Characterization of a Novel Rice Dynamic Narrow-Rolled Leaf Mutant with Deficiencies in Aromatic Amino Acids
Abstract
1. Introduction
2. Results
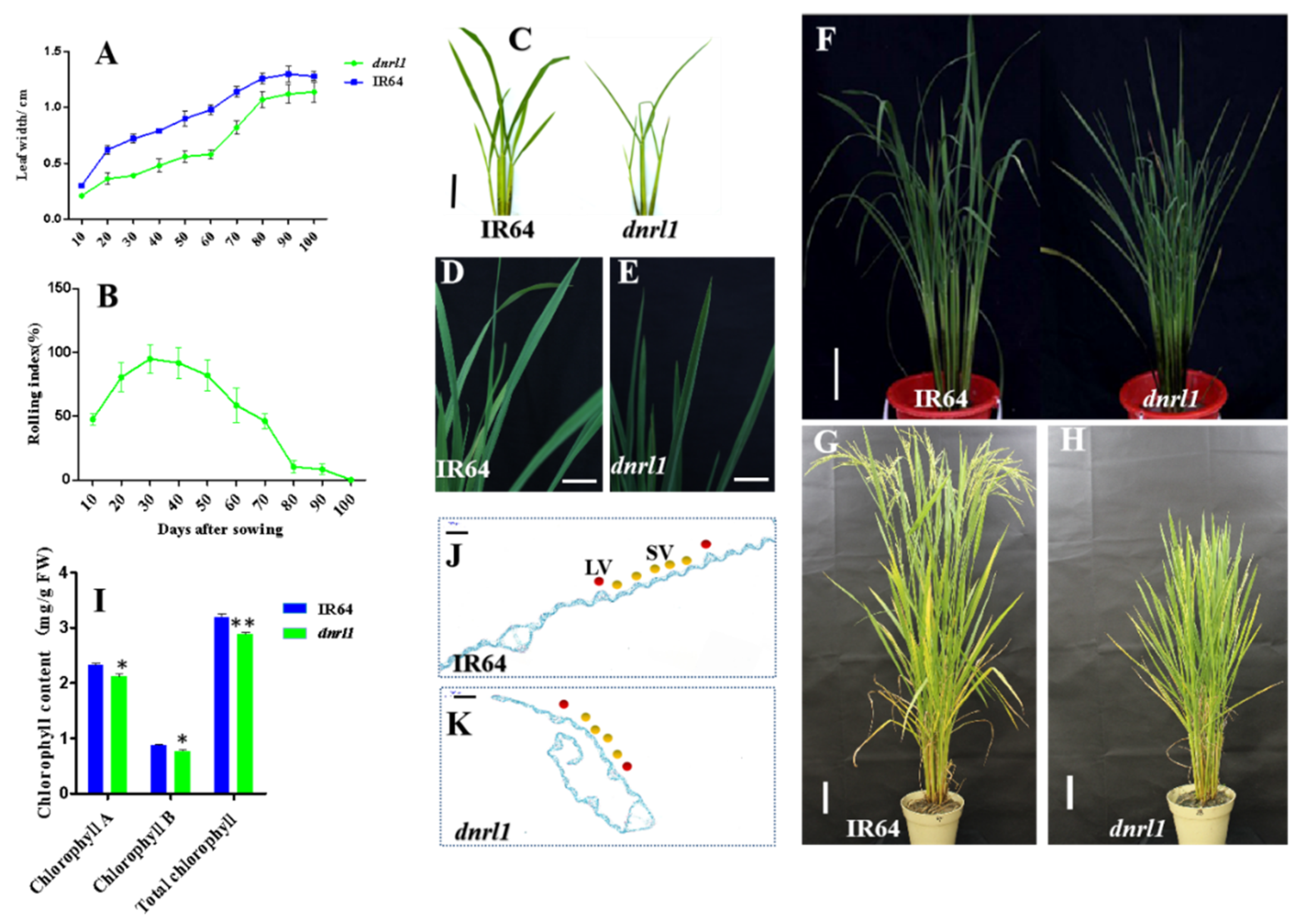
2.1. Phenotype of dnrl1 Mutant
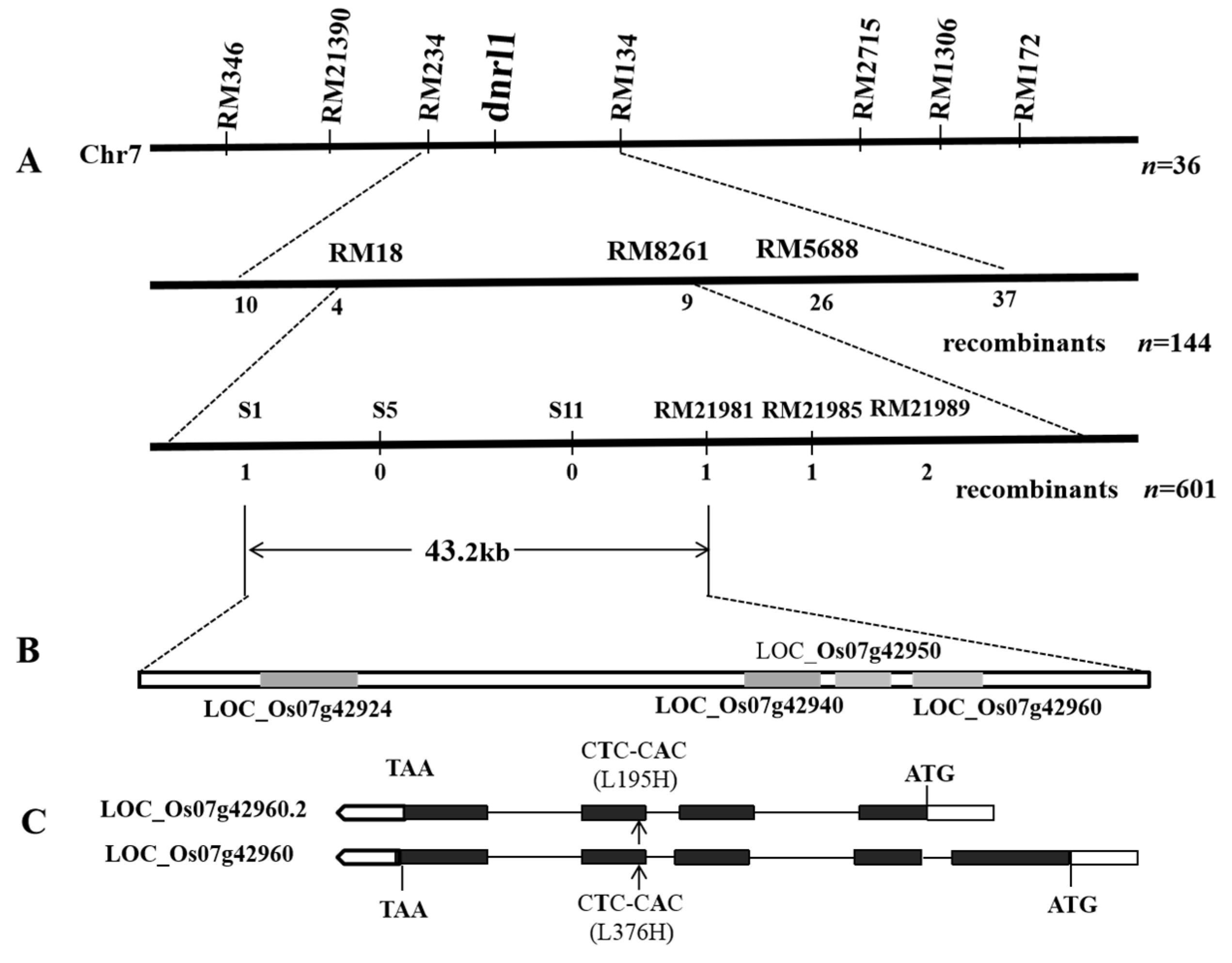
2.2. Genetic Control and Fine Mapping of DNRL1
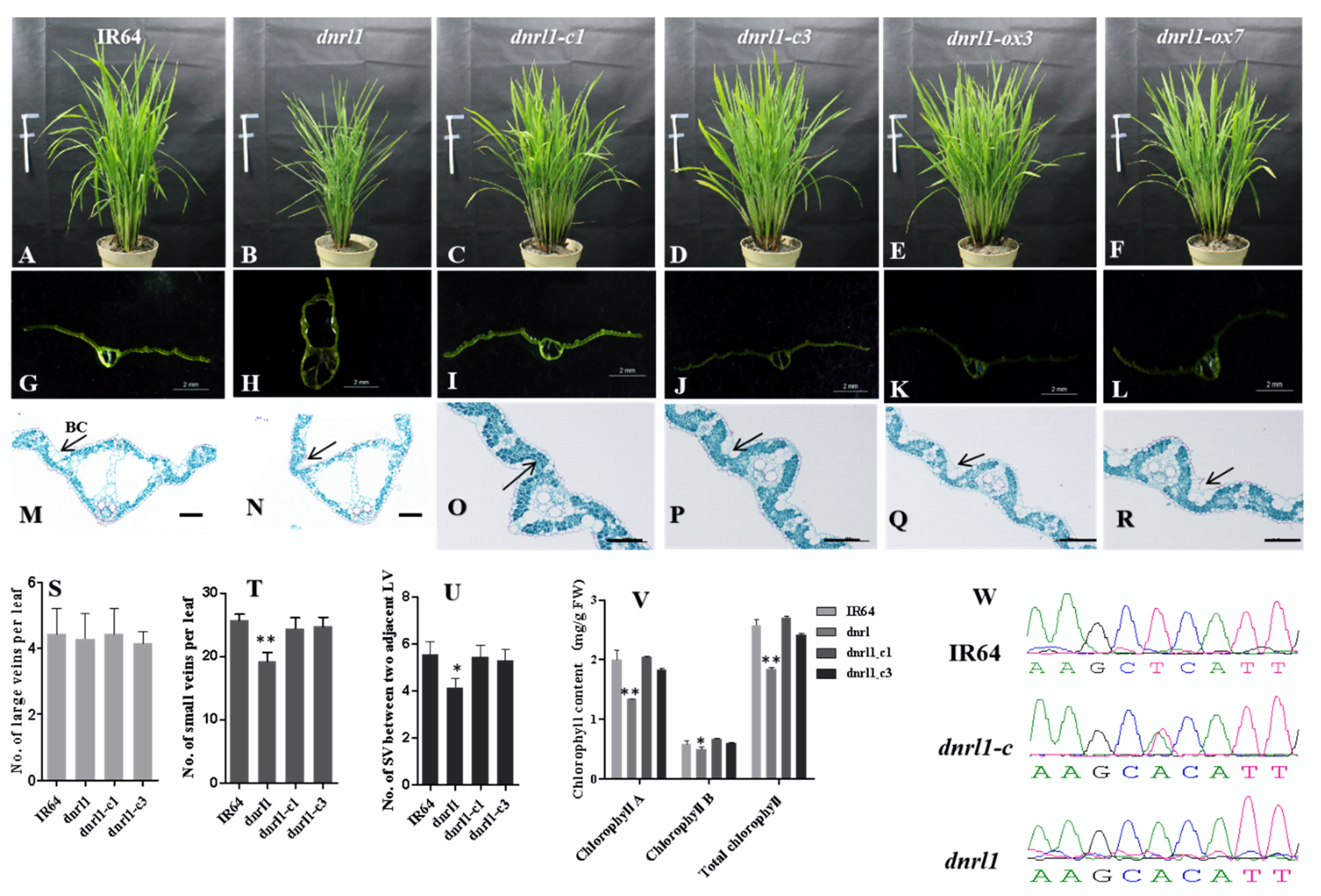
2.3. DNRL1 Complements the dnrl1 Phenotype
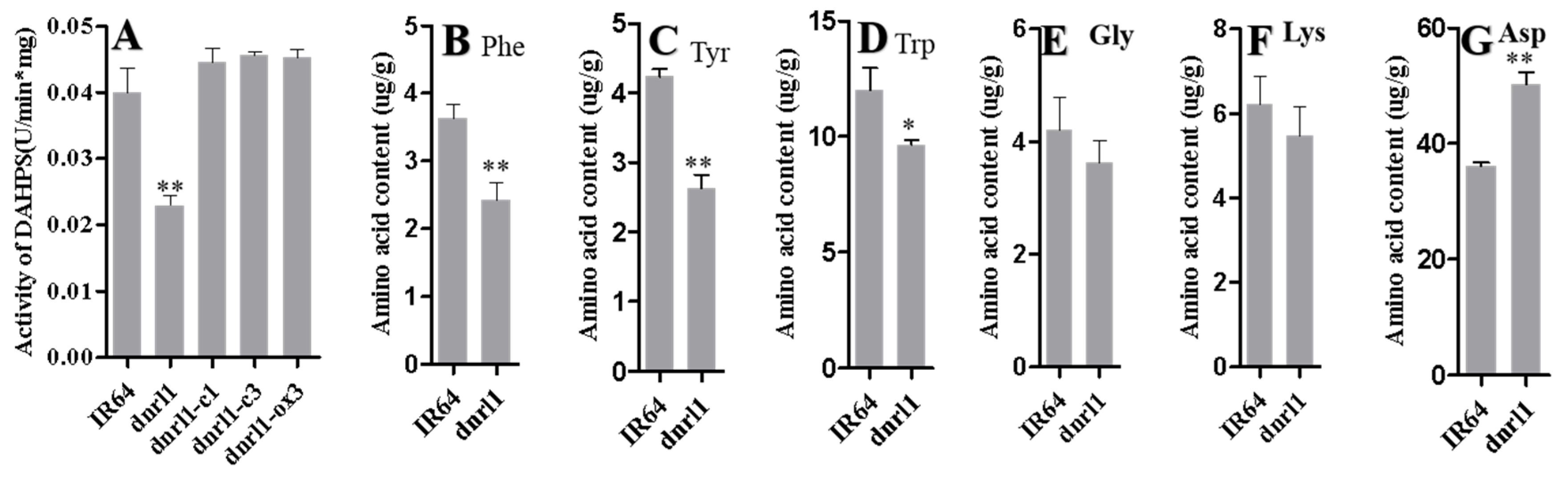
2.4. DNRL1 Encodes a DAHP Synth II Enzyme and Modulates Aromatic Amino Acid Synthesis
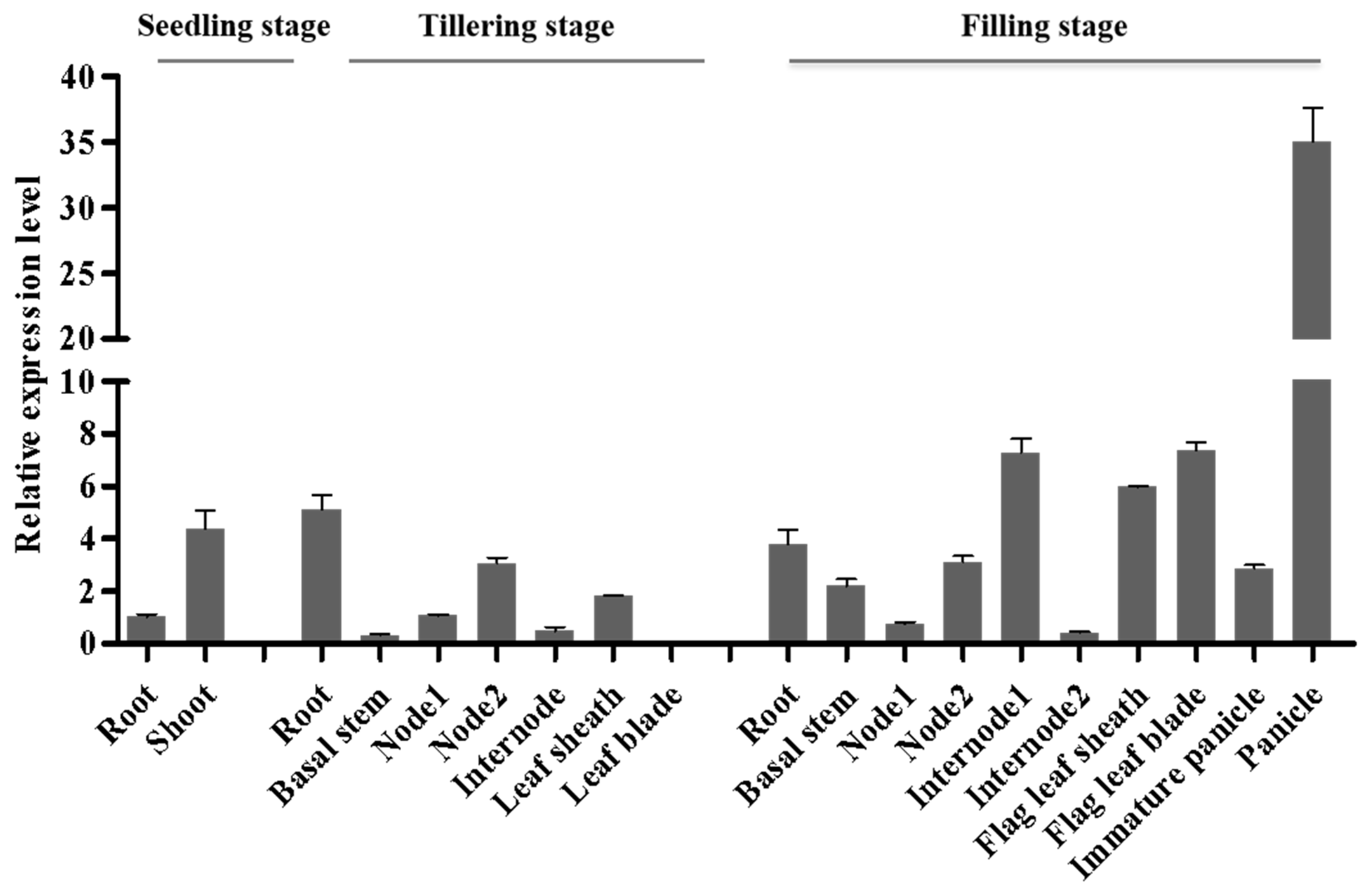
2.5. DNRL1 Expresses Constitutively
2.6. DNRL1-GFP Localizes to Chloroplasts
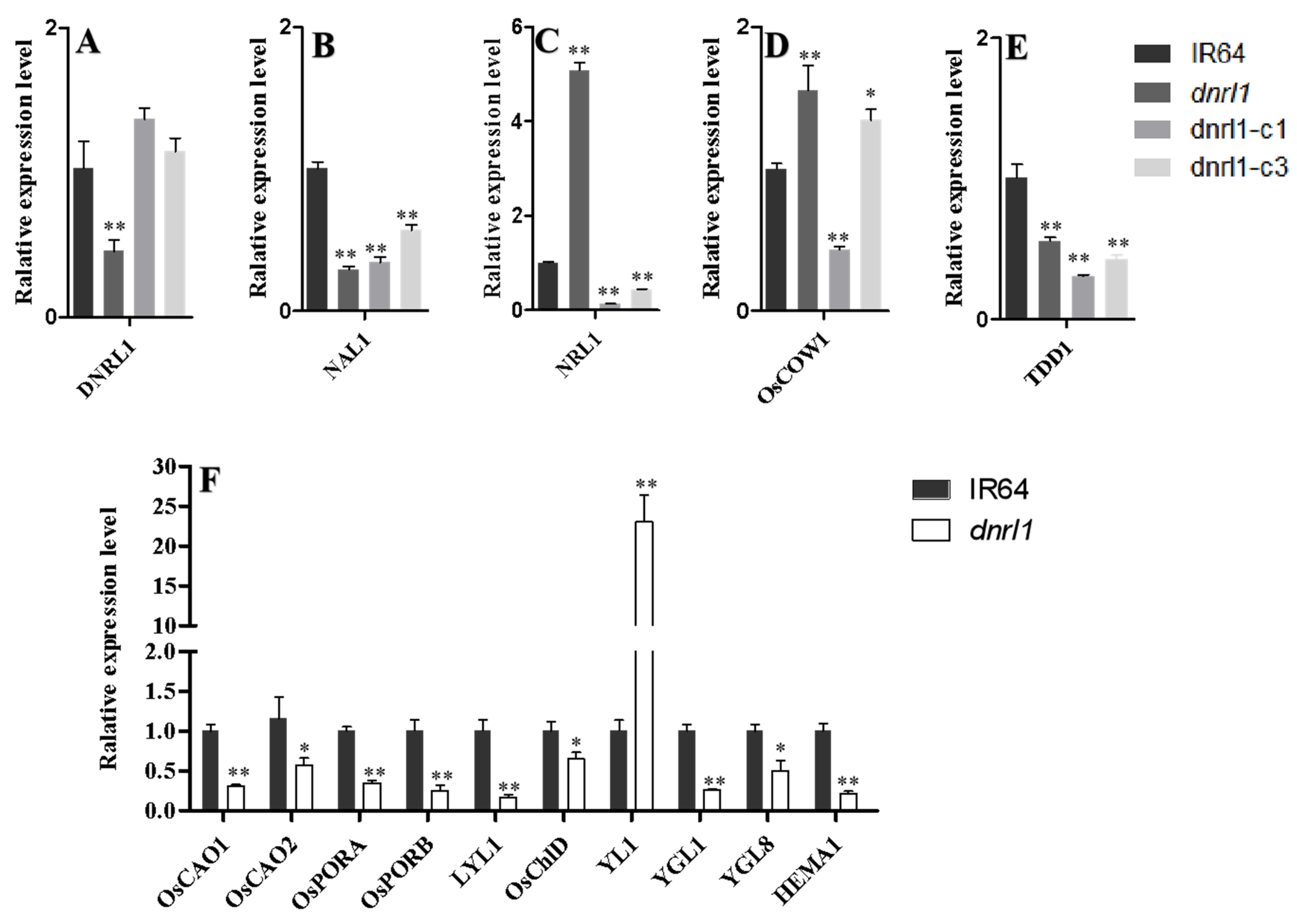
2.7. Altered Expression of Leaf Morphology and Chlorophyll Biosynthesis-Associated Genes in dnrl1
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Map-Based Cloning of DNRL1
4.3. Genetic Complementation and Over-Expression Analysis
4.4. Measurement of Chlorophyll Content
4.5. Quantitative Reverse Transcription PCR (qRT-PCR)
4.6. Subcellular Localization
4.7. Determination of Amino Acid Content
4.8. Assay of DAHPS Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsukaya, H. Mechanism of leaf-shape determination. Annu. Rev. Plant Biol. 2006, 57, 477–496. [Google Scholar] [CrossRef]
- Micol, J. Leaf development: Time to turn over a new leaf? Curr. Opin. Plant Biol. 2009, 12, 9–16. [Google Scholar] [CrossRef]
- Yuan, L. Hybrid rice breeding for super high yield. Hybrid Rice 1997, 12, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Pang, J.; Xie, H.; Qian, M.; Xu, B.; Cai, Y.; Peng, X. Research progress on rice narrow-leaf mutant associated gene. Mol. Plant Breed. 2017, 15, 4879–4887. [Google Scholar]
- Liu, B.; Li, P.; Li, X.; Liu, C.; Cao, S.; Chu, C.; Cao, X. Loss of function of OsDCL1 affects MicroRNA accumulation and causes developmental defects in rice. Plant Physiol. 2005, 139, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, M.; Tang, M.; Dong, B.; Wu, D.; Zhang, Z.; Zhou, B. Repression of microrna biogenesis by silencing of OsDCL1 activates the basal resistance to Magnaporthe oryzae in rice. Plant Sci. 2015, 237, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoo, S.; Zhang, H.; Pandeya, D.; Koh, H.; Hwang, J.; Kim, G.; Paek, N. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Q.; Zhu, X.; Qian, Q.; Xue, H. Shallot-like1 is a kanadi transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 2009, 21, 719–735. [Google Scholar] [CrossRef]
- Wu, C.; Fu, Y.; Hu, G.; Si, H.; Cheng, S.; Liu, W. Isolation and characterization of a rice mutant with narrow and rolled leaves. Planta 2010, 232, 313–324. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, L.; Zeng, D.; Gao, Z.; Guo, L.; Fang, Y.; Zhang, G.; Dong, G.; Yan, M.; Liu, J.; et al. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol. Biol. 2010, 73, 283–292. [Google Scholar] [CrossRef]
- Li, M.; Xiong, G.; Li, R.; Cui, J.; Tang, D.; Zhang, B.; Pauly, M.; Cheng, Z.; Zhou, Y. Rice cellulose synthase-like d4 is essential for normal cell-wall biosynthesis and plant growth. Plant J. 2009, 60, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Eiguchi, M.; Hibara, K.; Ito, J.; Nagato, Y. Rice slender leaf 1 gene encodes cellulose synthase-like d4 and is specifically expressed in m-phase cells to regulate cell proliferation. J. Exp. Bot. 2013, 64, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Lin, Z.; Li, Q.; Wu, H.; Xiang, C.; Wang, J. Dnl1, encodes cellulose synthase-like d4, is a major qtl for plant height and leaf width in rice (Oryza sativa L.). Biochem. Biophys. Res. Commun. 2015, 457, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Liu, Y.; Zhang, F.; Song, Y.; Wang, Z.; Peng, Y.; Sun, Z. OsCD1 encodes a putative member of the cellulose synthase-liked sub-family and is essential for rice plant architecture and growth. Plant Biotechnol. J. 2011, 9, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, C.; Hu, G.; Xing, L.; Qian, W.; Si, H.; Sun, Z.; Wang, X.; Fu, Y.; Liu, W. Characterization and fine mapping of a novel rice narrow leaf mutant nal9. J. Integr. Plant Biol. 2013, 55, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fei, G.; Wu, C.; Wu, F.; Sun, Y.; Chen, M.; Ren, Y.; Zhou, K.; Cheng, Z.; Wang, J.; et al. A rice virescent-yellow leaf mutant reveals new insights into the role and assembly of plastid caseinolytic protease in higher plants. Plant Physiol. 2013, 162, 1867–1880. [Google Scholar] [CrossRef]
- Woo, Y.; Park, H.; Su’udi, M.; Yang, J.; Park, J.; Back, K.; Park, Y.; An, G. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol. Biol. 2007, 65, 125–136. [Google Scholar] [CrossRef]
- Qi, J.; Qian, Q.; Bu, Q.; Li, S.; Chen, Q.; Sun, J.; Liang, W.; Zhou, Y.; Chu, C.; Li, X.; et al. Mutation of the rice narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol. 2008, 147, 1947–1959. [Google Scholar] [CrossRef]
- Jiang, D.; Fang, J.; Lou, L.; Zhao, J.; Yuan, S.; Yin, L.; Sun, W.; Peng, L.; Guo, B.; Li, X. Characterization of a null allelic mutant of the rice nal1 gene reveals its role in regulating cell division. PLoS ONE 2015, 10, e0118169. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, Y.; Liu, F.; Chen, Q.; Qi, J. Narrow leaf 1 (NAL1) regulates leaf shape by affecting cell expansion in rice (Oryza sativa L.). Biochem. Biophys. Res. Commun. 2019, 516, 957–962. [Google Scholar] [CrossRef]
- Fumika, C.; Yukiko, Y.; Toshihiro, K.; Yutaka, S.; Hiro-Yuki, H. Genetic analysis of rice mutants responsible for narrow leaf phenotype and reduced vein number. Genes Genet. Syst. 2016, 91, 235–240. [Google Scholar]
- Fujino, K.; Matsuda, Y.; Ozawa, K.; Nishimura, T.; Koshiba, T.; Fraaije, M.; Sekiguchi, H. Narrow leaf 7 controls leaf shape mediated by auxin in rice. Mol. Genet. Genom. 2008, 279, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sazuka, T.; Kamiya, N.; Nishimura, T.; Ohmae, K.; Sato, Y.; Imamura1, K.; Nagato, Y.; Koshiba, T.; Nagamura, Y.; Ashikari1, M.; et al. A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos. Plant J. 2009, 60, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Jiang, L.; Wang, J.; Zhang, X.; Guo, X.; Wu, C.; Wan, J. A detailed analysis of the leaf rolling mutant sll2 reveals complex nature in regulation of bulliform cell development in rice (Oryza sativa L.). Plant Biol. 2015, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, L.; Liu, F.; Wu, Y.; Zhu, Z.; Sun, C.; Tan, L. Narrow and rolled leaf 2 regulates leaf shape, male fertility, and seed size in rice. J. Integr. Plant Biol. 2016, 58, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Liu, K.; Tang, D.; Sun, M.; Li, Y.; Shen, Y.; Du, G.; Cheng, Z. Semi-rolled leaf2 modulates rice leaf rolling by regulating abaxial side cell differentiation. J. Exp. Bot. 2016, 67, 2139–2150. [Google Scholar] [CrossRef]
- Ma, L.; Sang, X.; Zhang, T.; Yu, Z.; Li, Y.; Zhao, F.; Wang, Z.; Wang, Y.; Yu, P.; Wang, N.; et al. Abnormal vascular bundles regulates cell proliferation and procambium cell establishment during aerial organ development in rice. New Phytol. 2017, 213, 275–286. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.; Lu, S.; Chitsaz, F.; Derbyshire, M.; Geer, R.; Gonzales, N.; et al. Cdd/sparcle: Functional classification of proteins via subfamily domain architectures. Nucl. Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Sato, K.; Mase, K.; Nakano, Y.; Nishikubo, N.; Sugita, R.; Tsuboi, Y.; Kajita, S.; Zhou, J.; Kitano, H.; Katayama, Y. 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase is regulated for the accumulation of polysaccharide-linked hydroxycinnamoyl esters in rice (Oryza sativa L.) internode cell walls. Plant Cell Rep. 2006, 25, 676–688. [Google Scholar] [CrossRef]
- Chen, W.; Sheng, Z.; Cai, Y.; Li, Q.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Tang, S.; Wang, J.; et al. Rice morphogenesis and chlorophyll accumulation is regulated by the protein encoded by NRL3 and its interaction with NAL9. Front. Plant Sci. 2019, 10, 175. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Qian, Q.; Zhang, G. Research advance in molecule regulation mechanism of leaf morphogenesis in Rice (Oryza sativa L.). Acta Agron. Sin. 2013, 39, 767–774, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Corea, O.; Ki, C.; Cardenas, C.; Kim, S.; Brewer, S.; Patten, A.; Davin, L.; Lewis, N. Arogenate dehydratase isoenzymes profoundly and differentially modulate carbon flux into lignins. J. Biol. Chem. 2012, 287, 11446–11459. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Ohmori, Y.; Takebayashi, Y.; Sakakibara, H.; Hirano, H. WUSCHEL-RELATED HOMEOBOX4 acts as a key regulator in early leaf development in rice. PLoS Genet. 2018, 14, e1007365. [Google Scholar] [CrossRef]
- He, P.; Wang, X.; Zhang, X.; Jiang, Y.; Tian, W.; Zhang, X.; Li, Y.; Sun, Y.; Xie, J.; Ni, J.; et al. Short and narrow flag leaf1, a GATA zinc finger domain-containing protein, regulates flag leaf size in rice (Oryza sativa). BMC Plant Biol. 2018, 18, 273. [Google Scholar] [CrossRef]
- Wu, J.; Wu, C.; Lei, C.; Baraoidan, M.; Bordeos, A.; Madamba, M.; Ramos-Pamplona, M.; Mauleon, R.; Portugal, A.; Ulat, V.; et al. Chemical and irradiation induced mutants of indica rice IR64 for forward and reverse genetics. Plant Mol. Biol. 2005, 59, 85–97. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, K. A simple method for isolation of rice DNA. Chin. J. Rice Sci. 1992, 6, 47–48. [Google Scholar]
- Shi, Y.; Chen, J.; Liu, W.; Huang, Q.; Shen, B.; Leung, H.; Wu, J. Genetic analysis and gene mapping of a new rolled-leaf mutant in rice (Oryza sativa L.). Sci. China Ser. C Life Sci. 2009, 52, 885–890. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.; Livak, K. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Chen, Y.; Zhang, L.; Fu, X.; Wei, Q.; Grierson, D.; Zhou, Y.; Huang, Y.; Dong, F.; Yang, Z. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 2016, 6, 23685. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Li, J. A new method for enzyme assay of the activity of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase. J. Southwest Agri. Univ. (Nat. Sci.) 2006, 28, 40–44. [Google Scholar]

| Cross | F1 Phenotype | F2 | P(3:1) | χ2 0.05 | ||
|---|---|---|---|---|---|---|
| Total No. of Plants | No. of Flat Leaf Plants | No. of Narrow Rolled-Leaf Plants | ||||
| dnrll/IR64 | Flat leaf | 833 | 632 | 201 | 0.56 | 0.34 |
| dnrll/Moroberekan | Flat leaf | 2353 | 1752 | 601 | 0.54 | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Shi, Y.; Zhang, X.; Xu, X.; Wu, J.-L. Characterization of a Novel Rice Dynamic Narrow-Rolled Leaf Mutant with Deficiencies in Aromatic Amino Acids. Int. J. Mol. Sci. 2020, 21, 1521. https://doi.org/10.3390/ijms21041521
Wang H, Shi Y, Zhang X, Xu X, Wu J-L. Characterization of a Novel Rice Dynamic Narrow-Rolled Leaf Mutant with Deficiencies in Aromatic Amino Acids. International Journal of Molecular Sciences. 2020; 21(4):1521. https://doi.org/10.3390/ijms21041521
Chicago/Turabian StyleWang, Huimei, Yongfeng Shi, Xiaobo Zhang, Xia Xu, and Jian-Li Wu. 2020. "Characterization of a Novel Rice Dynamic Narrow-Rolled Leaf Mutant with Deficiencies in Aromatic Amino Acids" International Journal of Molecular Sciences 21, no. 4: 1521. https://doi.org/10.3390/ijms21041521
APA StyleWang, H., Shi, Y., Zhang, X., Xu, X., & Wu, J.-L. (2020). Characterization of a Novel Rice Dynamic Narrow-Rolled Leaf Mutant with Deficiencies in Aromatic Amino Acids. International Journal of Molecular Sciences, 21(4), 1521. https://doi.org/10.3390/ijms21041521






