Association between Five Common Plasminogen Activator Inhibitor-1 (PAI-1) Gene Polymorphisms and Colorectal Cancer Susceptibility
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of Colorectal Cancer and Controls
2.2. PAI-1 -675 and PAI-1 +11053 Gene Polymorphisms are Associated with Colorectal Cancer Risk
2.3. PAI-1 Gene Polymorphism and Patient Clinicopathological Factors Affect CRC Susceptibility
2.4. The Five Combined PAI-1 Polymorphisms Have Different Effects on CRC Occurrence
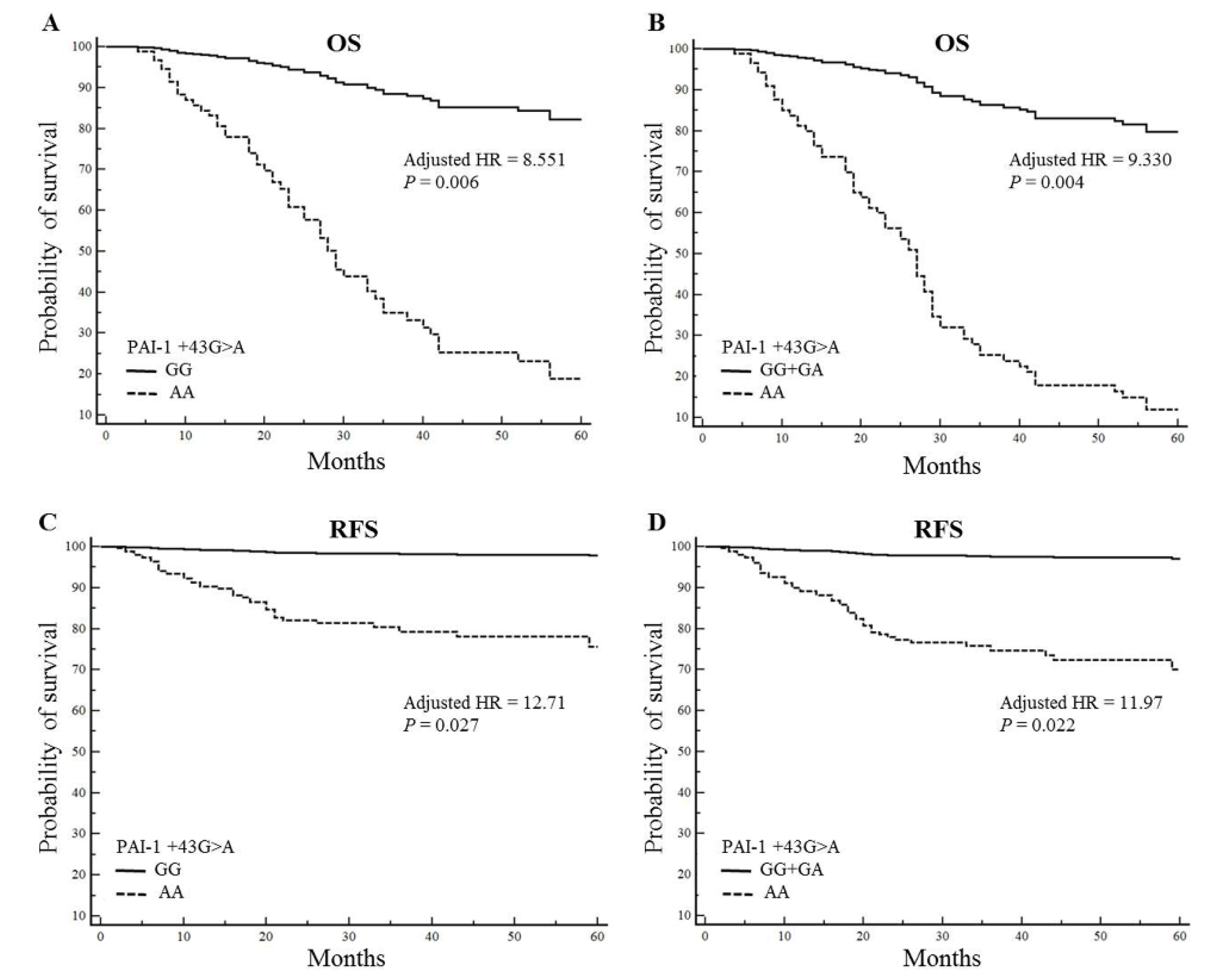
2.5. The PAI-1 +43AA Genotype is Associated with Poor Survival
3. Discussion
4. Materials and Methods
4.1. Population and Clinical Samples
4.2. Polymorphism Analysis
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Oh, C.M.; Kong, H.J.; Cho, H.S.; Lee, D.H.; Lee, K.H. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res. Treat. 2015, 47, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sheth, K.R.; Clary, B.M. Management of hepatic metastases from colorectal cancer. Clin. Colon Rectal Surg. 2005, 18, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R.; Collen, D. Mechanisms of physiological fibrinolysis. Baillieres Clin. Haematol. 1995, 8, 277–290. [Google Scholar] [CrossRef]
- Dellas, C.; Loskutoff, D.J. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb. Haemost. 2005, 93, 631–640. [Google Scholar] [CrossRef]
- Lijnen, H.R. Pleiotropic functions of plasminogen activator inhibitor-1. J. Thromb. Haemost. 2005, 3, 35–45. [Google Scholar] [CrossRef]
- Fersching, D.M.; Nagel, D.; Siegele, B.; Salat, C.; Heinemann, V.; Holdenrieder, S.; Stoetzer, O.J. Apoptosis-related biomarkers sFAS, MIF, ICAM-1 and PAI-1 in serum of breast cancer patients undergoing neoadjuvant chemotherapy. Anticancer Res. 2012, 32, 2047–2058. [Google Scholar]
- Han BNakamura MMori, I.; Nakamura, Y.; Kakudo, K. Urokinase-type plasminogen activator system and breast cancer (Review). Oncol. Rep. 2005, 14, 105–112. [Google Scholar]
- Pepper, M.S. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1104–1117. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; D’Armiento, M. The urokinase plasminogen activator system: A target for anti-cancer therapy. Curr. Cancer Drug Targets 2009, 9, 32–71. [Google Scholar] [CrossRef]
- McMahon, B.; Kwaan, H.C. The plasminogen activator system and cancer. Pathophysiol. Haemost. Thromb. 2008, 36, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Schleef, R.R.; Loskutoff, D.J. Fibrinolytic system of vascular endothelial cells. Role of plasminogen activator inhibitors. Pathophysiol. Haemost. Thromb. 1988, 18, 328–341. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, M.N.; Elena, T.; Antonio, C.; Roberta, L.; Giovanni, D.M. Homocysteine and arterial thrombosis: Challenge and opportunity. Thromb. Haemost. 2010, 103, 942–961. [Google Scholar] [CrossRef]
- Akpek, M.; Mehmet, G.K.; Huseyin, U.; Mikail, Y.; Nihat, K.; Ozgur, G.; Orhan, D.; Idris, A.; Deniz, E.; Omer, S.; et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis 2011, 219, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Vanizor, K.B.; Asim, O.; Gülseren, C.; Hüseyin, A.U.; Yunus, E.Y.; Ahmet, A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clin. Chim. Acta 2003, 332, 23–30. [Google Scholar] [CrossRef]
- Tofler, G.H.; Ralph, B.D.; Paul, F.J.; Andrew, G.B.; Peter, W.F.W.; Izabela, L.; Murray, A.M.; Jacob, S. Association between increased homocysteine levels and impaired fibrinolytic potential: Potential mechanism for cardiovascular risk. Thromb Haemost. 2002, 88, 799–804. [Google Scholar]
- Andreasen, P.A. PAI-1—A potential therapeutic target in cancer. Curr Drug Targets. 2007, 8, 1030–1041. [Google Scholar] [CrossRef]
- Stefansson, S.; McMahon, G.A.; Petitclerc, E.; Lawrence, D.A. Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr. Pharm. Des. 2003, 9, 1545–1564. [Google Scholar] [CrossRef]
- Langenskiold, M.; Holmdahl, L.; Angenete, E.; Falk, P.; Nordgren, S.; Ivarsson, M.L. Differential prognostic impact of uPA and PAI-1 in colon and rectal cancer. Tumour. Biol. 2009, 30, 210–220. [Google Scholar] [CrossRef]
- Angenete, E.; Langenskiöld, M.; Palmgren, I.; Falk, P.; Oresland, T.; Ivarsson, M.L. uPA and PAI-1 in rectal cancer--relationship to radiotherapy and clinical outcome. J. Surg. Res. 2009, 153, 46–53. [Google Scholar] [CrossRef]
- Stegnar, M.; Uhrin, P.; Peternel, P.; Mavri, A.; Salobir-Pajnic, B.; Stare, J.; Binder, B.R. The 4G/5G sequence polymorphism in the promoter of plasminogen activator inhibitor-1 (PAI-1) gene: Relationship to plasma PAI-1 level in venous thromboembolism. Thromb. Haemost. 1998, 79, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Saut, N.; Alessi, M.C.; Yudkin, J.S.; Margaglione, M.; Minno, G.D.; Hamsten, A.; Humphries, S.E.; Tregouet, D.A.; Juhan-Vague, I. Association of plasminogen activator inhibitor (PAI)-1 (SERPINE1) SNPs with myocardial infarction, plasma PAI-1, and metabolic parameters: The HIFMECH study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.J.; Wiman, B.; Hamsten, A.; Green, F.; Humphries, S.; Henney, A.M. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J. Biol. Chem. 1993, 268, 10739–10745. [Google Scholar] [PubMed]
- Ma, Z.; Jhun, B.; Jung, S.Y.; Oh, C.K. Binding of upstream stimulatory factor 1 to the E-box regulates the 4G/5G polymorphism-dependent plasminogen activator inhibitor 1 expression in mast cells. J. Allergy Clin. Immunol. 2008, 121, 1006–1012. [Google Scholar] [CrossRef]
- Ma, Z.; Paek, D.; Oh, C.K. Plasminogen activator inhibitor-1 and asthma: Role in the pathogenesis and molecular regulation. Clin. Exp. Allergy 2009, 39, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Hultman, K.; Tjärnlund-Wolf, A.; Odeberg, J.; Eriksson, P.; Jern, C. Allele-specific transcription of the PAI-1 gene in human astrocytes. Thromb. Haemost. 2010, 104, 998–1008. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.D., Jr.; Rich, S.S.; Jenny, N.S.; Tracy, R.P.; Haffner, S.M. Promoter (4G/5G) plasminogen activator inhibitor-1 genotype and plasminogen activator inhibitor-1 levels in blacks, Hispanics, and non-Hispanic whites: The Insulin Resistance Atherosclerosis Study. Circulation 2003, 107, 2422–2427. [Google Scholar] [CrossRef]
- Sogutlu Sari, E.; Yazici, A.; Eser, B.; Kazim Erol, M.; Kilic, A.; Samet Ermis, S.; Koytak, A.; Akşit, H.; Yakut, T. The prevalence of 4G/5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in central serous chorioretinopathy and its association with plasma PAI-1 levels. Cutan. Ocul. Toxicol. 2014, 33, 270–274. [Google Scholar] [CrossRef]
- Lin, S.; Huiya, Z.; Bo, L.; Wei, W.; Yongmei, G. The plasminogen activator inhibitor-1 (PAI-1) gene -844 A/G and -675 4G/5G promoter polymorphism significantly influences plasma PAI-1 levels in women with polycystic ovary syndrome. Endocrine 2009, 36, 503–509. [Google Scholar] [CrossRef]
- Forsti, A.; Lei, H.; Tavelin, B.; Enquist, K.; Palmqvist, R.; Altieri, A.; Hallmans, G.; Hemminki, K.; Lenner, P. Polymorphisms in the genes of the urokinase plasminogen activation system in relation to colorectal cancer. Ann. Oncol. 2007, 18, 1990–1994. [Google Scholar] [CrossRef] [PubMed]
- Verspaget, H.W.; Sier, C.F.; Ganesh, S.; Griffioen, G.; Lamers, C.B. Prognostic value of plasminogen activators and their inhibitors in colorectal cancer. Eur. J. Cancer 1995, 31, 1105–1109. [Google Scholar] [CrossRef]
- Abe, J.; Urano, T.; Konno, H.; Erhan, Y.; Tanaka, T.; Nishino, N.; Takada, A.; Nakamura, S. Larger and more invasive colorectal carcinoma contains larger amounts of plasminogen activator inhibitor type 1 and its relative ratio over urokinase receptor correlates well with tumor size. Cancer 1999, 86, 2602–2611. [Google Scholar] [CrossRef]
- Sakakibara, T.; Hibi, K.; Koike, M.; Fujiwara, M.; Kodera, Y.; Ito, K.; Nakao, A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br. J. Cancer 2005, 93, 799–803. [Google Scholar] [CrossRef]
- Loktionov, A.; Watson, M.A.; Stebbings, W.S.; Speakman, C.T.; Bingham, S.A. Plasminogen activator inhibitor-1 gene polymorphism and colorectal cancer risk and prognosis. Cancer Lett. 2003, 189, 189–196. [Google Scholar] [CrossRef]
- Vossen, C.Y.; Hoffmeister, M.; Chang-Claude, J.C.; Rosendaal, F.R.; Brenner, H. Clotting factor gene polymorphisms and colorectal cancer risk. J. Clin. Oncol. 2011, 29, 1722–1727. [Google Scholar] [CrossRef]
- Eriksson, P.; Kallin, B.; van ‘t Hooft, F.M.; Bavenholm, P.; Hamsten, A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc. Natl. Acad. Sci. USA 1995, 92, 1851–1855. [Google Scholar] [CrossRef]
- Dano, K.; Romer, J.; Nielsen, B.S.; Bjorn, S.; Pyke, C.; Rygaard, J.; Lund, L.R. Cancer invasion and tissue remodeling—Cooperation of protease systems and cell types. APMIS 1999, 107, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, P.A.; Kjoller, L.; Christensen, L.; Duffy, M.J. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 1997, 72, 1–22. [Google Scholar] [CrossRef]
- Isogai, C.; Laug, W.E.; Shimada, H.; Declerck, P.J.; Stins, M.F.; Durden, D.L.; Erdreich-Epstein, A.; DeClerck, Y.A. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001, 61, 5587–5594. [Google Scholar] [PubMed]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Wong, M.K.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef]
- Devy, L.; Blacher, S.; Grignet-Debrus, C.; Bajou, K.; Masson, V.; Gerard, R.D.; Gils, A.; Carmeliet, G.; Carmeliet, P.; Declerck, P.J.; et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Noël, A.; Gerard, R.D.; Masson, V.; Brunner, N.; Holst-Hansen, C.; Skobe, M.; Fusenig, N.E.; Carmeliet, P.; Collen, D.; et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–928. [Google Scholar] [CrossRef]
- Gutierrez, L.S.; Schulman, A.; Brito-Robinson, T.; Noria, F.; Ploplis, V.A.; Castellino, F.J. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000, 60, 5839–5847. [Google Scholar] [PubMed]
- Chazaud, B.; Ricoux, R.; Christov, C.; Plonquet, A.; Gherardi, R.K.; Barlovatz-Meimon, G. Promigratory effect of plasminogen activator inhibitor-1 on invasive breast cancer cell populations. Am. J. Pathol. 2002, 160, 237–246. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Wang, J.; Svoboda, K.; Declerck, P.J. Plasminogen activator inhibitor 1 may promote tumour growth through inhibition of apoptosis. Br. J. Cancer. 2000, 82, 1702–1708. [Google Scholar] [CrossRef]
- Marchand, A.; Proust, C.; Morange, P.E.; Lompré, A.M.; Trégouët, D.A. miR-421 and miR-30c inhibit SERPINE 1gene expression in human endothelial cells. PLoS ONE 2012, 7, e44532. [Google Scholar] [CrossRef]
- Park, G.; Son, B.; Kang, J.H.; Lee, S.; Jeon, J.; Kim, J.H.; Yi, G.R.; Youn, H.S.; Moon, C.; Nam, S.Y.; et al. LDR-induced miR-30a and miR-30b target the PAI-1 pathway to control adverse effects of NSCLC radiotherapy. Mol. Ther. 2019, 27, 342–354. [Google Scholar] [CrossRef]
- Wei, Y.; He, Z.; Jun, D.; Chao, L.; Zhongfu, Z.; Xuezheng, L. MicroRNA-30b is involved in the pathological process of diabetes mellitus induced by pancreatic cancer by regulating plasminogen activator inhibitor-1. Biotechnol. Biotechol. Equip. 2019, 33, 1741–1749. [Google Scholar] [CrossRef]
- Ryu, C.S.; Sakong, J.H.; Ahn, E.H.; Kim, J.O.; Ko, D.E.; Kim, J.H.; Lee, W.S.; Kim, N.K. Association study of the three functional polymorphisms (TAS2R46G>A, OR4C16G>A, and OR4X1A>T) with recurrent pregnancy loss. Genes Genom. 2019, 41, 61–70. [Google Scholar] [CrossRef]
| Characteristic | Control | Case | p | Colon | p | Rectum | p |
|---|---|---|---|---|---|---|---|
| N | 416 | 459 | 268 | 191 | |||
| Age (mean ± SD) | 61.04 ± 11.44 | 61.52 ± 12.63 | 0.56 | 61.00 ± 12.96 | 0.966 | 61.79 ± 12.12 | 0.47 |
| Gender (male), n (%) | 173 (41.6) | 215 (48.0) | 0.25 | 124 (47.1) | 0.394 | 91 (49.2) | 0.30 |
| Anti-HTN drug or BP ≥ 140/90 mmHg, n (%) | 186 (44.7) | 284 (63.4) | 0.01 | 163 (62.0) | 0.016 | 121 (65.4) | 0.01 |
| Anti-DM drug or FBS ≥ 126 mg/dl, n (%) | 61 (14.7) | 159 (35.5) | <0.01 | 91 (34.6) | <0.0001 | 68 (36.8) | <0.01 |
| Homocysteine (µmol/L) | 9.78 ± 4.22 | 10.47 ± 7.85 | 0.13 | 10.25 ± 8.20 | 0.349 | 10.70 ± 7.40 | 0.07 |
| Folate (ng/mL) | 9.10 ± 8.05 | 7.82 ± 6.35 | 0.02 | 7.82 ± 5.97 | 0.039 | 7.80 ± 6.93 | 0.08 |
| Triglycerides (mg/dl) | 143.78 ± 87.50 | 126.41 ± 77.42 | 0.01 | 126.65 ± 81.04 | 0.016 | 125.70 ± 73.21 | 0.02 |
| Body mass index (kg/m2) | 24.26 ± 3.36 | 23.09 ± 3.17 | <0.01 | 22.91 ± 3.28 | <0.0001 | 23.28 ± 2.99 | 0.01 |
| Tumor size | |||||||
| <5 cm | - | 189 (42.2) | - | 101 (38.4) | - | 88 (47.6) | - |
| ≥5 cm | - | 270 (60.3) | - | 167 (63.5) | - | 103 (55.7) | - |
| TNM stage, n (%) | |||||||
| I | - | 45 (10.0) | - | 23 (8.7) | - | 22 (11.9) | - |
| II | - | 183 (40.8) | - | 113 (43.3) | - | 69 (37.3) | - |
| III | - | 183 (40.8) | - | 102 (38.8) | - | 81 (43.8) | - |
| IV | - | 48 (10.7) | - | 29 (11.0) | - | 19 (10.3) | - |
| Genotype | Controls (n = 416) | CRC Patients (n = 459) | AOR (95% CI) * | pa | pb |
|---|---|---|---|---|---|
| PAI-1 -844G > A | |||||
| GG | 136 (32.7) | 154 (33.6) | 1.000 (reference) | ||
| GA | 199 (47.8) | 230 (50.1) | 1.000 (0.730–1.370) | 1.00 | 1.00 |
| AA | 81 (19.5) | 75 (16.3) | 0.779 (0.508–1.195) | 0.25 | 0.42 |
| Dominant (GG vs. GA + AA) | 0.937 (0.695–1.262) | 0.67 | 0.83 | ||
| Recessive (GG + GA vs. AA) | 0.806 (0.559–1.164) | 0.25 | 0.42 | ||
| HWE-P | 0.592 | 0.415 | |||
| PAI-1-675 4G > 5G | |||||
| 4G4G | 180 (43.3) | 171 (37.3) | 1.000 (reference) | ||
| 4G5G | 180 (43.3) | 206 (44.9) | 1.212 (0.890–1.651) | 0.22 | 1.00 |
| 5G5G | 56 (13.5) | 82 (17.9) | 1.556 (1.012–2.391) | 0.04 | 0.11 |
| Dominant (4G4G vs. 4G5G + 5G5G) | 1.284 (0.963–1.714) | 0.09 | 0.45 | ||
| Recessive (4G4G + 4G5G vs. 5G5G) | 1.385 (0.938–2.044) | 0.10 | 0.26 | ||
| HWE-P | 0.306 | 0.128 | |||
| PAI-1 +43G > A | |||||
| GG | 335 (80.5) | 375 (81.7) | 1.000 (reference) | ||
| GA | 75 (18.0) | 70 (15.3) | 0.890 (0.612–1.296) | 0.54 | 1.00 |
| AA | 6 (1.4) | 14 (3.1) | 0.647 (0.155–2.694) | 0.55 | 0.69 |
| Dominant (GG vs. GA + AA) | 0.875 (0.606–1.261) | 0.47 | 0.79 | ||
| Recessive (GG + GA vs. AA) | 0.670 (0.161–2.779) | 0.58 | 0.73 | ||
| HWE-P | 0.447 | 0.851 | |||
| PAI-1 +9785G > A | |||||
| GG | 383 (92.1) | 417 (90.8) | 1.000 (reference) | ||
| GA | 31 (7.5) | 42 (9.2) | 1.079 (0.629–1.849) | 0.78 | 1.00 |
| AA | 2 (0.5) | 0 (0.0) | N/A | 1.00 | 1.00 |
| Dominant (GG vs. GA + AA) | 1.000 (0.588–1.700) | 1.00 | 1.00 | ||
| Recessive (GG + GA vs. AA) | N/A | 1.00 | 1.00 | ||
| HWE-P | 0.124 | 0.329 | |||
| PAI-1 +11053T > G | |||||
| TT | 107 (25.7) | 133 (29.0) | 1.000 (reference) | ||
| TG | 204 (49.0) | 241 (52.5) | 0.966 (0.692–1.349) | 0.84 | 1.00 |
| GG | 105 (25.2) | 85 (18.5) | 0.620 (0.413–0.932) | 0.02 | 0.11 |
| Dominant (TT vs. TG + GG) | 0.850 (0.620–1.165) | 0.31 | 0.78 | ||
| Recessive (TT + TG vs. GG) | 0.662 (0.469–0.933) | 0.02 | 0.10 | ||
| HWE-P | 0.695 | 0.200 |
| Variable | PAI-1 -844GA | PAI-1 -675 4G5G + 5G5G | PAI-1 +43GA + AA | PAI-1 9785GA | PAI-1 11053TT + TG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | p a | p b | AOR (95% CI) | p a | p b | AOR (95% CI) | p a | p b | AOR (95% CI) | p a | p b | AOR (95% CI) | p a | p b | |
| Age | |||||||||||||||
| <61 years | 1.273 (0.801–2.023) | 0.31 | 0.38 | 1.942 (1.247–3.024) | 0.01 | 0.02 | 0.889 (0.528–1.495) | 0.66 | 0.66 | 0.633 (0.297–1.349) | 0.24 | 0.38 | 0.563 (0.333–0.950) | 0.03 | 0.08 |
| ≥61 years | 0.814 (0.517–1.280) | 0.37 | 0.62 | 1.034 (0.692–1.544) | 0.87 | 0.87 | 0.872 (0.509–1.494) | 0.62 | 0.77 | 2.467 (1.028–5.920) | 0.04 | 0.22 | 0.741 (0.462–1.188) | 0.21 | 0.53 |
| Gender | |||||||||||||||
| Male | 0.881 (0.536–1.447) | 0.62 | 0.77 | 1.313 (0.824–2.093) | 0.25 | 0.74 | 1.029 (0.568–1.862) | 0.93 | 0.93 | 1.306 (0.483–3.532) | 0.60 | 0.77 | 0.752 (0.440–1.285) | 0.30 | 0.74 |
| Female | 1.141 (0.745–1.748) | 0.55 | 0.68 | 1.437 (0.980–2.106) | 0.06 | 0.16 | 0.765 (0.472–1.241) | 0.28 | 0.46 | 1.123 (0.572–2.206) | 0.74 | 0.74 | 0.600 (0.378–0.954) | 0.03 | 0.16 |
| HTN | |||||||||||||||
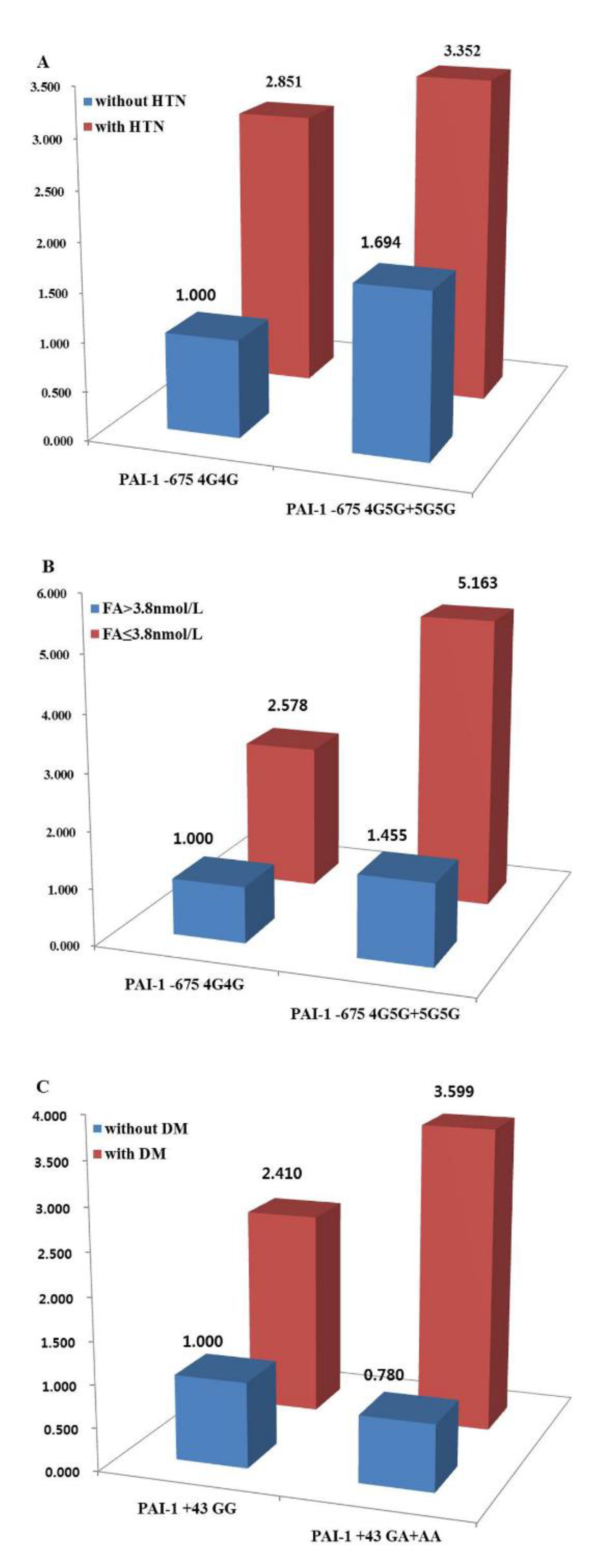
| without | 1.367 (0.852–2.194) | 0.20 | 0.33 | 1.694 (1.093–2.627) | 0.02 | 0.10 | 0.980 (0.575–1.669) | 0.94 | 0.94 | 1.395 (0.670–2.907) | 0.37 | 0.47 | 0.668 (0.398–1.120) | 0.13 | 0.32 |
| with | 0.793 (0.501–1.255) | 0.32 | 0.67 | 1.138 (0.755–1.715) | 0.54 | 0.67 | 0.838 (0.493–1.424) | 0.51 | 0.67 | 0.942 (0.415–2.139) | 0.89 | 0.89 | 0.618 (0.382–1.001) | 0.05 | 0.25 |
| DM | |||||||||||||||
| without | 1.236 (0.861–1.776) | 0.25 | 0.31 | 1.464 (1.051–2.039) | 0.02 | 0.06 | 0.780 (0.516–1.179) | 0.24 | 0.31 | 1.268 (0.693–2.320) | 0.44 | 0.44 | 0.633 (0.425–0.942) | 0.02 | 0.06 |
| with | 0.410 (0.180–0.937) | 0.03 | 0.17 | 1.017 (0.521–1.986) | 0.96 | 0.96 | 1.445 (0.525–3.978) | 0.48 | 0.96 | 0.829 (0.223–3.076) | 0.78 | 0.96 | 0.820 (0.383–1.758) | 0.61 | 0.96 |
| Smoking | |||||||||||||||
| without | 1.070 (0.732–1.564) | 0.73 | 0.73 | 1.351 (0.962–1.898) | 0.08 | 0.21 | 0.798 (0.516–1.234) | 0.31 | 0.41 | 1.405 (0.710–2.780) | 0.33 | 0.41 | 0.679 (0.455–1.014) | 0.06 | 0.21 |
| with | 0.839 (0.454–1.552) | 0.58 | 0.74 | 1.527 (0.837–2.787) | 0.17 | 0.47 | 1.130 (0.554–2.307) | 0.74 | 0.74 | 0.771 (0.270–2.202) | 0.63 | 0.74 | 0.616 (0.301–1.263) | 0.19 | 0.47 |
| * Folate | |||||||||||||||
| ≥3.8 ng/mL | 1.065 (0.733–1.546) | 0.74 | 0.74 | 1.449 (1.030–2.039) | 0.03 | 0.12 | 0.878 (0.572–1.347) | 0.55 | 0.74 | 1.164 (0.623–2.175) | 0.63 | 0.74 | 0.669 (0.448–0.999) | 0.05 | 0.12 |
| <3.8 ng/mL | 0.669 (0.256–1.750) | 0.41 | 0.79 | 1.783 (0.760–4.186) | 0.18 | 0.79 | 0.652 (0.202–2.103) | 0.47 | 0.79 | N/A | 0.99 | 0.99 | 1.183 (0.448–3.121) | 0.74 | 0.92 |
| ** Homocysteine | |||||||||||||||
| <13.2 µmol/L | 0.911 (0.633–1.312) | 0.62 | 0.63 | 1.630 (1.163–2.285) | 0.01 | 0.03 | 0.743 (0.480–1.151) | 0.18 | 0.31 | 1.168 (0.626–2.179) | 0.63 | 0.63 | 0.681 (0.461–1.005) | 0.05 | 0.13 |
| ≥13.2 µmol/L | 1.875 (0.739–4.757) | 0.19 | 0.79 | 0.895 (0.390–2.052) | 0.79 | 0.79 | 1.275 (0.450–3.616) | 0.65 | 0.79 | 0.644 (0.080–5.201) | 0.68 | 0.79 | 1.200 (0.455–3.163) | 0.71 | 0.79 |
| BMI | |||||||||||||||
| <25 kg/m2 | 0.832 (0.529–1.308) | 0.43 | 0.65 | 1.413 (0.935–2.135) | 0.10 | 0.25 | 1.044 (0.615–1.772) | 0.87 | 0.87 | 1.318 (0.565–3.071) | 0.52 | 0.65 | 0.672 (0.420–1.075) | 0.10 | 0.25 |
| ≥25 kg/m2 | 1.061 (0.546–2.063) | 0.86 | 0.86 | 1.333 (0.715–2.488) | 0.37 | 0.70 | 1.211 (0.523–2.803) | 0.66 | 0.82 | 1.596 (0.513–4.963) | 0.42 | 0.70 | 0.420 (0.192–0.920) | 0.03 | 0.15 |
| * VB12 | |||||||||||||||
| ≥368 mg | 0.843 (0.304–2.339) | 0.74 | 0.74 | 0.724 (0.312–1.679) | 0.45 | 0.74 | 0.436 (0.098–1.944) | 0.28 | 0.74 | 0.643 (0.079–5.260) | 0.68 | 0.74 | 0.562 (0.184–1.711) | 0.31 | 0.74 |
| <368 mg | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Triglycerides | |||||||||||||||
| <126.65 mg/dL | 1.039 (0.648–1.664) | 0.88 | 0.88 | 1.532 (1.010–2.323) | 0.05 | 0.23 | 0.866 (0.512–1.465) | 0.59 | 0.74 | 0.599 (0.237–1.513) | 0.28 | 0.46 | 0.657 (0.401–1.078) | 0.10 | 0.24 |
| ≥126.65 mg/dL | 1.087 (0.644–1.836) | 0.76 | 0.92 | 1.025 (0.628–1.672) | 0.92 | 0.92 | 0.807 (0.424–1.536) | 0.51 | 0.92 | 1.172 (0.516–2.659) | 0.71 | 0.92 | 0.697 (0.396–1.224) | 0.21 | 0.92 |
| Cholesterol | |||||||||||||||
| <150 mg/dL | 0.747 (0.265–2.107) | 0.58 | 0.95 | 1.083 (0.417–2.812) | 0.87 | 0.95 | 0.618 (0.165–2.318) | 0.48 | 0.95 | 1.048 (0.243–4.524) | 0.95 | 0.95 | 1.316 (0.471–3.675) | 0.60 | 0.95 |
| ≥150 mg/dL | 1.157 (0.722–1.854) | 0.55 | 0.68 | 1.254 (0.814–1.931) | 0.31 | 0.51 | 1.088 (0.638–1.855) | 0.76 | 0.76 | 1.612 (0.761–3.413) | 0.21 | 0.51 | 0.560 (0.328–0.958) | 0.03 | 0.17 |
| HDL | |||||||||||||||
| >42.75 mg/dL | 1.159 (0.568–2.365) | 0.69 | 0.69 | 1.899 (1.010–3.569) | 0.05 | 0.12 | 1.526 (0.688–3.384) | 0.30 | 0.37 | 0.383 (0.107–1.368) | 0.14 | 0.23 | 0.436 (0.218–0.874) | 0.02 | 0.10 |
| ≤42.75 mg/dL | 0.656 (0.347–1.239) | 0.19 | 0.69 | 0.907 (0.502–1.641) | 0.75 | 0.88 | 0.945 (0.443–2.016) | 0.88 | 0.88 | 1.298 (0.411–4.099) | 0.66 | 0.88 | 0.691 (0.355–1.343) | 0.28 | 0.69 |
| LDL | |||||||||||||||
| <130 mg/dL | 0.908 (0.464–1.779) | 0.78 | 0.78 | 1.358 (0.714–2.585) | 0.35 | 0.78 | 1.141 (0.496–2.625) | 0.76 | 0.78 | 0.805 (0.247–2.630) | 0.72 | 0.78 | 0.593 (0.293–1.199) | 0.15 | 0.73 |
| ≥130 mg/dL | 0.238 (0.033–1.721) | 0.16 | 0.39 | 1.553 (0.273–8.853) | 0.62 | 0.78 | 4.339 (0.662–28.419) | 0.13 | 0.39 | 3.547 (0.228–55.280) | 0.37 | 0.61 | 1.227 (0.201–7.503) | 0.83 | 0.83 |
| Genotype | Controls (2n = 832) | Cases (2n = 918) | AOR (95% CI) | pa | pb |
|---|---|---|---|---|---|
| PAI-1 -844G>A/-675 4G > 5G/+43G > A/+9785G > A/+11053T > G | |||||
| G-4G-G-G-T | 84 (10.1) | 99 (10.8) | 1.000 (reference) | ||
| G-4G-G-G-G | 68 (8.2) | 81 (8.8) | 1.011 (0.655–1.560) | 1.00 | 1.00 |
| G-4G-G-A-T | 6 (0.7) | 3 (0.3) | 0.424 (0.103–1.749) | 0.31 | 0.59 |
| G-4G-G-A-G | 1 (0.1) | 1 (0.1) | 0.849 (0.052–13.78) | 1.00 | 1.00 |
| G-4G-A-G-T | 17 (2.0) | 10 (1.1) | 0.499 (0.217–1.149) | 0.10 | 0.48 |
| G-4G-A-G-G | 9 (1.1) | 0 (0.0) | 0.045 (0.003–0.780) | 0.01 | 0.01 |
| G-4G-A-A-G | 0 (0.0) | 3 (0.3) | 5.945 (0.303–116.8) | 0.25 | 0.53 |
| G-5G-G-G-T | 190 (22.8) | 224 (24.4) | 1.000 (0.706–1.418) | 1.00 | 1.00 |
| G-5G-G-G-G | 44 (5.3) | 36 (3.9) | 0.694 (0.410–1.177) | 0.18 | 0.53 |
| G-5G-G-A-T | 19 (2.3) | 26 (2.8) | 1.161 (0.601–2.245) | 0.74 | 0.94 |
| G-5G-G-A-G | 3 (0.4) | 1 (0.1) | 0.283 (0.029–2.772) | 0.34 | 0.60 |
| G-5G-A-G-T | 30 (3.6) | 54 (5.9) | 1.527 (0.897–2.602) | 0.14 | 0.53 |
| G-5G-A-G-G | 0 (0.0) | 1 (0.1) | 2.548 (0.102–63.42) | 1.00 | 1.00 |
| G-5G-A-A-T | 1 (0.1) | 0 (0.0) | 0.283 (0.011–7.046) | 0.46 | 0.72 |
| A-4G-G-G-T | 61 (7.3) | 67 (7.3) | 0.932 (0.593–1.466) | 0.82 | 0.99 |
| A-4G-G-G-G | 261 (31.4) | 271 (29.5) | 0.881 (0.629–1.234) | 0.49 | 0.72 |
| A-4G-G-A-T | 3 (0.4) | 2 (0.2) | 0.566 (0.092–3.467) | 0.66 | 0.90 |
| A-4G-G-A-G | 0 (0.0) | 3 (0.3) | 5.945 (0.303–116.8) | 0.25 | 0.53 |
| A-4G-A-G-T | 0 (0.0) | 5 (0.5) | 9.342 (0.509–171.5) | 0.07 | 0.38 |
| A-4G-A-G-G | 28 (3.4) | 4 (0.4) | 0.121 (0.041–0.360) | 0.01 | 0.01 |
| A-4G-A-A-T | 2 (0.2) | 0 (0.0) | 0.170 (0.008–3.590) | 0.22 | 0.53 |
| A-5G-G-G-T | 5 (0.6) | 22 (2.4) | 3.733 (1.354–10.29) | 0.01 | 0.05 |
| A-5G-G-G-G | 0 (0.0) | 3 (0.3) | 5.945 (0.303–116.8) | 0.25 | 0.53 |
| A-5G-A-G-G | 0 (0.0) | 2 (0.2) | 4.246 (0.201–89.74) | 0.50 | 0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; An, H.J.; Kim, J.O.; Jun, H.H.; Kim, W.R.; Kim, E.J.; Oh, D.; Kim, J.W.; Kim, N.K. Association between Five Common Plasminogen Activator Inhibitor-1 (PAI-1) Gene Polymorphisms and Colorectal Cancer Susceptibility. Int. J. Mol. Sci. 2020, 21, 4334. https://doi.org/10.3390/ijms21124334
Oh J, An HJ, Kim JO, Jun HH, Kim WR, Kim EJ, Oh D, Kim JW, Kim NK. Association between Five Common Plasminogen Activator Inhibitor-1 (PAI-1) Gene Polymorphisms and Colorectal Cancer Susceptibility. International Journal of Molecular Sciences. 2020; 21(12):4334. https://doi.org/10.3390/ijms21124334
Chicago/Turabian StyleOh, Jisu, Hui Jeong An, Jung Oh Kim, Hak Hoon Jun, Woo Ram Kim, Eo Jin Kim, Doyeun Oh, Jong Woo Kim, and Nam Keun Kim. 2020. "Association between Five Common Plasminogen Activator Inhibitor-1 (PAI-1) Gene Polymorphisms and Colorectal Cancer Susceptibility" International Journal of Molecular Sciences 21, no. 12: 4334. https://doi.org/10.3390/ijms21124334
APA StyleOh, J., An, H. J., Kim, J. O., Jun, H. H., Kim, W. R., Kim, E. J., Oh, D., Kim, J. W., & Kim, N. K. (2020). Association between Five Common Plasminogen Activator Inhibitor-1 (PAI-1) Gene Polymorphisms and Colorectal Cancer Susceptibility. International Journal of Molecular Sciences, 21(12), 4334. https://doi.org/10.3390/ijms21124334





