Abstract
Hydrogen is the ultimate vector for a carbon-free, sustainable green-energy. While being the most promising candidate to serve this purpose, hydrogen inherits a series of characteristics making it particularly difficult to handle, store, transport and use in a safe manner. The researchers’ attention has thus shifted to storing hydrogen in its more manageable forms: the light metal hydrides and related derivatives (ammonia-borane, tetrahydridoborates/borohydrides, tetrahydridoaluminates/alanates or reactive hydride composites). Even then, the thermodynamic and kinetic behavior faces either too high energy barriers or sluggish kinetics (or both), and an efficient tool to overcome these issues is through nanoconfinement. Nanoconfined energy storage materials are the current state-of-the-art approach regarding hydrogen storage field, and the current review aims to summarize the most recent progress in this intriguing field. The latest reviews concerning H2 production and storage are discussed, and the shift from bulk to nanomaterials is described in the context of physical and chemical aspects of nanoconfinement effects in the obtained nanocomposites. The types of hosts used for hydrogen materials are divided in classes of substances, the mean of hydride inclusion in said hosts and the classes of hydrogen storage materials are presented with their most recent trends and future prospects.
1. Introduction
The 21st century has been marked by tremendously important technological breakthroughs, yet the massive expansion of industrialization has led to a deepening scarcity and skyrocketing prices of fossil fuels and energy raw materials, concomitant with a continual atmospheric pollution [1]. In the context of ever-increasing energy demands and the serious downsides of using fossil fuels, hydrogen has emerged over the past decades as a true and relevant promise of a carbon-free, green energy source for the world. However, hydrogen has a very low boiling point (20.4 K) at 1 atm, which severely restricts its use in the native form, except in some high pressure, cryogenic tanks that pose themselves additional energetic costs and safety risks regarding charging, transport and storing [1]. To circumvent the downfalls of using molecular dihydrogen (H2), scientists have turned their attention and research focus on hydrogen-containing compounds, in the form of metal hydrides and related materials, which in turn feature higher thermal stability, safer handling, no fuel loss upon storage and overall produce the cleanest energy known today. The fuel of the future should ideally produce no carbon-containing by-products, exhibiting time- and property-related endurance over 1500 dehydrogenation-rehydrogenation cycles, and most importantly, all of this while featuring a gravimetric weight percentage of at least 5.5 wt.% (DOE’s target set for 2025) [1,2,3,4,5,6]. The use of fossil fuels will eventually be phased-out and an energy-friendly alternative with no carbon emissions must be brought forward. Hydrogen can generate roughly three times more energy than gasoline (33.3 vs. 11.1 kWh/kg), and can be produced by thermochemical, electrolytic, solar splitting of water or by means of biological reactions involving bacteria and algae microorganisms [1]. The near-future (2025) targets for hydrogen storage systems require a system gravimetric capacity of 5.5 wt.%, volumetric capacity 0.04 kg H2/L, a hydrogen delivery temperature range of −40…+85 °C, a delivery pressure of five bar, a very fast refill time (3–5 min) and a high purity hydrogen production of 99.97% [4,5,6]. Broadly speaking, the hydrogen storage methods are divided in physisorption-based (fast kinetics, storage capacity dependent on support surface area and pore volume, weak Van der Waals interatomic forces) and chemisorption-based (somewhat slower kinetics of desorption/absorption, storage depends on chemical composition of the material, strong chemical bonds) [7,8]. The chemisorption method is the preferred one of the two, as it binds hydrogen through chemical bonds rather than weaker interatomic forces therefore ensuring a reliable hydrogen storage capacity and comprise of metal hydrides, tetrahydridoborates, tetrahydridoaluminates and metal amides.
The topic of hydrogen storage materials has been recently reviewed by a series of articles. Lai et al., have summed up the characteristics of potential hydrogen storage materials and established guidelines that new storage materials should obey for viable applicability in storage tanks [9]. An overview of hydrogen economy and appropriate recommendations was discussed by Abe et al. [10]. The state-of-the-art of boron-nitrogen compounds for energy storage was reviewed by Kumar et al. [11] and Hagemann [12]. The solid-state materials used for hydrogen storage have been addressed by Lee et al. [13], Hadjixenophontos et al. [14], Broom and Hirscher [15], Comanescu [16], Kharbachi et al. [17], Zheng et al. [18] and He et al. [19], among others. The role of highly dispersed catalysts on hydrogen storage materials [20] and the topologically engineered materials serving for energy conversion and storage [21] have also been very recently reviewed, while the critical issue of accurately describing hydrogen sorption properties of materials has been highlighted by Broom et al. [15].
Among hydrogen storage materials, metal hydrides have gained increased popularity [5,6,7,8,22,23,24,25,26] and channeled many research groups to corroborate hydrogen sorption data to formulate general design principles for these materials [27,28], or to tackle the imminent need to expand current knowledge to production of large-scale hydrogen storage facilities [29]. While many advances have been made in the field of metal hydrides for hydrogen storage applications, the high thermal stability, sluggish kinetics and poor reversibility of hydrogen release/uptake have shifted researchers’ attention towards nanoconfined hydrides that seem to alleviate some of these drawbacks, affording reversible, high gravimetric and volumetric hydrogen content at moderate temperatures [8,24,25].
The current review aims to tackle the current trend of employing nanoconfinements as a reliable tool to tune kinetic and thermodynamic behavior of hydride materials used for energy storage applications, and covers roughly the past five years.
2. Characterization Methods: Old, New, and Their Pitfalls
Traditionally, hydrogen storage materials follow a typical characterization protocol involving structural (XRD), elemental (XPS), morphological (SEM, TEM, N2 sorption isotherms) and recording of hydrogenation data (PCI curves) [8]. Recently, a fundamental issue regarding elucidation of local environment of hydrogen in energy materials has revealed fast sample spinning 1H NMR high-resolution spectroscopy as an appropriate tool to quantitatively characterize hydrogenated TiZrNi quasicrystals [30]. Kweon et al., showed by employing fast-spinning NMR spectroscopy that neutral hydrogen is surrounded by metal atoms shifting gradually from Zr to Ti and then Ni with increasing hydrogen content [30]. 1H magic-angle spinning (MAS) NMR spectra has shown real promise for tuning electronic characteristics in a Ba-Ti oxyhydride, and could become a tool to investigate hydrogen occupation in the vicinity of the nuclei (negative Knight shift, indicative of interaction of conduction band electrons and probe nucleus) [31]. A potential downside of using this technique is the high sensitivity to sample temperature, which was shown to increase due to fast rotor spinning (10–35 Hz), with a direct effect on main peak width change. Thus, additional precautions need to be undertaken to account for the effect of sample temperature increase when using fast spinning NMR spectroscopy [31].
Correct understanding of interfacial phenomena occurring during hydrogen storage is now termed as hydrogen spillover effect (HSPE). First discovered in 1964, it describes the migration of hydrogen atoms produced by H2 decomposition on an active site, and it allows for a more insightful view on the dynamic behavior of hydrogen in energy storage materials [7]. While molecular orbital energy computations showed unfavorable energy for H atom spillover on non-reducible supports, recent studies have shown that HSPE is indeed possible on inert supports such as siloxanic materials (SiO2) [7]. This bears a direct effect on hydrogen storage materials such as metal hydrides confined in mesoporous silica supports, where the spillover distance is limited to very short distances of ~10 nm [7].
Interestingly, developing tools to characterize metal hydrides during hydrogenation cycles has led to a summary of soft (X-ray absorption, XAS; X-ray emission spectroscopy, XES; resonant inelastic soft X-ray scattering, RIXS, X-ray photoelectron spectroscopy, XPS) and hard (X-ray diffraction, XRD) X-ray techniques used to this end (Figure 1) [32]. Soft X-rat techniques (100–5000 eV) are particularly appealing for tracking mechanistic behavior and intermediate product formation during hydrogenation studies, with direct influence over hydrogen storage capacity. XAS measurements for instance are bulk or surface-sensitive, and show 3d transition metal (TM) L-edges corresponding to transition of a 2p electron to an unoccupied 3d orbital, hence enabling monitoring of oxidation state changes during hydrogen release (+n...0) and uptake (0…+n) [32]. Similarly, TM-catalyzed alanates (2 mol%-catalyzed NaAlH4) showed in XAS measurements the Al and Na K-edge and Ti L-edge consistent with a Ti-like state throughout the hydrogen release/uptake cycles, but with clear differences in Al state, which may undergo various intermediate states (Al/NaAlH4/Na3AlH6) [32]. Quasi-elastic neutron scattering (QENS) studies have been undertaken to establish hydrogen dynamics in nanoscale sodium alanate NaAlH4 and showed that fitting QENS to a Lorentzian function can yield two dynamic states of hydrogen and concluded that even at 77 °C there is a high percentage (18%) of mobile hydrogen atoms in the nano-NaAlH4 [33].
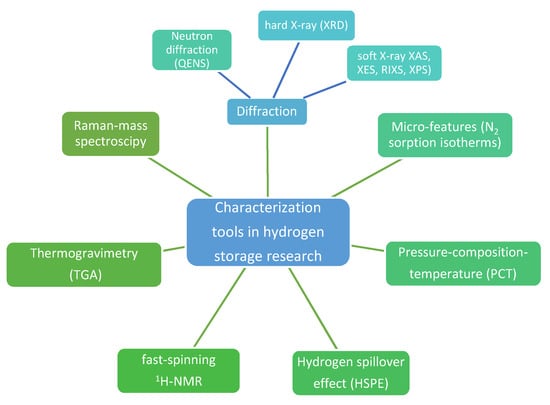
Figure 1.
Main investigation methods used for characterization of hydrogen storage materials.
As an alternative method to the conventional pressure-composition-temperature (PCT) method typically used to characterize thermodynamic parameters for hydride-based systems, a less complex investigation method has been described for MgH2-based materials: thermogravimetric analysis (TGA) [34]. This method relies on cycling the hydride under a flowing gas of constant hydrogen partial pressure, and the TGA curves are further analyzed using the van’t Hoff equation to obtain the absorption/desorption enthalpies, which in the case of VTiCr-catalyzed Mg/MgH2 materials, showed good agreement with traditional PCT results [34]. Other recent research established a nano-Pd patched surface of Pd80Co20 to afford one of the most sensitive optical hydrogen sensors (fast response of <3 s, high accuracy of <5%, and very low limit of detection of 2.5 ppm) [35]. Employing interpretable machine learning could also help formulate general design principles for intermetallic hydride-based systems being used to validate limited data from the HydPARK experimental metal hydride database and stressing the recommendation for experimental groups to report ΔH, ΔS, Peq, T and Vcell [27].
Valero-Pedraza et al., have characterized the hydrogen release form ammonia borane nanoconfined in mesoporous silica by means of Raman-mass spectroscopy, which confirmed hydrogen release from AB at lower temperatures, fewer BNHx gaseous fragments in nanoconfined samples and a lack of polyiminoborane formation during thermolysis [36]. The study also pointed out to silica-hydride interactions, which were identifiable based on modifications in the Raman spectra [36].
However, analysis of the literature data also points out to several weaknesses in applying traditional characterization methods that have not yet been tuned for current nanosized materials [15,34,37,38]. For instance, AB (ammonia borane) hydrogenation studies showed many inconsistencies [38]. By assessing TGA data in the literature, Petit and Demirci urge caution when evaluating ammonia borane weight loss (and consequently hydrogen release), as this was found to be highly dependent on the operation conditions (semi-closed/open reactor) and were shown to erroneously indicate a different hydrogen release temperature onset and hydrogen wt.% [38].
Surrey et al., conducted a critical review of a paper discussing electron microscopy observation of elementary steps in MgH2 release mechanisms [37]. In this work, they debunked the general assumption that TEM microscopy can be used, as such, without further testing methodology adjustment in the case of hydrogen storage materials such as MgH2. The issue was serious, as it led initial authors to misinterpret TEM observations, by disregarding the key aspect of electron beam induced dehydrogenation of MgH2 [37]. In a cascade chain of errors, the beam-induced heat producing dehydrogenation also led to a false interpretation of SAD (selected area diffraction) data, which only showed hollow MgO shells deprived of Mg-core, an effect actually ascribed to the nanoscale Kirkendall effect. As a result, it was apparent that the sample actually measured did not even contain MgH2 any longer [37].
In line with the issues raised above, Broom and Hirscher discussed the necessary steps for reproducible results in hydrogen storage research [15].
3. Bulk vs. Nanomaterials
After its first inclusion on the research outlook of scientists worldwide in 1996, nano-sized hydrides have known a wide expansion, mainly due to several important kinetic and thermodynamic improvements of nanoconfinement over their bulk counterparts [4,8,14,16,18,21,22,23,27,28,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Over time, nanoconfinement has emerged as a reliable tool for tuning not only thermodynamic and kinetic behavior at nanoscale, but also for altering reaction pathways, lowering or even suppressing side-reactions and side-products, while also affording better size control of the particles over several hydrogen release/uptake cycles (Figure 2).
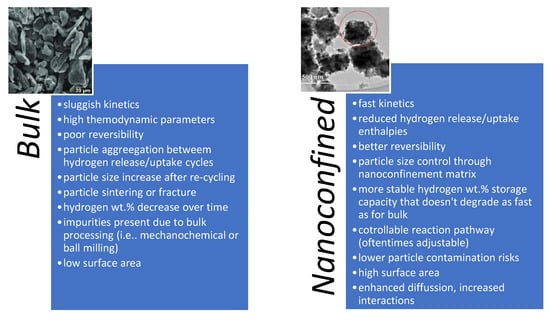
Figure 2.
Main features of bulk and nanoconfined materials for hydrogen storage; exemplified for the case of an overly-studied hydride, MgH2. (inset reprinted/adapted with permission from Ref. [65]. 2022, Elsevier).
3.1. Physical and Chemical Aspects of Nanoconfinement Effects
Nanoconfinement of active hydride species inside a porous host bears a number of physical and chemical implications [1,7,8,22,25,27,41,52,53,54,55,56,59,60,61,62,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
3.2. Nanocomposites
The proper term for describing the materials resulting from the nanoconfinement of active hydride source into a nanoporous matrix is nanocomposite [4,41,52,54,60,66,68,69,70,77,78].
4. Types of Hosts Used as Hydride Matrix
4.1. Siloxanic Materials (MCM-41, SBA-15, SBA-48, etc.)
Although some complex hydride materials (e.g., complex metal borohydrides) are plagued by an undesirable reaction with the porous host above the hydride melting temperature with formation of silicates [16], mesoporous silica is still used in several studies concerning nanoconfinement effects in hydrogen storage materials [74,87,88,89,90] (Table 1).

Table 1.
Recent progress in silica-based supports for nanoconfined hydrogen storage.
When LiBH4 was used as borohydride source in a mesoporous silica host, the reaction occurring during borohydride melting is a two-step process yielding lithium metasilicate, Li2SiO3, and ultimately lithium orthosilicate, Li4SiO4 (Equation (1)) [16]. This reaction is a downside of using nanoporous siloxanic supports for borohydride nanoconfinement, as it consumes the hydride material in an irreversible side-reaction (Equation (1)).
Confining LiBH4 by a melt impregnation technique in nanoporous silica MCM-41 (1D, dpore < 2 nm) or SBA-15 (2D-ordered pore structure, dpore = 5, 7 and 8 nm) of different pore sizes reveals an interesting interfacial effect governing Li+ and BH4− ion mobility [87]. Using solid-state NMR (1H, 6Li, 7Li and 11B), Lambregts et al., showed that, as a result of nanoconfinement, two distinct fractions of LiBH4 coexist and this is a temperature-dependent equilibrium (Equation (2)):
The high mobility LiBH4 is located near silica pore walls, whereas LiBH4 of lower mobility is located towards the pore’s core; the theoretical wall thickness was estimated based on a core-shell model LiBH4@SBA-15, as . The dynamic layer thickness is temperature-dependent, and increases from 0.5 nm (30 °C) to 1.2 nm (110 °C). Here again the results of calorimetric data were found to overestimate the highly-mobile LiBH4 layer thickness (1.9 nm), pointing out the need for care when deriving the same parameter from different techniques [87]. While 6,7Li NMR spectra was too complex for unequivocal deconvolution, 1H and 11B NMR spectra clearly show two components throughout the investigated temperature range (30–130 °C), consistent with the two LiBH4 fractions of different ion mobility [87].
Melt impregnation of NaBH4 in MCM-41 at 560 °C led to a drastic surface area decrease from 1110.9 m2 g−1 (pristine MCM-41) to 3.5 m2 g−1 (nanocomposite NaBH4@MCM-41), and to a 78% pore filling attested by pore volume decrease (1.02 cm3 g−1 to 0.02 cm3 g−1) [74]. Interestingly, some amount of sodium perborate NaBO4 resulting from unavoidable oxidation of the borohydride with silanol (Si-OH) groups is the main additional phase detected by XRD, confirming no significant additional phases due to melt impregnation at >500 °C. The dehydrogenation onset peak for NaBH4 was reduced by nanoconfinement from 550 °C (bulk) to 520 °C (nanocomposite) [74]. Due to the insulating nature of boron oxide phase (NaBO4), the ionic conductivity did not improve the same way it does for LiBH4, and remained largely the same (7.4 × 10−10 S cm−1). This 10-fold increase in ionic conductivity that only lasts up to 70 °C for the nanocomposite is attributed to the presence of larger dodecaborate ions B12H122− whose distinct presence was signaled in 11B NMR spectra by an additional sharp peak at −15.58 ppm (NaBH4@MCM-41) vs. −41.95 ppm (for pristine BH4-) (Figure 3) [74].
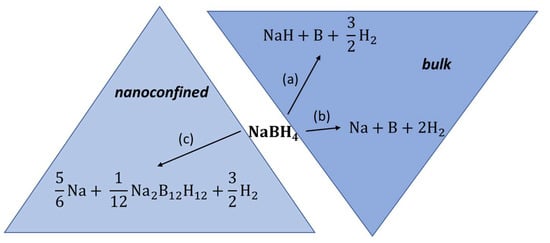
Figure 3.
Possible decomposition pathways for bulk NaBH4 (a,b) and for melt-impregnated, nanoconfined NaBH4 (c).
The organic–inorganic hybrid poly(acryalamide)-grafted mesoporous silica nanoparticles (PAM-MSN) have been evaluated as functionalized nanoporous hosts for tuning hydrogen release/uptake behavior in ammonia borane (AB), which started to desorb hydrogen in the said nanocomposite at a lower temperature with respect to pristine AB, which was further enhanced by functionalization of the mesoporous silica shell with carboxylic -COOH groups [88].
2D-ordered mesoporous silica of cylindrical pores (SBA-15) was successfully used by Yang et al., for enhancing the ionic conductivity of a mixed-anion borohydride, Li2(BH4)(NH2). By following a melt infiltration procedure, the Li-ion conductivity was increased in Li2(BH4)(NH2)@SBA-15 to 5 × 10−3 S cm−1 at 55 °C [89]. A marked kinetic improvement of hydrogen release (ΔT = 70 °C) was recently reported by Rueda et al., by confinement of ammonia borane (AB) in silica aerogel by simultaneous aerogel drying and AB gas antisolvent precipitation using compressed CO2, and achieving a weight AB loading of up to 60 wt.% [90].
4.2. Carbonaceous Materials (C-Replica of Mesoporous Silica, C-NTs, C-Foam, C-Spheres, Graphene, Graphene Oxide GO, Reduced Graphene Oxide r-GO)
Given the chemically-sensitive interaction between silanol (Si-OH) and borohydride (BH4-) groups and the subsequent oxidation reaction, the election of mesoporous silica as a host for loading borohydride materials seems less feasible. Therefore, many research studies have shifted their focus towards carbon-based materials, which do not exhibit such a drawback. Many forms of carbonaceous matrix have been employed: C-replica of mesoporous silica, C-NTs, C-foam, C-spheres, graphene, graphene oxide GO, reduced graphene oxide r-GO etc. (Table 2) [40,42,53,65,69,70,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146].

Table 2.
Recent progress in carbonaceous-based supports for nanoconfined hydrogen storage.
4.3. Metal-Organic Frameworks (MOFs) and Functionalized-MOFs
Metal-organic frameworks (MOFs) have recently been utilized as hosts for metal hydrides, due to their tunable porosity, stability and enhancement of kinetic and thermodynamic properties of hydrogen storage materials (Table 3). Their functionalization with appropriate groups/molecules opens new doors in energy storage field, being able to bypass side-reactions, alter significantly the reaction pathway, and afford a better reversible material in hydrogen release/uptake studies [39,40,68,86,147,148,149,150,151,152,153,154,155,156,157].

Table 3.
Recent progress in MOF-based nanoconfined hydrogen storage systems.
4.4. Main Group and TM (Transition Metal)-Oxides, Sulfides and Nitrides
Various metal oxides and nitrides of metals (main group and TM) have been employed as hosts for hydrogen storage materials [91,95,101,106,147,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172]. Embedding active hydrogen-storage systems into inert nanoscaffolds has been used in the past, but reports on shells of the sulfide type are rare in the scientific literature [80]. In fact, the only recent report is that of MgH2 nanoconfined in chemically-inert shells of CoS nano-boxes [80] (Table 4).

Table 4.
Recent progress in oxides, sulfides and nitrides-based hosts for nanoconfined hydrogen storage systems.
4.5. Metal Component/Host
Several reports have been published where the host is an actual metal matrix, usually one that is highly active in hydrogenation studies (Table 5) [40,68,94,112,121,122,138,163,164,165,166,167,168,169,170,171].

Table 5.
Recent progress in oxides, sulfides and nitrides-based hosts for nanoconfined hydrogen storage systems.
4.6. Gas Selective-Permeable Polymers
Attempts to restrict oxygen and moisture exposure of active hydrogenation sites in hydride materials have been made through the engineered approach of covering the hydride materials with a layer of H2-permeable polymer [88,127,156,173,174,175]. This approach proved to be very successful, provided that the hydride coverage was indeed complete (Table 6).

Table 6.
Examples of gas-selective H2-permeable polymers used as covering shells for hydrogen storage systems.
4.7. MXene
Ongoing recent trends in developing novel systems for energy storage have incorporated MXene materials with a 2D structure, as promising hydride hosts [94,156,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194]. While only few examples are currently available, it is foreseeable that MXenes will grow to become a mainstream storage matrix for nanoconfined hydride-based materials (Table 7).

Table 7.
Examples of MXenes used as hosts for hydrogen storage systems.
4.8. Catalytic Effects of Doping the Host and/or Substitution of the Hydride Species
Improvements on hydrogen release/uptake cycles have often been explored in conjunction with utilization of catalysts used to either dope the host, or the hydride material. This strategy is based on formation of active sites for hydrogenation reaction to occur, or is sometimes ascribed to the formation of a reactive intermediate species [19,68,92,102,111,112,113,117,125,128,151,160,161,163,195,196,197]. In addition, cation substitution or anion substitution in complex hydrides has been employed to reduce energy barriers and improve overall recyclability of the hydride materials (Table 8).

Table 8.
Examples of host decoration/doping and hydride substitution in nanosized systems.
4.9. (Nano)Catalyst Addition
The overall enhancement of kinetic and thermodynamic parameters can be tuned by utilization of catalysts. This is usually implemented to improve behavior of systems that already show promising results including recyclability (Table 9) [19,20,34,43,57,65,68,77,82,92,102,108,113,118,120,125,131,132,134,136,139,143,147,158,160,166,167,168,172,183,184,185,186,187,188,189,190,191,192,194,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212]. Due to the greater applicability of this approach in the past few years, the Table 9 summarizes them based on classes of substances and their corresponding characteristics.

Table 9.
Examples of recent advances using nanocatalysts to improve kinetic and thermodynamic properties of hydride-based systems in hydrogenation studies.
5. Inclusion Methods of Hydride Materials into Appropriate Host—State-of-the-Art and Limitations
5.1. Direct Synthesis
Starting from a commercially-available borohydride (such as LiBH4, NaBH4 etc.) and the corresponding salt of the metal (MClx), various novel borohydrides have been synthesized via the metathesis reaction (double exchange) (Equation (3)) [8,16].
Other approaches start from the organometallic precursor of the metal, which undergoes reduction (with H2 or another reductant, such as LiNp) typically after impregnation into the porous host. (Equation (4))
5.2. Infiltration Methods
5.2.1. Melt Infiltration
Melt infiltration of complex hydrides has widely been used to introduce the active hydride material into nanoporous hosts. This technique has the advantage of requiring no solvent (so it consists of less steps), but the hydride material must have a lower melting temperature, and the infiltration is carried out under H2 pressure in order to avoid the onset of dehydrogenation reaction.
5.2.2. Solvent Infiltration
Solvent infiltration has become the method of choice as it achieves pore filling of the porous scaffold at temperatures that are near ambient, provided that a suitable solvent for the material has been identified. This is typically an issue, as solubility data on complex hydrides are rather scarce, and usually their solubility in ether-like solvents is limited [16].
5.2.3. Solvent-Assisted Ball-Milling
Nanoconfinement of hydride-based materials in nanoporous hosts has the potential advantage of bypassing the slow kinetics of their bulk counterparts, thus enabling a shorter refueling time, in pursuit of the DOE’s current targets [5,6]. Very high surface area supports (MOFs, activated carbons) afford good hydrogen sorption capacities, but since the adsorption is mainly governed by physisorption, it is only relevant at 77 K. At this low temperature, a rough estimation (Chahine’s rule) is that for pressures that would occupy all adsorption sites (exceeding 20 bar), the expected storage capacity is ~1 wt.%/500 m2g−1 and scales proportional to the specific surface area [8]. Ball milling (with or without a solvent) can introduce the hydride material into the porosity of the employed scaffold. The process is energy-intensive and can proceed with an important increase in the local sample temperature, and therefore the process is carried out in steps (for instance, 20 min milling followed by a 10 min pause allowing controlled cooling).
6. Metal Hydrides and Their Recent Nanoconfinement Studies
Pristine metal hydrides have recently been comprehensively reviewed, and the results show promising trends upon nanoconfinement [213].
6.1. LiH
Alkali metal hydrides have been used for catalytic reactions, but have attracted attention due to their lightweight characteristics, as well as the high hydrogen gravimetric content. However, their high thermal stability makes them less attractive in their pure form; LiH, for instance, melts at 689 °C and decomposes at 720 °C into Li and H2 (Equation (5)). Alkali metal hydrides have unusually high decomposition temperatures due to their salt-like nature (LiH, mp = 698 °C; NaH, mp = 638 °C; KH, mp ~ 400 °C with K vaporizing in H2 current). Given their high decomposition temperature, alkali metal hydrides require kinetic and thermodynamic destabilization (Table 10).

Table 10.
Hydrogen storage features of nanosized LiH materials.
Recently, a series of strategies have been utilized to produce nanosized LiH, but not all attempts dealt with hydrogen storage applications [114,133,198,214,215,216], and some utilizing LiH-containing nanocomposites for their Li-storage capacity in a Co(OH)2-LiH novel anode material [217]. Even when dealing with potential hydrogen storage materials like LiH + MgB2, studies have focused on the phase-evolution process and XPS tracking thereof, rather than collection of hydrogen storage data [198]. Still, XPS data pointed to presence of LiBH4, Mg(3−x)/2Lix(BH4)x or Li-borate species present on account of multiple LiH-containing peaks identified [198]. At near-surface regions, LiBH4 or mixed Li-Mg borohydrides can form at 100 °C below the threshold for hydrogenation of MgB2; expectedly, LiBH4 production scales with the LiH in the starting composite (Equation (6)) [198].
Sun et al., have shown that harnessing the plasmonic thermal heating effect of Au nanoparticles could lead to light-induced dehydrogenation of nanocomposites Au@LiH, which showed a 3.4 wt.% loss ascribed to dehydrogenation content [214]. The Au NPs dispersed on the surface of LiH, Mg or NaAlH4 all showed marked improvements in hydrogenation studies. The preparation of Au/LiH composites involved LiH suspension in THF under sonication and overnight stirring at 500 rpm, after which a THF solution of HAuCl4 was added and stirring continued for an additional 24 h, leading to the Au/LiH material after centrifugation and overnight drying by Schlenk line technique. Hydrogen absorption was carried out under 14.8 atm H2, while desorption was conducted under 0.2 atm pressure, utilizing Xe lamp illumination affording 100 °C local temperature [214].
Overcoming kinetic and thermodynamic barriers in the complex Li-N-H system (Equation (7)) led White et al., to study the Li3N effect on the LiNH2 + 2LiH composite behavior [215]. On this occasion, a kinetic analysis showed the rate-limiting step is the formation of H2 (g) at the surface of the core-shell structure Li2NH@Li3N [215]. Again, the use of TEM measurements was shown to be inappropriate for LiNH2 materials, due to decomposition upon prolonged electron beam exposure. The equilibria shown in Equation (7) already occur upon the exposure of Li3N to 10 bar H2 (200 °C, 2 h), but not at one bar H2, which only altered the α-to-β ration of Li3N [215].
Considering the gravimetric hydrogen densities required by DOE standards, LiH, MgH2 and AlH3 are the main binary systems proposed to date [216]. Silicon doping of LiH has shown a drastic reduction in decomposition temperature (ΔT = 230 K), and could store up to 5 wt.% H2 with release at 490 °C [216]. A nanostructured electrode of Co(OH)2 and silica was recently employed in Li-conductivity studies and showed the formation of active LiH species, although the material was not investigated for its hydrogen storage properties [217].
A series of Li-based materials was investigated by Xia et al., who grafted on graphene LiH by in situ reduction in nBuLi with H2 (110 °C, 50 atm), producing LiH@G. This nanocomposite LiH@G was further treated with B2H6 or AB/THF, and novel LiBH4@G and LiNH2BH3@G nanocomposites were thus obtained (Equation (8)) [114].
The 2D LiH nanosheets were about 2 nm thick and afforded a 6.8 wt.% H2 storage when loaded at 50 wt.% in the said graphene-based nanocomposite, which withstood structural integrity upon further hydride-to-borohydride transformation (Figure 4) [114].
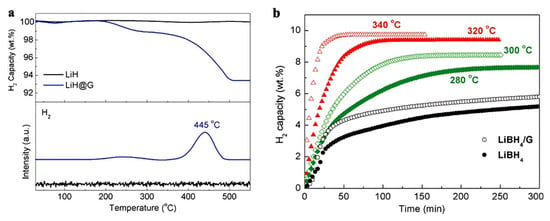
Figure 4.
TG of as-prepared LiH@G (a), and dehydrogenation isotherm of LiBH4@G (b). Reprinted/adapted with permission from Ref. [114]. 2017, Wiley, under CC BY 4.0 license.
Using HSAG (high surface area graphite) as scaffold, Wang et al., showed a 1.9 wt.% hydrogen storage at 200 °C for the composite LiH@HSAG, with reversible behavior at 300 °C and 60 bar H2 (Equation (9)) [133].
The morphology was tracked by SEM analysis and XRD diffraction, while hydrogenation data confirmed the modest 1.9 wt.% hydrogen storage by TGA (Figure 5).
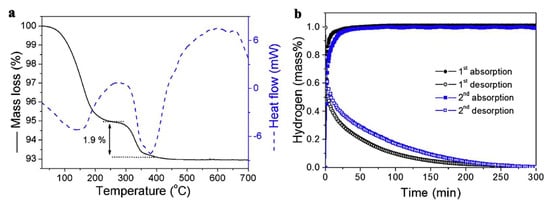
Figure 5.
TGA/DSC for LiH@HSAG (a) and hydrogen desorption/absorption kinetics for LiH@HSAG at 350 °C (b). Reprinted/adapted with permission from Ref. [133]. 2016, Elsevier.
This nanoconfinement approach in high surface area carbon (HSAG) of pore size 2–20 nm showed a high thermodynamic improvement, allowing for hydrogen release at 340 °C in LiH@HSAG rather than at the high 680 °C for pristine LiH [133].
6.2. MgH2
Due to its wide availability in nature, low cost, high gravimetric (7.6 wt.%) and volumetric (110 g/L) hydrogen storage capacities, the binary hydride, MgH2, is arguably the most studied metal hydride and Mg-based materials have been investigated exhaustively by a variety of research groups (Table 11) [34,43,47,53,55,57,61,65,77,78,80,92,94,95,98,102,103,109,110,115,118,121,122,124,130,134,137,146,147,149,158,168,169,170,172,177,196,198,201,202,204,205,208,209,210,211,212,214,216,218,219,220,221,222,223,224,225]. Synergistic effects of additives have been reviewed in the recent past: effect of nano-sized TMs (Ni, Cu, Fe, Co); salt addition in composites like MgH2 + 10 wt.% LaCl3; alloy formation Mg-La, Mg-Ni; incorporation in FeS2 nanospheres; dispersion effect of Nb2O5 catalysis; TiF3/TiO2/TiN/TiMn2 or Ti3C2 superior catalytic effects; Ni-based materials Mg@Ni binary nanocomposite; Mg2Ni alloy; Mg2NiH4; Ni@MgH2; NiB2/NiS/Ni3C/NiO/Ni3N/Ni2P; or carbon-based support influence (1D, 2D, 3D, graphene G, graphene oxide GO, MWCNT, etc.) (Table 11) [43,57,61,198,218,219,220,224]. The supporting role of a variety of carbonaceous hosts for MgH2 storage properties has been reviewed by Han et al., who underlined the added structural stability, catalytic effect and nanosizing structuring on metal hydrides, and magnesium hydride in particular [110]. Le et al., have recently viewed (2021) the nanoconfinement effects on H2-storage characteristics of MgH2 (and LiBH4) [47]. While conventional pressure-composition-temperature (PCT) data is time-consuming, an easier thermogravimetric analysis (TGA) was introduced by Zhou et al., to reliably determine abs./des. equilibrium temperature, and by using van’t Hoff equation to deduce reaction enthalpies and entropies: ΔHabs = 79.8 kJ mol−1, ΔSabs = 141.1 J mol−1K−1, and ΔHdes = 76.5 kJ mol−1, ΔSdes = 142.2 J mol−1K−1 for 5 at% VTiCr-catalyzed MgH2 [34]. Some research produced nano-assemblies MgH2@G (G = graphene) that was investigated as a material for high-performance Li-ion batteries (GMH composite with 50% MgH2 has reversible 946 mAhg-1 at 100 mAh-1 after 100 cycles) [103]. The necessity to better predict the behavior of Mg-containing clusters (MgnHm) that emerge as the Mg/MgHx (m < 2n) system matures, has led to a machine-learning (M-L) interatomic potential evaluation for Mg-H systems [223]. Wang and Huang have shown that the ML approach is able to accurately describe the diffusion coefficients and the Arrhenius type temperature dependence for 128 < t < 427 °C, a temperature range relevant for the Mg/MgH2 system in both pristine and nanoconfined conditions [223]. The diffusivity of H2 through Pd NPs deposited on Mg film has revealed that unlike the H2-impermeable MgO protective native film, the Pd-Mg interface can act as portals for hydrogenation of the Mg film [212].

Table 11.
Hydrogen storage features of nanosized MgH2 -based materials.
Jia et al., have utilized a Ni- or N-doped carbon scaffold for MgH2 nanoconfinement [92]. The carbonaceous support features high surface area, pore volume and narrow PSD (pore size distribution) and constitutes a C-replica of the mesoporous 2D-silica, SBA-15. Two Ni-loadings have been investigated: MgH2@xNi-CMK-3 (x = 1 and 5). Expectedly, the higher Ni-containing sample MgH2@5wt.%Ni-CMK-3 showed 7.5 wt.% storing capacity, whereas the MgH2@1wt.%Ni-CMK-3 and MgH2@ N-CMK-3 showed 6.5 wt.% hydrogen (Figure 6). The behavior of the nanocomposites has been investigated at 200, 250, 280 and 300 °C, and showed marked improvement scaling with temperature; at 300 °C, all three nanocomposites absorb 6 wt.% in 10 min (6.5 wt.% in 2h). The samples were degassed for 2 h at 350 °C prior to conducting absorption measurements (Figure 6) [92].
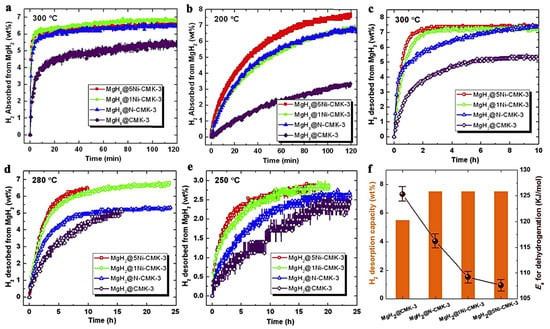
Figure 6.
Hydrogenation kinetics of MgH2@CMK-3, MgH2@N-CMK-3 and MgH2@xNi-CMK-3 (x = 1 and 5) at 300 °C (a) and 200 °C (b) and under 19.74 atm. H2 backpressure. Hydrogen desorption profiles of the four investigated samples at 300 °C (c), 280 °C (d), 250 °C (vacuum, p < 0.01 atm) (e). Dehydrogenation of nanocomposites within two hours at 300 °C and corresponding desorption activation energies. Ea,des (f). Reprinted/adapted with permission from Ref. [92]. 2017, Elsevier.
The enhancement in kinetics was obvious; van’t Hoff plot analysis revealed systematic decrease of the activation energy barrier in the order: MgH2@CMK-3 (125.3 ± 2.1 kJ mol−1) > MgH2@N-CMK-3 (116.2 ± 1.8 kJ mol−1) > MgH2@1Ni-CMK-3 (109.2 ± 1.3 kJ mol−1) > MgH2@5Ni-CMK-3 (107.6 ± 1.2 kJ mol−1) [92].
Zhang et al., have dispersed TM-oxides (TiO2 in particular) on amorphous carbon to achieve excellent, reversible hydrogen storage capacity, releasing in 10 min. at 275 °C, 6.5 wt.% hydrogen (85.5% that of pristine MgH2) (Figure 7) [95]. Notably, the activation energies for desorption (Ea,des) and absorption (Ea,abs) have been considerably reduced compared to bulk magnesium hydride (Figure 7a). In a multi-fold enhancement strategy, the MgH2 was first dispersed on carbon (MgH2 + C), which showed modest improvements (<1 wt.% H2) over MgH2 bulk with no dehydrogenation in the same timespan (Figure 7c), TiO2 was used as additive for MgH2 to yield composites of MgH2 + TiO2 NPs, which surprisingly released ~6 wt.% H2 in 10 min [95]. Driven by these enhancements, nanocomposites of the type MgH2 + TiO2 SCNPs/AC were synthesized, which further improved hydrogen release/uptake: even at 50 °C, over the course of 20 min, ~1.5 wt.% H2 is released, whereas at 125 °C (~4.8 wt.%) and at 200 °C (6.5 wt.%) the kinetics is sped up considerably (Figure 7c–e). The rehydrogenation occurs within 5 min at 200 °C, and full recovery of the hydrogen storage capacity is achieved (6.5 wt.%). In addition, no appreciable hydrogen storage loss was recorded up to the 10th cycle (Figure 7f ) [95].
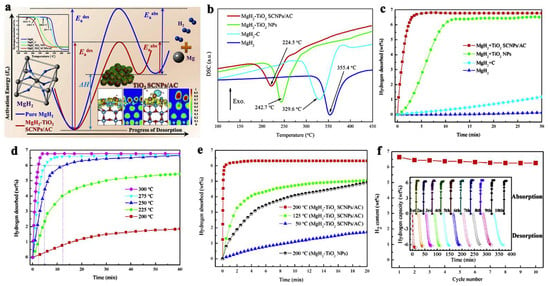
Figure 7.
Schematic mechanistic approach in catalytic behavior of MgH2-TiO2 SCNPs/AC (a). DSC (b) of the investigated samples: ball-milled MgH2, MgH2-C, MgH2-TiO2 NPs and MgH2-TiO2 SCNPs/AC. Isothermal desorption curves of the four investigated samples at 300 °C (c); Isothermal desorption curves of MgH2-TiO2 SCNPs/AC and MgH2-TiO2 NPs at various temperatures in the range 50…300 °C (d,e); confirmation of reversible hydrogen storage capacity of MgH2-TiO2 SCNPs/AC at 300 °C recharging pressure of 50 bar H2 (f). Reprinted/adapted with permission from Ref. [95]. 2019, Elsevier.
Using an FeCo nanocatalyst (mean size of 50 nm), Yang et al., synthesized composites MgH2 + nano-FeCo able to recharge to 6.7 wt.% hydrogen in one minute at 300 °C, and could desorb 6 wt.% (9.5 min, 300 °C) (Figure 8) [201]. In fact, even treatment under H2 backpressure at 150 °C produced 3.5 wt.% absorption in 10 min (Figure 8b). This highlights the importance of catalyst chosen, but also its morphology (nanosheets in the case of FeCo-nano). Plotting the Arrhenius equation also yielded the apparent activation energies: Ea,des = 65.3 ± 4.7 kJ mol−1 (60 kJ mol−1 reduction from pristine MgH2), and the absorption energy Ea,abs = 53.4 ± 1.0 kJ mol−1 (Figure 8d). Gratifyingly, the FeCo-catalyzed magnesium hydride composite was able to rehydrogenate fully and was tracked over the course of 10 hydrogen release/uptake cycles (Figure 8h) [201].
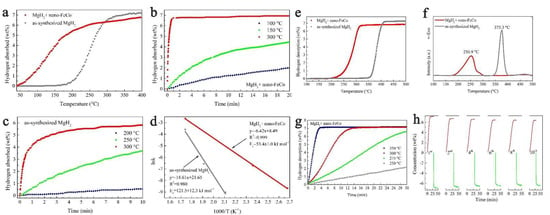
Figure 8.
Non-isothermal hydrogenation curves (a); isothermal hydrogenation curves at different temperatures (b,c) and the corresponding Arrhenius plot of MgH2 with and without nano-FeCo (d); non-isothermal dehydrogenation curves (e); DSC curves with a heating rate of 5 °C min−1 of MgH2 with and without nano-FeCo (f); isothermal dehydrogenation curves of MgH2 + nano-FeCo composite at 250, 275, 300, 350 °C (g); dehydrogenation (in red) and rehydrogenation (in green) curves of MgH2 + nano-FeCo composite in the 1st, 2nd, 4th, 6th, 8th and 10th cycle (h). Reprinted/adapted with permission from Ref. [201]. 2019, Royal Society of Chemistry.
The thermodynamic predictions that smaller size NPs will show the most important destabilization, Zhang et al., have produced ultrafine MgH2 that was able to release and recharge hydrogen under ambient temperature, with a very high hydrogen storage capacity of 6.7 wt.% (Figure 9) [222]. This capacity was checked over 50 cycles, and showed virtually the same high-capacity behavior (Figure 9). The conditions employed for reversible behavior were 360 min at rt (6.7 wt.%), or 60 min at 85 °C (6.7 wt.%), under 30 bar H2. This unexpectedly high storage capacity (65.6 g H2/L) surpasses even DOE’s requirement (50 gH2/L), and was possible solely on account of well-designed, size-restriction of MgH2 to nanoscale [222].
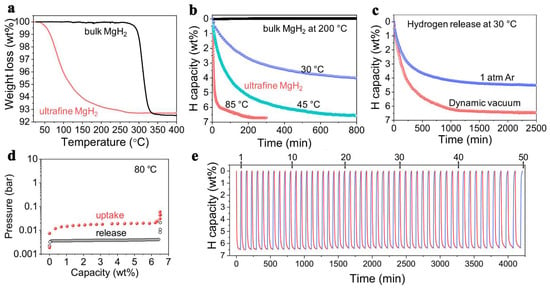
Figure 9.
TGA (a), isothermal TGA dehydrogenation (b), isothermal TGA dehydrogenation under different conditions (c), PCI curves measured at 80 °C (d); cycling stability of non-confined ultrafine MgH2 (e). Reprinted/adapted with permission from Ref. [220]. 2018, Elsevier B.V.
Using a nanoflake Ni catalyst, Yang et al., have synthesized MgH2 + 5 wt.% Ni, composites able to store 6.7 wt.% hydrogen (des., 300 °C, in 3 min) (Figure 10). The absorption was also very fast, achieving 4.6 wt.% at 125 °C in 20 min, under 29.6 atm H2 [202]. The results also translate into much lowered activation energies (Arrhenius plot): Ea,des = 71 kJ mol−1; Ea,abs = 28.03 kJ mol−1.
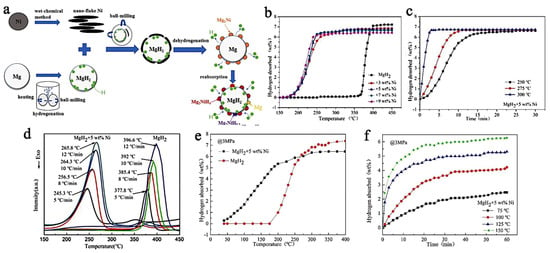
Figure 10.
Preparation and reaction evolution in the MgH2+Ni composite (a); the rising temperature dehydrogenation curve (b); Isothermal dehydrogenation curves of MgH2 + 5 wt.% Ni at different temperatures (c); DSC curves of MgH2 + 5 wt.% Ni at different rates of increasing temperature (d); Non-isothermal hydrogenation curves of MgH2 with and without 5 wt.% Ni (e); Isothermal hydrogen absorption curves at different temperatures of MgH2 + 5 wt.% Ni (f). Reprinted/adapted with permission from Ref. [202]. 2021, Royal Society of Chemistry.
These results have been explained by means of the intermediate Mg2Ni intermediate, which is an intermetallic well-known in the Mg-Ni systems, and which absorbs rapidly H2 to form Mg2NiH4. This functions as an effective “hydrogen pump” (Figure 10a) (Equation (10)) [202].
Decomposition of nBu2Mg typically used as an organometallic precursor to Mg/MgH2 NPs can follow two different steps, depending on the reaction temperature (Equations (11) and (12)).
However small it might be, nanosized matter in general is also more reactive towards various gases and substrates, and Mg/MgH2 coupled system is no exception. Previous examples have overcome this downside by either pressing the nano-powders into pellets, or capping them with other reagents. There are however many reports where MgH2 has been introduced in the porosity of a carbonaceous host, such as the 3D activated carbon utilized by Shinde et al., to achieve a reversible hydrogen storage of 6.63 wt.% (Figure 11) [137]. Not only was the nanocomposite MgH2@3D-C storing hydrogen under relatively mild conditions 6.63 wt.% (five minutes, 180 °C), but the desorption was likewise fast (6.55 wt.%, 75 min, 180 °C), and perhaps more importantly, the nanoconfined MgH2 was air-stable thanks to the protective carbon shell [137]. To the observed enhanced kinetics and improved thermodynamic behavior contribute decisively the transition metal dispersed into the 3D carbon: NI>Co>Fe. Running in a continuous regime, the nanocomposite was able to cycle for about 435 h (more than 18 days), without a palpable decrease in the hydrogenation storage capacity (Figure 11) [137].
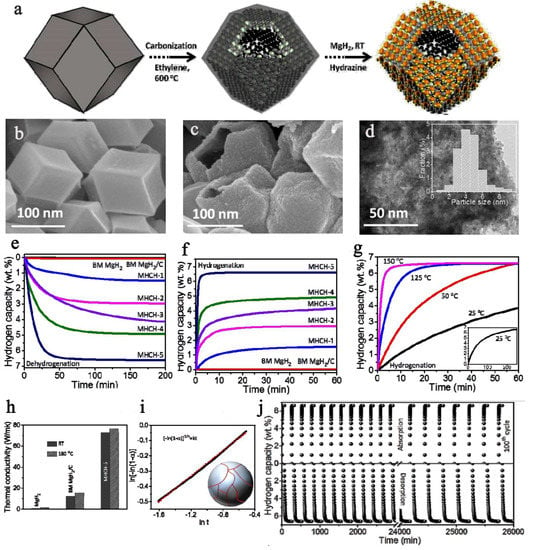
Figure 11.
(a) Schematics displaying the self-assembled MgH2 on three-dimensional metal interacted carbon. (b) SEM image of prepared metal-interacted 3-D carbon; (c) SEM, (d) TEM images of the MHCH-5; (e) dehydrogenation of the as-synthesized MHCH samples at 180 °C in comparison to ball-milled MgH2 and MgH2/C; (f) Isothermal hydrogenation; (g) Hydrogen absorption of the MHCH-5 for different temperatures—the inset (g) shows the hydrogen absorption property of the MHCH-5 at 25 °C, over a long time period; hydrogenation and dehydrogenation were performed under hydrogen pressures of 10 bar and 0.01 bar, respectively; (h) Thermal conductivity variation in MHCH-5, MgH2, and ball-milled MgH2/C for ambient temperature and 180 °C; (i) The growth mechanism of MgH2 in MHCH samples correlating with a Johnson–Mehl–Avrami model. (j) Reversible hydrogen (under 10 bar H2 pressure) and dehydrogenation (under 0.01 bar H2 pressure) performance of the MHCH-5 at 180 °C. Reprinted/adapted with permission from Ref. [137]. 2017, The Royal Society of Chemistry; RSC Pub.
While typically reduction in nBu2Mg infiltrated into a nanoporous host to afford MgH2 NPs is carried out in heterogeneous conditions (under H2 pressure), Shinde used a mixed reductant system: TEA ((HOCH2CH2)3N)/NH2NH2 hydrazine to reduce Mg(II) to Mg(0) [137]. The synthetic procedure is nicely followed in Figure 11, and in this case, both scanning electron microscopy (SEM) and transmission electron microscopy (TEM) could be used for characterization, since the electron beam no longer hits directly the MgH2 NPs; thus, the risk of in-situ decomposition during data acquisition is minimized (Figure 11). The hydrogen storage capacity exceeds 6 wt.% in case of Ni-NPs deposited in the 3D-AC (MHCH-5), confirming the beneficial and synergistic role of Ni when used in conjunction with MgH2. The plausible intermediate Mg2Ni forms the coupled system Mg2Ni/Mg2NiH4 during hydrogenation, and this can be held responsible for the superior cycling behavior in case of MgH2@3D-AC (MHCH)-5(Ni), whereas this type of intermetallic is not common for Co or Fe [137].
The self-assembled MgH2 NPs are well embedded into the carbonaceous host, which plays a critical role in the overall performance of MHCH-5. It is implied, based on the thermal conductivity data (Figure 11h), that the carbon shell is important. The high thermal conductivity (70 W/mK), many times higher than that of MgH2 NPs themselves, induces a lower temperature gradient in the sample and a high heat transfer coefficient, thus contributing to the exemplary behavior of the sample during hydrogenation cycling [137].
6.3. AlH3
Alane (AlH3) is a metastable hydride, stabilized by the Al proneness to combine with oxygen and form a µm layer of Al2O3 ensuring chemical passivation. In bulk, AlH3 decomposes at 100–150 °C and the kinetics are reasonably fast, but the high H2 pressure required to achieve reversibility (10 GPa, 600 °C, 24 h; 10 GPa at 25 °C or 6 GPa at 300–380 °C by other accounts are all very high pressures) remains a hard obstacle to overcome (Table 12). Even so, mitigation of this drawback has been attempted by means of nanoconfinement [40,44,51,109,125,203,206,216,226,227,228,229,230,231,232,233]. Some results are pure theoretical results concerning the catalytic activity of nano-AlH3 [229] in the decomposition of 1,3,5,7-Tetranitro-1,3,5,7-tetrazocane, with simulated evolution of Al-clusters during the reaction [228], or decomposition of CH3NO2/nano-AlH3 composite [232].

Table 12.
Hydrogen storage features of nanosized AlH3 materials.
The energetic bottleneck in hydrogenation of Al is the high activation barrier of H2 dissociation over the Al surface, therefore catalysts have been employed to lower this barrier by using TM dopants like Sc, V, Ti or Nb [229].
The reaction of LiH and AlCl3 was shown to be greatly sped up by using a 0.1 molar TiF3, when the final product obtained after five hours milling under Ar pressure was a nanocomposite of composition α-AlH3/LiCl-TiF3 [203]. Duan et al., have shown the critical role of TiF3 that acted as a seed crystal for α-AlH3. The pressure was also a crucial factor, as running the reaction under lower gas pressure only led to Al metal formation, without the envisioned hydridic phase (Equation (13)) [203].
However, thermodynamic data showed a Gibbs free energy for the expected α-AlH3 formation of ΔG = −269 kJ mol−1, therefore thermodynamically possible at 298 K [203]. Furthermore, tracking the reaction by solid-state 27Al NMR spectra has shown the complex behavior of the reactive mixture (Figure 12) (Equation (14)).
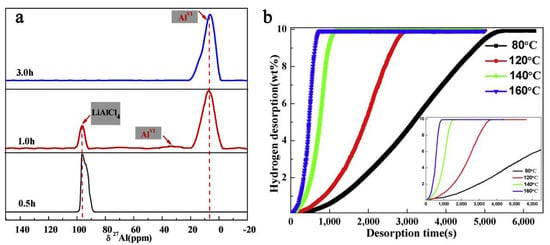
Figure 12.
Solid state 27Al NMR for reactive mixture LiH/AlCl3/TiF3 (3:1:0.1) after ball milling for 0.5 h, one hour and three hours (a); hydrogen desorption curve for final composite α-AlH3/LiCl-TiF3 at temperatures 80 °C, 120 °C, 140 °C and 160 °C (inset shown for α-AlH3/LiCl without TiF3 addition) (b). Reprinted/adapted with permission from Ref. [203]. 2019, Elsevier B.V.
The kinetics are vastly improved, and raising the temperature above 120 °C allows for complete dehydrogenation in roughly 10 min (Figure 12).
After five hours of ball milling under Ar pressure and dehydrogenation at 160 °C for 600 s, the final composite (Figure 13) shows nanosized AlH3 (mean size of α-AlH3 was 45 nm, without traces of agglomerates).
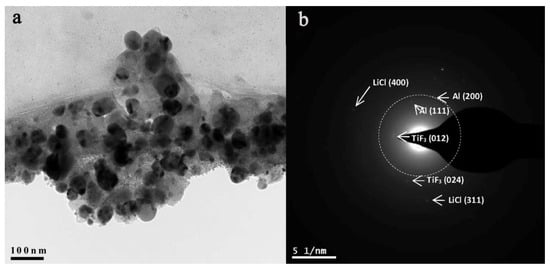
Figure 13.
TEM image of α-AlH3/LiCl-TiF3 after dehydrogenation for 600 s at 160 °C (bright field TEM, (a); ED pattern, (b)). Reprinted/adapted with permission from Ref. [203]. 2019, Elsevier B.V.
The phase composition already shows formation of Al, consistent with the dehydrogenation reaction that had occurred. The report also highlighted the important role of the fluoride additive, as TiF3 reduced Ea of H-desorption to 52.1 kJ/mol [203].
Nanoconfinement of alane in a Cr-based MOF (MIL-101) with Al-doping has led to a nanocomposite able to store and recharge at 298 K (ambient) and 100 bar H2, 17.4 mg H2/g (equivalent to 1.74 wt.% H2) [40]. The introduction of alane inside the MIL-101 pores was made via solvent infiltration from a THF solution of AlH3. In fact, the pristine MOF MIL-101 (3148 m2 g−1, 2.19 cm3g−1 and 2.5–3 nm pores) was shown to store 0.55 wt.% H2 under the same conditions. The hydrogen release profile from the investigated samples shows the improvement of nanoconfinement of AlH3 in MOF pores over the hydrogen release performance (Figure 14) [40].
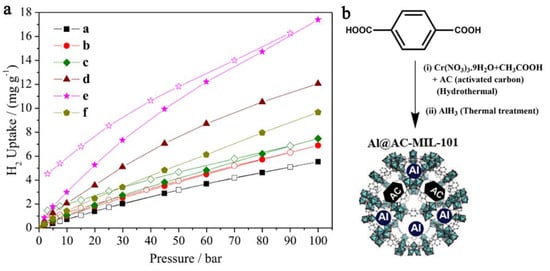
Figure 14.
Hydrogen adsorption–desorption isotherms for (a) MIL-101; (b) AC-MIL-101; (c) AL@MIL-101; (d) Al@AC-MIL-101-A; (e) Al@AC-MIL-101-B; (f) Al@AC-MIL-101-C at 298K and pressures up to 100 bar H2 (closed symbols-Adsorption; open symbols: Desorption) (a). AlH3 introduction into MIL = 101 (b). Reprinted/adapted with permission from Ref. [40]. 2017, Elsevier Inc.
The gravimetric storage capacity (17.4 mg H2 g−1 composite) was rather low considering DOE’s goals, due to the inability to increase Al-doping of the framework without crystallinity loss, and the role of AC additive became apparent in order to enhance hydrogen interaction with confined Al NPs [40].
In an attempt to improve upon previous results, Duan switched the nano-host to MWCNT (multi-walled carbon nanotubes) of high pore textural characteristics (550 m2 g−1, 6–8 nm diameter) and obtained by ball-milling xMgH2 + AlH3 (x = 1–4) nanocomposites MgH2/AlH3@CNT of crystal size 40–60 nm that released 8.2 wt.% H2 at 200 °C (60 min), and recharged to 5.61 wt.% H2 at 250 °C (10 min) (Figure 15) [109].
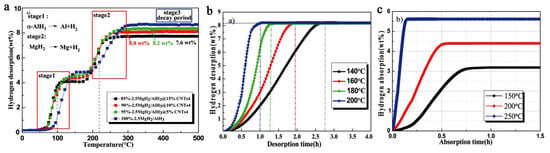
Figure 15.
TPD (temperature programmed desorption) of 85%, 90% and 95%- 2.5MgH2/AlH3/CNTs4 and 100%-2.5MgH2/AlH3 (ball milling, BPR 20:1, 200 rpm, 1 h) (a); dehydrogenation isotherm of 95%-2.5MgH2/AlH3/CNTs4 under 10−2 Pa pressure custom vacuum system (b); Isothermal rehydrogenation curves of 95%-2.5MgH2/AlH3@CNTs4 at different temperatures under 5 MPa H2 pressure (c) [109]. Reprinted/adapted with permission from Ref. [109]. 2021, Royal Society of Chemistry.
The Al metal produced in the first dehydrogenation stage of the composite (Figure 16) will react with MgH2 not yet dehydrogenated, to yield an intermetallic phase of Al12Mg17, which was confirmed by XRD data (Equation (15)).
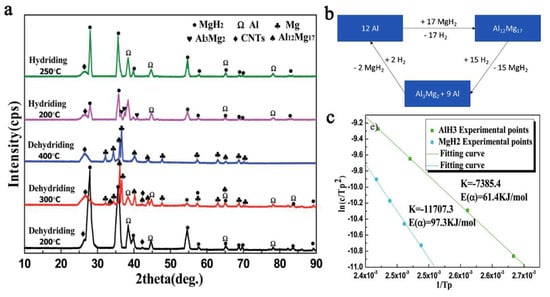
Figure 16.
XRD pattern for 95%- 2.5MgH2/AlH3/CNTs4 after dehydrogenation at temperatures 200…400 °C (a); Al-tracking throughout the proposed mechanism, based on reaction data from ref. [109] (b) and Kissinger plot for deduction of Ea for hydrogenation of MgH2 and AlH3 (c) [109]. Reprinted/adapted with permission from Ref. [109]. 2021, Royal Society of Chemistry.
The reactions involved in the mechanistic proposal of the authors also allowed computation of the apparent activation energies (by Kissinger plot), which were of 97.3 kJ mol−1 for MgH2 and 61.4 kJ mol−1 for AlH3 (Figure 16c).
Wang et al., showed the potential of nanosizing by introducing (injection in HSAG of Et2O solution of freshly-made AlH3 from metathesis of LiAlH4 and AlCl3) [44]. Considering the 14 wt.% loading with AlH3 in the composite AlH3@HSAG (by ICP-OES), the expected hydrogen capacity was 1.4 wt.%. However, only 15% of the Al behaved reversibly and thus only an overall 0.25 wt.% storage could be attributed to the nanoconfined AlH3 [44]. Interestingly, during sample preparation, the composite was heated at 65 °C under Ar to yield α-AlH3 polymorph and minimize spontaneous decomposition of AlH3 [44]. Either way, the reduction in dehydrogenation onset to ~60° (60…270 °C with a peak at 165 °C) shows the effect of nanosizing, effectively reducing hydrogen release by 50 °C [44].
Recently, using a triazine framework functionalized with bipyridine groups, CTF-bipy, a reversible behavior of alane in AlH3@CTF-bipy nanocomposite was observed at 700 bar H2 and 60 °C (although incomplete; Al signals still show in 27Al MAS-NMR) (Figure 17) [51].
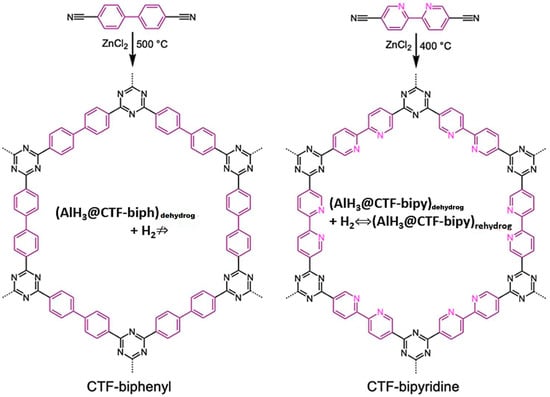
Figure 17.
Construction of triazine-type CTF-biph and CTF-bipy used for alane nanoconfinement. Reversibility was only achieved for AlH3@CTF-bipy, presumably due to Al-complexation to N-atoms of bipyridyl moieties (shown inside the CTF frame). Reprinted/adapted with permission from [51].
The EELS spectra of AlH3@CTF-biph and AlH3@CTF-bipy confirm that both contained aluminum, thus AlH3 introduction in the CTF-based frame was achieved. However, inherent oxidation had also occurred so the Al2O3 presence was also recorded by EELS data [51]. Although alane introduction into CTF-biph and CTF-bipy porosity was confirmed by N2 sorption isotherms (Figure 18), there was no reversibility in the case where CTF-biph was used as host [51].
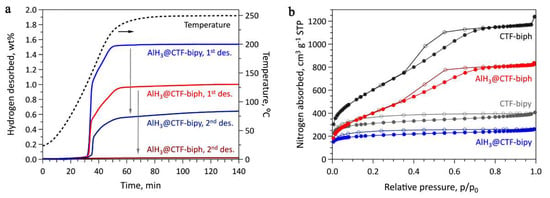
Figure 18.
(a) Sievert data for CTF-based supported alane; (b) N2 sorption isotherms at 77 K for CTF-biph, AlH3@CTF-biph, CTF-bipy, and AlH3@CTF-bipy. Reprinted/adapted with permission from Ref. [51]. 2021, Wiley-VCH GmbH.
Indeed, no reversibility was recorded in the absence of bipyridine groups (CTF-biph; biph = biphenyl), so the amino-functionality grafted on the covalent framework of the host was considered mandatory to achieve reversibility. This aspect was confirmed through DFT computations showing AlH3 or higher clusters—(AlH3)2, (AlH3)3, or (AlH3)4—coordinating the 2 N-atoms of the bipyridine group. However, the reversible H2 storage decreased from 1.44 wt.% (1st cycle) to 0.57 wt.% (4th cycle) [51].
Ball milling of a light metal nitride (Li3N) with AlH3 showed that a weakening of the Al-H bond is produced as a result of the milling process (a shift in XPS maximum), and that the hydrogen capacity decreases with the Li3N fraction: 9.04 wt.% (0.95AlH3-0.05Li3N), 8.71 wt.% (0.9AlH3-0.1Li3N) and 7.85 wt.% (0.85AlH3-0.15Li3N), compared to the ball milled pure AlH3 (9.86 wt.%) (Figure 19) [206].
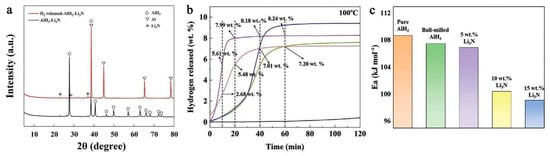
Figure 19.
The XRD pattern (0.9AlH3-0.1Li3N)dehydrog (a), the hydrogen release profile under isothermal conditions (100 °C) of (1 − x)AlH3-xLi3N (x = 0, 0.05, 0.1, 0.15) (b), and the calculated apparent activation energy (c). Reprinted/adapted with permission from Ref. [206]. 2022, Wiley-VCH GmbH.
Figure 19b shows the isothermal dehydrogenation of (1 − x)AlH3-xLi3N (x = 0.05, 0.1, 0.15) at 100 °C, confirming a decrease in H2 wt.% with the content of Li3N. The XRD pattern confirms that the sole dehydrogenation product of the composite is metallic Al (Figure 19a). The onset of dehydrogenation was conveniently reduced to 66.8 °C (0.95AlH3-0.05Li3N), thus approaching an operating regime suitable for FCEs. The beneficial role of lithium amide was confirmed by the apparent Ea which is strongly reduced (Figure 19c) [206].
6.4. TM-Hydrides
While main group metal hydrides are attractive due to metal abundance and low atomic weight of the metal (so higher wt.% H2 storage capacity), some TM (transition metals) have also been recently investigated by employing nanosizing effects (Table 13) [79,97,169,200,212,216,234]. The simplest and most classical model system to study TM-H interaction is the Pd-H system [200,234]. While the gravimetric storage capacity is too low for it to be considered for vehicular applications, the nature of Pd…H interaction has shed new light on thermodynamic predictions in Pd NPs forming PdHx, estimating cluster expansion, phase boundaries Pd/Pd…H, phase transitions (>400 K) and interfacial free energies by using DFT method [200,234]. Pd is often thought of as being able to absorb H2 like a sponge, reversibly absorbing more than 1000 times its own volume. In short, interaction of H2 with palladium comprises of H-H dissociation in atomic [H], diffusion of [H] into Pdbulk, where it occupies the free interstitial sites in fcc lattice of Pd, forming either an α-phase PdHx (x < 0.03, rt) or the hydridic β-phase PdHx (x > 0.03) [200]. The catalytic role of Ph-hydride has been recently harnessed in a complex Pd hydride CaPdH2, for semi-hydrogenation of CnH2n−2 (alkynes) to CnH2n (alkenes) [79].

Table 13.
Hydrogen storage features of nanosized (TM) Hx materials.
Rizo-Acosta et al., have addressed the issue of Mg/MgH2 slow kinetics by the addition of ETM (early transition metals: ETM = Sc, Y, Ti, Zr, V, Nb) to nanostructured MgH2 in a one-pot, mechanochemical reaction [169]. The influence of the milling time (0…120 min) over hydrogen wt.% storage capacity (Figure 20a) and absorption rate (Figure 20b) at 573 K has been studied and reveals that using 0.95 MgH2 –0.05 VH2, a 7.3 wt.% hydrogen uptake is registered, even higher than the experimental value for MgH2 (7.6 wt.% theoretical, 7.1 wt.% experimental) [169]. Moreover, the absorption rate is the fastest for 0.95 MgH2 –0.05 TiH2, with a shoulder in the sigmoidal shape due to (ETM)Hx formation, and varies in the order Y < V < Ti < Nb < Sc < Zr (Figure 20b). These hydrides (ScH2, YH3, TiH2, ZrH2, VH, NbH) are stable under experimental hydrogenation conditions and have a crystal size of ~10 nm, acting as effective catalysts for dehydrogenation (recombination of H atoms) and rehydrogenation (MgH2 nucleation due to MgH2/(ETM)Hx interface energies).
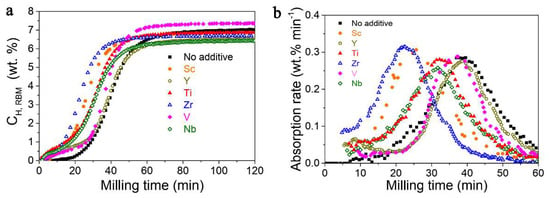
Figure 20.
(a) Hydrogen uptake curves for 0.95 MgH2−0.05 (ETM)Hx during reactive ball milling; (b) absorption rate as derivative of hydrogen uptake curves. Reprinted/adapted with permission from Ref. [169]. 2019, Royal Society of Chemistry.
Notably, the reductive synthesis (300 °C, 7.89 atm H2) yields stabilization of the lower oxidation states of ETM, and mostly (ETM)H2 are produced, except for YH3 which affords the slowest desorption rate (0.06 wt.% min−1, 1 wt.% hydrogen release in 15 min under 0.296 atm H2). The best result was obtained for 0.95 MgH2−0.05 VH, when 6.1 wt.% (90% of the maximum) hydrogen was desorbed in 15 min (Figure 21) [169].
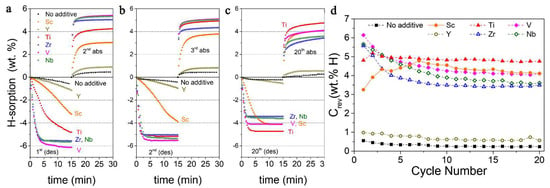
Figure 21.
Hydrogen sorption curves recorded during the 2nd (a), 3rd (b) and 20th (c) absorption cycle for as-synthesized nanocomposites. Evolution of reversible hydrogen storing capacity with number of cycles (d). Reprinted/adapted with permission from Ref. [169]. 2019, Royal Society of Chemistry.
The most stable reversible capacity during cycling was achieved for 0.95 MgH2−0.05 TiH2 nanocomposite, which shows fast kinetics and does not fall below 4.8 wt.% even after 20 cycles (Figure 21). Additionally, no Mg-ETM-H ternary phases were observed [169].
A series of notable advances have been observed for complex hydrides as well, although their details are beyond the scope of this review. In short, metal tetrahydridoaluminates/alanates [14,51]: LiAlH4 [54,81,96,99,101,111,113,135,166,178], NaAlH4 [33,45,50,69,74,82,127,128,129,167,214,234,235], tetrahydridoborates/borohydrides [3,12,14,42]: LiBH4 [42,45,47,54,56,70,78,81,87,89,91,93,99,100,104,106,108,120,123,130,134,135,161,216,230,231,236,237,238,239,240], NaBH4 [49,74,216,236,241], Mg(BH4)2 [42,55,61,70,105,126,132,151,159,207,216,220,224,236,242,243], Ca(BH4)2 [116,216,236,243] and (TM)(BH4)x [150,216], ammonia-borane NH3BH3 [36,38,63,64,75,85,86,88,90,139,140,141,142,143,144,153,154,156,162,171,175,197,213,244,245,246,247,248,249] and RCH reactive hydride composites [45,54,78,91,99,130,134,173,215,240,250] have been recently explored and improved thermodynamic and/or kinetic parameters have been reported [107,145,226,251,252,253].
7. Conclusions and Outlook
The urgency of a green, renewable and sustainable fuel to replace fossil fuels is more stringent today than ever. The metal hydrides constitute materials that possess intrinsically high gravimetric and volumetric hydrogen storage capacities, but their sluggish kinetics and poor thermodynamics still constitute an obstacle for the wide acceptance of their use in the fuel of the future. However, various strategies have been recently explored, and perhaps the most returns derive from basic shifts in thinking: oriented growth of MgH2 on catalytically active substrates; size-reduction in metal hydrides to few nm when thermodynamic destabilization works best; or usage of new class of catalysts of 2D-structure (MXenes)—they have all showed unexpectedly good results. There is clearly room for improvement in the fascinating field of metal hydrides, and research efforts ought to concentrate on improving nanoparticle system design, careful consideration of the incorporating matrix and selected hydrogenation/dehydrogenation catalysts, from both an economic and a feasibility point of view. Given the raw material scarcity but also reactivity and particular characteristics of some complex hydrides (like volatility of Al(BH4)3, or extreme toxicity of Be(BH4)2 etc.), the optimal hydrogen storage material will likely be based on magnesium nanoconfined in a carbonaceous host and/or catalyzed by Ti-based catalysts (such as TiO2, TiO, or MXenes). The realistic application of metal hydride systems is conditioned by a number of factors: (i) the discovery of a material that displays a reliably-reversible behavior in hydrogenation studies; (ii) consistent performance across hundreds of H2-absorption/desorption cycles; (iii) lower activation energies and consequently faster absorption/desorption kinetics and improved thermodynamics; (iv) consistently fast kinetics for fast refueling; (v) thermodynamic stability and material integrity to afford safe storage in a fuel tank; (vi) reasonable resistance to air and/or moisture; (vii) synthesis route moderately easy and preferably comprising of few steps; (viii) access to sufficient raw materials and limit amount of CRM (critical raw materials) used; (ix) reliable scaling-up of the lab demonstrator to a multi-KW tank capable to drive a vehicle for 500 km or more; (x) strong safety precautions and technological parameters implementation to afford a tank capable to store, release and withstand high H2 pressures (of more than 100 atm). Within this framework, the EU directives to limit CRM usage is expected to drive the research towards more-abundant metal sources such as Mg or Al (Mg was also included in the list of CRM from 2020, although currently it can be obtained in enough quantities). Noble metal catalysis (like Pd) will probably not become a commercial way of speeding up hydrogen delivery or the recharging of hydride-based fuels due to the associated cost. Other catalysts like MXenes can be produced on a larger scale, but the Ti-based material could also face soon shortages.
Nanoconfinement still offers general improvements across the board for hydride-based materials, but the choice of host is limited—among the classes of hosts presented in the current review, the most promising are carbonaceous frameworks and MOFs. Carbon-based materials can be tailored morphologically for hydride inclusion, and their cost is modest; however, this must be considered with care since a zero-carbon policy might imply soon that carbon should not be used as a host any longer. Even though it releases no CO2 in the atmosphere; there will be an associated cost with treatment of the end-of-life C-based fuel, and so the carbon footprint will not be negligible.
Considering these material, performance, safety and cost restrictions, the final choice for a viable, sustainable hydride-based material is a delicate one and only validation through a scaling-up proven in an operational environment could confirm whether it can be used on a large-scale tank for vehicular applications and afterwards adopted by industry. The ultimate goal is, without a doubt, to approach as much as possible the reversible, theoretical hydrogen capacity, and this is a joint venture of all the above considerations.
Funding
This work was supported by the Romanian Ministry of Research and Innovation through the Project PN-III-P1-1.1-TE-2021-1657 (TE 84), Core Program PN19-03 (contract no. PN21N/2019), and PN-III-P2-2.1-PED-2019-4816 within PNCD III. The fee for open access publication was supported from the project 35PFE/2021, funded by the Romanian Ministry of Research, Innovation and Digitization.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The fee for open access publication was supported from the project 35PFE/2021, funded by the Romanian Ministry of Research, Innovation and Digitization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agarwal, A.K.; Martínez, A.G.; Kalwar, A.; Valera, H. Energy, Environment, and Sustainability—Advanced Combustion for Sustainable Transport; Springer Nature: Singapore, 2022; pp. 299–330. [Google Scholar] [CrossRef]
- Černý, R.; Brighi, F.M.M. Metal hydroborates: From hydrogen stores to solid electrolytes. J. Alloys Compd. 2022, 895, 162659. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, 1902757. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; White, J.L.; Kang, S.Y.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef] [PubMed]
- US DOE Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles. Available online: https://www.energy.gov/eere/fuelcells/downloads/target-explanation-document-onboard-hydrogen-storage-light-duty-fuel-cell (accessed on 10 May 2022).
- US DOE Hydrogen & Fuel Cells Program. Available online: https://www.hydrogen.energy.gov/ (accessed on 10 May 2022).
- Shen, H.; Li, H.; Yang, Z.; Li, C. Magic of hydrogen spillover: Understanding and application. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Fernandez, J.R.A.; Leardini, F.; Aguey-Zinsou, K.-F. How to Design Hydrogen Storage Materials? Fundamentals, Synthesis, and Storage Tanks. Adv. Sustain. Syst. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kumar, R.; Karkamkar, A.; Bowden, M.; Autrey, T. Solid-state hydrogen rich boron–nitrogen compounds for energy storage. Chem. Soc. Rev. 2019, 48, 5350–5380. [Google Scholar] [CrossRef]
- Hagemann, H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules 2021, 26, 7425. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F.; et al. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef]
- Broom, D.P.; Hirscher, M. Improving Reproducibility in Hydrogen Storage Material Research. ChemPhysChem 2021, 22, 2141–2157. [Google Scholar] [CrossRef] [PubMed]
- Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. [Google Scholar] [CrossRef]
- Kharbachi, A.E.; Dematteis, E.M.; Shinzato, K.; Stevenson, S.C.; Bannenberg, L.J.; Heere, M.; Zlotea, C.; Szilágyi, P.Á.; Bonnet, J.-P.; Grochala, W.; et al. Metal Hydrides and Related Materials. Energy Carriers for Novel Hydrogen and Electrochemical Storage. J. Phys. Chem. C 2020, 124, 7599–7607. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage. AAAS Res. 2021, 2021, 3750689. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Cao, H.; Chen, P. The Roles of Alkali/Alkaline Earth Metals in the Materials Design and Development for Hydrogen Storage. Acc. Mater. Res. 2021, 2, 726–738. [Google Scholar] [CrossRef]
- Huang, Y.; An, C.; Zhang, Q.; Zang, L.; Shao, H.; Liu, Y.; Zhang, Y.; Yuan, H.; Wang, C.; Wang, Y. Cost-effective Mechanochemical Synthesis of Highly Dispersed Supported Transition Metal Catalysts for Hydrogen Storage. Nano Energy 2021, 80, 105535. [Google Scholar] [CrossRef]
- Li, Z.; Wei, B. Topological materials and topologically engineered materials: Properties, synthesis, and applications for energy conversion and storage. J. Mater. Chem. A 2021, 9, 1297–1313. [Google Scholar] [CrossRef]
- Wood, B.C.; Heo, T.W.; Kang, S.Y.; Wan, L.F.; Li, S. Beyond Idealized Models of Nanoscale Metal Hydrides for Hydrogen Storage. Ind. Eng. Chem. Res. 2020, 59, 5786–5796. [Google Scholar] [CrossRef]
- Callini, E.; Atakli, Z.Ö.K.; Hauback, B.C.; Orimo, S.-i.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjorvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Ngene, P.; Longo, A.; Mooij, L.; Bras, W.; Dam, B. Metal-hydrogen systems with an exceptionally large and tunable thermodynamic destabilization. Nat. Commun. 2017, 8, 1846. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Witman, M.; Ling, S.; Grant, D.M.; Walker, G.S.; Agarwal, S.; Stavila, V.; Allendorf, M.D. Extracting an Empirical Intermetallic Hydride Design Principle from Limited Data via Interpretable Machine Learning. J. Phys. Chem. Lett. 2020, 11, 40–47. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.W.; Huang, Z. Editorial: Metal Hydride-Based Energy Storage and Conversion Materials. Front. Chem. 2020, 8, 675. [Google Scholar] [CrossRef]
- Andersson, J.; Gronkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Kweon, J.J.; Kim, H.-I.; Lee, S.-h.; Kim, J.; Lee, S.K. Quantitative probing of hydrogen environments in quasicrystals by high-resolution NMR spectroscopy. Acta Mater. 2022, 226, 117657. [Google Scholar] [CrossRef]
- Misaki, T.; Oikawa, I.; Takamura, H. Negative Knight Shift in Ba-Ti Oxyhydride: An Indication of the Multiple Hydrogen Occupation. Chem. Mater. 2019, 31, 18–7178. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Jeong, S.; White, J.; Feng, X.; Cho, E.S.; Stavila, V.; Allendorf, M.; Urban, J.; Guo, J. In-situ/operando X-ray characterization of metal hydrides. ChemPhysChem 2019, 20, 1261–1271. [Google Scholar] [CrossRef]
- NaraseGowda, S.; Brown, C.M.; Tyagi, M.; Jenkins, T.; Dobbins, T.A. Quasi-Elastic Neutron Scattering Studies of Hydrogen Dynamics for Nanoconfined NaAlH4. J. Phys. Chem. C 2016, 120, 14863–14873. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, Y.; Bowman, R.C., Jr.; Zhang, J.; Liu, H.; Sun, P.; Fang, Z.Z. A high throughput dynamic method for characterizing thermodynamic properties of catalyzed magnesium hydrides by thermogravimetric analysis. Phys. Chem. Chem. Phys. 2021, 23, 15374. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.M.; Pham, M.T.; Guin, T.; Madhogaria, R.P.; Phan, M.-H.; Larsen, G.K.; Nguyen, T.D. Sub-second and ppm-level optical sensing of hydrogen using templated control of nano-hydride geometry and composition. Nat. Commun. 2021, 12, 2414. [Google Scholar] [CrossRef] [PubMed]
- Valero-Pedraza, M.J.; Gascón, V.; Carreón, M.A.; Leardini, F.; Ares, J.R.; Martín, Á.; Sánchez-Sánchez, M.; Bañares, M.A. Operando Raman-mass spectrometry investigation of hydrogen release by thermolysis of ammonia borane confined in mesoporous materials. Microporous Mesoporous Mater. 2016, 226, 454–465. [Google Scholar] [CrossRef]
- Surrey, A.; Nielsch, K.; Rellinghaus, B. Comments on “Evidence of the hydrogen release mechanism in bulk MgH2”. Sci. Rep. 2017, 7, 44216. [Google Scholar] [CrossRef]
- Petit, J.-F.; Demirci, U.B. Discrepancy in the thermal decomposition/dehydrogenation of ammonia borane screened by thermogravimetric analysis. Int. J. Hydrogen Energy 2019, 44, 14201–14206. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wang, C.-Y. Insight into the Catalytic Effects of Open Metal Sites in Metal−Organic Frameworks on Hydride Dehydrogenation via Nanoconfinement. ACS Sustain. Chem. Eng. 2019, 7, 16013–16025. [Google Scholar] [CrossRef]
- Prabhakaran, P.K.; Catoire, L.; Deschamps, J. Aluminium doping composite metal-organic framework by alane nanoconfinement: Impact on the room temperature hydrogen uptake. Microporous Mesoporous Mater. 2017, 243, 214–220. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Fagg, D.P. 5—Nanostructured advanced materials for hydrogen storage. In Nanomaterials for Sustainable Energy and Environmental Remediation—Materials Today; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–163. [Google Scholar] [CrossRef]
- Lai, Q.; Yang, Y.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in hollow carbon spheres: Melt infiltration versus solvent impregnation for enhanced hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 23225–23238. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int. J. Hydrogen Energy 2018, 43, 1545–1565. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Aguey-Zinsou, K.-F. Hydrogen storage properties of nanoconfined aluminium hydride (AlH3). Chem. Eng. Sci. 2019, 194, 64–70. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4. Crystals 2016, 6, 70. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Martín, Á. Innovative methods to enhance the properties of solid hydrogen storage materials based on hydrides through nanoconfinement: A review. J. Supercrit. Fluids 2018, 141, 198–217. [Google Scholar] [CrossRef]
- Le, T.T.; Pistidda, C.; Nguyen, V.H.; Singh, P.; Raizada, P.; Klassen, T.; Dornheim, M. Nanoconfinement effects on hydrogen storage properties of MgH2 and LiBH4. Int. J. Hydrogen Energy 2021, 46, 23723–23736. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhou, X.; Elimelech, M.; Kim, J.-H. Environmental Applications of Engineered Materials with Nanoconfinement. ACS EST Eng. 2021, 1, 706–724. [Google Scholar] [CrossRef]
- Salman, M.S.; Rawal, A.; Aguey-Zinsou, K.-F. Tunable NaBH4 Nanostructures Revealing Structure-Dependent Hydrogen Release. Adv. Energy Sustain. Res. 2021, 2, 2100063. [Google Scholar] [CrossRef]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]
- Stavila, V.; Li, S.; Dun, C.; Marple, M.A.T.; Mason, H.E.; Snider, J.L.; Reynolds III, J.E.; Gabaly, F.E.; Sugar, J.D.; Spataru, C.D.; et al. Defying Thermodynamics: Stabilization of Alane Within Covalent Triazine Frameworks for Reversible Hydrogen Storage. Angew. Chem. Int. Ed. 2021, 60, 25815–25824. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Nanocomposites for Environmental and Energy Applications. Nanomaterials 2021, 11, 345. [Google Scholar] [CrossRef]
- Huen, P.; Paskevicius, M.; Richter, B.; Ravnsbæk, D.B.; Jensen, T.R. Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling. Inorganics 2017, 5, 57. [Google Scholar] [CrossRef]
- Wood, B.C.; Stavila, V.; Poonyayant, N.; Heo, T.W.; Ray, K.G.; Klebanoff, L.E.; Udovic, T.J.; Lee, J.R.I.; Angboonpong, N.; Sugar, J.D.; et al. Nanointerface-Driven Reversible Hydrogen Storage in the Nanoconfined Li–N–H System. Adv. Mater. Interfaces 2017, 4, 1600803. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ray, K.G.; Jørgensen, M.; Mattox, T.M.; Cowgill, D.F.; Eshelman, H.V.; Sawvel, A.M.; Snider, J.L.; York, W.; Wijeratne, P.; et al. Nanoscale Mg–B via Surfactant Ball Milling of MgB2: Morphology, Composition, and Improved Hydrogen Storage Properties. J. Phys. Chem. C 2020, 124, 21761–21771. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; de Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Rabkin, E.; Skripnyuk, V.; Estrin, Y. Ultrafine-Grained Magnesium Alloys for Hydrogen Storage Obtained by Severe Plastic Deformation. Front. Mater. 2019, 6, 240. [Google Scholar] [CrossRef]
- Stavila, V.; Klebanoff, L.E. Nanostructured Metal Amides and Nitrides for Hydrogen Storage. U.S. Patent No. US10000377B1, 19 June 2018. Available online: https://patents.google.com/patent/US10000377B1/en (accessed on 16 May 2022).
- Li, L.; Huang, Y.; An, C.; Wang, Y. Lightweight hydrides nanocomposites for hydrogen storage: Challenges, progress and prospects. Sci. China Mater. 2019, 62, 1597–1625. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Callini, E.; Aguey-Zinsou, K.-F.; Ahuja, R.; Ares, J.R.; Bals, S.; Biliškov, N.; Chakraborty, S.; Charalambopoulou, G.; Chaudhary, A.-L.; Cuevas, F.; et al. Nanostructured materials for solid-state hydrogen storage: A review of the achievement of COST Action MP1103. Int. J. Hydrogen Energy 2016, 41, 14404–14428. [Google Scholar] [CrossRef]
- Turani-Belloto, K.; Castilla-Martinez, C.A.; Cot, D.; Petit, E.; Benarib, S.; Demirci, U.B. Nanosized ammonia borane for solid-state hydrogen storage: Outcomes, limitations, challenges and opportunities. Int. J. Hydrogen Energy 2021, 46, 7351–7370. [Google Scholar] [CrossRef]
- Valero-Pedraza, M.-J.; Cot, D.; Petit, E.; Aguey-Zinsou, K.-F.; Alauzun, J.G.; Demirci, U.B. Ammonia Borane Nanospheres for Hydrogen Storage. ACS Appl. Nano Mater. 2019, 2, 1129–1138. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Liu, J.; Ma, Z.; Zhang, J.; Liu, Y.; Li, Y.; Li, L. Enhancing hydrogen storage properties of MgH2 by core-shell CoNi@C. J. Alloys Compd. 2021, 862, 158004. [Google Scholar] [CrossRef]
- de Kort, L.M.; Gulino, V.; de Jongh, P.E.; Ngene, P. Ionic conductivity in complex metal hydride-based nanocomposite materials: The impact of nanostructuring and nanocomposite formation. J. Alloys Compd. 2022, 901, 163474. [Google Scholar] [CrossRef]
- Pasquini, L. Design of Nanomaterials for Hydrogen Storage. Energies 2020, 13, 3503. [Google Scholar] [CrossRef]
- Malouche, A.; Zlotea, C.; Szilágyi, P.Á. Interactions of hydrogen with Pd@MOF composites. ChemPhysChem 2019, 20, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- EHuang, Y.; Shao, H.; Zhang, Q.; Zang, L.; Guo, H.; Liu, Y.; Jiao, L.; Yuan, H.; Wang, Y. Layer-by-layer uniformly confined Graphene-NaAlH4 composites and hydrogen storage performance. Int. J. Hydrogen Energy 2020, 45, 28116–28122. [Google Scholar] [CrossRef]
- Zheng, J.; Yao, Z.; Xiao, X.; Wang, X.; He, J.; Chen, M.; Cheng, H.; Zhang, L.; Chen, L. Enhanced hydrogen storage properties of high-loading nanoconfined LiBH4-Mg(BH4)2 composites with porous hollow carbon nanospheres. Int. J. Hydrogen Energy 2021, 46, 852–864. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Zou, Y.; Chu, H.; Li, B.; Zhang, K. Enhanced Hydrogen Storage/Sensing of Metal Hydrides by Nano-modification. Mater. Today Nano 2020, 9, 100071. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Moury, R.; Ould-Amara, S.; Demirci, U.B. Destabilization of Boron-Based Compounds for Hydrogen Storage in the Solid-State: Recent Advances. Energies 2021, 14, 7003. [Google Scholar] [CrossRef]
- Luo, X.; Rawal, A.; Aguey-Zinsou, K.-F. Investigating the Factors Affecting the Ionic Conduction in Nanoconfined NaBH4. Inorganics 2021, 9, 2. [Google Scholar] [CrossRef]
- Biswas, P.; Ghildiyal, P.; Kwon, H.; Wang, H.; Alibay, Z.; Xu, F.; Wang, Y.; Wong, B.M.; Zachariah, M.R. Rerouting Pathways of Solid-State Ammonia Borane Energy Release. J. Phys. Chem. C 2022, 126, 48–57. [Google Scholar] [CrossRef]
- Bannenberg, L.J.; Boshuizen, B.; Nugroho, F.A.A.; Schreuders, H. Hydrogenation Kinetics of Metal Hydride Catalytic Layers. ACS Appl. Mater. Interfaces 2021, 13, 52530–52541. [Google Scholar] [CrossRef] [PubMed]
- El-Eskandarany, M.S.; Al-Ajmi, F.; Banyan, M.; Al-Duweesh, A. Synergetic effect of reactive ball milling and cold pressing on enhancing the hydrogen storage behavior of nanocomposite MgH2/10 wt% TiMn2 binary system. Int. J. Hydrogen Energy 2019, 44, 26428–26443. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; He, Y.; Yao, Z.; Ye, X.; Kou, H.; Chen, C.; Huang, T.; Fan, X.; Chen, L. Probing an intermediate state by X-ray absorption near-edge structure in nickel-doped 2LiBH4-MgH2 reactive hydride composite at moderate temperature. Mater. Today Nano 2020, 12, 100090. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, R.; Guo, J.; Qin, C.; Xiong, Z.; Yan, H.; Gao, W.; Pei, Q.; Wu, A.; Chen, P. Enabling Semihydrogenation of Alkynes to Alkenes by Using a Calcium Palladium Complex Hydride. J. Am. Chem. Soc. 2021, 143, 20891–20897. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Panda, S.; Zhang, Q.; Sun, F.; Khan, D.; Ding, W.; Zou, J. Improving hydrogen sorption performances of MgH2 through nanoconfinement in a mesoporous CoS nano-boxes scaffold. J. Chem. Eng. 2021, 406, 126790. [Google Scholar] [CrossRef]
- Ngene, P.; Verkuijlen, M.H.W.; Barre, C.; Kentgens, A.P.M.; de Jongh, P.E. Reversible Li-insertion in nanoscaffolds: A promising strategy to alter the hydrogen sorption properties of Li-based complex hydrides. Nano Energy 2016, 22, 169–178. [Google Scholar] [CrossRef][Green Version]
- Paskevicius, M.; Filsø, U.; Karimi, F.; Puszkiel, J.; Pranzas, P.K.; Pistidda, C.; Hoell, A.; Welter, E.; Schreyer, A.; Klassen, T.; et al. Cyclic stability and structure of nanoconfined Ti-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 4159–4167. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Navarrete, A.; Sturm, G.; Link, G.; Rueda, M.; Stefanidis, G.; Martín, A. Release of hydrogen from nanoconfined hydrides by application of microwaves. J. Power Sources 2017, 353, 131–137. [Google Scholar] [CrossRef]
- Pasquini, L. The effects of nanostructure on the hydrogen sorption properties of magnesium-based metallic compounds: A review. Crystals 2018, 8, 106. [Google Scholar] [CrossRef]
- Liu, W.; Stoddart, J.F. Emergent behavior in nanoconfined molecular containers. Chem. 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Lambregts, S.F.H.; van Eck, E.R.H.; Suwarno; Ngene, P.; de Jongh, P.E.; Kentgens, A.P.M. Phase Behavior and Ion Dynamics of Nanoconfined LiBH4 in Silica. J. Phys. Chem. C 2019, 123, 25559–25569. [Google Scholar] [CrossRef]
- Mishra, S.; Kang, P.-C.; Guo, R.-F.; Wang, C.-Y.; Nebhani, L. Combined Effect of Functionality and Pore Size on Dehydrogenation of Ammonia Borane via Its Nanoconfinement in Polyacrylamide-Grafted Organically Modified Mesoporous Silica. ACS Appl. Energy Mater. 2021, 4, 6585–6598. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, F.; Liu, Y.; Zhang, Y.; Wang, X.; Pang, Y.; Zheng, S. Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries. Nanomaterials 2021, 11, 946. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Segovia, J.J.; Martín, Á. Improvement of the kinetics of hydrogen release from ammonia borane confined in silica aerogel. Microporous Mesoporous Mater. 2017, 237, 189–200. [Google Scholar] [CrossRef]
- de Kort, L.M.; Harmel, J.; de Jongh, P.E.; Ngene, P. The effect of nanoscaffold porosity and surface chemistry on the Li-ion conductivity of LiBH4-LiNH2/metal oxide nanocomposites. J. Mater. Chem. A 2020, 8, 20687–20697. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Carbon scaffold modified by metal (Ni) or non-metal (N) to enhance hydrogen storage of MgH2 through nanoconfinement. Int. J. Hydrogen Energy 2017, 42, 22933–22941. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Liu, Y.; Zhang, L.; Hu, J.; Gao, M.; Pan, H. A Unique Double-Layered Carbon Nanobowl-Confined Lithium Borohydride for Highly Reversible Hydrogen Storage. Small 2020, 16, 2001963. [Google Scholar] [CrossRef]
- Hu, M.; Xie, X.; Chen, M.; Zhu, C.; Liu, T. TiCX-decorated Mg nanoparticles confined in carbon shell: Preparation and catalytic mechanism for hydrogen storage. J. Alloys Compd. 2020, 817, 152813. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Mao, J.; Lan, Z.; Huang, X.; Lu, Y.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Synergistic catalysis in monodispersed transition metal oxide nanoparticles anchored on amorphous carbon for excellent low-temperature dehydrogenation of magnesium hydride. Mater. Today Energy 2019, 12, 146–154. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Zlotea, C.; Oumellal, Y.; Berrú, J.J.S.; Aguey-Zinsou, K.-F. On the feasibility of the bottom-up synthesis of Mg2CoH5 nanoparticles supported on a porous carbon and their hydrogen desorption behaviour. Nano-Struct. Nano-Objects 2018, 16, 144–150. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, G.; Chen, J.; Zhang, B.; Li, Q.; Yu, X. One-step uniform growth of magnesium hydride nanoparticles on graphene. Prog. Nat. Sci. Mater. Int. 2017, 27, 81–87. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Liu, H.; Yan, M. Enhanced hydrogen storage properties of 2LiBH4-LiAlH4 nanoconfined in resorcinol formaldehyde carbon aerogel. J. Alloys Compd. 2017, 726, 525–531. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, H.-z.; Gao, S.-c.; Wang, X.-h. Enhanced dehydrogenation kinetic properties and hydrogen storage reversibility of LiBH4 confined in activated charcoal. Trans. Nonferrous Met. Soc. China 2018, 28, 1618–1625. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, F.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.; Li, X.; Ren, S.; Zhou, S.; Zhang, H.; Li, Y.; Han, S. Improved hydrogen storage properties of MgH2 by nickel@nitrogen-doped carbon spheres. Dalton Trans. 2020, 49, 3495–3502. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, G.; Sun, D.; Fang, F.; Yu, X. Magnesium Hydride Nanoparticles Self-Assembled on Graphene as Anode Material for High-Performance Lithium-Ion Batteries. ACS Nano 2018, 12, 3816–3824. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Xian, K.; Li, Z.; Shen, Y.; Yao, Z.; Liu, Y.; Pan, H. LiBH4 Nanoconfined in Porous Hollow Carbon Nanospheres with High Loading, Low Dehydrogenation Temperature, Superior Kinetics, and Favorable Reversibility. ACS Appl. Energy Mater. 2020, 3, 3928–3938. [Google Scholar] [CrossRef]
- Aditya, M.V.V.S.; Panda, S.; Tatiparti, S.S.V. Boron from net charge acceptor to donor and its effect on hydrogen uptake by novel Mg-B-electrochemically synthesized reduced graphene oxide. Sci. Rep. 2021, 11, 10995. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Yao, Z.; Liu, Y.; Wu, M.; Li, Z.; Liu, Y.; Sun, W.; Pan, H. A nanoconfined-LiBH4 system using a unique multifunctional porous scaffold of carbon wrapped ultrafine Fe3O4 skeleton for reversible hydrogen storage with high capacity. Chem. Eng. J. 2022, 428, 131056. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Lin, H.; Liu, Y.; Zhang, Y.; Li, S.; Ma, Z.; Li, L. Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement. Adv. Mater. 2017, 29, 1700760. [Google Scholar] [CrossRef] [PubMed]
- Sitthiwet, C.; Thiangviriya, S.; Thaweelap, N.; Meethom, S.; Kaewsuwan, D.; Chanlek, N.; Utke, R. Hydrogen sorption and permeability of compacted LiBH4 nanoconfined into activated carbon nanofibers impregnated with TiO2. J. Phys. Chem. Solids 2017, 110, 344–353. [Google Scholar] [CrossRef]
- Duan, C.; Wu, M.; Cao, Y.; Fu, D.; Zhang, Y.; Su, Z.; Sun, Z.; Wu, Y. Novel core–shell structured MgH2/AlH3@CNT nanocomposites with extremely high dehydriding–rehydriding properties derived from nanoconfinement. J. Mater. Chem. A 2021, 9, 10921. [Google Scholar] [CrossRef]
- Han, D.J.; Bang, K.R.; Cho, H.; Cho, E.S. Effect of carbon nanoscaffolds on hydrogen storage performance of magnesium hydride. Korean J. Chem. Eng. 2020, 37, 1306–1316. [Google Scholar] [CrossRef]
- Cho, Y.J.; Li, S.; Snider, J.L.; Marple, M.A.T.; Strange, N.A.; Sugar, J.D.; Gabaly, F.E.; Schneemann, A.; Kang, S.; Kang, M.-h.; et al. Reversing the Irreversible: Thermodynamic Stabilization of LiAlH4 Nanoconfined Within a Nitrogen-Doped Carbon Host. ACS Nano 2021, 15, 10163–10174. [Google Scholar] [CrossRef]
- Gasnier, A.; Luguet, M.; Pereira, A.G.; Troiani, H.; Zampieri, G.; Gennari, F.C. Entanglement of N-doped graphene in resorcinol-formaldehyde: Effect over nanoconfined LiBH4 for hydrogen storage. Carbon 2019, 147, 284–294. [Google Scholar] [CrossRef]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Fang, F.; Sun, D.; Li, X.; Guo, Z.; Yu, X. Oxygen-free Layer-by-Layer Assembly of Lithiated Composites on Graphene for Advanced Hydrogen Storage. Adv. Sci. 2016, 4, 1600257. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Zang, L.; Guo, H.; Jiao, L.; Yuan, H.; Wang, Y. Highly Dispersed MgH2 Nanoparticle–Graphene Nanosheet Composites for Hydrogen Storage. ACS Appl. Nano Mater. 2019, 2, 3828–3835. [Google Scholar] [CrossRef]
- Comanescu, C.; Capurso, G.; Maddalena, A. Nanoconfinement in activated mesoporous carbon of calcium borohydride for improved reversible hydrogen storage. Nanotechnology 2012, 23, 385401. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.F.; Cho, E.S.; Marangoni, T.; Shea, P.; Kang, S.Y.; Rogers, C.; Zaia, E.; Cloke, R.R.; Wood, B.C.; Fischer, F.R.; et al. Edge-Functionalized Graphene Nanoribbon Encapsulation to Enhance Stability and Control Kinetics of Hydrogen Storage Materials. Chem. Mater. 2019, 31, 2960–2970. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Yao, Z.; Xian, K.; Wu, M.; Liu, Y.; Sun, W.; Pan, H. High-loading, ultrafine Ni nanoparticles dispersed on porous hollow carbon nanospheres for fast (de)hydrogenation kinetics of MgH2. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Morse, J.R.; Zugell, D.A.; Patterson, E.; Baldwin, J.W.; Willauer, H.D. Hydrogenated graphene: Important material properties regarding its application for hydrogen storage. J. Power Sources 2021, 494, 229734. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, W.; Ren, Z.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Nano-synergy enables highly reversible storage of 9.2 wt% hydrogen at mild conditions with lithium borohydride. Nano Energy 2021, 83, 105839. [Google Scholar] [CrossRef]
- Han, D.J.; Kim, S.; Cho, E.S. Revealing the role of defects in graphene oxide in the evolution of magnesium nanocrystals and the resulting effects on hydrogen storage. J. Mater. Chem. A 2021, 9, 9875. [Google Scholar] [CrossRef]
- Dun, C.; Jeong, S.; Kwon, D.-H.; Kang, S.Y.; Stavila, V.; Zhang, Z.; Lee, J.-W.; Mattox, T.M.; Heo, T.W.; Wood, B.C.; et al. Hydrogen Storage Performance of Preferentially Oriented Mg/rGO Hybrids. Chem. Mater. 2022, 34, 2963–2971. [Google Scholar] [CrossRef]
- Martínez, A.A.; Gasnier, A.; Gennari, F.C. Pore Filling of a Carbon Matrix by Melt-Impregnated LiBH4. J. Phys. Chem. C 2022, 126, 66–78. [Google Scholar] [CrossRef]
- Cho, H.; Hyeon, S.; Park, H.; Kim, J.; Cho, E.S. Ultrathin Magnesium Nanosheet for Improved Hydrogen Storage with Fishbone Shaped One-Dimensional Carbon Matrix. ACS Appl. Energy Mater. 2020, 3, 8143–8149. [Google Scholar] [CrossRef]
- Ko, Y.; Lombardo, L.; Li, M.; Oveisi, E.; Yang, H.; Zuüttel, A. Interfacial Effect between Aluminum-Based Complex Hydrides and Nickel-Containing Porous Carbon Sheets. ACS Appl. Energy Mater. 2020, 3, 9685–9695. [Google Scholar] [CrossRef]
- Jeong, S.; Heo, T.W.; Oktawiec, J.; Shi, R.; Kang, S.Y.; White, J.L.; Schneemann, A.; Zaia, E.W.; Wan, L.F.; Ray, K.G.; et al. A Mechanistic Analysis of Phase Evolution and Hydrogen Storage Behavior in Nanocrystalline Mg(BH4)2 within Reduced Graphene Oxide. ACS Nano 2020, 14, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, C.A.G.; Moreira, B.R.; de Oliveira, A.D.; Passador, F.R.; de Almeida Neto, G.R.; Leiva, D.R.; Pessan, L.A. Development of polymer nanocomposites with sodium alanate for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 5337–5346. [Google Scholar] [CrossRef]
- Carr, C.L.; Jayawardana, W.; Zou, H.; White, J.L.; Gabaly, F.E.; Conradi, M.S.; Stavila, V.; Allendorf, M.D.; Majzoub, E.H. Anomalous H2 Desorption Rate of NaAlH4 Confined in Nitrogen-Doped Nanoporous Carbon Frameworks. Chem. Mater. 2018, 30, 2930–2938. [Google Scholar] [CrossRef]
- Do, H.W.; Kim, H.; Cho, E.S. Enhanced hydrogen storage kinetics and air stability of nanoconfined NaAlH4 in graphene oxide framework. RSC Adv. 2021, 11, 32533. [Google Scholar] [CrossRef] [PubMed]
- Dansirima, P.; Thiangviriya, S.; Plerdsranoy, P.; Utke, O.; Utke, R. Small hydrogen storage tank filled with 2LiBH4-MgH2 nanoconfined in activated carbon: Reaction mechanisms and performances. Int. J. Hydrogen Energy 2019, 44, 10752–10762. [Google Scholar] [CrossRef]
- Magnin, Y.; Villermaux, E.; Amara, H.; Bichara, C.; Pellenq, R.J.M. Morphology control of metallic nanoparticles supported on carbon substrates in catalytic conditions. Carbon 2020, 159, 504–511. [Google Scholar] [CrossRef]
- Wahab, M.A.; Young, D.J.; Karim, A.; Fawzia, S.; Beltramini, J.N. Low-temperature hydrogen desorption from Mg(BH4)2 catalysed by ultrafine Ni nanoparticles in a mesoporous carbon matrix. Int. J. Hydrogen Energy 2016, 41, 20573–20582. [Google Scholar] [CrossRef]
- Wang, L.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Direct and reversible hydrogen storage of lithium hydride (LiH) nanoconfined in high surface area graphite. Int. J. Hydrogen Energy 2016, 41, 18088–18094. [Google Scholar] [CrossRef]
- Utke, R.; Thiangviriya, S.; Javadian, P.; Jensen, T.R.; Milanese, C.; Klassen, T.; Dornheim, M. 2LiBH4–MgH2 nanoconfined into carbon aerogel scaffold impregnated with ZrCl4 for reversible hydrogen storage. Mater. Chem. Phys. 2016, 169, 136–141. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Javadian, P.; Jensen, N.D.; Nielsen, U.G.; Jensen, T.R.; Utke, R. Compaction of LiBH4-LiAlH4 nanoconfined in activated carbon nanofibers: Dehydrogenation kinetics, reversibility, and mechanical stability during cycling. Int. J. Hydrogen Energy 2017, 42, 1036–1047. [Google Scholar] [CrossRef]
- Pandey, A.P.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Singh, S.; Verma, S.K.; Shaneeth, M.; Sekkar, V.; Srivastava, O.N. Hydrogen storage properties of carbon aerogel synthesized by ambient pressure drying using new catalyst triethylamine. Int. J. Hydrogen Energy 2020, 45, 30818–30827. [Google Scholar] [CrossRef]
- Shinde, S.S.; Kim, D.H.; Yu, J.Y.; Lee, J.H. Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage. Nanoscale 2017, 9, 7094–7103. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Aloni, S.; Liu, Y.-S.; Guo, J.; Urban, J.J. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage. Nat. Commun. 2016, 7, 10804. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.H.; Wu, S.Y.; Chang, Y.H.; Narwane, M.; Chen, B.H.; Liu, W.L.; Kurniawan, D.; Chiang, W.H.; Lin, C.H.; Chuang, Y.C.; et al. Mechanistic Insight into the Synergetic Interaction of Ammonia Borane and Water on ZIF-67-Derived Co@Porous Carbon for Controlled Generation of Dihydrogen. ACS Appl. Mater. Interfaces 2021, 13, 47465–47477. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ouyang, L.; Felderhoff, M.; Zhu, M. Low temperature dehydrogenation properties of ammonia borane within carbon nanotube arrays: A synergistic effect of nanoconfinement and alane. RSC Adv. 2020, 10, 19027–19033. [Google Scholar] [CrossRef] [PubMed]
- So, S.H.; Jang, J.H.; Sung, S.J.; Yang, S.J.; Nam, K.T.; Park, C.R. Demonstration of the nanosize effect of carbon nanomaterials on the dehydrogenation temperature of ammonia borane. Nanoscale Adv. 2019, 1, 4697–4703. [Google Scholar] [CrossRef]
- Champet, S.; van den Berg, J.; Szczesny, R.; Godula-Jopek, A.; Gregory, D.H. Nano-inclusion in one step: Spontaneous ice-templating of porous hierarchical nanocomposites for selective hydrogen release. Sustain. Energy Fuels 2019, 3, 396–400. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Liu, M.; Cui, J.; Wang, Z.; Yu, P. Enhanced dehydrogenation performance of ammonia borane con-catalyzed by novel TiO2(B) nanoparticles and bio-derived carbon with well-organized pores. Int. J. Hydrogen Energy 2020, 45, 28070–28077. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.; Chen, B.; Liu, Z.; Xia, Q.; Zhu, Y.; Xia, Y. Improved hydrogen release from ammonia borane confined in microporous carbon with narrow pore size distribution. J. Mater. Chem. A 2017, 5, 15395–15400. [Google Scholar] [CrossRef]
- Fang, L.; Feng, J.J.; Shi, X.; Si, T.; Song, Y.; Jia, H.; Li, Y.; Li, H.-W.; Zhang, Q. Turn Bulks into 0D, 1D and 2D Metallic Nanomaterials by Selective Aqueous Corrosion. Chem. Commun. 2019, 55, 10476–10479. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Z.; Gao, M.; Hu, J.; Du, F.; Yao, J.; Pan, H.; Liu, Y. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sources 2018, 398, 183–192. [Google Scholar] [CrossRef]
- Zhang, L.; Nyahuma, F.M.; Zhang, H.; Cheng, C.; Zheng, J.; Wu, F.; Chen, L. Metal organic framework supported niobium pentoxide nanoparticles with exceptional catalytic effect on hydrogen storage behavior of MgH2. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Wang, T.C.; White, J.L.; Bie, B.; Deng, H.; Edgington, J.; Sugar, J.D.; Stavila, V.; Allendorf, M.D. Design Rules for Metal-Organic Framework Stability in High-Pressure Hydrogen Environments. ChemPhysChem 2019, 20, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Q.; Panda, S.; Zhu, W.; Sun, F.; Khan, D.; Dong, J.; Ding, W.; Zou, J. In-situ catalyzed and nanoconfined magnesium hydride nanocrystals in a Ni-MOF scaffold for hydrogen storage. Sustain. Energy Fuels 2020, 4, 4694–4703. [Google Scholar] [CrossRef]
- Callini, E.; Szilagyi, P.A.; Paskevicius, M.; Stadie, N.P.; Rehault, J.; Buckley, C.E.; Borgschulte, A.; Zuttel, A. Stabilization of volatile Ti(BH4)3 by nanoconfinement in a metal–organic framework. Chem. Sci. 2016, 7, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Wan, L.F.; Lipton, A.S.; Liu, Y.-S.; Snider, J.L.; Baker, A.A.; Sugar, J.D.; Spataru, C.D.; Guo, J.; Autrey, T.S.; et al. Nanoconfinement of Molecular Magnesium Borohydride Captured in a Bipyridine-Functionalized Metal−Organic Framework. ACS Nano 2020, 14, 10294–10304. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Snurr, R.Q. Molecular modelling and machine learning for high-throughput screening of metal-organic frameworks for hydrogen storage. Mol. Simul. 2019, 45, 1069–1081. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Liao, C.-W.; Chang, Y.-W.; Chang, B.K.; Wang, H.; Li, J.; Wang, C.-Y. Influence of Metal−Organic Framework Porosity on Hydrogen Generation from Nanoconfined Ammonia Borane. J. Phys. Chem. C 2017, 121, 27369–27378. [Google Scholar] [CrossRef]
- Peil, S.; Wisser, D.; Stähle, M.; Roßmann, P.K.; Avadhut, Y.S.; Hartmann, M. Hydrogen Release from Ammonia Borane Nanoconfined in Metal−Organic Frameworks with MIL-53 Topology. J. Phys. Chem. C 2021, 125, 9990–10000. [Google Scholar] [CrossRef]
- Ullman, A.M.; Brown, J.W.; Foster, M.E.; Leonard, F.; Leong, K.; Stavila, V.; Allendorf, M.D. Transforming MOFs for Energy Applications Using the Guest@MOF Concept. Inorg. Chem. 2016, 55, 7233–7249. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, F.; Ma, J. Nanoconfinement engineering for enhanced adsorption of carbon materials, metal–organic frameworks, mesoporous silica, MXenes and porous organic polymers: A review. Environ. Chem. Lett. 2022, 20, 563–595. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, Z.; Huang, X.; Liu, H.; Guo, J. Study on catalytic effect and mechanism of MOF (MOF = ZIF-8, ZIF-67, MOF-74) on hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2019, 44, 28863–28873. [Google Scholar] [CrossRef]
- Huang, T.; Huang, X.; Hu, C.; Wang, J.; Liu, H.; Ma, Z.; Zou, J.; Ding, W. Enhancing hydrogen storage properties of MgH2 through addition of Ni/CoMoO4 nanorods. Mater. Today Energy 2021, 19, 100613. [Google Scholar] [CrossRef]
- Leick, N.; Strange, N.A.; Schneemann, A.; Stavila, V.; Gross, K.; Washton, N.; Settle, A.; Martinez, M.B.; Gennett, T.; Christensen, S.T. Al2O3 Atomic Layer Deposition on Nanostructured γ-Mg(BH4)2 for H2 Storage. ACS Appl. Energy Mater. 2021, 4, 1150–1162. [Google Scholar] [CrossRef]
- Habibi, P.; Vlugt, T.J.H.; Dey, P.; Moultos, O.A. Reversible Hydrogen Storage in Metal-Decorated Honeycomb Borophene Oxide. ACS Appl. Mater. Interfaces 2021, 13, 43233–43240. [Google Scholar] [CrossRef]
- Zettl, R.; Gombotz, M.; Clarkson, D.; Greenbaum, S.G.; Ngene, P.; de Jongh, P.E.; Wilkening, H.M.R. Li-Ion Diffusion in Nanoconfined LiBH4-LiI/Al2O3: From 2D Bulk Transport to 3D Long-Range Interfacial Dynamics. ACS Appl. Mater. Interfaces 2020, 12, 38570–38583. [Google Scholar] [CrossRef]
- Salameh, C.; Moussa, G.; Bruma, A.; Fantozzi, G.; Malo, S.; Miele, P.; Demirci, U.B.; Bernard, S. Robust 3D Boron Nitride Nanoscaffolds for Remarkable Hydrogen Storage Capacity from Ammonia Borane. Energy Technol. 2018, 6, 570–577. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Liu, Y.-S.; Shea, P.T.; Kang, S.Y.; Zaia, E.W.; Park, J.Y.; Chuang, Y.-D.; Yuk, J.M.; Zhou, X.; et al. Hierarchically Controlled Inside-Out Doping of Mg Nanocomposites for Moderate Temperature Hydrogen Storage. Adv. Funct. Mater. 2017, 27, 1704316. [Google Scholar] [CrossRef]
- Fan, J.; Cui, X.; Yu, S.; Gu, L.; Zhang, Q.; Meng, F.; Peng, Z.; Ma, L.; Ma, J.-Y.; Qi, K.; et al. Interstitial Hydrogen Atom Modulation to Boost Hydrogen Evolution in Pd-Based Alloy Nanoparticles. ACS Nano 2019, 13, 12987–12995. [Google Scholar] [CrossRef]
- Abbas, A.; Rajagopal, V.; Huang, S.J. Magnesium metal matrix composites and their applications. In Magnesium Alloys; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.Z.; Zhang, Y.; Liu, D.M.; Wang, C.Y.; Si, T.Z.; Li, Y.T.; Zhang, Q.A. Coupling of nanoconfinement with metallic catalysis in supported NaAlH4 for low-temperature hydrogen storage. J. Power Sources 2021, 491, 229611. [Google Scholar] [CrossRef]
- Ianni, E.; Sofianos, M.V.; Rowles, M.R.; Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Synthesis of NaAlH4/Al composites and their applications in hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 17309–17317. [Google Scholar] [CrossRef]
- Khan, D.; Zou, J.; Pan, M.; Ma, Z.; Zhu, W.; Huang, T.; Zeng, X.; Ding, W. Hydrogen storage properties of nanostructured 2MgH2-Co powders: The effect of high-pressure compression. Int. J. Hydrogen Energy 2019, 44, 15146–15158. [Google Scholar] [CrossRef]
- Rizo-Acosta, P.; Cuevas, F.; Latroche, M. Hydrides of early transition metals as catalysts and grain growth inhibitors for enhanced reversible hydrogen storage in nanostructured magnesium. J. Mater. Chem. A 2019, 7, 23064–23075. [Google Scholar] [CrossRef]
- Patelli, N.; Migliori, A.; Morandi, V.; Pasquini, L. Interfaces within biphasic nanoparticles give a boost to magnesium-based hydrogen storage. Nano Energy 2020, 72, 104654. [Google Scholar] [CrossRef]
- Lai, Q.; Rawal, A.; Quadir, M.Z.; Cazorla, C.; Demirci, U.B.; Aguey-Zinsou, K.-F. Nanosizing Ammonia Borane with Nickel: A Path toward the Direct Hydrogen Release and Uptake of B-N-H Systems. Adv. Sustain. Syst. 2018, 2, 1700122. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Zhu, Y.; Zhao, Y.; Lin, H.; Zhang, Y.; Li, H.; Zhang, J.; Liu, Y.; Gao, W.; et al. Crystal-facet-dependent catalysis of anatase TiO2 on hydrogen storage of MgH2. J. Alloys Compd. 2020, 822, 153553. [Google Scholar] [CrossRef]
- Le, T.T.; Pistidda, C.; Abetz, C.; Georgopanos, P.; Garroni, S.; Capurso, G.; Milanese, C.; Puszkiel, J.; Dornheim, M.; Abetz, V.; et al. Enhanced Stability of Li-RHC Embedded in an Adaptive TPX™ Polymer Scaffold. Materials 2020, 13, 991. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Darmadi, I.; Cusinato, L.; Susarrey-Arce, A.; Schreuders, H.; Bannenberg, L.J.; da Silva Fanta, A.B.; Kadkhodazadeh, S.; Wagner, J.B.; Antosiewicz, T.J.; et al. Metal–polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef]
- Ploszajski, A.R.; Billing, M.; Cockcroft, J.K.; Skipper, N.T. Crystalline structure of an ammonia borane–polyethylene oxide cocrystal: A material investigated for its hydrogen storage potential. CrystEngComm 2018, 20, 4436–4440. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of synthesis, characteristics, and environmental applications of MXene: A comprehensive review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines 2021, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, H.; Sun, Y.; Sun, L.; Xu, F.; Sun, S.; Zhang, G.; Huang, P.; Du, Y.; Wang, J.; et al. Dehybridization effect in improved dehydrogenation of LiAlH4 by doping with two-dimensional Ti3C2. Mater. Today Nano 2019, 8, 100054. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of two-dimension MXene Ti3C2 on hydrogen storage performances of MgH2-LiAlH4 composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Zhu, Y.; Li, S.; Zhang, J.; Li, L. Excellent catalytic activity of a two-dimensional Nb4C3Tx (MXene) on hydrogen storage of MgH2. Appl. Surf. Sci. 2019, 493, 431440. [Google Scholar] [CrossRef]
- Yadav, A.; Dashora, A.; Patel, N.; Miotello, A.; Press, M.; Kothari, D.C. Study of 2D MXene Cr2C material for hydrogen storage using density functional theory. Appl. Surf. Sci. 2016, 389, 88–95. [Google Scholar] [CrossRef]
- Wu, R.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved hydrogen storage properties of NaAlH4 doped with 2D titanium carbide. J. Power Sources 2016, 327, 519–525. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Z.; Zhang, M.; Gao, M.; Hu, J.; Du, F.; Liu, Y.; Pan, H. A novel solid-solution MXene (Ti0.5V0.5)3C2 with high catalytic activity for hydrogen storage in MgH2. Materialia 2018, 1, 114–120. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, D.; Liu, X.; Fan, G.; Liu, B. Improving the hydrogen storage performance of lithium borohydride by Ti3C2 MXene. Int. J. Hydrogen Energy 2019, 44, 29297–29303. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, X.; Zheng, J.; Chen, M.; Chen, L. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Feng, X.; Yuan, J.; Lv, Y.; Liu, B.; Huang, H.; Zhang, B.; Yan, Y.; Han, S.; Wu, Y. Improvement of desorption performance of Mg(BH4)2 by two-dimensional Ti3C2 MXene addition. Int. J. Hydrogen Energy 2020, 45, 16654–16662. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, Y.; Chen, Y.; Liu, X.; Liu, B.; Han, S. Two-dimensional C@TiO2/Ti3C2 composite with superior catalytic performance for NaAlH4. Int. J. Hydrogen Energy 2020, 45, 21666–21675. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, H.; Xiao, X.; Chen, M.; Chen, L. Enhanced low temperature hydrogen desorption properties and mechanism of Mg(BH4)2 composited with 2D MXene. Int. J. Hydrogen Energy 2019, 44, 24292–24300. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Ren, Z.; Liu, Y.; Hu, J.; Li, H.; Gao, M.; Pan, H.; Liu, Y. In situ formed ultrafine NbTi nanocrystals from a NbTiC solid-solution MXene for hydrogen storage in MgH2. J. Mater. Chem. A 2019, 7, 14244–14252. [Google Scholar] [CrossRef]
- Xian, K.X.; Gao, M.; Li, Z.; Gu, J.; Shen, Y.; Wang, S.; Yao, Z.; Liu, Y.; Pan, H. Superior Kinetic and Cyclic Performance of a 2D Titanium Carbide Incorporated 2LiH + MgB2 Composite toward Highly Reversible Hydrogen Storage. ACS Appl. Energy Mater. 2019, 2, 4853–4864. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalytic effect of sandwich-like Ti3C2/TiO2(A)-C on hydrogen storage performance of MgH2, Nanotechnology 2020, 31, 115404. Nanotechnology 2020, 31, 115404. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Chen, W.; Jiao, Z.; Ma, S. Reversible hydrogen storage behaviors of Ti2N MXenes predicted by first-principles calculations. J. Mater. Sci. 2019, 54, 493–505. [Google Scholar] [CrossRef]
- Veit, R.D.; Farber, R.G.; Sitaraman, N.S.; Arias, T.A.; Sibener, S.J. Suppression of nano-hydride growth on Nb(100) due to nitrogen doping. J. Chem. Phys. 2020, 152, 214703. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, Z.; Zhu, Y.; Zhang, J.; Li, L. Magnesium Nanoparticles with Pd Decoration for Hydrogen Storage. Front. Chem. 2020, 7, 949. [Google Scholar] [CrossRef]
- Feng, Y.F.; Zhou, X.; Yang, J.-h.; Gao, X.; Yin, L.; Zhao, Y.; Zhang, B. Encapsulation of Ammonia Borane in Pd/Halloysite Nanotubes for Efficient Thermal Dehydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 2122–2129. [Google Scholar] [CrossRef]
- Snider, J.L.; Mattox, T.M.; Liu, Y.-S.; Wan, L.F.; Wijeratne, P.; Allendorf, M.D.; Stavila, V.; Wood, B.C.; Klebanoff, L.E. The influence of LiH and TiH2 on hydrogen storage in MgB2 II. XPS study of surface and near-surface phenomena. Int. J. Hydrogen Energy 2022, 47, 403–419. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Zhang, H.; Xia, G.; Sun, D.; Yu, X. Heterostructures Built in Metal Hydrides for Advanced Hydrogen Storage Reversibility. Adv. Mater. 2020, 32, 2002647. [Google Scholar] [CrossRef] [PubMed]
- Dekura, S.; Kobayashi, H.; Kusada, K.; Kitagawa, H. Hydrogen and Storage Properties of Palladium and Related Nanomaterials: Size, Shape, Alloying, and Metal–Organic Framework Coating Effects. ChemPhysChem 2019, 20, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ji, L.; Yan, N.; Sun, Z.; Lu, X.; Zhang, L.; Zhu, X.; Chen, L. Superior catalytic effect of FeCo nanosheets on MgH2 for hydrogen storage. Dalton Trans. 2019, 48, 12699–12706. [Google Scholar] [CrossRef]
- Yang, X.; Hou, Q.; Yua, L.; Zhang, J. Improvement of the hydrogen storage characteristics of MgH2 with a flake Ni nano-catalyst composite. Dalton Trans. 2021, 50, 1797. [Google Scholar] [CrossRef]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J.; Youngblood, J. An efficient mechanochemical synthesis of alpha-aluminum hydride: Synergistic effect of TiF3 on the crystallization rate and selective formation of alpha-aluminum hydride polymorph. J. Hazard. Mater. 2019, 373, 141–151. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; Zhang, W.; Fan, X.; Zhang, L.; Cheng, C.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Transition metal (Co, Ni) nanoparticles wrapped with carbon and their superior catalytic activities for the reversible hydrogen storage of magnesium hydride. Phys. Chem. Chem. Phys. 2017, 19, 4019–4029. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, J.; Song, Y. Catalytic mechanisms of TiH2 thin layer on dehydrogenation behavior of fluorite-type MgH2: A first principles study. Int. J. Hydrogen Energy 2020, 45, 21600–21610. [Google Scholar] [CrossRef]
- Zhao, S.; Liang, L.; Liu, B.; Wang, L.; Liang, F. Superior Dehydrogenation Performance of α-AlH3 Catalyzed by Li3N: Realizing 8.0 wt.% Capacity at 100 °C. Small 2022, 18, 2107983. [Google Scholar] [CrossRef]
- Dun, C.; Jeong, S.; Liu, Y.-S.; Leick, N.; Mattox, T.M.; Guo, J.; Lee, J.-W.; Gennett, T.; Stavila, V.; Urban, J.J. Additive Destabilization of Porous Magnesium Borohydride Framework with Core-Shell Structure. Small 2021, 17, 2101989. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, L.; Fu, Y.; Wang, W.; Wang, Y.; Bi, J.; Li, Y.; Han, S. Enhanced kinetics of MgH2 via in situ formed catalysts derived from MgCCo1.5Ni1.5. J. Alloys Compd. 2020, 822, 153621. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, Y.; Wang, Y.; Bi, J.; Zhang, L.; Peng, D.; Li, Y.; Han, S. MgCNi3 prepared by powder metallurgy for improved hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2019, 44, 8347–8356. [Google Scholar] [CrossRef]
- Ding, X.; Ding, H.; Song, Y.; Xiang, C.; Li, Y.; Zhang, Q. Activity-Tuning of Supported Co–Ni Nanocatalysts via Composition and Morphology for Hydrogen Storage in MgH2. Front. Chem. 2020, 7, 937. [Google Scholar] [CrossRef]
- Patelli, N.; Migliori, A.; Pasquini, L. Reversible metal-hydride transformation in Mg-Ti-H nanoparticles at remarkably low temperatures. Chem. Phys. Chem. 2019, 20, 1325–1333. [Google Scholar] [CrossRef]
- Kumar, S.; Pavloudis, T.; Singh, V.; Nguyen, H.; Steinhauer, S.; Pursell, C.; Clemens, B.; Kioseoglou, J.; Grammatikopoulos, P.; Sowwan, M. Hydrogen Flux through Size Selected Pd Nanoparticles into Underlying Mg Nanofilms. Adv. Energy Mater. 2018, 8, 1701326. [Google Scholar] [CrossRef]
- Rossin, A.; Tuci, G.; Luconi, L.; Giambastiani, G. Metal–Organic Frameworks as Heterogeneous Catalysts in Hydrogen Production from Lightweight Inorganic Hydrides. ACS Catal. 2017, 7, 5035–5045. [Google Scholar] [CrossRef]
- Sun, Y.; Aguey-Zinsou, K.-F. Light-activated hydrogen storage in Mg, LiH and NaAlH4. ChemPlusChem 2018, 83, 904–908. [Google Scholar] [CrossRef]
- White, J.L.; Baker, A.A.; Marcus, M.A.; Snider, J.L.; Wang, T.C.; Lee, J.R.I.; Kilcoyne, D.A.L.; Allendorf, M.D.; Stavila, V.; Gabaly, F.E. The Inside-Outs of Metal Hydride Dehydrogenation: Imaging the Phase Evolution of the Li-N-H Hydrogen Storage System. Adv. Mater. Interfaces 2020, 7, 1901905. [Google Scholar] [CrossRef]
- Bannenberg, L.J.; Heere, M.; Benzidi, H.; Montero, J.; Dematteis, E.M.; Suwarno, S.; Jaron, T.; Winny, M.; Orłowski, P.A.; Wegner, W.; et al. Metal (boro-) hydrides for high energy density storage and relevant emerging technologies. Int. J. Hydrogen Energy 2020, 45, 33687–33730. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.I.; Jang, Y.; Balasubramanian, M.; Lee, W.; Park, G.O.; Park, S.B.; Yoo, J.; Hong, J.S.; Choi, Y.-S.; et al. Exceptional Lithium Storage in a Co(OH)2 Anode: Hydride Formation. ACS Nano 2018, 12, 2909–2921. [Google Scholar] [CrossRef] [PubMed]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y. Recent advances in improving performances of the lightweight complex hydrides Li-Mg-N-H system. Prog. Nat. Sci. 2017, 27, 21–33. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Yao, L.; Xu, C.; Liu, Y.; Li, L. State of the art multi-strategy improvement of Mg-based hydrides for hydrogen storage. J. Alloys Compd. 2019, 782, 796–823. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019, 9, 9907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydride. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Wang, N.; Huang, S. Molecular dynamics study on magnesium hydride nanoclusters with machine-learning interatomic potential. Phys. Rev. B 2020, 102, 094111. [Google Scholar] [CrossRef]
- Gigante, A.; Leick, N.; Lipton, A.S.; Tran, B.; Strange, N.A.; Bowden, M.; Martinez, M.B.; Moury, R.; Gennett, T.; Hagemann, H.; et al. Thermal Conversion of Unsolvated Mg(B3H8)2 to BH4− in the Presence of MgH2. ACS Appl. Energy Mater. 2021, 4, 3737–3747. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A Mater. Sci. Process. 2016, 122, 97. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Duan, C.; Su, Z.; Cao, Y.; Hu, L.; Fu, D.; Ma, J.; Zhang, Y. Synthesis of core-shell α-AlH3@Al(OH)3 nanocomposite with improved low-temperature dehydriding properties by mechanochemical mixing and ionic liquid treatment. J. Clean. Prod. 2021, 283, 124635. [Google Scholar] [CrossRef]
- Zhao, Y.; Mei, Z.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H. Thermal Decomposition Mechanism of 1,3,5,7-Tetranitro-1,3,5,7-tetrazocane Accelerated by Nano-Aluminum Hydride (AlH3): ReaxFF-Lg Molecular Dynamics Simulation. ACS Omega 2020, 5, 23193–23200. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Zhu, M. AlH3 as a hydrogen storage material: Recent advances, prospects and challenges. Rare Met. 2021, 40, 3337–3356. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Zhang, L.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Single-pot solvothermal strategy toward support-free nanostructured LiBH4 featuring 12 wt% reversible hydrogen storage at 400 °C. Chem. Eng. J. 2022, 428, 132566. [Google Scholar] [CrossRef]
- Xian, K.; Nie, B.; Li, Z.; Gao, M.; Li, Z.; Shang, C.; Liu, Y.; Guo, Z.; Pan, H. TiO2 decorated porous carbonaceous network structures offer confinement, catalysis and thermal conductivity for effective hydrogen storage of LiBH4. Chem. Eng. J. 2021, 407, 127156. [Google Scholar] [CrossRef]
- Wang, X.-K.; Zhao, Y.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H. Atomic perspective revealing for combustion evolution of nitromethane/nano-aluminum hydride composite. J. Mol. Graph. Model. 2021, 108, 107987. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum hydride for solid-state hydrogen storage: Structure, synthesis, thermodynamics, kinetics, and regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Rahm, J.M.; Löfgren, J.; Erhart, P. Quantitative predictions of thermodynamic hysteresis: Temperature-dependent character of the phase transition in Pd–H. Acta Mater. 2022, 227, 117697. [Google Scholar] [CrossRef]
- Huen, P.; Peru, F.; Charalambopoulou, G.; Steriotis, T.A.; Jensen, T.R.; Ravnsbæk, D.B. Nanoconfined NaAlH4 Conversion Electrodes for Li Batteries. ACS Omega 2017, 2, 1956–1967. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pistidda, C.; Dornheim, M.; Baricco, M. Exploring Ternary and Quaternary Mixtures in the LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 System. ChemPhysChem 2019, 20, 1348–1359. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Huang, Z.; Li, H.-W.; Gao, M.; Pan, H.; Liu, Y. Recent Development of Lithium Borohydride-Based Materials for Hydrogen Storage. Adv. Energy Sustain. Res. 2021, 2, 2100073. [Google Scholar] [CrossRef]
- Wu, R.; Ren, Z.; Zhang, X.; Lu, Y.; Li, H.; Gao, M.; Pan, H.; Liu, Y. Nanosheet-like Lithium Borohydride Hydrate with 10 wt % Hydrogen Release at 70 °C as a Chemical Hydrogen Storage Candidate. J. Phys. Chem. Lett. 2019, 10, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for Hydrogen Storage: Destabilization, Additive, and Nanoconfinement Approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef]
- Bergemann, N.; Pistidda, C.; Uptmoor, M.; Milanese, C.; Santoru, A.; Emmler, T.; Puszkiel, J.; Dornheim, M.; Klassen, T. A new mutually destabilized reactive hydride system: LiBH4–Mg2NiH4. J. Energy Chem. 2019, 34, 240–254. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.-F. Controlling the growth of NaBH4 nanoparticles for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 2054–2067. [Google Scholar] [CrossRef]
- White, J.L.; Strange, N.A.; Sugar, J.D.; Snider, J.L.; Schneemann, A.; Lipton, A.S.; Toney, M.F.; Allendorf, M.D.; Stavila, V. Melting of Magnesium Borohydride under High Hydrogen Pressure: Thermodynamic Stability and Effects of Nanoconfinement. Chem. Mater. 2020, 32, 5604–5615. [Google Scholar] [CrossRef]
- Albanese, E.; Corno, M.; Baricco, M.; Civalleri, B. Simulation of nanosizing effects in the decomposition of Ca(BH4)2 through atomistic thin film models. Res. Chem. Intermed. 2021, 47, 345–356. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Demirci, U.B. Mechanistic insights into the thermal decomposition of ammonia borane, a material studied for chemical hydrogen storage. Inorg. Chem. Front. 2021, 8, 1900–1930. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Diaz, L.B.; Hanlon, J.M.; Bielewski, M.; Milewska, A.; Gregory, D.H. Ammonia Borane Based Nanocomposites as Solid-State Hydrogen Stores for Portable Power Applications. Energy Technol. 2018, 6, 583–594. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Jiang, L. 1D Nanoconfined Ordered-Assembly Reaction. Adv. Mater. Interfaces 2019, 6, 1900104. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Ali, N.A.; Sazelee, N.A.; Ismail, M. An overview of reactive hydride composite (RHC) for solid-state hydrogen storage materials. Int. J. Hydrogen Energy 2021, 46, 31674–31698. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J. Pitfalls in the characterisation of the hydrogen sorption properties of materials. Int. J. Hydrogen Energy 2017, 42, 29320–29343. [Google Scholar] [CrossRef]
- Wang, X.; Song, S.; Zhang, H. A redox interaction-engaged strategy for multicomponent nanomaterials. Chem. Soc. Rev. 2020, 49, 736–764. [Google Scholar] [CrossRef]
- Wu, P.; Tan, S.; Moon, J.; Yan, Z.; Fung, V.; Li, N.; Yang, S.-Z.; Cheng, Y.; Abney, C.W.; Wu, Z.; et al. Harnessing strong metal–support interactions via a reverse route. Nat. Commun. 2020, 11, 3042. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).