Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis
Abstract
1. Introduction
2. Results
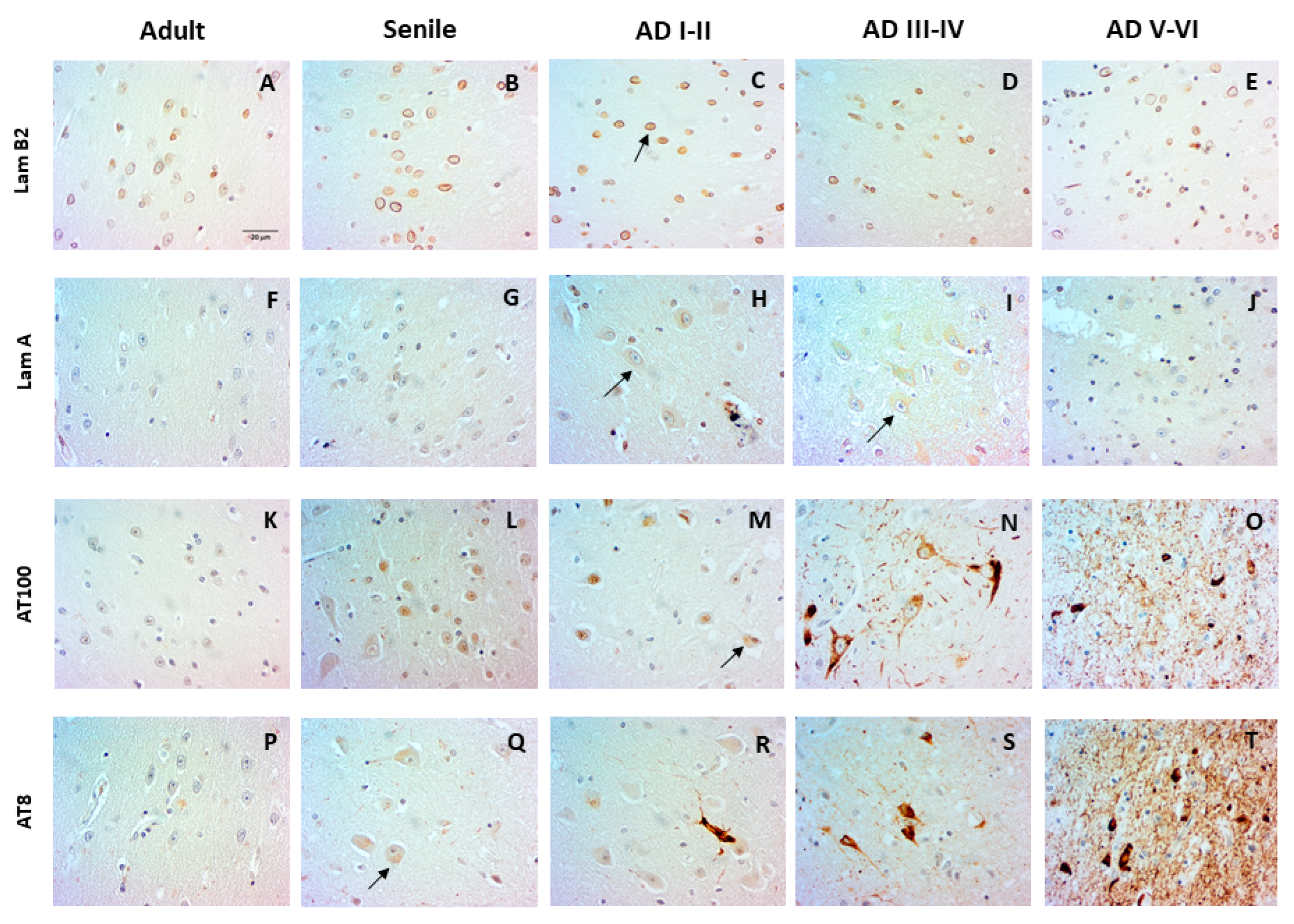
2.1. Nuclear Changes in the Entorhinal Cortex at Early AD Stages
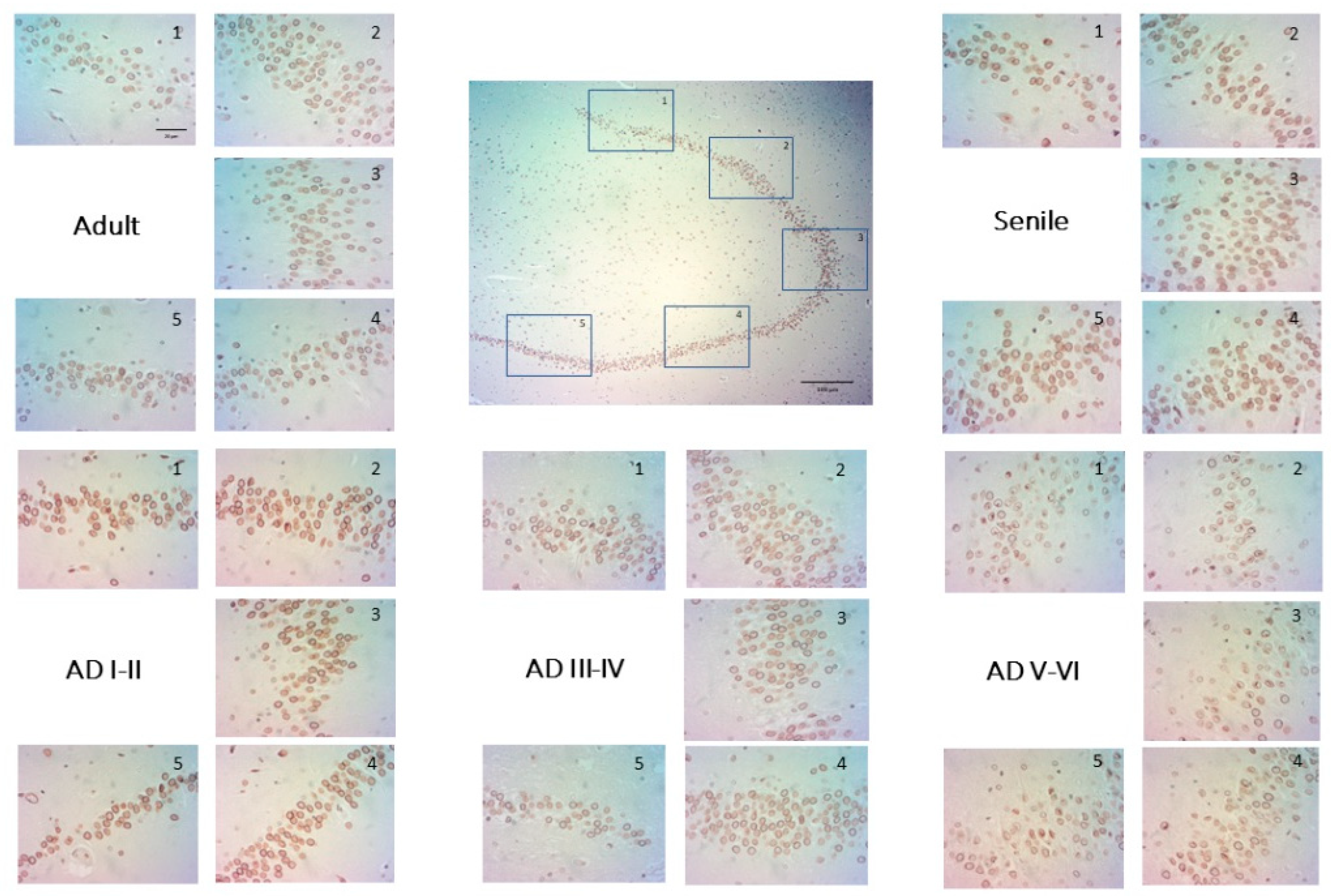
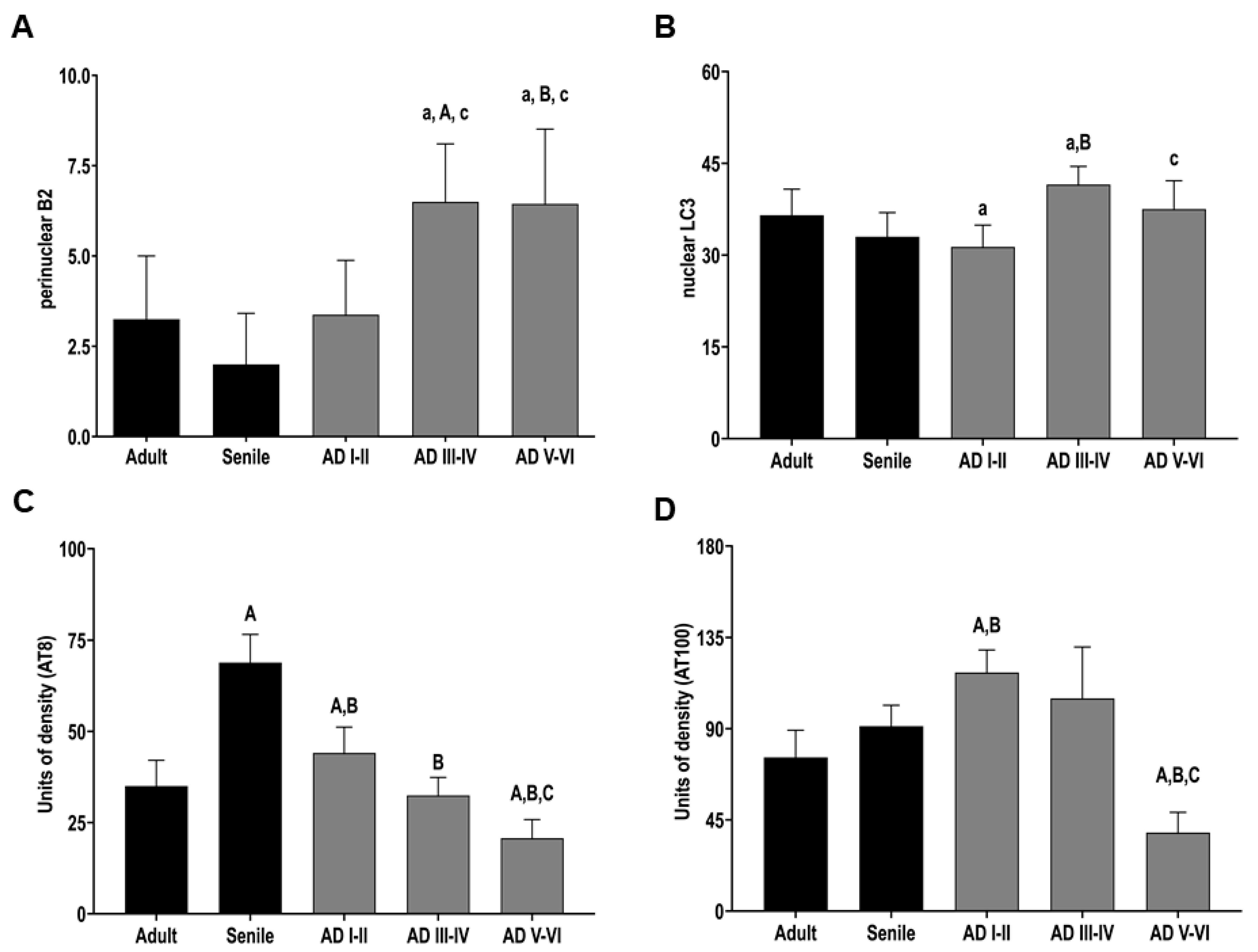
2.2. The Distribution of Lamin B2 Uncovers Two Populations of DG Granular Neurons
2.3. Phosphorylated Tau (AT100 and AT8) Is Absent in the DG Cell Nucleus at Intermediate and Late AD Stages
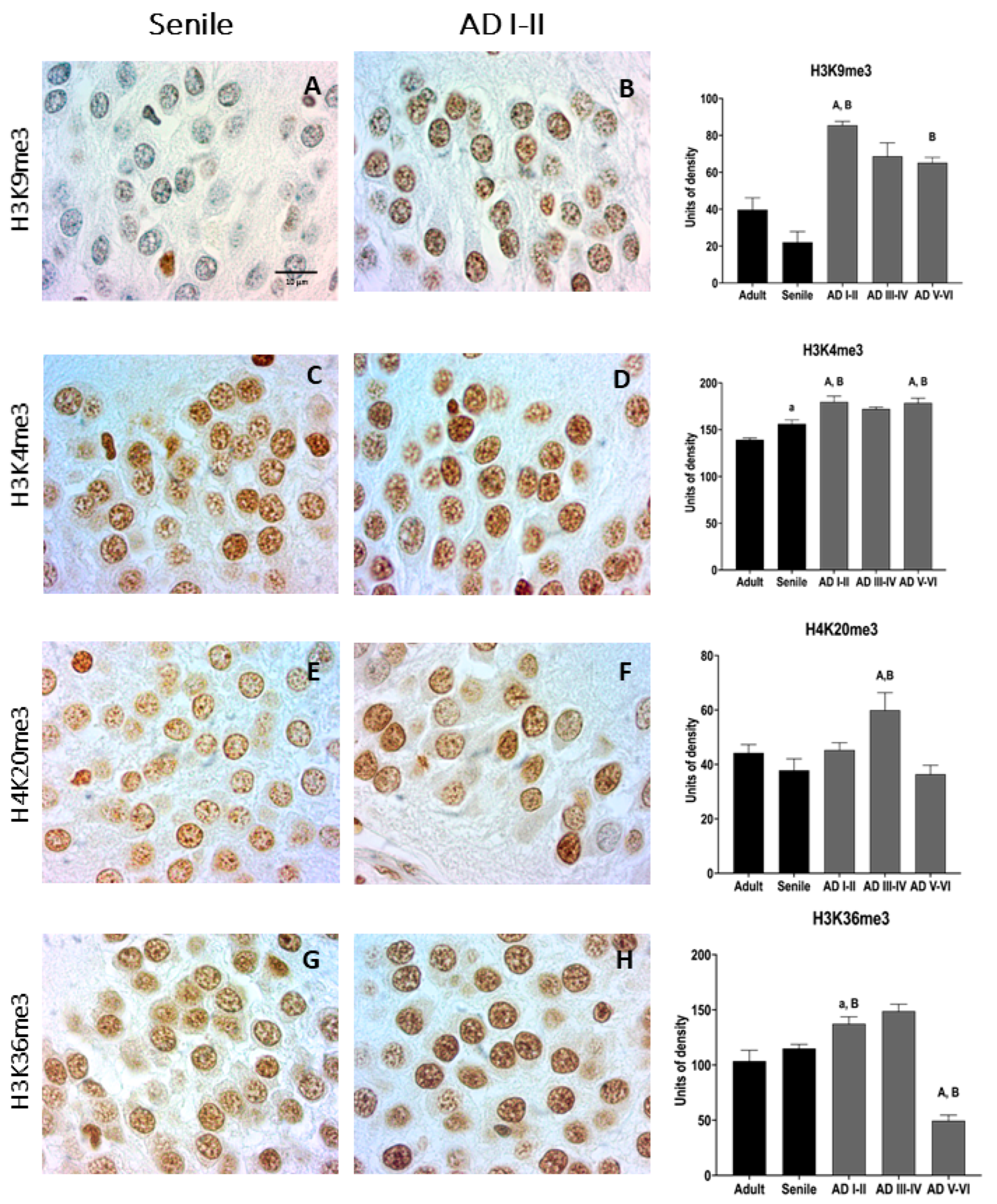
2.4. AD Granular Neurons Show Drastic Changes in Epigenetic Chromatin Markers
2.5. Intermediate and Late AD Stages Are Characterized by Increased Nuclear and Perinuclear Autophagy Marker (LC3)
3. Discussion
3.1. Similarities between Changes in CA1 and Entorhinal Cortex Pyramidal Cells in AD
3.2. The Dynamic Neuronal Nature of DG through Development and Aging
3.3. Heterochromatin Markers and Nuclear Tau: Hallmarks of Early AD in Granular Cells
3.4. Increased Nuclear Autophagy in Late AD Stages Supports Biphasic Neurogenesis Changes in AD
4. Materials and Methods
4.1. Human Brain Samples and Immunohistochemistry
4.2. Image Acquisition and Analysis
4.3. Data Analysis
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AHN | adult human neurogenesis |
| AT8 | Tau protein phosphorylated at Ser202/Thr205 |
| AT100 | Tau protein phosphorylated at Thr212/Ser214 |
| BrDU | 5-bromo-2′-deoxyuridine, proliferation marker |
| CA1 | Ammon’s horn region 1 (hippocampal region with pyramidal cells) |
| CA3 | Ammon’s horn region 3 (hippocampal region with pyramidal cells) |
| CR | calretinin protein, neurogenesis marker |
| ChIP | chromatin immunoprecipitation |
| DCX | doublecortin protein, neurogenesis marker |
| DG | dentate gyrus |
| DRPLA | dentatorubral-pallidoluysian atrophy |
| DSBs | double strand breaks |
| EC | entorhinal cortex |
| GCL | granule cell layer |
| H3K9me3 | trymethylated Histone 3 protein, heterochromatin marker |
| H4K20me3 | trymethylated Histone 4 protein, heterochromatin marker |
| H3K4me3 | trymethylated Histone 3 protein euchromatin marker |
| H3K36me3 | trymethylated Histone 3 protein euchromatin marker |
| iDG | immature dentate gyrus |
| iNs | induced neurons |
| Ki67 | proliferation marker |
| LADs | lamin associated domains |
| LC3 | autophagy marker (protein MAP1LC3B) |
| MAPT | Tau protein |
| NADs | nucleolus-associated domains |
| NL | nuclear lamin |
| NFT | neurofibrillary tangles |
| NSC | neural stem cells |
| PolyQ | polyglutamine repeats-associated disease |
| SGZ | subgranular zone |
References
- McEwen, B.S. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001, 933, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Bender, A.R. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus 2016, 26, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Delgorio, P.L.; Hiscox, L.V. Effect of Aging on the Viscoelastic Properties of Hippocampal Subfields Assessed with High-Resolution MR Elastography. Cereb. Cortex 2021, 31, 2799–2811. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Bernstein, H. DNA damage as the primary cause of aging. Q. Rev. Biol. 1981, 56, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Gaubatz, J.W.; Tan, B.H. Aging affects the levels of DNA damage in postmitotic cells. Ann. N. Y. Acad. Sci. 1994, 719, 97–107. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Van Remmen, H. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. USA 2001, 98, 10469–10474. [Google Scholar] [CrossRef]
- Karanjawala, Z.E.; Lieber, M.R. DNA damage and aging. Mech. Ageing Dev. 2004, 125, 405–416. [Google Scholar]
- Rutten, B.P.; Schmitz, C. The aging brain: Accumulation of DNA damage or neuron loss? Neurobiol. Aging. 2007, 28, 91–98. [Google Scholar] [CrossRef]
- Feser, J.; Tyler, J. Chromatin structure as a mediator of aging. FEBS Lett. 2011, 585, 2041–2048. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear Genomic Instability and Aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef]
- Verheijen, B.M.; Vermulst, M.; van Leeuwen, F.W. Somatic mutations in neurons during aging and neurodegeneration. Acta Neuropathol. 2018, 135, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Alonso, E.; Hernández-Vivanco, A.; Walton, C.C.; Perea, G.; Frade, J.M. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci. Rep. 2018, 8, 14316. [Google Scholar] [CrossRef] [PubMed]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef] [PubMed]
- Herrup, K. Reimagining Alzheimer’s disease--an age-based hypothesis. J. Neurosci. 2010, 30, 16755–16762. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog. Neurobiol. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Frade, J.M.; Ovejero-Benito, M.C. Neuronal cell cycle: The neuron itself and its circumstances. Cell Cycle 2015, 14, 712–720. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Mertens, J.; Herdy, J.R. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 2021, 28, 1533–1548. [Google Scholar] [CrossRef]
- Liu, R.M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [PubMed]
- de Flores, R.; La Joie, R.; Chételat, G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 2015, 309, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.G.; Schuff, N. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2010, 31, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Pantel, J. Neuroimaging of hippocampal atrophy in early recognition of Alzheimer’s disease—A critical appraisal after two decades of research. Psychiatry Res. Neuroimaging 2016, 247, 71–78. [Google Scholar] [CrossRef]
- Madusanka, N.; Choi, H.K.; So, J.H.; Choi, B.K.; Park, H.G. One-year Follow-up Study of Hippocampal Subfield Atrophy in Alzheimer’s Disease and Normal Aging. Curr. Med. Imaging Rev. 2019, 15, 699–709. [Google Scholar] [CrossRef]
- Knowles, W.D. Normal anatomy and neurophysiology of the hippocampal formation. J. Clin. Neurophysiol. 1992, 9, 252–263. [Google Scholar] [CrossRef]
- Alkadhi, K.A. Cellular and Molecular Differences Between Area CA1 and the Dentate Gyrus of the Hippocampus. Mol. Neurobiol. 2019, 56, 6566–6580. [Google Scholar] [CrossRef]
- Gil, L.; Niño, S.A.; Chi-Ahumada, E.; Rodríguez-Leyva, I.; Guerrero, C.; Rebolledo, A.B.; Arias, J.A.; Jiménez-Capdeville, M.E. Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2020, 21, 1841. [Google Scholar] [CrossRef]
- Gil, L.; Niño, S.A.; Capdeville, G.; Jiménez-Capdeville, M.E. Aging and Alzheimer’s disease connection: Nuclear Tau and lamin A. Neurosci. Lett. 2021, 749, 135741. [Google Scholar] [CrossRef]
- Méndez-López, I.; Blanco-Luquin, I.; Sánchez-Ruiz de Gordoa, J.; Urdánoz-Casado, A.; Roldán, M.; Acha, B.; Echavarri, C.; Zelaya, V.; Jericó, I.; Mendioroz, M. Hippocampal LMNA Gene Expression is Increased in Late-Stage Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 878. [Google Scholar] [CrossRef]
- Nagy, Z.; Esiri, M.M.; Cato, A.M.; Smith, A.D. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 1997, 94, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Regalado-Reyes, M.; Furcila, D.; Hernández, F.; Ávila, J.; DeFelipe, J.; León-Espinosa, G. Phospho-Tau Changes in the Human CA1 During Alzheimer’s Disease Progression. J. Alzheimer’s Dis. 2019, 69, 277–288. [Google Scholar] [CrossRef]
- Antón-Fernández, A.; Vallés-Saiz, L.; Avila, J.; Hernández, F. Neuronal nuclear tau and neurodegeneration. Neuroscience 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ortega, K.; Garcia-Esparcia, P.; Gil, L.; Lucas, J.J.; Ferrer, I. Altered machinery of protein synthesis in Alzheimer’s: From the nucleolus to the ribosome. Brain Pathol. 2016, 26, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Götz, J.; Feany, M.B. Connecting the dots between tau dysfunction and neurodegeneration. Trends Cell Biol. 2015, 25, 46–53. [Google Scholar] [CrossRef]
- Cornelison, G.L.; Levy, S.A.; Jenson, T.; Frost, B. Tau-induced nuclear envelope invagination causes a toxic accumulation of mRNA in Drosophila. Aging Cell 2019, 18, e12847. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Bardai, F.H. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr. Biol. 2016, 26, 129–136. [Google Scholar] [CrossRef]
- Frost, B. Alzheimer’s disease: An acquired neurodegenerative laminopathy. Nucleus 2016, 7, 275–283. [Google Scholar] [CrossRef]
- Klein, H.U.; McCabe, C. Epigenome-wide study uncovers large-scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat. Neurosci. 2019, 22, 37–46. [Google Scholar] [CrossRef]
- Gil, L.; Niño, S.A. Phospho-Tau and Chromatin Landscapes in Early and Late Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10283. [Google Scholar] [CrossRef]
- Nativio, R.; Donahue, G. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Mansuroglu, Z.; Benhelli-Mokrani, H. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci. Rep. 2016, 6, 33047. [Google Scholar] [CrossRef] [PubMed]
- Benhelli-Mokrani, H.; Mansuroglu, Z. Genome-wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions. Nucleic Acids Res. 2018, 46, 11405–11422. [Google Scholar] [CrossRef] [PubMed]
- Rico, T.; Gilles, M.; Chauderlier, A.; Comptdaer, T.; Magnez, R.; Chwastyniak, M.; Drobecq, H.; Pinet, F.; Thuru, X.; Buée, L.; et al. Tau Stabilizes Chromatin Compaction. Front. Cell Dev. Biol. 2021, 9, 740550. [Google Scholar] [CrossRef]
- Patzke, N.; Spocter, M.A.; Karlsson, K.E.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct. Funct. 2015, 220, 361–383. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Arellano, J.I.; Harding, B.; Thomas, J.L. Adult Human Hippocampus: No New Neurons in Sight. Cereb. Cortex 2018, 28, 2479–2481. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.L.; Milenkovic, I.; Rasika, S.; et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb. Cortex 2018, 28, 2458–2478. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human hippocampal neurogenesis persists in aged adults and alzheimer’s disease patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef]
- Opendak, M.; Gould, E. Adult neurogenesis: A substrate for experience-dependent change. Trends Cogn. Sci. 2015, 19, 151–161. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Tronel, S.; Lemaire, V. Influence of ontogenetic age on the role of dentate granule neurons. Brain Struct. Funct. 2015, 220, 645–661. [Google Scholar] [CrossRef]
- Laplagne, D.A.; Espósito, M.S.; Piatti, V.C.; Morgenstern, N.A.; Zhao, C.; van Praag, H.; Gage, F.H.; Schinder, A.F. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006, 4, e409. [Google Scholar] [CrossRef]
- Nakashiba, T.; Cushman, J.D.; Pelkey, K.A.; Renaudineau, S.; Buhl, D.L.; McHugh, T.J.; Rodriguez Barrera, V.; Chittajallu, R.; Iwamoto, K.S.; McBain, C.J.; et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 2012, 149, 188–201. [Google Scholar] [CrossRef]
- Kerloch, T.; Clavreul, S.; Goron, A.; Abrous, D.N.; Pacary, E. Dentate Granule Neurons Generated During Perinatal Life Display Distinct Morphological Features Compared with Later-Born Neurons in the Mouse Hippocampus. Cereb. Cortex 2019, 29, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.D.; Espinueva, D.F.; Seib, D.R.; Ash, A.M.; Cooke, M.B.; Cahil, S.P.; O’Leary, T.P.; Kwan, S.S.; Snyder, J.S. Adult-Born Hippocampal Neurons Undergo Extended Development and Are Morphologically Distinct from Neonatally-Born Neurons. J. Neurosci. 2020, 40, 5740–5756. [Google Scholar] [CrossRef] [PubMed]
- Masachs, N.; Charrier, V.; Farrugia, F.; Lemaire, V.; Blin, N.; Mazier, W.; Tronel, S.; Montaron, M.F.; Ge, S.; Marsicano, G.; et al. The temporal origin of dentate granule neurons dictates their role in spatial memory. Mol. Psychiatry 2021, 26, 7130–7140. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 1990, 301, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.A.; Altman, J. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 1993, 14, 83–144. [Google Scholar] [PubMed]
- Lopez-Rojas, J.; Kreutz, M.R. Mature granule cells of the dentate gyrus—Passive bystanders or principal performers in hippocampal function? Neurosci. Biobehav. Rev. 2016, 64, 167–174. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Ben Abdallah, N.M.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 2010, 31, 151–161. [Google Scholar] [CrossRef]
- Lazic, S.E. Modeling hippocampal neurogenesis across the lifespan in seven species. Neurobiol. Aging 2012, 33, 1664–1671. [Google Scholar] [CrossRef]
- Tanapat, P.; Hastings, N.B.; Reeves, A.J.; Gould, E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 1999, 19, 5792–5801. [Google Scholar] [CrossRef]
- Brown, J.; Cooper-Kuhn, C.M.; Kempermann, G.; Van Praag, H.; Winkler, J.; Gage, F.H.; Kuhn, H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003, 17, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.T. In vivo imaging of dendritic pruning in dentate granule cells. Nat. Neurosci. 2016, 19, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J. Neurosci. Res. 2002, 70, 327–334. [Google Scholar] [CrossRef]
- Kohler, S.J.; Williams, N.I.; Stanton, G.B.; Cameron, J.L.; Greenough, W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA 2011, 108, 10326–10331. [Google Scholar] [CrossRef]
- Ngwenya, L.B.; Heyworth, N.C.; Shwe, Y.; Moore, T.L.; Rosene, D.L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef]
- Geinisman, Y.; de Toledo-Morrell, L.; Morrell, F. Loss of perforated synapses in the dentate gyrus: Morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. USA 1986, 83, 3027–3031. [Google Scholar] [CrossRef]
- Yassa, M.A.; Stark, S.M.; Bakker, A.; Albert, M.S.; Gallagher, M.; Stark, C.E. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 2010, 51, 1242–1252. [Google Scholar] [CrossRef]
- Seki, T. Understanding the Real State of Human Adult Hippocampal Neurogenesis from Studies of Rodents and Non-human Primates. Front. Neurosci. 2020, 14, 839. [Google Scholar] [CrossRef]
- Zhao, C.; Teng, E.M. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Denoth-Lippuner, A.; Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 2021, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Herrup, K. Post-mitotic role of the cell cycle machinery. Curr. Opin. Cell Biol. 2013, 25, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Suberbielle, E.; Sanchez, P.E.; Kravitz, A.V.; Wang, X.; Ho, K.; Eilertson, K.; Devidze, N.; Kreitzer, A.C.; Mucke, L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 2013, 16, 613–621. [Google Scholar] [CrossRef]
- Zada, D.; Bronshtein, I.; Lerer-Goldshtein, T.; Garini, Y.; Appelbaum, L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat. Commun. 2019, 10, 895. [Google Scholar] [CrossRef]
- Shanbhag, N.M.; Evans, M.D.; Mao, W.; Nana, A.L.; Seeley, W.W.; Adame, A.; Rissman, R.A.; Masliah, E.; Mucke, L. Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 77. [Google Scholar] [CrossRef]
- Thadathil, N.; Delotterie, D.F.; Xiao, J.; Hori, R.; McDonald, M.P.; Khan, M.M. DNA Double-Strand Break Accumulation in Alzheimer’s Disease: Evidence from Experimental Models and Postmortem Human Brains. Mol. Neurobiol. 2021, 58, 118–131. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Eckermann, M.; Schmitzer, B.; van der Meer, F.; Franz, J.; Hansen, O.; Stadelmann, C.; Salditt, T. Three-dimensional virtual histology of the human hippocampus based on phase-contrast computed tomography. Proc. Natl. Acad. Sci. USA 2021, 118, e2113835118. [Google Scholar] [CrossRef]
- Dou, Z.; Xu, C.; Donahue, G.; Shimi, T.; Pan, J.A.; Zhu, J.; Ivanov, A.; Capell, B.C.; Drake, A.M.; Shah, P.P.; et al. Autophagy mediates degradation of nuclear lamina. Nature 2015, 527, 105–109. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.M.; Lee, J.; Kumar, A.; Lee, S.; Orenstein, S.J.; Nixon, R.A. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur. J. Neurosci. 2013, 37, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Bordi, M.; Berg, M.J.; Mohan, P.S.; Peterhoff, C.M.; Alldred, M.J.; Che, S.; Ginsberg, S.D.; Nixon, R.A. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 2016, 12, 2467–2483. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Hernández, N.; Sproul, A.A.; Yu, W.H. Alzheimer’s disease and the autophagic-lysosomal system. Neurosci. Lett. 2019, 697, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, J.; Sigmon, H.C.; Sloan, R.P.; Brickman, A.M.; Provenzano, F.A.; Small, S.A. Brain regions vulnerable and resistant to aging without Alzheimer’s disease. PLoS ONE 2020, 15, e0234255. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Federico, C.; Pinedo, F.; Bruno, F.; Rebolledo, A.B.; Montoya, J.J.; Olazabal, I.M.; Ferrer, I.; Saccone, S. Aging dependent effect of nuclear tau. Brain Res. 2017, 1677, 129–137. [Google Scholar] [CrossRef]
- Bukar Maina, M.; Al-Hilaly, Y.K.; Serpell, L.C. Nuclear Tau and Its Potential Role in Alzheimer’s Disease. Biomolecules 2016, 6, 9. [Google Scholar] [CrossRef]
- Galas, M.C.; Bonnefoy, E.; Buee, L.; Lefebvre, B. Emerging Connections Between Tau and Nucleic Acids. Adv. Exp. Med. Biol. 2019, 1184, 135–143. [Google Scholar]
- Kruman, I.I.; Wersto, R.P.; Cardozo-Pelaez, F.; Smilenov, L.; Chan, S.L.; Chrest, F.J.; Emokpae, R., Jr.; Gorospe, M.; Mattson, M.P. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 2004, 41, 549–561. [Google Scholar] [CrossRef]
- Yang, Y.; Herrup, K. Cell division in the CNS: Protective response or lethal event in post-Mitotic neurons? Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 457–466. [Google Scholar] [CrossRef]
- Raina, A.K.; Zhu, X.; Rottkamp, C.A.; Monteiro, M.; Takeda, A.; Smith, M.A. Cyclin’ toward dementia: Cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J. Neurosci. Res. 2000, 61, 128–133. [Google Scholar] [CrossRef]
- Yang, Y.; Geldmacher, D.S.; Herrup, K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J. Neurosci. 2001, 21, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mufson, E.J.; Herrup, K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J. Neurosci. 2003, 23, 2557–2563. [Google Scholar] [CrossRef]
- Currais, A.; Hortobágyi, T.; Soriano, S. The neuronal cell cycle as a mechanism of pathogenesis in Alzheimer’s disease. Aging 2009, 1, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Coffinier, C.; Choe, Y.; Beigneux, A.P.; Davies, B.S.; Yang, S.H.; Barnes, R.H., 2nd; Hong, J.; Sun, T.; Pleasure, S.J.; et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. USA 2012, 109, E423–E431. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, E.C.; Gerace, L. The stability of the nuclear lamina polymer changes with the composition of lamin subtypes according to their individual binding strengths. J. Biol. Chem. 2004, 279, 42811–44281. [Google Scholar] [CrossRef]
- Simon, D.N.; Wilson, K.L. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat. Rev. Mol. Cell Biol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell. 2019, 49, 920–935.e5. [Google Scholar] [CrossRef]
- Muramatsu, R.; Ikegaya, Y.; Matsuki, N.; Koyama, R. Neonatally born granule cells numerically dominate adult mice dentate gyrus. Neuroscience 2007, 148, 593–598. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011, 345, 1–19. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.K.; Eadie, B.D.; Ernst, C.; Christie, B.R. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 2006, 16, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Redila, V.A.; Olson, A.K.; Swann, S.E.; Mohades, G.; Webber, A.J.; Weinberg, J.; Christie, B.R. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus 2006, 16, 305–311. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Belarbi, K.; Arellano, C.; Ferguson, R.; Jopson, T.; Rosi, S. Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav. Immun. 2012, 26, 18–23. [Google Scholar] [CrossRef]
- Yuan, T.F.; Li, J.; Arias-Carrion, O. Evidence of adult neurogenesis in non-human primates and human. Cell Tissue Res. 2014, 358, 17–23. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport 1995, 6, 2479–2482. [Google Scholar] [CrossRef]
- West, M.J. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging 1993, 14, 287–293. [Google Scholar] [CrossRef]
- Mani, R.B.; Lohr, J.B.; Jeste, D.V. Hippocampal pyramidal cells and aging in the human: A quantitative study of neuronal loss in sectors CA1 to CA4. Exp. Neurol. 1986, 94, 29–40. [Google Scholar] [CrossRef]
- Fukuda, S.; Kato, F.; Tozuka, Y.; Yamaguchi, M.; Miyamoto, Y.; Hisatsune, T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 2003, 23, 9357–9366. [Google Scholar] [CrossRef] [PubMed]
- Maslov, A.Y.; Barone, T.A.; Plunkett, R.J.; Pruitt, S.C. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004, 24, 1726–1733. [Google Scholar] [CrossRef]
- Kronenberg, G.; Reuter, K.; Steiner, B.; Brandt, M.D.; Jessberger, S.; Yamaguchi, M.; Kempermann, G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003, 467, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Seri, B.; García-Verdugo, J.M.; Collado-Morente, L.; McEwen, B.S.; Alvarez-Buylla, A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004, 478, 359–378, Erratum in J. Comp. Neurol. 2004, 20, 427. [Google Scholar] [CrossRef]
- Brandt, M.D.; Jessberger, S.; Steiner, B.; Kronenberg, G.; Reuter, K.; Bick-Sander, A.; von der Behrens, W.; Kempermann, G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell Neurosci. 2003, 24, 603–613. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Torres-Alemán, I.; Trejo, J.L. Pronounced individual variation in the response to the stimulatory action of exercise on immature hippocampal neurons. Hippocampus 2006, 16, 480–490. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Takamori, Y.; Tamura, Y.; Kataoka, Y.; Cui, Y.; Seo, S.; Kanazawa, T.; Kurokawa, K.; Yamada, H. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur. J. Neurosci. 2007, 25, 1653–1662. [Google Scholar] [CrossRef]
- Kill, I.R.; Hutchison, C.J. S-phase phosphorylation of lamin B2. FEBS Lett. 1995, 377, 26–30. [Google Scholar] [CrossRef]
- Sen Gupta, A.; Sengupta, K. Lamin B2 Modulates Nucleolar Morphology, Dynamics, and Function. Mol. Cell Biol. 2017, 37, e00274-17. [Google Scholar] [CrossRef]
- Ehninger, D.; Kempermann, G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008, 331, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA repair, genome stability, and aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Lodato, M.A.; Rodin, R.E.; Bohrson, C.L.; Coulter, M.E.; Barton, A.R.; Kwon, M.; Sherman, M.A.; Vitzthum, C.M.; Luquette, L.J.; Yandava, C.N.; et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 2018, 359, 555–559. [Google Scholar] [CrossRef]
- Baquero, J.; Varriano, S.; Ordonez, M.; Kuczaj, P.; Murphy, M.R.; Aruggoda, G.; Lundine, D.; Morozova, V.; Makki, A.E.; Alonso, A.D.C.; et al. Nuclear Tau, p53 and Pin1 Regulate PARN-Mediated Deadenylation and Gene Expression. Front. Mol. Neurosci. 2019, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Asada-Utsugi, M.; Uemura, K.; Ayaki, T.; TUemura, M.; Minamiyama, S.; Hikiami, R.; Morimura, T.; Shodai, A.; Ueki, T.; Takahashi, R.; et al. Failure of DNA double-strand break repair by tau mediates Alzheimer’s disease pathology in vitro. Commun. Biol. 2022, 5, 358. [Google Scholar] [CrossRef]
- Brasnjevic, I.; Hof, P.R.; Steinbusch, H.W.; Schmitz, C. Accumulation of nuclear DNA damage or neuron loss: Molecular basis for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases. DNA Repair 2008, 7, 1087–1097. [Google Scholar] [CrossRef]
- Montavon, T.; Shukeir, N.; Erikson, G.; Engist, B.; Onishi-Seebacher, M.; Ryan, D.; Musa, Y.; Mittler, G.; Meyer, A.G.; Genoud, C.; et al. Complete loss of H3K9 methylation dissolves mouse heterochromatin organization. Nat. Commun. 2021, 12, 4359. [Google Scholar] [CrossRef]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733.e12. [Google Scholar] [CrossRef]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 2020, 11, 1545. [Google Scholar] [CrossRef]
- Padeken, J.; Zeller, P.; Towbin, B.; Katic, I.; Kalck, V.; Methot, S.P.; Gasser, S.M. Synergistic lethality between BRCA1 and H3K9me2 loss reflects satellite derepression. Genes Dev. 2019, 33, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Colmenares, S.U.; Karpen, G.H. Heterochromatin: Guardian of the Genome. Annu. Rev. Cell Dev. Biol. 2018, 34, 265–288. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Gundersen, H.J. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 1990, 296, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Gallagher, M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. USA 1996, 93, 9926–9930. [Google Scholar] [CrossRef]
- Gazzaley, A.H.; Benson, D.L.; Huntley, G.W.; Morrison, J.H. Differential subcellular regulation of NMDAR1 protein and mRNA in dendrites of dentate gyrus granule cells after perforant path transection. J. Neurosci. 1997, 17, 2006–2017. [Google Scholar] [CrossRef]
- Merrill, D.A.; Chiba, A.A.; Tuszynski, M.H. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp. Neurol. 2001, 438, 445–456. [Google Scholar] [CrossRef]
- Scheibel, M.E.; Lindsay, R.D.; Tomiyasu, U.; Scheibel, A.B. Progressive dendritic changes in aging human cortex. Exp. Neurol. 1975, 47, 392–403. [Google Scholar] [CrossRef]
- Barnes, C.A. Normal aging: Regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994, 17, 13–18. [Google Scholar] [CrossRef]
- Wu, R.; Terry, A.V.; Singh, P.B.; Gilbert, D.M. Differential subnuclear localization and replication timing of histone H3 lysine 9 methylation states. Mol. Biol. Cell 2005, 16, 2872–2881. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, J.; Hyeon, S.J.; Cho, H.; Hwang, Y.J.; Shin, J.Y.; McKee, A.C.; Kowall, N.W.; Kim, J.I.; Stein, T.D.; et al. Epigenome signatures landscaped by histone H3K9me3 are associated with the synaptic dysfunction in Alzheimer’s disease. Aging Cell 2020, 19, e13153. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, J.; Hagerty, S.W.; Soh, B.Y.; McAlpin, S.E.; Cormier, K.A.; Smith, K.M.; Ferrante, R.J. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 19176–19181. [Google Scholar] [CrossRef] [PubMed]
- Bulut-Karslioglu, A.; De La Rosa-Velázquez, I.A.; Ramirez, F.; Barenboim, M.; Onishi-Seebacher, M.; Arand, J.; Galán, C.; Winter, G.E.; Engist, B.; Gerle, B.; et al. Suv39h-dependent H3K9me3 marks intact retrotransposons and silences LINE elements in mouse embryonic stem cells. Mol. Cell. 2014, 55, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Matellán, A.; Alcazar, N.; Hernández, F.; Serrano, M.; Ávila, J. In vivo reprogramming ameliorates aging features in dentate gyrus cells and improves memory in mice. Stem Cell Rep. 2020, 15, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Schewe, J.C.; Normann, S.; Brüstle, O.; Schramm, J.; Elger, C.E.; Wiestler, O.D. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 2001, 11, 311–321. [Google Scholar] [CrossRef]
- Dempsey, R.J.; Kalluri, H.S. Ischemia-induced neurogenesis: Role of growth factors. Neurosurg. Clin. 2007, 18, 183–190. [Google Scholar] [CrossRef]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef]
- Cho, K.O.; Lybrand, Z.R.; Ito, N.; Brulet, R.; Tafacory, F.; Zhang, L.; Good, L.; Ure, K.; Kernie, S.G.; Birnbaum, S.G.; et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015, 6, 6606. [Google Scholar] [CrossRef]
- Ribak, C.E.; Dashtipour, K. Neuroplasticity in the smashed dentate gyrus of the epileptic brain. Prog. Brain Res. 2002, 136, 319–328. [Google Scholar] [PubMed]
- Kunze, A.; Grass, S.; Witte, O.W.; Yamaguchi, M.; Kempermann, G.; Redecker, C. Proliferative response of distinct hippocampal progenitor cell populations after cortical infarcts in the adult brain. Neurobiol. Dis. 2006, 21, 324–332. [Google Scholar] [CrossRef]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 343–347. [Google Scholar] [CrossRef]
- Perry, E.K.; Johnson, M.; Ekonomou, A.; Perry, R.H.; Ballard, C.; Attems, J. Neurogenic abnormalities in Alzheimer’s disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol. Dis. 2012, 47, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Zhang, Y.; Luo, H.; Zhu, S.; Yang, Y.; Zhao, T.; Wu, J.; Huang, Y.; Kong, J.; et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus 2009, 19, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Nicola, D.; Suzzi, S.; Vargas-Caballero, M.; Fransen, N.L.; Al-Malki, H.; Cebrian-Silla, A.; Garcia-Verdugo, J.M.; Riecken, K.; Fehse, B.; Perry, V.H. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 2014, 137, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Ucar, D.; Mahmoudi, S.; Karra, K.; Wong, E.D.; Devarajan, K.; Daugherty, A.C.; Kundaje, A.B.; Mancini, E.; et al. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 2014, 158, 673–688. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Pallas-Bazarra, N.; Bolós, M.; Terreros-Roncal, J.; Ávila, J.; Llorens-Martín, M. Untold New Beginnings: Adult Hippocampal Neurogenesis and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S497–S505. [Google Scholar] [CrossRef] [PubMed]
- Thurston, V.C.; Pena, P.; Pestell, R.; Binder, L.I. Nucleolar localization of the microtubule-associated protein tau in neuroblastomas using sense and anti-sense transfection strategies. Cell Motil. Cytoskelet. 1997, 38, 100–110. [Google Scholar] [CrossRef]
- Walton, N.M.; Shin, R.; Tajinda, K.; Heusner, C.L.; Kogan, J.H.; Miyake, S.; Chen, Q.; Tamura, K.; Matsumoto, M. Adult neurogenesis transiently generates oxidative stress. PLoS ONE 2012, 7, e35264. [Google Scholar] [CrossRef]
- Hester, M.S.; Danzer, S.C. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J. Neurosci. 2013, 33, 8926–8936. [Google Scholar] [CrossRef]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- Shetty, A.K.; Hattiangady, B.; Rao, M.S.; Shuai, B. Neurogenesis response of middle-aged hippocampus to acute seizure activity. PLoS ONE 2012, 7, e43286. [Google Scholar] [CrossRef]
- Scharfman, H.E.; Gray, W.P. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia 2007, 48, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.E.; Hayashi, Y.K.; Bonne, G.; Arimura, T.; Noguchi, S.; Nonaka, I.; Nishino, I. Autophagic degradation of nuclear components in mammalian cells. Autophagy 2009, 5, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Kroemer, G. Autophagy Mediates Tumor Suppression via Cellular Senescence. Trends Cell Biol. 2016, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Ivanov, A.; Adams, P.D.; Berger, S.L. Mammalian autophagy degrades nuclear constituents in response to tumorigenic stress. Autophagy 2016, 12, 1416–1417. [Google Scholar] [CrossRef]
- Frake, R.A.; Ricketts, T.; Menzies, F.M.; Rubinsztein, D.C. Autophagy and neurodegeneration. J. Clin. Investig. 2015, 125, 65–74. [Google Scholar] [CrossRef]
- Reddy, P.H.; Oliver, D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef]
- Baron, O.; Boudi, A.; Dias, C.; Schilling, M.; Nölle, A.; Vizcay-Barrena, G.; Rattray, I.; Jungbluth, H.; Scheper, W.; Fleck, R.A.; et al. Stall in Canonical Autophagy-Lysosome Pathways Prompts Nucleophagy-Based Nuclear Breakdown in Neurodegeneration. Curr. Biol. 2017, 27, 3626–3642.e6. [Google Scholar] [CrossRef]
- Moreno-Blas, D.; Gorostieta-Salas, E.; Pommer-Alba, A.; Muciño-Hernández, G.; Gerónimo-Olvera, C.; Maciel-Barón, L.A.; Konigsberg, M.; Massieu, L.; Castro-Obregón, S. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging 2019, 11, 6175–6198. [Google Scholar] [CrossRef]
- Ranade, D.; Koul, S.; Thompson, J.; Prasad, K.B.; Sengupta, K. Chromosomal aneuploidies induced upon Lamin B2 depletion are mislocalized in the interphase nucleus. Chromosoma 2017, 126, 223–244. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Waldau, B.; Shetty, A.K. Behavior of neural stem cells in the Alzheimer brain. Cell Mol. Life Sci. 2008, 65, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.G.; Borges, K.; Dingledine, R. Quantitative transcriptional neuroanatomy of the rat hippocampus: Evidence for wide-ranging, pathway-specific heterogeneity among three principal cell layers. Hippocampus 2009, 19, 253–264. [Google Scholar] [CrossRef]
- Zeier, Z.; Madorsky, I.; Xu, Y.; Ogle, W.O.; Notterpek, L.; Foster, T.C. Gene expression in the hippocampus: Regionally specific effects of aging and caloric restriction. Mech. Ageing Dev. 2011, 132, 8–19. [Google Scholar] [CrossRef][Green Version]
- Shimizu, H.; Mizuguchi, A.; Aoki, M. Differential responses between CA1 pyramidal cells and granule cells to ischemic insult in rat hippocampal slices. Neurosci. Lett. 1996, 203, 195–198. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Stringer, J.L.; Alkadhi, K.A. Combination of hypothyroidism and stress abolishes early LTP in the CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res. 2001, 922, 250–260. [Google Scholar] [CrossRef]
- Yao, H.; Huang, Y.H.; Liu, Z.W.; Wan, Q.; Ding, A.S.; Zhao, B.; Fan, M.; Wang, F.Z. The different responses to anoxia in cultured CA1 and DG neurons from newborn rats. Sheng Li Xue Bao Acta Physiol. Sin. 1998, 50, 61–66. [Google Scholar]
- Daval, J.L.; Pourié, G.; Grojean, S.; Lièvre, V.; Strazielle, C.; Blaise, S.; Vert, P. Neonatal hypoxia triggers transient apoptosis followed by neurogenesis in the rat CA1 hippocampus. Pediatr. Res. 2004, 55, 561–567. [Google Scholar] [CrossRef]
- Hsu, J.C.; Zhang, Y.; Takagi, N.; Gurd, J.W.; Wallace, M.C.; Zhang, L.; Eubanks, J.H. Decreased expression and functionality of NMDA receptor complexes persist in the CA1, but not in the dentate gyrus after transient cerebral ischemia. J. Cereb. Blood Flow Metab. 1998, 18, 768–775. [Google Scholar] [CrossRef]
- Choi, S.H.; Tanzi, R.E. Is Alzheimer’s Disease a Neurogenesis Disorder? Cell Stem Cell 2019, 25, 7–8. [Google Scholar] [CrossRef]
- Cuartero, M.I.; de la Parra, J. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J. Clin. Investig. 2019, 129, 1536–1550. [Google Scholar] [CrossRef] [PubMed]
- Briley, D.; Ghirardi, V. Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci. Rep. 2016, 6, 27812. [Google Scholar] [CrossRef] [PubMed]


| Antibody (Clone)/Supplier/Catalog Number/Manufacture |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, L.; Chi-Ahumada, E.; Niño, S.A.; Capdeville, G.; Méndez-Torres, A.M.; Guerrero, C.; Rebolledo, A.B.; Olazabal, I.M.; Jiménez-Capdeville, M.E. Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis. Int. J. Mol. Sci. 2022, 23, 12873. https://doi.org/10.3390/ijms232112873
Gil L, Chi-Ahumada E, Niño SA, Capdeville G, Méndez-Torres AM, Guerrero C, Rebolledo AB, Olazabal IM, Jiménez-Capdeville ME. Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis. International Journal of Molecular Sciences. 2022; 23(21):12873. https://doi.org/10.3390/ijms232112873
Chicago/Turabian StyleGil, Laura, Erika Chi-Ahumada, Sandra A. Niño, Gabriela Capdeville, Areli M. Méndez-Torres, Carmen Guerrero, Ana B. Rebolledo, Isabel M. Olazabal, and María E. Jiménez-Capdeville. 2022. "Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis" International Journal of Molecular Sciences 23, no. 21: 12873. https://doi.org/10.3390/ijms232112873
APA StyleGil, L., Chi-Ahumada, E., Niño, S. A., Capdeville, G., Méndez-Torres, A. M., Guerrero, C., Rebolledo, A. B., Olazabal, I. M., & Jiménez-Capdeville, M. E. (2022). Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis. International Journal of Molecular Sciences, 23(21), 12873. https://doi.org/10.3390/ijms232112873







