Tumor-Stroma Crosstalk Enhances REG3A Expressions that Drive the Progression of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
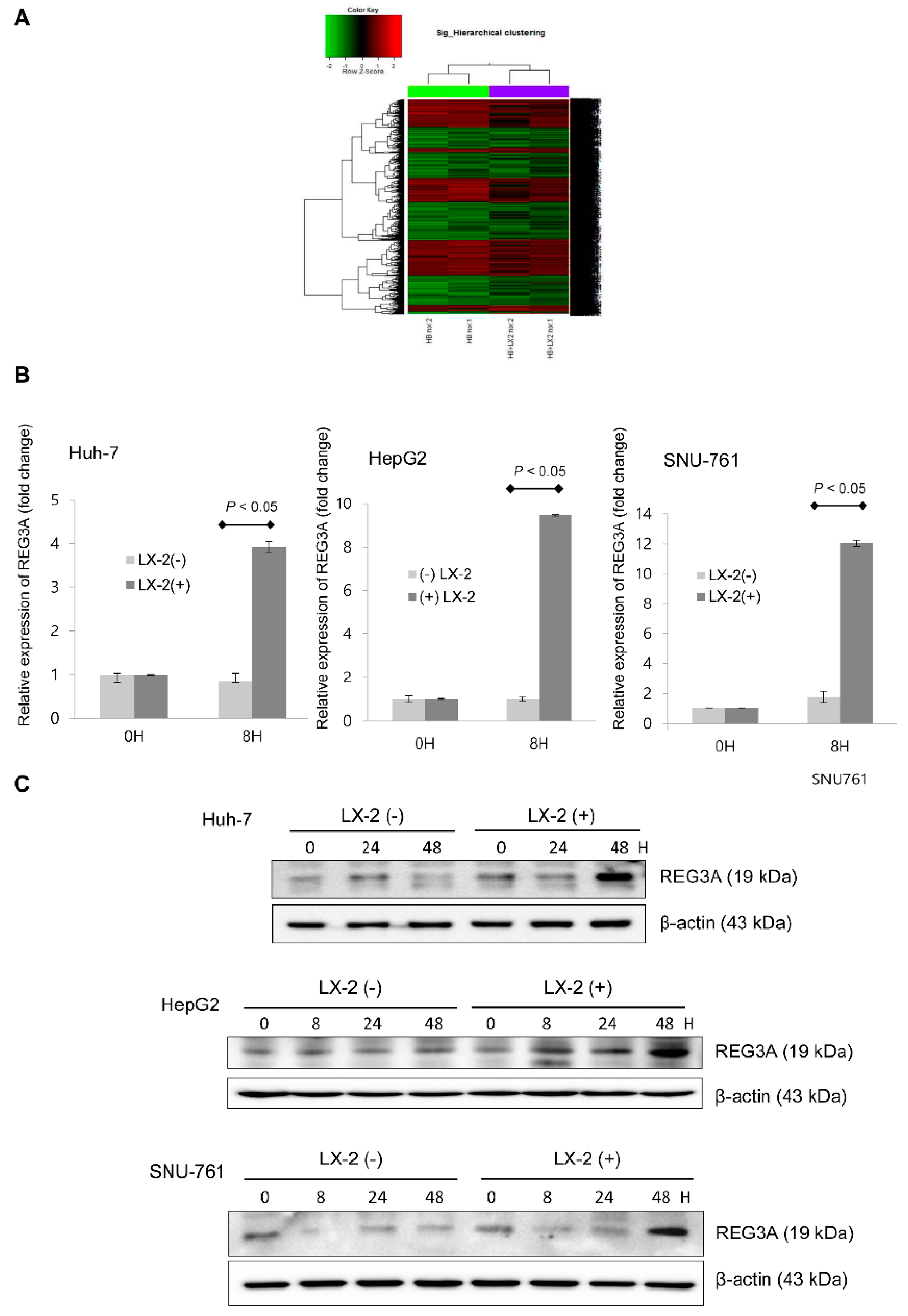
2.1. Coculturing HCC Cells and HSCs Enhanced REG3A Expression
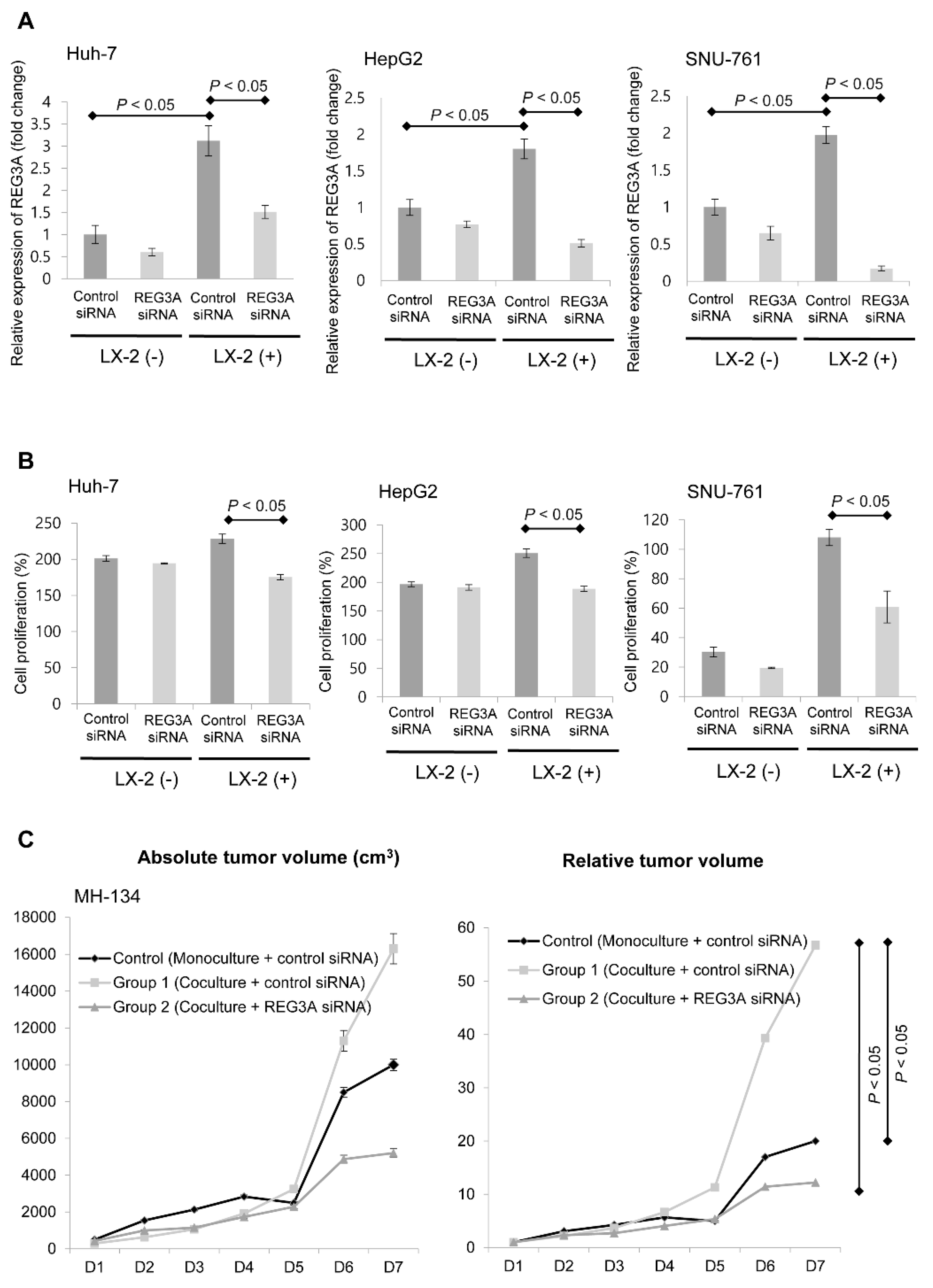
2.2. Modulation of REG3A in Cocultured HCC Cells Showed Anti-Tumor Effects In Vitro and In Vivo
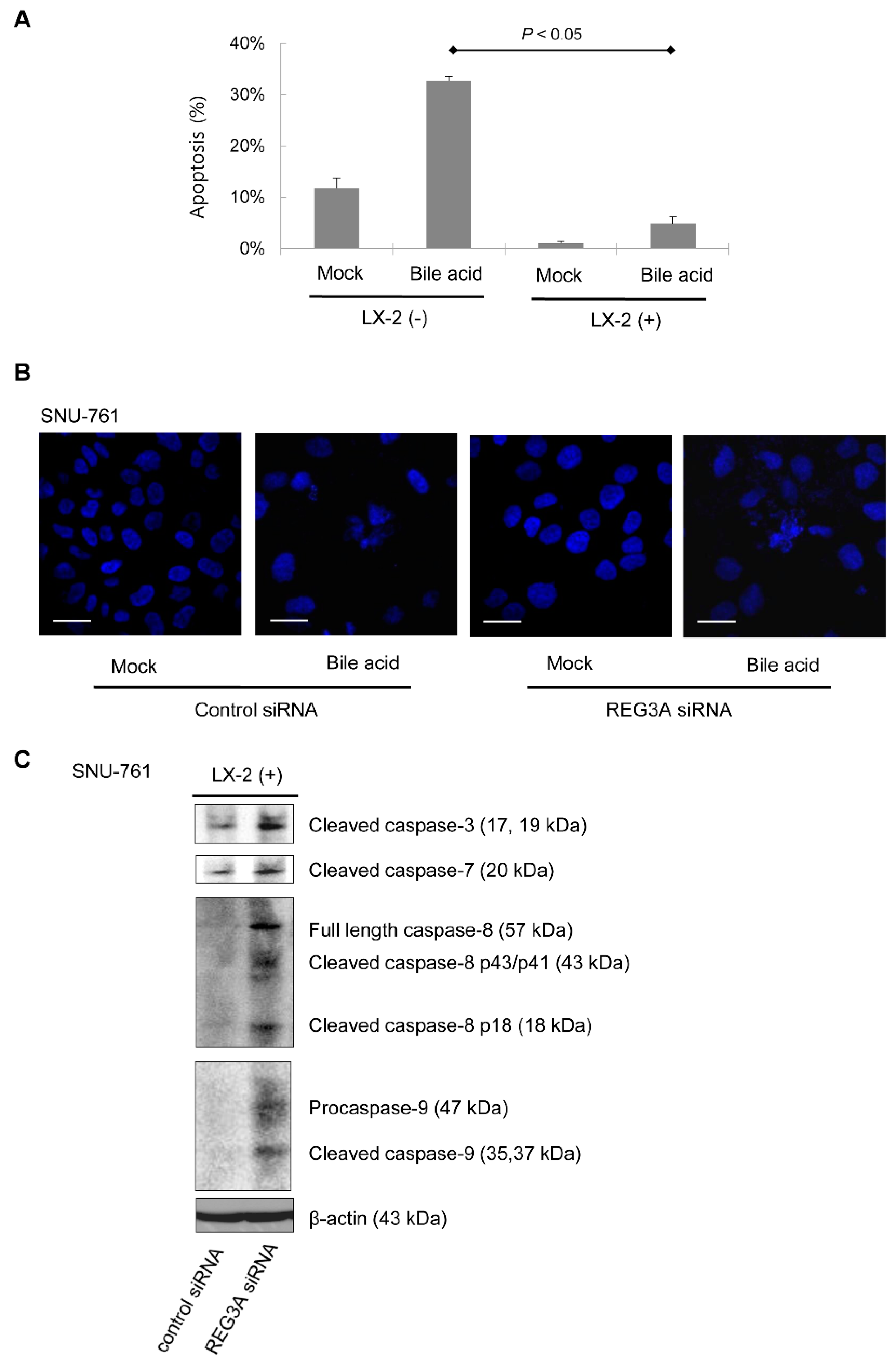
2.3. Downregulation of REG3A Decreased Bile Acid-Induced HCC Cell Apoptosis
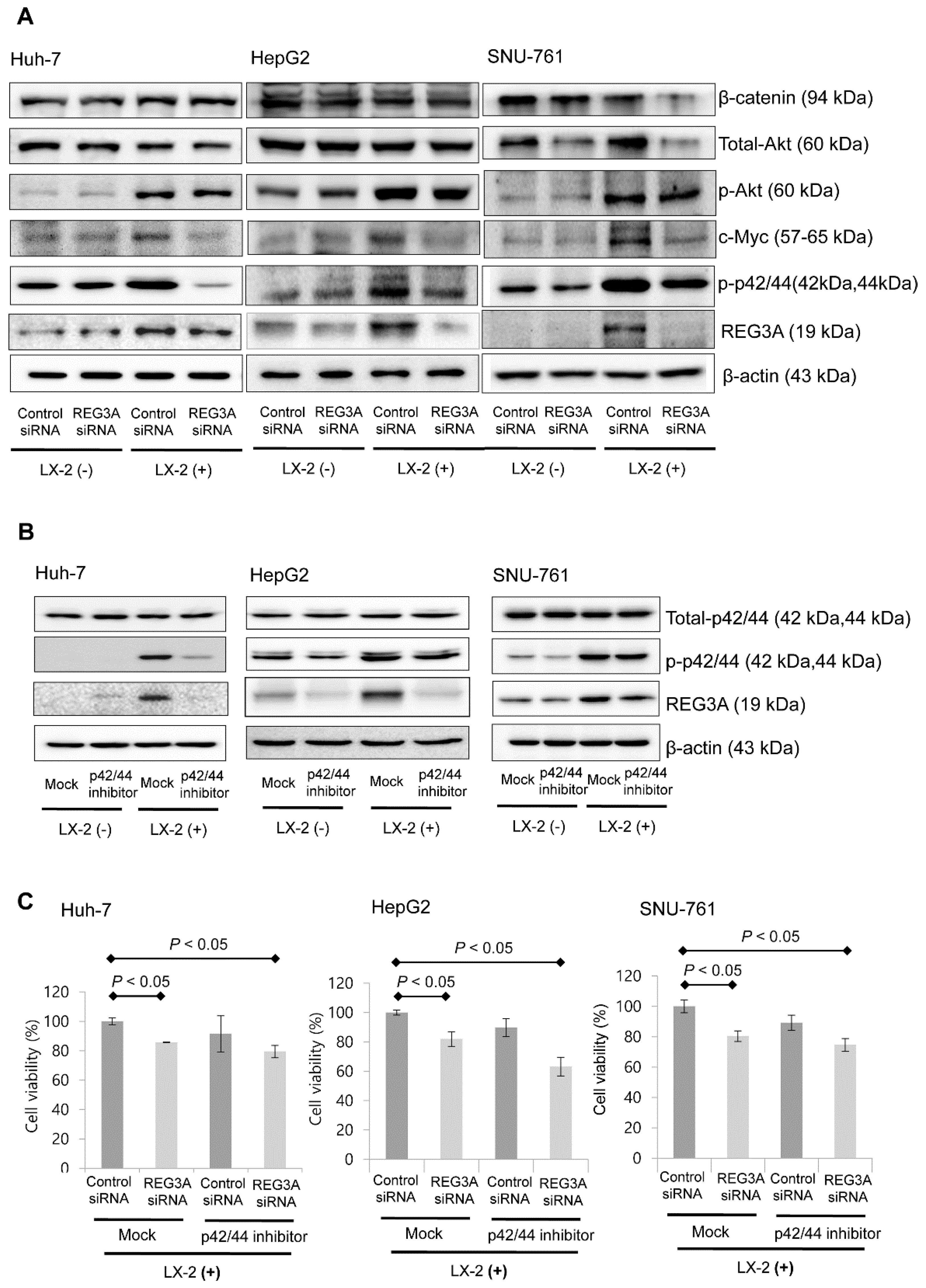
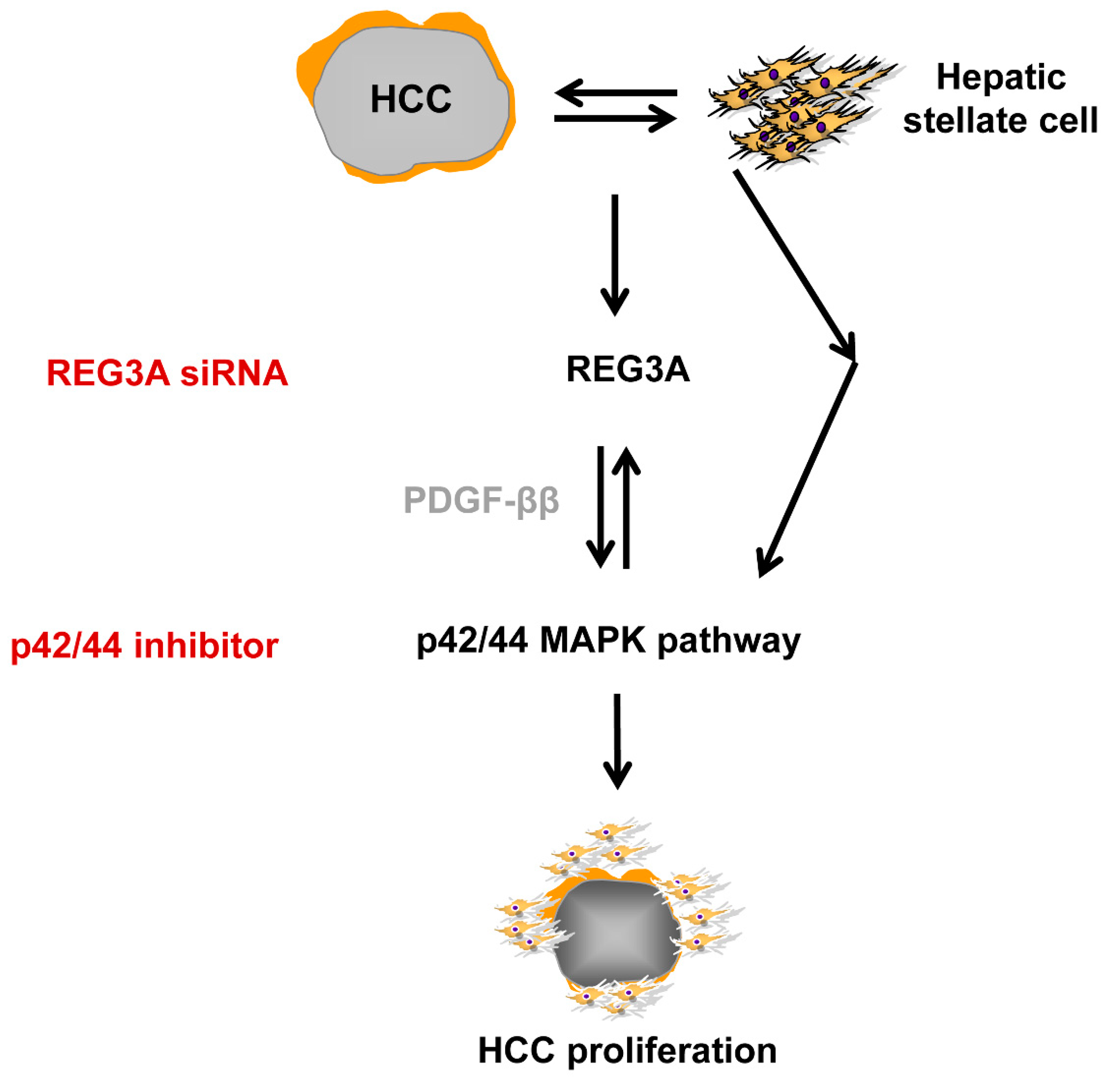
2.4. The Antitumor Mechanism of REG3A in HCC Cells Cocultured with HSCs: p42/44 Pathway
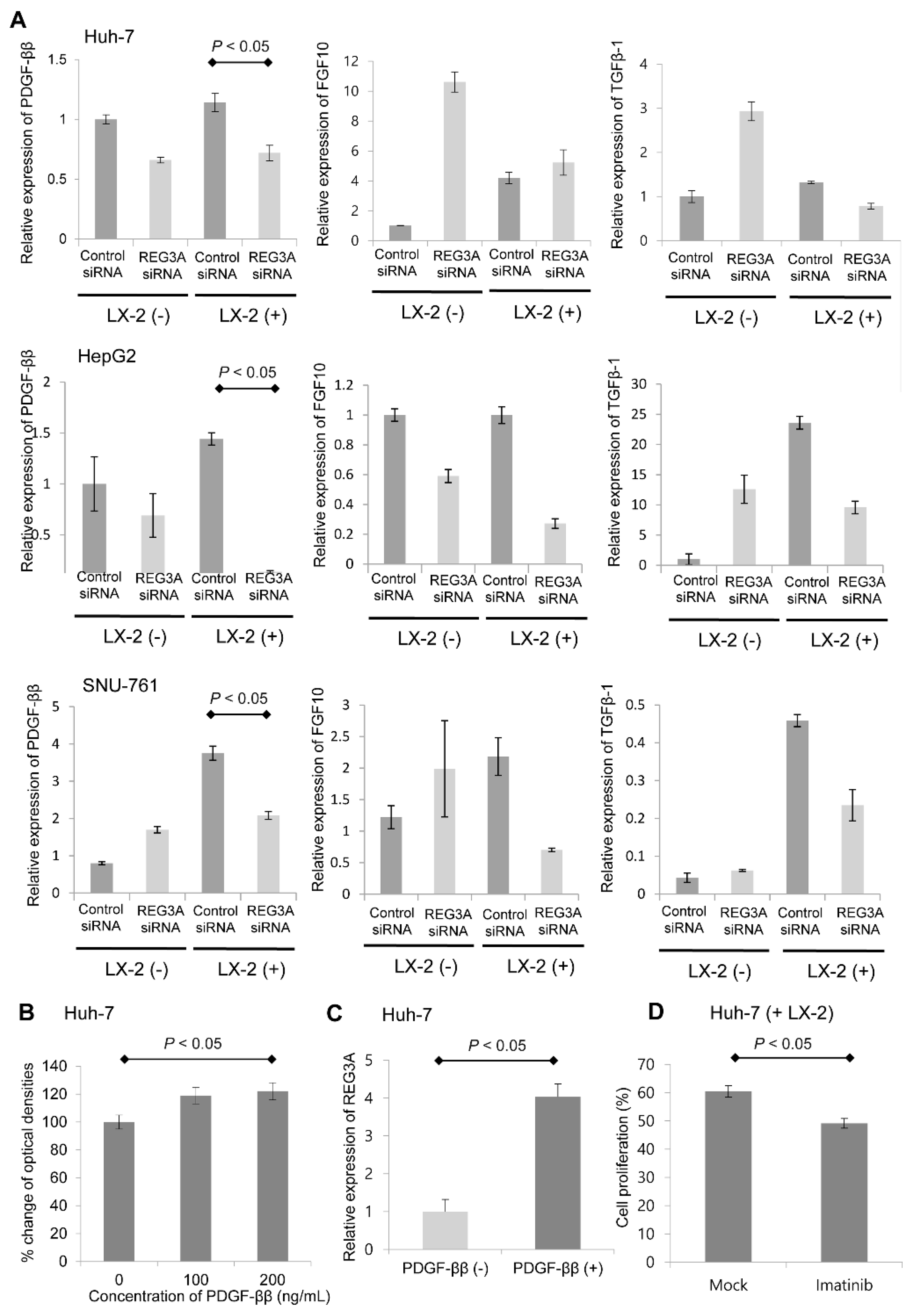
2.5. Crosstalk-Induced REG3A Upregulation was Modulated by PDGF-ββ
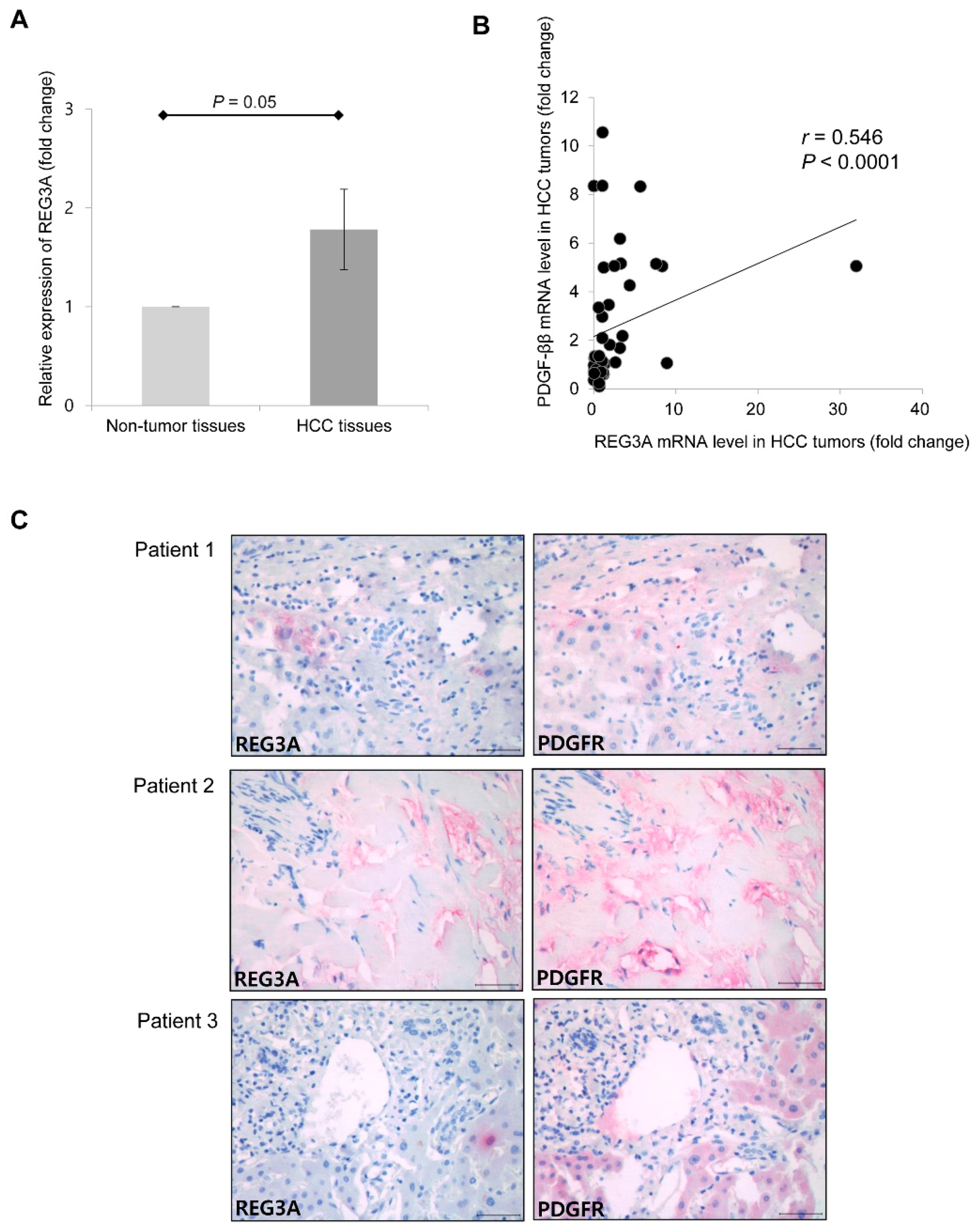
2.6. Upregulated mRNA Expression of REG3A was Correlated with the Expression of PDGF-ββ in Human HCC Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Coculture
4.2. Cell Proliferation Analysis (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay)
4.3. Apoptosis Analysis
4.4. cDNA Microarray Analysis
4.5. Small Interfering RNA (siRNA) Transfection
4.6. In Vivo Subcutaneous Xenograft Model
4.7. Immunoblot Analysis
4.8. Real-Time Polymerase Chain Reaction (qPCR) Analysis
4.9. Statistical Analyses
4.10. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| HSC | hepatic stellate cell |
| cDNA | complementary DNA |
| siRNA | small interfering RNA |
| WME | William’s medium E |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ELISA | enzyme-linked immunosorbent assay |
| PCR | polymerase chain reaction |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| SD | standard deviation |
| REG3A | regenerating gene protein 3 |
| FGF10 | fibroblast growth factor 10 |
| TGF-β1 | transforming growth factor-β1 |
| PDGF | platelet-derived growth factor |
| PI3K | phosphoinositide 3-kinase |
| MAPK | mitogen-activated protein kinase |
References
- El-Serag, H.B. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology 2004, 127, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Memeo, R.; de’Angelis, N.; de Blasi, V.; Cherkaoui, Z.; Brunetti, O.; Longo, V.; Piardi, T.; Sommacale, D.; Marescaux, J.; Mutter, D.; et al. Innovative surgical approaches for hepatocellular carcinoma. World J. Hepatol. 2016, 8, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Lin, D.Y.; Park, J.W.; Kudo, M.; Qin, S.; Chung, H.C.; Song, X.; Xu, J.; Poggi, G. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.H.; Heo, J.; Xu, J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Gansukh, B.; Shia, J.; Kalin, M.; Katz, S.; Abad, L. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J. Hepatol. 2014, 60, 319–324. [Google Scholar] [CrossRef]
- Lee, J.S. The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 220–229. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, H.J.; Kim, K.M.; Yu, E.S.; Kim, K.H.; Kim, S.M.; Kim, T.W.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; et al. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 60–70. [Google Scholar] [CrossRef][Green Version]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Macias-Barragan, J. Update on the pathophysiology of liver fibrosis. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 459–472. [Google Scholar] [CrossRef]
- Bruix, J.; Boix, L.; Sala, M.; Llovet, J.M. Focus on hepatocellular carcinoma. Cancer Cell 2004, 5, 215–219. [Google Scholar] [CrossRef]
- Zhu, B.; Lin, N.; Zhang, M.; Zhu, Y.; Cheng, H.; Chen, S.; Ling, Y.; Pan, W.; Xu, R. Activated hepatic stellate cells promote angiogenesis via interleukin-8 in hepatocellular carcinoma. J. Transl Med. 2015, 13, 365. [Google Scholar] [CrossRef]
- Cho, Y.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y.; Yoon, J.H. Hypoxia Enhances Tumor-Stroma Crosstalk that Drives the Progression of Hepatocellular Carcinoma. Dig. Dis. Sci. 2016, 61, 2568–2577. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Friedman, S.L. Cytokines and fibrogenesis. Semin. Liver Dis. 1999, 19, 129–140. [Google Scholar] [CrossRef]
- Fukushima, N.; Koopmann, J.; Sato, N.; Prasad, N.; Carvalho, R.; Leach, S.D.; Hruban, R.H.; Goggins, M. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod. Pathol. 2005, 18, 779–787. [Google Scholar] [CrossRef]
- Ye, Y.; Xiao, L.; Wang, S.J.; Yue, W.; Yin, Q.S.; Sun, M.Y.; Xia, W.; Shao, Z.Y.; Zhang, H. Up-regulation of REG3A in colorectal cancer cells confers proliferation and correlates with colorectal cancer risk. Oncotarget 2016, 7, 3921–3933. [Google Scholar] [CrossRef]
- Choi, B.; Suh, Y.; Kim, W.H.; Christa, L.; Park, J.; Bae, C.D. Downregulation of regenerating islet-derived 3 alpha (REG3A) in primary human gastric adenocarcinomas. Exp. Mol. Med. 2007, 39, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Via, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene Expression Comparison between the Lymph Node-Positive and—Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Keim, V.; Iovanna, J.L.; Rohr, G.; Usadel, K.H.; Dagorn, J.C. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology 1991, 100, 775–782. [Google Scholar] [CrossRef]
- Lasserre, C.; Simon, M.T.; Ishikawa, H.; Diriong, S.; Nguyen, V.C.; Christa, L.; Vernier, P.; Brechot, C. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur. J. Biochem. 1994, 224, 29–38. [Google Scholar] [CrossRef]
- Christa, L.; Carnot, F.; Simon, M.T.; Levavasseur, F.; Stinnakre, M.G.; Lasserre, C.; Thepot, D.; Clement, B.; Devinoy, E.; Brechot, C. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am. J. Physiol. 1996, 271, G993–G1002. [Google Scholar] [CrossRef]
- Ortiz, E.M.; Dusetti, N.J.; Vasseur, S.; Malka, D.; Bodeker, H.; Dagorn, J.C.; Iovanna, J.L. The pancreatitis-associated protein is induced by free radicals in AR4-2J cells and confers cell resistance to apoptosis. Gastroenterology 1998, 114, 808–816. [Google Scholar] [CrossRef]
- Cavard, C.; Terris, B.; Grimber, G.; Christa, L.; Audard, V.; Radenen-Bussiere, B.; Simon, M.T.; Renard, C.A.; Buendia, M.A.; Perret, C. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene 2006, 25, 599–608. [Google Scholar] [CrossRef]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8(+) T cell subset with suppressive function in myeloma patients. Oncoimmunology 2019, 8, e1486949. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Sole, M.; et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Dika, I.E.; Abou-Alfa, G.K. Treatment options after sorafenib failure in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gedaly, R.; Angulo, P.; Hundley, J.; Daily, M.F.; Chen, C.; Evers, B.M. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J. Surg. Res. 2012, 176, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Berasain, C. Making sorafenib irresistible: In vivo screening for mechanisms of therapy resistance in hepatocellular carcinoma hits on Mapk14. Hepatology 2015, 61, 1755–1757. [Google Scholar] [CrossRef]
- Friemel, J.; Rechsteiner, M.; Frick, L.; Bohm, F.; Struckmann, K.; Egger, M.; Moch, H.; Heikenwalder, M.; Weber, A. Intratumor heterogeneity in hepatocellular carcinoma. Clin. Cancer Res. 2015, 21, 1951–1961. [Google Scholar] [CrossRef]
- Gnoni, A.; Santini, D.; Scartozzi, M.; Russo, A.; Licchetta, A.; Palmieri, V.; Lupo, L.; Faloppi, L.; Palasciano, G.; Memeo, V.; et al. Hepatocellular carcinoma treatment over sorafenib: Epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin. Ther. Targets 2015, 19, 1623–1635. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Taketa, K.; Miyano, K.; Yamane, T.; Sato, J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982, 42, 3858–3863. [Google Scholar]
- Lee, J.H.; Ku, J.L.; Park, Y.J.; Lee, K.U.; Kim, W.H.; Park, J.G. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J. Gastroenterol. 1999, 5, 289–295. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’Byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Park, M.J.; Kim, K.; Park, J.-Y.; Kim, J.; Kim, W.; Yoon, J.-H. Tumor-Stroma Crosstalk Enhances REG3A Expressions that Drive the Progression of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 472. https://doi.org/10.3390/ijms21020472
Cho Y, Park MJ, Kim K, Park J-Y, Kim J, Kim W, Yoon J-H. Tumor-Stroma Crosstalk Enhances REG3A Expressions that Drive the Progression of Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2020; 21(2):472. https://doi.org/10.3390/ijms21020472
Chicago/Turabian StyleCho, Yuri, Min Ji Park, Koeun Kim, Jae-Young Park, Jihye Kim, Wonjin Kim, and Jung-Hwan Yoon. 2020. "Tumor-Stroma Crosstalk Enhances REG3A Expressions that Drive the Progression of Hepatocellular Carcinoma" International Journal of Molecular Sciences 21, no. 2: 472. https://doi.org/10.3390/ijms21020472
APA StyleCho, Y., Park, M. J., Kim, K., Park, J.-Y., Kim, J., Kim, W., & Yoon, J.-H. (2020). Tumor-Stroma Crosstalk Enhances REG3A Expressions that Drive the Progression of Hepatocellular Carcinoma. International Journal of Molecular Sciences, 21(2), 472. https://doi.org/10.3390/ijms21020472




