Abstract
Sugarcane (Saccharum spp.) is an important sugar and energy crop worldwide. As a core regulator of the salicylic acid (SA) signaling pathway, nonexpressor of pathogenesis-related genes 1 (NPR1) plays a significant role in the response of the plant to biotic and abiotic stresses. However, there is currently no report on the NPR1-like gene family in sugarcane. In this study, a total of 18 NPR1-like genes were identified in Saccharum spontaneum and classified into three clades (clade I, II, and III). The cis-elements predicted in the promotors revealed that the sugarcane NPR1-like genes may be involved in various phytohormones and stress responses. RNA sequencing and quantitative real-time PCR analysis demonstrated that NPR1-like genes were differentially expressed in sugarcane tissues and under Sporisorium scitamineum stress. In addition, a novel ShNPR1 gene from Saccharum spp. hybrid ROC22 was isolated by homologous cloning and validated to be a nuclear-localized clade II member. The ShNPR1 gene was constitutively expressed in all the sugarcane tissues, with the highest expression level in the leaf and the lowest in the bud. The expression level of ShNPR1 was decreased by the plant hormones salicylic acid (SA) and abscisic acid (ABA). Additionally, the transient expression showed that the ShNPR1 gene plays a positive role in Nicotiana benthamiana plants’ defense response to Ralstonia solanacearum and Fusarium solani var. coeruleum. This study provided comprehensive information for the NPR1-like family in sugarcane, which should be helpful for functional characterization of sugarcane NPR1-like genes in the future.
1. Introduction
The nonexpressor of pathogenesis-related genes 1, also known as noninducible immunity protein 1, salicylic acid (SA) insensitive 1, or pathogenesis-related (PR) gene, is essential for the plant response to pathogen challenge [1,2,3]. NPR1 plays a significant role in the establishments of systemic acquired resistance (SAR) and induced systemic resistance (ISR) [4]. The NPR1 gene was first discovered from the Arabidopsis thaliana npr1 mutant in the research on the plant SAR signaling pathway [1,5]. Previous studies have shown that in the npr1 mutant, the gene encoding the PR protein was not expressed, and the SAR reaction cannot be activated, thereby failing to develop disease resistance [1,2,5]. As a key regulator of SA signaling transduction in plants, NPR1 is located downstream of the SA signaling pathway and upstream of the PR protein gene. NPR1 is the intersection of multiple resistance signaling pathways and plays an important role in regulating the overall disease resistance of plants [4,6].
At present, two to six NPR1-like genes have been found in the plant species. In Arabidopsis alone, five additional NPR1-like genes (AtNPR2, AtNPR3, AtNPR4, AtNPR5/AtBOP1, and AtNPR6/AtBOP2) have been described [7,8,9,10]. The encoding proteins for these NPR1-like genes contain two well-documented domains, including ankyrin repeats and BTB/POZ (broad complex, tramtrack, bric a brac/pox virus, and zinc finger) [5,8,11,12]. Phylogenetic analysis revealed that the NPR1-like gene family can be classified into three clades [13,14]. As reported, each clade of the NPR1-like gene family appears to have its own set of functional requirements [5,9,15]. Clade I members (AtNPR1 and AtNPR2) are involved with positive SAR regulation and clade II members (AtNPR3 and AtNPR4) are negatively involved in SAR regulation [5,15], while clade III members (AtBOP1 and AtBOP2) are related to the growth and development of plant leaves and flowers [8]. Most of the peptides encoded by the PR genes are effectors directly involved in the defense against pathogens [16]. Once infected by pathogens, the accumulation of SA in plants can cause changes in the redox state of plant cells, resulting in the cleavage of disulfide bonds between NPR1 oligomer molecules in the cytoplasm into monomers and their transfer to the nucleus [17]. NPR1 is phosphorylated upon entry into the nucleus and is subsequently degraded by the 26S proteasome after cullin 3-mediated ubiquitination [12]. Only the phosphorylated NPR1 can activate SAR [18]. The key role of the NPR1 gene in plant disease resistance, especially in the establishment of SAR, has been confirmed by much research [6,19]. Overexpression of the NPR1 gene in Oryza sativa [20], Citrus reticulata Blanco [21], BraSsica campestris [22], GoSsypium spp [23], Vitis vinifera [24], and Triticum aestivum [25] can effectively improve plant resistance to rice sheath blight, ulcer disease, Pseudomonas syringae, cotton root rot, powdery mildew, and gibberellic disease. Thus, NPR1, as the main regulator of plant defense signals, plays a significant role in a wide range of defense responses [6].
Sugarcane (Saccharum spp.), which belongs to monocotyledonous plants of the Gramineae genus Saccharum, is an important global sugar and biofuel feedstock crop in more than 110 tropical and subtropical countries [26]. As with other crops, sugarcane production is affected by a variety of adverse environmental conditions, including drought, cold, and disease, especially fungus [26,27]. Screening sugarcane genes in response to adversity stress will provide a basis for the cultivation of excellent sugarcane varieties with stress resistance [26]. Therefore, the use of biotechnology and genetic engineering technology can speed up the process of sugarcane breeding and improve the quality of sugarcane varieties. It has been confirmed that various plant species overexpressing NPR1 or its orthologs can enhance disease resistance to several kinds of pathogens [24,28,29]. However, a systematic investigation of the evolution and biological function of the NPR1-like genes in Saccharum has not yet been reported. In the present study, 18 SsNPR genes were identified from the genome of S. spontaneum [30]. Their classification, gene structure, motif composition, chromosomal distribution, evolution, cis-regulatory elements (CREs), and synteny analysis were performed. In addition, the expression profiles of NPR1-like genes in different sugarcane tissues and under S. scitamineum stress were measured using RNA sequencing (RNA-seq) and quantitative real-time PCR (qRT-PCR). Furthermore, a clade II NPR1-like gene, ShNPR1, was successfully cloned from sugarcane variety ROC22. The sequence characteristics, subcellular localization, tissue-specific expression, gene expression patterns under different stresses, and transient expression of ShNPR1 in Nicotiana benthamiana after inoculation with the tobacco pathogens Ralstonia solanacearum and Fusarium solani var. coeruleum were analyzed. This study provides a reference for systematically understanding the characteristics of the sugarcane NPR1-like gene family and the identification of its function in disease resistance.
2. Results
2.1. Identification and Characterization of the NPR1-like Gene Family
A total of 18 NPR1-like gene sequences were identified in the S. spontaneum genome. These genes that belong to alleles were designated as the same name followed by the letters “a”, “b”, “c”, and “d”, and duplicated genes were designated as the same name followed by the letters “e”, “f”, and “g” [30] (Table S1). Among these 18 SsNPR genes (SsNPR1–5), SsNPR2 and SsNPR3 had four alleles (SsNPR2a/2b/2c/2d and SsNPR3a/3b/3c/3d), and SsNPR1 and SsNPR4 had two alleles (SsNPR1a/1b and SsNPR4a/4b). Additionally, SsNPR5e, SsNPR5f, and SsNPR5g all originated from dispersed duplications. The encoded SsNPR proteins were 429-647 amino acid (aa) residues in length, with the molecular weight (MW) ranging from 45.42 kDa to 70.57 kDa. Their isoelectric points (pIs) varied from 5.48 to 6.68, and the instability index was all greater than 40. In addition, all these SsNPRs were predicted to be acidic proteins and located in the nucleus (Table S1). The secondary structure of the 18 SsNPR proteins was mainly composed of an α-helix (43.98–56.57%), irregular curl (32.87–43.39%), and extended chain (5.75–9.19%), but lacked a β-sheet (Table S2).
2.2. Phylogenetic Analysis of the NPR1-like Gene Family
To explore the evolutionary relationship of the SsNPR1-like gene family, NPR1-like protein sequences from five monocots and nine dicots were used to construct the phylogenetic tree (Table S3). Figure 1 showed that these NPR1-like proteins were divided into three clades, including clade I, II, and III. SsNPR3a/3b/3c/3d were classified into clade I, including AtNPR1 and AtNPR2, which were reported as positive regulators of SAR [15]. SsNPR1a/1b and SsNPR2a/2b/2c/2d were classified into clade II, among which AtNPR3 and AtNPR4 served as negative regulators of SAR [10]. The other eight SsNPR proteins (SsNPR4a/4b and SsNPR5a/5b/5c/5e/5f/5g) were classified into clade III, along with AtNPR5/AtBOP1 and AtNPR5/AtBOP2, which participated in the organ determinacy and symmetry [8,31]. The NPRs of monocotyledonous and dicotyledonous plants were not clustered separately, indicating that the NPR sequences were conserved among plant species.
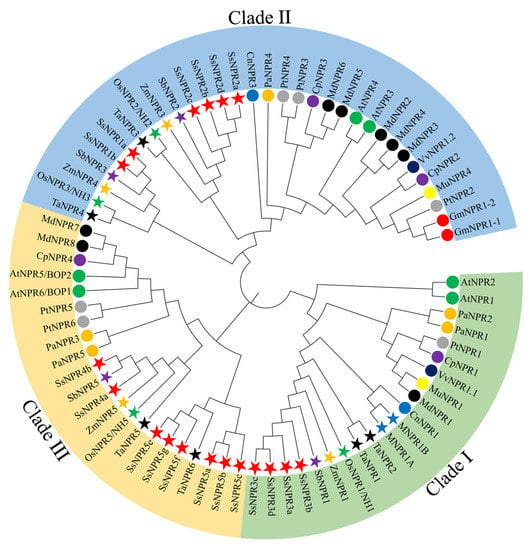
Figure 1.
Phylogenetic analysis of NPR1 homologous proteins from 15 plant species. The MEGA X with the neighbor-joining method was used to conduct the phylogenetic tree. NPR1-related proteins came from five monocot species (Musa acuminate (M), Oryza sativa (Os), Sorghum bicolor (Sb), Triticum aestivum (Ta), and Zea mays (Zm)) and nine dicot species (Arabidopsis thaliana (At), Carica papaya (Cp), Cocos nucifera (Cn), Glycine max (Gm), Malus domestica (Md), Morus multicaulis (Mm), Persea americana (Pa), Populus trichocarpa (Pt), and Vitis vinifera (Vv)). All the GenBank accession numbers of the NPR1 homologous proteins are listed in Table S3. Three major clades are distinguished with three colors, and the NPR1 homologous proteins from different plant species are indicated by different colors of circles and stars.
2.3. Gene Structures and Conserved Motifs of the NPR1-like Gene Family
To further understand the potential functions of SsNPR1-like genes, their conserved motifs and gene structures were analyzed. A total of 10 different conserved motifs were observed in the 18 NPR1-like proteins (Figure 2 and Table S4). NPR1-like members that belonged to the same clade had similar types, arrangements, and number of motifs. All these NPR1-like proteins contained motifs 1, 2, 3, 4, and 5. Interestingly, there were eight types of motifs in all three clades, but their motif composition was not the same. For example, motifs 6 and 7 were absent in clades I and II, while motifs 8 and 10 were not contained in clade III. Furthermore, the exon–intron organizations showed that the exon numbers were from two to four in the NPR1-like proteins. As expected, most of the NPR1-like genes in the same clade had similar patterns of exon–intron distributions and positions. These NPR1-like genes in clades I and II had four exons. Six NPR1-like genes in clade III (SsNPR4a/4b and SsNPR5a/5c/5e/5f) presented similar exon–intron structures. Most members of the NPR1-like genes in different clades had similar gene structures, indicating that NPR1-like sequences were conserved in evolution.
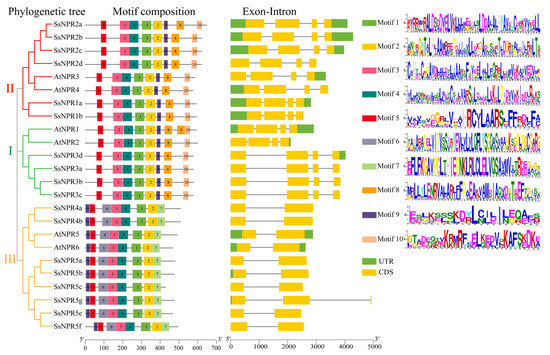
Figure 2.
Phylogenetic tree, conserved motif, and gene structure of the NPR1-like proteins in Saccharum spontaneum and Arabidopsis. Different colors on the phylogenetic tree represent different clades of the NPR1-like gene family and different colored boxes indicate different motifs (motif 1–10). The noncoding sequences, exons, and introns are shown as green boxes, yellow boxes, and black lines in the exon–intron structures, respectively. The length of proteins and genes is estimated using the scale at the bottom.
2.4. Chromosomal Location, Gene Duplications, and Synteny Analysis of the NPR1-like Gene Family
As illustrated in Figure 3, the distribution of 18 SsNPR1-like genes on the 12 S. spontaneum chromosomes was uneven. Chromosome 3D contained three SsNPR1-like genes, and Chromosomes 3A, 3B, and 3C each contained two SsNPR1-like genes. The remaining eight chromosomes each contained one SsNPR1-like gene (Figure 3A). A total of 14 segmental duplication gene pairs in 12 SsNPR1-likes were observed, of which 11 pairs (78.57%) occurred between alleles and three pairs (21.43%) occurred between nonalleles (Figure 3A and Table S5). The nonsynonymous (Ka) and synonymous (Ks) nucleotide substitution rates and the Ka/Ks of these SsNPR1-like gene pairs were calculated (Table S5). The results showed that among the 13 of 14 SsNPR1-likes, Ka/Ks was <1, and the remaining one SsNPR1-like gene pair (SsNPR2b/SsNPR2c) did not have a value. Therefore, purifying selection might be the primary pressure that preserves the function of SsNPR1-likes. Furthermore, to explore the expansion mechanisms of the NPR1-like genes, the whole-genome duplication (WGD)/segmental, and dispersed, proximal and tandem events were analyzed using chromosomal information of S. spontaneum. The result showed that 12 WGD/fragment repetitive genes (66.67%), three scattered repetitive genes (16.67%), and three single copies (16.67%) were detected in the 18 sugarcane NPR1-like genes (Figure 3B and Table S6).
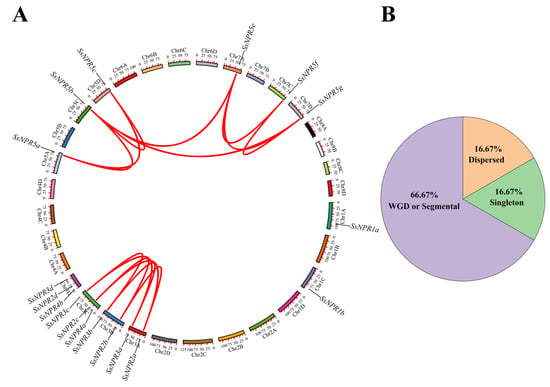
Figure 3.
Evolutionary history of NPR1-like genes in Saccharum spontaneum. (A) Synteny analysis of the NPR1-like genes in S. spontaneum. The red lines represent the SsNPR1-likes that have been replicated. (B) The distribution of gene duplications among SsNPR genes. WGD represents the whole-genome duplication.
2.5. Promoter Analysis of the NPR1-like Genes
The promoter regions of the SsNPR1-like genes from S. spontaneum were submitted to the PlantCARE database to search the cis-elements. Four types of CREs, which were related to light, plant growth/development, hormone, and stress-related responses, were predicted in the 2000-bp promoters of the 18 NPR1-like genes (Figure 4, Tables S7 and S8). Promoters of the 18 NPR1-like genes all contained light response elements. The abscisic acid (ABA) and methyl jasmonate (MeJA) response elements existed in the promoters of 100% and 88.89% NPR1-like genes. In addition, the promoters of twelve (66.67%), five (27.78%), and three (16.67%) NPR1-like genes contained auxin, SA response, and gibberellin (GA) elements, respectively (Table S8). Elements related to the stress response, such as anoxic-induction elements (AREs) or GC-motifs (enhance anoxic specific inducibility), MYB binding sites, which are involved in drought-inducibility (MBSs), low-temperature-responsive elements (LTRs), and defense and stress response elements (TC-rich repeats), were found in the promoters of ten (55.56%), eight (44.44%), eight (44.44%), and three (16.67%) NPR1-like genes, respectively. The SsNPR3a promoter contained a MYB binding site, while the SsNPR5g/5e/5f promoters contained a defense and stress-responsive element. Regarding plant growth and development, promoters of nine (50%) NPR1-like genes contained a CAT-box, which is a cis-acting regulatory element related to meristem expression. Promoters of five (27.78%) and four (22.22%) NPR1-like genes may have been involved in zein metabolism regulation and circadian control. Promoters of three (16.67%) NPR1-like genes (SsNPR4a/4b/5g) contained an element of seed-specific regulation. These results indicate that the NPR1-like genes may be widely involved in the response to various stressors and in the regulation of plant growth and development.
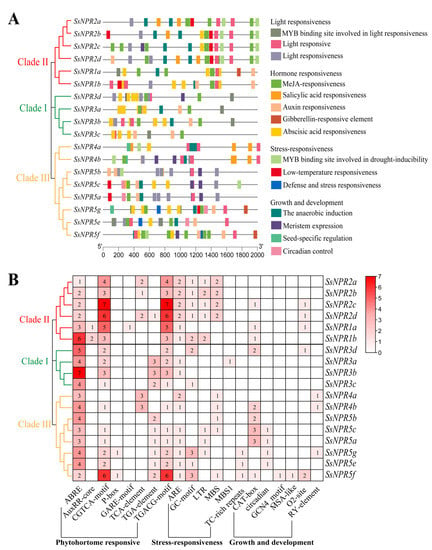
Figure 4.
Cis-regulatory element (CRE) analysis of NPR1-like gene family. (A) The location distribution of different kinds of CREs in the promoter region. Different colored boxes correspond to different kinds of CREs and some CREs may overlap with other CREs. (B) The number of CREs in the promoter region of the NPR1-like genes. The number of each CRE is shown in the heatmap box, and blank indicates that there was no corresponding CRE. Different colors on the phylogenetic tree represent different groups of the NPR1-like gene family.
2.6. Tissue-Specific Expression of NPR1-like Genes and Their Expression Profiles during the Interaction between Sugarcane and Smut Pathogen
Among the 18 NPR1-like genes, 12 showed expression (FPKM > 0) in all sugarcane tissues based on the transcriptome data. The number of NPR1-like genes expressed (FPKM > 0) in the sugarcane epidermis, stem pith, root, leaf, and bud was 18, 15, 13, 17, and 18, respectively. In clade II, the expression level of SsNPR1b in the five ROC22 tissues was higher than that of the other SsNPR1-likes, indicating that this gene may play an important role in sugarcane growth and development (Figure 5A). SsNPR2a/2b/2c/2d exhibited the highest transcript abundance in the leaf than those in the other tissues. In clade I, the expression level of NPR1-like genes in the epidermis was the highest. However, the expression level of all members in clade I was lower than that in clade II. In clade III, the expression level of SsNPR5a/5b/5c/5e/5f/5g exhibited low or undetectable expressions (FPKM < 1) in the stem pith, root, and leaf, while the expression level of all genes was the highest in the bud.
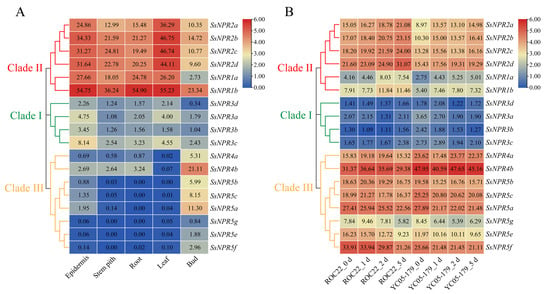
Figure 5.
Expression profile of NPR1-like genes in various tissues of sugarcane and under smut pathogen stress. (A) Expression profile of NPR1-like genes in different tissues of sugarcane hybrid ROC22. (B) Expression profile of NPR1-like genes in the interaction between different sugarcane genotypes and Sporisorium scitamineum. ROC22_0 d/1 d/2 d/5 d represents the sugarcane smut-susceptible variety ROC22 under S. scitamineum treatment for 0 d, 1 d, 2 d, and 5 d, respectively. YC05–179_0 d/1 d/2 d/5 d represents the sugarcane smut-resistant variety YC05–179 under S. scitamineum treatment for 0 d, 1 d, 2 d, and 5 d, respectively. The clustering tree on the left side of the figure is constructed using MEGA X with the neighbor method, and different colors on the phylogenetic tree represent different NPR1-like clades. The expression heatmap of NPR1-like genes is constructed by TBtools with the transcript level transformed by log2 (FPKM). The FPKM is the number of fragments per kilobase of transcript per million mapped, and its value is displayed in each heatmap box.
The expression pattern of NPR1-like genes during the interaction between sugarcane smut-susceptible variety ROC22/-resistant variety YC05–179 and smut pathogen for 0-5 d was analyzed (Figure 5B). The SsNPR3a/3b/3c/3d genes in clade II exhibited a low expression level (FPKM < 4) under the S. scitamineum stress. The expression level of clade II genes showed an upward trend, while the expression of SsNPR4a/4b and SsNPR5a/5b/5c/5e/5f/5g in clade III had a downward trend. It is worth noting that the expression level of clade II genes in the susceptible variety was higher than that in the resistant one, indicating that the regulatory pattern of the genes in clade II may be negative in the interaction between sugarcane and smut pathogen. In addition, in clade III, the expression level of SsNPR5e/5f in the resistant variety was lower than that in the susceptible one, while the expression trend of SsMYC4b was the opposite. The results indicate that all these SsNPR1-likes can be induced by the smut pathogen infection, and their expression patterns in the infected sugarcane-resistant and -susceptible varieties were different.
2.7. Cloning and Sequence Analysis of the ShNPR1 Gene
The expression of clade II gene members increased with time during the interaction between sugarcane smut susceptible variety ROC22/resistant variety YC05-179 and smut pathogen, suggesting that members of clade II respond positively to smut pathogen infection. Furthermore, the cDNA sequence of the homologous gene of SsNPR2b belonging to clade II gene members was successfully cloned from the sugarcane hybrid ROC22 leaves and termed ShNPR1 (GenBank accession no. ON737832). The ORF length of ShNPR1 was 1866 bp and encoded 621 amino acids, which contained 16 cysteine residues. The amino acid sequence similarity between ShNPR1 and SsNPR2b was as high as 99.80% (Table S9). The ShNPR1 protein contained a nuclear localization signal at the C-terminus, an N-terminal BTB/POZ domain, a NPR1-like C-terminal region, and ankyrin repeats in the central region (Figure S1).
Multiple sequence alignments were performed on ShNPR1, SsNPR1-likes, and AtNPR1-likes (AtNPR1 to AtNPR6) with known functions to check the conservatism of residues, domains, and motifs (Figure 6). We found that npr1-2 (Cys150Tyr), nim1-2 (His300Tyr), and npr1-1 (His334Tyr) functional sites in AtNPR1 were completely conserved in all 25 NPR1-like proteins. C82, C216, and C156 cysteine residues in AtNPR1, which participated in its oligomer-monomer transition [19], were also highly conservative in NPR1-like proteins. There was a BTB/POZ domain in the N-terminal and an ankyrin conserved domain in the middle of the Saccharum and Arabidopsis NPR1-like proteins. In addition, NPR1-like proteins belonging to clades I and II have a nuclear localization signal (NLS) at the C-terminus.
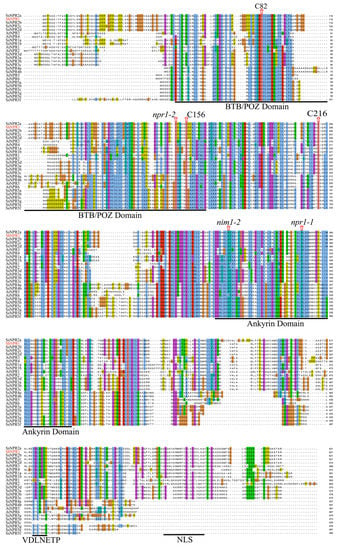
Figure 6.
Multiple alignments of amino acid sequences of NPR1-like proteins in Saccharum and Arabidopsis thaliana. BTB/POZ, ankyrin conserved domains, EAR-like repression motif (VDLNETP), and nuclear localization signal (NLS) are underlined in black. npr1-1, npr1-2, and nim1-2 in AtNPR1 mutation sites, and the highly conserved cysteines residues (C82, C216, and C156) in AtNPR1 are shown by red arrows.
2.8. ShNPR1 Was a Nuclear Localized Protein
To understand the subcellular localization of the ShNPR1 protein, ShNPR1::GFP fusion proteins were expressed transiently in N. benthamiana leaf cells. In the control group, which contained an empty vector pFAST-R05 (35S::GFP) (the green fluorescent protein gene followed the ccdB gene, which contained a stop codon), no fluorescence was observed after Agrobacterium-mediated transformation and transient expression in N. benthamiana leaves (Figure 7). However, the fluorescence was observed in the nucleus of the lower epidermal cells of the 35S::ShNPR1::GFP leaves. Based on the Plant-mPloc predicted program, this result was in accordance with that in sequence analysis, which suggests that ShNPR1 was mainly localized to the nucleus. These findings prove that ShNPR1 is a nuclear localized protein.
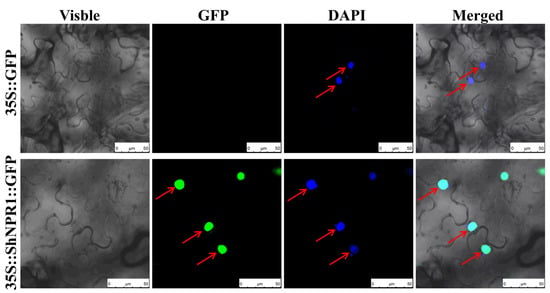
Figure 7.
Subcellular location of ShNPR1 protein in Nicotiana benthamiana lower epidermal cells. Images of epidermal cells were captured using visible light, green fluorescence, blue fluorescence, and merged light. 35S::GFP, the Agrobacterium tumefaciens strain carried the empty vector pFAST-R05; 35S::ShNPR1::GFP, the A. tumefaciens strain carried the recombinant vector pFAST-R05-ShNPR1; pFAST-R05 expresses a gene fused to a green fluorescent protein gene (GFP), which followed the ccdB gene (with a stop codon); red arrows indicate nucleus. DAPI, 4′, 6-diamidino-2-phenylindole. Bar = 50 µm.
2.9. ShNPR1 Was Constitutively Expressed in Sugarcane Tissues
The relative expression level of the ShNPR1 gene was detected in the 10-month-old ROC22 root, bud, leaf, stem pith, and stem epidermis by qRT-PCR (Figure 8). The results indicated that this gene was constitutively expressed in all these tissues. The lowest expression level of ShNPR1 occurred in the bud, while its relative expression level in the leaf and epidermis was 5.34- and 2.20-fold higher than that in the bud, respectively.
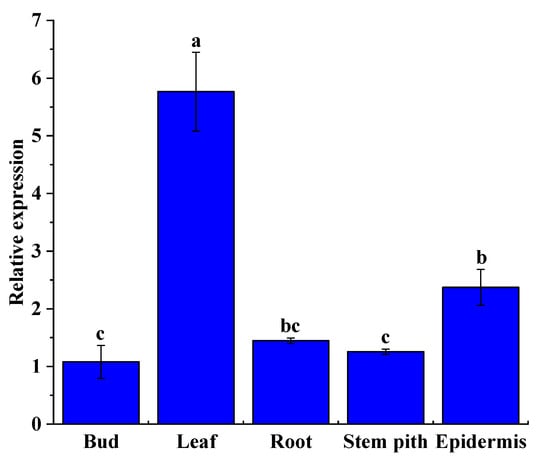
Figure 8.
Tissue-specific expression analysis of ShNPR1 in 10-month-old sugarcane ROC22 plants by qRT-PCR analysis. The expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for data normalization. The bars represent the mean ± standard error (n = 3). Different lowercase letters on the top of the bars indicate the significant differences determined by Duncan’s new multiple range test (p-value < 0.05).
2.10. Sporisorium Scitamineum, ABA, SA, and MeJA Triggered ShNPR1 Activation
The qRT-PCR method was used to analyze the expression patterns of the ShNPR1 gene under different exogenous stresses. The transcripts of ShNPR1 were detected in the interaction between two different sugarcane varieties and S. scitamineum (Figure 9A). As compared to the control, the gene expression level of ShNPR1 was significantly increased in the sugarcane smut-resistant variety YT96-86 at 3 days post-inoculation (dpi), but was significantly decreased in the sugarcane smut-susceptible variety ROC22 at 1 and 3 dpi. Under 100 μM ABA stress, there was a downregulation of ShNPR1 at 3 and 24 h, whereas the highest expression was observed at 6 h, which was 2.09-fold higher than the control (Figure 9B). After 5 mM SA treatment, the gene expression level of ShNPR1 was stabilized from 0 to 3 h, while its transcript abundance significantly reduced at 12 to 24 h (Figure 9C). Under 25 μM MeJA stress for 3 h, the gene expression level of ShNPR1 was 2.16-fold higher than that of the control, following which it dropped to the control level at 12 and 24 h (Figure 9D).
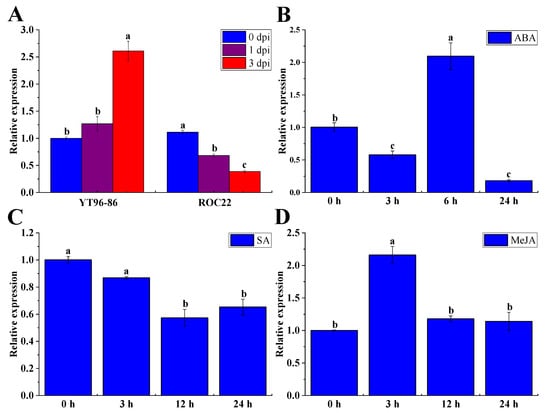
Figure 9.
Expression patterns of ShNPR1 in different stress conditions by qRT-PCR analysis. (A) Relative expression of ShNPR1 in sugarcane after inoculation with Sporisorium scitamineum. YT96-86 was a sugarcane smut-resistant variety. ROC22 was a sugarcane smut-susceptible variety. dpi: days post-inoculation. (B–D) Relative expression of ShNPR1 in 4-month-old ROC22 plantlets under abscisic acid (ABA), salicylic acid (SA), and methyl jasmonate (MeJA) stresses. The leaf samples treated with SA and MeJA were harvested at 0 h, 3 h, 12 h, and 24 h, while those treated with ABA were collected at 0 h, 3 h, 6 h, and 24 h. The expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for data normalization. The bars represent the mean ± standard error (n = 3). Different lowercase letters on the top of the bars indicate the significant differences determined by Duncan’s new multiple range test (p-value < 0.05).
2.11. Transient Expression of ShNPR1 in Nicotiana benthamiana Induced Plant Immune Response
The overexpression plasmid pEarleyGate 203 (35S::00) and the recombinant plasmid pEarleyGate 203-ShNPR1 (35S::ShNPR1) carried by A. tumefaciens were transiently overexpressed in the leaves of N. benthamiana. The immune effect induced by transient overexpression of ShNPR1 was analyzed by phenotypic observation, lesion area statistics, 3,3-diaminobenzidine (DAB) staining, and expression level analysis of immune-related marker genes in N. benthamiana (Figure 10).
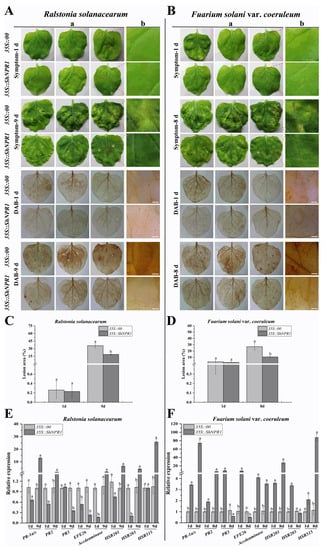
Figure 10.
The immune effect of transient overexpression of ShNPR1 in Nicotiana benthamiana after inoculation with Ralstonia solanacearum and Fuarium solani var. coeruleum. (A) The disease symptoms and 3,3-diaminobenzidine (DAB) staining of N. benthamiana leaves at 1 day and 9 days post-inoculation with R. solanacearum. (B) The disease symptoms and DAB staining of N. benthamiana leaves at 1 day and 8 days post-inoculation with F. solani var. coeruleum. (C) Lesion area of leaves after infection with R. solanacearum. (D) Lesion area of leaves after infection with F. solani var. coeruleum. (E) Expression of eight tobacco-immune-related marker genes in the N. benthamiana leaves 1 day and 9 days after inoculation with R. solanacearum. (F) Expression of eight tobacco-immune-related marker genes in the N. benthamiana leaves 1 day and 8 days after inoculation with F. solani var. coeruleum. The tobacco-immune-related marker genes, namely salicylic-acid-pathway-related genes NtPR-1a/c, NtPR2, and NtPR3; ethylene synthesis-dependent genes NtEFE26 and NtAccdeaminase; hypersensitive reaction marker genes NtHSR201, NtHSR203, and NtHSR515. NtEF-1α was used for data normalization. (a) and (b) represent images taken with the Canon camera and a microscope (Bar = 2 mm), respectively. 35S::00, the Agrobacterium tumefaciens strain carried the empty vector pEarleyGate 203; 35S::ShNPR1, the A. tumefaciens strain carried the recombinant vector pEarleyGate 203-ShNPR1. The bars represent the mean ± standard error (n = 3). Different lowercase letters on the top of the bars indicate the significant differences determined by Duncan’s new multiple range test (p-value < 0.05).
There was no obvious difference in the symptom and DAB staining in 35S::00 and 35S::ShNPR1 leaves infected by R. solanacearum and F. solani var. coeruleum on one day. However, 35S::00 and 35S::ShNPR1 leaves that had been infected with R. solanacearum for nine days curled, wilted, and displayed white disease symptoms. Compared to 35S::00 leaves, this phenotype was weaker in 35S::ShNPR1 leaves (Figure 10A). Similarly, eight days after infection with F. solani var. coeruleum, leaves of 35S::00 with curled and wilted symptoms were more serious than that of 35S::ShNPR1 leaves (Figure 10B). After challenge by F. solani var. coeruleum and R. solanacearum for eight and nine days, the lesion areas of 35S::ShNPR1 leaves were all significantly lower than those of 35S::00 (Figure 10C,D). Furthermore, the DAB results demonstrated that the staining color in the ShNPR1-overexpressed tobacco leaves was lighter than that in the 35S::00 leaves after being infected by R. solanacearum for nine days and F. solani var. coeruleum for eight days (Figure 10A,B).
At one-day after post inoculation with R. solanacearum, the qRT-PCR analysis indicated that the expression level of four immune-related genes in 35S::ShNPR1 leaves, namely the SA-pathway-related gene NtPR2, ethylene (ET) synthesis-dependent genes NtEFE26 and NtAccdeaminase, and hypersensitive response (HR) marker gene NtHSR203, was significantly lower than that in the control group (Figure 10E). However, on nine days of inoculation with R. solanacearum, the expression level of SA-pathway-related gene NtPR-1a/c and HR marker genes NtHSR201 and NtHSR515 was significantly higher than that in the control group by 13.00-, 6.53-, and 24.61-fold, respectively (Figure 10E). Furthermore, the expression level of the seven immune-related genes (NtPR-1a/c, NtPR2, NtPR3, NtEFE26, NtAccdeaminase, NtHSR201, and NtHSR203) in the 35S::ShNPR1 leaves was increased at one day after inoculation with F. solani var. coeruleum. Their expression level in the 35S::ShNPR1 leaves was 3.39-, 1.89-, 8.56-, 7.55-, 4.09-, 3.57-, and 3.35-fold that observed in the control, respectively (Figure 10F). Eight days after inoculation with F. solani var. coeruleum, the expression level of NtPR-1a/c, NtPR2, NtAccdeaminase, NtHSR201, and NtHSR515 was all increased, and was 74.01-, 6.65-, 3.29-, 27.42-, and 76.38-fold that of the control, respectively (Figure 10F).
Based on these above results, overexpression of ShNPR1 in N. benthamiana leaves enhances resistance to R. solanacearum and F. solani var. coeruleum infections, which was accompanied by the changing expression level of immune-related genes.
3. Discussion
Previous studies have shown that the NPR1-like genes not only take part in regulating plant growth and organ development, but also play vital roles in plant defense signal transduction pathways [12,15]. However, no systematic study on the NPR1-like genes has been conducted in Saccharum. In this study, the characteristics of the NPR1-like gene family in sugarcane and the regulatory role of ShNPR1 in response to pathogen stress were analyzed, which should set a foundation for further functional identification of the NPR1-like genes in Saccharum.
In this study, 18 NPR1-like homologous genes with Arabidopsis AtNPRs were identified from the genome of S. spontaneum and were unevenly distributed (4, 6, and 8) among three clades (I, II, and III) in the phylogenetic tree (Figure 1). Moreover, three duplicate genes of SsNPR1 were found in clade III, but none in clades I and II (Table S1). Due to the fact that five monocots (Saccharum, O. sativa, Sorghum bicolor, T. aestivum, and Zea mays) and five dicots (A. thaliana, Carica papaya, Malus domestica, Persea Americana, and Populus trichocarpa) have at least one member of the NPR1-like protein in each of the three clades (Table S3), the ancient duplication events resulting in functional divergence of NPR1-like genes may occur prior to the monocot-dicot split. Furthermore, SsNPR members in the same clade shared similar gene structures, conserved domains, and motifs presented in the Arabidopsis NPR1-like sequences (Figure 2), suggesting that their orthologs probably display similar biological functions in Saccharum. These findings indicate that the NPR1-like genes are highly conserved in numerous plant species.
WGDs or polyploidy was an important driving force in the evolution of organisms [32]. After WGDs, a proper balance in signaling and regulatory networks is maintained, while other types of duplication events (e.g., local, tandem, segmental, and aneuploidy) leave genes out of balance to varying degrees [33]. In this study, single copy, dispersed duplication, and WGD were all found in the NPR1-like gene family, and the expansion of the SsNPR gene family occurred mainly through WGD (Table S6). Synteny analysis revealed that the NPR1-like gene family in S. spontanum has experienced at least two rounds of WGDs (Figure 3), and the gene duplication events of NPR1-like genes in the monocots and dicots occurred after the divergence of monocots and dicots (Figure 1). Here, 13 out of 14 pairs of SsNPR duplicated genes have a Ka/Ks < 1 (Table S5), indicating that the replicated NPR1-like genes may be subject to strong selection pressure for purification.
Promoter cis-elements are essential for gene regulation [34]. MeJA and ABA both are important regulators in developmental processes and plant defense responses [35,36]. Previous studies showed that a similar antagonistic relationship exists between ABA and NPR1, e.g., ABA can inhibit the expression of the NPR1 gene [37,38]. In our study, there were many stress-related and phytohormone-responsive cis-elements in the SsNPR promoters (Figure 4 and Table S8). Interestingly, each of the NPR promoters in Saccharum contained an ABRE cis-element, which is involved in ABA responsiveness (Figure 4). This suggests that the ABRE element may be involved in the regulation of NPR1 by ABA. Furthermore, more than 88% of the promoter regions in the NPR1-like genes contained MeJA-responsive elements (CGTCA-motif and TGACG-motif) (Figure 4B), suggesting that NPR1-like genes may also involve a series of sugarcane physiological responses mediated by MeJA.
The nuclear localization signal in NPR1-like genes is closely related to its ability to timely and effectively induce the expression of defense genes [39]. AtNPR1C82A and AtNPR1C216A mutants cause NPR1 to depolymerize into monomers, localize in the nucleus, and stimulate the expression of defense genes in Arabidopsis [19]. Amino acid alignment analysis and subcellular location results showed that the ShNPR1 protein contained these two functional sites (Figure 6) and was localized in the nucleus (Figure 7). SA production results in an increase in the thioredoxins leading to the reduction of C156 and disassembly of the NPR1 oligomer [17,19]. Monomeric NPR1 is then translocated to the nucleus via a bipartite NLS where it induces the expression of PR1 [40,41]. C156 is a highly conserved cysteines residue in NPR proteins (Figure 6), indicating that C156 may be involved in the formation of the NPR oligomer to regulate the expression of the PR gene.
The expression abundance of the NPR1-like gene in different plant tissues is not consistent [13,42]. There are 19 BjuNPR genes detected in the roots, stems, leaf, pod, and flowers of B. juncea var. tumida [42]. Different BjuNPR genes expressed in different tissues of B. juncea var. tumida, for example, BjuNPR4-B, exhibited a specific high expression in all tissues, while BjuNPR6-A was not expressed in the root [42]. Robert et al. [13] found that the expression of Persea americana PaNPR was higher in mature tissues than that in younger ones. In this study, ShNPR1 expressed constitutively in the sugarcane tissues, with the highest expression level in the leaf and the lowest level in the bud (Figure 8). Interestingly, the tissue-specific expression patterns of sugarcane NPR1-like genes in different evolutionary branches were different (Figure 5A). These results indicate that NPR1-like genes may play different roles in the regulation of plant growth and development.
Previous research has shown that NPR1-like genes can be regulated by biotic stress [20,43,44]. Molla et al. [20] found that overexpressing AtNPR1 in rice causes an enhancement in tolerance to sheath blight disease. Overexpression of the MpNPR1 gene enhanced the resistance of Malus pumila to fire injury and fungal disease, and activated the expression of a series of downstream disease-resistance-related genes [43]. The Gossypium hirsutum GhNPR1 gene was induced by MeJA, SA, and ET, and played a defensive role in Fusarium oxysporum and Xanthomonas campestris infections [44]. Here, after inoculation with the smut pathogen, the ShNPR1 transcript abundance significantly increased in the smut-resistant variety YT96-86, but was the opposite in the smut-susceptible variety ROC22 (Figure 9A), suggesting that ShNPR1 may be a positive responsive component of smut resistance in sugarcane. Phytohormones, such as SA, MeJA, ET, and ABA, play an essential role in the regulation of plant immune responses to pathogens [45]. NPR1 is not only a receptor of SA but also a regulator of SAR, which plays a vital role in SA/JA signaling crosstalk [46]. In addition, SA-mediated redox modulation also plays an important role in the SA-mediated attenuation of the JA signaling pathway [47]. In this study, we found that SA reduced transcript levels of ShNPR1 in Saccharum (Figure 3A), which was contrary to what was observed in Persea americana [13]. This result seems to be host-specific. On the other hand, after exogenous treatment with JA, the expression of NPR1 increased first and then decreased, and similar results were also observed in banana plants [48]; however, a contradictory result was seen in Brassica juncea [49]. This difference in NPR1 expression patterns may highlight the response diversity in monocotyledonous and dicotyledonous.
Yu et al. [25] found that overexpression of the Secale cereale NPR1 gene could improve fusarium head blight resistance in wheat. Ramineni et al. [50] reported that the expression level of the PR1 gene in the BjNPR1-overexpressing transgenic plants was significantly higher than that in the untransformed plants after being infected by Sclerospora graminicola. The hybrid lily ‘Sorbonne’ LhSorNPR1 positively regulated the defense response of Arabidopsis to P. syringae pv. tomato DC3000 by elevating the transcript levels of SA-associated genes (PR1, PR2, and PR5) [51]. The overexpression of BjNPR1 showed enhanced resistance to Alternaria brassicae and Erysiphe cruciferarum as there was a delay in symptoms and a reduced disease severity [49]. Similarly, in our study, the control leaves post-inoculated with R. solanacearum or F. solani var. coeruleum exhibited more severe disease symptoms than the leaves that overexpressed ShNPR1 (Figure 10A–D). In addition, the transcript abundance of the tested tobacco-immune-related marker genes, especially SA-pathway-related genes and HR marker genes, was higher in the 35S::ShNPR1 leaves than that in the control (Figure 10E,F). These results reveal that the ShNPR1 gene plays a positive role in plant defense responses, and the overexpression of ShNPR1 in N. benthamiana leaves enhances resistance to R. solanacearum and F. solani var. coeruleum infections.
In summary, the ShNPR1 gene may be involved in plant SA, JA, and HR signal regulatory networks and its expression can be induced by exogenous hormones ABA, SA, and MeJA. Although it is obvious that ShNPR1 acts as an essential part of the biotic signaling pathway, further studies should be conducted to verify the function of the ShNPR1 gene in stable transgenic plants.
4. Materials and Methods
4.1. Plant Materials and Treatments
Two different sugarcane genotypes, including smut-resistant variety YT96-86 and smut-susceptible variety ROC22, were provided by the Key Laboratory of Sugarcane Biology and Genetics and Breeding, Ministry of Agriculture and Rural Affairs (Fuzhou, China). These two materials were used to detect the expression level of ShNPR1 in sugarcane infected with the smut pathogen. Ten-month-old sugarcane stalks were selected from the two varieties and cultured at 32 °C. After the buds germinated to 1–2 cm in length, an acupuncture inoculation method with 5 × 106 mL−1 (containing 0.01% Tween-20, v/v) of smut pathogen suspension was used to inoculate the sugarcane buds, and the control group was inoculated with sterile water (containing 0.01% Tween-20, v/v) [52]. All inoculated materials were grown at 28 °C with a photoperiod of 16 h light and 8 h darkness. Five buds at time points of 0, 1, and 3 dpi were harvested and immediately frozen in liquid nitrogen, and stored at −80 °C until use.
For the analysis of the expression patterns of ShNPR1 under different exogenous stresses, four-month-old ROC22 tissue culture seedlings were cultivated in water for about 10 d. The leaves of the seedlings were sprayed with 100 µM ABA, 5 mM SA (containing 0.01% Tween-20, v/v), and 25 µM MeJA (containing 0.1% ethanol and 0.05% Tween-20, v/v), respectively [53,54]. The leaf samples treated with SA and MeJA were harvested at 0, 3, 12, and 24 h, while those treated with ABA were collected at 0, 3, 6, and 12 h. As for the different sugarcane tissues, the root, bud, +1 leaf, stem epidermis, and stem pith were sampled from nine healthy and mature 10-month-old ROC22 plants [54]. All samples were immediately frozen in liquid nitrogen after being harvested and stored at −80 °C.
4.2. Identification and Characterization of the NPR1-like Gene Family
In order to identify the NPR1-like gene family in Saccharum, the genome of sugarcane ancestor S. spontaneum AP85-441 (http://www.life.illinois.edu/ming/downloads/Spontaneum_genome/) (accessed on 10 June 2021) [30] was selected. The hidden Markov model profile of BTB.hmm (Pfam: PF00651) was obtained from the Pfam database (http://pfam.xfam.org/) (accessed on 13 June 2021) [55]. The putative NPR1-like proteins identified by HMMER v3 software [56] were submitted to the CDD database (https://www.ncbi.nlm.nih.gov/cdd) (accessed on 13 June 2021) and BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (accessed on 13 June 2021) to check for the N-terminal BTB/POZ domain and ANK repeats [14]. The basic properties including MW, pI, the grand average of hydropathicity (GRAVY), and instability index were analyzed using the online ExPASy-ProtParam tool (http://web.expasy.org/protparam/) (accessed on 2 July 2021). The subcellular localization and secondary structure of NPR1-like proteins were predicted using Plant-mPloc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) (accessed on 5 July 2021) and Prabi (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_server.html) (accessed on 10 July 2021), respectively.
4.3. Analysis of Phylogenetic, Gene Structure, Motif Composition, Gene Duplication, and Synteny
The NPR1-related protein sequences of O. sativa (Os), Z. mays (Zm), S. bicolor (Sb), Musa acuminate (M), T. aestivum (Ta), A. thaliana (At), C. papaya (Cp), Cocos nucifera (Cn), Morus multicaulis (Mu), Glycine max (Gm), M. domestica (Mp), Vitis vinifera (Vv), P. trichocarpa (Pt), and P. americana (Pa) were downloaded from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) (accessed on 5 October 2021) (Table S3). The full-length proteins from Saccharum and 14 other plant species were aligned by MUSCLE within MEGA X [57]. The phylogenetic tree was constructed by MEGA X with the neighbor-joining (NJ) method and the parameters of complete deletion, Poisson correction, and 1000 bootstrap replicates [57]. Then, the phylogenetic tree was displayed by ITOL v6 (https://itol.embl.de/) (accessed on 10 October 2021).
The Multiple Expectation Maximization for Motif Elicitation online program (http://meme-suite.org/tools/meme) (accessed on 18 October 2021) was applied to identify the conserved motifs of NPR1-like proteins [58]. Gene structures of the identified SsNPR1-like gene IDs were extracted from the GFF3 file in the genome data. TBtools was used to display the phylogenetic tree, conserved motifs, and gene structures [59]. The Multiple Collinearity Scan toolkit (MCScanX) was used to analyze the duplication pattern and synteny of the NPR1-like genes [60]. TBtools was used to calculate the values of Ka/Ks between orthologous gene pairs [59].
4.4. Cis-Acting Regulatory Elements Analysis in the Promoter Regions
The promoter regions, 2 kb of the genomic DNA sequence upstream of the translation start site of each sugarcane NPR1-like gene, were identified by searching the genome of S. spontaneum. These promoter sequences were then submitted to the PlantCARE online program (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 25 October 2021) to identify cis-regulatory elements [61]. Then, the results were visualized using TBtools [59].
4.5. Expression Profiles of Sugarcane NPR1-like Genes Based on the Available Transcriptome Datasets
The bud, stem pith, epidermis, leaf, and root of 10-month-old ROC22 sugarcane plants were collected and frozen in liquid nitrogen. The experiment has three biological replicates, and each biological replicate contains three samples. According to the transcriptome data reported by Que et al. [62], the buds of ROC22 (sugarcane smut-susceptible variety) and Saccharum spp. hybrid YC05–179 (sugarcane smut-resistant variety) were taken after being infected with S. scitamineum for 0, 1, 2, and 5 days. Based on the reference genome of S. spontaneum [30], the above RNA samples were sent to BMK Biotechnology Co, Ltd. (Beijing, China) for RNA-seq analysis. The resulting raw data were treated by FASTP and HISAT2 programs, respectively, to improve the sequence quality and map the sequence data to the reference genome. The expression profiles of NPR1-like genes in different sugarcane tissues and under S. scitamineum stress were transformed by log2 FPKM (the number of fragments per kilobase of transcript per million mapped in the transcriptome data). The heat map was constructed with TBtools [59].
4.6. Gene Cloning and Bioinformatic Analysis of ShNPR1
Using the first-strand cDNA of ROC22 leaves as a template and the ShNPR1-cDNA (Table S10) as a primer, the cDNA sequence of ShNPR1 was cloned. The PCR program temperature conditions were 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 54 °C for 45 s, and 72 °C for 2 min, and an elongation step at 72 °C for 10 min. The PCR product was constructed into the cloning vector Blunt-Zero (Transgen Biotech, China, Beijing) and transformed into Escherichia coli DH5α. The positive recombinant vector Blunt-Zero-ShNPR1 was obtained after sequencing (Fuzhou Shangya Biotechnology Co., Ltd., Fuzhou, China). ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) (accessed on 12 November 2021) and the conserved domains database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 13 November 2021) were used to predict the ORF and the conserved domain of the ShNPR1 gene. The sequences were aligned using the Clustal Omega program (https://www.ebi.ac.uk/Tools/msa/clustalo/) (accessed on 5 March 2022) and visualized in Jalview Version 2.11.2.2. DNAMAN 6.0 software was used to perform sequence homology analysis.
4.7. Subcellular Localization Analysis of ShNPR1
The primer ShNPR1-gate (Table S10) was used to amplify the ShNPR1 ORF (without the stop codon). This fragment was then ligated to the subcellular localization vector pFAST-R05 by using gateway technology. After sequencing verification, a positive recombinant plasmid pFAST-R05-ShNPR1 was obtained. To confirm the subcellular location of the ShNPR1 protein, the A. tumefaciens GV3101 cells containing the pFAST-R05-ShNPR1-GFP vector or pFAST-R05-GFP vector were centrifuged and resuspended by the induction medium (10 mM MES, 10 mM magnesium chloride (MgCl2), and 200 μM acetosyringone, pH 5.0–5.4) at an OD600 of 0.8 [63]. Then, they were injected into N. benthamiana leaves. The infected plants were cultured for 2 d at 28 °C (16 h of light and 8 h of darkness). In addition, 10 μg/mL of 4′, 6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. Subcellular localization results were observed under laser scanning confocal microscopy (Leica TCS SP8, Wetzlar, Germany) using a 10× lens, a 488 nm green fluorescence excitation wavelength, and a 458 nm chromatin DAPI filter.
4.8. Quantifification of ShNPR1 Expression by qRT-PCR Analysis
On the NCBI online software, the specific qRT-PCR primers ShNPR1-QF/R (Table S10) were designed based on the ShNPR1 gene sequence. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal reference [64]. The 10-fold diluent cDNAs of different sugarcane tissues (root, bud, leaf, stem pith, and stem epidermis) and samples treated with ABA, SA, and MeJA were used as the qRT-PCR templates. RNA extraction with Trizol® Reagent (Invitrogen, Carlsbad, CA, USA) and qRT-PCR analysis with ChamQ™ Universal SYBR@ qPCR Master Mix were referenced to our previous study [65]. The 20 μL reaction system of qRT-PCR contained 10 μL of SYBR Green Master Mix, 0.4 μL of 10 μM forward primer, 0.4 μL of 10 μM reverse primer, 1.0 μL of 10× diluted cDNA template, and 8.2 μL of sterile distilled water. The qRT-PCR procedure was 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. After that, the melting curves were analyzed. The 2−ΔΔCt method was used to calculate the qRT-PCR data [66]. The significance level of the data was analyzed using DPS 9.50 software, and graphs were plotted using Origin 8.0 software. All these data points were mean ± standard error (n = 3). Bars superscripted by different lowercase letters indicate significant differences, as determined by Duncan’s new multiple range test (p-value < 0.05).
4.9. Transient Expression of ShNPR1 in Nicotiana benthamiana
The plant overexpression vector pEarleyGate 203-ShNPR1 was constructed using the gateway technology. The empty vector pEarleyGate 203 and the recombinant vector pEarleyGate 203-ShNPR1 carried by A. tumefaciens GV3101 were transiently overexpressed in the leaves of four-week-old N. benthamiana [67]. The one-day overexpression N. benthamiana leaves were inoculated with tobacco R. solanacearum and F. solani var. coeruleum according to the method of Ren et al. [65]. All materials were kept at 28 °C for 16 h of light and 8 h of darkness, and phenotypic alterations were noted. The Adobe Photoshop CS5 software was used to analyze the leaf lesion area. In addition, the leaves inoculated with R. solanacearum for 1 d and 9 d, or inoculated with F. solani var. coeruleum for 1 d and 8 d were collected for DAB histochemical detection to test hydrogen peroxide (H2O2) accumulation and qRT-PCR analysis of the expression level of the tobacco-immune-related marker genes. These tobacco-immune-related marker genes included the HR marker genes NtHSR201, NtHSR203, and NtHSR515 [68]; SA-related genes NtPR-1a/c, NtPR2, and NtPR3 [69]; ET synthesis-dependent genes NtEFE26 and NtAccdeaminase [70] (Table S10). NtEF-1α was used as an internal reference gene (Table S10).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23147984/s1.
Author Contributions
Conceptualization, S.Z., Y.S. and Y.Q.; methodology, Y.S. and Y.Q.; software, S.Z. and D.W. validation, S.Z., L.Q., Z.Z., J.Z., W.Z., A.F. and S.Y.; writing—original draft preparation, S.Z. and Y.S; writing—review and editing, Y.S. and Y.Q.; supervision, Y.S and Y.Q.; project administration, Y.S. and Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2019YFD1000500), State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (SKLCUSA-b202201), Natural Science Foundation of Fujian Province, China (2020J01591 and 2015J06006), and China Agriculture Research System of MOF and MARA (CARS-17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Weihua Su for his help in bioinformatics analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, H.; Bowling, S.A.; Gordon, A.S.; Dong, X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J.; Rogers, E.E.; Ausubel, F.M. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 1996, 143, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.R.T.S.; Tsui, F.; Klessig, D.F. Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol. Plant Microbe Interact. 1997, 10, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, C.; Jane, G.; Joseph, D.C.; Sigrid, V.; Xinnian, D. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar]
- Dong, X. NPR1, all things considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef]
- Ha, C.M.; Jun, J.H.; Nam, H.G.; Fletcher, J.C. BLADE-ON-PETIOLE1 encodes a BTB/POZ domain protein required for leaf morphogenesis in Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 1361–1370. [Google Scholar] [CrossRef]
- Hepworth, S.R.; Zhang, Y.; McKim, S.; Li, X.; Haughn, G.W. BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis. Plant Cell 2005, 17, 1434–1448. [Google Scholar] [CrossRef]
- Liu, G.; Holub, E.B.; Alonso, J.M.; Ecker, J.R.; Fobert, P.R. An Arabidopsis NPR1-like gene, NPR4, is required for disease resistance. Plant J. 2005, 41, 304–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.T.; Qu, N.; Zhao, Q.; Bi, D.; Li, X. Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J. 2006, 48, 647–656. [Google Scholar] [CrossRef]
- Bardwell, V.J.; Treisman, R. The POZ domain: A conserved protein-protein interaction motif. Genes Dev. 1994, 8, 1664–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoel, S.H.; Mou, Z.; Tada, Y.; Spivey, N.W.; Genschik, P.; Dong, X. Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 2009, 137, 860–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Mahomed, W.; Reeksting, B.J.; Engelbrecht, J.; Ibarra-Laclette, E.; van den Berg, N. Phylogenetic and expression analysis of the NPR1-like gene family from Persea americana (Mill.). Front. Plant Sci. 2015, 6, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, Z.; Niu, X.; Xu, Q.; Yang, L. Genome-wide identification and analysis of the NPR1-like gene family in bread wheat and its relatives. Int. J. Mol. Sci. 2019, 20, 5974. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Li, X.; Dong, X. Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 6531–6536. [Google Scholar] [CrossRef] [Green Version]
- Faugeron-Girard, C.; Gloaguen, V.; Koci, R.; Celerier, J.; Raynaud, A.; Moine, C. Use of a Pleurotus ostreatus complex cell wall extract as elicitor of plant defenses: From greenhouse to field trial. Molecules 2020, 25, 1094. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Molla, K.A.; Karmakar, S.; Chanda, P.K.; Sarkar, S.N.; Datta, S.K.; Datta, K. Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci. 2016, 250, 105–114. [Google Scholar] [CrossRef]
- Zhang, X.; Francis, M.I.; Dawson, W.O.; Graham, J.H.; Orbović, V.; Triplett, E.W.; Mou, Z. Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur. J. Plant Pathol. 2010, 128, 91–100. [Google Scholar] [CrossRef]
- Potlakayala, S.D.; DeLong, C.; Sharpe, A.; Fobert, P.R. Conservation of NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1 function between Arabidopsis thaliana and Brassica napus. Physiol. Mol. Plant Pathol. 2007, 71, 174–183. [Google Scholar] [CrossRef]
- Kumar, V.; Joshi, S.G.; Bell, A.A.; Rathore, K.S. Enhanced resistance against Thielaviopsis basicola in transgenic cotton plants expressing Arabidopsis NPR1 gene. Transgenic Res. 2013, 22, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Le Henanff, G.; Farine, S.; Kieffer-Mazet, F.; Miclot, A.-S.; Heitz, T.; Mestre, P.; Bertsch, C.; Chong, J. Vitis vinifera VvNPR1.1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta 2011, 234, 405–417. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, X.; Yao, J.; Zhou, M.; Ma, H. Resistance against Fusarium head blight in transgenic wheat plants expressing the ScNPR 1 gene. J. Phytopathol. 2017, 165, 223–231. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.; Lu, G.; Zeng, R.; Que, Y. Sugarcane ratooning ability: Research status, shortcomings, and prospects. Biology 2021, 10, 1052. [Google Scholar] [CrossRef]
- Rajput, M.A.; Rajput, N.A.; Syed, R.N.; Lodhi, A.M.; Que, Y. Sugarcane smut: Current knowledge and the way forward for management. J. Fungi 2021, 7, 1095. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Li, Q.; Zhu, Z.; Lou, Y.; Wang, L.; Wang, J.; Wang, M.; Li, Q.; Yang, D.; et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 2007, 5, 313–324. [Google Scholar] [CrossRef]
- Wally, O.; Jayaraj, J.; Punja, Z.K. Broad-spectrum disease resistance to necrotrophic and biotrophic pathogens in transgenic carrots (Daucus carota L.) expressing an Arabidopsis NPR1 gene. Planta 2009, 231, 131–141. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- McKim, S.M.; Stenvik, G.E.; Butenko, M.A.; Kristiansen, W.; Cho, S.K.; Hepworth, S.R.; Aalen, R.B.; Haughn, G.W. The BLADE-ON-PETIOLE genes are essential for abscission zone formation in Arabidopsis. Development 2008, 135, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Li, J.; Tang, H.; Paterson, A.H. Integrated syntenic and phylogenomic analyses reveal an ancient genome duplication in monocots. Plant Cell 2014, 26, 2792–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.M.; Komatsu, S. Proteomic analysis of rice leaf sheath during drought stress. J. Proteome Res. 2006, 5, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Jong-Joo, C.; Yang, D.C. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar]
- Sean, R.C.; Pedro, L.R.; Ruth, R.F.; Suzanne, R.A. Abscisic Acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar]
- Inaba, T.; Nagano, Y.; Sakakibara, T.; Sasaki, Y. Identification of a cis-regulatory element involved in phytochrome down-regulated expression of the pea small GTPase gene pra2. Plant Physiol. 1999, 120, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Hu, Y.; Zhang, J.; Huguet-Tapia, J.C.; Block, A.K.; Park, S.; Sapkota, S.; Liu, Z.; Liu, S.; White, F.F. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc. Natl. Acad. Sci. USA 2019, 116, 20938–20946. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.; Tian, M.; Klessig, D.F. Salicylic acid binds NPR3 and NPR4 to regulate NPR1-dependent defense responses. Cell Res. 2012, 22, 1631–1633. [Google Scholar] [CrossRef]
- Kinkema, M.; Fan, W.; Dong, X. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 2000, 12, 2339–2350. [Google Scholar] [CrossRef] [Green Version]
- Maier, F.; Zwicker, S.; Huckelhoven, A.; Meissner, M.; Funk, J.; Pfitzner, A.J.; Pfitzner, U.M. NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEINS1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol. Plant Pathol. 2011, 12, 73–91. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Z.; Zhang, Z.; Cai, Z.; Liao, J.; Tan, Q.; Xiang, M.; Chang, L.; Xu, D.; Tian, Q.; et al. Genome-wide identification and analysis of NPR family genes in Brassica juncea var. tumida. Gene 2020, 769, 145210. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Jin, Q.; Borejsza-Wysocka, E.E.; He, S.Y.; Aldwinckle, H.S. Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus x domestica. Mol. Plant Microbe Interact. 2007, 20, 1568–1580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Cheng, C.; Gao, Q.; Liu, J.; Guo, X. Molecular cloning and characterization of GhNPR1, a gene implicated in pathogen responses from cotton (Gossypium hirsutum L.). Biosci. Rep. 2008, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Barbara, N.K.; David, M.B. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Despres, C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Endah, R.; Beyene, G.; Kiggundu, A.; van den Berg, N.; Schlüter, U.; Kunert, K.; Chikwamba, R. Elicitor and Fusarium-induced expression of NPR1-like genes in banana. Plant Physiol. Biochem. 2008, 46, 1007–1014. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Mehari, H.; Meena, R.P.; Bhat, J.A.; Yadav, P.; Papalou, P.; Rawat, S.; Grover, A. Overexpression of NPR1 in Brassica juncea confers broad spectrum resistance to fungal pathogens. Front. Plant Sci. 2017, 8, 1693. [Google Scholar] [CrossRef] [Green Version]
- Ramineni, R.; Sadumpati, V.; Khareedu, V.R.; Vudem, D.R. Transgenic pearl millet male fertility restorer line (ICMP451) and hybrid (ICMH451) expressing Brassica juncea nonexpressor of pathogenesis related genes 1 (BjNPR1) exhibit resistance to downy mildew disease. PLoS ONE 2014, 9, e90839. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Zhang, Y.; Wang, Y.; Yang, G.; Yang, L.; Wang, L.; Wang, R.; Xie, Z. Overexpression of LhSorNPR1, a NPR1-like gene from the oriental hybrid lily ‘Sorbonne’, conferred enhanced resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Physiol. Mol. Biol. Plants 2017, 23, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Fu, X.; Huang, N.; Zhong, Z.; Su, W.; Lin, W.; Cui, H.; Que, Y. A sugarcane smut fungus effector simulates the host endogenous elicitor peptide to suppress plant immunity. New Phytol. 2022, 233, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, N.; Wang, L.; Ling, H.; Sun, T.; Ahmad, W.; Muhammad, K.; Guo, J.; Xu, L.; Gao, S.; et al. A novel L-ascorbate peroxidase 6 gene, ScAPX6, plays an important role in the regulation of response to biotic and abiotic stresses in sugarcane. Front. Plant Sci. 2017, 8, 2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Guo, J.; Ling, H.; Chen, S.; Wang, S.; Xu, L.; Allan, A.C.; Que, Y. Isolation of a novel peroxisomal catalase gene from sugarcane, which is responsive to biotic and abiotic stresses. PLoS ONE 2014, 9, e84426. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Su, Y.; Guo, J.; Wu, Q.; Xu, L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Yu, Q.; Li, Z.; Liu, F.; Su, W.; Zhang, C.; Ling, H.; Luo, J.; Su, Y.; Que, Y. A PIP-mediated osmotic stress signaling cascade plays a positive role in the salt tolerance of sugarcane. BMC Plant Biol. 2021, 21, 589. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Wu, Q.; Guo, J.; Xu, L.; Que, Y. Comprehensive selection of reference genes for gene expression normalization in sugarcane by real time quantitative RT-PCR. PLoS ONE 2014, 9, e97469. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zou, W.; Feng, J.; Zhang, C.; Su, W.; Zhao, Z.; Wang, D.; Sun, T.; Wang, W.; Cen, G.; et al. Characterization of the sugarcane MYC gene family and the negative regulatory role of ShMYC4 in response to pathogen stress. Ind. Crop. Prod. 2022, 176, 114406. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 2012, 24, 1675–1690. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.I.; Kim, Y.H.; Kim, B.R.; Lee, S.Y.; Lim, C.K.; Hur, J.H.; Lee, J.Y. Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol. Cells 2007, 24, 232–239. [Google Scholar]
- Brogue, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef]
- Chen, N.; Goodwin, P.H.; Hsiang, T. The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J. Exp. Bot. 2003, 54, 2449–2456. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).