Abstract
The composition and organization of the plasma membrane play important functional and regulatory roles in integrin signaling, which direct many physiological and pathological processes, such as development, wound healing, immunity, thrombosis, and cancer metastasis. Membranes are comprised of regions that are thick or thin owing to spontaneous partitioning of long-chain saturated lipids from short-chain polyunsaturated lipids into domains defined as ordered and liquid-disorder domains, respectively. Liquid-ordered domains are typically 100 nm in diameter and sometimes referred to as lipid rafts. We posit that integrin β senses membrane thickness and that mechanical force on the membrane regulates integrin activation through membrane thinning. This review examines what we know about the nature and mechanism of the interaction of integrins with the plasma membrane and its effects on regulating integrins and its binding partners.
1. Introduction
Biomembranes are a key component of life, as they allow the formation of compartments to provide conditions required for biochemical reactions, and also provide an interaction platform for a multitude of key cellular processes. The membranes consist of phospholipids, glycolipids, sterols, and proteins, arranged in a lipid bilayer. The bilayer architecture is a consequence of the amphipathic character of its constituents, which in an aqueous environment with polar regions oriented towards the aqueous phase and hydrophobic regions facing each other.
The concept of the bilayer was first postulated in 1935 [1] and then replaced with the fluid mosaic model in 1972 [2]. The latter model is in large part still accepted and describes biomembranes as fluid objects where lipids and proteins are in motion and can freely diffuse along the plane of the lipid bilayer. One main adjustment to the fluid mosaic model of biomembranes is the fact that they have a very high protein content [3,4]. This molecular crowding is increasingly described to occur also in soluble states [5] and drives the separation of phases of distinct contents. The non-homogenous distribution of proteins within the membrane is amplified further through interactions on either side of the membrane.
Islands with distinct compositions compared to the rest of the fluid mosaic biomembrane are considered membrane microdomains. Lipid rafts, wherein the spontaneous partitioning of long-chain saturated lipids from short-chain polyunsaturated lipids results in thicker liquid-ordered and thinner liquid-disordered domains, respectively [6,7], as noted by Hansen [8], are the best-characterized membrane microdomain. The fact that lipid rafts do not solubilize in nonionic detergents like triton X-100 at 4 °C and are thus called detergent-resistant membranes [9,10,11] makes them especially difficult to study.
Lipid rafts are rich in cholesterol and sphingolipids as well as in a variety of proteins [12,13], including lipid-linked proteins such as glycosylphosphatidylinositol-anchored proteins and signaling molecules. Thus, they provide an essential platform for cell signaling processes [10] and play important roles in the regulation of cell adhesion to the extracellular matrix and associated cell migration by providing a scaffold that concentrates adaptor and scaffolding proteins, as well as the actin cytoskeleton, effectors, kinases, and receptors to trigger cancer signaling events [14] (Figure 1). By concentrating signaling molecules such as the src family of non-receptor tyrosine kinases as well as the small GTPase rac1, lipid raft microdomains regulate the extracellular matrix-mediated cell migration and direct signaling pathways of cell division, cell shape, cell motility, and cell adhesion [10,13,15,16,17,18,19,20,21,22,23,24,25]. Specifically, lipid rafts organize signaling molecules and provide platforms for cell adhesion signaling. Several studies show that integrins are associated with lipid rafts [26,27,28] and this interaction is important for triggering signaling cascades upon cell attachment to the extracellular matrix.

Figure 1.
Schematic of lipid raft organization. The asymmetric plasma membrane contains phospholipids, glycosphingolipids, cholesterol, and protein receptors that are organized in the thicker lipid microdomains. These lipid rafts compartmentalize cellular processes and signal transduction by organizing and concentrating signaling molecules to more favorably interact with protein receptors as well as effectors. Lipid rafts float freely in the plasma membrane while being packed tighter and more ordered compared to non-raft regions.
Cell adhesion to the extracellular matrix is mediated by the integrin transmembrane receptor, a heterodimer composed of an α and a β subunit. Integrins attach to extracellular matrix components, such as collagen or fibronectin, and assemble a large focal adhesion protein complex that links the adhesion sites to the actin cytoskeleton. When bound to the extracellular matrix, integrins transmit signals within the cell that control cell spreading, retraction, migration, and proliferation. These signals drive many physiological and pathological processes, such as development, wound healing, immunity, thrombosis, and cancer metastasis.
In this review we discuss the role of lipid rafts in integrin-mediated cell adhesion and how lipid rafts can affect integrin structure and signaling. Although the importance of lipid rafts in cell adhesion has long been recognized, the lack of mechanistic insights has prevented a clear view of how lipid rafts are linked to integrin function. Here we focus on recent findings that have helped to postulate a detailed mechanistic model that can explain how lipid rafts selectively include activated integrin receptors and hence provide a platform for active integrin signaling.
2. Cytoskeletal Rearrangements
The F-actin cytoskeleton interacts and controls many structural and functional aspects of the membrane lipid rafts. For example, integrin α5 translocation and activation were prevented by the disruption of the F-actin-based cytoskeleton and knockdown of caveolin-1. Lipid rafts play a role in many cellular processes including cell proliferation [29,30,31] and act as a sorting platform for proteins, especially those involved in cancer (ovarian, prostate, and renal cell carcinoma). Their association with the actin cytoskeleton drives tumor progression. Thus, molecules that interfere with the assembly of the actin cytoskeleton with lipid rafts might have anticancer activities. In general, the cytoskeletal rearrangement that stabilizes lipid rafts and the recruitment of cytoskeletal proteins to the ordered microdomains facilitates increased intermolecular interactions that are crucial during the development of cancer. Since cholesterol and saturated sphingolipids increase the rigidity of lipid rafts, their protein compartmentalization might stabilize interactions of raft components [32]. Tumor suppressors that regulate lipid rafts might reduce the attachment of the cytoskeleton to the membrane [33]. One such tumor suppressor, the protein that is responsible for neurofibromatosis 2, termed merlin (or schwannomin or neurofibromin 2), disrupts such membrane interactions with the actin cytoskeleton [34,35]. The inactive closed merlin conformation is found to be associated with non-raft regions of the plasma membrane, while merlin activation by severing the merlin head-tail intramolecular interaction seems to be associated with lipid rafts [36] (Figure 2).
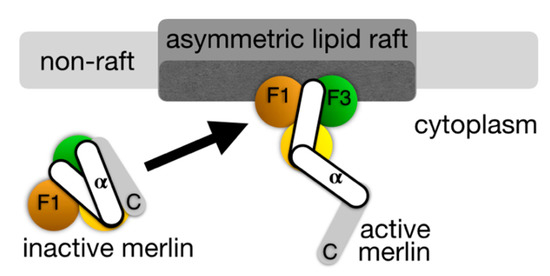
Figure 2.
Proposed merlin activation mechanism in lipid rafts. Merlin belongs to the ezrin-radixin-moesin (ERM) family of proteins that are characterized by their N-terminal four point one, ezrin, radixin, moesin (FERM) head domain, a central α-helical domain and a C-terminal tail domain that binds to the actin cytoskeleton. The FERM domain is comprised of three subdomains, F1, F2, and F3 that are arranged in a cloverleaf-like structure (depicted spectrally; F1, orange; F2, yellow; F3, green). The C-terminal domain of merlin differs from other ERM proteins and does not contain an F-actin binding domain. Left, the tumor suppressor protein merlin is inactive in its closed conformer. Right, upon binding to the PI(4,5)P2 that is found in lipid rafts, merlin is activated by severing its head-tail interaction, thereby resulting in its open conformation and tumor suppressor functions [36].
3. Integrin Structure
Integrins are single-pass αβ heterodimeric transmembrane receptors that are composed of a large extracellular ligand-binding domain and short cytoplasmic tail domains (Figure 3A) [37]. Their single transmembrane α-helices engage in intermolecular interactions when integrin is in its resting state. Upon activation with extracellular ligands (Figure 3B,C), integrins change from their low-affinity binding to their high-affinity binding state, which modifies cell adhesion [38].
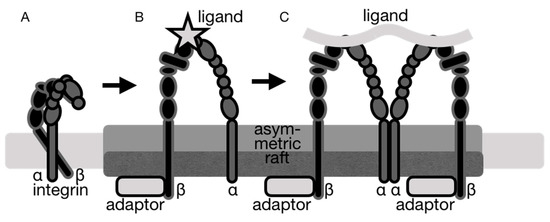
Figure 3.
Integrin adhesion and signaling are linked to the recruitment of integrins to lipid rafts. Several integrins have been found in lipid rafts, whereby the activated form of integrin preferably localizes to cholesterol-enriched lipid rafts. The cholesterol-rich membrane domains cluster integrin at focal adhesions, which regulates integrin activation. (A), inactive integrin localized to non-raft regions of the plasma membrane. (B), activated integrin localizes to lipid rafts. (C), integrin clustering at focal adhesions in lipid rafts.
Structural studies on many integrins showed that this integrin exists in a continuous conformational equilibrium ranging from a compact conformation to a fully extended conformer with the cytoplasmic tail domains separated upon binding to extracellular ligands [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. High-resolution structural data are perhaps more available for the inactive integrin conformer and for the transmembrane domains, where we know that the longer transmembrane α-helix of the β subunit is tilted relative to the shorter α-helix of the α subunit that it binds to [61] (Figure 3A). In the active integrin conformation, it is thought that the angle of the transmembrane α-helix of the β subunit is distinct from that of its tilt seen in the inactive when bound to the α subunit [62]. Hinging movements of the integrin transmembrane domains during integrin activation were speculated over two decades ago [40,41,44,63,64]. For example, movements of the membrane proximal region in and out of the membrane were suggested to provide a venue for integrin signaling [65]. The nuclear magnetic resonance (NMR) structure of the transmembrane domain of integrin β3 in phospholipid bicelles and detergent micelles revealed that it forms a 30-residue α-helix that is embedded in the hydrophobic bicelle core. The length of this transmembrane α-helix suggested a pronounced tilt within a typical lipid bilayer, whereby the charged lysine (residue 716) snorkels out of the lipid core while hydrophobic residues (residues 717 through 721), in particular leucines, remain immersed in the membrane. This NMR structure of the transmembrane domains of the non-covalently-associated integrin αIIbβ3 in small bicelles also revealed that a so-called inner membrane clasp (IMC) stabilizes the integrin heterodimer at the intracellular side, while this role is performed by the ectodomain and outer membrane clasp (OMC) on the extracellular side [66]. The structure suggested a straight α-helix for integrin α and an α-helix tilt of ~25° for integrin β. The α-helix tilt of integrin β might control bidirectional transmembrane signaling and changes in the thickness of the membrane seem to affect integrin signaling by modulating the tilt angle.
We posit that thicker membranes might force integrin repartitioning. The membrane bilayer clearly plays a role in the integrin activation process [67]. For example, the binding of cholesterol to glycosylphosphatidylinositol-anchored proteins and lymphocyte function-associated antigen 1 (LFA-1) integrin (also known as integrin αLβ2 or CD11a/CD18) was visualized by single-molecule near-field optical microscopy in immune cells [68]. Additionally, upon binding to lipid raft components, the integrin conformation is altered [37,69,70] and thus function. For example, active integrin α4 of integrin α4β1 bound cholesterol to then mediate adhesion of T lymphocytes [71].
4. Integrin Signaling
Integrins are bidirectional transmembrane signaling and adhesion metalloprotein receptors involved in cell survival, cell migration, cell proliferation, and cell differentiation [72,73]. Integrins play key roles in hematopoiesis, vascular development, immune and inflammatory responses, as well as hemostasis and arterial thrombosis. By binding to the extracellular matrix, integrins regulate cellular responses to several physical and chemical cues [74] that control a variety of biological processes. The binding of integrins to extracellular ligands is stabilized by the binding to intracellular scaffolding proteins such as vinculin or talin, thereby linking integrins to the actin cytoskeleton and mediating mechano-transduction [75,76].
Integrins signal in both directions with distinct biological outcomes (Figure 4). Inside-out integrin signaling is initiated by the binding of an intracellular activator. For example, the binding of talin or kindlins to the integrin β cytoplasmic tail domain leads to conformational changes that activate integrins by increasing the integrin affinity for extracellular ligands. The resulting strong interactions between integrins and the extracellular matrix enable integrins to transmit forces for extracellular matrix remodeling and assembly as well as cell migration.
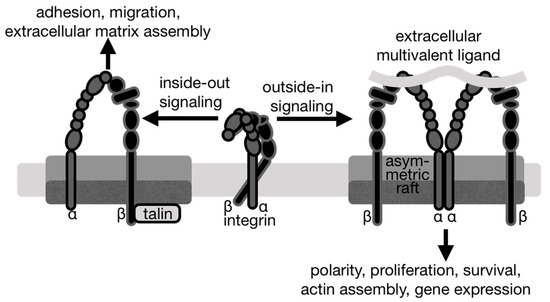
Figure 4.
Integrins signal in both directions with different outcomes. Left, inside-out integrin signaling is initiated by the binding of an intracellular activator such as talin or kindlin to the integrin β cytoplasmic tail domain. This leads to conformational changes that activate integrins by increasing the integrin affinity for extracellular ligands. The resulting strong interactions between integrins and the extracellular matrix enable integrins to transmit forces for extracellular matrix remodeling and assembly as well as cell migration. Center, in its inactive, low-affinity state, the integrin is in a bent conformation. Right, during traditional outside-in signaling, multivalent extracellular ligands bind to integrins, thereby causing a conformational change and integrin clustering.
In contrast, during traditional outside-in signaling, extracellular ligands bind to integrins, causing a conformational change and integrin clustering due to the multivalent nature of the ligands. Both inside-out and outside-in signaling lead to the regulation of cell polarity, cell survival, and cell proliferation, as well as gene expression and the remodeling of the actin cytoskeleton [77]. There are several proteins that interact with integrins within lipid rafts and an important question remains, how mechanistically lipid rafts affect these processes.
5. Lipid Rafts in Integrin Partitioning
Lipid rafts are involved in integrin-mediated signal transduction pathways initiated by cell adhesion [16,78,79], as well as in integrin clustering at focal adhesions, which regulates integrin activation (38). Lipid rafts regulate integrin signaling by partitioning activated integrins in microdomains where they form specific interactions with upstream and downstream signaling molecules [80,81,82]. The recruitment of integrins α4β1 and αLβ2 to lipid raft domains occurs specifically during inside-out signaling, upon binding to and activation by extracellular matrix ligands [71]. These integrins are excluded from lipid rafts without stimulation but mobilized to the lipid raft compartment upon stimulation [71]. In oligodendrocytes, activation of integrin α6β1 with manganese increases integrin α6β1 concentration in lipid rafts [83]. Lipid rafts might sequester activated integrins by providing a more favorable membrane environment for the distinct conformation of activated integrins since it is the activated form of integrin that preferentially localizes to the cholesterol-enriched membrane lipid rafts [83] (Figure 3 and Figure 4). While little is known about the interaction of the integrin transmembrane α-helices with membrane lipids, an invariant lysine at the C-terminus of both integrin subunits seems to be important for the lateral mobility of integrins in the membrane [65].
Several integrins have been found in lipid rafts, such as integrins LFA-1 [26,71], αVβ3 [27], α6β4 [84], and β1 [85,86], also in α6β1 [87]. Platelet integrin αIIbβ3 partitions in the 1,2-dioleoyl-sn-glycero-3-phosphocholine-rich liquid-disordered phase and is excluded from the cholesterol- and sphingomyelin-rich liquid-ordered phase [88]. Interestingly, activated platelets form lipid rafts that act as foci and integrate adhesion and signaling molecules [89]. In leukocytes, integrin activation leads to lipid raft association, which again has a role in cell adhesion [71]. Further, integrin α5 translocates into lipid rafts upon activation [90]. In rat fibroblasts, changes of phospholipids, cholesterol levels, and membrane fluidity reduced binding of integrin α5β1 to fibronectin at focal adhesions [91]. In T cells, integrins α4β1 and αLβ2 colocalize with the ganglioside lipid raft marker [71]. Cell surface integrins have also been shown to localize to lipid rafts [16,26,27,71]. Integrins α6β4 and αLβ2 are enriched in lipid rafts, whereby integrin α6β4 localized in rafts promotes movement of integrin α6β4 to the rafts [92].
Loss of cell adhesion alters the localization and raft partitioning of many non-raft molecules [93], including integrins [16,26,27,71]. In migrating cells, lipid rafts preferentially localize at the leading edges where new integrin-mediated adhesion to the extracellular matrix sites is occurring [94]. For example, during tumor cell migration of melanoma cells, lipid rafts control lamellipodia formation through the cytoskeleton-mediated recruitment of integrins β1 and β3 to the leading edge [95]. Additionally, lipid rafts might play a crucial role in platelet-derived growth factor receptor α-mediated regulation of differentiation based on integrin partitioning. For example, in oligodendrocytes, the platelet-derived growth factor receptor α binds to integrin αVβ3, thereby promoting proliferation. Upon differentiation, most of platelet-derived growth factor receptor α localizes to rafts, where it binds to integrin α6β1, thereby switching to survival [87]. Further, integrin-mediated adhesion signaling appears to be involved in various causes of neural injury [96]. Integrin αVβ3 is upregulated in astrocytes upon neural injury and inflammation, leading to astrocyte activation and migration via αVβ3 integrin interactions with the receptor thymocyte differentiation antigen 1 (Thy-1)/cluster of differentiation 90 (CD90) in neurons [97]. In this process, αVβ3 integrin restricts Thy-1/CD90 into nanoclusters, which activates neural signaling via RhoA and Rho-associated protein kinase (ROCK) to induce altered actin dynamics and neurite retraction [98].
6. Integrins and Lipid Raft Components
Integrin-mediated cell adhesion requires intact membrane domains and is dependent on the lipid raft components such as cholesterol, phospholipids, sphingolipids, glycosylphosphatidylinositol-anchored proteins, and the actin cytoskeleton [18,99], as well as divalent cations such as calcium and manganese [100]. Despite decades of studies on integrin activation, the roles that the lipids play in the plasma membrane, in integrin inside-out and outside-in activation steps, have not been fully studied, probably due to the difficulty of working with detergent-resistance membranes. Biochemical experiments might be aided by the use of lipid compositions that more closely resemble the platelet cell membrane by having biomimetic lipid compositions of the platelet membrane [101,102]. In recent years, some insights into the interactions between integrins and lipid raft components have been described. The first lipid ligand for integrin, oxysterol 25-hydrocholesterol, was recently shown to bind to integrins α5β1 and αVβ3, but this ligand binds to the integrin headpiece, thereby activating integrin and the focal adhesion kinase pathway [103]. Caveolin-1, the major lipid raft marker, was also found in high concentrations in integrin α6β4 fractions, an interaction which contributed to tissue regeneration via the lipid raft-mediated integrin signaling pathway [104].
7. The Role of Cholesterol
Cholesterol is found in both leaflets of the membrane bilayer [105]. Its concentration directly influences integrins such as integrins αVβ3 and α5β1 [27,91] by affecting both adhesion and signaling by integrins. There is a strong correlation between membrane cholesterol and integrin signaling and adhesion, given that integrin assembly at cellular focal adhesions is dependent on the membrane cholesterol level [16]. While focal adhesions are highly enriched in cholesterol, cell detachment internalizes lipid rafts and decreases lipid order and the membrane cholesterol level. Further, a synthetic bile acid-phospholipid conjugate that inhibits integrin causes integrin internalization via lipid rafts [106]. Indeed, integrin β1 colocalizes with cholesterol in lipid rafts while ionizing radiation separates integrin β1 from cholesterol rafts [107]. Clearly, integrin adhesion and signaling correlates with the recruitment of these membrane proteins to lipid rafts.
Integrin-mediated cell adhesion to the extracellular matrix regulates the localization of cholesterol-enriched vesicles to the membrane [93], resulting in altered membrane composition. The high concentration of cholesterol in focal adhesions increases the membrane order, and adhesion sites in general have a similarly high membrane order as seen in rafts [108] and a higher order compared to caveolae [108]. Cell detachment triggers the internalization of cholesterol accompanied by decreased lipid order and plasma membrane cholesterol concentrations, as well as the lipid raft marker, glycophosphatidylinositol-anchored protein [16]. Further, during heart disease and metabolic disorders, cholesterol increases in the tissue [109,110,111,112] and this likely is affecting integrin signaling through lipid raft regulation [111].
Integrin sequestration changes are also dependent on cholesterol concentrations in lipid rafts [113]. Ligands and bilayer asymmetry influence the sequestering of integrin into rafts and suggest a role of lipid packing and bilayer thickness that characterize the liquid-ordered and liquid-disordered domains in integrin sequestering. For example, integrin αVβ3 is sequestered into the liquid-disorder region in the absence of ligands but into the liquid-ordered domains upon binding to vitronectin. Despite the clear significance of cholesterol in integrin sequestering and recruitment during raft-mediated integrin adhesion and signaling, little is known about this biophysical regulation, probably due to the challenges attributed to the size and transient nature of raft domains in the plasma membrane [22,114].
Recent data showed that systematic changes in the membrane cholesterol concentration impact the sequestration of integrin αVβ3 in coexisting liquid-ordered and liquid-disordered domains, thus being qualitatively distinct in sequestering in the absence and presence of its native ligand vitronectin [113]. Nevertheless, the role of cholesterol in integrin sequestration and recruitment during lipid membrane raft-mediated integrin signaling and adhesions remains poorly understood, perhaps partly due to the small size and transient nature of raft domains [22,114].
8. Integrin-Glycosphingolipids Interactions
Mixtures of cholesterol, 1,2-dioleoyl-sn-glycero-3-phosphocholine, and dipalmitoylphosphatidylcholine form coexisting liquid-ordered and liquid-disordered domains, whereby molar concentrations influence integrin αVβ3 sequestration regardless of its ligand vitronectin and without clustering [113]. Significantly, focal adhesions have high concentrations of cholesterol [108] and can thus play a role in regulating integrin function [16]. Additionally, focal adhesions sense force and alter in size upon environmental stimuli during cell spreading and cell migration, whereby integrins diffuse into and out of focal adhesions [115,116,117].
Glycosphingolipids that are mainly in the outer leaflet also modulate integrin activity [118], whereby galacto-gluco-ceramide-mono-N-acetyl neuramic acid directly binds to integrin α5β1. Clustering of ganglioside-enriched lipid rafts regulate the activity of integrin β1 [119]. Further, integrin α5 was found to be the protein most elevated from lipid rafts of endothelial cells that was exposed to oscillatory shear stress, which also increased the level of activated integrin α5 regulated by membrane cholesterol and fluidity [120,121]. Lipid raft molecules dissociate upon cholesterol removal [122]. In vivo studies suggested a mechano-transduction mechanism that is integrin- and raft-dependent [120].
9. Role of Raft Thickness
In addition to native ligands, the bilayer asymmetry also influences the sequestration of integrins in raft-mimicking liquid mixtures [123,124]. The bilayer asymmetry dependency during integrin sequestration demonstrates the significance in the different lipid packing and bilayer thickness between liquid-ordered and liquid-disordered domains. As discussed above, cholesterol might regulate integrin distribution by changing the bilayer thickness and lipid packing densities in liquid-ordered and liquid-disordered domains. The cholesterol-mediated integrin sequestration is due to the bilayer thickness of coexisting liquid-ordered and liquid-disordered domains, whereby cholesterol might regulate the distribution of integrin αVβ3 by altering the bilayer thickness and lipid packing densities of the liquid-disordered and liquid-ordered domains [113].
The greater thickness of the ordered lipid structure in the raft domain compared to the non-raft membrane could be more favorable for the activated integrin transmembrane domain structure. Thereby, lipid rafts might stabilize the activated integrin conformation. In analogy with the immune system and the T cell receptor activating components of the signaling complex pre-assembled in the lipid rafts, these microdomains might provide a favorable environment in integrin clustering and maintaining downstream signaling [125].
The lipid bilayer separates its components laterally [6] and the domain formation is controlled by the bilayer thickness mismatch [126,127]. Cholesterol enhances lipid packing and bilayer thickness, whereby 29 mol% of cholesterol in a 1,2-dioleoyl-sn-glycero-3-phosphocholine bilayer increases its thickness by 2.8 Å [128]. This affects the hydrophobic matching conditions between the thicknesses of hydrophobic membranes versus the transmembrane region of membrane proteins. The regions of thick and thin membranes within the plasma membrane result from spontaneous partitioning of long-chain saturated lipids from short-chain polyunsaturated lipids into liquid-ordered and liquid-disordered domains [6,7]. While the reorganization of lipid phases and integrins plays a role in cell adhesion [6,71], it is unclear whether the liquid-ordered or liquid-disordered phases drive integrin reorganization. Integrin imaging by direct stochastic optical reconstruction microscopy (dSTORM) and stimulated emission depletion (STED) showed that the active and inactive forms of integrin β1 are localized to distinct types of clustering, but with which lipid domains the integrins were associated was not determined [129]. Since the membrane order is decreased by the detachment of integrin-mediated adhesion from a substrate [93,108], adhesion likely causes the transition from the liquid-ordered or liquid-disordered phase (Figure 3). In the absence of vitronectin, integrin αVβ3 shows a preference for liquid-disordered domains [113], which suggests a significance of domain-specific lipid packing as a biophysical regulator of integrin sequestration. Indeed, incorporation of membrane-spanning proteins into the cholesterol-enriched microdomains is energetically unfavorable [130] and not due to the hydrophobic mismatch, since the thickness of the integrin α and β transmembrane α-helices are 31.6 ± 3.4 and 30.0 ± 3.6 Å, respectively (https://opm.phar.umich.edu/), which is similar to the hydrophobic thickness of the liquid-disordered phase (33 ± 1 Å). However, upon binding to vitronectin, integrin αVβ3 translocates to the liquid-ordered domain, which is not due to the lipid packing differences, as here the hydrophobic mismatch is significant with the liquid-ordered thickness of 38 ± 1 Å [131] representing the biophysical mechanism of integrin sequestration. Thus, integrins might adjust their structure to overcome the hydrophobic mismatch as seen, for example, for rhodopsin, which adjusts its conformation or oligomerizes according to the bilayer thickness [132]. Interestingly, mutations of integrin αIIbβ3 that affect integrin activation [63] have perturbed transmembrane α-helix packing with crossing angles of the two integrin transmembrane α-helices 10° for the mutant, compared to 35° for the wild type [133].
Integrin α5β1 plays important physiological roles in metabolic syndrome and coronary heart disease. For example, inflammation activates β1 to cause contraction of endothelial cells and vascular leakage [134]. Inflammation also causes an influx of cholesterol, which potently alters lipid raft structures [111] and the thickness of the membrane. The transmembrane interactions of the α and β subunits keep integrin in its inactive, low affinity conformer [49,63,135,136]. In this inter-subunit interaction, integrin β is tilted by at least 25° [62,66,137,138] (Figure 3 and Figure 4). For integrin β, the thicker rafts might accommodate activated integrin by decreasing the integrin β transmembrane tilt [139]. However, this mechanism does not seem possible for integrin α, which is already almost completely straight in its inactive conformer [66,138]. Therefore, integrin α might instead overcome its hydrophobic mismatch in the thicker rafts by oligomerization (Figure 3 and Figure 4). Indeed, transmission electron microscopy of purified integrin αIIbβ3 showed clear dimers and trimer when incubated with activating manganese, which were not seen when incubated with calcium [140]. An integrin αIIb homodimer model confirmed this possibility [141] and a peptide of its transmembrane region oligomerizes in sodium dodecyl sulfate-polyacrylamide gel electrophoresis [142]. Integrin αα and ββ homodimers were found in zwitterionic or acidic micelles [143] as well as in biological membranes [141,143,144], which might function in integrin clustering by binding to multivalent ligands [49,145].
In general, the dimerization of transmembrane α-helices is energetically driven by the hydrophobic mismatch [146]. For example, the thickness of the lipid bilayer influences the monomer-dimer equilibrium of glycophorin A that dimerizes most efficiently under hydrophobic matching conditions [147]. That study further showed that cholesterol promoted self-association of transmembrane α-helices by affecting the order of the lipid acyl chains. Indeed, the lipid acyl chain order generally determines the strength and stability of transmembrane helix-helix interactions [140]. Alternatively, clustering in itself might drive the ordering of lipids and thus create a lipid raft, given that areas of higher lipid order would tend to fuse and thereby favoring the clustering of signaling molecules [83]. While the role of anionic lipids in inside-out signaling is starting to be appreciated, the molecular mechanisms remain controversial [148,149,150].
10. Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2) Lipid Microdomains
Another form of lipid organization occurs with negatively charged phospholipids, such as phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), which plays key roles in cell adhesion. PI(4,5)P2 is present in the inner leaflet of the plasma membrane at relatively low concentrations overall (~1% in average), but is enriched at focal adhesion sites by the enzyme phosphatidylinositol 4-phosphate 5-kinase type Iγ (PIP5KIγ) [151,152]. The longer PIP5KIγ668 isoform (human numbering) contains additional C-terminal residues that target the enzyme to focal adhesions by interacting with the talin head domain [153,154]. In the presence of bivalent cations or basic peptides, PI(4,5)P2 can cluster into microdomains with a very high PI(4,5)P2 content [155,156]. Formation of PI(4,5)P2 microdomains was demonstrated in model membrane systems in the presence of calcium and to a lesser extent with magnesium [155,157], but has also been observed in cells [158,159]. Using super-resolution stimulated emission depletion microscopy, PI(4,5)P2 microdomains were observed in pheochromocytoma (PC) 12 cells associated with the SNAP receptor protein syntaxin-1A and were found to have a size of approximately 70 nm and an estimated PI(4,5)P2 content as high as 82% [159].
How these PI(4,5)P2 microdomains interact or coexist with cholesterol-rich lipid rafts has been a matter of debate. Initial studies suggested that PI(4,5)P2 partitions into lipid rafts based on the fact that depletion of cholesterol lead to delocalization of PI(4,5)P2 microdomains [160]. This view was supported by the fact that several signaling events are apparently triggered by both lipid rafts and associated PI(4,5)P2 microdomains [161,162]. However, other studies have refuted the existence of PI(4,5)P2 inside lipid rafts [163], arguing that the unsaturated acyl chains found in PI(4,5)P2 would indeed not seem compatible with an ordered lipid raft domain. Nevertheless, multiple studies show that cholesterol promotes PI(4,5)P2 clustering into microdomains [164,165,166]. The exact mechanism for this is unclear, but altering the charge state of PI(4,5)P2, providing hydrogen bonds to the PI(4,5)P2 headgroup, or altering the lateral mobility of PI(4,5)P2 have been suggested as possible reasons [164,165,167].
Several of the core focal adhesion proteins interact with PI(4,5)P2, including talin, vinculin, and focal adhesion kinase [148,168,169,170,171,172,173]. In all these cases, PI(4,5)P2 binding induces conformational changes, which result in enhanced adhesion strength by talin and vinculin [149,174] or increased adhesion signaling via focal adhesion kinase [175,176]. As discussed above for lipid rafts, an intriguing question is whether PI(4,5)P2 microdomains may play a role in integrin clustering and/or assembly and stabilization of the mature focal adhesion complex. This might occur either via colocalizing focal adhesion proteins to PI(4,5)P2 membrane domains due to the high local density of PI(4,5)P2, or by inducing more stable protein assemblies, such as oligomeric states of focal adhesion proteins. Interestingly, PI(4,5)P2 is found to induce oligomerization and clustering in various systems also outside focal adhesions, including the adhesion molecule CD44 or the serotonin transporter SERT [177,178]. In focal adhesions, PI(4,5)P2 is shown to promote oligomerization of vinculin and focal adhesion kinase. In the case of vinculin, PI(4,5)P2 bridges vinculin molecules via different binding sites to promote higher-order oligomers [170,171,173], whereas for focal adhesion kinase, binding to PI(4,5)P2 induces conformational changes that promote focal adhesion kinase oligomers via protein/protein interactions [175]. Whether such effects can contribute to integrin clustering is unclear, but based on observations discussed above, the presence of high density PI(4,5)P2 microdomains can be expected to have important roles in adhesion stability, maturation, and signaling via a number of mechanisms, such as promoting colocalization, oligomerization, and conformational changes in a number of focal adhesion proteins.
11. The Role of Integrin Acylation
In general, acylation (addition of myristol or palmitoyl) but not prenylation (addition of farnesyl or geranyl-geranyl) targets proteins to lipid rafts [179,180]. Myristoyl or prenyl group attachment mainly results in higher affinity for membranes and promotes intramolecular and intermolecular protein-protein interactions [181]. While geranylgeranyl can anchor proteins in the membrane, farnesyl cannot [181]. For example, integrin β4 is palmitoylated on several cysteines (residues 732, 736, 738, 742) at the membrane-proximal segment of the β4 tail [84,182,183,184], residues that are not conserved in platelet integrin β3 [62], which is important for membrane binding and lipid raft localization [181] and is necessary for integrin α6β4 incorporation into lipid rafts where it interacts with growth factor receptors and enhances invasiveness of cancer cells [84,185,186]. Further, palmitoylation of integrins α3, β4, and α6 affects their interaction with tetraspanin [185,187].
Integrins α3β1 and α6β1 are not palmitoylated [84] and might associate with tetraspanins [188] which are palmitoylated and could promote integrin incorporation into lipid rafts [84]. The concept that a palmitoylated protein moves between a ganglioside (GM1) raft and PI(4,5)P2 domains was first described by Hansen [189]. While palmitoylation of integrin β4 is necessary for integrin α6β4 incorporation into lipid rafts, it is not required for its binding to laminin-5 nor assembly of hemidesmosomes or adhesion [84]. This again suggests that the membrane plays a role in integrin activation. More studies are required to better understand the role and mechanism of acylation of integrins during integrin activation.
12. Conclusions and Future Directions
Lipid rafts regulate signaling pathways such as integrin signaling by partitioning activated integrins in these microdomains where they form specific interactions with upstream and downstream signaling molecules. Lipid rafts might sequester activated integrins by providing a more favorable membrane environment for the distinct conformation of activated integrins. Two decades ago, tilting of the transmembrane domain of inactive integrins was predicted and then confirmed experimentally [61]. At the same time, a slight movement of one or both integrin transmembrane domains in and out of the membrane with interacting proteins to mask exposed integrin transmembrane regions was already noted as a mechanism for integrin activation [65,190]. However, despite decades of studies on integrin activation, the roles that the lipids in the plasma membrane play in these inside-out and outside-in activation steps are still not fully understood. The complex lipid/protein environment in lipid rafts is challenging to reconstitute in vitro, hence no high-resolution structural information is currently available that could provide detailed mechanistic insight into lipid-mediated integrin activation. Future advances that will help to better understand the lipid raft structure and what exactly stabilizes them together with the recent revolution in cryogenic electron microscopy are likely to provide such insights at atomic detail. Such studies will be directly relevant to our understanding of complex diseases in humans.
Funding
D.L. acknowledges support from the Spanish Ministry of Science, Innovation and Universities for the Spanish State Research Agency Retos Grant RTI2018-099318-B-I00, cofunded by the European Regional Development Fund (FEDER). T.I. is supported by grants from the National Institute of Health, the Department of Defense, The National Science Foundation (NSF-RAPID award MCB-2033939, using funds provided by the 2020 CARES Act), and by start-up funds provided to The Scripps Research Institute from the State of Florida.
Acknowledgments
We are grateful for many insightful discussions with Scott Hansen, Marina Primi Candido, and Rangarajan Erumbi (all from The Scripps Research Institute). To keep this review succinct, we apologize for not discussing all research that contributed to providing advances in this topic. This is publication number 29873 from The Scripps Research Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Danielli, J.F.; D’avson, H. A contribition to the theory of permeability of thin films. J. Cell. Comp. Physiol. 1935, 5, 495–508. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Engelman, N.M. Membranes are more mosaic than fluid. Nature 2005, 438, 578–580. [Google Scholar] [CrossRef]
- Goni, F.M. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1467–1476. [Google Scholar] [CrossRef]
- Walter, H.; Brooks, D.E. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995, 361, 135–139. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Boil. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The Fluid—Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40years. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.N.; Pavel, M.A.; Wang, H.; Hansen, S.B. Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183091. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure of Detergent-Resistant Membrane Domains: Does Phase Separation Occur in Biological Membranes? Biochem. Biophys. Res. Commun. 1997, 240, 1–7. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Schroeder, R.J.; Ahmed, S.N.; Zhu, Y.; London, E.; Brown, D.A. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Boil. Chem. 1998, 273, 1150–1157. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and Function of Sphingolipid- and Cholesterol-rich Membrane Rafts. J. Boil. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Boil. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T.K. Paradigm Shift of the Plasma Membrane Concept from the Two-Dimensional Continuum Fluid to the Partitioned Fluid: High-Speed Single-Molecule Tracking of Membrane Molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Boil. 1998, 14, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.A.; Alderson, N.B.; Kiosses, W.B.; Chiang, H.-H.; Anderson, R.G.W.; Schwartz, M.A. Integrins Regulate Rac Targeting by Internalization of Membrane Domains. Science 2004, 303, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Golub, T.; Wacha, S.; Caroni, P. Spatial and temporal control of signaling through lipid rafts. Curr. Opin. Neurobiol. 2004, 14, 542–550. [Google Scholar] [CrossRef]
- Jacobson, K.; Mouritsen, O.G.; Anderson, R.G.W. Lipid rafts: At a crossroad between cell biology and physics. Nature 2007, 9, 7–14. [Google Scholar] [CrossRef]
- Viola, A.; Gupta, N. Tether and trap: Regulation of membrane-raft dynamics by actin-binding proteins. Nat. Rev. Immunol. 2007, 7, 889–896. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Gerner-Smidt, K.; Ivanov, A.I.; Nusrat, A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am. J. Physiol. Liver Physiol. 2008, 295, G965–G976. [Google Scholar] [CrossRef]
- Oneyama, C.; Hikita, T.; Enya, K.; Dobenecker, M.-W.; Saito, K.; Nada, S.; Tarakhovsky, A.; Okada, M. The Lipid Raft-Anchored Adaptor Protein Cbp Controls the Oncogenic Potential of c-Src. Mol. Cell 2008, 30, 426–436. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2009, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, K.; Uronen, R.-L.; Blom, T.; Li, S.; Bittman, R.; Lappalainen, P.; Peränen, J.; Raposo, G.; Ikonen, E.; Uronen, R.-L. LDL Cholesterol Recycles to the Plasma Membrane via a Rab8a-Myosin5b-Actin-Dependent Membrane Transport Route. Dev. Cell 2013, 27, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Moissoglu, K.; Kiessling, V.; Wan, C.; Hoffman, B.D.; Norambuena, A.; Tamm, L.K.; Schwartz, M.A. Regulation of Rac1 translocation and activation by membrane domains and their boundaries. J. Cell Sci. 2014, 127, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.F. Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J. Cell Biol. 2015, 211, 173–190. [Google Scholar] [CrossRef]
- Krauss, K.; Altevogt, P. Integrin Leukocyte Function-associated Antigen-1-mediated Cell Binding Can Be Activated by Clustering of Membrane Rafts. J. Boil. Chem. 1999, 274, 36921–36927. [Google Scholar] [CrossRef]
- Green, J.M. Role of cholesterol in formation and function of a signaling complex involving alphavbeta3, integrin-associated protein (CD47), and heterotrimeric G proteins. J. Cell Biol. 1999, 146, 673–682. [Google Scholar] [CrossRef]
- Bodin, S.; Utton, M.A.; Noble, W.; Hill, J.E.; Anderton, B.H.; Hanger, D.P. Integrin-dependent interaction of lipid rafts with the actin cytoskeleton in activated human platelets. J. Cell Sci. 2005, 118, 759–769. [Google Scholar] [CrossRef][Green Version]
- Jeon, J.H.; Kim, S.K.; Kim, H.J.; Chang, J.; Ahn, C.M.; Chang, Y.S. Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer 2010, 69, 165–171. [Google Scholar] [CrossRef]
- Murai, T. The Role of Lipid Rafts in Cancer Cell Adhesion and Migration. Int. J. Cell Boil. 2012, 2012, 763283. [Google Scholar] [CrossRef]
- Wang, R. Lipid raft regulates the initial spreading of melanoma A375 cells by modulating beta1 integrin clustering. Int. J. Biochem. Cell Biol. 2013, 45, 1679–1689. [Google Scholar] [CrossRef]
- Orädd, G.; Shahedi, V.; Lindblom, G. Effect of sterol structure on the bending rigidity of lipid membranes: A 2H NMR transverse relaxation study. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Inder, K.L.; Shah, A.; Cristino, A.S.; McKie, A.B.; Gabra, H.; Davis, M.J.; Hill, M.M. Integrative Analysis of Subcellular Quantitative Proteomics Studies Reveals Functional Cytoskeleton Membrane–Lipid Raft Interactions in Cancer. J. Proteome Res. 2016, 15, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Stickney, J.T.; Bacon, W.C.; Rojas, M.; Ratner, N.; Ip, W. Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res. 2004, 64, 2717–2724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McClatchey, A.I.; Giovannini, M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005, 19, 2265–2277. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Mandati, V.; Zheng, J.; Sharff, A.J.; Bricogne, G.; Griffin, P.R.; Kissil, J.; Izard, T. Lipid binding promotes the open conformation and tumor-suppressive activity of neurofibromin 2. Nat. Commun. 2018, 9, 1338. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Banno, A.; Ginsberg, M.H. Integrin activation. Biochem. Soc. Trans. 2008, 36, 229–234. [Google Scholar] [CrossRef]
- Lee, J.-O.; Rieu, P.; Arnaout, M.; Liddington, R. Crystal structure of the A domain from the a subunit of integrin CR3 (CD11 b/CD18). Cell 1995, 80, 631–638. [Google Scholar] [CrossRef]
- Lu, C.; Takagi, J.; Springer, T.A. Association of the Membrane Proximal Regions of the α and β Subunit Cytoplasmic Domains Constrains an Integrin in the Inactive State. J. Boil. Chem. 2001, 276, 14642–14648. [Google Scholar] [CrossRef]
- Takagi, J.; Erickson, H.P.; Springer, T.A. C-terminal opening mimics ‘inside-out’ activation of integrin alpha5beta1. Nat. Genet. 2001, 8, 412–416. [Google Scholar] [CrossRef]
- Xiong, J.-P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal Structure of the Extracellular Segment of Integrin alpha Vbeta 3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-P.; Stehle, T.; Zhang, R.; Joachimiak, A.; Frech, M.; Goodman, S.L.; Arnaout, M.A. Crystal Structure of the Extracellular Segment of Integrin alpha Vbeta 3 in Complex with an Arg-Gly-Asp Ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Petre, B.M.; Walz, T.; Springer, T.A. Global Conformational Rearrangements in Integrin Extracellular Domains in Outside-In and Inside-Out Signaling. Cell 2002, 110, 599–611. [Google Scholar] [CrossRef]
- Arnaout, M.A. Integrin structure: New twists and turns in dynamic cell adhesion. Immunol. Rev. 2002, 186, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Carman, C.V.; Springer, T.A. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science 2003, 301, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Springer, T.A. Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 2003, 15, 547–556. [Google Scholar] [CrossRef]
- Xiong, J.-P.; Stehle, T.; Goodman, S.L.; Arnaout, M.A. A Novel Adaptation of the Integrin PSI Domain Revealed from Its Crystal Structure. J. Boil. Chem. 2004, 279, 40252–40254. [Google Scholar] [CrossRef]
- Luo, B.-H.; Springer, T.A.; Takagi, J. A Specific Interface between Integrin Transmembrane Helices and Affinity for Ligand. PLoS Boil. 2004, 2, e153. [Google Scholar] [CrossRef]
- Arnaout, M.A.; Mahalingam, B.; Xiong, J.-P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Boil. 2005, 21, 381–410. [Google Scholar] [CrossRef]
- Adair, B.D. Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J. Cell Biol. 2005, 168, 1109–1118. [Google Scholar] [CrossRef]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, M.A.; Goodman, S.L.; Xiong, J.P. Structure and mechanics of integrin-based cell adhesion. Curr. Opin. Cell Biol. 2007, 19, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.P. Crystal structure of the complete integrin alphaVbeta3 ectodomain plus an alpha/beta transmembrane fragment. J. Cell. Biol. 2009, 186, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Eng, E.T. Intact alphaIIbbeta3 integrin is extended after activation as measured by solution X-ray scattering and electron microscopy. J. Biol. Chem. 2011, 286, 35218–35226. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, B.; Ajroud, K.; Alonso, J.L.; Anand, S.; Adair, B.D.; Horenstein, A.L.; Malavasi, F.; Xiong, J.-P.; Arnaout, M.A. Stable coordination of the inhibitory Ca2+ ion at the metal ion-dependent adhesion site in integrin CD11b/CD18 by an antibody-derived ligand aspartate: Implications for integrin regulation and structure-based drug design. J. Immunol. 2011, 187, 6393–6401. [Google Scholar] [CrossRef]
- Adair, B.D.; Xiong, J.-P.; Alonso, J.L.; Hyman, B.T.; Arnaout, M.A. EM Structure of the Ectodomain of Integrin CD11b/CD18 and Localization of Its Ligand-Binding Site Relative to the Plasma Membrane. PLoS ONE 2013, 8, e57951. [Google Scholar] [CrossRef]
- Dai, A.; Ye, F.; Taylor, D.W.; Hu, G.; Ginsberg, M.H.; Taylor, K.A. The Structure of a Full-length Membrane-embedded Integrin Bound to a Physiological Ligand. J. Boil. Chem. 2015, 290, 27168–27175. [Google Scholar] [CrossRef]
- Xu, X.P. Three-Dimensional Structures of Full-Length, Membrane-Embedded Human αIIbβ3 Integrin Complexes. Biophys. J. 2016, 110, 798–809. [Google Scholar] [CrossRef]
- Cormier, A. Cryo-EM structure of the alphavbeta8 integrin reveals a mechanism for stabilizing integrin extension. Nat. Struct. Mol. Biol. 2018, 25, 698–704. [Google Scholar] [CrossRef]
- Lau, T.-L.; Dua, V.; Ulmer, T.S. Structure of the Integrin αIIb Transmembrane Segment. J. Boil. Chem. 2008, 283, 16162–16168. [Google Scholar] [CrossRef]
- Kim, C.; Schmidt, T.; Cho, E.-G.; Ye, F.; Ulmer, T.S.; Ginsberg, M.H. Basic amino-acid side chains regulate transmembrane integrin signalling. Nature 2011, 481, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.E.; Diaz-Gonzalez, F.; Leong, L.; Wu, C.; A McDonald, J.; Shattil, S.J.; Ginsberg, M.H. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Boil. Chem. 1996, 271, 6571–6574. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, K.E. Transmembrane signal transduction of the alpha (IIb) beta (3) integrin. Protein Sci. 2002, 11, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Nilsson, I.; Von Heijne, G.; Johansson, S. Determination of the Border between the Transmembrane and Cytoplasmic Domains of Human Integrin Subunits. J. Boil. Chem. 1999, 274, 37030–37034. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.L. The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009, 28, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Hanein, D.; Volkmann, N. Conformational Equilibrium of Human Platelet Integrin Investigated by Three-Dimensional Electron Cryo-Microscopy; Springer Science and Business Media LLC: Berlin, Germany, 2018; Volume 87, pp. 353–363. [Google Scholar]
- Van Zanten, T.S.; Cambi, A.; Koopman, M.; Joosten, B.; Figdor, C.; Garcia-Parajo, M.F. Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc. Natl. Acad. Sci. USA 2009, 106, 18557–18562. [Google Scholar] [CrossRef] [PubMed]
- Van Kooyk, Y.; Figdor, C. Avidity regulation of integrins: The driving force in leukocyte adhesion. Curr. Opin. Cell Boil. 2000, 12, 542–547. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2009, 339, 269–280. [Google Scholar] [CrossRef]
- Leitinger, B.; Hogg, N. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 2002, 115, 963–972. [Google Scholar]
- Howe, A.; Aplin, A.; Alahari, S.K.; Juliano, R.L. Integrin signaling and cell growth control. Curr. Opin. Cell Boil. 1998, 10, 220–231. [Google Scholar] [CrossRef]
- Rossi, F.; Yozgat, Y.; De Stanchina, E.; Veach, D.; Clarkson, B.; Manova, K.; Giancotti, F.G.; Antonescu, C.R.; Besmer, P. Imatinib upregulates compensatory integrin signaling in a mouse model of gastrointestinal stromal tumor and is more effective when combined with dasatinib. Mol. Cancer Res. 2010, 8, 1271–1283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Boil. 2011, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Boil. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Nagasato, A.I.; Yamashita, H.; Matsuo, M.; Ueda, K.; Kioka, N. The distribution of vinculin to lipid rafts plays an important role in sensing stiffness of extracellular matrix. Biosci. Biotechnol. Biochem. 2017, 81, 1136–1147. [Google Scholar] [CrossRef]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Boil. 2010, 11, 288–300. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Eng, C.H.; Schlaepfer, D.D.; Marcantonio, E.E.; Gundersen, G.G. Localized Stabilization of Microtubules by Integrin- and FAK-Facilitated Rho Signaling. Science 2004, 303, 836–839. [Google Scholar] [CrossRef]
- Guan, J.-L. Cell biology: Integrins, Rafts, Rac, and Rho. Science 2004, 303, 773–774. [Google Scholar] [CrossRef]
- De Deyne, P.G.; O’Neill, A.; Resneck, W.G.; Dmytrenko, G.M.; Pumplin, D.W.; Bloch, R.J. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J. Cell Sci. 1998, 111, 2729–2740. [Google Scholar]
- Milner, R. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J. Cell Sci. 1999, 112, 4271–4279. [Google Scholar]
- Felsenfeld, D.P.; Schwartzberg, P.L.; Venegas, A.; Tse, R.; Sheetz, M.P. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nature 1999, 1, 200–206. [Google Scholar] [CrossRef]
- Decker, L.; Ffrench-Constant, C. Lipid Rafts and Integrin Activation Regulate Oligodendrocyte Survival. J. Neurosci. 2004, 24, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Gagnoux-Palacios, L. Compartmentalization of integrin alpha6beta4 signaling in lipid rafts. J. Cell Biol. 2003, 162, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.F. The integrins alpha3beta1 and alpha6beta1 physically and functionally associate with CD36 in human melanoma cells. Requirement for the extracellular domain OF CD36. J. Biol. Chem. 2000, 275, 35264–35275. [Google Scholar] [CrossRef] [PubMed]
- Claas, C.; Stipp, C.S.; Hemler, M.E. Evaluation of Prototype Transmembrane 4 Superfamily Protein Complexes and Their Relation to Lipid Rafts. J. Boil. Chem. 2000, 276, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Baron, W.; Decker, L.; Colognato, H.; Ffrench-Constant, C. Regulation of Integrin Growth Factor Interactions in Oligodendrocytes by Lipid Raft Microdomains. Curr. Boil. 2003, 13, 151–155. [Google Scholar] [CrossRef]
- Gaul, V. The lateral diffusion and fibrinogen induced clustering of platelet integrin alphaIIbbeta3 reconstituted into physiologically mimetic GUVs. Integr. Biol. 2015, 7, 402–411. [Google Scholar] [CrossRef]
- Hussain, N.F.; Siegel, A.P.; Ge, Y.; Jordan, R.; Naumann, C.A. Bilayer Asymmetry Influences Integrin Sequestering in Raft-Mimicking Lipid Mixtures. Biophys. J. 2013, 104, 2212–2221. [Google Scholar] [CrossRef][Green Version]
- Sun, H. Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the beta1-integrin-mediated signaling pathway. Biomaterials 2015, 55, 84–95. [Google Scholar] [CrossRef]
- Gopalakrishna, P.; Chaubey, S.K.; Manogaran, P.S.; Pande, G. Modulation of alpha5beta1 integrin functions by the phospholipid and cholesterol contents of cell membranes. J. Cell. Biochem. 2000, 77, 517–528. [Google Scholar] [CrossRef]
- Leitinger, N.; Huber, J.; Rizza, C.; Mechtcheriakova, D.; Bochkov, V.; Koshelnick, Y.; Berliner, J.A.; Binder, B.R.; Goepfert, T.M.; McCarthy, M.; et al. The isoprostane 8-iso-PGF 2α stimulates endothelial cells to bind monocytes: Difference to thromboxane-mediated endothelial activation. FASEB J. 2001, 15, 1254–1256. [Google Scholar] [CrossRef]
- Norambuena, A.; Schwartz, M.A. Effects of integrin-mediated cell adhesion on plasma membrane lipid raft components and signaling. Mol. Boil. Cell 2011, 22, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Manes, S.; Del Real, G.; LaCalle, R.A.; Lucas, P.; Gómez-Moutón, C.; Sánchez-Palomino, S.; Delgado, R.; Alcamí, J.; Mira, E.; Martínez-A, C. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000, 1, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Wang, R.; Zeng, X. Lipid rafts regulate the lamellipodia formation of melanoma A375 cells via actin cytoskeleton-mediated recruitment of beta1 and beta3 integrin. Oncol. Lett. 2018, 16, 6540–6546. [Google Scholar] [PubMed]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Bill, R.M.; Conner, A.C.; Heath, P.R.; Conner, M.T. Transcriptome Analysis of Gene Expression Provides New Insights into the Effect of Mild Therapeutic Hypothermia on Primary Human Cortical Astrocytes Cultured under Hypoxia. Front. Cell. Neurosci. 2017, 11, 386. [Google Scholar] [CrossRef]
- Lagos-Cabre, R. Alphavbeta3 Integrin regulates astrocyte reactivity. J. Neuroinflamm. 2017, 14, 194. [Google Scholar] [CrossRef]
- Maldonado, H. Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 243–254. [Google Scholar] [CrossRef]
- Pande, G. The role of membrane lipids in regulation of integrin functions. Curr. Opin. Cell Boil. 2000, 12, 569–574. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J. The regulation of integrin function by divalent cations. Cell Adhes. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Murphy, R.C.; Watson, S.P. Platelet lipidomics: Modern day perspective on lipid discovery and characterization in platelets. Circ. Res. 2014, 114, 1185–1203. [Google Scholar] [CrossRef]
- Green, S.M.; Padula, M.P.; Marks, D.C.; Johnson, L. The Lipid Composition of Platelets and the Impact of Storage: An Overview. Transfus. Med. Rev. 2020, 34, 108–116. [Google Scholar] [CrossRef]
- Pokharel, S.M.; Shil, N.K.; Gc, J.B.; Colburn, Z.T.; Tsai, S.-Y.; Segovia, J.A.; Chang, T.-H.; Bandyopadhyay, S.; Natesan, S.; Jones, J.; et al. Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nat. Commun. 2019, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S. Netrin-1-Induced Stem Cell Bioactivity Contributes to the Regeneration of Injured Tissues via the Lipid Raft-Dependent Integrin alpha6beta4 Signaling Pathway. Sci. Rep. 2016, 6, 37526. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Burger, K.; Fahrenholz, F. Cholesterol as Modulator of Receptor Function. Biochemistry 1997, 36, 10959–10974. [Google Scholar] [CrossRef]
- Su, J.; Gan-Schreier, H.; Goeppert, B.; Chamulitrat, W.; Stremmel, W.; Pathil, A. Bivalent Ligand UDCA-LPE Inhibits Pro-Fibrogenic Integrin Signalling by Inducing Lipid Raft-Mediated Internalization. Int. J. Mol. Sci. 2018, 19, 3254. [Google Scholar] [CrossRef] [PubMed]
- Babel, L. Lipid-rafts remain stable even after ionizing radiation induced disintegration of beta1 integrin containing focal adhesions. BMC Res. Notes 2017, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K.; Le Lay, S.; Balasubramanian, N.; Schwartz, M.A. Integrin-mediated adhesion regulates membrane order. J. Cell Boil. 2006, 174, 725–734. [Google Scholar] [CrossRef]
- Linton, M.R.F. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Ed.; MDText. com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Hansen, S.B. The Role of High Cholesterol in Age-Related COVID19 Lethality. bioRxiv 2020. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Boil. 2019, 21, 225–245. [Google Scholar] [CrossRef]
- Ge, Y.; Gao, J.; Jordan, R.; Naumann, C.A. Changes in Cholesterol Level Alter Integrin Sequestration in Raft-Mimicking Lipid Mixtures. Biophys. J. 2018, 114, 158–167. [Google Scholar] [CrossRef]
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Boil. 2006, 7, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Patla, I.; Volberg, T.; Elad, N.; Hirschfeld-Warneken, V.; Grashoff, C.; Fässler, R.; Spatz, J.P.; Geiger, B.; Medalia, O. Dissecting the molecular architecture of integrin adhesion sites by cryo-electron tomography. Nature 2010, 12, 909–915. [Google Scholar] [CrossRef]
- Shibata, A.C.E.; Chen, L.H.; Nagai, R.; Ishidate, F.; Chadda, R.; Miwa, Y.; Naruse, K.; Shirai, Y.M.; Fujiwara, T.K.; Kusumi, A.; et al. Rac1 recruitment to the archipelago structure of the focal adhesion through the fluid membrane as revealed by single-molecule analysis. Cytoskeleton 2013, 70, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Geiger, C.; Agustar, H.K.; Compaoré, G.; Coulibaly, B.; Sié, A.; Becher, H.; Lanzer, M.; Jaenisch, T. Declining malaria parasite prevalence and trends of asymptomatic parasitaemia in a seasonal transmission setting in north-western Burkina Faso between 2000 and 2009–2012. Malar. J. 2013, 12, 27. [Google Scholar] [CrossRef]
- Yates, A.J.; Rampersaud, A. Sphingolipids as Receptor Modulators: An Overview. Ann. N. Y. Acad. Sci. 1998, 845, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.S.; Brown, W.S.; Woodside, D.G.; Vanderslice, P.; McIntyre, B.W. Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics. Immunol. Cell Boil. 2009, 87, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, Y.; Gu, M.; Zhang, L.; Li, D.; Li, H.; Chien, S.; Shyy, J.Y.-J.; Zhu, Y. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2016, 113, 769–774. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Chen, Z.; Yan, M.; Li, B.; Lv, H.; Wang, C.; Xiang, S.; Shi, L.; Zhu, Y.; et al. Coupling of Integrin alpha5 to Annexin A2 by Flow Drives Endothelial Activation. Circ. Res. 2020. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Caiolfa, V.R.; Zamai, M.; Malengo, G.; Andolfo, A.; Madsen, C.D.; Sutin, J.; Digman, M.A.; Gratton, E.; Blasi, F.; Sidenius, N. Monomer–dimer dynamics and distribution of GPI-anchored uPAR are determined by cell surface protein assemblies. J. Cell Boil. 2007, 179, 1067–1082. [Google Scholar] [CrossRef]
- Ge, Y.; Siegel, A.P.; Jordan, R.; Naumann, C.A. Ligand Binding Alters Dimerization and Sequestering of Urokinase Receptors in Raft-Mimicking Lipid Mixtures. Biophys. J. 2014, 107, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Drevot, P.; Langlet, C.; Guo, X.; Bernard, A.; Colard, O.; Chauvin, J.; Lasserre, R.; He, H.-T. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002, 21, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Petruzielo, R.S.; Pan, J.; Drazba, P.; Kučerka, N.; Standaert, R.; Feigenson, G.W.; Katsaras, J. Bilayer Thickness Mismatch Controls Domain Size in Model Membranes. J. Am. Chem. Soc. 2013, 135, 6853–6859. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Boil. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Pencer, J.; Nieh, M.-P.; Katsaras, J. Influence of cholesterol on the bilayer properties of monounsaturated phosphatidylcholine unilamellar vesicles. Eur. Phys. J. E 2007, 23, 247–254. [Google Scholar] [CrossRef]
- Spiess, M. Active and inactive beta1 integrins segregate into distinct nanoclusters in focal adhesions. J. Cell Biol. 2018, 217, 1929–1940. [Google Scholar] [CrossRef]
- Greisen, P.; Lum, K.; Ashrafuzzaman, M.; Greathouse, D.V.; Andersen, O.S.; Lundbaek, J.A.; Lundbæk, J.A. Linear rate-equilibrium relations arising from ion channel-bilayer energetic coupling. Proc. Natl. Acad. Sci. USA 2011, 108, 12717–12722. [Google Scholar] [CrossRef]
- Gandhavadi, M.; Allende, D.; Vidal, A.; Simon, S.; McIntosh, T. Structure, Composition, and Peptide Binding Properties of Detergent Soluble Bilayers and Detergent Resistant Rafts. Biophys. J. 2002, 82, 1469–1482. [Google Scholar] [CrossRef][Green Version]
- Soubias, O.; Teague, W.E.; Hines, K.G.; Gawrisch, K. Rhodopsin/Lipid Hydrophobic Matching—Rhodopsin Oligomerization and Function. Biophys. J. 2015, 108, 1125–1132. [Google Scholar] [CrossRef]
- Kalli, A.C.; Campbell, I.D.; Sansom, M.S. Multiscale simulations suggest a mechanism for integrin inside-out activation. Proc. Natl. Acad. Sci. USA 2011, 108, 11890–11895. [Google Scholar] [CrossRef]
- Hakanpaa, L. Targeting beta1-integrin inhibits vascular leakage in endotoxemia. Proc. Natl. Acad. Sci. USA 2018, 115, E6467–E6476. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.W. Transmembrane domain helix packing stabilizes integrin alphaIIbbeta 3 in the low affinity state. J. Biol. Chem. 2005, 280, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.W.; Kulp, D.W.; Span, L.M.; DeGrado, J.L.; Billings, P.C.; Senes, A.; Bennett, J.S.; DeGrado, W.F. Consensus motif for integrin transmembrane helix association. Proc. Natl. Acad. Sci. USA 2009, 107, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Killian, J. Snorkeling of lysine side chains in transmembrane helices: How easy can it get? FEBS Lett. 2003, 544, 69–73. [Google Scholar] [CrossRef]
- Lau, T.-L.; Partridge, A.W.; Ginsberg, M.H.; Ulmer, T.S. Structure of the Integrin β3 Transmembrane Segment in Phospholipid Bicelles and Detergent Micelles. Biochemistry 2008, 47, 4008–4016. [Google Scholar] [CrossRef]
- Lagarrigue, F.; Kim, C.; Ginsberg, M.H. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 2016, 128, 479–487. [Google Scholar] [CrossRef]
- Li, R.; Mitra, N.; Gratkowski, H.; Vilaire, G.; Litvinov, R.; Nagasami, C.; Weisel, J.W.; Lear, J.D.; DeGrado, W.F.; Bennett, J.S. Activation of Integrin alphaIIbbeta3 by Modulation of Transmembrane Helix Associations. Science 2003, 300, 795–798. [Google Scholar] [CrossRef]
- Li, R.; Gorelik, R.; Nanda, V.; Law, P.B.; Lear, J.D.; DeGrado, W.F.; Bennett, J.S. Dimerization of the Transmembrane Domain of Integrin αIIb Subunit in Cell Membranes. J. Boil. Chem. 2004, 279, 26666–26673. [Google Scholar] [CrossRef]
- Parthasarathy, K.; Lin, X.; Tan, S.M.; Law, S.A.; Torres, J. Transmembrane helices that form two opposite homodimeric interactions: An asparagine scan study of αM and β2 integrins. Protein Sci. 2008, 17, 930–938. [Google Scholar] [CrossRef]
- Li, R. Oligomerization of the integrin alphaIIbbeta3: Roles of the transmembrane and cytoplasmic domains. Proc. Natl. Acad. Sci. USA 2001, 98, 12462–12467. [Google Scholar] [CrossRef]
- Schneider, D.; Engelman, D.M. Involvement of transmembrane domain interactions in signal transduction by alpha/beta integrins. J. Biol. Chem. 2004, 279, 9840–9846. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Metcalf, U.G.; Gorelik, R.; Li, R.; Mitra, N.; Nanda, V.; Law, P.B.; Lear, J.D.; DeGrado, W.F.; Bennett, J.S. A push-pull mechanism for regulating integrin function. Proc. Natl. Acad. Sci. USA 2005, 102, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Energetics of Hydrophobic Matching in Lipid-Protein Interactions. Biophys. J. 2008, 94, 3996–4013. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, V.; Schneider, D. The membrane environment modulates self-association of the human GpA TM domain—Implications for membrane protein folding and transmembrane signaling. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Martel, V.; Racaud-Sultan, C.; Dupe, S.; Marie, C.; Paulhe, F.; Galmiche, A.; Block, M.R.; Albiges-Rizo, C. Conformation, Localization, and Integrin Binding of Talin Depend on Its Interaction with Phosphoinositides. J. Boil. Chem. 2001, 276, 21217–21227. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F. New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J. Cell Biol. 2009, 187, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Anthis, N.J.; Wegener, K.L.; Ye, F.; Kim, C.; Goult, B.T.; Lowe, E.D.; Vakonakis, I.; Bate, N.; Critchley, D.R.; Ginsberg, M.H.; et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009, 28, 3623–3632. [Google Scholar] [CrossRef]
- Di Paolo, G. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 γ by the FERM domain of talin. Nature 2002, 420, 85–89. [Google Scholar] [CrossRef]
- Ling, K. Type I γ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002, 420, 89–93. [Google Scholar] [CrossRef]
- De Pereda, J.M. Structural basis for phosphatidylinositol phosphate kinase type Igamma binding to talin at focal adhesions. J. Biol. Chem. 2005, 280, 8381–8386. [Google Scholar] [CrossRef]
- Kong, X. Structural basis for the phosphorylation-regulated focal adhesion targeting of type Igamma phosphatidylinositol phosphate kinase (PIPKIgamma) by talin. J. Mol. Biol. 2006, 359, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Slochower, D.R.; Wang, Y.-H.; Tourdot, R.W.; Radhakrishnan, R.; Janmey, P.A. Counterion-mediated pattern formation in membranes containing anionic lipids. Adv. Colloid Interface Sci. 2014, 208, 177–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, D.A. PIP2Clustering: From model membranes to cells. Chem. Phys. Lipids 2015, 192, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Christian, D.A.; Wang, Y.-H.; Madara, J.J.; Discher, D.; Janmey, P.A.; Ilya, L. Calcium-Dependent Lateral Organization in Phosphatidylinositol 4,5-Bisphosphate (PIP2)- and Cholesterol-Containing Monolayers. Biochemistry 2009, 48, 8241–8248. [Google Scholar] [CrossRef]
- James, D.J.; Khodthong, C.; Kowalchyk, J.A.; Martin, T.F.J. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J. Cell Boil. 2008, 182, 355–366. [Google Scholar] [CrossRef]
- Bogaart, G.V.D.; Meyenberg, K.; Risselada, H.J.; Amin, H.; Willig, K.I.; Hubrich, B.E.; Dier, M.; Hell, S.W.; Grubmüller, H.; Diederichsen, U.; et al. Membrane protein sequestering by ionic protein–lipid interactions. Nature 2011, 479, 552–555. [Google Scholar] [CrossRef]
- Pike, L.J.; Casey, L. Localization and Turnover of Phosphatidylinositol 4,5-Bisphosphate in Caveolin-enriched Membrane Domains. J. Boil. Chem. 1996, 271, 26453–26456. [Google Scholar] [CrossRef]
- Pike, L.J.; Miller, J.M. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Boil. Chem. 1998, 273, 22298–22304. [Google Scholar] [CrossRef]
- Johnson, C.M.; Chichili, G.R.; Rodgers, W. Compartmentalization of Phosphatidylinositol 4,5-Bisphosphate Signaling Evidenced Using Targeted Phosphatases. J. Boil. Chem. 2008, 283, 29920–29928. [Google Scholar] [CrossRef]
- Van Rheenen, J.; Achame, E.M.; Janssen, H.; Calafat, J.; Jalink, K. PIP2 signaling in lipid domains: A critical re-evaluation. EMBO J. 2005, 24, 1664–1673. [Google Scholar] [CrossRef]
- Graber, Z.T.; Gericke, A.; Kooijman, E.E. Phosphatidylinositol-4,5-bisphosphate ionization in the presence of cholesterol, calcium or magnesium ions. Chem. Phys. Lipids 2014, 182, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Redfern, R.; Isler, Y.; Ross, A.H.; Gericke, A. Cholesterol stabilizes fluid phosphoinositide domains. Chem. Phys. Lipids 2014, 182, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Vogt, V.M.; Feigenson, G.W. Multivalent Cation-Bridged PI(4,5)P2 Clusters Form at Very Low Concentrations. Biophys. J. 2018, 114, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Kwik, J.; Boyle, S.; Fooksman, D.; Margolis, L.; Sheetz, M.P.; Edidin, M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA 2003, 100, 13964–13969. [Google Scholar] [CrossRef]
- Cai, X.; Lietha, D.; Ceccarelli, D.F.; Karginov, A.V.; Rajfur, Z.; Jacobson, K.; Hahn, K.M.; Eck, M.J.; Schaller, M.D. Spatial and Temporal Regulation of Focal Adhesion Kinase Activity in Living Cells. Mol. Cell. Boil. 2007, 28, 201–214. [Google Scholar] [CrossRef]
- Palmer, S.M. Lipid binding to the tail domain of vinculin: Specificity and the role of the N and C termini. J. Biol. Chem. 2009, 284, 7223–7231. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Rangarajan, E.S.; Patil, D.N.; George, E.M.; Brown, D.T.; Izard, T. Lipid binding promotes oligomerization and focal adhesion activity of vinculin. J. Cell Boil. 2014, 207, 643–656. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Patil, D.N.; Rangarajan, E.S.; Rader, C.; Izard, T. Lipid-Directed Vinculin Dimerization. Biochemistry 2015, 54, 2758–2768. [Google Scholar] [CrossRef]
- Izard, T.; Brown, D.T. Mechanisms and Functions of Vinculin Interactions with Phospholipids at Cell Adhesion Sites. J. Boil. Chem. 2016, 291, 2548–2555. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Rangarajan, E.S.; Brown, D.T.; Izard, T. Differential lipid binding of vinculin isoforms promotes quasi-equivalent dimerization. Proc. Natl. Acad. Sci. USA 2016, 113, 9539–9544. [Google Scholar] [CrossRef]
- Gilmore, A.P.; Burridge, K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature 1996, 381, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Goñi, G.M.; Epifano, C.; Boskovic, J.; Camacho-Artacho, M.; Zhou, J.; Bronowska, A.; Martín, M.T.; Eck, M.J.; Kremer, L.; Gräter, F.; et al. Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc. Natl. Acad. Sci. USA 2014, 111, E3177–E3186. [Google Scholar] [CrossRef] [PubMed]
- Acebrón, I.; Righetto, R.D.; Schoenherr, C.; de Buhr, S.; Redondo, R.; Culley, J.; Rodríguez, C.F.; Daday, C.; Biyani, N.; Llorca, O.; et al. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020, in press. [Google Scholar]
- Chen, X.; Khajeh, J.A.; Ju, J.H.; Gupta, Y.K.; Stanley, C.B.; Do, C.; Heller, W.T.; Aggarwal, A.K.; Callaway, D.J.E.; Bu, Z. Phosphatidylinositol 4,5-Bisphosphate Clusters the Cell Adhesion Molecule CD44 and Assembles a Specific CD44-Ezrin Heterocomplex, as Revealed by Small Angle Neutron Scattering. J. Boil. Chem. 2015, 290, 6639–6652. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, A.; Hofmaier, T.; Klotzsch, E.; Kudlacek, O.; Stockner, T.; Sitte, H.H.; Schütz, G.J. Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter. Nat. Commun. 2017, 8, 14089. [Google Scholar] [CrossRef]
- Bussell, K. Destination lipid rafts. Nat. Rev. Mol. Cell Boil. 2002, 3, 399. [Google Scholar] [CrossRef]
- Zacharias, D.A.; Violin, J.D.; Newton, A.C.; Tsien, R.Y. Partitioning of Lipid-Modified Monomeric GFPs into Membrane Microdomains of Live Cells. Science 2002, 296, 913–916. [Google Scholar] [CrossRef]
- Resh, M.D. Covalent lipid modifications of proteins. Curr. Boil. 2013, 23, R431–R435. [Google Scholar] [CrossRef]
- Rabinovitz, I.; Tsomo, L.; Mercurio, A.M. Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes. Mol. Cell. Boil. 2004, 24, 4351–4360. [Google Scholar] [CrossRef]
- Dutta, U.; Shaw, L.M. A key tyrosine (Y1494) in the beta4 integrin regulates multiple signaling pathways important for tumor development and progression. Cancer Res. 2008, 68, 8779–8787. [Google Scholar] [CrossRef]
- Germain, E.C.; Santos, T.M.; Rabinovitz, I. Phosphorylation of a novel site on the β4 integrin at the trailing edge of migrating cells promotes hemidesmosome disassembly. Mol. Biol. Cell 2009, 20, 56–67. [Google Scholar] [CrossRef]
- Yang, X.; Kovalenko, O.V.; Tang, W.; Claas, C.; Stipp, C.S.; Hemler, M.E. Palmitoylation supports assembly and function of integrin–tetraspanin complexes. J. Cell Boil. 2004, 167, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Chung, J. Curcumin inhibition of the functional interaction between integrin alpha6beta4 and the epidermal growth factor receptor. Mol. Cancer Ther. 2011, 10, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Ragan, M.A. Palmitoylation: A protein S-acylation with implications for breast cancer. NPJ Breast Cancer 2016, 2, 16028. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Specific tetraspanin functions. J. Cell Boil. 2001, 155, 1103–1108. [Google Scholar] [CrossRef]
- Robinson, C.V.; Rohacs, T.; Hansen, S.B. Tools for Understanding Nanoscale Lipid Regulation of Ion Channels. Trends Biochem. Sci. 2019, 44, 795–806. [Google Scholar] [CrossRef]
- Stefansson, A.; Armulik, A.; Nilsson, I.; Von Heijne, G.; Johansson, S. Determination of N- and C-terminal Borders of the Transmembrane Domain of Integrin Subunits. J. Boil. Chem. 2004, 279, 21200–21205. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).