Therapeutic Ultrasound Halts Progression of Chronic Kidney Disease In Vivo via the Regulation of Markers Associated with Renal Epithelial–Mesenchymal Transition and Senescence
Abstract
1. Introduction
2. Results
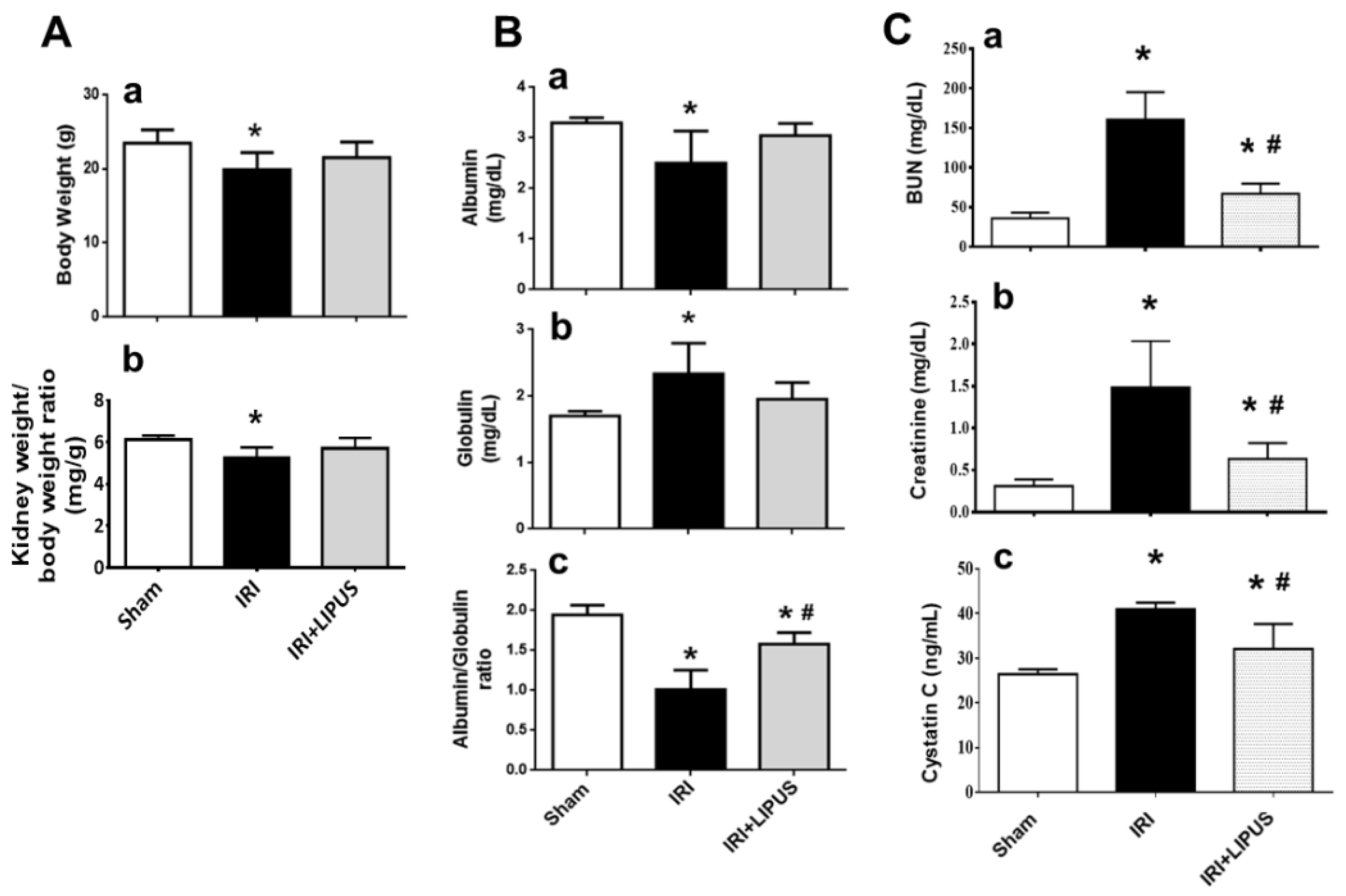
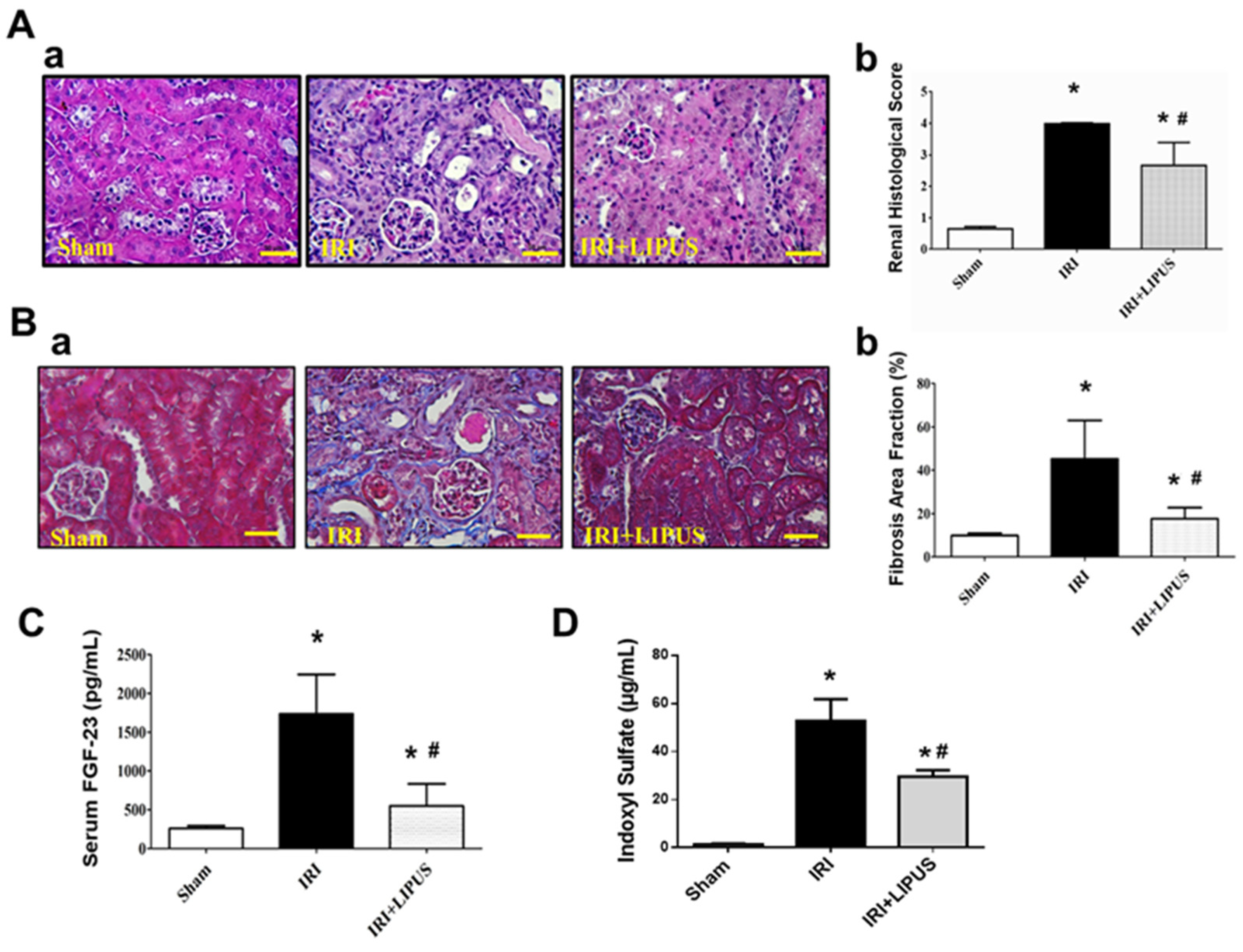
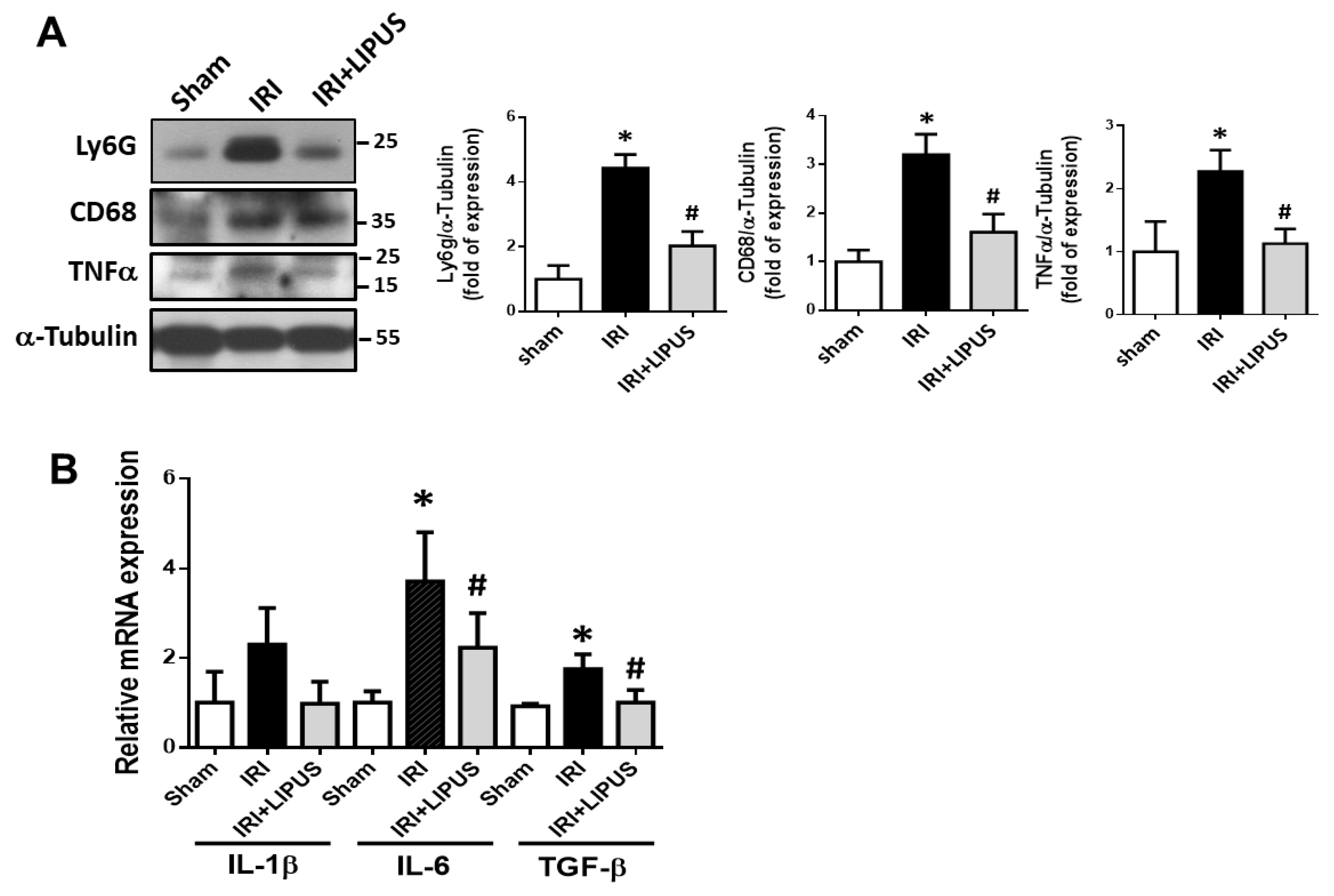
2.1. LIPUS Alleviated Changes in Body Weight, Serum Biochemistry, and Renal Injury in IRI-CKD Mouse Models
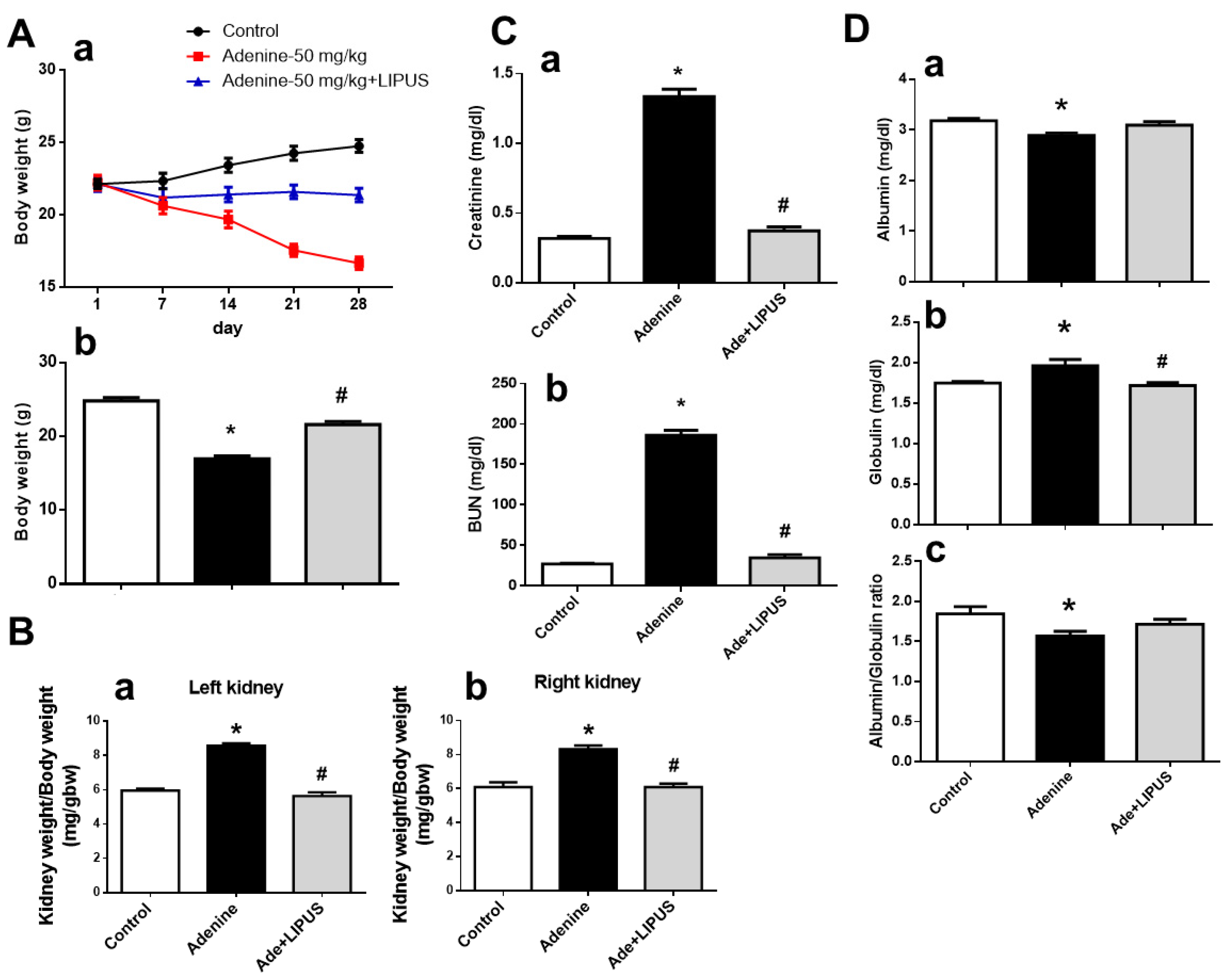
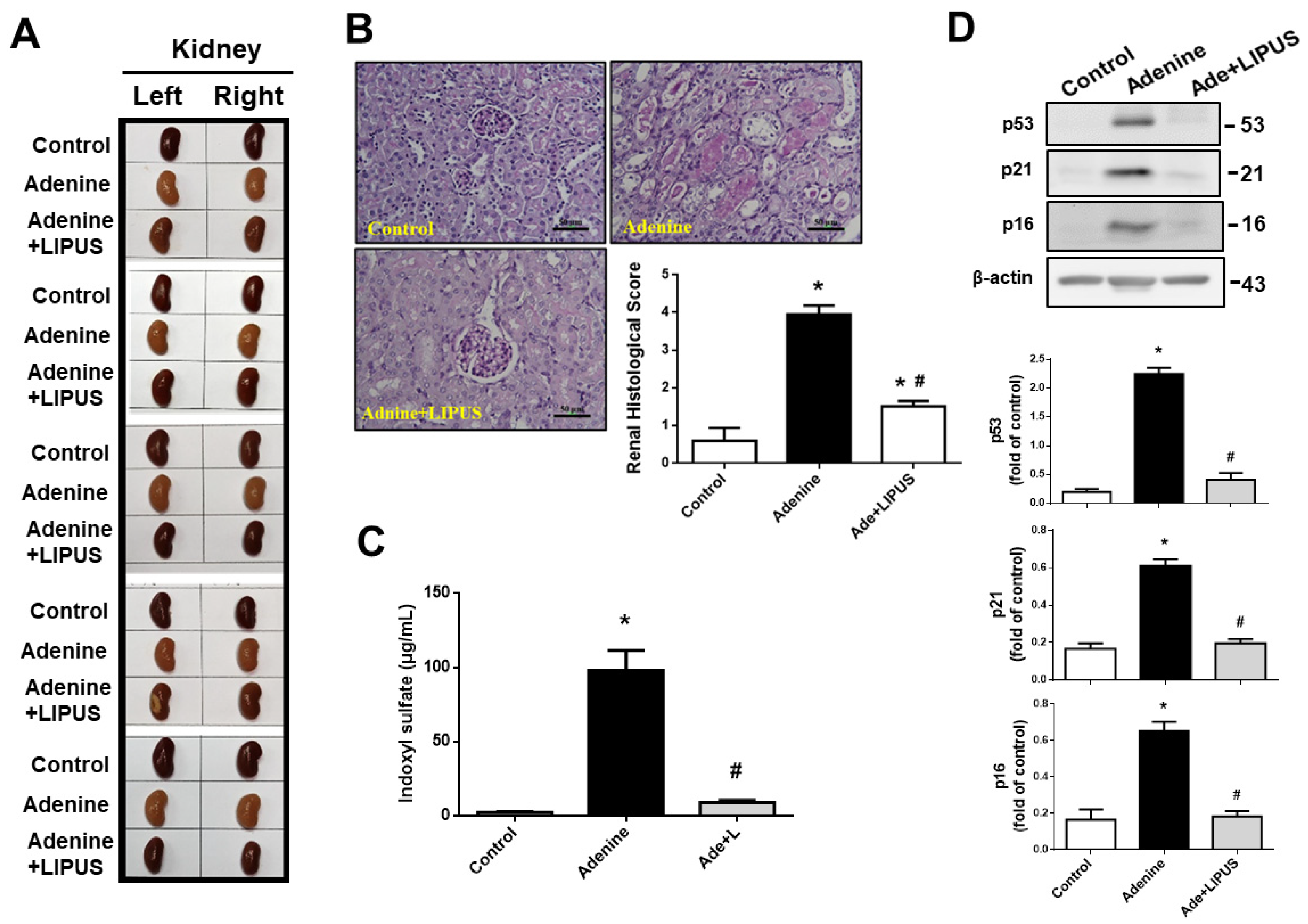
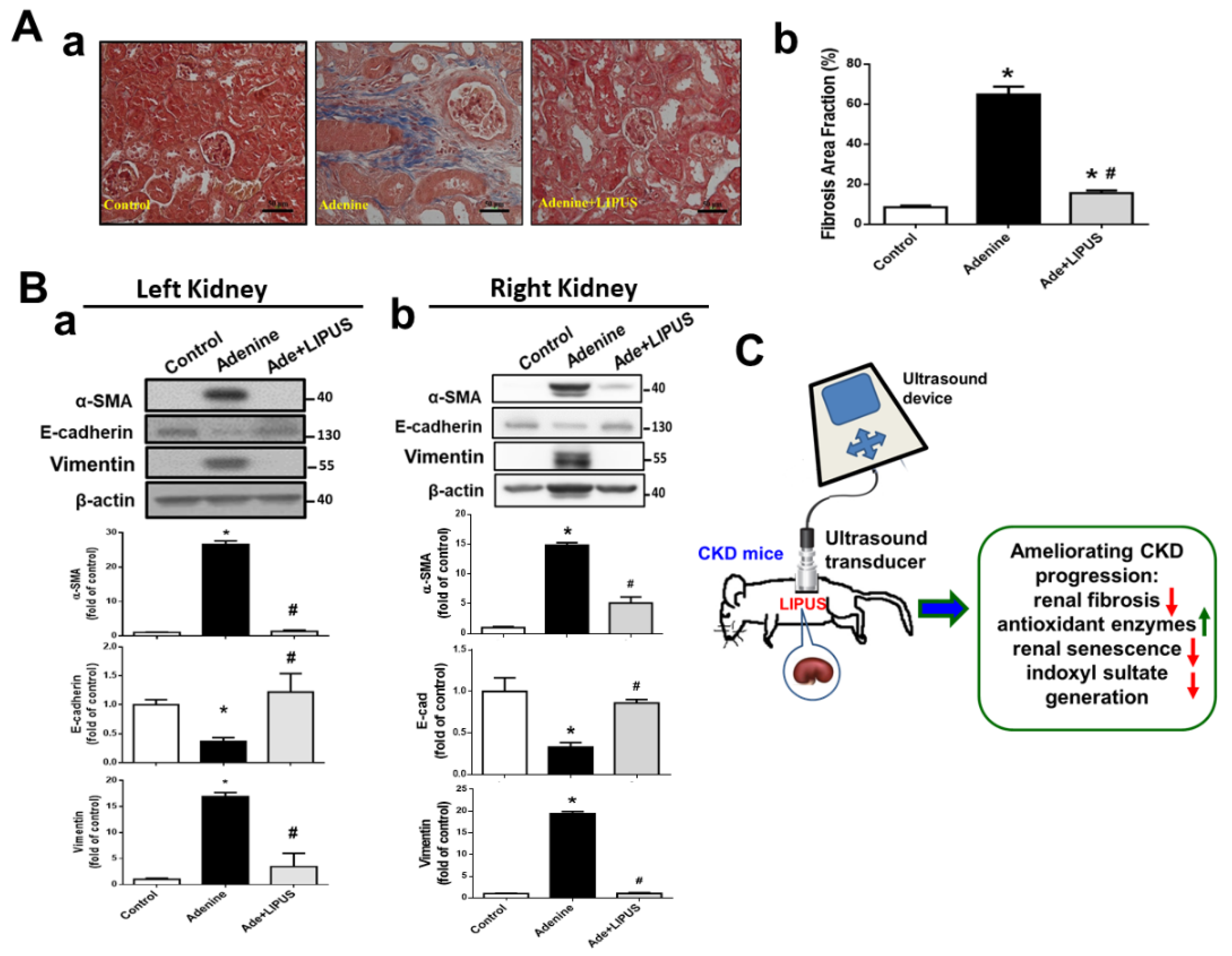
2.2. LIPUS Alleviated Changes in Body Weight, Serum Biochemistry, and Renal Injury in Adenine-Induced CKD Mouse Models
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.1.1. IRI-Induced CKD Model
4.1.2. Adenine-Induced CKD Model
4.2. LIPUS Treatment
4.3. Serum Biochemistry Analysis
4.4. Detection of Serum Indoxyl Sulfate
4.5. Histology Analysis
4.6. Immunoblotting
4.7. Real-Time Quantitative PCR (qPCR)
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mak, R.H.; Cheung, W.; Cone, R.D.; Marks, D.L. Mechanisms of disease: Cytokine and adipokine signaling in uremic cachexia. Nature clinical practice. Nephrology 2006, 2, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.R. The stimulation of bone growth by ultrasound. Arch. Orthop. Trauma. Surg. 1983, 101, 153–159. [Google Scholar] [CrossRef]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J. Bone Jt. Surg. 1994, 76, 26–34. [Google Scholar] [CrossRef]
- Ogata, T.; Ito, K.; Shindo, T.; Hatanaka, K.; Eguchi, K.; Kurosawa, R.; Kagaya, Y.; Monma, Y.; Ichijo, S.; Taki, H.; et al. Low-intensity pulsed ultrasound enhances angiogenesis and ameliorates contractile dysfunction of pressure-overloaded heart in mice. PLoS ONE 2017, 12, e0185555. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, K.; Ito, K.; Aizawa, K.; Shindo, T.; Nishimiya, K.; Hasebe, Y.; Tuburaya, R.; Hasegawa, H.; Yasuda, S.; Kanai, H.; et al. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS ONE 2014, 9, e104863. [Google Scholar] [CrossRef]
- Tang, L.; Li, N.; Jian, W.; Kang, Y.; Yin, B.; Sun, S.; Guo, J.; Sun, L.; Ta, D. Low-intensity pulsed ultrasound prevents muscle atrophy induced by type 1 diabetes in rats. Skelet. Muscle 2017, 7, 29. [Google Scholar] [CrossRef]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Forbes, J.M.; Hewitson, T.D.; Becker, G.J.; Jones, C.L. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000, 57, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Huang, L.; Ye, H.; Bajwa, A.; Chattrabhuti, K.; Lee, S.; Klibanov, A.L.; Kalantari, K.; Rosin, D.L.; Okusa, M.D. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol. 2013, 24, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Loh, J.Z.; Yang, T.H.; Huang, K.T.; Wu, C.T.; Guan, S.S.; Liu, S.H.; Hung, K.Y. Prevention of acute kidney injury by low intensity pulsed ultrasound via anti-inflammation and anti-apoptosis. Sci. Rep. 2020, 10, 14317. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Yamazaki, D.; Sufiun, A.; Kitada, K.; Hitomi, H.; Nakano, D.; Nishiyama, A. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS ONE 2018, 13, e0192531. [Google Scholar] [CrossRef] [PubMed]
- Harwood, R.; Bridge, J.; Ressel, L.; Scarfe, L.; Sharkey, J.; Czanner, G.; Kalra, P.A.; Odudu, A.; Kenny, S.; Wilm, B.; et al. Murine models of renal ischemia reperfusion injury: An opportunity for refinement using noninvasive monitoring methods. Physiol. Rep. 2022, 10, e15211. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef]
- Gao, L.; Zhong, X.; Jin, J.; Li, J.; Meng, X.M. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct. Target. Ther. 2020, 5, 9. [Google Scholar] [CrossRef]
- Basile, D.P.; Rovak, J.M.; Martin, D.R.; Hammerman, M.R. Increased transforming growth factor-beta 1 expression in regenerating rat renal tubules following ischemic injury. Am. J. Physiol. 1996, 270, F500–F509. [Google Scholar] [CrossRef]
- Gewin, L. The many talents of transforming growth factor-β in the kidney. Curr. Opin. Nephrol. Hypertens. 2019, 28, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.A.; McCarty, J.H. TGF-β activation and signaling in angiogenesis. In Physiologic and Pathologic Angiogenesis-Signaling Mechanisms and Targeted Therapy; Simionescu, D., Simionescu, A., Eds.; InTechOpen: London, UK, 2007. [Google Scholar] [CrossRef]
- Kinashi, H.; Ito, Y.; Sun, T.; Katsuno, T.; Takei, Y. Roles of the TGF-β–VEGF-C pathway in fibrosis-related lymphangiogenesis. Int. J. Mol. Sci. 2018, 19, 2487. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, O.; López-Hernández, F.J.; López-Novoa, J.M. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010, 77, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Holle, J.; Kirchner, M.; Okun, J.; Bayazit, A.K.; Obrycki, L.; Canpolat, N.; Bulut, I.K.; Azukaitis, K.; Duzova, A.; Ranchin, B.; et al. Serum indoxyl sulfate concentrations associate with progression of chronic kidney disease in children. PLoS ONE 2020, 15, e0240446. [Google Scholar] [CrossRef]
- Yang, K.; Du, C.; Wang, X.; Li, F.; Xu, Y.; Wang, S.; Chen, S.; Chen, F.; Shen, M.; Chen, M.; et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood 2017, 129, 2667–2679. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Brunori, G.; Kopple, J.D.; Traverso, G.; Panarello, G.; Enia, G.; Strippoli, P.; De Vecchi, A.; Querques, M.; Viglino, G. Cross-sectional comparison of malnutrition in continuous ambulatory peritoneal dialysis and hemodialysis patients. Am. J. Kidney Dis. 1995, 26, 475–486. [Google Scholar] [CrossRef]
- Wu, P.P.; Hsieh, Y.P.; Kor, C.T.; Chiu, P.F. Association between albumin-globulin ratio and mortality in patients with chronic kidney disease. J. Clin. Med. 2019, 8, 1991. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Quarles, L.D. Fibroblast growth factor-23: What we know, what we don’t know, and what we need to know. Nephrol. Dial. Transplant. 2013, 28, 2228–2236. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sugiyama, H.; Wang, D.H.; Toda, N.; Maeshima, Y.; Yamasaki, Y.; Masuoka, N.; Yamada, M.; Kira, S.; Makino, H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 2005, 68, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Kim, J.; Westphal, S.N.; Long, K.E.; Padanilam, B.J. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J. Am. Soc. Nephrol. 2014, 25, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Guo, X.; Che, L.; Guan, X.; Wu, B.; Lu, R.; Zhu, M.; Pang, H.; Yan, Y.; Ni, Z.; et al. Klotho reduces necroptosis by targeting oxidative stress involved in renal ischemic-reperfusion injury. Cell. Physiol. Biochem. 2018, 45, 2268–2282. [Google Scholar] [CrossRef]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Renal and extrarenal actions of Klotho. Semin. Nephrol. 2013, 33, 118–129. [Google Scholar] [CrossRef]
- Fan, H.; Yang, H.C.; You, L.; Wang, Y.Y.; He, W.J.; Hao, C.M. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [CrossRef]
- Ponnusamy, M.; Zhou, X.; Yan, Y.; Tang, J.; Tolbert, E.; Zhao, T.C.; Gong, R.; Zhuang, S. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J. Pharmacol. Exp. Ther. 2014, 350, 243–256. [Google Scholar] [CrossRef]
- Muratsubaki, S.; Kuno, A.; Tanno, M.; Miki, T.; Yano, T.; Sugawara, H.; Shibata, S.; Abe, K.; Ishikawa, S.; Ohno, K.; et al. Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes. Sci. Rep. 2017, 7, 5311. [Google Scholar] [CrossRef]
- Tsuruta, J.K.; Dayton, P.A.; Gallippi, C.M.; O’Rand, M.G.; Streicker, M.A.; Gessner, R.C.; Gregory, T.S.; Silva, E.J.; Hamil, K.G.; Moser, G.J.; et al. Therapeutic ultrasound as a potential male contraceptive: Power, frequency and temperature required to deplete rat testes of meiotic cells and epididymides of sperm determined using a commercially available system. Reprod. Biol. Endocrinol. 2012, 10, 7. [Google Scholar] [CrossRef]
- Kito, Y.; Saigo, C.; Takeuchi, T. Novel transgenic mouse model of polycystic kidney disease. Am. J. Pathol. 2017, 187, 1916–1922. [Google Scholar] [CrossRef]
- Gigliotti, J.C.; Huang, L.; Bajwa, A.; Ye, H.; Mace, E.H.; Hossack, J.A.; Kalantari, K.; Inoue, T.; Rosin, D.L.; Okusa, M.D. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J. Am. Soc. Nephrol. 2015, 26, 2470–2481. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Wang, C.-C.; Loh, J.-Z.; Chiang, T.-C.; Weng, T.-I.; Chan, D.-C.; Hung, K.-Y.; Chiang, C.-K.; Liu, S.-H. Therapeutic Ultrasound Halts Progression of Chronic Kidney Disease In Vivo via the Regulation of Markers Associated with Renal Epithelial–Mesenchymal Transition and Senescence. Int. J. Mol. Sci. 2022, 23, 13387. https://doi.org/10.3390/ijms232113387
Lin C-Y, Wang C-C, Loh J-Z, Chiang T-C, Weng T-I, Chan D-C, Hung K-Y, Chiang C-K, Liu S-H. Therapeutic Ultrasound Halts Progression of Chronic Kidney Disease In Vivo via the Regulation of Markers Associated with Renal Epithelial–Mesenchymal Transition and Senescence. International Journal of Molecular Sciences. 2022; 23(21):13387. https://doi.org/10.3390/ijms232113387
Chicago/Turabian StyleLin, Chen-Yu, Ching-Chia Wang, Jui-Zhi Loh, Tsai-Chen Chiang, Te-I Weng, Ding-Cheng Chan, Kuan-Yu Hung, Chih-Kang Chiang, and Shing-Hwa Liu. 2022. "Therapeutic Ultrasound Halts Progression of Chronic Kidney Disease In Vivo via the Regulation of Markers Associated with Renal Epithelial–Mesenchymal Transition and Senescence" International Journal of Molecular Sciences 23, no. 21: 13387. https://doi.org/10.3390/ijms232113387
APA StyleLin, C.-Y., Wang, C.-C., Loh, J.-Z., Chiang, T.-C., Weng, T.-I., Chan, D.-C., Hung, K.-Y., Chiang, C.-K., & Liu, S.-H. (2022). Therapeutic Ultrasound Halts Progression of Chronic Kidney Disease In Vivo via the Regulation of Markers Associated with Renal Epithelial–Mesenchymal Transition and Senescence. International Journal of Molecular Sciences, 23(21), 13387. https://doi.org/10.3390/ijms232113387






