Genome-Wide Identification of OSC Gene Family and Potential Function in the Synthesis of Ursane- and Oleanane-Type Triterpene in Momordica charantia
Abstract
:1. Introduction
2. Results
2.1. Identification of OSC Gene Family in Bitter Gourd
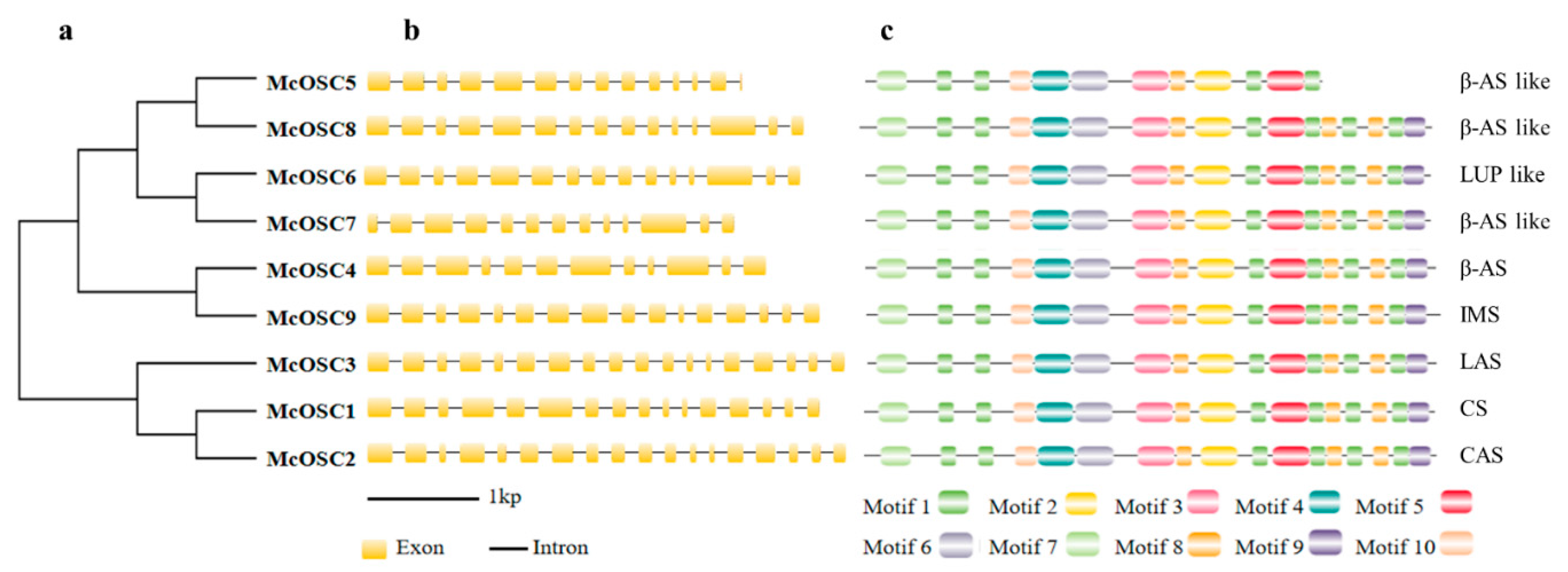
2.2. Phylogenetic Relationships, Exon–Intron Structure, and Chromosome Location Analysis
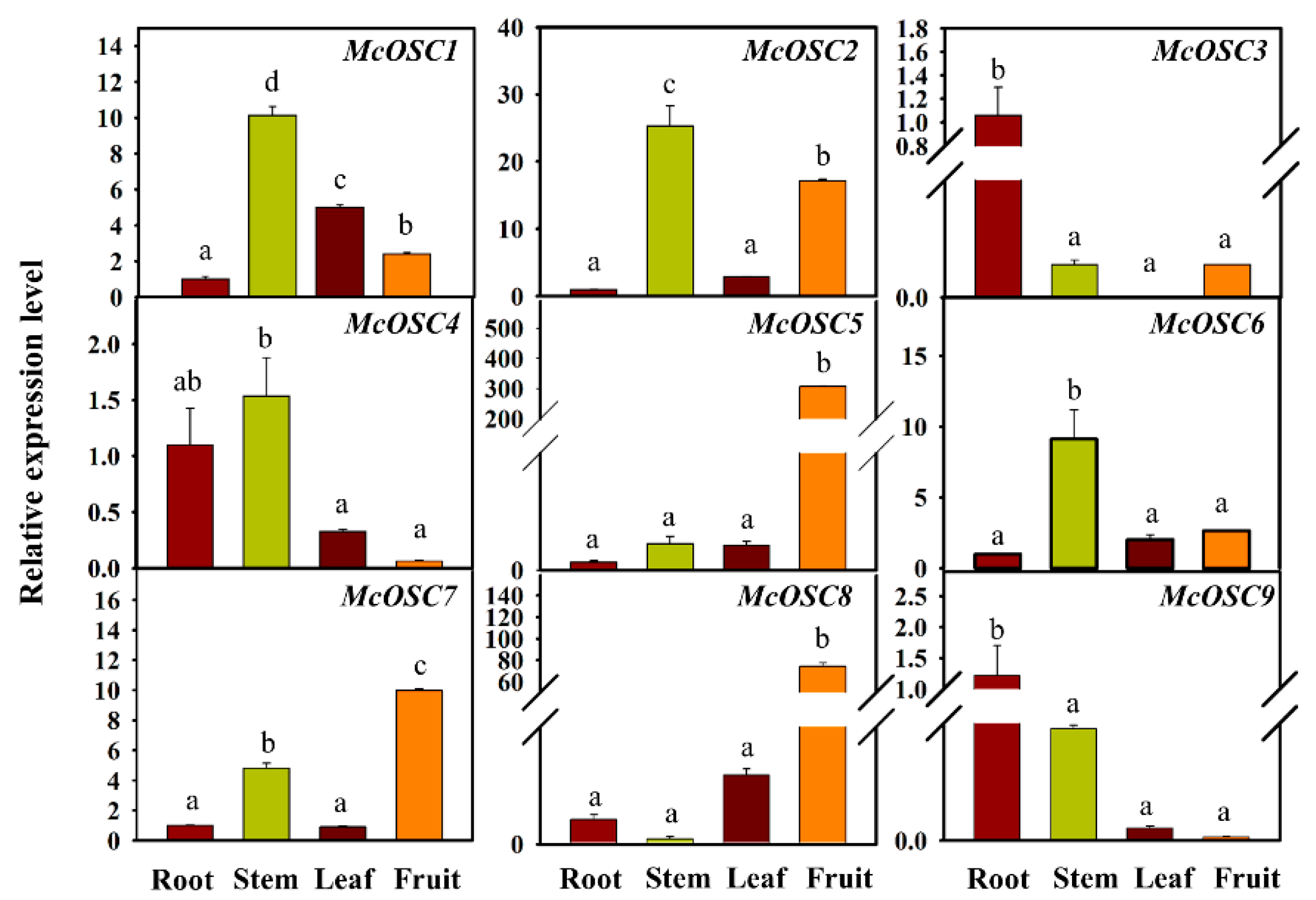
2.3. Transcript Profiles of Bitter Gourd OSC Genes in Different Tissues
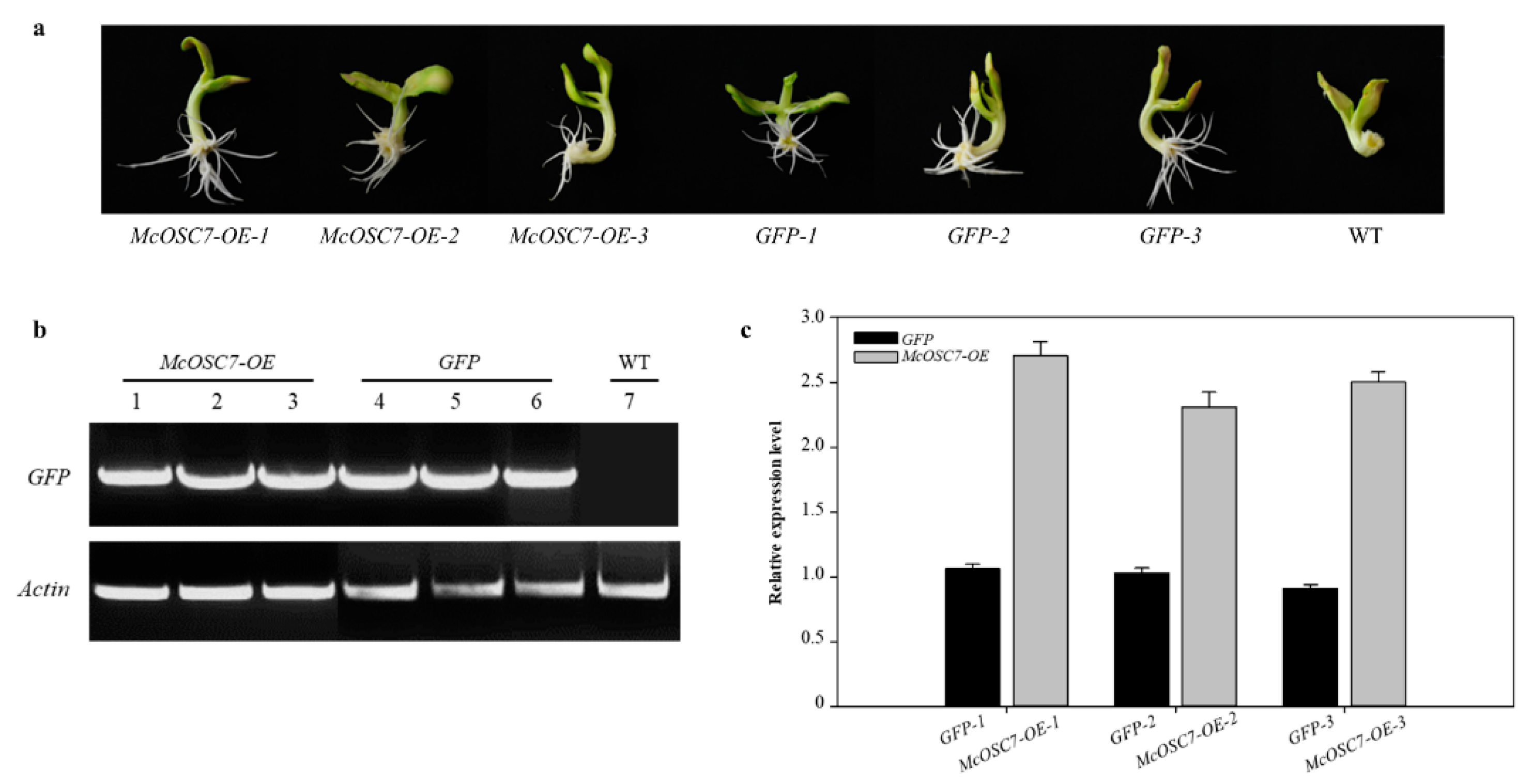
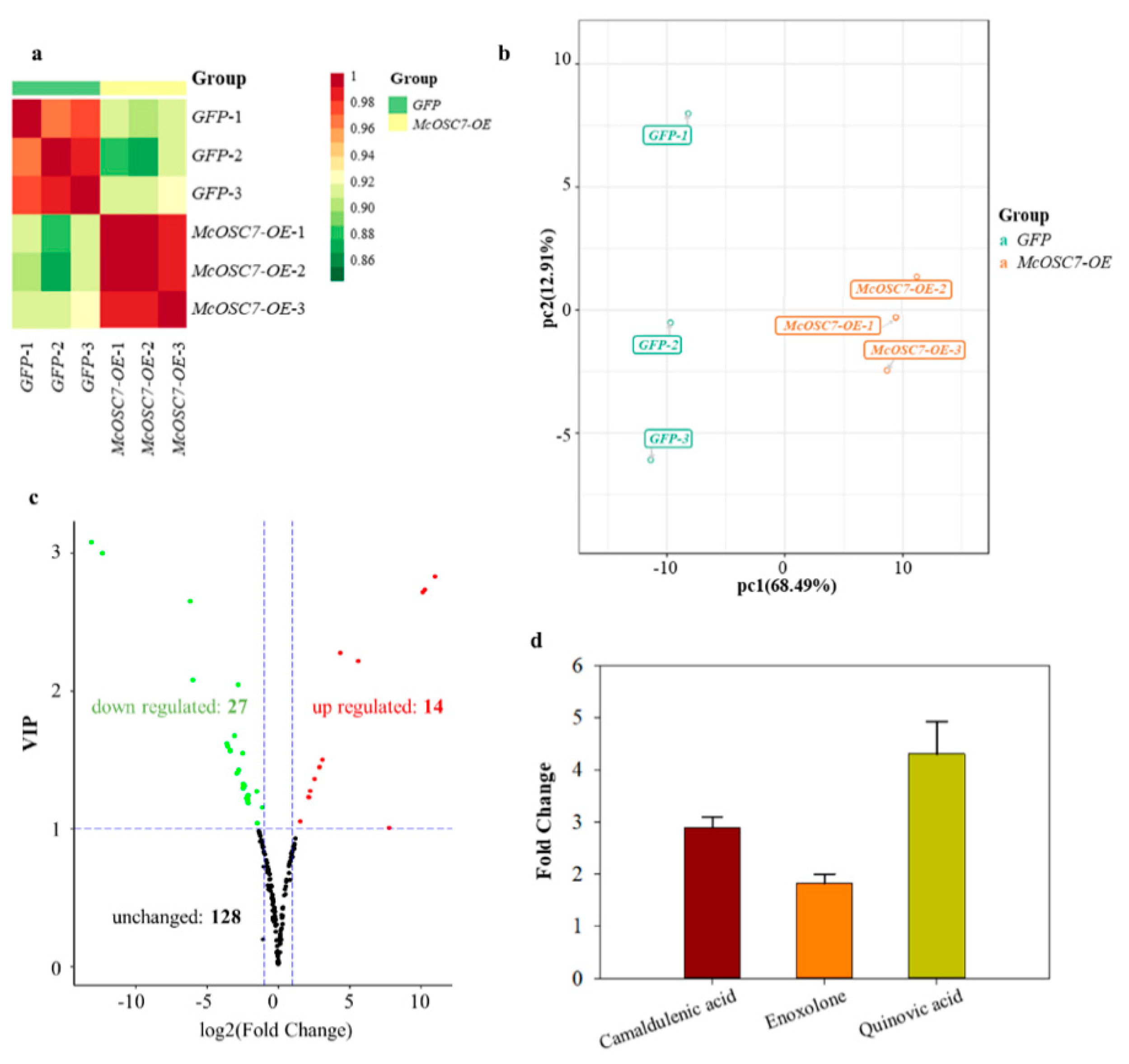
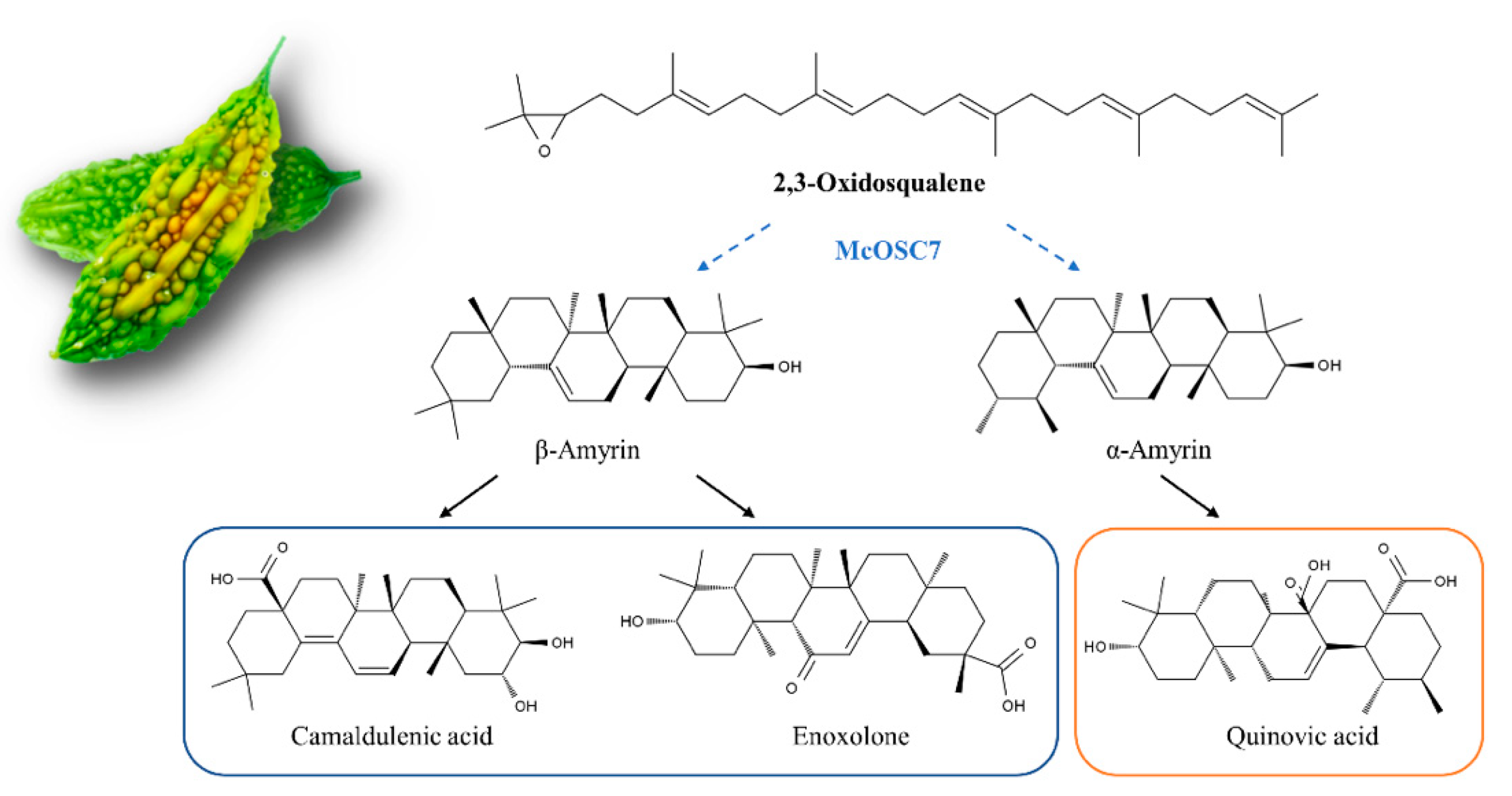
2.4. Widely Targeted Metabolomics Analysis of Transformed Hairy Roots of Bitter Gourd
3. Discussion
4. Materials and Methods
4.1. Identification of OSC Gene Family in Bitter Gourd
4.2. Phylogenetic Relationships, Exon–Intron Structure Analysis, and Chromosome Location
4.3. Plant Materials
4.4. RNA Extraction and qRT-PCR
4.5. Agrobacterium-Mediated Transgenic Hairy Roots of Bitter Gourd
4.6. Widely Targeted Metabolic Profiling and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSC | oxidosqualene cyclase |
| qRT-PCR | quantitative real-time PCR |
| cDNA | coding DNA |
| Tm | melting temperatures |
| TMT | timentin |
| HPLC-MS | high-performance liquid chromatography—mass spectrometry |
| MS | murashige and skoog |
| ESI | electrospray ionization |
| Q TRAP | triple quadrupole linear ion trap mass spectrometer |
References
- Thimmappa, R.; Geisler, K.; Louveau, T.; O′Maille, P.; Osbourn, A. Triterpene Biosynthesis in Plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ali, R.; Parveen, R.; Najmi, A.K.; Ahmad, S. Pharmacological Evidences for Cytotoxic and Antitumor Properties of Boswellic Acids from Boswellia Serrata. J. Ethnopharmacol. 2016, 191, 315–323. [Google Scholar] [CrossRef]
- Forestier, E.; Romero-Segura, C.; Pateraki, I.; Centeno, E.; Compagnon, V.; Preiss, M.; Berna, A.; Boronat, A.; Bach, T.J.; Darnet, S.; et al. Distinct Triterpene Synthases in the Laticifers of Euphorbia lathyris. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Coloma, A.; Lopez-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-Based Plant Defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Sawai, S.; Saito, K. Triterpenoid Biosynthesis and Engineering in Plants. Front. Plant Sci. 2011, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of Plant (Tri)Terpenoids: From Metabolic Engineering of Plants to Synthetic Biology Invivo and Invitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Van Montagu, M.; Pedreno, M.A.; Martins, J.C.; Thevelein, J.M.; Goossens, A. Combinatorial Biosynthesis of Sapogenins and Saponins in Saccharomyces cerevisiae Using a C-16 Alpha Hydroxylase from Bupleurum falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, J.; Stephenson, M.J.; Miettinen, K.; Brouwer, B.; Leveau, A.; Brett, P.; Goss, R.J.M.; Goossens, A.; O′Connell, M.A.; Osbourn, A. A Translational Synthetic Biology Platform for Rapid Access to Gram-Scale Quantities of Novel Drug-Like Molecules. Metab. Eng. 2017, 42, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive Amyrin Synthases for Efficient Alpha-Amyrin Synthesis in Engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef]
- Vranova, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the Origins of Triterpenoid Skeletal Diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Duan, L.; Liu, D.; Guo, J.; Ge, S.; Dicks, J.; Omaille, P.; Osbourn, A.; Qi, X. Divergent Evolution of Oxidosqualene Cyclases in Plants. New Phytol. 2012, 193, 1022–1038. [Google Scholar] [CrossRef]
- Seki, H.; Tamura, K.; Muranaka, T. P450s and Ugts: Key Players in the Structural Diversity of Triterpenoid Saponins. Plant Cell Physiol. 2015, 56, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Fukushinna, E.O.; Shimizu, Y.; Seki, H.; Fujisawa, Y.; Ishimoto, M.; Osakabe, K.; Osakabe, Y.; Muranaka, T. Lotus Japonicus Triterpenoid Profile and Characterization of the CYP716A51 and LjCYP93E1 Genes Involved in Their Biosynthesis in Planta. Plant Cell Physiol. 2019, 60, 2496–2509. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Masuda, K.; Ebizuka, Y. Mutational Studies on Triterpene Syntheses: Engineering Lupeol Synthase into Beta-Amyrin Synthase. J. Am. Chem. Soc. 2000, 122, 6816–6824. [Google Scholar] [CrossRef]
- Shibuya, M.; Adachi, S.; Ebizuka, Y. Cucurbitadienol Synthase, the First Committed Enzyme for Cucurbitacin Biosynthesis, Is a Distinct Enzyme from Cycloartenol Synthase for Phytosterol Biosynthesis. Tetrahedron 2004, 60, 6995–7003. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Zhao, Z.; Wang, J.; Li, J.; Xin, W.; Xia, G.; Xiang, F. Cloning and Functional Analysis of a Beta-Amyrin Synthase Gene Associated with Oleanolic Acid Biosynthesis in Gentiana Straminea Maxim. Biol. Pharm. Bull. 2009, 32, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Grover, J.K.; Yadav, S.P. Pharmacological Actions and Potential Uses of Momordica Charantia: A Review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. Food Prospects and Nutraceutical Attributes of Momordica Species: A Potential Tropical Bioresources—A Review. Food Sci. Hum. Wellness 2014, 3, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.M.; Vazquez, R.D.P.; Fernandez-Arche, A.; Ruiz-Gutierrez, V. Supressive Effect of Maslinic Acid from Pomace Olive Oil on Oxidative Stress and Cytokine Production in Stimulated Murine Macrophages. Free. Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant Effects of Maslinic Acid in Livers, Hearts and Kidneys of Streptozotocin-Induced Diabetic Rats: Effects on Kidney Function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-l.; Yang, H.-t.; Lee, H.-l.; Yin, M.-c. Protective Effects of Maslinic Acid against Alcohol-Induced Acute Liver Injury in Mice. Food Chem. Toxicol. 2014, 74, 149–155. [Google Scholar] [CrossRef]
- Wang, Y.-y.; Diao, B.-z.; Zhong, L.-h.; Lu, B.-l.; Cheng, Y.; Yu, L.; Zhu, L.-y. Maslinic Acid Protects against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Injury in Mice. Microb. Pathog. 2018, 119, 49–53. [Google Scholar] [CrossRef]
- Takase, S.; Kera, K.; Hirao, Y.; Hosouchi, T.; Kotake, Y.; Nagashima, Y.; Mannen, K.; Suzuki, H.; Kushiro, T. Identification of Triterpene Biosynthetic Genes from Momordica Charantia Using Rna-Seq Analysis. Biosci. Biotechnol. Biochem. 2019, 83, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chen, Y.-C.; Tseng, S.-W.; Liu, Y.-T.; Wen, H.-Y.; Li, W.-H.; Huang, C.-Y.; Ko, C.-Y.; Wang, T.-T.; Wu, T.-K. The Cysteine 703 to Isoleucine or Histidine Mutation of the Oxidosqualene-Lanosterol Cyclase from Saccharomyces Cerevisiae Generates an Iridal-Type Triterpenoid. Biochimie 2012, 94, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. Chimeric Triterpene Synthase. A Possible Model for Multifunctional Triterpene Synthase. J. Am. Chem. Soc. 1999, 121, 1208–1216. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Khan, A.L.; Rehman, N.U.; Csuk, R. Biosynthetic Diversity in Triterpene Cyclization within the Boswellia Genus. Phytochemistry 2021, 184, 1–23. [Google Scholar] [CrossRef]
- Shang, Y.; Huang, S. Multi-Omics Data-Driven Investigations of Metabolic Diversity of Plant Triterpenoids. Plant J. 2019, 97, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Bao, J.; Guo, J.; Ding, Q.; Lu, J.; Huang, M.; Wang, Y. Biological Activities and Potential Molecular Targets of Cucurbitacins: A Focus on Cancer. Anti-Cancer Drugs 2012, 23, 777–787. [Google Scholar] [CrossRef]
- Xue, Z.; Tan, Z.; Huang, A.; Zhou, Y.; Sun, J.; Wang, X.; Thimmappa, R.B.; Stephenson, M.J.; Osbourn, A.; Qi, X. Identification of Key Amino Acid Residues Determining Product Specificity of 2,3-Oxidosqualene Cyclase in Oryza Species. New Phytol. 2018, 218, 1076–1088. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, J.J.; Kjer, K.M.; Duckett, C.N.; Tallamy, D.W. Convergent Evolution of Cucurbitacin Feeding in Spatially Isolated Rootworm Taxa (Coleoptera Chrysomelidae; Galerucinae, Luperini). Mol. Phylogenet. Evol. 2003, 29, 161–175. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Hisatsune, A.; Katsuki, H.; Miyata, T.; Yokomizo, K.; Isohama, Y. Glycyrrhizin Attenuates Mucus Production by Inhibition of MUC5AC mRNA Expression in Vivo and in Vitro. J. Pharmacol. Sci. 2010, 113, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Z.-q.; Song, J.; Liu, Q.-m.; Wang, C.; Huang, Z.; Chu, L.; Liang, H.-f.; Zhang, B.-x.; Chen, X.-p. 18 Beta-Glycyrrhetinic-Acid-Mediated Unfolded Protein Response Induces Autophagy and Apoptosis in Hepatocellular Carcinoma. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Ye, H.; Li, C.; Wang, H.; Liu, B.; Zhang, Y. Molecular Characterization of the Pentacyclic Triterpenoid Biosynthetic Pathway in Catharanthus Roseus. Planta 2012, 236, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Han, Y.; Zhang, J. Cloning of 2,3-Oxidosqualene Cyclase Gene from Panax quinquefolius and Its Distribution Pattern. Lishizhen Med. Mater. Med. Res. 2013, 24, 1052–1055. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. Gsds 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. Meme: Discovering and Analyzing DNA and Protein Sequence Motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Y.; Luo, S.; Wang, L.; Huang, R.; Wen, Q.; Han, X.; Miao, N.; Cheng, J.; Liu, Z.; et al. Whole-Genome Sequencing Provides Insights into the Genetic Diversity and Domestication of Bitter Gourd Momordica Spp. Hortic. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Lodeiro, S.; Schulz-Gasch, T.; Matsuda, S.P.T. Enzyme Redesign: Two Mutations Cooperate to Convert Cycloartenol Synthase into an Accurate Lanosterol Synthase. J. Am. Chem. Soc. 2005, 127, 14132–14133. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, A.; Soler, M.; Dupas, A.; Ladouce, N.; Silva-Martins, G.; Martinez, Y.; Lapierre, C.; Franche, C.; Truchet, I.; Grima-Pettenati, J. Eucalyptus Hairy Roots, a Fast, Efficient and Versatile Tool to Explore Function and Expression of Genes Involved in Wood Formation. Plant Biotechnol. J. 2016, 14, 1381–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, W.; Li, D.; Li, X.; Gao, Y.; Li, W.; Li, H.; Liu, J.; Liu, H.; Chen, W.; Luo, J.; et al. Metabolome-Based Genome-Wide Association Study of Maize Kernel Leads to Novel Biochemical Insights. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.A.; Hou, X.; Han, K.; Khan, N.; Dong, H.; Saqib, M.; Zhang, Z.; Naseri, E.; Hu, C. Lipidomic Study Reveals the Effect of Morphological Variation and Other Metabolite Interactions on the Lipid Composition in Various Cultivars of Bok Choy. Biochem. Biophys. Res. Commun. 2018, 506, 755–764. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography-Mass Spectrometry, Xcms, and Chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Yang, Y.; Li, Y.; Yin, X.; Chen, Z.; Yang, D.; Yang, Y.; Yang, Y.; Yang, X. Genome-Wide Identification of OSC Gene Family and Potential Function in the Synthesis of Ursane- and Oleanane-Type Triterpene in Momordica charantia. Int. J. Mol. Sci. 2022, 23, 196. https://doi.org/10.3390/ijms23010196
Han Y, Yang Y, Li Y, Yin X, Chen Z, Yang D, Yang Y, Yang Y, Yang X. Genome-Wide Identification of OSC Gene Family and Potential Function in the Synthesis of Ursane- and Oleanane-Type Triterpene in Momordica charantia. International Journal of Molecular Sciences. 2022; 23(1):196. https://doi.org/10.3390/ijms23010196
Chicago/Turabian StyleHan, Yutong, Ya Yang, Yan Li, Xin Yin, Zhiyu Chen, Danni Yang, Yongping Yang, Yunqiang Yang, and Xuefei Yang. 2022. "Genome-Wide Identification of OSC Gene Family and Potential Function in the Synthesis of Ursane- and Oleanane-Type Triterpene in Momordica charantia" International Journal of Molecular Sciences 23, no. 1: 196. https://doi.org/10.3390/ijms23010196
APA StyleHan, Y., Yang, Y., Li, Y., Yin, X., Chen, Z., Yang, D., Yang, Y., Yang, Y., & Yang, X. (2022). Genome-Wide Identification of OSC Gene Family and Potential Function in the Synthesis of Ursane- and Oleanane-Type Triterpene in Momordica charantia. International Journal of Molecular Sciences, 23(1), 196. https://doi.org/10.3390/ijms23010196





