Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hematopoietic Stem Cell Transplantation (HSCT)
2.3. MHC-Matched HSCT Models
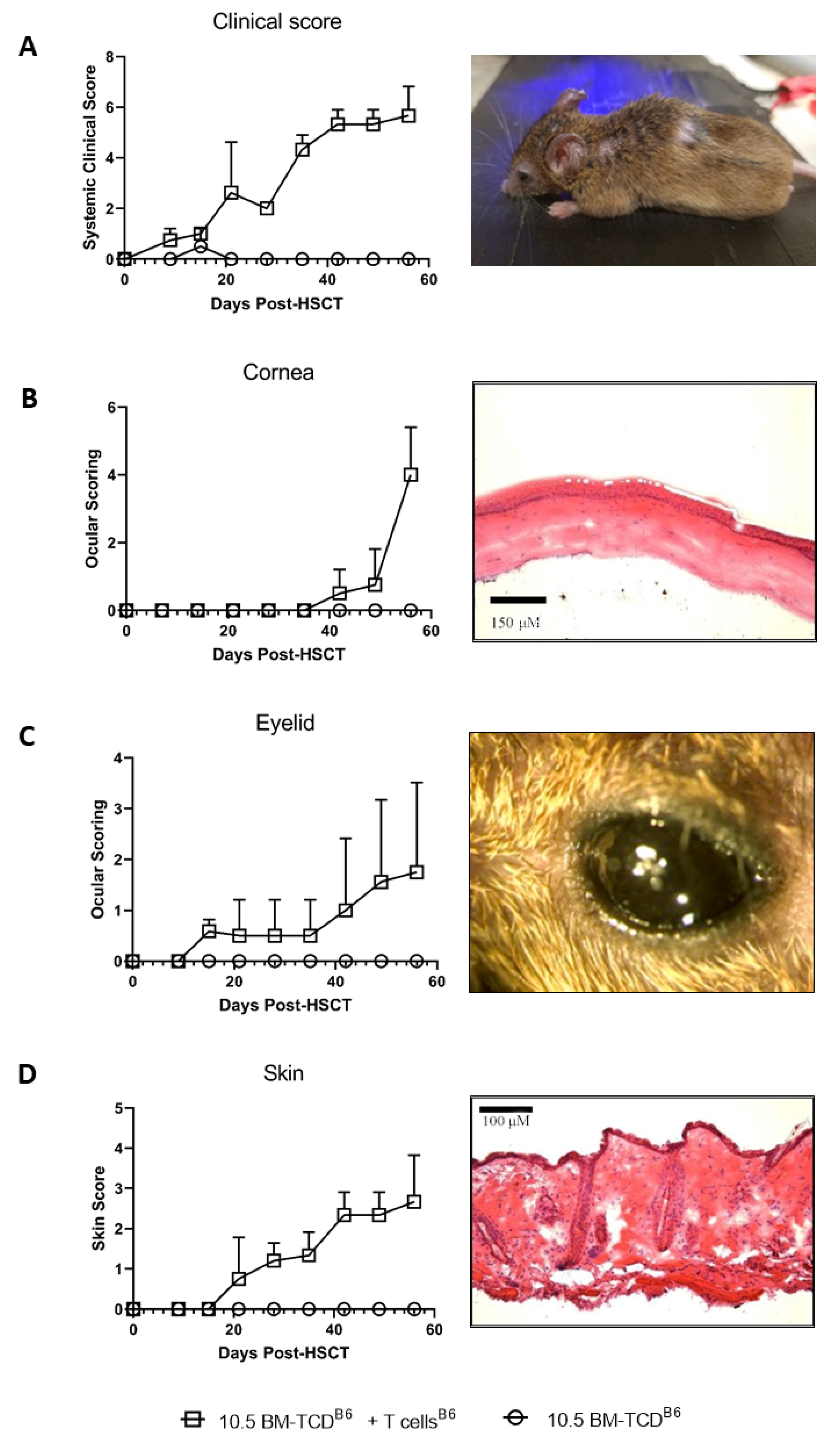
2.4. Clinical Evaluation of Systemic and Ocular Graft vs. Host Disease
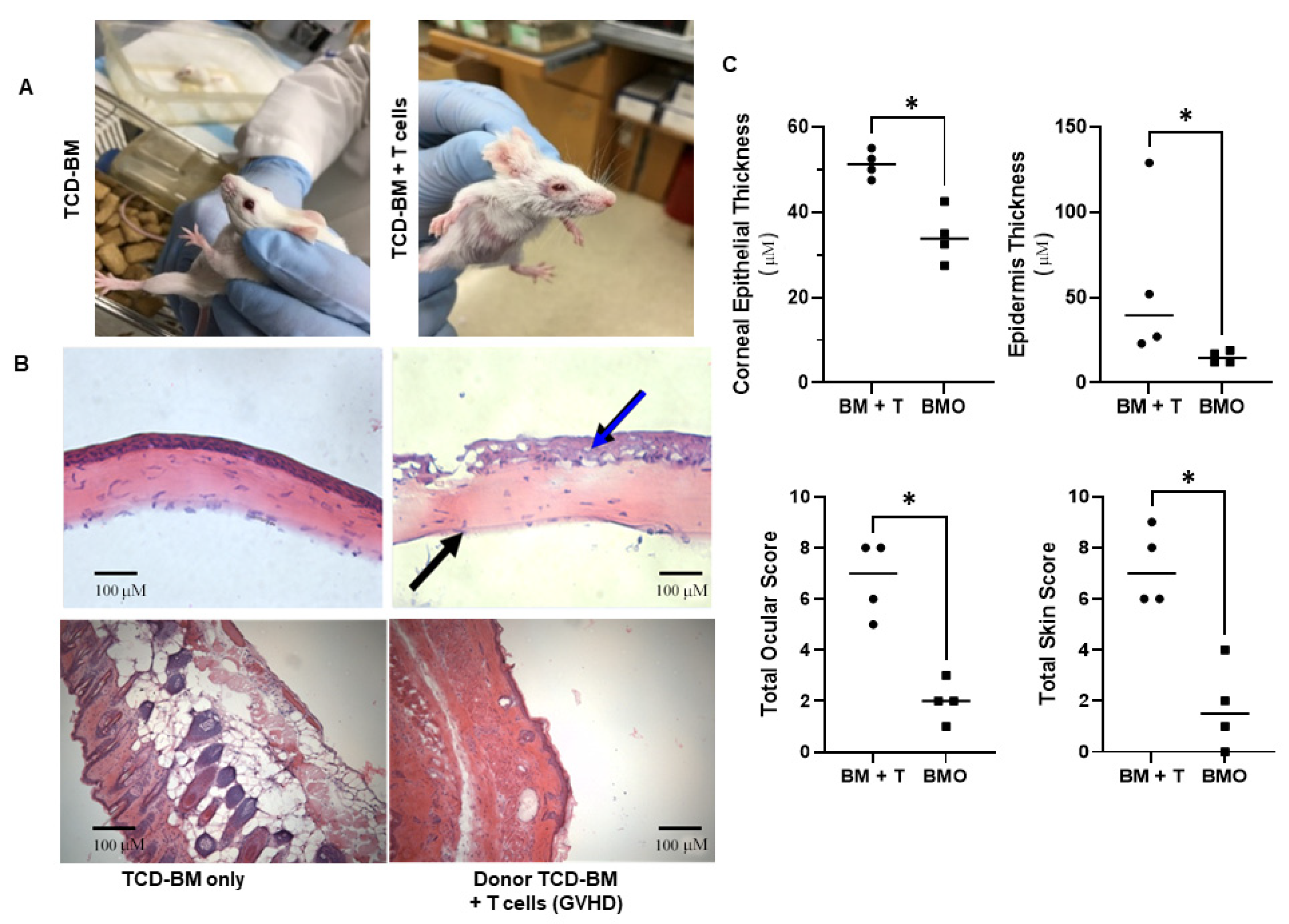
2.5. Histology and Analyses of Corneal and Cutaneous Pathological Changes
2.6. Details of Staining and Sectioning
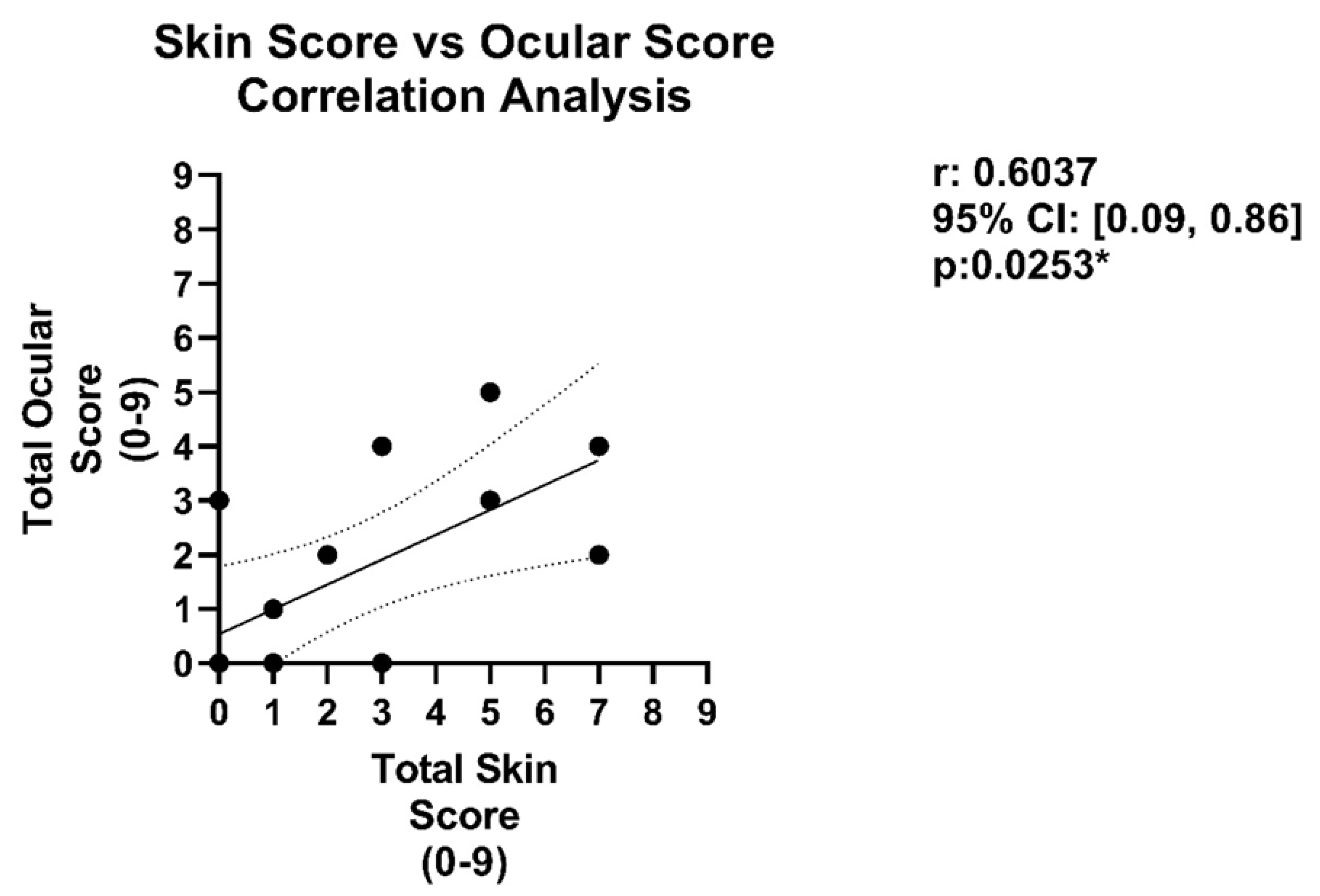
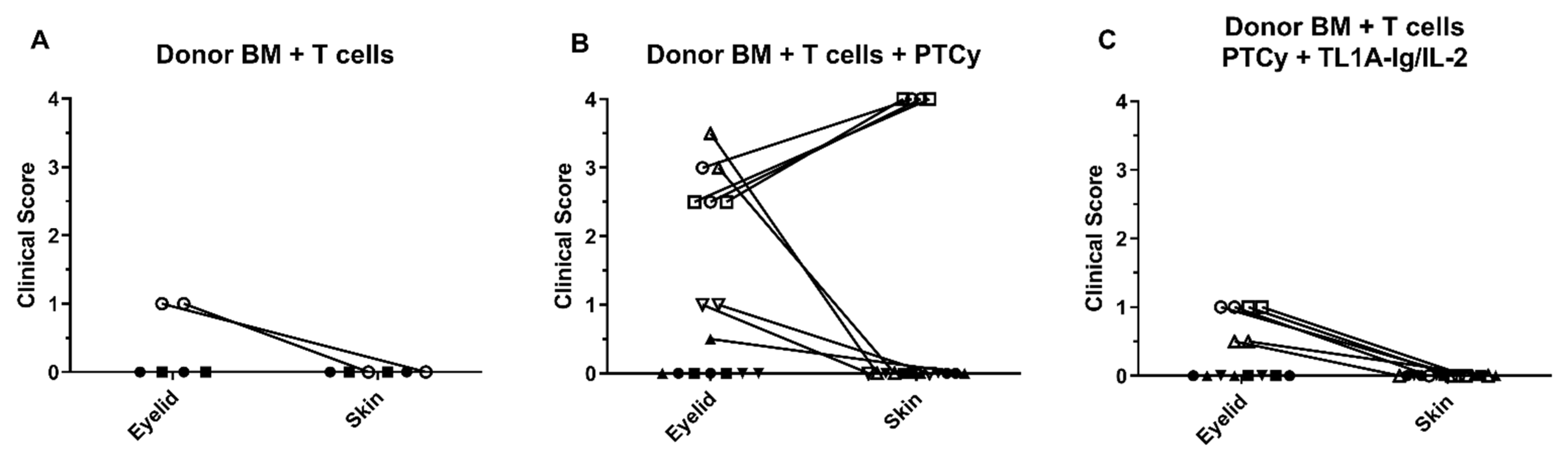
2.7. Disease Comparison and Statistical Analyses
3. Results
3.1. Kinetic Analysis of Changes in the Eye and Skin following Pre-Clinical MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants
3.2. Analysis of Ocular and Cutaneous Changes following MHC-Mismatched Allogeneic Hematopoietic Stem Cell Transplants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.W.; Sykes, M. Emerging concepts in haematopoietic cell transplantation. Nat. Rev. Immunol. 2012, 12, 403–416. [Google Scholar] [CrossRef]
- Martin, P.J.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Flowers, M.E. Treatment of chronic graft-versus-host disease: Past, present and future. Korean J. Hematol. 2011, 46, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, R.G.; Ross, D.B.; Barreras, H.; Herretes, S.; Podack, E.R.; Komanduri, K.V.; Perez, V.L.; Levy, R.B. The allure and peril of hematopoietic stem cell transplantation: Overcoming immune challenges to improve success. Immunol. Res. 2013, 57, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Riemens, A.; Boome, L.T.; Imhof, S.; Kuball, J.; Rothova, A. Current insights into ocular graft-versus-host disease. Curr. Opin. Ophthalmol. 2010, 21, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Hessen, M.; Akpek, E.K. Ocular graft-versus-host disease. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 540–547. [Google Scholar] [CrossRef]
- Tabbara, K.F.; Al-Ghamdi, A.; Al-Mohareb, F.; Ayas, M.; Chaudhri, N.; Al-Sharif, F.; Al-Zahrani, H.; Mohammed, S.Y.; Nassar, A.; Aljurf, M. Ocular Findings after Allogeneic Hematopoietic Stem Cell Transplantation. Ophthalmology 2009, 116, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.W.; Jabs, D.A.; Tutschka, P.J.; Green, W.R.; Santos, G.W. The Eye in Bone Marrow Transplantation. I. Clinical study. Arch. Ophthalmol. 1983, 101, 580–584. [Google Scholar] [CrossRef]
- Franklin, R.M.; Kenyon, K.R.; Tutschka, P.J.; Saral, R.; Green, W.R.; Santos, G.W. Ocular Manifestations of Graft-vs-Host Disease. Ophthalmology 1983, 90, 4–13. [Google Scholar] [CrossRef]
- Na, K.-S.; Yoo, Y.-S.; Mok, J.W.; Lee, J.W.; Joo, C.-K. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015, 50, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Lamey, P.-J.; Lundy, F.; Al-Hashimi, I. Sjögren’s syndrome: A condition with features of chronic graft-versus-host disease: Does duct cell adhesion or permeability play a role in pathogenesis? Med. Hypotheses 2004, 62, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Marcellus, D.C.; Altomonte, V.L.; Farmer, E.R.; Horn, T.D.; Freemer, C.S.; Grant, J.; Vogelsang, G.B. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood 1999, 93, 66–70. [Google Scholar] [CrossRef]
- Herretes, S.; Ross, D.B.; Duffort, S.; Barreras, H.; Yaohong, T.; Saeed, A.M.; Murillo, J.C.; Komanduri, K.V.; Levy, R.B.; Perez, V.L. Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants. Investig. Opthalmology Vis. Sci. 2015, 56, 2348–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, V.L.; Barsam, A.; Duffort, S.; Urbieta, M.; Barreras, H.; Lightbourn, C.; Komanduri, K.V.; Levy, R.B. Novel Scoring Criteria for the Evaluation of Ocular Graft-versus-Host Disease in a Preclinical Allogeneic Hematopoietic Stem Cell Transplantation Animal Model. Biol. Blood Marrow Transplant. 2016, 22, 1765–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, R.L.; Pérez-Simón, J.A.; Caballero-Velazquez, T.; Flores, T.; Carrancio, S.; Herrero, C.; Blanco, B.; Gutierrez-Cosio, S.; Cañete-Campos, C.; González, F.C.; et al. Limbus Damage in Ocular Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2011, 17, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, E.; Ogawa, Y.; Matsumoto, Y.; Kawakita, T.; Shimmura, S.; Tsubota, K. Four cases of corneal perforation in patients with chronic graft-versus-host disease. Mol. Vis. 2011, 17, 598–606. [Google Scholar]
- Kim, S.K. Update on ocular graft versus host disease. Curr. Opin. Ophthalmol. 2006, 17, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Ntoukas, T.; Cursiefen, C.; Westekemper, H.; Eberwein, P.; Reinhard, T.; Bertz, H.; Nepp, J.; Lawitschka, A.; Heiligenhaus, A.; Seitz, B.; et al. Diagnosis and Treatment of Ocular Chronic Graft-Versus-Host Disease: Report From the German–Austrian–Swiss Consensus Conference on Clinical Practice in Chronic GVHD. Cornea 2012, 31, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.; Bader, C.S.; Barreras, H.; Copsel, S.; Pfeiffer, B.J.; Lightbourn, C.O.; Altman, N.H.; Komanduri, K.V.; Levy, R.B. Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Ross, D.B.; Panoskaltsis-Mortari, A.; Kanakry, C.G.; Blazar, B.R.; Levy, R.B.; Luznik, L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014, 124, 2131–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, D.; Barreras, H.; Bader, C.S.; Copsel, S.; Lightbourn, C.O.; Pfeiffer, B.J.; Altman, N.H.; Podack, E.R.; Komanduri, K.V.; Levy, R.B. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lightbourn, C.O.; Wolf, D.; Copsel, S.N.; Wang, Y.; Pfeiffer, B.J.; Barreras, H.; Bader, C.S.; Komanduri, K.V.; Perez, V.L.; Levy, R.B. Use of Post-transplant Cyclophosphamide Treatment to Build a Tolerance Platform to Prevent Liquid and Solid Organ Allograft Rejection. Front. Immunol. 2021, 12, 636789. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.; Du, J.; Veenstra, R.G.; Reichenbach, D.K.; Panoskaltsis-Mortari, A.; Taylor, P.A.; Freeman, G.J.; Serody, J.S.; Murphy, W.J.; Munn, D.H.; et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood 2014, 123, 3988–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radojcic, V.; Pletneva, M.A.; Yen, H.-R.; Ivcevic, S.; Panoskaltsis-Mortari, A.; Gilliam, A.C.; Drake, C.G.; Blazar, B.R.; Luznik, L. STAT3 Signaling in CD4+T Cells Is Critical for the Pathogenesis of Chronic Sclerodermatous Graft-Versus-Host Disease in a Murine Model. J. Immunol. 2010, 184, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.E.; McNiff, J.; Yan, J.; Doyle, H.; Mamula, M.; Shlomchik, M.; Shlomchik, W.D. Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Investig. 2003, 112, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Cooke, K.; Kobzik, L.; Martin, T.; Brewer, J.; Delmonte, J.J.; Crawford, J.; Ferrara, J. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 1996, 88, 3230–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copsel, S.; Wolf, D.; Kale, B.; Barreras, H.; Lightbourn, C.O.; Bader, C.S.; Alperstein, W.; Altman, N.H.; Komanduri, K.V.; Levy, R.B. Very Low Numbers of CD4+ FoxP3+ Tregs Expanded in Donors via TL1A-Ig and Low-Dose IL-2 Exhibit a Distinct Activation/Functional Profile and Suppress GVHD in a Preclinical Model. Biol. Blood Marrow Transplant. 2018, 24, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Cutler, C.S.; Koreth, J.; Ritz, J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood 2017, 129, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.; Kiladjian, J.-J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK Inhibition with Ruxolitinib versus Best Available Therapy for Myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Spoerl, S.; Mathew, N.R.; Bscheider, M.; Schmitt-Graeff, A.; Chen, S.; Mueller, T.; Verbeek, M.; Fischer, J.; Otten, V.; Schimckl, M.; et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014, 123, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Perales, M.-A.; Schroeder, M.A.; Ali, H.; Shah, N.N.; Chen, Y.-B.; Fazal, S.; Dawkins, F.W.; Arbushites, M.C.; Tian, C.; et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): A multicenter, open-label phase 2 trial. Blood 2020, 135, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Lazaryan, A.; Bachier, C.R.; Salhotra, A.; Weisdorf, D.J.; Zoghi, B.; Essell, J.; Green, L.; Schueller, O.; Patel, J.; et al. ROCK2 Inhibition With Belumosudil (KD025) for the Treatment of Chronic Graft-Versus-Host Disease. J. Clin. Oncol. 2021, 39, 1888–1898. [Google Scholar] [CrossRef]
- Jacobsohn, D.A.; Kurland, B.F.; Pidala, J.; Inamoto, Y.; Chai, X.; Palmer, J.M.; Arai, S.; Arora, M.; Jagasia, M.; Cutler, C.; et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: Results from the Chronic GVHD Consortium. Blood 2012, 120, 2545–2552. [Google Scholar] [CrossRef] [Green Version]
- Martires, K.J.; Baird, K.; Steinberg, S.M.; Grkovic, L.; Joe, G.O.; Williams, K.M.; Mitchell, S.A.; Datiles, M.; Hakim, F.T.; Pavletic, S.Z.; et al. Sclerotic-type chronic GVHD of the skin: Clinical risk factors, laboratory markers, and burden of disease. Blood 2011, 118, 4250–4257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitko, C.L.; Pidala, J.; Schoemans, H.M.; Lawitschka, A.; Flowers, M.E.; Cowen, E.W.; Tkaczyk, E.; Farhadfar, N.; Jain, S.; Steven, P.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant. Cell. Ther. 2021, 27, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Radojcic, V.; Lafyatis, R.; Cinar, R.; Rosenstein, R.K.; Cowen, E.W.; Cheng, G.-S.; Sheshadri, A.; Bergeron, A.; Williams, K.M.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transplant. Cell. Ther. 2021, 27, 817–835. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Thomas, E.D.; Strob, R.; Clift, R.A.; Fefer, A.; Johnson, F.L.; Neimean, P.E.; Lerner, K.G.; Glucksberg, H.; Buckner, C.D. Bone-marrow transplantation (second of two parts). N. Engl. J. Med. 1975, 292, 895–902. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.-P.; Liu, Q.-F.; Xu, L.-P.; Liu, K.-Y.; Zhang, X.-H.; Yu, W.-J.; Xu, Y.; Huang, F.; Huang, X.-J. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J. Hematol. Oncol. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Donor Strain | Recipient Strain | Genetic Disparity |
|---|---|---|
| B6 (H2b) | C3H.SW (H2b) | MHC Matched 1 |
| LP/J (H2b) | B6 (H2b) | MHC Matched |
| B10.D2 (H2d) | BALB/c (H2d) | MHC Matched |
| B6 (H2b) | B10.BR (H2k) | MHC Mismatched 2 |
| B6 (H2b) | BALB/c (H2d) | MHC Mismatched |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, R.B.; Mousa, H.M.; Lightbourn, C.O.; Shiuey, E.J.; Latoni, D.; Duffort, S.; Flynn, R.; Du, J.; Barreras, H.; Zaiken, M.; et al. Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease. Int. J. Mol. Sci. 2022, 23, 184. https://doi.org/10.3390/ijms23010184
Levy RB, Mousa HM, Lightbourn CO, Shiuey EJ, Latoni D, Duffort S, Flynn R, Du J, Barreras H, Zaiken M, et al. Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease. International Journal of Molecular Sciences. 2022; 23(1):184. https://doi.org/10.3390/ijms23010184
Chicago/Turabian StyleLevy, Robert B., Hazem M. Mousa, Casey O. Lightbourn, Eric J. Shiuey, David Latoni, Stephanie Duffort, Ryan Flynn, Jing Du, Henry Barreras, Michael Zaiken, and et al. 2022. "Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease" International Journal of Molecular Sciences 23, no. 1: 184. https://doi.org/10.3390/ijms23010184
APA StyleLevy, R. B., Mousa, H. M., Lightbourn, C. O., Shiuey, E. J., Latoni, D., Duffort, S., Flynn, R., Du, J., Barreras, H., Zaiken, M., Paz, K., Blazar, B. R., & Perez, V. L. (2022). Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease. International Journal of Molecular Sciences, 23(1), 184. https://doi.org/10.3390/ijms23010184







