Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes
Abstract
:1. Introduction
2. Results
2.1. Chemical Structure and Elemental Analysis of the Synthesized Powders
- d—crystallite size (nm)
- λ—wavelength of used radiation (nm)
- β—full width at half maximum (FWHM) of the peak (radians)
- θ—the diffraction angle of the corresponding reflection (°).
- χ—the degree of crystallinity
- K—constant (for hydroxyapatite it is equal to 0.24)
- β(002)—full width in a half minimum (FWHM)for (002) reflection (°).
2.2. Ultrastructure, Porosity and Mechanical Strength of the Granules
2.3. Study of Silver and Gallium Ions Release from Granules
2.4. Cytotoxicity Studies of Powders and Granules
2.5. Results of Antibacterial Activity Studies
2.5.1. Preliminary Studies
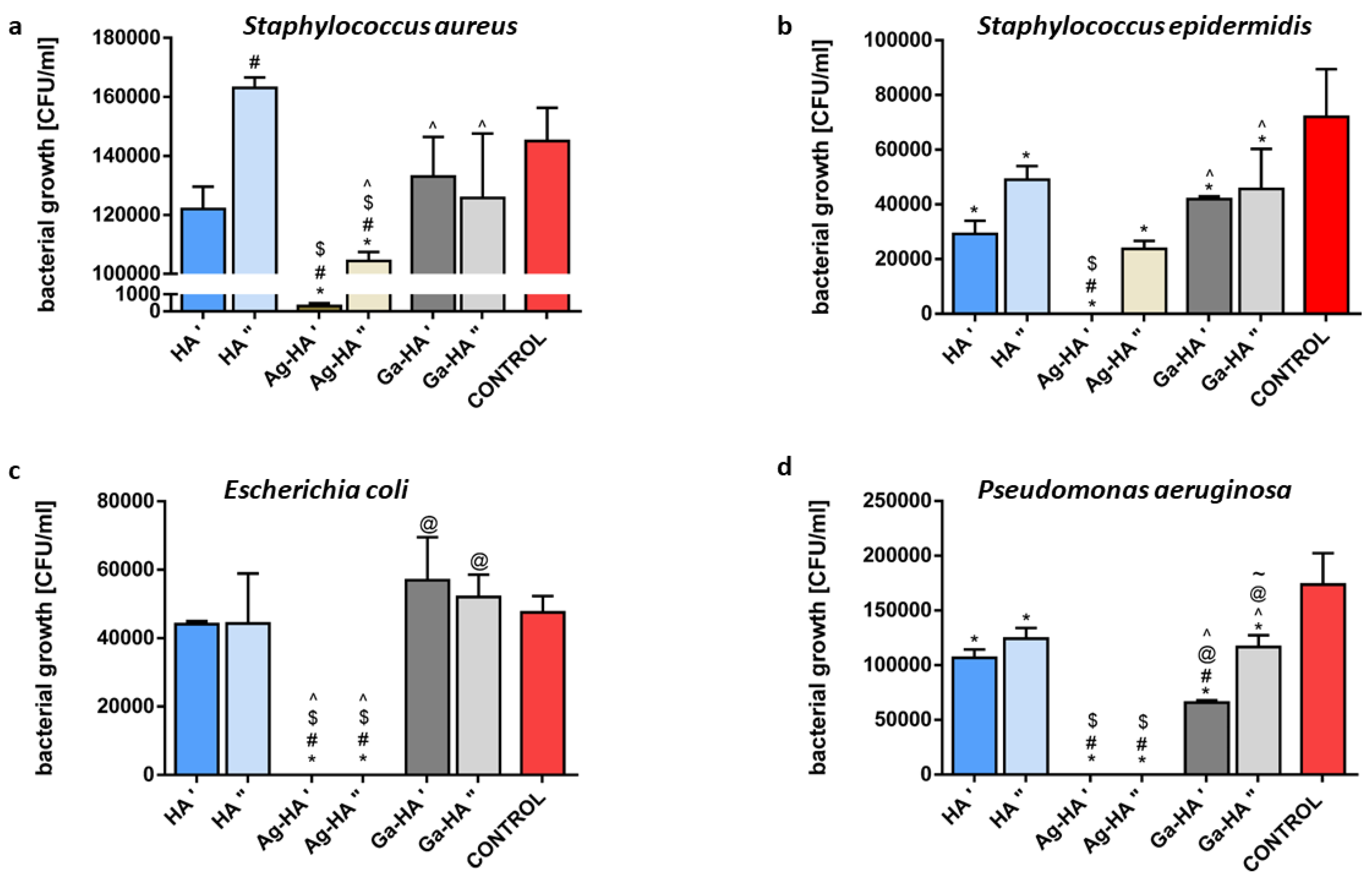
2.5.2. Antibacterial Activity of Powders
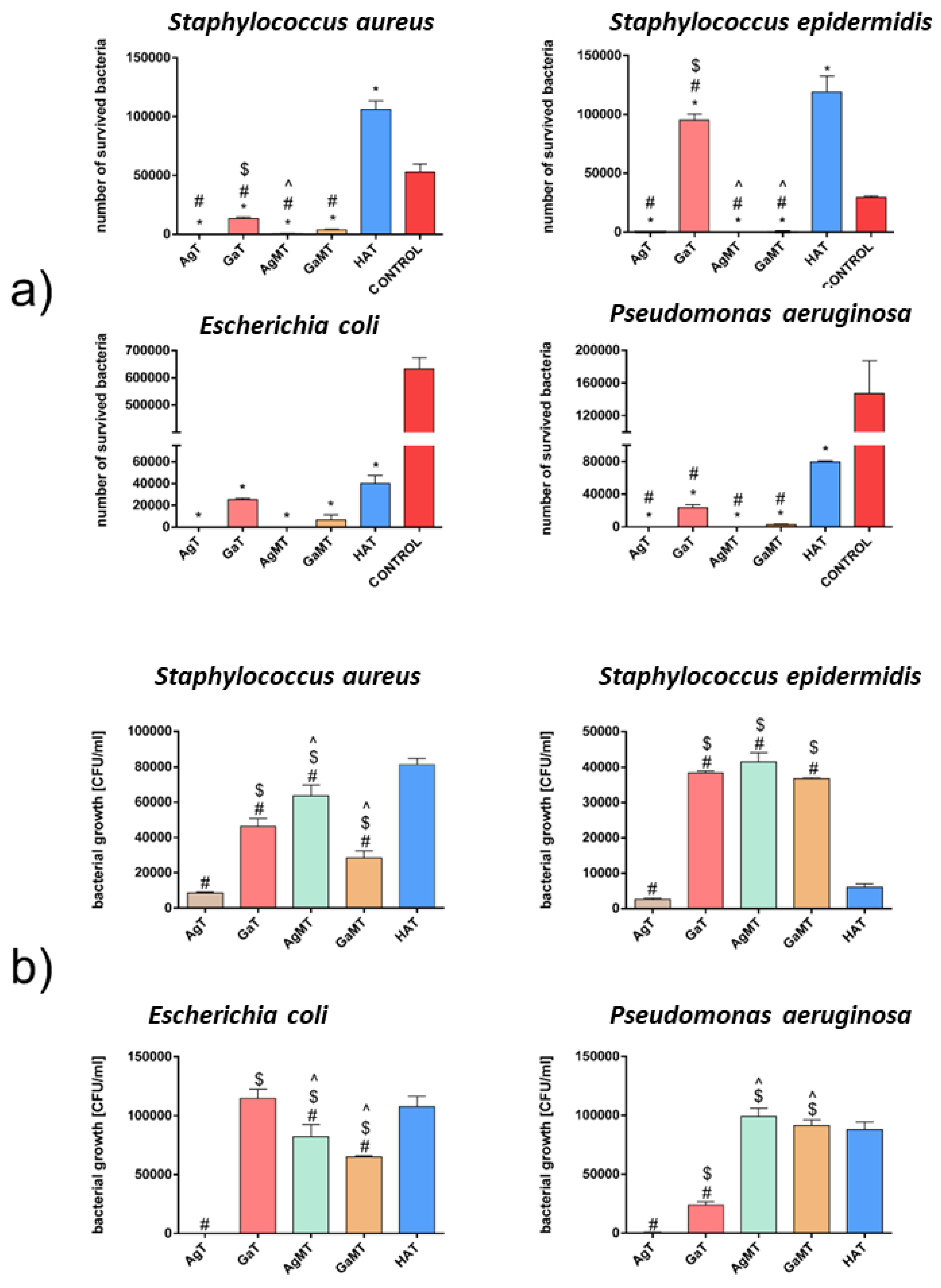
2.5.3. Antibacterial Activity of Granules
3. Materials and Methods
3.1. Synthesis of Silver- or Gallium-Containing Hydroxyapatite Powders
3.2. Preparation of Microgranules
3.3. Preparation of Composite Granules
3.4. Physicochemical Analysis of Ag-HA and Ga-HA
3.5. Physicochemical Analysis of the Granules
- PC—capillary pressure
- σ—mercury interfacial tension
- θ—contact angle
- r—pore radius.
3.6. In Vitro Cytotoxicity Studies
3.7. Antibacterial Activity Studies
3.7.1. Strains and Maintenance
3.7.2. Agar Plate Test
3.7.3. Antibacterial Activity Test (AATCC Test Method 100-2004 “Antibacterial Finishes on Textile Materials: Assessment of Developed from American Association of Textile Chemists and Colorists”)
3.7.4. Bacterial Adhesion Test
3.7.5. Direct Contact with Powders Test
3.7.6. Antibacterial Activity in Sample Extracts
3.7.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, S.; Zhang, Y.-W.; Zhou, R.-B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Echazú, M.I.; Perna, O.; Olivetti, C.E.; Antezana, P.E.; Municoy, S.; Tuttolomondo, M.V.; Galdopórpora, J.M.; Alvarez, G.S.; Olmedo, D.G.; Desimone, M.F. Recent Advances in Synthetic and Natural Biomaterials-Based Therapy for Bone Defects. Macromol. Biosci. 2022, 22, 2100383. [Google Scholar] [CrossRef]
- Ambrosio, L.; Raucci, M.G.; Vadalà, G.; Ambrosio, L.; Papalia, R.; Denaro, V. Innovative Biomaterials for the Treatment of Bone Cancer. Int. J. Mol. Sci. 2021, 22, 8214. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections: The new CDC guidelines. J. Am. Acad. Pas 2018, 31, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Søreide, K.; Ansari, D. Surgical Infections and Antibiotic Stewardship: In Need for New Directions. Scand. J. Surg. 2021, 110, 110–112. [Google Scholar] [CrossRef] [Green Version]
- Spina, N.T.; Aleem, I.S.; Nassr, A.; Lawrence, B.D. Surgical site infections in spine surgery: Preoperative prevention strategies to minimize risk. Glob. Spine J. 2018, 8, 31S–36S. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention Control. Healthcare-Associated Infections: Surgical Site Infections; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Aminzare, M.; Eskandari, A.; Baroonian, M.; Berenov, A.; Hesabi, Z.R.; Taheri, M.; Sadrnezhaad, S. Hydroxyapatite nanocomposites: Synthesis, sintering and mechanical properties. Ceram. Int. 2013, 39, 2197–2206. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. Rsc Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, properties and applications of dense hydroxyapatite: A review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [Green Version]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Basu, S.; Basu, B. Doped biphasic calcium phosphate: Synthesis and structure. J. Asian Ceram. Soc. 2019, 7, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Ratnayake, J.T.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Calhoun, J.H.; Mader, J.T. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clin. Orthop. Relat. Res. (1976–2007) 2002, 401, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abram, S.L.; Fromm, K.M. Handling (nano) silver as antimicrobial agent: Therapeutic window, dissolution dynamics, detection methods and molecular interactions. Chem.–A Eur. J. 2020, 26, 10948–10971. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- McRee, A.E. Therapeutic review: Silver. J. Exot. Pet Med. 2015, 2, 240–244. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Trujillo, N.A.; Oldinski, R.A.; Ma, H.; Bryers, J.D.; Williams, J.D.; Popat, K.C. Antibacterial effects of silver-doped hydroxyapatite thin films sputter deposited on titanium. Mater. Sci. Eng. C 2012, 32, 2135–2144. [Google Scholar] [CrossRef]
- Dubnika, A.; Zalite, V. Preparation and characterization of porous Ag doped hydroxyapatite bioceramic scaffolds. Ceram. Int. 2014, 40, 9923–9930. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Salma, I.; Reinis, A.; Poca, L.; Berzina-Cimdina, L. Evaluation of the physical and antimicrobial properties of silver doped hydroxyapatite depending on the preparation method. J. Mater. Sci. Mater. Med. 2014, 25, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C 2015, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Łapa, A.; Cresswell, M.; Campbell, I.; Jackson, P.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Gallium-and cerium-doped phosphate glasses with antibacterial properties for medical applications. Adv. Eng. Mater. 2020, 22, 1901577. [Google Scholar] [CrossRef] [Green Version]
- Verron, E.; Masson, M.; Khoshniat, S.; Duplomb, L.; Wittrant, Y.; Baud’Huin, M.; Badran, Z.; Bujoli, B.; Janvier, P.; Scimeca, J.C. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br. J. Pharmacol. 2010, 159, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Strazic Geljic, I.; Melis, N.; Boukhechba, F.; Schaub, S.; Mellier, C.; Janvier, P.; Laugier, J.P.; Bouler, J.M.; Verron, E.; Scimeca, J.C. Gallium enhances reconstructive properties of a calcium phosphate bone biomaterial. J. Tissue Eng. Regen. Med. 2018, 12, e854–e866. [Google Scholar] [CrossRef]
- Melnikov, P.; Matos, M.d.F.C.; Malzac, A.; Teixeira, A.R.; de Albuquerque, D.M. Evaluation of in vitro toxicity of hydroxyapatite doped with gallium. Mater. Lett. 2019, 253, 343–345. [Google Scholar] [CrossRef]
- Cassino, P.C.; Rosseti, L.S.; Ayala, O.I.; Martines, M.A.U.; Portugual, L.C.; Oliveira, C.G.d.; Silva, I.S.; Caldas, R.d.A. Potencial of different hydroxyapatites as biomaterials in the bone remodeling. Acta Cir. Bras. 2018, 33, 816–823. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga (iii)-containing hydroxyapatite with antibacterial activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Melnikov, P.; Teixeira, A.; Malzac, A.; Coelho, M.d.B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Sampath Kumar, T.; Madhumathi, K.; Rubaiya, Y.; Doble, M. Dual mode antibacterial activity of ion substituted calcium phosphate nanocarriers for bone infections. Front. Bioeng. Biotechnol. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, L.; Deng, Y.; Zhang, Y.; Zhou, Q.; Shi, Q.; Zhong, S.; Sun, Y.; Yang, Z.; Sun, M.; Politis, C. Silver-doped biphasic calcium phosphate/alginate microclusters with antibacterial property and controlled doxorubicin delivery. J. Appl. Polym. Sci. 2021, 138, 50433. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Hydroxylapatite nanoparticles: Fabrication methods and medical applications. Sci. Technol. Adv. Mater. 2012, 13, 064103. [Google Scholar] [CrossRef] [Green Version]
- Kolmas, J.; Kalinowski, E.; Wojtowicz, A.; Kolodziejski, W. Mid-infrared reflectance microspectroscopy of human molars: Chemical comparison of the dentin–enamel junction with the adjacent tissues. J. Mol. Struct. 2010, 966, 113–121. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, R.; Zhuo, X.; Li, Z.; Huang, Y.; Ma, L.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y. Incorporation of silver and strontium in hydroxyapatite coating on titanium surface for enhanced antibacterial and biological properties. Mater. Sci. Eng. C 2017, 71, 852–861. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 286–291. [Google Scholar] [CrossRef]
- Ragab, H.; Ibrahim, F.; Abdallah, F.; Al-Ghamdi, A.A.; El-Tantawy, F.; Radwan, N.; Yakuphanoglu, F. Synthesis and in vitro antibacterial properties of hydroxyapatite nanoparticles. IOSR J. Pharm. Biol. Sci. 2014, 9, 77–85. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of hydroxyapatite with antibacterial properties using a microwave-assisted combustion method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [Green Version]
- Jahangir, M.U.; Islam, F.; Wong, S.Y.; Jahan, R.A.; Matin, M.A.; Li, X.; Arafat, M.T. Comparative analysis and antibacterial properties of thermally sintered apatites with varied processing conditions. J. Am. Ceram. Soc. 2021, 104, 1023–1039. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Ithakisiou, G.-N.; Van Putten, J.P.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides Against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertesvåg, H. Alginate-modifying enzymes: Biological roles and biotechnological uses. Front. Microbiol. 2015, 6, 523. [Google Scholar] [PubMed] [Green Version]
- Pajor, K.; Pajchel, Ł.; Zgadzaj, A.; Piotrowska, U.; Kolmas, J. Modifications of hydroxyapatite by gallium and silver ions—physicochemical characterization, cytotoxicity and antibacterial evaluation. Int. J. Mol. Sci. 2020, 21, 5006. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, K.H.; You, C.K.; Rautray, T.R.; Kwon, T.Y. Synthesis of spherical hydroxyapatite granules with interconnected pore channels using camphene emulsion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 150–157. [Google Scholar] [CrossRef]
- Liu, M.; Wu, J.; Gan, Y.; Hanaor, D.A.; Chen, C. Evaporation limited radial capillary penetration in porous media. Langmuir 2016, 32, 9899–9904. [Google Scholar] [CrossRef]
- EN ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity (ISO 10993-5:2009), Annex A Neutral Red Uptake (NRU) Cytotoxicity Test. International Organization for Standardization: Geneva, Switzerland, 2009.
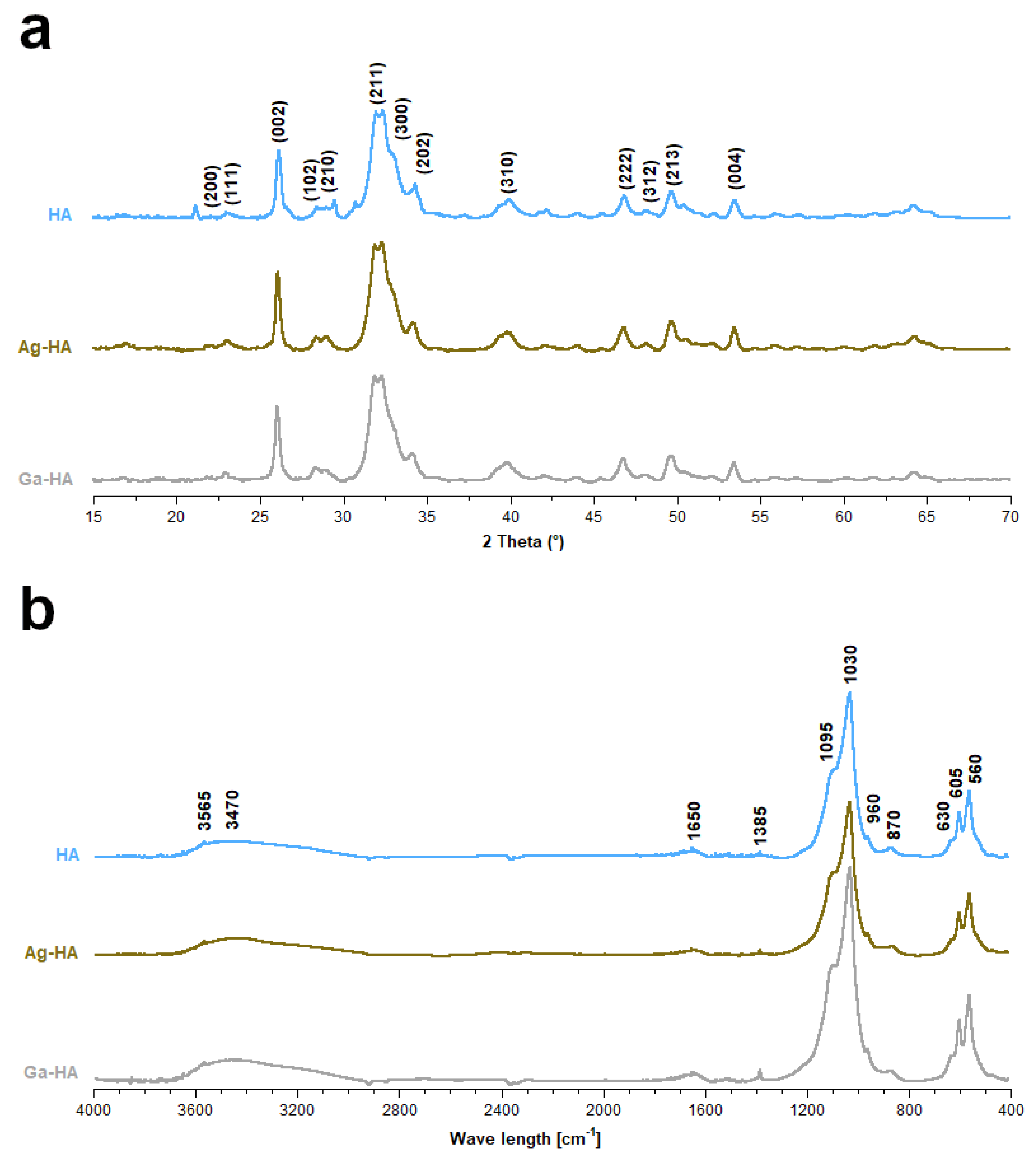
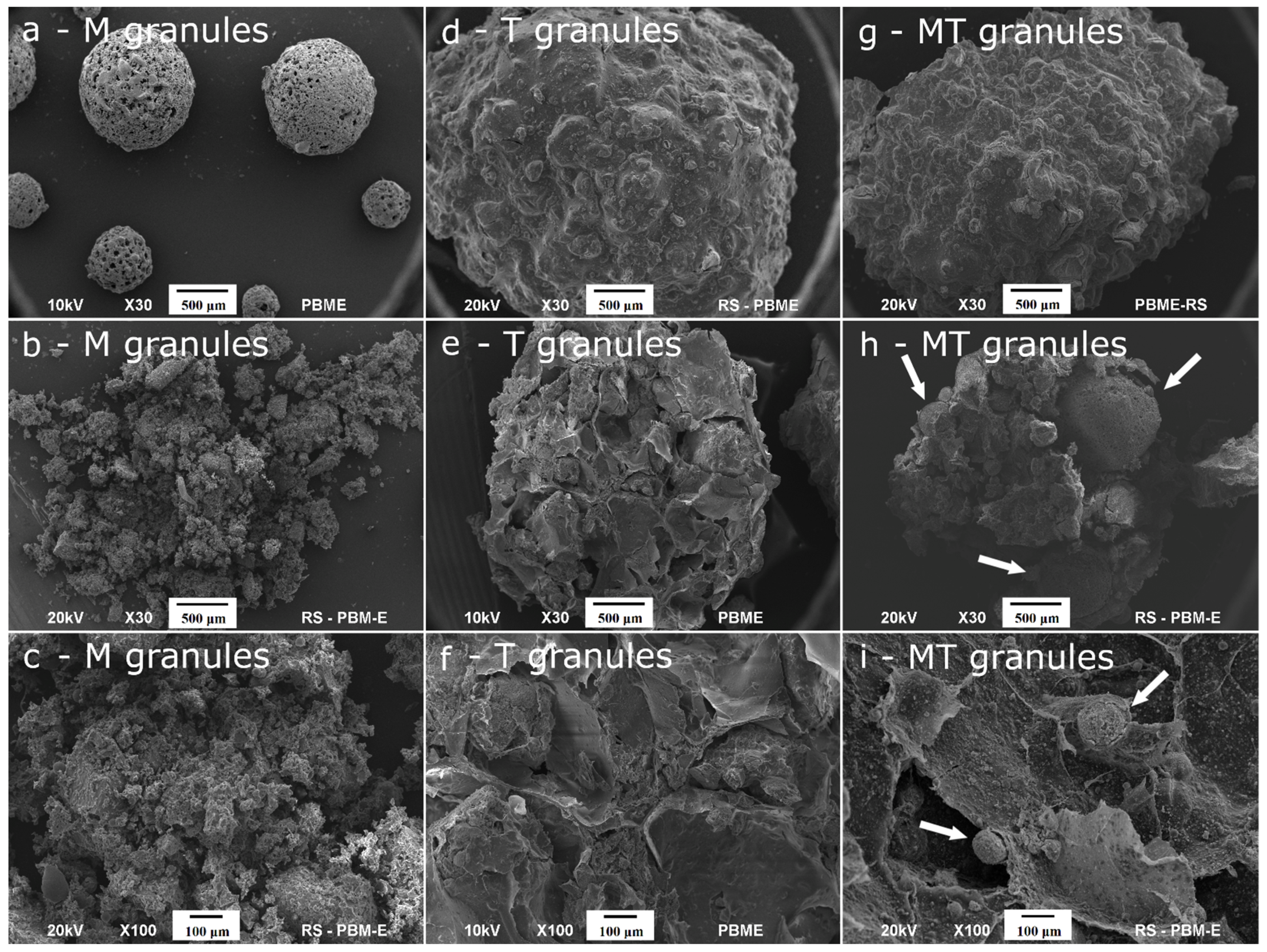
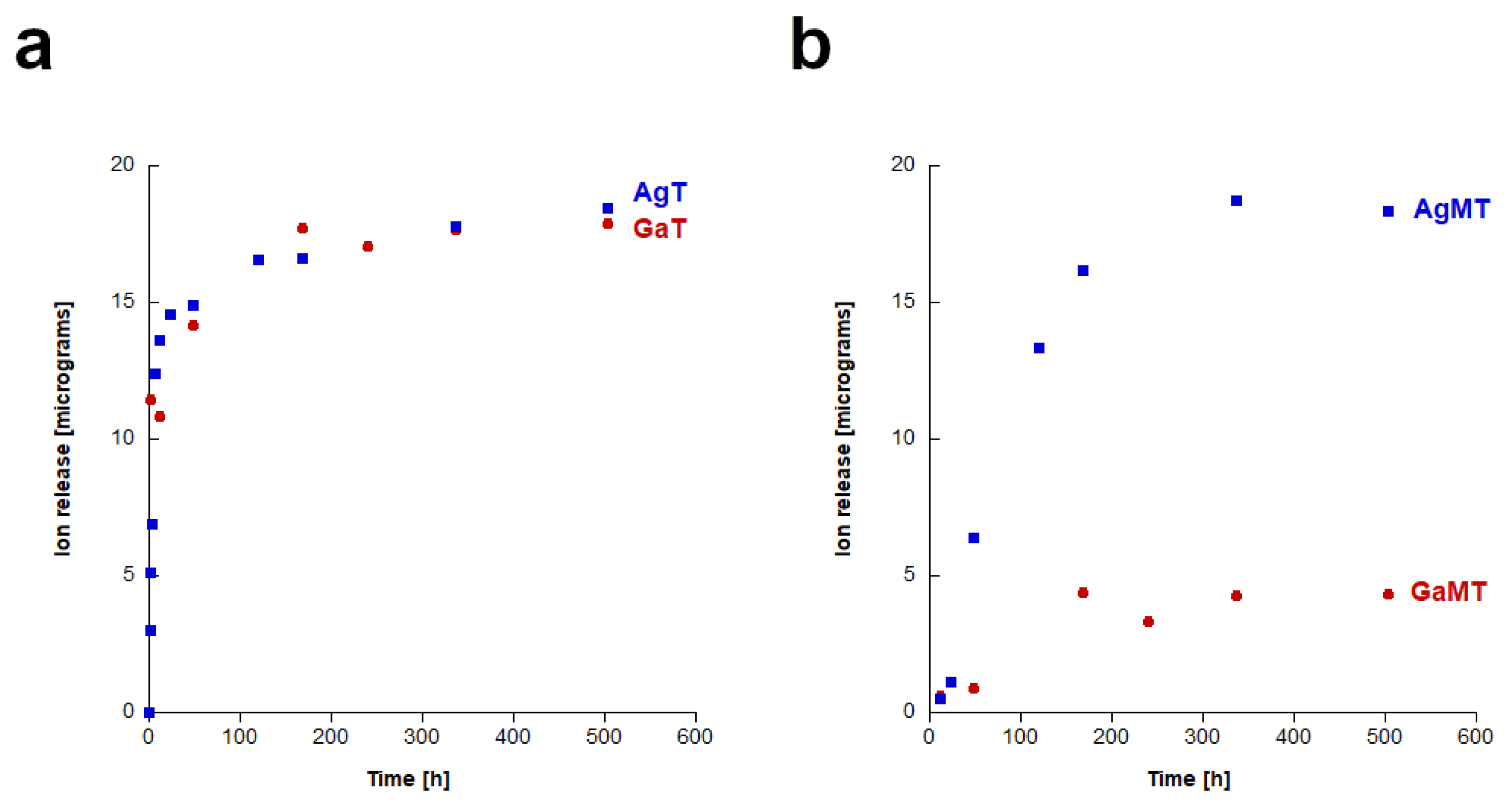
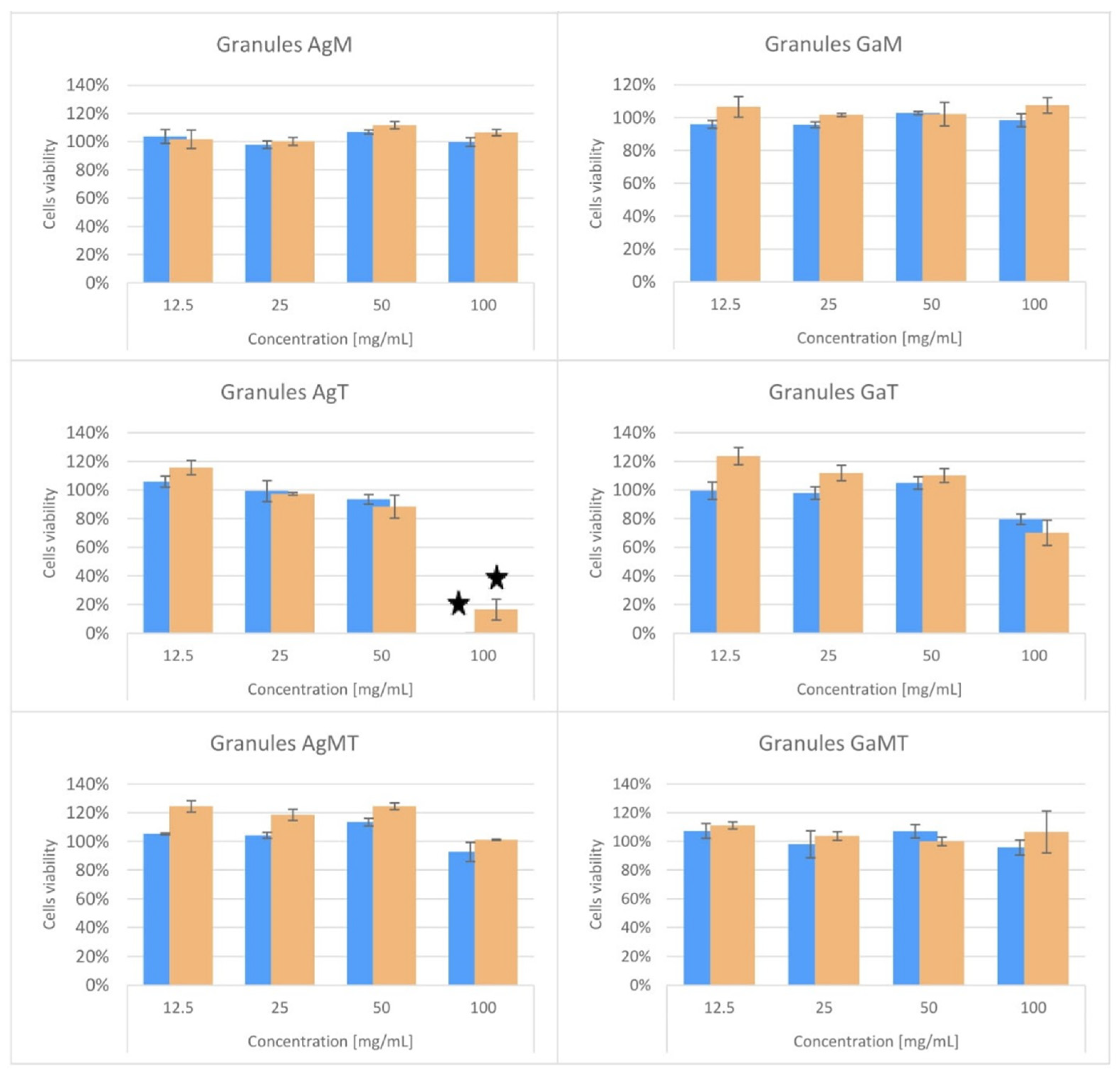
| Ag-HA | Ga-HA | |
|---|---|---|
| Crystallinity index (CI) | 0.45 | 0.39 |
| Crystallite size—c-axis (nm) | 27 ± 2 | 26 ± 3 |
| Crystallite size—a-axis (nm) | 6 ± 2 | 7 ± 2 |
| Ionic dopant content (wt%) | 0.46 | 0.41 |
| Sample | Total Volume of Pores (cm3/g) | Porosity of Granules (%) | SSA of Pores (m2/g) | Volume of Mesopores (cm3/g) | Percentage of Mesopores (%) | Average Diameter of Pores (nm) | Apparent Density of Granules (g/cm3) | Average Mechanical Strength (N/granule) |
|---|---|---|---|---|---|---|---|---|
| GaT | 0.87 | 65 | 79 | 0.32 | 37 | 40 | 0.8 | 34 |
| GaMT | 0.28 | 36 | 21 | 0.06 | 22 | 35 | 1.3 | 110 |
| AgT | 0.94 | 65 | 55 | 0.23 | 25 | 65 | 0.7 | 37 |
| AgMT | 0.56 | 55 | 42 | 0.12 | 22 | 45 | 1.0 | 15 |
| Sample | Cells Viability ± SD (%) | IC50 (mg/mL) | Classification |
|---|---|---|---|
| HA powder | 101 ± 2 | N | Non-cytotoxic |
| Ag-HA powder | 0 ± 0 | 75 | Cytotoxic |
| Ga-HA powder | 80 ± 10 | N | Non-cytotoxic |
| AgM granules | 100 ± 3 | N | Non-cytotoxic |
| GaM granules | 98 ± 4 | N | Non-cytotoxic |
| AgT granules | 0 ± 0 | 61 | Cytotoxic |
| GaT granules | 80 ± 4 | N | Non-cytotoxic |
| AgMT granules | 93 ± 7 | N | Non-cytotoxic |
| GaMT granules | 96 ± 5 | N | Non-cytotoxic |
| LT | 0 ± 0 | <10 | Cytotoxic |
| PE | 102 ± 7 | N | Non-cytotoxic |
| Sample | Cells Viability ± SD (%) | IC50 (mg/mL) | Classification |
|---|---|---|---|
| Ag-HA powder | 0 ± 0 | 59 | Cytotoxic |
| Ga-HA powder | 37 ± 9 | 86 | Cytotoxic |
| AgM granules | 107 ± 2 | N | Non-cytotoxic |
| GaM granules | 108 ± 5 | N | Non-cytotoxic |
| AgT granules | 17 ± 7 | 74 | Cytotoxic |
| GaT granules | 70 ± 9 | N | Non-cytotoxic |
| AgMT granules | 101 ± 0 | N | Non-cytotoxic |
| GaMT granules | 107 ± 15 | N | Non-cytotoxic |
| LT | 0 ± 0 | <10 | Cytotoxic |
| PE | 102 ± 7 | N | Non-cytotoxic |
| Sample | Zones of Bacterial Growth Inhibition (mm) | |||
|---|---|---|---|---|
| Staphylococcus aureus | Staphylococcus epidermidis | Escherichia coli | Pseudomonas aeruginosa | |
| HA | 0 | 0 | 0 | 0 |
| Ag-HA | 12 | 13 | 11 | 12 |
| Ga-HA | 0 | 0 | 0 | 0 |
| AgM | 0 | 0 | 0 | 0 |
| GaM | 0 | 0 | 0 | 0 |
| AgT | 10 | 10 | 8 | 10 |
| GaT | 0 | 0 | 0 | 0 |
| AgMT | 0 | 0 | 0 | 0 |
| GaMT | 0 | 0 | 0 | 0 |
| Granules | Fabrication method | Comment |
|---|---|---|
| AgM | Camphene emulsion | Microgranules containing Ag-HA |
| GaM | Camphene emulsion | Microgranules containing Ga-HA |
| HAT | Alginate cross-linking | Composite granules containing HA |
| AgT | Alginate cross-linking | Composite granules containing Ag-HA |
| GaT | Alginate cross-linking | Composite granules containing Ga-HA |
| AgMT | Alginate cross-linking | Composite granules containing AgM and HA |
| GaMT | Alginate cross-linking | Composite granules containing GaM and HA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajor, K.; Michalicha, A.; Belcarz, A.; Pajchel, L.; Zgadzaj, A.; Wojas, F.; Kolmas, J. Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. Int. J. Mol. Sci. 2022, 23, 7102. https://doi.org/10.3390/ijms23137102
Pajor K, Michalicha A, Belcarz A, Pajchel L, Zgadzaj A, Wojas F, Kolmas J. Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. International Journal of Molecular Sciences. 2022; 23(13):7102. https://doi.org/10.3390/ijms23137102
Chicago/Turabian StylePajor, Kamil, Anna Michalicha, Anna Belcarz, Lukasz Pajchel, Anna Zgadzaj, Filip Wojas, and Joanna Kolmas. 2022. "Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes" International Journal of Molecular Sciences 23, no. 13: 7102. https://doi.org/10.3390/ijms23137102
APA StylePajor, K., Michalicha, A., Belcarz, A., Pajchel, L., Zgadzaj, A., Wojas, F., & Kolmas, J. (2022). Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. International Journal of Molecular Sciences, 23(13), 7102. https://doi.org/10.3390/ijms23137102







