Abstract
Loss of the flagellum was an important step in the evolution of fungi. The flagellated fungi of the phylum Olpidiomycota are the closest relative of the non-flagellated terrestrial fungi. There are genes encoding proteins, the occurrence of which shows a strong correlation with the incidence of the flagellum. One of these gene/protein families is “TPPP-like proteins” whose main feature is the presence of the p25alpha domain. The functional link between TPPP and flagellum has also been shown. Most of the phyla of flagellated fungi have been known to contain TPPP-like proteins but Olpidiomycota was an exception. This study demonstrates that Olpidium bornovanus, similarly to some fungi of Chytridiomycota and Blastocladiomycota, has a “fungal-type” TPPP characterized by the presence of two (a complete and an incomplete) p25alpha domains.
1. Introduction
The early-branching fungi (i.e., which are near to the root of the tree) reproduce through the production of motile zoospores, propelled by a single, posteriorly oriented flagellum and are dependent on aquatic environment for dispersal [1]. However, more evolved terrestrial fungi “lost the flagellated zoospore stage and invented means to disperse the spores aerially” [2]. Loss of the flagellum was an important step in the evolution of fungi and was interpreted to be associated with the terrestrial radiation of non-flagellated fungi [3]. The loss of the flagellum might have either occurred once [4] or at least four times [5]. However, recent data by Chang et al. [2] support the first scenario. According to the recent classification by Tedersoo et al. [6], which adopts 18 phyla in Fungi, flagellated fungi can be found in seven phyla: Rozellomycota (alias Cryptomicota), Aphelidiomycota, Neocallimastigomycota, Monoblepharomycota, Chytridiomycota, Blastocladiomycota and Olpidiomycota.
Comparative genomic studies [7,8] revealed the existence of the so-called flagellar or ‘ciliary’ genes and proteins, which are present in all eukaryotic organism possessing flagella. Some of these genes/proteins belong to the family of TPPP-like proteins containing at least one p25alpha domain [9,10], which consists of about 160 amino acids [11,12]. (TPPP refers to the term “tubulin polymerization promoting protein” [13].) The p25alpha domain (Pfam05517, IPR008907) generally does not occur in non-flagellated species. The functional connection between TPPP and flagellum was proven in Chlamydomonas reinhardtii, a biflagellated green alga [14]. Its TPPP ortholog, FAP265 protein, can be found in the flagella, and is essential in its formation, as shown by using FAP265 null mutants [14]. TPPP-like proteins can be grouped according to two characteristics: (i) the length of their p25alpha domain, which can be long, short, truncated or partial; and (ii) the presence or absence of other type of domains [10]. (For example, apicortin contains both partial p25alpha and DCX domains [15].) Recently, a TPPP-form present only in Fungi has also been identified [16]. (See later).
A novel study has revealed that as expected, most of the flagellated fungi contain TPPP-like proteins [16]. Phyla Rozellomycota (Cryptomycota), Neocallimastigomycota, Monoblepharomycota, Chytridiomycota and Blastocladiomycota possess these proteins; however, they were not found in Aphelidiomycota and Olpidiomycota, probably due to the lack of enough genomic and proteomic data, either in general (NCBI) or in special (Mycocosm) databases. The Olpidiomycota contains only a single genus Olpidium which was placed among Zygomycota, species of which are non-flagellated [1,17]. It was suggested [16] that the apparent hiatus of TPPP-like proteins in Olpidium is due to its incomplete sequencing caused by technical problems [18]. However, a recent analysis has shown that in contrast to the above mentioned two studies where Olpidium was placed within non-flagellated terrestrial fungi, Olpidium is their sister group [2]. This topology substantiates a single loss of the flagellum rather than its multiple losses among the fungi [2]. The repositioning was possible since the authors of that study successfully sequenced the Olpidium bornovanus which provided sufficient data for the analysis. In this paper, I show that O. bornovanus has at least one TPPP-like (p25alpha domain containing) protein by analyzing sequence data recently made available on the NCBI website.
2. Results
TPPP-like proteins are characterized by the presence of the p25alpha domain [10]. It starts generally with a LxxxF(Y)xxFxxF sequence. The C-terminal part of the domain contains a very characteristic “Rossman-like” sequence, GxGxGxxGR (Figure 1) [10]. These proteins can be grouped on the basis of the length and completeness of the p25alpha domain and the presence of another kind of domain(s) [10]. A special, “fungal-type” TPPP, which contains both a full and a partial (C-terminal) p25alpha domain, is present only in certain Fungi (Figure 1) [16].
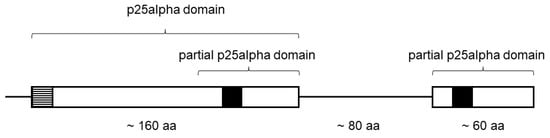
Figure 1.
Schematic structure of a fungal-type TPPP. The positions of the Rossmann-like motifs (GXGXGXXGR) are indicated by black squares. The dashed line square indicates the LxxxF(Y)xxFxxF sequence. aa- amino acids.
Blast analyses in NCBI databases (https://www.ncbi.nlm.nih.gov/protein/ and https://www.ncbi.nlm.nih.gov/nuccore, accessed on 4 November 2021) revealed the presence of p25alpha domain containing proteins and nucleotides in O. bornovanus. Two partial hypothetical proteins, KAG5460860 and KAG5458366, and two WGS (whole genome shotgun) sequences, JAEFCI010004592 and JAEFCI010008581, were found. KAG5460860 and KAG5458366 includes 64 and 163 amino acids, respectively. The amino acid sequences of KAG5460860 and KAG5458366 correspond partly to the JAEFCI010004592 and JAEFCI010008581 WGS sequences, respectively. JAEFCI010008581 encodes the C-terminal part of the KAG5458366 protein since the nucleotide bases coding the last amino acid of the protein are followed by a stop codon. The N-terminal half is missing and cannot be completed based on this nucleotide sequence. It seems that the beginning of this partial protein corresponds to a real exon boundary since the TPX65513 protein of Chytriomyces confervae (a fungal type TPPP) has an exon boundary exactly at this position.
However, the translation of the JAEFCI010004592 sequence indicated that the partial sequence of the KAG5460860 hypothetical protein can be completed, at least partly. At the C-terminal end of the partial protein it can be done with certainty (nucleotides 2230-2253) (Figure 2), but not at the N-terminus. KAG5460860 starts with a methionine coded by nucleotides 2038-2040 of JAEFCI010004592. The previous nucleotides were translated manually (Figure 2). Two parts of the translated sequence resulted in amino acid sequences which are highly homologous to known fungal-type proteins (cf. Figure 3). One of such sequences can be found immediately before the starting methionine and coded by nucleotides 1960–2037; the other one is coded by nucleotides 1683–1733 (Figure 2). There is no homology in the middle region (nucleotides 1734–1959). It should be noted that the numbers of nucleotides (226) in this intermediate region cannot be divided by three, there is a phase shift here between the two coding regions, so it is very likely that these nucleotides represent an intron. It is common in most fungal-type TPPPs that the first ~30–40 amino acids are encoded by an exon separated by a phase 1 intron from the second exon (in all members of the classes of Spizellomycetes and Rhizophydiomycetes, and also in Paraphysoderma sedebokerense), which can also be the case here (Figure 4). However, the very N-terminal part of the first exon is missing; the homologous translated sequence starts with the fifth amino acid of the p25alpha domain, and no initiation codon can be identified. The N-terminus may be encoded by another exon, but we cannot say for sure.

Figure 2.
Partial sequence of Olpidium bornovanus isolate S191 BJ554k121_659286, whole genome shotgun sequence (GenBank: JAEFCI010004592.1). Numbers indicate the order of its nucleotides. Black background indicates the nucleotides corresponding to the KAG5460860, hypothetical protein. Bold capital letters show the amino acid sequence of this protein. Gray background indicates nucleotides whose translation are shown in Figure 3. The corresponding amino acids are shown with lower case italic letters. The possible positions of the initiation codon (atg) are labeled by bold letters.
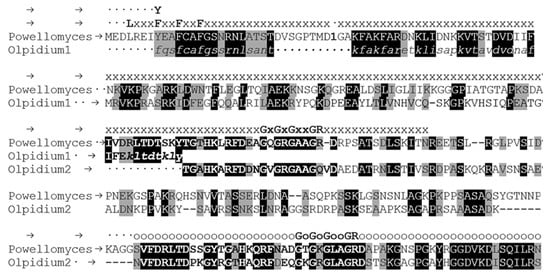
Figure 3.
Alignment of Olpidium bornovanus and Powellomyces hirtus proteins by Clustal Omega [19]. Olpidium1—O. bornovanus KAG5460860, hypothetical protein, partial. Olpidium2—O. bornovanus KAG5458366, hypothetical protein, partial. Powellomyces—P. hirtus TPX57673, hypothetical protein. Lower case italic letters stand for amino acids of Olpidium1 obtained by manual translation of JAEFCI010004592 whole genome shotgun sequence. Bold letters stand for sequences being present in duplicates in the proteins. Identical and biochemically similar amino acids in both proteins are labeled by black and grey background, respectively. Letters ‘x’ and ‘o’ indicate the p25alpha and partial p25alpha domains, respectively. The “Rossman-like” sequences, GXGXGXXGR, and the LXXF(Y)XXFXXF sequence at the beginning of the p25alpha domain are also shown. The position of the first intron (phase 1) of the P. hirtus protein is marked by number 1. (The glycine is coded by the last nucleotide of the first exon and the first two nucleotide of the second one).


Figure 4.
Multiple alignment (manually refined) of the p25alpha domains of fungal-type TPPPs by Clustal Omega [20]. The N-termini (amino acids before the p25alpha domain) and the interdomain parts are not included in the alignment. Amino acids that are identical and biochemically similar in at least two thirds of the proteins are labeled by black and grey background, respectively. The “Rossman-like” sequences, GXGXGXXGR, are also shown. The positions of a phase 1 intron of some proteins and that of a phase 0 intron of Chytriomyces confervae TPX65513 protein are marked by number 1 and 0, respectively. Asterisk (*) indicates that 39 amino acids of Enthel1467718 protein of Entophlyctis helioformis following the arginin (R) are not shown for clarity. Question marks (?) indicate the positions of the tentative introns of Olpidium bornovanus TPPP. The Accession Numbers of fungal proteins are listed in Table 1.
The possible starting methionine may be encoded by triplets 1505–1507 or 1511–1513 (Figure 2). There are examples of an intron with such a position; in Rhizoclosmatium globosum ORY45507 and Obelidium mucronatum Obemuc1859513 fungal-type TPPPs; there are phase 1 introns here in both cases (Figure 4). Thus O. bornovanus TPPP is encoded probably by four exons. Based on the combination of the partly supplemented sequence of KAG5460860 and the sequence of KAG5458366, a more complete but still an incomplete hypothetical protein sequence can be produced, the very N-terminal part of which is still absent (Figure 3). Using this sequence as query in BLASTP search of the NCBI website, fungal-type TPPPs of various fungi were obtained as best hits (Table 2). Each of them belongs to the Chytridiomycota phylum. TPX57673 protein of P. hirtus was found as the most similar one. The alignment of the Olpidium and the Powellomyces proteins is shown in Figure 3. The Olpidium protein is a “fungal-type” TPPP as well since it contains the last part of the p25alpha domain in duplicate.
An abundant source of fungal sequences is the Mycocosm webpage [19] (https://mycocosm.jgi.doe.gov/mycocosm/home, accessed on 12 November 2021), which contains a great amount of additional data in comparison with the NCBI page. Thus, fungal-type TPPPs from this site were also compared with the Olpidium one (Figure 4). These proteins show a significant homology in their p25alpha domains, which does not hold in the interdomain part the length of which is also different by species. The Rossmann-like sequence is absent in some cases in the first (Catenaria, Blastocladiella, Globomyces, Synchytrium) or the second p25alpha domain (Batrachochytrium) but not in O. bornovanus (Figure 4).
| Species | Accession No. 1 |
|---|---|
| Allomyces macrogynus | KNE68590 |
| Batrachochytrium dendrobatidis JAM81 | XP_006680205 |
| Batrachochytrium salamandrivorans | KAH6573313 |
| Blastocladiella britannica | Blabri126943 |
| Blyttiomyces helicus | RKO89614 |
| Caulochytrium protostelioides | RKP02545 |
| Catenaria anguillulae 1 | ORZ33943 |
| Catenaria anguillulae 2 | ORZ35986 |
| Chytriomyces confervae 1 | TPX65513 |
| Chytriomyces confervae 2 | TPX72533 |
| Chytriomyces lagenaria 1 | Chylag1383254 |
| Chytriomyces lagenaria 2 | Chylag1491303 |
| Cladochytrium polystomum 1 | Clapol11821589 |
| Cladochytrium polystomum 2 | Clapol11869731 |
| Cladochytrium replicatum | Clarep11774182 |
| Entophlyctis helioformis | Enthel1467718 |
| Fimicolochytrium jonesii | Fimjon1566472 |
| Gaertneriomyces semiglobifer | Gaesem1531638 |
| Geranomyces variabilis | Gervar1417039 |
| Globomyces pollinis-pini | Glopol1609812 |
| Gorgonomyces haynaldii | Gorhay1188404 |
| Obelidium mucronatum 1 | Obemuc1832726 |
| Obelidium mucronatum 2 | Obemuc1859513 |
| Olpidium bornovanus | KAG5460860 + KAG5458366 |
| Paraphysoderma sedebokerense | Parsed11082034 |
| Powellomyces hirtus | TPX57673 |
| Rhizoclosmatium globosum 1 | Rhihy1315321 |
| Rhizoclosmatium globosum 2 | ORY45507 |
| Spizellomyces punctatus | XP_016604112 |
| Synchytrium endobioticum | TPX44587 |
| Synchytrium microbalum | XP_031024160 |
| Triparticalcar arcticum | Triarc1169044 |
1 Uppercase and lowercase accession numbers stand for NCBI and Mycocosm identifiers, respectively.
Based on the multiple alignment of fungal-type TPPPs, phylogenetic analysis was carried out and a phylogenetic tree of fungal-type TPPPs was constructed using Bayesian method (Figure 5). Olpidium, not surprisingly, is separated from all other TPPPs which form three clades corresponding to the known phylogeny. One is the TPPPs of the phylum Blastocladiomycota, the second is those of the phylum Chytridiomycota, the third clade corresponds to a special group of paralogous TPPPs which are present only in certain families of Chytridiomycota (Chytridiomycetes and Cladochytriomycetes) [16]. Within the clades, the species phylogeny is valid, the sub-clades correspond to the various families: Blastocladiomycetes and Physodermatomycetes within Blastocladiomycota; Rhizophydiomycetes, Cladochytriomycetes, Synchytriomycetes and Spizellomycetes within Chytridiomycota. Here, TPPPs of Chytridiomycetes and Cladochytriomycetes form a common sub-clade as found earlier [16].
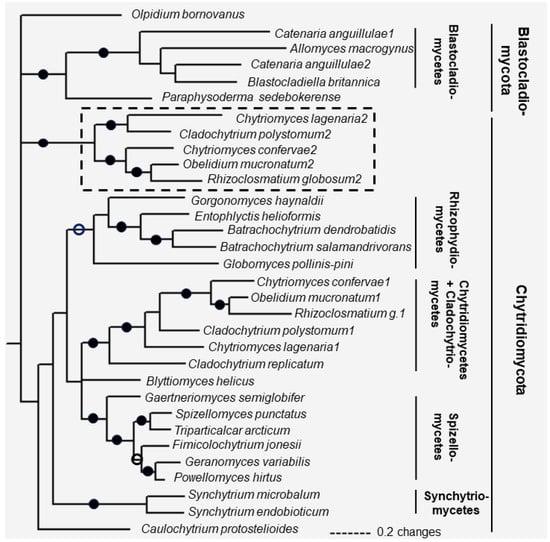
Figure 5.
Phylogenetic tree of fungal-type TPPPs constructed by Bayesian analysis. Number of generations was 2.4 × 10−6. Full and open circles at a node indicate that the branch was supported by maximal Bayesian posterior probability (BPP) and ≥0.95 BPP, respectively. All the other branches were supported by BPP ≥ 50%. The box with dotted lines includes fungal-type TPPP paralogs being present only in Chytridiomycetes and Cladochytriomycetes. The Accession Numbers of fungal proteins are listed in Table 1.

Table 2.
Best protein hits when using Olpidium bornovanus KAG5460860 + KAG5458366 as a query.
Table 2.
Best protein hits when using Olpidium bornovanus KAG5460860 + KAG5458366 as a query.
| Species | Max Score | Total Score | Query Cover | E Value 1 | Percent Identy | Length | Accession |
|---|---|---|---|---|---|---|---|
| Olpidium bornovanus | 322 | 322 | 61% | 5 × 10−109 | 100.00% | 163 | KAG5458366 |
| Powellomyces hirtus | 186 | 186 | 100% | 1 × 10−53 | 42.60% | 303 | TPX57673 |
| Spizellomyces punctatus | 179 | 179 | 100% | 9 × 10−51 | 41.07% | 315 | XP_016604112 |
| Spizellomyces sp. ‘palustris’ | 172 | 172 | 100% | 6 ×10−48 | 38.54% | 336 | TPX61118 |
| Caulochytrium protostelioides | 171 | 171 | 100% | 8 × 10−48 | 37.50% | 319 | RKP02545 |
| Chytriomyces confervae | 160 | 160 | 100% | 2 × 10−43 | 35.88% | 335 | TPX65513 |
| Chytriomyces confervae | 155 | 155 | 100% | 2 × 10−41 | 36.30% | 335 | TPX65886 |
| Synchytrium endobioticum | 150 | 150 | 99% | 1 × 10−39 | 38.01% | 338 | TPX44587 |
| Olpidium bornovanus | 139 | 139 | 24% | 2 × 10−38 | 100.00% | 64 | KAG5460860 |
| Synchytrium microbalum | 146 | 146 | 99% | 2 × 10−38 | 36.26% | 286 | XP_031024160 |
| Batrachochytrium salamandrivorans | 124 | 124 | 85% | 5 × 10−30 | 36.22% | 286 | KAH6573313 |
| Batrachochytrium dendrobatidis JAM81 | 120 | 168 | 92% | 2 × 10−28 | 36.23% | 289 | XP_006680205 |
| Batrachochytrium dendrobatidis JEL423 | 120 | 168 | 92% | 3 × 10−28 | 36.23% | 299 | OAJ42613 |
| Rhizoclosmatium globosum | 119 | 119 | 99% | 3 × 10−28 | 36.23% | 262 | ORY45507 |
| Chytriomyces confervae | 117 | 117 | 90% | 9 × 10−28 | 35.00% | 255 | TPX72533 |
| Chytriomyces confervae | 111 | 160 | 72% | 4 × 10−26 | 40.44% | 183 | TPX78276 |
1 E-value is the measure of likeliness that sequence similarity is not by random chance. An E-value smaller than 1 × 10−50 includes database matches of very high quality. Blast hits with E-value smaller than 0.01 can still be considered as good hit for homology matches.
3. Conclusions
All the phyla of the flagellated fungi contain species with TPPP-like proteins except Aphelidiomycota; however, it can be expected that, similar to the case of Olpidiomycota, this deficit will disappear with a progress in sequencing of the members of the phylum. The occurrence of these proteins in Fungi varies according to phyla; the most early branching ones, Rozellomycota, Neocallimastigomycota, and Monoblepharomycota, do not contain the fungal-type TPPPs but other kinds of proteins of this family, such as apicortin, short- and long-type TPPPs [16]. In Chytridiomycota, which is the most well-known phylum of flagellated Fungi, all these proteins can be found, beside the most common fungal-type TPPP. Blastocladiomycota and Olpidiomycota seem to include species that possess only the fungal-type TPPP, featured by the presence of two (a complete and an incomplete) p25alpha domains. It is an open question whether this special TPPP was lost in the phyla closer to the root of the fungal phylogenetic tree, or whether they appeared only in more developed phyla. TPPP-like proteins, in general, are known to stabilize microtubules [13,21]. This protein is indispensable for the proper functioning of the flagellum, a microtubule-based organelle, in C. reinhardtii [14]. It has been shown that the amino acid sequences responsible for binding to microtubule are located at the C-terminus of the p25alpha domain [22,23]. Thus, the fungal-type TPPP contains two microtubule binding sites (cf. Figure 1), which may result in a stronger interaction. Whether it does cause a functional advantage requires further investigation. Through experimental work it can be verified whether TPPP is localized in the flagellum and the microtubule-TPPP interaction occurs in fungi, including Olpidium. However, the occurrence of a p25alpha domain-containing protein in O. bornovanus further strengthens the correlation suggested earlier [9,16] between the incidence of this domain and that of the eukaryotic flagellum.
4. Methods
Database homology search. It was carried out with an NCBI Blast search [24] (http://www.ncbi.nlm.nih.gov/BLAST/, accessed on 12 November 2021): sequences of various fungal proteins (e.g., B. dendrobatidis XP_006680205, C. confervae TPX65513, P. hirtus TPX57673, S. punctatus XP_016604112) containing p25alpha-domain were used as queries against protein and nucleotide databases to find similar sequences in Olpidium using BLASTP and TBLASTN analyses, respectively.
Phylogenetic analysis. Multiple alignments of sequences were done by the Clustal Omega program [20]. Bayesian analysis using MrBayes v3.1.2 [25] was also performed to construct phylogenetic trees. Default priors and the WAG model [26] were used assuming equal rates across sites. Two independent analyses were run with three heated and one cold chain (temperature parameter 0.2) for generations as indicated in the Figure legends, with a sampling frequency of 0.01 and the first 25% of generations were discarded as burn-in. The two runs were convergent. The phylogenetic trees were drawn using the program Drawgram of the PHYLIP package version 3.696 [27].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232213927/s1. Data S1: Sequence alignment used for phylogenetic tree construction in nexus file and phylogenetic tree in con file.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the paper and in the Supplementary Materials.
Acknowledgments
The author thanks Judit Oláh for the careful reading of the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- James, T.Y.; Letcher, P.M.; Longcore, J.E.; Mozley-Standridge, S.E.; Porter, D.; Powell, M.J.; Griffith, G.W.; Vilgalys, R. A molecular phylogeny of the flagellated fungi Chytridiomycota. and description of a new phylum Blastocladiomycota. Mycologia 2006, 98, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Rochon, D.; Sekimoto, S.; Wang, Y.; Chovatia, M.; Sandor, L.; Salamov, A.; Grigoriev, I.V.; Stajich, J.E.; Spatafora, J.W. Genome-scale phylogenetic analyses confirm Olpidium as the closest living zoosporic fungus to the non-flagellated, terrestrial fungi. Sci. Rep. 2021, 11, 3217. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Liu, Y.J.; Hodson, M.C.; Hall, B.D. Loss of the flagellum happened only once in the fungal lineage: Phylogenetic structure of kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evol. Biol. 2006, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Maer, A.M.; Koundakjian, E.; Polyanovsky, A.; Keil, T.; Subramaniam, S.; Zuker, C.S. Decoding cilia function: Defining specialized genes required for compartmentalized cilia biogenesis. Cell 2004, 117, 527–539. [Google Scholar] [CrossRef]
- Li, J.B.; Gerdes, J.M.; Haycraft, C.J.; Fan, Y.; Teslovich, T.M.; May-Simera, H.; Li, H.; Blacque, O.E.; Li, L.; Leitch, C.C.; et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 2004, 117, 541–552. [Google Scholar] [CrossRef]
- Orosz, F.; Ovádi, J. TPPP orthologs are ciliary proteins. FEBS Lett. 2008, 582, 3757–3764. [Google Scholar] [CrossRef]
- Orosz, F. A new protein superfamily: TPPP-like proteins. PLoS ONE 2012, 7, e49276. [Google Scholar] [CrossRef]
- Hlavanda, E.; Kovács, J.; Oláh, J.; Orosz, F.; Medzihradszky, K.F.; Ovádi, J. Brain-specific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry 2002, 41, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Vincze, O.; Tőkési, N.; Oláh, J.; Hlavanda, E.; Zotter, Á.; Horváth, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; et al. Tubulin polymerization promoting proteins (TPPPs): Members of a new family with distinct structures and functions. Biochemistry 2006, 45, 13818–13826. [Google Scholar] [CrossRef] [PubMed]
- Tirián, L.; Hlavanda, E.; Oláh, J.; Horváth, I.; Orosz, F.; Szabó, B.; Kovács, J.; Szabad, J.; Ovádi, J. TPPP/p25 promotes tubulin assemblies and blocks mitotic spindle formation. Proc. Nat. Acad. Sci. USA 2003, 100, 13976–13981. [Google Scholar] [CrossRef] [PubMed]
- Tammana, D.; Tammana, T.V.S. Chlamydomonas FAP265 is a tubulin polymerization promoting protein, essential for flagellar reassembly and hatching of daughter cells from the sporangium. PLoS ONE 2017, 12, e0185108. [Google Scholar] [CrossRef]
- Orosz, F. Apicortin, a unique protein, with a putative cytoskeletal role, shared only by apicomplexan parasites and the placozoan Trichoplax adhaerens. Infect. Genet. Evol. 2009, 9, 1275–1286. [Google Scholar] [CrossRef]
- Orosz, F. On the TPPP-like proteins of flagellated Fungi. Fungal Biol. 2021, 125, 357–367. [Google Scholar] [CrossRef]
- Sekimoto, S.; Rochon, D.; Long, J.E.; Dee, J.M.; Berbee, M.L. A multigene phylogeny of Olpidium and its implications for early fungal evolution. BMC Evol. Biol. 2011, 11, 331. [Google Scholar] [CrossRef]
- Lay, C.Y.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenicity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Leung, J.M.; Nagayasu, E.; Hwang, Y.C.; Liu, J.; Pierce, P.G.; Phan, I.Q.; Prentice, R.A.; Murray, J.M.; Hu, K. A doublecortin-domain protein of Toxoplasma and its orthologues bind to and modify the structure and organization of tubulin polymers. BMC Mol. Cell Biol. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Tőkési, N.; Oláh, J.; Hlavanda, E.; Szunyogh, S.; Szabó, A.; Babos, F.; Magyar, A.; Lehotzky, A.; Vass, E.; Ovádi, J. Identification of motives mediating alternative functions of the neomorphic moonlighting TPPP/p25. Biochim. Biophys. Acta 2014, 1842, 547–557. [Google Scholar] [CrossRef]
- Oláh, J.; Szénási, T.; Szabó, A.; Kovács, K.; Lőw, P.; Stifanic, M.; Orosz, F. Tubulin binding and polymerization promoting properties of TPPP proteins are evolutionarily conserved. Biochemistry 2017, 56, 1017–1024. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucl. Acid Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixture models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. PHYLIP Phylogeny Inference Package, version 3.696; Department of Genome Sciences and Department of Biology University of Washington: Seattle, WA, USA, 2008; Available online: http://evolution.genetics.washington.edu/phylip.html (accessed on 13 August 2008).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).