfaces is discussed.
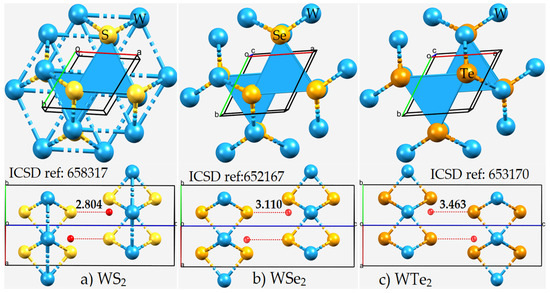
Figure 3.
The top and side views of the unit-cells of (a) WS2, (b) WSe2, and (c) WTe2 in the 2H-phase, consisting of hexagonal triatomic layers, Ch–W–Ch, with a plane of metal atoms bound to two planes of chalcogen atoms (see Figure 1). The Inorganic Crystal Structure Database (ICSD) references to the structures are given. The distance between the W-bonded Ch site in an WCh6 unit in a monolayer and the centroid of the triangular face formed by three nearest Ch sites in the same unit in the neighboring layer (tiny red sphere) is shown in Å. Each structure is crystallized in the space group P63/mmc.
The question that arises then is where is the van der Waals gap that is often referred to as existing between the monolayers in a bilayer 2H system of the WCh2 crystals? Does this purported van der Waals gap actually exist? The simple answer is that there is no such van der Waals gap between the monolayers in the 2H phase of the WCh2 crystal. The Ch atoms, bonded to W in the two W–Ch2 monolayers forming the bilayer 2H system, overlap with each other in the interface region. This means there is already a penetration between the Ch atoms in the interlayer region (vide infra). This overlapping is readily appreciated when one relates the Ch···Ch interlayer bond distance with the sum of the van der Waals radii of interacting Ch atomic domains. The Bondi’s van der Waals radii of S, Se, and Te are 1.80, 1.90, and 2.0 Å, respectively [55]. Therefore, twice of each of these radii gives values of 3.60, 3.80, and 4.0 Å, which are slightly smaller or comparable to the interlayer distances of 3.357, 3.738, and 4.007 Å observed in the WS2, WSe2, and WTe2 crystals, respectively. Since the van der Waals radii for all atomic domains of the periodic table are associated with an error of approximately 0.2 Å [56], it seems apparent that there is penetration between the Ch atoms in the interfacial/interlayer region of the WCh2 systems.
In the case of WTe2, the interlayer Ch···Ch bond distance in the crystal geometry is 4.007 Å and is within the radii error limit of twice the van der Waals radius of Te. This suggests that there is no van der Waals gap between the layers in either of the three crystal systems, as the Ch sites of the two interacting monolayers are bonded with each other through weak attractive forces (vide infra). That there is no physical gap (only voids) between the monolayers of WCh2 is also evident from the space-filling model shown in Figure 4, in which, the S atoms of each of the two monolayers are not only just facing each other, but “kissing” [57,58,59]. Further evidence of mutual penetration between the bonded atomic basins that emerges from a QTAIM analysis is discussed below.
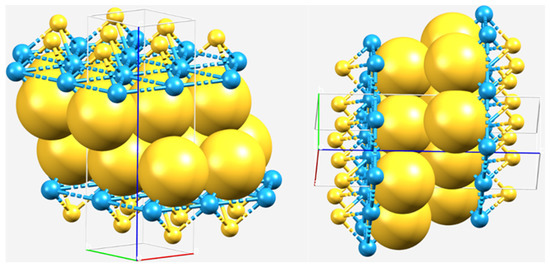
Figure 4.
Illustration of combined ball-and-stick and space-filling views of the gapless WS2 crystal system in the 2H-phase. The tungsten-bonded S atoms (large yellow spheres) belonging to two different monolayers are “kissing” each other in the interface region, thus making the interfacial/interlayer region gapless. This may be likened to the concept of “kissing spheres” in coordination chemistry [57,58,59].
2.2. The Nature of the Potential on the Electrostatic Surfaces of Isolated WCh2 Monomers
The MESP model [60] has been widely used to provide insight into the nucleophilic and electrophilic regions on the electrostatic surface of molecular entities [61,62,63,64,65,66]. Two key local descriptors of this model are VS,min and VS,max, the local most minimum and local most maximum of potential, respectively. Each can be positive or negative depending on the nature of the electron density depletion or accumulation at specific regions on the molecular surface [52,67,68]. When they are positive, the region is electrophilic, and when negative, it is nucleophilic [54,69,70]. These can show up on the same atom in a molecular entity; hence, the charge density on an atom in molecules may be anisotropic. Specifically, the local most maximum of potential, VS,max, on the surface of a bonded atom is commonly observed on the outer extension of a covalent bond, and the local most minimum of potential, VS,min, is commonly observed in a region dominated by π-density and lone-pair electron density [52,69] (i.e., around a covalently bonded atom in a molecule).
We used the 0.001 a.u. isodensity envelope, on which, we computed the potential on the electrostatic surfaces of the three WCh2 isolated monomers, the MESP graphs of which are shown in Figure 5. It is immediately evident that the surface of each isolated monomer comprises positive and negative regions; the charge density is indeed anisotropic in these species.
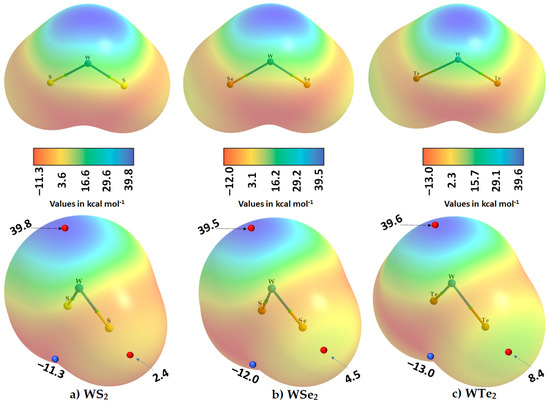
Figure 5.
Two different views (top and bottom) of the 0.001 a.u. isodensity envelope mapped potential on the electrostatic surface of the isolated WCh2 monomers: (a) WS2, (b) WSe2, and (c) WTe2, obtained with M06-2X/Aug-cc-pVTZ(-PP). The tiny red and blue spheres represent the local most maximum and minimum of potentials (VS,max and VS,min), respectively. Superimposed in each case is the QTAIM molecular graph.
The most prominent negative region shows up on the surface of the junction region between the two Ch atoms in WCh2. The largest of these is found on the surface of the WTe2 molecule, with VS,min = −13.0 kcal mol−1 (Figure 5c).
The outer portions of W, opposite to the Ch–W bond axes, are very much positive (deep blue regions), which is not unexpected given that the metal center has empty, electron-deficient dπ orbitals. The principal reason for the emergence of the blue region on the surface of W is that the chalcogenides pull the electron density towards the bonding region from the tungsten atom, thus leaving behind a strongly positive region on the surface of the metal at equilibrium (a σd hole). The strength of the σd hole on W is very similar in the three monomers: 39.8 kcal mol−1 in WS2, 39.5 kcal mol−1 in WSe2 and 39.6 kcal mol−1 in WTe2, which explains why W accepts multiple chalcogen-centered bonds in the WCh2 crystals (see Figure 2 and Figure 3). This also explains why the region on the surface between the W and two Ch atoms is calculated to be the most negative (shown in red in Figure 5).
By contrast, the axial portions of the Ch atoms bonded to tungsten are relatively more positive than the lateral portions that appear, firstly, along, and, secondly, around the W–Ch bond extensions. The first are nothing other than p-type σ-holes (σp); they decrease in magnitude in the order WTe2 (8.4 kcal mol−1) > WSe2 (4.5 kcal mol−1) > WS2 (2.4 kcal mol−1), a trend that is consistent with the calculated binding energy of the WCh2 dimers (vide infra).
We did not observe a VS,min on the lateral sites of the bonded Ch atoms in WCh2. This does not mean that they are not present, or neutral; they would be observable if a higher isodensity envelope, for example 0.002 a.u., can be used for computing the electrostatic potential. For instance, a calculation with this isodensity envelope resulted in a VS,min of −2.1 kcal mol−1 for the lateral portion of S in WS2. Clearly, the Ch···Ch bonding features observed in the crystal, shown in Figure 2, are the result of attraction between sites of dissimilar charge densities localized on the lateral and axial sites of the bonded Ch atomic basins; this is nothing other than the attraction between a Lewis acid and a Lewis base site. While the Ch···Ch bonds are significantly bent, we still characterize them as chalcogen bonds that follow a Type I topology. (A Type I bonding topology is generally observed when the angle of interaction ∠D–Ch···A lies between 90° and 140°, where D is the donor fragment bonded with Ch, and A is the acceptor site [54]).
2.3. The Topologies of the Electron Localization and the Local Orbital Locator
We carried out an analysis of the electron localization function (ELF) [71] and the local orbital locator (LOL) [72,73], which are built using the kinetic energy density [74,75,76], since the nature of the electron localization and delocalization in WCh2 is one of the main aims of this study. This is necessary to gain insight into the nature of bonding and non-bonding pairs in these systems, as has been carried out elsewhere for other systems [77,78,79]. As Jacobsen summarized in his study [80] that followed on from the work of Schmider and Becke [72,73], a covalent bond is expressed in LOL as a local maximum between the bound centers in which the LOL = ½ surface enclosing the maximum has an increasing concave shape in multiple bonds. The LOL attains large values (above ½) in regions where the electron density is dominated by a single localized orbital. Lone-pairs are resolved as local maxima in the center of a characteristically shaped LOL = ½ surface. We invoked the LOL analysis to see whether it can adequately detect the localized pairs of electrons, bonding or non-bonding, that are not only evident in the topologies of ELF but also in the L (the negative of the Laplacian of the charge density, L = −∇2ρ) of QTAIM, since the latter is homeomorphic to the former, with few exceptions. While the LOL analysis encloses a large number of critical points, we restricted ourselves to the (3,−3) attractors in the gradient vector field of LOL. As Jacobsen noted previously [80], the critical point concept was borrowed from QTAIM, which refers to each nucleus as a (3,−3) attractor [47]. LOL also features such attractors in the bonding region, and in molecular regions that encloses lone-pairs [80].
Our calculations indicate that each W–S bond in WS2 is characterized by one (3,−3) attractor, which represents locally maximal electron localization, for which, the ELF and LOL values corresponding to this attractor are 0.766 and 0.626, respectively. In addition, each Ch atom of the monomer features a single attractor expected of the valence lone-pair, with ELF and LOL values of 0.889 and 0.621, respectively, in the case of WS2. The tungsten center in the monomers is surrounded with a quintet of lone-pair attractors; two of them are along the outer extension of the two Ch–W axes (LOL and ELF values for each are 0.890 and 0.737, respectively). For WSe2, the ELF (and LOL) values are 0.688 (0.581), 0.811 (0.494), and 0.888 (0.735) for the (3,−3) attractors responsible for the W–Se bonds, Se’s lone-pair, and W’s lone-pair along the Se–W bond extensions, respectively. For WTe2, these values are 0.645 (0.556), 0.748 (0.441), and 0.887 (0.734), respectively. Other than the valence shell lone-pair attractor around the Ch atom noted above, there are two other lone-pair attractors located in close vicinity to each Ch atom in WCh2 molecules. This suggests that the ELF and LOL models are superior to the MESP model in identifying the detailed topology of bonding and non-bonding pairs in this type of molecule.
While the W–Ch bonds in WCh2 have a multiple bond character (vide infra), the LOL picture still shows one attractor for each bond in the monomer. This mirrors the results in the study of Jacobsen, who found a single attractor for both C–C and C=C bonds, with a larger LOL for the former of 0.822 compared to 0.776; a value of 0.721 was found for the triple bond in N2 [80]. When acetylene was included in that study, the trend was maintained, and the LOL for C≡C was lower than that calculated for C–C and C=C. The increase in bond multiplicity led to an increase in the delocalized bonding character, and a reduction in the value of LOL. The trend in the ELF values for these bonds are very similar to that of LOL; ELF values of 0.9685 (for C–C single bond in C2H6), 0.9412 (for C=C double bond in C2H4), and 0.8906 (for C≡C triple bond in C2H2) have been reported [80,81]. This trend in the decrease in LOL and ELF with an increase in bond order is similar to what we observed for the monomers of the WCh2 series, where the LOL between the bonded atomic basins systematically decreased as one passes from WS2 through WSe2 to WTe2.
The 2D maps of the ELF and LOL functions for the molecular plane defined by the two Ch atoms and one W atom for the three WCh2 monomers are shown in Figure 6. Based on its definition, the ELF should range between 0 and 1 [71,79]. ELF = 1 corresponds to perfect localization and ELF = ½ corresponds to an electron gas. An ELF value close to 1 means that electrons are localized, implying a covalent bond, a lone pair, or inner shells of the atom involved. The value range of LOL is identical to ELF, but in the color bar in Figure 6 (bottom), the range is limited to between 0 and 0.8, corresponding to bonding and non-bonding attractor profiles. As can be seen from the 2D maps of ELF, the core regions of each atomic domain in each of the three monomers feature circular localization domains and have high ELF and LOL values close to 0.9. No strong localization is evident in the bonding regions between W and Ch in the three monomers, consistent with the multiple character of the W–Ch bonds.
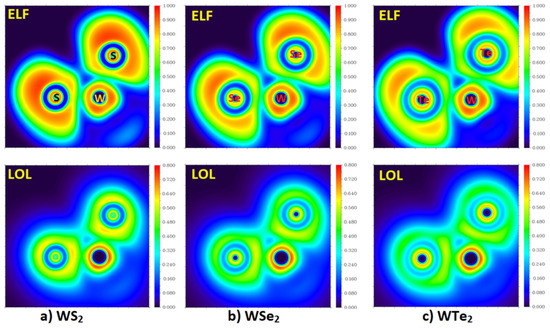
Figure 6.
M06-2X/Aug-cc-pVTZ(-PP) level 2D maps of ELF and LOL functions (Top and Bottom, respectively) obtained on the plane defined by the two Ch atoms and one W atom in isolated WCh2 (Ch = S, Se, Te) monomers: (a) WS2; (b) WSe2; and (c) WTe2.
2.4. Intermolecular Properties of (WCh2)2 Dimers, and Comparison with the WCh2 Crystals
The geometrically relaxed (WCh2)2 (Ch = S, Se, Te) dimers obtained with M06-2X/aug-cc-pVTZ(-PP) are shown in Figure 7. Table 1 summarizes the geometric, dipolar moment, and energetic properties of the three dimers, obtained using three different basis sets, including LANL08, def2-TZVPPD, and aug-cc-pVTZ(-PP). In addition, the corresponding results of M06-2X-D3/LANL08 are included, in which Grimme’s dispersion with the original D3 damping function was invoked to see the effect of dispersion on the properties of the dimers. As can be seen from Table 1, there are no remarkable changes in any of the properties when the effect of dispersion was incorporated with M06-2X. The comparison between M06-2X and M06-2X-D3 shows that, for the (WCh2)2 (Ch = S, Se) dimers, the Ch···Ch bond distances increased marginally by 0.003 Å, whereas, for (WTe2)2, the Te···Te bond distance decreased by 0.001 Å. The ∠W1–Ch2···Ch6 and ∠W4–Ch6···Ch2 angles increased by 0.2° for the first two dimers and remained unchanged for the latter. The ∆E and ∆E(BSSE) both increased by 0.1 kcal mol−1, yet no change was noticeable in the dipole moments of all three dimers. This indicates that the effect of dispersion on the dimer properties is very marginal when it is incorporated with the M06-2X functional.
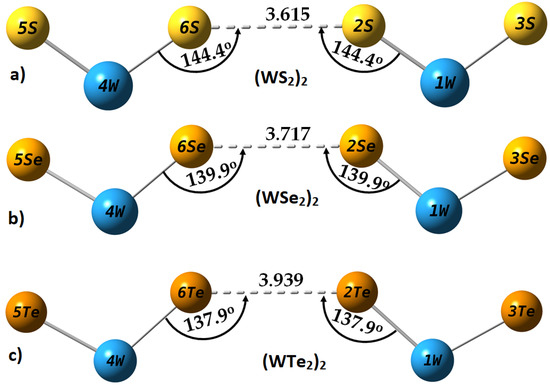
Figure 7.
M06-2X/Aug-cc-pVTZ(-PP) computed fully relaxed geometries of (WCh2)2 (Ch = S, Se, Te) dimers, showing a Type I topology of non-linear Ch···Ch bonding links between the interacting monomers: (a) (WS2)2; (b) (WSe2)2; and (c) (WTe2)2. Selected bond lengths and bond angles are in Å and degrees, respectively. Atomic numbering and labeling are shown in each case.

Table 1.
M06-2X computed geometric, electronic, and energetic properties of (WCh2)2 (Ch = S, Se, Te) dimers, obtained with three different basis sets a,b.
Among the three basis sets tested, the best Ch···Ch intermolecular bond distances (when compared to the values obtained in the crystalline state, Figure 2) were obtained with the LANL08 functional. The def2-TZVPPD and Aug-cc-pVTZ(-PP) basis sets underestimate them to a small extent. However, the angles of the interaction, ∠W1–Ch2···Ch6 and ∠W4–Ch6···Ch2, are better reproduced by def2-TZVPPD and Aug-cc-pVTZ(-PP), and they are underestimated by LANL08. This is not surprising, since angles are more sensitive to the presence or absence of a d-polarization function in the basis set. We conclude that the Aug-cc-pVTZ(-PP) basis set performs best in obtaining reasonable estimates of r(Ch···Ch) and ∠W-Ch···Ch for all three (WCh2)2 dimers.
An inspection of the ∆E and ∆E(BSSE) values in Table 1 shows that the average BSSE energy is largest for the LANL08 basis set, and smallest with Aug-cc-pVTZ(-PP). This is why the ∆E(BSSE) is markedly smaller than ∆E for each of the three dimers with M06-2X/LANL08 and M06-2X-D3/LANL08. Nevertheless, the ∆E(BSSE) values obtained with the highest basis set applied suggest that the Ch···Ch interactions in the three (WCh2)2 dimers are of van der Waals type, since interaction energies of van der Waals complexes are generally close to, or below, −1.0 kcal mol−1, and are smaller than weak interactions in weakly bound complexes (interaction energies between −1.0 and −4.0 kcal mol−1) [82,83,84].
To determine the validity of this conclusion, we computed the binding energies of all three dimers using MP2(full), B97-D3(BJ), PW6B95-D3(BJ), and B3LYP-D3(BJ) using the M06-2X/Aug-cc-pVTZ(-PP) computed equilibrium geometries. The results are compared with Table 2.

Table 2.
Computed uncorrected and BSSE-corrected binding energies (∆E and ∆E(BSSE), respectively) of the (WCh2)2 (Ch = S, Se, Te) dimers, obtained with several methods in conjunction with the Aug-cc-pVTZ(-PP) basis set a.
The values of ∆E and ∆E(BSSE) obtained with all of these four methods are larger than those computed with M06-2X and M06-2X-D3, suggesting that dispersion indeed plays an important role in elevating the complexation energies of the three dimers. The BSSE in energy is calculated to be very large with the post-Hartree–Fock method, MP2(full), and the trend in ∆E and ∆E(BSSE) across the dimer series is not systematic. That is not the case with all the DFT functionals used, which give a systematic increase in the bonding energy when passing from (WS2)2 to (WSe2)2 to (WTe2)2. This is also observed for ∆E(BSSE) as expected, given that Te is significantly more polarizable and less electronegative than Se, which, in turn, is more polarizable and less electronegative than S.
With the exception of M06-2X and M06-2X-D3, all other computational methods employed give ∆E(BSSE) close to, or larger than, −2.0 kcal mol−1 for (WTe2)2, showing that this dimer is not a van der Waals system. That may also be the case with (WSe2)2, since ∆E(BSSE) gives the predicted values of −1.16, −1.48, and −1.73 kcal mol−1 with PW6B95-D3(BJ), B97-D3(BJ), and B3LYP-D3(BJ), respectively. (MP2(full) predicts a spurious ∆E(BSSE) of −3.66 kcal mol−1, larger than that of (WTe2)2). By contrast, and despite the S···S intermolecular distance in (WS2)2 being significantly smaller than the Se···Se and Te···Te in (WSe2)2 and (WTe2)2, respectively (see Table 1 and Figure 2 for bond distances), the conclusion remains that (WS2)2 is a van der Waals system.
2.5. Charge Density Topological Properties of (WCh2)2 Dimers
The QTAIM description of bonding interactions present in each (WCh2)2 dimer can be rationalized from the molecular graphs shown in Figure 8. As expected, they all show bond paths (line in atom color) and bond critical points (bcp, tiny spheres in green) between bonded atomic basins, indicative of chemical bonding between them [85]. QTAIM’s bond path descriptors do not just identify atom–atom links between molecular domains [86], but also allow for the recognition of various other interactions [87,88]; for example, between delocalized bonds in one molecular entity and the positive or negative site in another entity, as observed, for example, in Ti bonding to hydrocarbons [89].
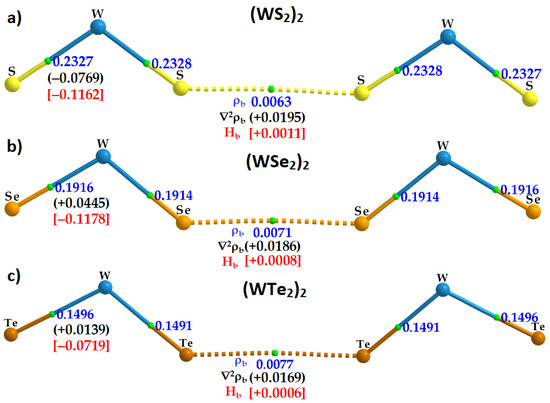
Figure 8.
M06-2X/Aug-cc-pVTZ(-PP) computed QTAIM-based molecular graphs, presenting a description of intra- and inter-molecular interactions in WCH2 dimers: (a) (WS2)2; (b) (WSe2)2; and (c) (WTe2)2. Shown are the charge density (ρb/a.u.; numbers in blue), the Laplacian of the charge density (∇2ρb/a.u.; numbers in black), and the total energy density (Hb/a.u.; numbers in red) at the Ch···Ch bond critical points. The former property is also depicted at the W–Ch bond critical points. Bond paths and bond critical points are shown as lines in atom color and tiny spheres in green between bonded atomic basins, respectively. Large spheres represent atomic domains for each dimer.
The charge density ρb at the Ch···Ch bcp is smallest, 0.0063 a.u., for (WS2)2 and largest, 0.0077 a.u., for (WTe2)2. Interestingly, the trend in the ρb values is concordant with the BSSE-corrected binding energies calculated for the three (WCh2)2 dimers (see Table 1 and Table 2 for ∆E(BSSE)), suggesting that the charge density at the Ch···Ch bcp is indeed a measure of its bond strength.
Obviously, ρb at a Ch···Ch bcp is significantly smaller than that at a W–Ch bcp; the first are due to weak interactions between Ch atoms and the latter are the consequence of ionic bonds with some covalency. The small but non-zero values of ρb at the bcp of the Ch···Ch bonds are an indication of a bonded interaction signaled by QTAIM. The MESP model does not provide a way of quantifying the interaction. It does, however, enable one to see whether there is an atom–atom overlapping in the intermolecular region. This is indeed evident in Figure 9, which shows the way bonded atomic domains responsible for the formation of the (WCh2)2 dimers are linked with each other.
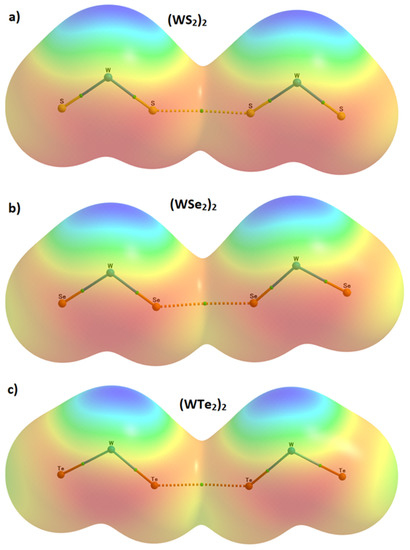
Figure 9.
The 0.001 a.u. isodensity envelope mapped potential on the molecular surface of the three dimers, overlaid in the QTAIM bond paths and critical points, obtained using M06-2X/Aug-cc-pVTZ(-PP): (a) (WS2)2; (b) (WSe2)2; (c) (WTe2)2, suggesting no van der Waals gap existing between bonded chalcogen atoms in the intermolecular region since they are overlapped with each other. Color codes: red (−11.0 a.u.); yellow (+2.6 a.u.), green (+16.1 a.u.); cyan (+29.7 a.u.); and blue (+43.3 a.u.); 1 a.u. = 627.5 kcal mol−1.
For quantum chemical systems in the gas phase, and within the framework of QTAIM, the van der Waals isosurface of a molecular entity is often defined as the ρ = 0.001 a.u. density envelope. The closest distance between a nucleus in a molecular entity and its van der Waals surface is called a “non-bonded atomic radius” [90]. Thus, for a non-covalently interacting pair of atoms A and B, the difference between the bond length of A···B and the sum of their non-bonded radii is regarded as the “mutual penetration distance” [91,92] between the van der Waals sphere of bonded atomic basins. It has been argued that this is a necessary and sufficient condition to identify hydrogen bonding in complex systems [93], and is transferable to other interactions. On this basis, in the case of (WS2)2, (WSe2)2, and (WTe2)2, the non-bonded radii of S2/S6, Se2/Se6, and Te2/Te6 atomic domains (see Figure 7) are calculated to be 2.134, 2.218, and 2.386 Å, respectively; twice these non-bonded radii are 4.268, 4.436, and 4.772 Å, respectively. Clearly, the difference between the calculated Ch2···Ch6 bond distance (see Table 1) and the twice non-bonded radius is 0.653, 0.719, and 0.834 Å for the (WS2)2, (WSe2)2, and (WTe2)2 dimers, respectively, suggesting that the mutual penetration between the two Ch sites responsible for the intermolecular region is strongest in (WTe2)2. Since the larger the mutual penetration distance between bonded atomic basins, the stronger the interaction between them, this accords with the trend observed in the binding energy of the dimers (Table 1 and Table 2).
The sign of the Laplacian of the charge density, ∇2ρb, at a bcp is often used to determine whether a bonding interaction is of a closed-shell (∇2ρb > 0) or open-shell (∇2ρb < 0) type [47,93]. A closed-shell interaction is observed in molecular entities that have non-covalent interactions associated with charge-density-depleted regions (for example, halogen bond, hydrogen bond, chalcogen bond, and pnictogen bond, van der Waals interaction); an open-shell interaction is synonymous covalent, polar covalent, or dative covalent bonds. A purely ionic bond—does such a thing exist? [94]—would be a closed-shell interaction.
As expected, we found ∇2ρb > 0 at the Ch···Ch bcps of the (WCh2)2 dimers, with the largest value of 0.0195 a.u. for (WS2)2 and the smallest, +0.0169 a.u., (WTe2)2. ∇2ρb = −0.0769 a.u. at the W–Ch bcps of the (WS2)2 dimer, whereas ∇2ρb > 0 at the W–Ch bcps for the (WCh2)2 (Ch = Se, Te) dimers. This suggests that the W–S bonds in (WS2)2 are significantly more covalent than the W–Ch bonds in (WCh2)2 (Ch = Se, Te). That polar covalent bonds have ∇2ρb > 0 is not surprising [95,96], and our view on the polar covalent nature of the W–Ch bonds is in accordance with a similar conclusion arrived at for Mo–Ch bonds in MoCh2 crystals [97].
This conclusion above can be verified by considering the sign and magnitude of the total energy density, Hb, which is the sum of the potential and gradient kinetic energy densities, Vb and Gb, at the bcps. In QTAIM, when the potential energy density significantly dominates over the gradient kinetic energy density at the bond bcp, then the Hb < 0, which is indicative of an open-shell interaction. When the gradient kinetic energy density dominates at the bcp, Hb > 0, the signature of a closed-shell interaction is revealed.
Our results show that Hb > 0 for the Ch···Ch interactions in the three (WCh2)2 dimers; they are of the closed-shell type and can therefore be regarded as non-covalent interactions. On the other hand, the W–Ch bonds in the three dimers have Hb < 0, and become less negative in the order (WS2)2 > (WSe2)2 > (WTe2)2. These results are suggestive of W–Ch ionic bonds with a distinctive covalent character. Indeed, the combined signature ∇2ρb > 0 and Hb < 0 suggest a mixed ionic and covalent character that increases with a decrease in the electronegativity, and an increase in the polarizability of the chalcogen across the series from S through Se to Te.
The atomic charge is an informative quantum mechanical property of atoms in molecules [89] and crystals [98]. The results of our QTAIM calculations show that the integrated charge on each chalcogen site of the interacting WCh2 unit responsible for the dimer is negative; the charge on each of S, Se, and Te are −0.388, −0.240, and −0.075 e for (WS2)2, (WSe2)2, and (WTe2)2, respectively, with a decrease in negative charge paralleling the decrease in the electronegativity of the chalcogen. The corresponding charge on W is 0.782, 0.483, and 0.147 e, respectively. These are somewhat different from the values found for the same atoms in the isolated monomers (viz. 0.787 and −0.393 e on W and S in WS2; 0.485 and −0.243 e on W and Se in WSe2; and 0.146 and −0.073 e on W and Te in WTe2). Clearly, the dimer formation is accompanied by a rearrangement of charges, showing an effect of electrostatic polarization when the two monomers interact to form a dimer. Note that the magnitude of charges on the W and Ch atoms in the isolated monomers are substantially decreased compared to the formal charges +4.0 and −2.0 of W and Ch ions, respectively, showing that W becomes increasingly less positive, and Ch becomes increasing more positive. This implies that there is a significant transfer of charge from the W4+ ions to the Ch2− ions during the formation of the isolated monomers.
The rearrangement of charge under the circumstances given above has been noted in numerous occasions previously. For example, it was argued that M–Ch bonds in Mo-Ch2 crystals have significant covalent character, and a degree of polarity in the bond is caused by charge transfer from the metal to the chalcogen atoms [3,97,99,100]. In the case of WCh2, the electronegativity difference between W and Ch is 0.28, 0.10, and 0.07 for S, Se, and Te-containing systems, respectively; on this basis alone, one would conclude that the W–Ch bond is still a polar covalent bond, as has been deduced by others [101,102]. Although the two Ch atoms of the monomers that interact to form the dimer have the same charge, they still attract each other when forming the Ch···Ch bond. A similar conclusion can be arrived at by examining the nature of the electrostatic potentials of these interacting Ch atoms (vide supra). This reminds us of the concept of “like attracting like”—a concept that has been observed previously in a number of other systems [52,67,103,104,105].
A judgement on the polarity of a molecular domain can be made based on its dipole moment, μ. Our M06-2X/aug-cc-pVTZ(-PP) level calculation gave dipole moments of 2.73, 2.41, and 2.09 D for the monomers WS2, WSe2, and WTe2, respectively. Dimerization resulted in dipole moments of 5.30, 4.69, and 4.09 D for (WS2)2, (WSe2)2, and (WTe2)2, respectively. There is a clear enhancement of the dipole moment on the formation of the dimer, suggesting an increasing polarity of the dimers at equilibrium.
2.6. Charge Density Based Isosurface Topologies of (WCh2)2 Dimers
A number of charge-density-based approaches have been developed within the last decade and applied to many chemical systems to explore chemical bonding interactions [48,49,50,51,106,107]. They have not only enabled the validation of graphically illustrated bonding environments obtained from isosurface topologies that depict the interaction between atomic domains in localized and delocalized bonds, and between delocalized bonds and anions, cations, or molecular entities, but have also greatly assisted a more rigorous characterization of chemical bonding interactions in general. Among these approaches are the RDG, IGM-δg, DORI, and the recently proposed IRI-based NCI approach. Each has its limitations, and hence a joint application of these approaches to chemical systems may lead to a deeper understanding of the bonding involved between interacting atomic domains.
The RDG-based NCI characterization involves the plot of the sign(λ2) × ρ vs. RDG domains, where λ2 (λ1 ≤ λ2 ≤ λ3) is the second eigenvalue of the Hessian of the charge density matrix, and ρ is the charge density. RDG describes the reduced charge density gradient around the bond critical point region; the lower bound of RDG is zero and is reached whenever the charge density gradient vanishes at the bcp, a dimensionless quantity within the generalized gradient approximation of the exchange correlation term in DFT Hamiltonians [108,109].
The sign of λ2 can be positive or negative depending on whether it is associated with an eigenvector perpendicular to the bond path at a bcp, or whether it is associated with an eigenvector directed in the ring plane at a ring critical point [86]. Clearly, the sign of λ2 is one of the key parameters of the RDG-NCI approach, since it enables one to distinguish between supposedly attractive (λ2 < 0) and/or repulsive (λ2 > 0) interactions, and ρ quantifies the strength of that interaction. When the quantity sign(λ2) × ρ is mapped onto the RDG isosurface, both the nature and strength of the interactions within molecular entities become evident [51].
The sign(λ2) × ρ vs. RDG plots are shown in Figure 10 and Figure 11 for (WS2)2, (WSe2)2, and (WTe2)2, respectively. The circular isosurface volume appearing between two Ch atoms (Ch6 and Ch2) in these dimers clearly corresponds to low-density regions, as shown by the greenish spikes in the sign(λ2) × ρ vs. RDG plots in the region −0.01 a.u. < sign(λ2) × ρ < 0.0 a.u. In this region, the sign(λ2) is negative. These are the characteristics of non-covalently bonded Ch···Ch interactions in all three dimers. There is a second spike in each of the three sign(λ2) × ρ vs. RDG plots, where sign(λ2) is positive. Since these appear in the low-density regions, with 0.0 a.u. < sign(λ2) × ρ < 0.01 a.u., these are signatures of van der Waals interactions.
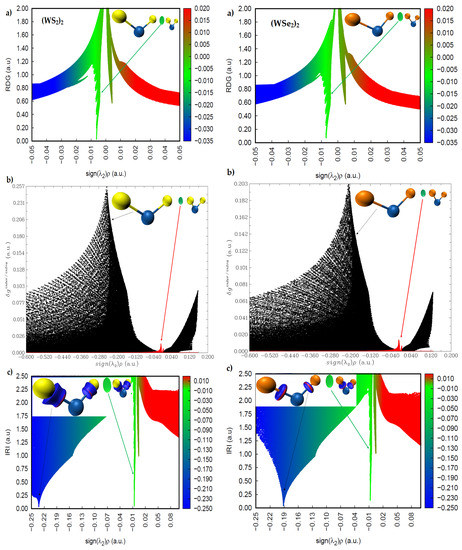
Figure 10.
Comparison of the (a) sign(λ2) × ρ vs. RDG plot with those of the (b) sign(λ2) × ρ vs. IGM (δginter/intra) and (c) sign(λ2) × ρ vs. IRI plots for left, (WS2)2; right, (WSe2)2, obtained with M06-2X/Aug-cc-pVTZ(-PP). RDG, IGM(δginter/intra), and IRI isosurface domains were obtained with isovalues of 0.4, 0.01, and 1.0 a.u., respectively.
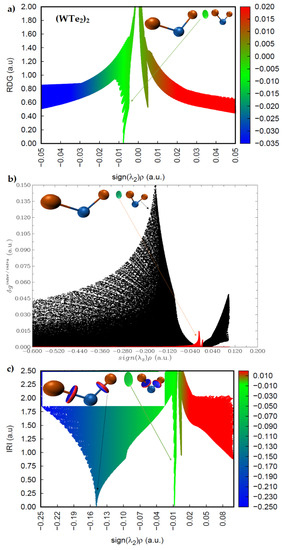
Figure 11.
Comparison of the (a) sign(λ2) × ρ vs. RDG plot with those of the (b) sign(λ2) × ρ vs. IGM (δginter/intra) and (c) sign(λ2) × ρ vs. IRI plots for (WTe2)2, obtained with M06-2X/Aug-cc-pVTZ(-PP). RDG, IGM(δginter/intra), and IRI isosurface domains were obtained with isovalues of 0.4, 0.01, and 1.0 a.u., respectively.
One of the major drawbacks of RDG is that the isosurfaces of interacting fragments become noisy in large complex molecules; this makes it difficult to rationalize the actual molecular fragments or entities that are responsible for the development of specific RDG isosurface domains [49,50,107,110]. The IGM model was proposed to overcome this shortcoming. It has an advantage over the RDG-NCI approach in that it provides a way of identifying and quantifying the net ED gradient attenuation due to interactions using real or promolecular density [49,50].
A descriptor of the model, δginter/intra, can be derived; it uniquely defines inter- or intra-molecular interaction regions. Figure 10b and Figure 11b show the sign(λ2) × ρ vs. IGM(δginter/intra) for the three dimers. The spikes colored black are due to intramolecular interactions (W–Ch bonds), and those colored red are intermolecular interactions. The first appear in the high-density regions, and the second in the low-density regions. The IGM-based isosurface volumes that show up between the Ch atoms in (WS2)2, (WSe2)2, and (WTe2)2 correspond to the weak red spikes, and the strength of the interaction increases in this order: (WS2)2 < (WSe2)2 < (WTe2)2.
The more recently proposed IRI model [48] has an advantage over these two models above. It can not only provide insight into the presence of likely intermolecular interactions between the atomic domains, but can also give insight into the intramolecular interactions with the framework of isolated molecules. IRI relies on the charge density and its gradient to identify the inter- and inter-molecular interactions in molecular entities; IRI is simply the ratio between the charge density and its gradient norm. In other words, IRI is the gradient norm of the electron density weighted by the scaled charge density [48]. Figure 10c and Figure 11c show sign(λ2) × ρ vs. IRI for the dimers (WS2)2 and (WSe2)2, respectively, whereas that of the dimer (WTe2)2 is shown in Figure 11c. Two types of isosurfaces are evident. Those colored blue (dumbbell- and circular-disc-shaped) are a consequence of covalent bonding between W and Ch atomic basins. Those with a greenish color signal are the non-covalently bonded atomic basins of Ch atoms. Both isosurfaces were obtained with an IRI isovalue of 1.0 a.u., which is rather different to values of 0.4 and 0.01 a.u. used for RDG-NCI and IGM-δg models, respectively, as each model requires a specific range of isovalues to generate isosurfaces of interest. The blue isosurfaces signify a significant accumulation of the charge density in the bonding region between W and Ch atomic basins, as expected for ionic bonding with some covalent character. The green isosurfaces indicate low-density regions, as similarly observed in the RDG and IGM-δg plots that appear between the non-covalently bonded Ch atoms. The spikes in the sign(λ2) × ρ vs. IRI plots corresponding to these isosurfaces are identical to those of the sign(λ2) × ρ vs. IGM(δginter/intra) plots, indicating that both models provide a similar insight into the atomic basins causing the interactions and their strengths.
Klein and coworkers have recently proposed the intrinsic bond strength index (IBSI) that emerges from the IGM formulation [106]. According to them, each chemical interaction (or bond) in a molecular entity has its own IGM-δgpair signature, and, hence, the IBSI index. This means that the IGM and its δg descriptor are a way forward to locally quantify the electron density interpenetration from wavefunction calculations. These workers correlated the IBSI index with a number of conventional bond orders (such as Mulliken, Wiberg, Mayer, delocalization index, or electron localization function—ELF), albeit with poor regression coefficients, and called IBSI a new complementary index that is related to the bond strength. Considering A (as W1Ch3Ch2) and B (as W4Ch5Ch6) as two fragments of the (WCh2)2 dimer system (see Figure 7 atomic numbering), we calculated the δg indices for the three dimers that quantify the contribution of an atom–atom pair to the interaction between the two fragments.
Our calculations gave δg indices that were the largest at 0.159, 0.188, and 0.232 a.u., for each Ch2 and Ch6 atom in (WS2)2, (WSe2)2, and (WTe2)2, respectively, which are the pair of atoms that are closest to each other. Accordingly, the pair Ch2···Ch6 have the largest δg indices of 0.135, 0.163, and 0.209 a.u. for the corresponding systems, respectively, suggesting that the two Te atoms contribute largely to the Te···Te interaction in (WTe2)2 compared to that contributed by the two S and two Se atoms for the S···S and Se···Se interactions in (WS2)2 and (WSe2)2, respectively. Similarly, the sum of the related IBSIW (IBSI for weak interaction) for Ch2/Ch6 was 1.114, 1.258, and 1.413 a.u./Å2, respectively. The IBSIW indices for the Ch2···Ch6 pair were 1.034, 1.179, and 1.349 a.u./Å2, respectively, where IBSIW for any atom–atom pair i and j is defined by Equation (1) and Equation (2).
where di,j is the distance between atoms i and j in Å.
Since the larger the value of the IBSIW index, the stronger the interaction, it is clear that the preference of stability follows the order of (WS2)2 < (WSe2)2 < (WTe2)2, and is in accordance with the trend in the observed binding energies of these dimers (Table 2).
2.7. Second Order Charge Transfer between the Monomers Forming (WCh2)2 Dimers and Bond Order
Finally, we note that the attractive interaction between the WCh2 monomers leading to the formation of homodimers can indeed be recognized by the second-order estimates between donor and acceptor orbitals in an NBO basis. Specifically, our calculations suggest that there are several weak hyperconjugative charge transfer interactions between the interacting molecules forming the (WS2)2 dimers. The dominant delocalizations involve BD(3)W1–Ch2→BD*(3)W4–Ch6, BD(3)W4–Ch6→BD*(3)W1–Ch2, LP(2)Ch2/LP(2)Ch6→BD*(3)W4–Ch6/ BD*(3)W1–Ch2, where LP(2), BD(3), and BD*(3) represent the second π-type lone-pair, dπ-type bonding, and anti-bonding-type orbitals, respectively. To give an example, the second order charge transfer delocalization energies E(2) for these interactions were found to be 0.8, 0.8, and 0.5 kcal mol−1 for (WTe2)2, respectively. These results suggest that there are mutual hyperconjugative charge transfer interactions between the two monomers when they form a dimer. That W–Ch bonds in isolated WCh2 and complexed (WCh2)2 have the triple bond character, which was involved in the charge transfer delocalization, was evidenced by a bond order analysis [111,112,113,114]. For instance, the M06-2X/Aug-cc-pVTZ(-PP) computed Meyer; Fuzzy; and QTAIM bond orders are 2.8; 2.9; 2.6 and 0.05; 0.22; 0.115 for W–Te and Te···Te interactions in (WTe2)2, respectively. Very similar results were obtained for the W–Se and Se···Se interactions in (WSe2)2 and W–S and S···S interactions in (WTe2)2. The bond orders suggest that the link between W and Ch is of a triple-bond character, whereas that between the two Ch atoms (Ch···Ch) of the two interacting monomers forming the dimers is of a single-bond character. We note that the Mayer bond order [115] essentially reflects the number of electrons shared by two interacting atoms, which is very similar to QTAIM’s bond order. The latter has been called the delocalization index (the number of electrons exchanged between a pair of two atomic basins bonded to each other [50,111,116]).
3. Molecular Models
Three V-shaped WCh2 monomers and the three (WCh2)2 dimers were examined in this study using the DFT-M06-2X functional. The construction of the dimers was made possible by inspecting the geometry of the interfacial region between the WCh2 layers, reported in the crystals of WCh2 in the solid state [2,4,117,118].
Due to the fact that we were specifically interested in a basic understanding of the nature of the interactions between Ch sites in the interfacial/interlayer region of WCh2 crystals, we did not explore any other dimers in the conformational space. However, we are interested in exploring this conformational space to identify the global minimum for each dimer, and then to examine the extent to which the nature of the bonding environment and bonding energies between the two monomers changes upon dimerization; we will discuss this elsewhere.
We have also limited our study to providing insight into the nature of the interfacial bonding in the 2H phase of the WCh2 crystals. This phase is semiconducting, and stable at room temperature. Reports show that another phase, the 1T phase, is highly unstable and converts back into the 2H phase with time and a variation in temperature. Metastable 1T(1T′) phases require higher formation energy compared to the thermodynamically stable 2H phase; thus, in standard chemical vapor deposition and vapor transport processes, the transition metal dichalcogenide materials normally grow in the 2H phases [15,119]. The crystal structure of the 2H phase of WS2 is shown in Figure 1.
4. Computational Methodology
Although transition metals in chemical systems often have multiconfigurational character [120], there have been several DFT studies [121,122,123] reported that utilized the M06-2X functional [42] for applications involving main-group thermochemistry, kinetics, noncovalent interactions, and electronic excitation energies to valence and Rydberg states. Wang et al. have demonstrated that the M06 and M06-2X hybrid metafunctionals have broad applicability, including transition metal systems [122]. As a result of this, we selected the M06-2X functional to model WCh2 monomers and (WCh2)2 dimers. We show that this functional reproduces the geometry of the intermolecular interactions found in the interfacial regions in the crystalline phase (cf. Figure 1 and Figure 2) very well. We selected three different basis sets (LANL08, def2-TZVPPD, and Aug-cc-pVTZ(-PP)—all available in the EMSL basis set exchange library [43]) to examine the extent to which the basis set size affects the intermolecular bond distances, bond angles, and other properties of the dimers examined. The basis set aug-cc-pVTZ(-PP) indicates that Aug-cc-pVTZ was centered on S and Aug-cc-pVTZ-PP was centered on Se, Te, and W. Tight convergence and ultrafine integration grids were chosen, recommended for DFT methods, and also the default option in Gaussian 16 [124]. The geometry optimization of each system was followed by a subsequent frequency calculation to identify the nature of the stationary or saddle points on the PES. The geometries of the monomers and dimers were all found to be true minima.
The binding energy, ∆E (Equation (3)), for the (WCh2)2 dimers was calculated using the supermolecular procedure proposed by Pople [125]. ∆E was obtained by subtracting the sum of the total electronic energies (ETsum(monomers)) of the respective isolated monomers forming the dimers from the total electronic energy of each dimer (ET(dimer)). The energies of the monomers, as in the fully relaxed dimer configurations, were utilized. The basis set superposition error (BSSE) was calculated using the counterpoise procedure of Boys and Bernardi [126], Equation (4), where E(BSSE) is the BSSE energy.
∆E = (ET(dimer)) – (ETsum(monomers))
∆E(BSSE) = ∆E + E(BSSE)
For reasons given in a following section, and because they are useful for the study of non-covalent interactions [127,128], we have also computed the BSSE-corrected ∆E of all three dimers using M06-2X-D3, MP2(full), B97-D3(BJ) [129], PW6B95-D3(BJ) [42,130], and B3LYP-D3(BJ) [131,132], where D3 is Grimme’s dispersion correction with the original D3 damping function [133], and D3(BJ) refers to the D3 version of Grimme’s dispersion with Becke–Johnson damping correction [130]. The fully relaxed M06-2X geometries of the dimers were used as the starting point for the latter five methods.
The topological properties (viz. the charge density, the Laplacian of the charge density and the total energy density at the bond critical points (ρb, ∇2ρb and Hb, respectively), the bond path, and the molecular graphs) were obtained using the AIMAll software [134]. The MESP graphs and the extrema of potentials were obtained using AIMAll and MultiWfn [110] software, respectively. The charge-density-based RDG [51], IRI [30], and IGM [50] isosurfaces that fingerprint intermolecular interactions between the monomers in the equilibrium geometries of the (WCh2)2 dimers were obtained using MultiWfn and VMD software [135], respectively. All of the electronic structure calculations were performed using Gaussian 16 [124].
5. Conclusions
In this study, we discussed the geometric, electronic, charge density topology, and energetic properties of (WCh2)2 (Ch = S, Se, Te) dimers examined at the M06-2X level of theory, in conjunction with three different basis sets: LANL08, def2-TZVPPD, and Aug-cc-pVTZ(-PP). We showed that, though each basis set has its own limitations, they all provided a very consistent Ch···Ch intermolecular bond distance for the three dimers examined and are well comparable with the interfacial Ch···Ch geometries observed for the WCh2 crystals in the room temperature 2H-phase. The overall performance in obtaining intermolecular bond distances and angles of approach leading to the formation of the three dimers is shown to be described well with the Dunning’s pseudopotential basis set Aug-cc-PVTZ(-PP). Although the M06-2X functional provided an intermolecular geometry in reasonable agreement with the experimentally established solid state geometry of WCh2, it underestimated the binding energies of the dimers. The effect of Grimme’s dispersion with the original D3 damping function had a negligible effect on the geometric, electronic, and energetic properties of the dimers, especially when it was incorporated with the M06-2X functional. However, the uncorrected and BSSE-corrected binding energies calculated on the M062X/Aug-cc-pVTZ(-PP) geometries of the dimers with MP2(full), B97-D3(BJ), PW6B95-D3(BJ), and B3LYP-D3(BJ) enabled us to demonstrate that (WSe2)2 and (WTe2)2 are weakly bonded dimers, and that (WS2)2 is a van der Waals system. That the (WS2)2 is a van der Waals system is a result of the attraction between the W-bound S atoms with positive electrostatic potentials along the W–S bond extensions in the isolated WS2 monomers, as has been observed for other systems before [52,84,103,104,105].
The QTAIM, IRI, IGM-δginter/intra, and RDG-based results associated with the charge density topological indicators have confirmed the occurrence of Ch···Ch attractive engagements leading to the formation of the (WCh2)2 dimers. The origin of the Ch···Ch interactions in (WCh2)2 was revealed by the second order hyperconjugative estimates of donor acceptor interactions in an NBO analysis, which showed that there are several weak charge transfer delocalizations between the interacting monomers at the dimer geometry, including, for example, LP(Ch)→BD*(3)W–Ch and BD(2/3)W–Ch→BD*(3)W–Ch. We therefore propose that the characteristics of the chalcogen–chalcogen bonding interactions observed in the (WCh2)2 dimers of tungsten sulfide, tungsten selenide, and tungsten telluride are prototypes for a basic understanding of the local interfacial/interlayer chemical bonding environment observed in the layered tungsten dichalcogenides in 2D, and that there is no van der Waals gap observed between the monolayers responsible for the interfacial/interlayer regions in bi-, tri-, and/or multi-layered tungsten dichalcogenides, but only voids.
Author Contributions
Conceptualization, project design, and project administration, P.R.V.; formal analysis and investigation, P.R.V. and A.V.; discussion: P.R.V., A.V., H.M.M. and K.Y.; supervision, P.R.V.; writing—original draft, P.R.V. and A.V.; writing—review and editing, P.R.V., H.M.M., A.V. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This research did not report any data.
Acknowledgments
This work was entirely conducted using the various computation and laboratory facilities provided by the University of Tokyo. P.R.V. is currently affiliated with the University of Witwatersrand (SA) and Institute of Innovation for Future Society of Nagoya University (Japan). A.V. is currently affiliated with AIST, Tsukuba 305-8560, Japan. K.Y. is currently affiliated with Kyoto University, ESICB, Kyoto, 615-8245, Japan. H.M.M. thanks the National Research Foundation, Pretoria, South Africa, and the University of the Witwatersrand for funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had absolutely no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wilson, J.A.; Yoffe, A.D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Mentzen, B.F.; Sienko, M.J. Preparation and X-ray study of mixed-anion tungsten dichalcogenides. Inorg. Chem. 1976, 15, 2198–2202. [Google Scholar] [CrossRef]
- Ataca, C.; Şahin, H.; Ciraci, S. Stable, Single-Layer MX2 Transition-Metal Oxides and Dichalcogenides in a Honeycomb-Like Structure. J. Phys Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Oliver, S.M.; Beams, R.; Krylyuk, S.; Kalish, I.; Singh, A.K.; Bruma, A.; Tavazza, F.; Joshi, J.; Stone, I.R.; Stranick, S.J.; et al. The structural phases and vibrational properties of Mo1−x WxTe2 alloys. 2D Mater. 2017, 4, 045008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duerloo, K.-A.N.; Li, Y.; Reed, E.J. Structural phase transitions in two-dimensional Mo- and W-dichalcogenide monolayers. Nat. Commun. 2014, 5, 4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güller, F.; Llois, A.M.; Goniakowski, J.; Noguera, C. Prediction of structural and metal-to-semiconductor phase transitions in nanoscale MoS2, WS2 and other transition metal dichalcogenide zigzag ribbons. Phys. Rev. B 2015, 91, 075407. [Google Scholar] [CrossRef]
- Dias, A.C.; Qu, F.; Azevedo, D.L.; Fu, J. Band structure of monolayer transition-metal dichalcogenides and topological properties of their nanoribbons: Next-nearest-neighbor hopping. Phys. Rev. B 2018, 98, 075202. [Google Scholar] [CrossRef]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Bai, X. Two-Dimensional Transition Metal Dichalcogenides: Synthesis, Biomedical Applications and Biosafety Evaluation. Front. Bioeng. Biotech. 2020, 8. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Chen, Q.; Miao, N.; Si, C.; Zhou, J.; Sun, Z. Quantifying the composition dependency of the ground-state structure, electronic property and phase-transition dynamics in ternary transition-metal-dichalcogenide monolayers. J. Mat. Chem. C 2020, 8, 721–733. [Google Scholar] [CrossRef]
- Fukuda, M.; Zhang, J.; Lee, Y.-T.; Ozaki, T. A structure map for AB2 type 2D materials using high-throughput DFT calculations. Mater. Adv. 2021, 2, 4392–4413. [Google Scholar] [CrossRef]
- Ju, L.; Qin, J.; Shi, L.; Yang, G.; Zhang, J.; Sun, L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Intercalated Layered Materials; Lévy, F. (Ed.) D. Reidel Publishing: Dordrecht, The Netherlands, 1979. [Google Scholar]
- Inorganic and Metallic Nanotubular Materials; Kijima, T. (Ed.) Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Sokolikova, M.S.; Mattevi, C. Direct synthesis of metastable phases of 2D transition metal dichalcogenides. Chem. Soc. Rev. 2020, 49, 3952–3980. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.; He, Q.; Tran, T.H.; Repaka, D.V.M.; Zhou, D.-D.; Sun, Y.; Xi, S.; Li, Y.; Chaturvedi, A.; Tan, C.; et al. Metastable 1T′-phase group VIB transition metal dichalcogenide crystals. Nature Mat. 2021, 20, 1113–1120. [Google Scholar] [CrossRef]
- Shi, W.; Ye, J.; Zhang, Y.; Suzuki, R.; Yoshida, M.; Miyazaki, J.; Inoue, N.; Saito, Y.; Iwasa, Y. Superconductivity Series in Transition Metal Dichalcogenides by Ionic Gating. Sci. Rep. 2015, 5, 12534. [Google Scholar] [CrossRef] [Green Version]
- Duong, D.L.; Yun, S.J.; Lee, Y.H. van der Waals Layered Materials: Opportunities and Challenges. ACS Nano 2017, 11, 11803–11830. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef]
- Constantinescu, G.; Kuc, A.; Heine, T. Stacking in Bulk and Bilayer Hexagonal Boron Nitride. Phys. Rev. Lett. 2013, 111, 036104. [Google Scholar] [CrossRef]
- He, J.; Hummer, K.; Franchini, C. Stacking effects on the electronic and optical properties of bilayer transition metal dichalcogenides MoS2, MoSe2, WS2 and WSe2. Phys. Rev. B 2014, 89, 075409. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Dai, J.; Yao, W.; Xiao, D.; Cui, X. Valley polarization in MoS2 monolayers by optical pumping. Nat. Nanotech 2012, 7, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Optics Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef] [PubMed]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide TS2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Yu, K.; Wen, Z.; Chen, J. Semiconducting graphene: Converting graphene from semimetal to semiconductor. Nanoscale 2013, 5, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, N.M.; Tang, W.; Rassay, S. Transition Metal Dichalcogenides Properties and Applications. In Semiconductors: Synthesis, Properties and Applications; Pech-Canul, M.I., Ravindra, N.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–396. [Google Scholar] [CrossRef]
- Liu, M.; Liu, W.; Liu, X.; Wang, Y.; Wei, Z. Application of transition metal dichalcogenides in mid-infrared fiber laser. Nano Select 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Anju, S.; Mohanan, P.V. Biomedical applications of transition metal dichalcogenides (TMDCs). Synth. Met. 2021, 271, 116610. [Google Scholar] [CrossRef]
- Kan, M.; Wang, B.; Lee, Y.H.; Sun, Q. A density functional theory study of the tunable structure, magnetism and metal-insulator phase transition in VS2 monolayers induced by in-plane biaxial strain. Nano Res. 2015, 8, 1348–1356. [Google Scholar] [CrossRef]
- Terrones, H.; López-Urías, F.; Terrones, M. Novel hetero-layered materials with tunable direct band gaps by sandwiching different metal disulfides and diselenides. Sci. Rep. 2013, 3, 1549. [Google Scholar] [CrossRef]
- Bastos, C.M.O.; Besse, R.; Da Silva, J.L.F.; Sipahi, G.M. Ab initio investigation of structural stability and exfoliation energies in transition metal dichalcogenides based on Ti-, V-, and Mo-group elements. Phys. Rev. Mater. 2019, 3, 044002. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-T.; Lu, L.-S.; Wang, D.; Huang, J.-K.; Li, M.-Y.; Chang, T.-R.; Chou, Y.-C.; Juang, Z.-Y.; Jeng, H.-T.; Li, L.-J.; et al. Evidence of indirect gap in monolayer WSe2. Nature Commun. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.K.; Das, R.; Mahadevan, P. Layer-Dependent Electronic Structure Changes in Transition Metal Dichalcogenides: The Microscopic Origin. ACS Omega 2020, 5, 15169–15176. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Drumm, D.W.; Russo, S.P.; Greentree, A.D. A study of size-dependent properties of MoS2 monolayer nanoflakes using density-functional theory. Sci. Rep. 2017, 7, 9775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Guillén, J.Á.; San-Jose, P.; Roldán, R. Electronic Band Structure of Transition Metal Dichalcogenides from Ab Initio and Slater–Koster Tight-Binding Model. Appl. Sci. 2016, 6, 284. [Google Scholar] [CrossRef] [Green Version]
- Khalatbari, H.; Vishkayi, S.I.; Oskouian, M.; Soleimani, H.R. Band structure engineering of NiS2 monolayer by transition metal doping. Sci. Rep. 2021, 11, 5779. [Google Scholar] [CrossRef] [PubMed]
- Förste, J.; Tepliakov, N.V.; Kruchinin, S.Y.; Lindlau, J.; Funk, V.; Förg, M.; Watanabe, K.; Taniguchi, T.; Baimuratov, A.S.; Högele, A. Exciton g-factors in monolayer and bilayer WSe2 from experiment and theory. Nature Commun. 2020, 11, 4539. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M.; Varadwaj, A.; Yamashita, K. Chalcogen···Chalcogen Bonding in Molybdenum Disulfide, Molybdenum Diselenide and Molybdenum Ditelluride Dimers as Prototypes for a Basic Understanding of the Local Interfacial Chemical Bonding Environment in 2D Layered Transition Metal Dichalcogenides. Inorganics 2022, 10, 11. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. A New Basis Set Exchange: An Open, Up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. Available online: https://www.basissetexchange.org/ (accessed on 12 October 2021). [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, R.G.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Glendening, E.E.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO (Natural Bond Orbital), 3.1; as Implemented in GAUSSIAN 03; Gaussian Inc.: Pittsburg, PA, USA, 2004. [Google Scholar]
- Reed, A.E.; Weinhold, F. On the role of d orbitals in sulfur hexafluoride. J. Am. Chem. Soc. 1986, 108, 3586–3593. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions. Chemistry–Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. The Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. ChemPhysChem 2018, 19, 724–735. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Is the Fluorine in Molecules Dispersive? Is Molecular Electrostatic Potential a Valid Property to Explore Fluorine-Centered Non-Covalent Interactions? Molecules 2019, 24, 379. [Google Scholar] [CrossRef] [Green Version]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Does Chlorine in CH3Cl Behave as a Genuine Halogen Bond Donor? Crystals 2020, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S. The use and misuse of van der Waals radii. Struct. Chem. 2021, 32, 623–629. [Google Scholar] [CrossRef]
- Trabesinger, A. How many kisses? Nature Phys. 2007, 3, 223. [Google Scholar] [CrossRef]
- Hermann, A.; Lein, M.; Schwerdtfeger, P. The Search for the Species with the Highest Coordination Number. Angew Chem. Int. Edn. 2007, 46, 2444–2447. [Google Scholar] [CrossRef] [PubMed]
- Trombach, L.; Schwerdtfeger, P. Gregory-Newton problem for kissing sticky spheres. Phys. Rev. E 2018, 98, 033311. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Janjić, G.V.; Zarić, S.D. σ-Hole interactions of covalently-bonded nitrogen, phosphorus and arsenic: A survey of crystal structures. Crystals 2014, 4, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Bauzá, A.; Seth, S.K.; Frontera, A. Molecular electrostatic potential and “atoms-in-molecules” analyses of the interplay between π-hole and lone pair···π/X–H···π/metal···π interactions. J. Comp. Chem. 2018, 39, 458–463. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Electrostatically enhanced F•••F interactions through hydrogen bonding, halogen bonding and metal coordination: An ab initio study. Phys. Chem. Chem. Phys. 2016, 18, 20381–20388. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri, R.; Ektefa, F. Exploring the Mechanism of Reactions of SiX3 and CX3 Radicals with Si20X20 Fullerenes (X = H, F): A Density Functional Study. J. Clust. Sci. 2016, 27, 1719–1728. [Google Scholar] [CrossRef]
- Loveday, O.; Echeverría, J. Methyl groups as widespread Lewis bases in noncovalent interactions. Nature Commun. 2021, 12, 5030. [Google Scholar] [CrossRef]
- Zhou, P.-P.; Qiu, W.-Y.; Liu, S.; Jin, N.-Z. Halogen as halogen-bonding donor and hydrogen-bonding acceptor simultaneously in ring-shaped H3N·X(Y)·HF (X = Cl, Br and Y = F, Cl, Br) Complexes. Phys. Chem. Chem. Phys. 2011, 13, 7408–7418. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Do surfaces of positive electrostatic potential on different halogen derivatives in molecules attract? like attracting like! J. Comput. Chem. 2018, 39, 343–350. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. A DFT assessment of some physical properties of iodine-centered halogen bonding and other non-covalent interactions in some experimentally reported crystal geometries. Phys. Chem. Chem. Phys. 2018, 20, 15316–15329. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Dong, W.; Wang, Y.; Li, Q. Noncovalent Interactions in Complexes Involving the Cyclic C2H2X (X=O, S, Se) Molecules and the Lewis Acids YF (Y=F, Cl, Br, H). ChemistrySelect 2019, 4, 9506–9515. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.-Y. Halogen bonding interaction of chloromethane withseveral nitrogen donating molecules: Addressing thenature of the chlorine surface σ-hole. Phys. Chem. Chem. Phys. 2014, 16, 19573–19589. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Schmider, H.L.; Becke, A.D. Chemical content of the kinetic energy density. J. Mol. Str. THEOCHEM 2000, 527, 51–61. [Google Scholar] [CrossRef]
- Schmider, H.L.; Becke, A.D. Two functions of the density matrix and their relation to the chemical bond. J. Chem. Phys. 2002, 116, 3184–3193. [Google Scholar] [CrossRef]
- Ruedenberg, K. The Physical Nature of the Chemical Bond. Rev. Mod. Phys. 1962, 34, 326–376. [Google Scholar] [CrossRef]
- Bacskay, G.B.; Nordholm, S.; Ruedenberg, K. The Virial Theorem and Covalent Bonding. J. Phys. Chem. A 2018, 122, 7880–7893. [Google Scholar] [CrossRef]
- Carpio-Martínez, P.; Barquera-Lozada, J.E.; Pendás, A.M.; Cortés-Guzmán, F. Laplacian of the Hamiltonian Kinetic Energy Density as an Indicator of Binding and Weak Interactions. ChemPhysChem 2020, 21, 194–203. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Wen, C.; Zhang, M.; Geng, Y.; Liu, X.; Su, Z. The Al≡Al triple bond in Al2X5+ and Al2X62+ (X = Li, Na) clusters with multiple alkali metal coordination. New J. Chem. 2020, 44, 21119–21124. [Google Scholar] [CrossRef]
- Gatti, C. Chemical bonding in crystals: New directions. Z. Kristall. Cryst. Mater. 2005, 220, 399–457. [Google Scholar] [CrossRef]
- Savin, A.; Jepsen, O.; Flad, J.; Andersen, O.K.; Preuss, H.; von Schnering, H.G. Electron Localization in Solid-State Structures of the Elements: The Diamond Structure. Angew. Chem. Intl. Ed. Eng. 1992, 31, 187–188. [Google Scholar] [CrossRef]
- Jacobsen, H. Localized-orbital locator (LOL) profiles of chemical bonding. Can. J. Chem. 2008, 86, 695–702. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Johnson, S.; Tang, T.H.; Popelier, P.L.A. The Electron Pair. J. Phys. Chem. 1996, 100, 15398–15415. [Google Scholar] [CrossRef]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1994. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001; Volume 19. [Google Scholar]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Hybrid organic–inorganic CH3NH3PbI3 perovskite building blocks: Revealing ultra-strong hydrogen bonding and mulliken inner complexes and their implications in materials design. J. Comput. Chem. 2017, 38, 2802–2818. [Google Scholar] [CrossRef]
- Cossard, A.; Desmarais, J.K.; Casassa, S.; Gatti, C.; Erba, A. Charge Density Analysis of Actinide Compounds from the Quantum Theory of Atoms in Molecules and Crystals. J. Phys. Chem. Lett. 2021, 12, 1862–1868. [Google Scholar] [CrossRef]
- Saleh , G.; Gatti , C.; Lo Presti, L.; Contreras-García, J. Revealing Non-covalent Interactions in Molecular Crystals through Their Experimental Electron Densities. Chem. Eur. J. 2012, 18, 15523–15536. [Google Scholar] [CrossRef]
- Hathwar, V.R.; Guru Row, T.N. Charge Density Analysis of Heterohalogen (Cl···F) and Homohalogen (F···F) Intermolecular Interactions in Molecular Crystals: Importance of the Extent of Polarizability. Cryst. Growth Des. 2011, 11, 1338–1346. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Guru Row, T.N. Experimental evidence for ‘carbon bonding’ in the solid state from charge density analysis. Chem. Commun. 2014, 50, 49–51. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Matta, C.F. Bonding to titanium. Inorg. Chem. 2001, 40, 5603–5611. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Otaki, H.; Ando, K. Atoms-in-molecules analysis of the effect of intermolecular interactions on dielectric properties in hydrogen-bonded material 5-bromo-9-hydroxyphenalenone. Int. J. Quant. Chem. 2013, 113, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Aljameedi, K.; Karton, A.; Jayatilaka, D.; Thomas, S.P. Bond orders for intermolecular interactions in crystals: Charge transfer, ionicity and the effect on intramolecular bonds. IUCrJ 2018, 5, 635–646. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Love, I. An AIM analysis of S,O bonds. J. Phys. Chem. A 2009, 113, 2640–2646. [Google Scholar] [CrossRef]
- Love, I. Characteristics of Multiple N,O Bonds. J. Phys. Chem. A 2006, 110, 10507–10512. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, J.; Zhu, C.; Miao, J.; Huang, Y.; Heinz, H. Interpretable molecular models for molybdenum disulfide and insight into selective peptide recognition. Chem. Sci. 2020, 11, 8708–8722. [Google Scholar] [CrossRef]
- Neal, S.N.; Li, S.; Birol, T.; Musfeldt, J.L. Chemical bonding and Born charge in 1T-HfS2. npj 2D Mater. Appl. 2021, 5, 45. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Zibouche, N.; Wahiduzzaman, M.; Oliveira, A.F.; Kuc, A.; Heine, T. Electromechanics in MoS2 and WS2: Nanotubes vs. monolayers. Sci. Rep. 2013, 3, 2961. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani-Asl, M.; Borini, S.; Kuc, A.; Heine, T. Strain-dependent modulation of conductivity in single-layer transition-metal dichalcogenides. Phys. Rev. B 2013, 87, 235434. [Google Scholar] [CrossRef] [Green Version]
- Vera-Hidalgo, M.; Giovanelli, E.; Navío, C.; Pérez, E.M. Mild Covalent Functionalization of Transition Metal Dichalcogenides with Maleimides: A “Click” Reaction for 2H-MoS2 and WS2. J. Am. Chem. Soc. 2019, 141, 3767–3771. [Google Scholar] [CrossRef]
- Ghatak, K.; Kang, K.N.; Yang, E.-H.; Datta, D. Controlled edge dependent stacking of WS2-WS2 Homo- and WS2-WSe2 Hetero-structures: A Computational Study. Sci. Rep. 2020, 10, 1648. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.-Y. Unusual bonding modes of perfluorobenzene in its polymeric (dimeric, trimeric and tetrameric) forms: Entirely negative fluorine interacting cooperatively with entirely negative fluorine. Phys. Chem. Chem. Phys. 2015, 17, 31624–31645. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, J.; Wang, Q.; Jena, P. Like charges attract? J. Phys. Chem. Lett. 2016, 7, 2689–2695. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Jin, B.-Y. Can an entirely negative fluorine in a molecule, viz. perfluorobenzene, interact attractively with the entirely negative site(s) on another molecule(s)? Like liking like! RSC Adv. 2016, 6, 19098–19110. [Google Scholar] [CrossRef]
- Klein, J.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. New Way for Probing Bond Strength. J. Phys. Chem. A 2020, 124, 1850–1860. [Google Scholar] [CrossRef]
- de Silva, P.; Corminboeuf, C. Simultaneous Visualization of Covalent and Noncovalent Interactions Using Regions of Density Overlap. J. Chem. Theory Comput. 2014, 10, 3745–3756. [Google Scholar] [CrossRef]
- Zupan, A.; Perdew, J.P.; Burke, K.; Causà, M. Density-gradient analysis for density functional theory: Application to atoms. Int. J. Quant. Chem. 1997, 61, 835–845. [Google Scholar] [CrossRef]
- Zupan, A.; Burke, K.; Ernzerhof, M.; Perdew, J.P. Distributions and averages of electron density parameters: Explaining the effects of gradient corrections. J. Chem. Phys. 1997, 106, 10184–10193. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Chen, F. A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Matito, E.; Poater, J.; Solà, M.; Duran, M.; Salvador, P. Comparison of the AIM delocalization index and the Mayer and Fuzzy atom bond orders. J. Phys. Chem. A 2005, 109, 9904–9910. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for ‘fuzzy’ atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Stephens, M.E. Spatial localization of the electronic pair and number distributions in molecules. J. Am. Chem. Soc. 1975, 97, 7391–7399. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Werstiuk, N.H. A practical and efficient method to calculate AIM localization and delocalization indices at post-HF levels of theory. J. Comp. Chem. 2003, 24, 379–385. [Google Scholar] [CrossRef]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Outeiral, C.; Vincent, M.A.; Martín Pendás, Á.; Popelier, P.L.A. Revitalizing the concept of bond order through delocalization measures in real space. Chem. Sci. 2018, 9, 5517–5529. [Google Scholar] [CrossRef] [Green Version]
- Brixner, L.H. Preparation and properties of the single crystalline AB2-type selenides and tellurides of niobium, tantalum, molybdenum and tungsten. J. Inorg. Nucl. Chem. 1962, 24, 257–263. [Google Scholar] [CrossRef]
- Yanaki, A.A.; Obolonchik, V.A. Preparation of transition-metal tellurides by means of hydrogen telluride. Inorg. Mater. 1973, 9, 1855–1858. [Google Scholar]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Carlson, R.K.; Truhlar, D.G.; Gagliardi, L. Multiconfiguration Pair-Density Functional Theory: A Fully Translated Gradient Approximation and Its Performance for Transition Metal Dimers and the Spectroscopy of Re2Cl82−. J. Chem. Theory Comp. 2015, 11, 4077–4085. [Google Scholar] [CrossRef]
- Duan, C.; Chen, S.; Taylor, M.G.; Liu, F.; Kulik, H.J. Machine learning to tame divergent density functional approximations: A new path to consensus materials design principles. Chem. Sci. 2021, 12, 13021–13036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Verma, P.; Jin, X.; Truhlar, D.G.; He, X. Revised M06 density functional for main-group and transition-metal chemistry. Proc. Nail. Acad. Sci. USA 2018, 115, 10257–10262. [Google Scholar] [CrossRef] [Green Version]
- Kepp, K.P. Accuracy of theoretical catalysis from a model of iron-catalyzed ammonia synthesis. Commun. Chem. 2018, 1, 63. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pople, J.A. The Lennard-Jones lecture. Intermolecular binding. Faraday Discuss. 1982, 73, 7–17. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A look at the density functional theory zoo with the advanced GMTKN55 database for general main group thermochemistry, kinetics and noncovalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef] [Green Version]
- Santra, G.; Martin, J.M.L. What Types of Chemical Problems Benefit from Density-Corrected DFT? A Probe Using an Extensive and Chemically Diverse Test Suite. J. Chem. Theory Comp. 2021, 17, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, J.F.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [Green Version]
- Keith, T.A. AIMAll (Version 19.10.12); TK Gristmill Software: Overland Park, KS, USA, 2016; Available online: https://aim.tkgristmill.com (accessed on 20 October 2021).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Molec. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).