Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis
Abstract
1. Introduction
2. Role of Inflammation in the Pathophysiology of Sepsis
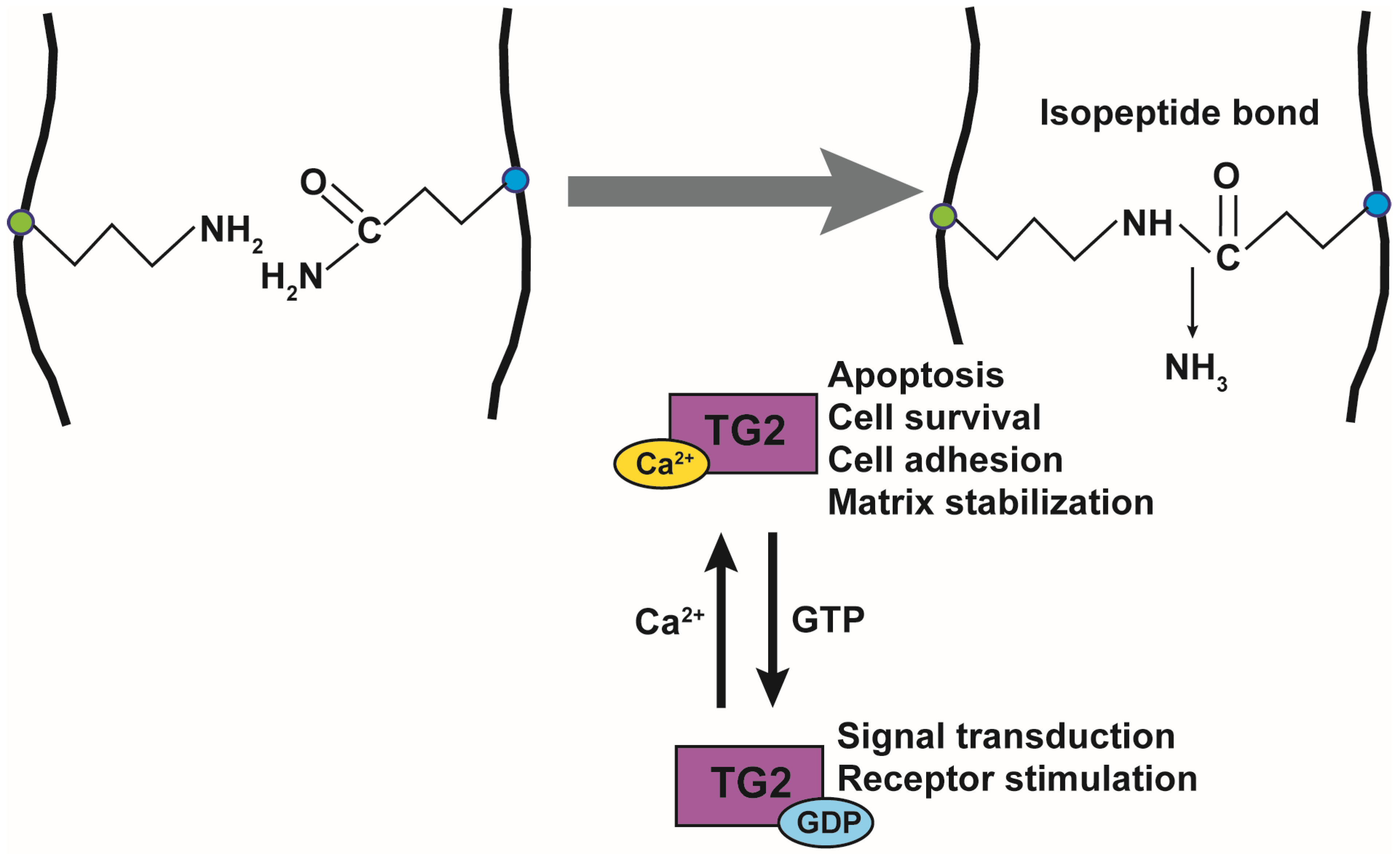
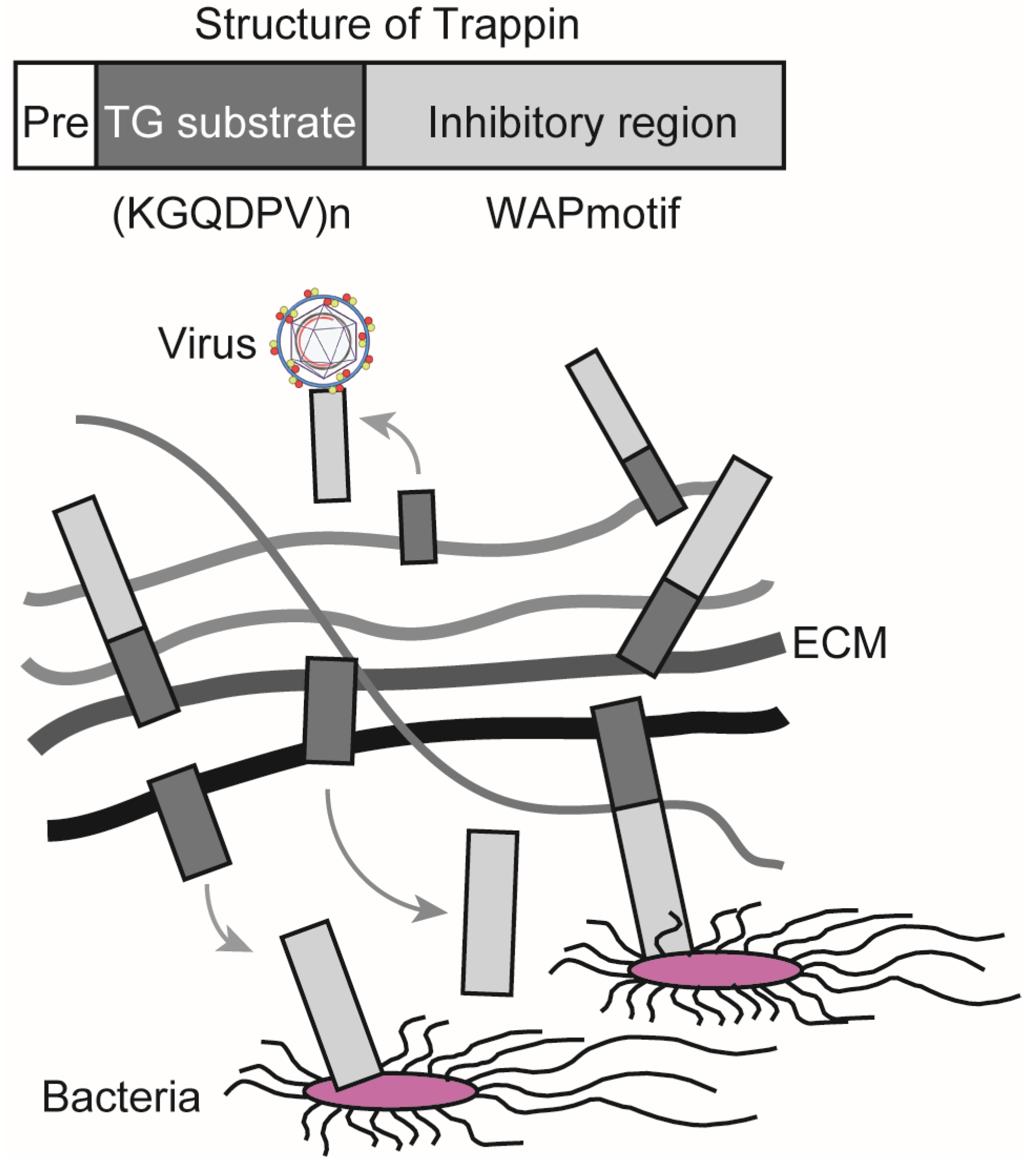
3. Physiological and Pathological Role of TG in Inflammation
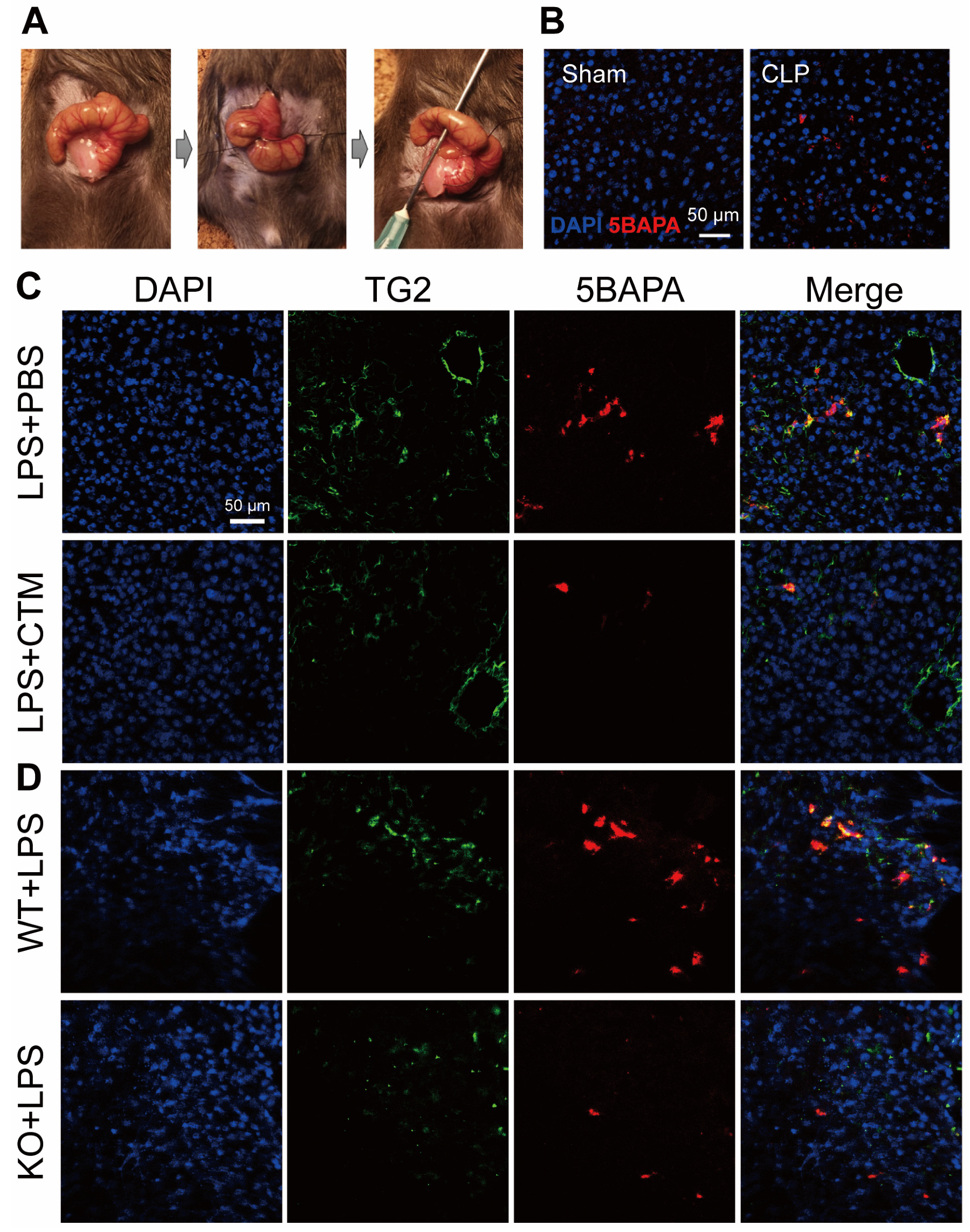
4. Targeting TG2 in Inflammation and Sepsis: Evidence from Knockout Mouse Models
5. Targeting TG2 in Inflammation and Sepsis: Evidence from Pharmacological Inhibition
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Machado, F.R. The Global Epidemiology of Sepsis. Does It Matter That We Know So Little? Am. J. Respir. Crit. Care Med. 2016, 193, 228–230. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Measuring the global burden of disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.R.; Payen, D. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.R.; Timsit, J.F.; Garrouste-Orgeas, M.; Français, A.; Vesin, A.; Descorps-Declere, A.; Dubois, Y.; Souweine, B.; Haouache, H.; Goldgran-Toledano, D.; et al. Outcomes in severe sepsis and patients with septic shock: Pathogen species and infection sites are not associated with mortality. Crit. Care Med. 2011, 39, 1886–1895. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Delano, M.J.; Ward, P.A. Sepsis-induced immune dysfunction: Can immune therapies reduce mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Mebazaa, A.; Laterre, P.F.; Russell, J.A.; Bergmann, A.; Gattinoni, L.; Gayat, E.; Harhay, M.O.; Hartmann, O.; Hein, F.; Kjolbye, A.L.; et al. Designing phase 3 sepsis trials: Application of learned experiences from critical care trials in acute heart failure. J. Intensive Care 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Peters van Ton, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Odii, B.O.; Coussons, P. Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family. Sci. World J. 2014, 2014, 714561. [Google Scholar] [CrossRef] [PubMed]
- Chrobok, N.L.; Sestito, C.; Wilhelmus, M.M.; Drukarch, B.; van Dam, A.M. Is monocyte- and macrophage-derived tissue transglutaminase involved in inflammatory processes? Amino Acids 2017, 49, 441–452. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef]
- Falasca, L.; Farrace, M.G.; Rinaldi, A.; Tuosto, L.; Melino, G.; Piacentini, M. Transglutaminase type II is involved in the pathogenesis of endotoxic shock. J. Immunol. 2008, 180, 2616–2624. [Google Scholar] [CrossRef]
- Jeong, E.M.; Son, Y.H.; Choi, Y.; Kim, J.H.; Lee, J.H.; Cho, S.Y.; Kim, I.G. Transglutaminase 2 is dispensable but required for the survival of mice in dextran sulfate sodium-induced colitis. Exp. Mol. Med. 2016, 48, e267. [Google Scholar] [CrossRef]
- Matic, I.; Sacchi, A.; Rinaldi, A.; Melino, G.; Khosla, C.; Falasca, L.; Piacentini, M. Characterization of transglutaminase type II role in dendritic cell differentiation and function. J. Leukoc. Biol. 2010, 88, 181–188. [Google Scholar] [CrossRef]
- Su, T.; Qin, X.Y.; Furutani, Y.; Yu, W.; Kojima, S. Imaging of the ex vivo transglutaminase activity in liver macrophages of sepsis mice. Anal. Biochem. 2020, 597, 113654. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Korponay-Szabó, I.; Király, R.; Sarang, Z.; Tsay, G.J. Transglutaminase 2 in human diseases. BioMedicine 2017, 7, 15. [Google Scholar] [CrossRef]
- Deutschman, C.S.; Tracey, K.J. Sepsis: Current dogma and new perspectives. Immunity 2014, 40, 463–475. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Opal, S.M.; van der Poll, T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, N.C.; Guo, R.F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar] [CrossRef]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Hu, D.; Ren, J.; Wang, G.; Gu, G.; Chen, J.; Zhou, B.; Liu, S.; Wu, X.; Li, J. Persistent inflammation-immunosuppression catabolism syndrome, a common manifestation of patients with enterocutaneous fistula in intensive care unit. J. Trauma Acute Care Surg. 2014, 76, 725–729. [Google Scholar] [CrossRef]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Rahman, O. Post Intensive Care Syndrome. In StatPearls; Copyright© 2021; StatPearls Publishing, LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Payen, D.; Faivre, V.; Miatello, J.; Leentjens, J.; Brumpt, C.; Tissières, P.; Dupuis, C.; Pickkers, P.; Lukaszewicz, A.C. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 2019, 19, 931. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ner-Gaon, H.; Varon, J.; Cullen, A.M.; Guo, J.; Choi, J.; Barragan-Bradford, D.; Higuera, A.; Pinilla-Vera, M.; Short, S.A.; et al. Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-γ in NK cells. J. Clin. Investig. 2020, 130, 3238–3252. [Google Scholar] [CrossRef] [PubMed]
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Yamashita, F.; Ishida-Yamamoto, A.; Yamada, K.; Kinoshita, C.; Fushiki, S.; Ueda, E.; Morishima, Y.; Tabata, K.; Yasuno, H.; et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proc. Natl. Acad. Sci. USA 1998, 95, 1044–1049. [Google Scholar] [CrossRef]
- Kuramoto, N.; Takizawa, T.; Takizawa, T.; Matsuki, M.; Morioka, H.; Robinson, J.M.; Yamanishi, K. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J. Clin. Investig. 2002, 109, 243–250. [Google Scholar] [CrossRef]
- Piro, M.C.; Ventura, A.; Smirnov, A.; Saggini, A.; Lena, A.M.; Mauriello, A.; Bianchi, L.; Melino, G.; Candi, E. Transglutaminase 3 Reduces the Severity of Psoriasis in Imiquimod-Treated Mouse Skin. Int. J. Mol. Sci. 2020, 21, 1566. [Google Scholar] [CrossRef]
- Candi, E.; Oddi, S.; Paradisi, A.; Terrinoni, A.; Ranalli, M.; Teofoli, P.; Citro, G.; Scarpato, S.; Puddu, P.; Melino, G. Expression of transglutaminase 5 in normal and pathologic human epidermis. J. Investig. Dermatol. 2002, 119, 670–677. [Google Scholar] [CrossRef]
- Furutani, Y.; Kato, A.; Fibriani, A.; Hirata, T.; Kawai, R.; Jeon, J.H.; Fujii, Y.; Kim, I.G.; Kojima, S.; Hirose, S. Identification, evolution, and regulation of expression of Guinea pig trappin with an unusually long transglutaminase substrate domain. J. Biol. Chem. 2005, 280, 20204–20215. [Google Scholar] [CrossRef]
- Mehta, K.; Eckert, R. Transglutaminases: Family of Enzymes with Diverse Functions; Karger: Basel, Switzerland, 2005; Volume 38, p. 253. [Google Scholar]
- Hitomi, K.; Kojima, S.; Fesus, L. Transglutaminases, Multiple Functional Modifiers and Targets for New Drug Discovery, 1st ed.; Springer: Tokyo, Japan, 2015; p. VIII, 391. [Google Scholar]
- Tatsukawa, H.; Furutani, Y.; Hitomi, K.; Kojima, S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016, 7, e2244. [Google Scholar] [CrossRef]
- Bellemare, A.; Vernoux, N.; Morisset, D.; Bourbonnais, Y. Human pre-elafin inhibits a Pseudomonas aeruginosa-secreted peptidase and prevents its proliferation in complex media. Antimicrob. Agents Chemother. 2008, 52, 483–490. [Google Scholar] [CrossRef]
- Drannik, A.G.; Nag, K.; Sallenave, J.M.; Rosenthal, K.L. Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes. J. Virol. 2013, 87, 7526–7538. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.; Ball, T.B.; Levinson, P.; Maranan, L.; Jaoko, W.; Wachihi, C.; Pak, B.J.; Podust, V.N.; Broliden, K.; Hirbod, T.; et al. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS 2009, 23, 1669–1677. [Google Scholar] [CrossRef]
- Drannik, A.G.; Nag, K.; Yao, X.D.; Henrick, B.M.; Jain, S.; Ball, T.B.; Plummer, F.A.; Wachihi, C.; Kimani, J.; Rosenthal, K.L. Anti-HIV-1 activity of elafin is more potent than its precursor’s, trappin-2, in genital epithelial cells. J. Virol. 2012, 86, 4599–4610. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Peyrotte, E.A.; Meyers, A.F.; Gajanayaka, N.; Ball, T.B.; Sandstrom, P.; Lavigne, C. Human rElafin Inhibits HIV-1 Replication in Its Natural Target Cells. Biores. Open Access 2013, 2, 128–137. [Google Scholar] [CrossRef]
- Furutani, Y.; Kato, A.; Yasue, H.; Alexander, L.J.; Beattie, C.W.; Hirose, S. Evolution of the trappin multigene family in the Suidae. J. Biochem. 1998, 124, 491–502. [Google Scholar] [CrossRef]
- Kato, A.; Rooney, A.P.; Furutani, Y.; Hirose, S. Evolution of trappin genes in mammals. BMC Evolut. Biol. 2010, 10, 31. [Google Scholar] [CrossRef]
- Aizarani, N.; Saviano, A.; Sagar; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef]
- Piacentini, M.; Baiocchini, A.; Del Nonno, F.; Melino, G.; Barlev, N.A.; Rossin, F.; D’Eletto, M.; Falasca, L. Non-alcoholic fatty liver disease severity is modulated by transglutaminase type 2. Cell Death Dis. 2018, 9, 257. [Google Scholar] [CrossRef]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Kuncio, G.S.; Tsyganskaya, M.; Zhu, J.; Liu, S.L.; Nagy, L.; Thomazy, V.; Davies, P.J.; Zern, M.A. TNF-alpha modulates expression of the tissue transglutaminase gene in liver cells. Am. J. Physiol. 1998, 274, G240–G245. [Google Scholar]
- Mirza, A.; Liu, S.L.; Frizell, E.; Zhu, J.; Maddukuri, S.; Martinez, J.; Davies, P.; Schwarting, R.; Norton, P.; Zern, M.A. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am. J. Physiol. 1997, 272, G281–G288. [Google Scholar] [CrossRef]
- Bijli, K.M.; Kanter, B.G.; Minhajuddin, M.; Leonard, A.; Xu, L.; Fazal, F.; Rahman, A. Regulation of endothelial cell inflammation and lung polymorphonuclear lymphocyte infiltration by transglutaminase 2. Shock 2014, 42, 562–569. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.S.; Choi, D.H.; Bang, M.S.; Han, T.R.; Joh, T.H.; Kim, S.Y. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J. Biol. Chem. 2004, 279, 53725–53735. [Google Scholar] [CrossRef]
- Lombardo, E.; Alvarez-Barrientos, A.; Maroto, B.; Boscá, L.; Knaus, U.G. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J. Immunol. 2007, 178, 3731–3739. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Mahidhara, R.; Billiar, T.R. Apoptosis in sepsis. Crit. Care Med. 2000, 28 (Suppl. S4), N105–N113. [Google Scholar] [CrossRef] [PubMed]
- Amendola, A.; Rodolfo, C.; Di Caro, A.; Ciccosanti, F.; Falasca, L.; Piacentini, M. “Tissue” transglutaminase expression in HIV-infected cells: An enzyme with an antiviral effect? Ann. N. Y. Acad. Sci. 2001, 946, 108–120. [Google Scholar] [CrossRef]
- Amendola, A.; Gougeon, M.L.; Poccia, F.; Bondurand, A.; Fesus, L.; Piacentini, M. Induction of “tissue” transglutaminase in HIV pathogenesis: Evidence for high rate of apoptosis of CD4+ T lymphocytes and accessory cells in lymphoid tissues. Proc. Natl. Acad. Sci. USA 1996, 93, 11057–11062. [Google Scholar] [CrossRef]
- Shrestha, R.; Tatsukawa, H.; Shrestha, R.; Ishibashi, N.; Matsuura, T.; Kagechika, H.; Kose, S.; Hitomi, K.; Imamoto, N.; Kojima, S. Molecular mechanism by which acyclic retinoid induces nuclear localization of transglutaminase 2 in human hepatocellular carcinoma cells. Cell Death Dis. 2015, 6, e2002. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Fukaya, Y.; Frampton, G.; Martinez-Fuentes, A.; Suzuki, K.; Kuo, T.F.; Nagatsuma, K.; Shimokado, K.; Okuno, M.; Wu, J.; et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 2009, 136, 1783–1795.e10. [Google Scholar] [CrossRef]
- Shrestha, R.; Shrestha, R.; Qin, X.Y.; Kuo, T.F.; Oshima, Y.; Iwatani, S.; Teraoka, R.; Fujii, K.; Hara, M.; Li, M.; et al. Fungus-derived hydroxyl radicals kill hepatic cells by enhancing nuclear transglutaminase. Sci. Rep. 2017, 7, 4746. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Fujii, S.; Shimizu, A.; Kagechika, H.; Kojima, S. Carboxylic Derivatives of Vitamin K2 Inhibit Hepatocellular Carcinoma Cell Growth through Caspase/Transglutaminase-Related Signaling Pathways. J. Nutr. Sci. Vitaminol. 2015, 61, 285–290. [Google Scholar] [CrossRef]
- Qin, X.Y.; Lu, J.; Cai, M.; Kojima, S. Arachidonic acid suppresses hepatic cell growth through ROS-mediated activation of transglutaminase. FEBS Open Bio 2018, 8, 1703–1710. [Google Scholar] [CrossRef]
- Qin, X.Y.; Su, T.; Kojima, S. Prevention of arachidonic acid-induced liver injury by controlling oxidative stress-mediated transglutaminase activation with garlic extracts. Exp. Ther. Med. 2020, 19, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Sarang, Z.; Molnár, P.; Németh, T.; Gomba, S.; Kardon, T.; Melino, G.; Cotecchia, S.; Fésüs, L.; Szondy, Z. Tissue transglutaminase (TG2) acting as G protein protects hepatocytes against Fas-mediated cell death in mice. Hepatology 2005, 42, 578–587. [Google Scholar] [CrossRef]
- Yoo, H.; Ahn, E.R.; Kim, S.J.; Lee, S.H.; Oh, S.H.; Kim, S.Y. Divergent results induced by different types of septic shock in transglutaminase 2 knockout mice. Amino Acids 2013, 44, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Aleanzi, M.; Demonte, A.M.; Esper, C.; Garcilazo, S.; Waggener, M. Celiac disease: Antibody recognition against native and selectively deamidated gliadin peptides. Clin. Chem. 2001, 47, 2023–2028. [Google Scholar] [CrossRef]
- Dieterich, W.; Laag, E.; Schöpper, H.; Volta, U.; Ferguson, A.; Gillett, H.; Riecken, E.O.; Schuppan, D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology 1998, 115, 1317–1321. [Google Scholar] [CrossRef]
- Fleckenstein, B.; Molberg, Ø.; Qiao, S.W.; Schmid, D.G.; von der Mülbe, F.; Elgstøen, K.; Jung, G.; Sollid, L.M. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J. Biol. Chem. 2002, 277, 34109–34116. [Google Scholar] [CrossRef]
- Doig, C.J.; Sutherland, L.R.; Sandham, J.D.; Fick, G.H.; Verhoef, M.; Meddings, J.B. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am. J. Respir. Crit. Care Med. 1998, 158, 444–451. [Google Scholar] [CrossRef]
- Forsberg, G.; Fahlgren, A.; Hörstedt, P.; Hammarström, S.; Hernell, O.; Hammarström, M.L. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am. J. Gastroenterol. 2004, 99, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Olén, O.; Bell, M.; Ekbom, A.; Montgomery, S.M. Coeliac disease and risk of sepsis. Gut 2008, 57, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E. Insights into Transglutaminase 2 Function Gained from Genetically Modified Animal Models; Transglutaminases; Hitomi, K., Kojima, S., Fesus, L., Eds.; Springer: Tokyo, Japan, 2015; pp. 83–115. [Google Scholar]
- De Laurenzi, V.; Melino, G. Gene disruption of tissue transglutaminase. Mol. Cell Biol. 2001, 21, 148–155. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, B.; Tahk, H.; Kim, D.H.; Ahn, E.R.; Choi, C.; Jeon, Y.; Park, S.Y.; Lee, H.; Oh, S.H.; et al. Transglutaminase 2 gene ablation protects against renal ischemic injury by blocking constant NF-κB activation. Biochem. Biophys. Res. Commun. 2010, 403, 479–484. [Google Scholar] [CrossRef]
- Nanda, N.; Iismaa, S.E.; Owens, W.A.; Husain, A.; Mackay, F.; Graham, R.M. Targeted inactivation of Gh/tissue transglutaminase II. J. Biol. Chem. 2001, 276, 20673–20678. [Google Scholar] [CrossRef]
- Iismaa, S.E.; Mearns, B.M.; Lorand, L.; Graham, R.M. Transglutaminases and disease: Lessons from genetically engineered mouse models and inherited disorders. Physiol. Rev. 2009, 89, 991–1023. [Google Scholar] [CrossRef]
- Deasey, S.; Shanmugasundaram, S.; Nurminskaya, M. Tissue-specific responses to loss of transglutaminase 2. Amino Acids 2013, 44, 179–187. [Google Scholar] [CrossRef]
- Johnson, K.B.; Petersen-Jones, H.; Thompson, J.M.; Hitomi, K.; Itoh, M.; Bakker, E.N.; Johnson, G.V.; Colak, G.; Watts, S.W. Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1355–H1366. [Google Scholar] [CrossRef]
- Oh, K.; Park, H.B.; Byoun, O.J.; Shin, D.M.; Jeong, E.M.; Kim, Y.W.; Kim, Y.S.; Melino, G.; Kim, I.G.; Lee, D.S. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J. Exp. Med. 2011, 208, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Park, H.B.; Seo, M.W.; Byoun, O.J.; Lee, D.S. Transglutaminase 2 exacerbates experimental autoimmune encephalomyelitis through positive regulation of encephalitogenic T cell differentiation and inflammation. Clin. Immunol. 2012, 145, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.U.; Cho, J.W.; Kim, S.Y.; Shin, J.H.; Ro, J.Y. Inflammatory mediators resulting from transglutaminase 2 expressed in mast cells contribute to the development of Parkinson’s disease in a mouse model. Toxicol. Appl. Pharmacol. 2018, 358, 10–22. [Google Scholar] [CrossRef]
- Van Strien, M.E.; de Vries, H.E.; Chrobok, N.L.; Bol, J.; Breve, J.J.P.; van der Pol, S.M.P.; Kooij, G.; van Buul, J.D.; Karpuj, M.; Steinman, L.; et al. Tissue Transglutaminase contributes to experimental multiple sclerosis pathogenesis and clinical outcome by promoting macrophage migration. Brain Behav. Immun. 2015, 50, 141–154. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, K.B.; Son, Y.H.; Shin, J.; Lee, J.H.; Kim, H.J.; Hong, A.Y.; Bae, H.W.; Kwon, M.A.; Lee, W.J.; et al. Transglutaminase 2 mediates UV-induced skin inflammation by enhancing inflammatory cytokine production. Cell Death Dis. 2017, 8, e3148. [Google Scholar] [CrossRef]
- Yen, J.H.; Lin, L.C.; Chen, M.C.; Sarang, Z.; Leong, P.Y.; Chang, I.C.; Hsu, J.D.; Chen, J.H.; Hsieh, Y.F.; Pallai, A.; et al. The metastatic tumor antigen 1-transglutaminase-2 pathway is involved in self-limitation of monosodium urate crystal-induced inflammation by upregulating TGF-β1. Arthritis Res. Ther. 2015, 17, 65. [Google Scholar] [CrossRef]
- Cassiman, D.; Libbrecht, L.; Desmet, V.; Denef, C.; Roskams, T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol. 2002, 36, 200–209. [Google Scholar] [CrossRef]
- Sághy, T.; Köröskényi, K.; Hegedűs, K.; Antal, M.; Bankó, C.; Bacsó, Z.; Papp, A.; Stienstra, R.; Szondy, Z. Loss of transglutaminase 2 sensitizes for diet-induced obesity-related inflammation and insulin resistance due to enhanced macrophage c-Src signaling. Cell Death Dis. 2019, 10, 439. [Google Scholar] [CrossRef]
- Soveg, F.; Abdala-Valencia, H.; Campbell, J.; Morales-Nebreda, L.; Mutlu, G.M.; Cook-Mills, J.M. Regulation of allergic lung inflammation by endothelial cell transglutaminase 2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L573–L583. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Delikatny, E.J.; Ahlqvist, J.; Capper, H.; Cooper, A.J. Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem. Pharmacol. 2005, 69, 961–970. [Google Scholar] [CrossRef]
- Palanski, B.A.; Khosla, C. Cystamine and Disulfiram Inhibit Human Transglutaminase 2 via an Oxidative Mechanism. Biochemistry 2018, 57, 3359–3363. [Google Scholar] [CrossRef]
- Oono, M.; Okado-Matsumoto, A.; Shodai, A.; Ido, A.; Ohta, Y.; Abe, K.; Ayaki, T.; Ito, H.; Takahashi, R.; Taniguchi, N.; et al. Transglutaminase 2 accelerates neuroinflammation in amyotrophic lateral sclerosis through interaction with misfolded superoxide dismutase 1. J. Neurochem. 2014, 128, 403–418. [Google Scholar] [CrossRef]
- Elli, L.; Ciulla, M.M.; Busca, G.; Roncoroni, L.; Maioli, C.; Ferrero, S.; Bardella, M.T.; Bonura, A.; Paliotti, R.; Terrani, C.; et al. Beneficial effects of treatment with transglutaminase inhibitor cystamine on the severity of inflammation in a rat model of inflammatory bowel disease. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 452–461. [Google Scholar] [CrossRef]
- Luciani, A.; Villella, V.R.; Esposito, S.; Brunetti-Pierri, N.; Medina, D.; Settembre, C.; Gavina, M.; Pulze, L.; Giardino, I.; Pettoello-Mantovani, M.; et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010, 12, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ji, X.; Tang, J.; Lin, G.; Xiao, L.; Liang, C.; Wang, M.; Su, F.; Ferrandon, D.; Li, Z. Positive Feedback Regulation between Transglutaminase 2 and Toll-Like Receptor 4 Signaling in Hepatic Stellate Cells Correlates with Liver Fibrosis Post Schistosoma japonicum Infection. Front. Immunol. 2017, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Khosla, C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol. Ther. 2007, 115, 232–245. [Google Scholar] [CrossRef]
- Lesort, M.; Lee, M.; Tucholski, J.; Johnson, G.V. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J. Biol. Chem. 2003, 278, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Keillor, J.W.; Apperley, K.Y.; Akbar, A. Inhibitors of tissue transglutaminase. Trends Pharmacol. Sci. 2015, 36, 32–40. [Google Scholar] [CrossRef]
- Akbar, A.; McNeil, N.M.R.; Albert, M.R.; Ta, V.; Adhikary, G.; Bourgeois, K.; Eckert, R.L.; Keillor, J.W. Structure-Activity Relationships of Potent, Targeted Covalent Inhibitors That Abolish Both the Transamidation and GTP Binding Activities of Human Tissue Transglutaminase. J. Med. Chem. 2017, 60, 7910–7927. [Google Scholar] [CrossRef]
- Kerr, C.; Szmacinski, H.; Fisher, M.L.; Nance, B.; Lakowicz, J.R.; Akbar, A.; Keillor, J.W.; Lok Wong, T.; Godoy-Ruiz, R.; Toth, E.A.; et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene 2017, 36, 2981–2990. [Google Scholar] [CrossRef]
- Jambrovics, K.; Uray, I.P.; Keillor, J.W.; Fésüs, L.; Balajthy, Z. Benefits of Combined All-Trans Retinoic Acid and Arsenic Trioxide Treatment of Acute Promyelocytic Leukemia Cells and Further Enhancement by Inhibition of Atypically Expressed Transglutaminase 2. Cancers 2020, 12, 648. [Google Scholar] [CrossRef]
| Experimental Evidence | Mechanistic Insight | Role of TG2 | Reference | |
|---|---|---|---|---|
| TG2 KO mice | ||||
| TG2 KO protects from LPS-induced septic shock and mortality | TG2 promotes NF-κΒ activation and DC differentiation | ⇧ Promoting | [19] [21] | |
| TG2 KO reduces LPS-induced EC inflammation and lung PMN infiltration | TG2 promotes DNA-binding and transcriptional activity of RelA/p65 and NF-κB activation | ⇧ Promoting | [67] | |
| TG2 KO exacerbates TNF-α-dependent septic shock and liver injury | TG2 inhibits TNF-α-induced expression of caspase 3 and cathepsin D | ⇩ Protective | [81] | |
| TG2 KO reduces inflammation and fibrosis after noninfectious pulmonary injury | TG2 induces the secretion of IL-6 in epithelial cells but not inflammatory cells and contributes to the effector phase of fibrogenesis under the control of TGF-β in fibroblasts | ⇧ Promoting | [96] | |
| TG2 KO ameliorates experimental autoimmune encephalomyelitis, which is an autoimmune disease model for multiple sclerosis | TG2 promotes differentiation of CD4(+) T cells into IL-17- or IFN-γ-producing cells andmacrophage migration into the central nervous system associated with the induction of RhoA GTPase activity, and iNOS and TNF-α production | ⇧ Promoting | [97] [99] | |
| TG2 KO decreasesneuroinflammation in MPTP-induced Parkinson’s disease model | TG2 promotesthe release of inflammatory mediators such as histamine, leukotrienes, and cytokines by mast cells in the substantia nigra | ⇧ Promoting | [98] | |
| TG2 KO reduces pro-inflammatory cytokine production in UV-irradiated keratinocytes | UV irradiation stimulates TG2 activity through phospholipase C-dependent endoplasmic reticulum calcium release | ⇧ Promoting | [100] | |
| TG2 KO increases hyper inflammatory responses in a peritonitis model | TG2 inhibits MSU crystal-induced IL-1β and TNF-α production in macrophages through a TGF-β-dependent pathway | ⇩ Protective | [101] | |
| TG2 inhibitors | ||||
| Cystamine | Cystamine inhibits LPS-induced liver injury | TG2 is mainly expressed and activated in midzonal F4/80/CD80+ M1 macrophages in the livers of septic mice | ⇧ Promoting | [22] |
| Cystamine inhibits neuroinflammation in amyotrophic lateral sclerosis | TG2 catalyzes the oligomerization of superoxide dismutase 1 and induces TNF-α, IL-1β, and nitric oxide in microglial cells | ⇧ Promoting | [107] | |
| Cystamine ameliorates TNBS-induced colitis in a rat model of inflammatory bowel disease | TG2 activity is associated with the production of mucosal TNF-α and serological IL-6 | ⇧ Promoting | [108] | |
| Cystamine rescues defective CFTR-induced cystic fibrosis | TG2 catalyzes the crosslinking of beclin 1, leading to the sequestration of phosphatidylinositol-3-kinase complex III, accumulation of p62, and aggresome formation | ⇧ Promoting | [109] | |
| NC9 | NC9 reduces pro-inflammatory cytokine production in combined ATRA and ATO-treated APL cells | TG2 leads to inflammation, which is probably due to reactive oxygen species production | ⇧ Promoting | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.; Qin, X.-Y.; Furutani, Y. Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis. Int. J. Mol. Sci. 2021, 22, 1897. https://doi.org/10.3390/ijms22041897
Su T, Qin X-Y, Furutani Y. Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis. International Journal of Molecular Sciences. 2021; 22(4):1897. https://doi.org/10.3390/ijms22041897
Chicago/Turabian StyleSu, Ting, Xian-Yang Qin, and Yutaka Furutani. 2021. "Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis" International Journal of Molecular Sciences 22, no. 4: 1897. https://doi.org/10.3390/ijms22041897
APA StyleSu, T., Qin, X.-Y., & Furutani, Y. (2021). Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis. International Journal of Molecular Sciences, 22(4), 1897. https://doi.org/10.3390/ijms22041897







