Assessing the Morphological and Behavioral Toxicity of Catechol Using Larval Zebrafish
Abstract
1. Introduction
2. Results
2.1. Determination of LC50 Values for Catechol on Zebrafish Larvae
2.2. Behavioral Effects of Catechol
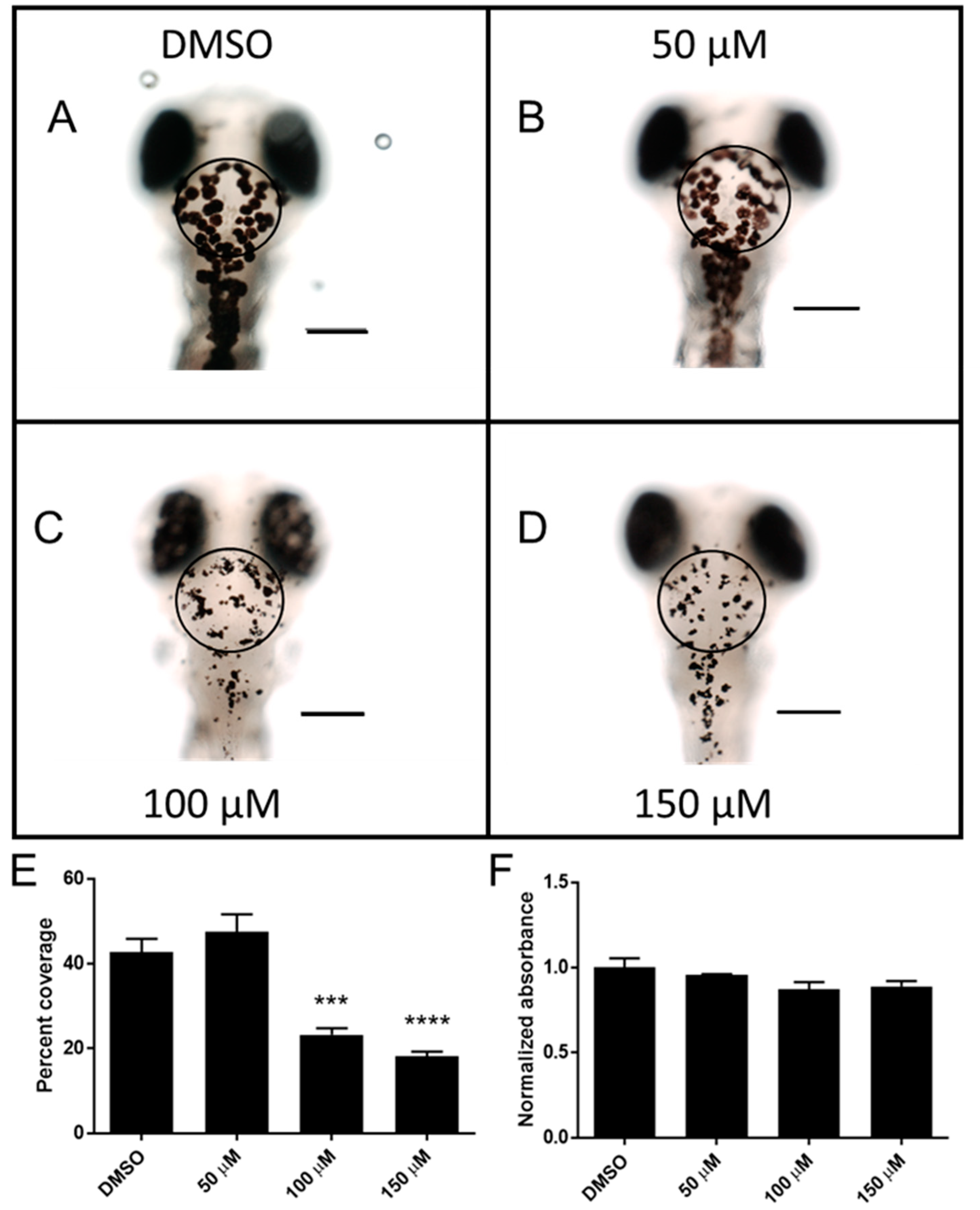
2.3. Catechol Affects Pigmentation in Zebrafish Larvae
2.4. Catechol Exposure Affects the Expression of Several Melanocyte-Related Genes
3. Discussion
4. Materials and Methods
4.1. Zebrafish Husbandry and Embryo Collection
4.2. Chemicals
4.3. Chemical Larval Exposure
4.4. Behavioral and Toxicity Testing
4.5. Melanin Quantification
4.6. QPCR Chemical Challenge
4.7. RNA Extraction and cDNA Synthesis
4.8. qPCR
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, D.; Pandey, J.; Tiwary, B.N.; Jain, R.K. A process optimization for bio-catalytic production of substituted catechols (3-nitrocatechol and 3-methylcatechol. BMC Biotechnol. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. In Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Lyon, France, 17–24 February 1998; IARC Monogr Eval Carcinog Risks Hum 1999. Volume 71, pp. 1–315. [Google Scholar]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Adsorption of catechol, resorcinol, hydroquinone, and their derivatives: A review. Int. J. Energy Environ. Eng. 2012, 3, 32. [Google Scholar] [CrossRef]
- Babic, S.; Cizmek, L.; Marsavelski, A.; Malev, O.; Pflieger, M.; Strunjak-Perovic, I.; Popovic, N.T.; Coz-Rakovac, R.; Trebse, P. Utilization of the zebrafish model to unravel the harmful effects of biomass burning during Amazonian wildfires. Sci. Rep. 2021, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mistri, R.; Ray, B.C. A rapid and selective method for simultaneous determination of six toxic phenolic compounds in mainstream cigarette smoke using single-drop microextraction followed by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 9265–9272. [Google Scholar] [CrossRef]
- Sepetdjian, E.; Abdul Halim, R.; Salman, R.; Jaroudi, E.; Shihadeh, A.; Saliba, N.A. Phenolic compounds in particles of mainstream waterpipe smoke. Nicotine Tob. Res. 2013, 15, 1107–1112. [Google Scholar] [CrossRef]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef]
- Sugumaran, M.; Umit, K.; Evans, J.; Muriph, R.; Ito, S.; Wakamatsu, K. Oxidative Oligomerization of DBL Catechol, a potential Cytotoxic Compound for Melanocytes, Reveals the Occurrence of Novel Ionic Diels-Alder Type Additions. Int. J. Mol. Sci. 2020, 21, 6774. [Google Scholar] [CrossRef]
- Hidekazu, Y.; Akiko, T.; Kazue, I.; Masahisa, U.; Hiroshi, U. Hair Dyeing by Using Catechinone Obtained from (+)-Catechin. J. Cosmet. Dermatol. Sci. Appl. 2012, 02, 158–163. [Google Scholar] [CrossRef][Green Version]
- Levay, G.; Bodell, W.J. Role of hydrogen peroxide in the formation of DNA adducts in HL-60 cells treated with benzene metabolites. Biochem. Biophys. Res. Commun. 1996, 222, 44–49. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.; Eggen, R.I. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Li, K.M.; Balu, N.; Saeed, M.; Devanesan, P.; Higginbotham, S.; Zhao, J.; Gross, M.L.; Rogan, E.G. Catechol ortho-quinones: The electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis 2002, 23, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Vadhanam, M.V.; Thaiparambil, J.; Gairola, C.G.; Gupta, R.C. Oxidative DNA adducts detected in vitro from redox activity of cigarette smoke constituents. Chem. Res. Toxicol. 2012, 25, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Ellis, L.D.; Soo, E.C.; Achenbach, J.C.; Morash, M.G.; Soanes, K.H. Use of the zebrafish larvae as a model to study cigarette smoke condensate toxicity. PLoS ONE 2014, 9, e115305. [Google Scholar] [CrossRef]
- Kluver, N.; Konig, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kuhne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Env. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef] [PubMed]
- Wagle, M.; Mathur, P.; Guo, S. Corticotropin-releasing factor critical for zebrafish camouflage behavior is regulated by light and sensitive to ethanol. J. Neurosci. 2011, 31, 214–224. [Google Scholar] [CrossRef] [PubMed]
- EPA. Catechol (Pyrocatechol); EPA: Washington, DC, USA, 2000; p. 4. [Google Scholar]
- Chen, Y.H.; Huang, Y.H.; Wen, C.C.; Wang, Y.H.; Chen, W.L.; Chen, L.C.; Tsay, H.J. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 2008, 30, 440–447. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotoxicol. Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R. The new age of chemical screening in zebrafish. Zebrafish 2010, 7, 1. [Google Scholar] [CrossRef]
- Mueller, K.P.; Neuhauss, S.C. Sunscreen for fish: Co-option of UV light protection for camouflage. PLoS ONE 2014, 9, e87372. [Google Scholar] [CrossRef]
- Polatoğlu, İ.; Karataş, D. Modeling of molecular interaction between catechol and tyrosinase by DFT. J. Mol. Struct. 2020, 1202, 127192. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Derm. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Battyani, Z.; Xerri, L.; Hassoun, J.; Bonerandi, J.J.; Grob, J.J. Tyrosinase gene expression in human tissues. Pigment. Cell Res. 1993, 6, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.D. Insights from zebrafish on human pigment cell disease and treatment. Dev. Dyn. 2017, 246, 889–896. [Google Scholar] [CrossRef]
- Fujii, R. The regulation of motile activity in fish chromatophores. Pigment. Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef]
- Johnson, S.L.; Nguyen, A.N.; Lister, J.A. mitfa is required at multiple stages of melanocyte differentiation but not to establish the melanocyte stem cell. Dev. Biol. 2011, 350, 405–413. [Google Scholar] [CrossRef]
- Baker, B.I. The role of melanin-concentrating hormone in color change. Ann. N. Y. Acad. Sci. 1993, 680, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.J.; Shuster, S. The association between the melanocyte-stimulating hormone receptor and the alpha 2-adrenoceptor on the Anolis melanophore. Br. J. Pharm. 1982, 75, 169–176. [Google Scholar] [CrossRef]
- Price, E.R.; Horstmann, M.A.; Wells, A.G.; Weilbaecher, K.N.; Takemoto, C.M.; Landis, M.W.; Fisher, D.E. alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J. Biol. Chem. 1998, 273, 33042–33047. [Google Scholar] [CrossRef]
- Swaminath, G.; Deupi, X.; Lee, T.W.; Zhu, W.; Thian, F.S.; Kobilka, T.S.; Kobilka, B. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J. Biol. Chem. 2005, 280, 22165–22171. [Google Scholar] [CrossRef]
- Johnson, S.L.; Africa, D.; Walker, C.; Weston, J.A. Genetic control of adult pigment stripe development in zebrafish. Dev. Biol. 1995, 167, 27–33. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio); Institute of Neuroscience, University of Oregon: Eugene, OR, USA, 2007; Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 17 July 2022).
- Lin, V.C.; Ding, H.Y.; Tsai, P.C.; Wu, J.Y.; Lu, Y.H.; Chang, T.S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morash, M.G.; Soanes, K.H.; Achenbach, J.C.; Ellis, L.D. Assessing the Morphological and Behavioral Toxicity of Catechol Using Larval Zebrafish. Int. J. Mol. Sci. 2022, 23, 7985. https://doi.org/10.3390/ijms23147985
Morash MG, Soanes KH, Achenbach JC, Ellis LD. Assessing the Morphological and Behavioral Toxicity of Catechol Using Larval Zebrafish. International Journal of Molecular Sciences. 2022; 23(14):7985. https://doi.org/10.3390/ijms23147985
Chicago/Turabian StyleMorash, Michael G., Kelly H. Soanes, John C. Achenbach, and Lee D. Ellis. 2022. "Assessing the Morphological and Behavioral Toxicity of Catechol Using Larval Zebrafish" International Journal of Molecular Sciences 23, no. 14: 7985. https://doi.org/10.3390/ijms23147985
APA StyleMorash, M. G., Soanes, K. H., Achenbach, J. C., & Ellis, L. D. (2022). Assessing the Morphological and Behavioral Toxicity of Catechol Using Larval Zebrafish. International Journal of Molecular Sciences, 23(14), 7985. https://doi.org/10.3390/ijms23147985





