VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms
Abstract
:1. Introduction
2. Results
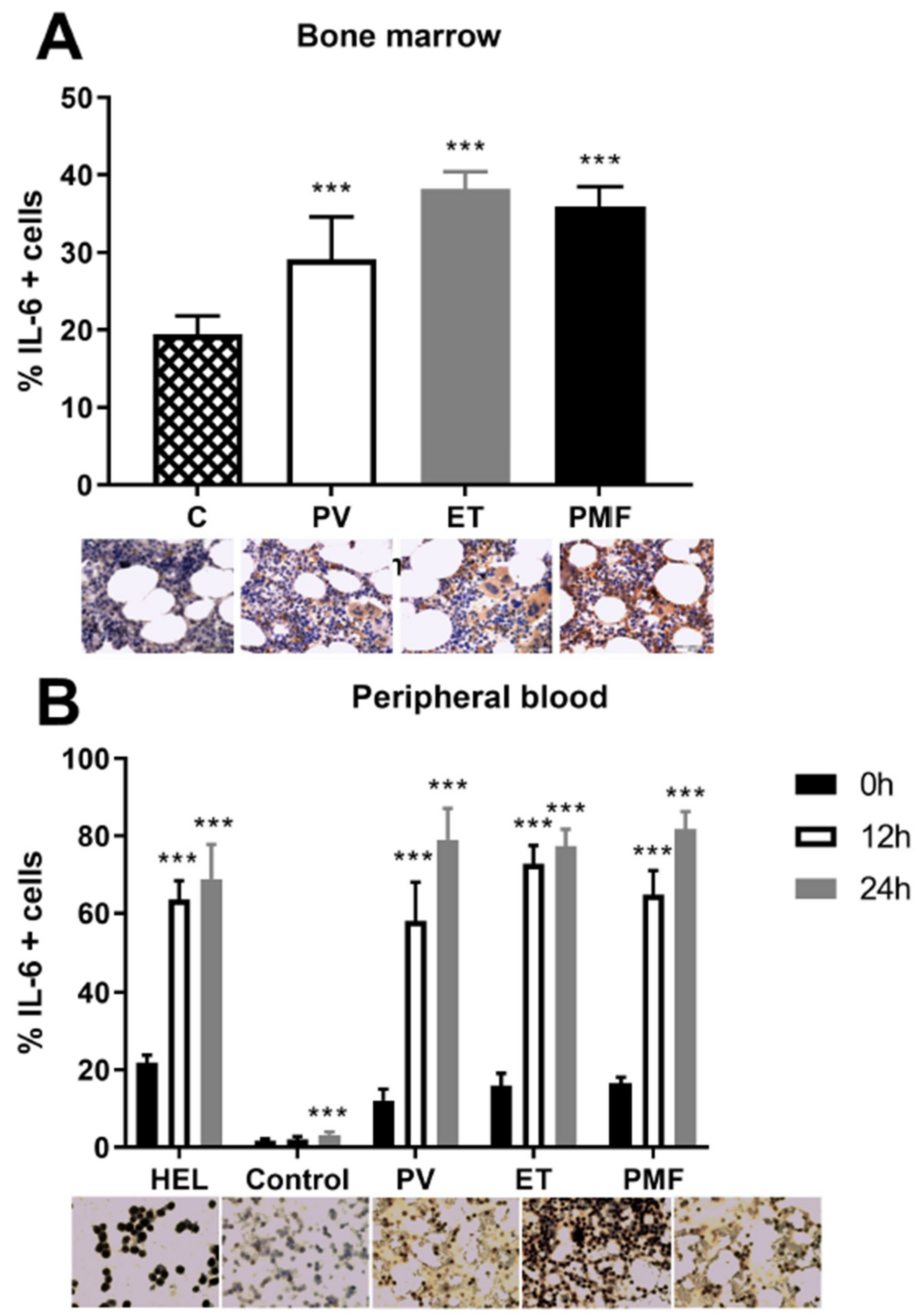
2.1. IL-6 Effect on Angiogenic Factors in Mononuclear Cells of MPN
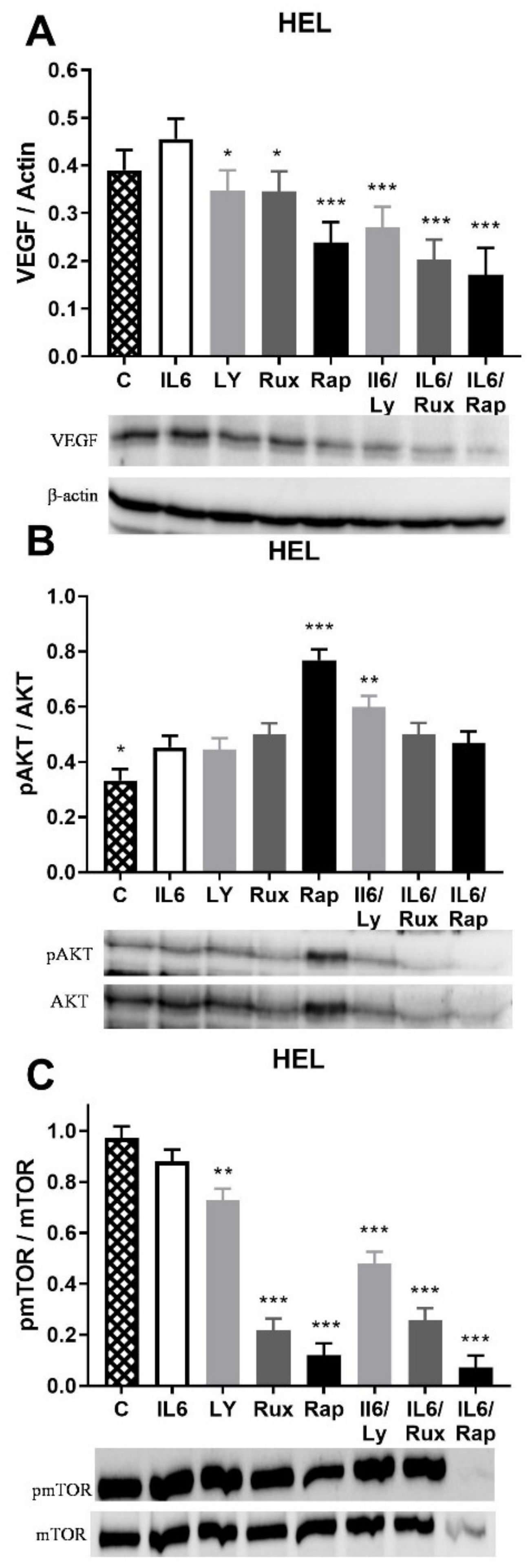
2.2. IL-6 Effect on Angiogenic Factors and Inflammation-Related Signaling Pathways in JAK2V617F Positive HEL Cells
2.3. VEGF Induction of Angiogenic Factors Gene Expression in MNC of MPN
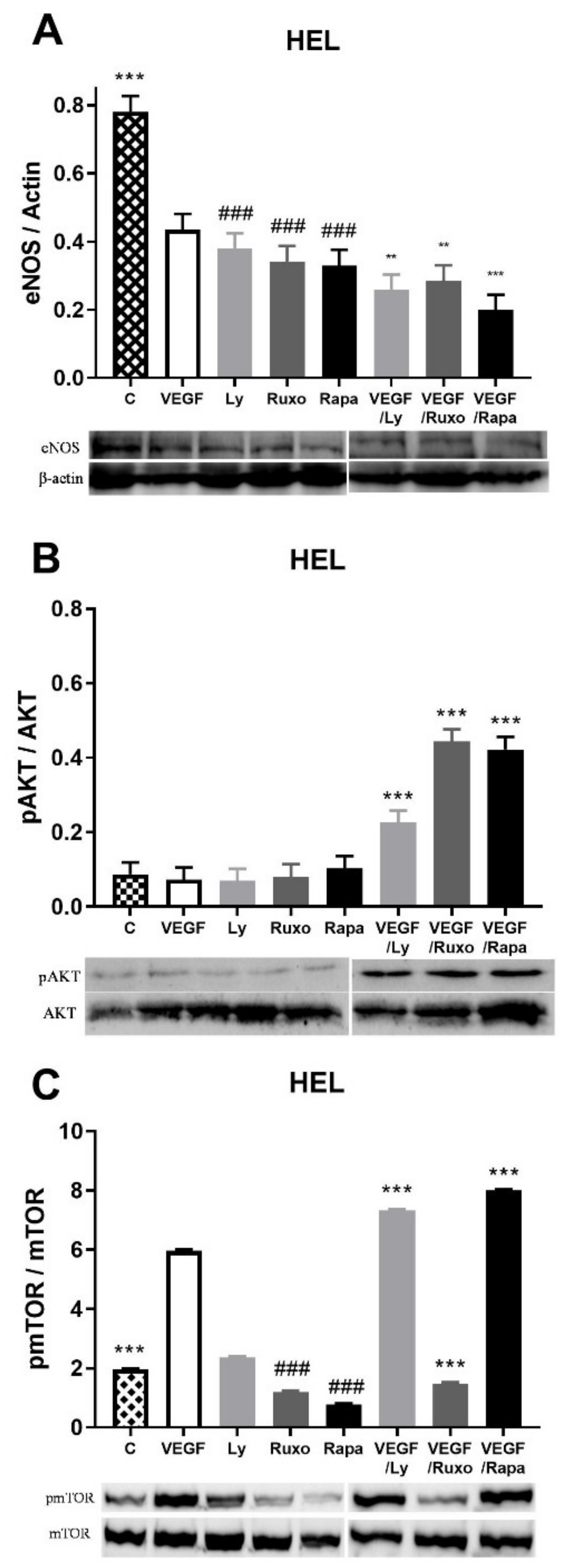
2.4. VEGF Induction of Angiogenic Factors and Inflammation-Related Signaling Pathways in HEL Cells
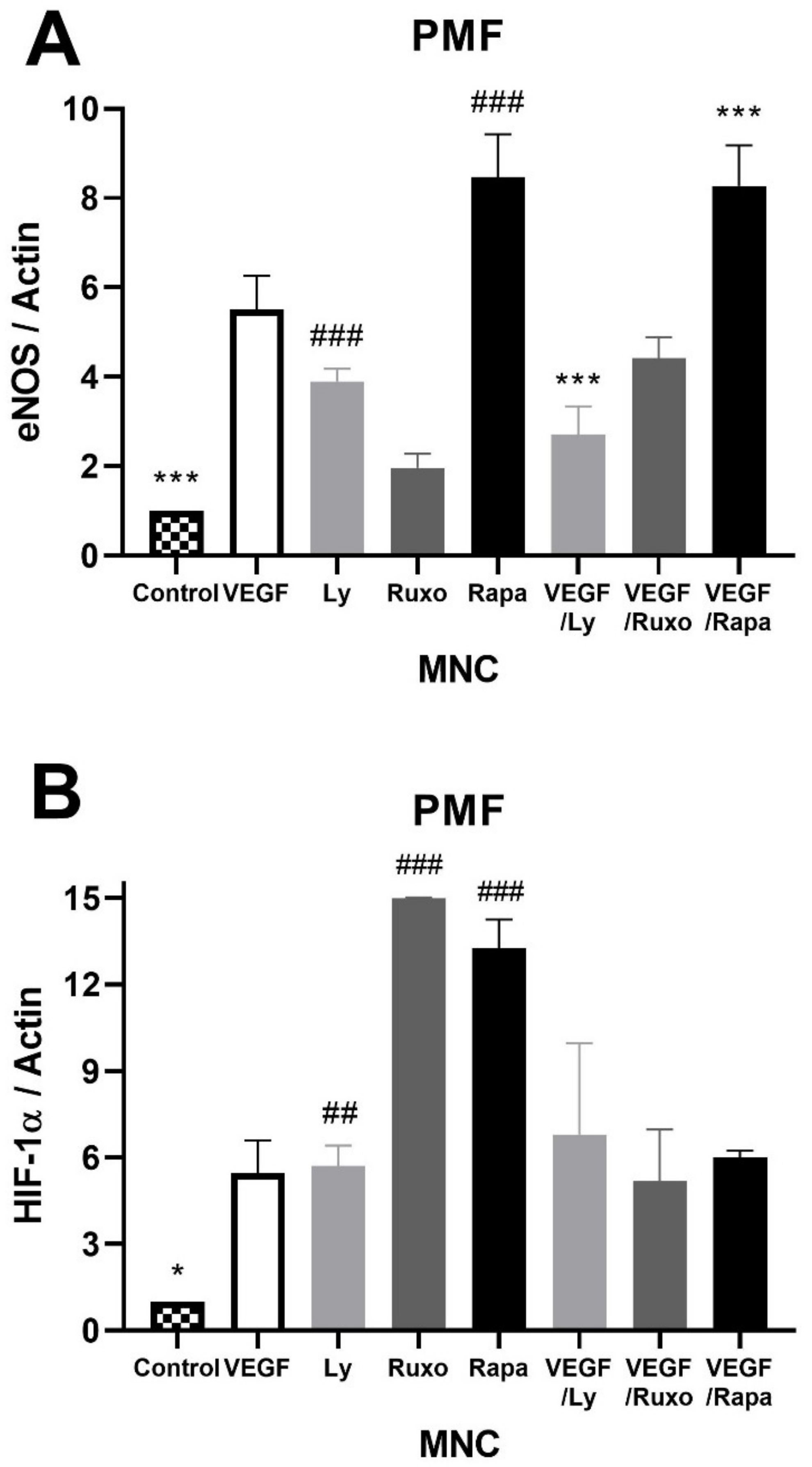
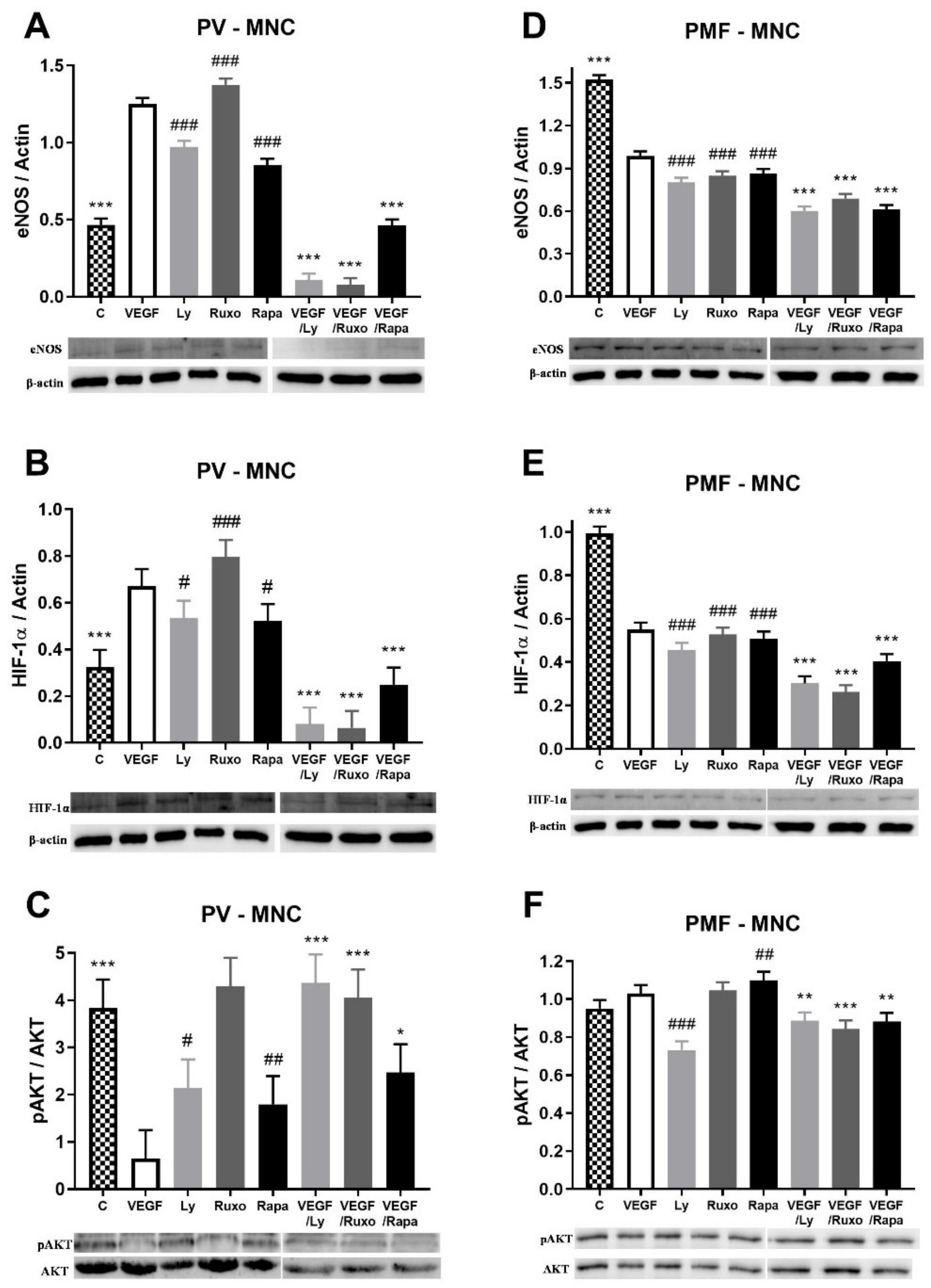
2.5. VEGF Induction of Angiogenic Factors and Inflammation-Related Signaling Pathways in MPN
2.6. VEGF Induction of IL-6 in MPN
3. Discussion
4. Materials and Methods
4.1. HEL 92.1.7 Cell Line
4.2. Patients
4.3. Western Blotting
4.4. Real-Time Quantitative PCR
4.5. Immunocytochemistry/Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medinger, M.; Skoda, R.; Gratwohl, A.; Theocharides, A.; Buser, A.; Heim, D.; Dirnhofer, S.; Tichelli, A.; Tzankov, A. Angiogenesis, and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: Correlation with clinical parameters and JAK2-V617F mutational status. Br. J. Haematol. 2009, 146, 150–157. [Google Scholar] [CrossRef]
- Gadomska, G.; Stankowska, K.; Boinska, J.; Ślusarz, R.; Tylicka, M.; Michalska, M.; Jachalska, A.; Rość, D. VEGF-A, sVEGFR-1, and sVEGFR-2 in BCR-ABL negative myeloproliferative neoplasms. Medicina (Kaunas) 2017, 53, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Fu, J.; Liu, G.; Zhang, L.; Xu, Q.; Wang, S.Y. Angiogenesis in JAK2 V617F positive myeloproliferative neoplasms and ruxolitinib decrease VEGF, HIF-1 enesis in JAK2 V617F positive cells. Leuk. Lymphoma 2018, 59, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, D.; Gotic, M.; Skoda, R.; Beleslin-Cokic, B.; Milic, N.; Mitrovic-Ajtic, O.; Nienhold, R.; Sefer, D.; Suboticki, T.; Buac, M.; et al. Bone marrow microvessel density and plasma angiogenic factors in myeloproliferative neoplasms: Clinicopathological and molecular correlations. Ann. Hematol. 2017, 96, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Subotički, T.; Mitrović Ajtić, O.; Beleslin-Čokić, B.B.; Nienhold, R.; Diklić, M.; Djikić, D.; Lekovic, D.; Bulat, T.; Marković, D.; Gotić, M.; et al. Angiogenic factors are increased in circulating granulocytes and CD34+ cells of myeloproliferative neoplasms. Mol. Carcinog. 2017, 56, 567–579. [Google Scholar] [CrossRef]
- Čokić, V.P.; Mitrović-Ajtić, O.; Beleslin-Čokić, B.B.; Marković, D.; Buač, M.; Diklić, M.; Kraguljac-Kurtović, N.; Damjanović, S.; Milenković, P.; Gotić, M.; et al. Proinflammatory Cytokine IL-6 and JAK-STAT Signaling Pathway in Myeloproliferative Neoplasms. Mediat. Inflamm. 2015, 2015, 453020. [Google Scholar] [CrossRef] [Green Version]
- Bartalucci, N.; Tozzi, L.; Bogani, C.; Martinelli, S.; Rotunno, G.; Villeval, J.L.; Vannucchi, A.M. Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J. Cell. Mol. Med. 2013, 17, 1385–1396. [Google Scholar] [CrossRef]
- Čokić, V.P.; Mossuz, P.; Han, J.; Socoro, N.; Beleslin-Čokić, B.B.; Mitrović, O.; Subotički, T.; Diklić, M.; Leković, D.; Gotić, M.; et al. Microarray and proteomic analyses of myeloproliferative neoplasms with a highlight on the mTOR signaling pathway. PLoS ONE 2015, 10, e0135463. [Google Scholar] [CrossRef] [Green Version]
- Hoermann, G.; Greiner, G.; Valent, P. Cytokine Regulation of Microenvironmental Cells in Myeloproliferative Neoplasms. Mediat. Inflamm. 2015, 2015, 869242. [Google Scholar] [CrossRef] [Green Version]
- Caine, G.J.; Lip, G.Y.; Stonelake, P.S.; Ryan, P.; Blann, A.D. Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb. Haemost. 2004, 92, 185–190. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory Cytokines in Vascular Dysfunction and Vascular Disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tefferi, A.; Vaidya, R.; Caramazza, D.; Finke, C.; Lasho, T.; Pardanani, A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: A comprehensive cytokine profiling study. J. Clin. Oncol. 2011, 29, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Gangat, N.; Jimma, T.; Finke, C.M.; Lasho, T.L.; Pardanani, A.; Tefferi, A. Plasma cytokines in polycythemia vera: Phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am. J. Hematol. 2012, 87, 1003–1005. [Google Scholar] [CrossRef]
- Pourcelot, E.; Trocme, C.; Mondet, J.; Bailly, S.; Toussaint, B.; Mossuz, P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: Clinical implications. Exp. Hematol. 2014, 42, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Li Volti, G.; Giallongo, C.; Spampinato, M.; Barbagallo, I.; Di Rosa, M.; Romano, A.; Avola, R.; Tibullo, D.; Palumbo, G.A. The Role of Inflammation and Inflammasome in Myeloproliferative Disease. J. Clin. Med. 2020, 9, 2334. [Google Scholar] [CrossRef]
- Gerber, H.P.; Ferrara, N. The role of VEGF in normal and neoplastic hematopoiesis. J. Mol. Med. 2003, 81, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Ahmed, N.; Hassan, H.T. Increased serum levels of vascular endothelial growth factor correlate with splenomegaly in polycythemia vera. Leuk. Res. 2002, 26, 1007–1010. [Google Scholar] [CrossRef]
- Panteli, K.; Bai, M.; Hatzimichael, E.; Zagorianakou, N.; Agnantis, N.J.; Bourantas, K. Serum levels, and bone marrow immunohistochemical expression of, vascular endothelial growth factor in patients with chronic myeloproliferative diseases. Hematology 2007, 12, 481–486. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, X. Cytokines frequently implicated in myeloproliferative neoplasms. Cytokine X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Gianelli, U.; Vener, C.; Raviele, P.R.; Savi, F.; Somalvico, F.; Calori, R.; Iurlo, A.; Radaelli, F.; Fermo, E.; Bucciarelli, P.; et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am. J. Clin. Pathol. 2007, 128, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.S.; Zhai, W.; Young, W.L.; Yang, G.Y. Interleukin-6 triggers human cerebral endothelial cells proliferation and migration: The role for KDR and MMP-9. Biochem. Biophys. Res. Commun. 2006, 342, 1396–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Ye, J.; Shen, F.; Zhu, Y.; Yeghiazarians, Y.; Zhu, W.; Chen, Y.; Lawton, M.T.; Young, W.L.; Yang, G.-Y. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J. Cereb. Blood Flow Metab. 2008, 28, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subotički, T.; Mitrović Ajtić, O.; Beleslin-Čokić, B.B.; Bjelica, S.; Djikić, D.; Diklić, M.; Leković, D.; Gotić, M.; Santibanez, J.F.; Noguchi, C.T.; et al. IL-6 stimulation of DNA replication is JAK1/2 mediated in cross-talk with hyperactivated ERK1/2 signaling. Cell Biol. Int. 2019, 43, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.; Smart, N.; Punn, A.; Jabr, R.; Marber, M.; Heads, R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell. Signal. 2013, 25, 898–909. [Google Scholar] [CrossRef] [Green Version]
- Hideshima, T.; Nakamura, N.; Chauhan, D.; Anderson, K.C. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 2001, 20, 5991–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleppe, M.; Kwak, M.; Koppikar, P.; Riester, M.; Keller, M.; Bastian, L.; Hricik, T.; Bhagwat, N.; McKenney, A.S.; Papalexi, E.; et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015, 5, 316–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawara, K.; Scott, H.; Emathinger, J.; Ide, A.; Fox, R.; Greiner, D.; LaJoie, D.; Hedeen, D.; Nandakumar, M.; Oler, A.J.; et al. Co-Expression of VEGF and IL-6 Family Cytokines is Associated with Decreased Survival in HER2 Negative Breast Cancer Patients: Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion. Transl. Oncol. 2019, 12, 245–255. [Google Scholar] [CrossRef]
- Bockhorn, J.; Dalton, R.; Nwachukwu, C.; Huang, S.; Prat, A.; Yee, K.; Chang, Y.-F.; Huo, D.; Wen, Y.; Swanson, K.E.; et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat. Commun. 2013, 4, 1393. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.E.; Martin, B.; Mellor, L.; Jacob, R.B.; Tawara, K.; McDougal, O.M.; Oxford, J.T.; Jorcyk, C.L. Oncostatin M binds to extracellular matrix in a bioactive conformation: Implications for inflammation and metastasis. Cytokine 2015, 72, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, S.; Fayard, B.; Weis, J.; Weissenberger, J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int. J. Cancer 2005, 115, 202–213. [Google Scholar] [CrossRef]
- Adachi, Y.; Aoki, C.; Yoshio-Hoshino, N.; Takayama, K.; Curiel, D.T.; Nishimoto, N. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int. J. Cancer 2006, 119, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.; Kulbe, H.; Chakravarty, P.; Leader, D.; Vassileva, V.; Leinster, D.A.; Thompson, R.; Schioppa, T.; Nemeth, J.; Vermeulen, J.; et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin. Cancer Res. 2011, 17, 6083–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subotički, T.; Mitrović Ajtić, O.; Živković, E.; Diklić, M.; Đikić, D.; Tošić, M.; Beleslin-Čokić, B.; Dragojević, T.; Gotić, M.; Santibanez, J.F.; et al. VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms. Int. J. Mol. Sci. 2021, 22, 6671. https://doi.org/10.3390/ijms22136671
Subotički T, Mitrović Ajtić O, Živković E, Diklić M, Đikić D, Tošić M, Beleslin-Čokić B, Dragojević T, Gotić M, Santibanez JF, et al. VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms. International Journal of Molecular Sciences. 2021; 22(13):6671. https://doi.org/10.3390/ijms22136671
Chicago/Turabian StyleSubotički, Tijana, Olivera Mitrović Ajtić, Emilija Živković, Miloš Diklić, Dragoslava Đikić, Milica Tošić, Bojana Beleslin-Čokić, Teodora Dragojević, Mirjana Gotić, Juan F. Santibanez, and et al. 2021. "VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms" International Journal of Molecular Sciences 22, no. 13: 6671. https://doi.org/10.3390/ijms22136671
APA StyleSubotički, T., Mitrović Ajtić, O., Živković, E., Diklić, M., Đikić, D., Tošić, M., Beleslin-Čokić, B., Dragojević, T., Gotić, M., Santibanez, J. F., & Čokić, V. (2021). VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms. International Journal of Molecular Sciences, 22(13), 6671. https://doi.org/10.3390/ijms22136671







