Abstract
Carbohydrates and their conjugates are the most abundant natural products, with diverse and highly important biological roles. Synthetic glycoconjugates are versatile tools used to probe biological systems and interfere with them. In an endeavor to provide an efficient route to glycomimetics comprising structurally diverse carbohydrate units, we describe herein a robust, stereoselective, multicomponent approach. Isopropylidene-protected carbohydrate-derived aldehydes and ketones were utilized in the Passerini reaction, giving different glycosylated structures in high yields and diastereoselectivities up to 90:10 diastereomeric ratio (d.r). Access to highly valuable building blocks based on α-hydroxy C-glycosyl acids or more complex systems was elaborated by simple post-condensation methodologies.
1. Introduction
Carbohydrates are a ubiquitous group of natural products that mediate numerous, highly diverse cellular events and, consequently, participate in development of various diseases. Synthetic carbohydrates and their derivatives are powerful tools in biology and medicinal chemistry with diverse applications ranging from diagnostics to vaccines and therapeutics [1,2,3,4]. Moreover, carbohydrates represent attractive chiral synthons susceptible to diverse modifications and transformations, a highly desirable feature in diversity-oriented synthesis [5] and in the synthesis of chiral catalyst systems [6] or of noncovalent systems built upon weak interactions [1]. Carbohydrates are still a relatively untapped pool of new therapeutics [7,8]; however, advances in the functional understanding of carbohydrate-involving biological processes enabled carbohydrates to take over a prominent role in drug design. A particular group of compounds, glycomimetics, has emerged in the response to the growing demand for highly specific structures aimed to probe biological processes or interfere with them [9]. Klebe and Diederich described structurally rigid and metabolically stable lin-benzoguanines functionalized with isopropylidene-protected carbohydrate moieties as superior ligands for tRNA-guanine transglycosylase, the enzyme associated with Shigella flexneri-caused infection [10]. According to the DrugBank [11], there are 14 approved drugs bearing the isopropylidene motif in their structure; however, such structures are generally neglected in drug development.
As a part of our ongoing project aimed to enrich the chemical space of natural product-like compounds, we have recently reported a strategy to access a library of densely functionalized glycomimetics. We utilized, for the first time, bis-isopropylidene-protected d-fructose-derived aldehyde 1 (Figure 1) in the Passerini reaction with various acids and isocyanides [12]. Although carbohydrate-derived aldehydes were reported to affect diastereoselectivity in multicomponent reactions (MCRs), observed stereoselectivity was highly influenced by OH-protecting groups (acetyl or benzyl protecting groups were frequently used) and other components in MCRs [13]. Contrary to that, all reactions performed with bis-isopropylidene-protected aldehyde 1 yielded glycomimetics in high yields and diastereoselectivities up to diastereomeric ratio (d.r.) 94:6. Therefore, to validate the methodology, in this work, we expanded the pool of carbohydrate-derived carbonyl compounds to obtain Passerini products adorned with structurally different carbohydrate units. We were also intrigued by the possible role of the rigid isorpopylidene protecting group(s) in the diastereoselective outcome of the reactions. Moreover, an important advantage of the selected carbohydrate derivatives is the fixed anomeric configuration, since the presence of multiple anomers often hampers the purification and characterization of carbohydrate-related compounds.
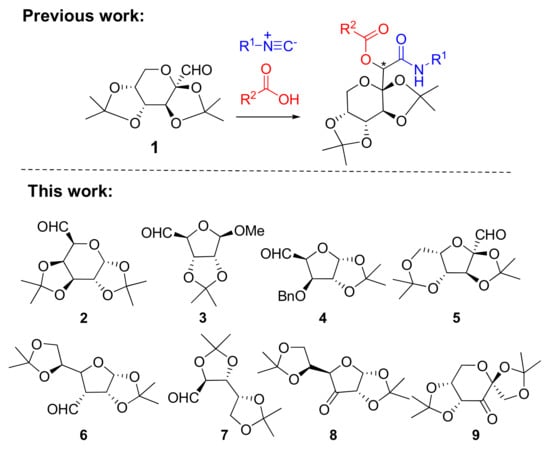
Figure 1.
The selection of isopropylidene-protected aldehydes and ketones used as carbonyl components in the Passerini reaction.
We selected commercially available hexoses and pentoses in pyranose, furanose, or open chain forms, easily convertible into aldehyde or keto-derivatives by oxidation with Dess−Martin periodinane. Six aldehydes were utilized in this study (Figure 1): 1,2:3,4-di-O-isopropylidene-α-d-galactopyranose-derived aldehyde 2, methyl-2,3-O-isopropylidene-β-d-ribofuranoside-derived aldehyde 3, 3-O-benzyl-1,2-O-isopropylidene-α-d-xylofuranose-derived aldehyde 4, 2,3:4,6-di-O-isopropylidene-α-l-sorbofuranose-derived aldehyde 5, 1,2:5,6-di-O-isopropylidene-α-d-allofuranose-derived aldehyde 6, and 2,3:4,6-di-O-isopropylidene-d-arabinose-derived aldehyde 7. In addition, to complement our selection of carbohydrate-related carbonyl components, two ketones were envisaged: derivatives 8 and 9, obtained from 1,2:5,6-di-O-isopropylidene-α-d-glucofuranose and 1,2:4,5-di-O-isopropylidene-β-d-fructopyranose, respectively (Figure 1).
2. Results and Discussion
Our initial experiments were performed with aldehyde 2, and a collection of examples is listed in Figure 2. All reactions were performed in dichloromethane (DCM) at room temperature until the consumption of the aldehyde, typically 6–24 h. Passerini reactions with acetic acid and cyclohexyl isocyanide, tert-butyl isocyanide, or methyl isocyanoacetate furnished the desired Passerini products 2a–c in very good yields (~80%, Figure 2). The ratio of two diastereoisomers, determined by 1H NMR spectroscopy of the reaction mixture, was 80:20 d.r. for compounds 2a and 2b and 70:30 d.r. for 2c. Next, we expanded the scope of the acid components by utilizing N-terminally protected amino acids. Gratifyingly, Boc-protected phenylalanine, leucine, and proline in the reaction with methyl isocyanoacetate produced depsitripeptides 2d–f in very good yields (58–80%). In earlier work from our group, we successfully used α-hydrazino acids in the Passerini reaction to obtain hydrazino depsipeptides, a novel class of backbone-extended peptidomimetics [14]. Therefore, we performed Passerini reactions with Nα-Cbz-, Nβ-Boc-protected hydrazino acids related to phenylalanine, leucine, and proline and obtained the corresponding hydrazino depsitripeptides in very good yields (53–74%). The ratio of two diastereoisomers, ~70:30 d.r. remained constant through all reactions performed with methyl isocyanoacetate, with the exception of 2f, for which lower diastereoselectivity can be attributed to a somewhat problematic purification. We managed to isolate the major diastereoisomer of compound 2a and prepared crystals suitable for X-ray analysis (Supplementary Materials). The configuration of the new stereocenter formed in the Passerini reaction was determined as S, as found for products obtained with fructose-derived aldehyde 1 [12].
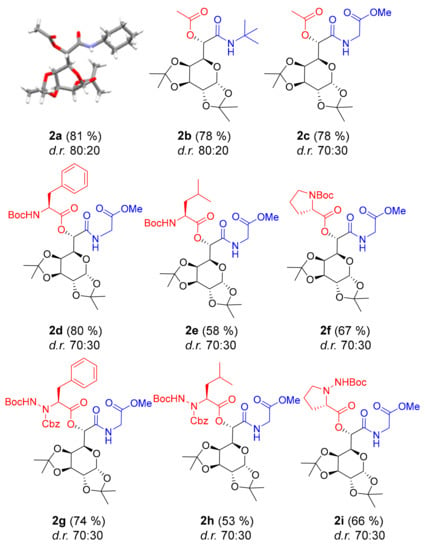
Figure 2.
Passerini products obtained with aldehyde 2. Isolated yields are given in parentheses. Diastereomeric ratios (d.r.) were determined by 1H NMR analysis of the reaction mixtures.
Encouraged by the highly consistent results obtained with aldehyde 2, we proceeded to examine the Passerini reaction with carbonyl compounds 3–9. In all further experiments, two groups of acid–isocyanide components were used: a “simple” acetic acid–cyclohexyl isocyanide combination and an “amino acid-derived” combination with Boc-protected phenylalanine as the acid component and methyl isocyanoacetate (glycine-derived isocyanide). Such selection enabled us to ascertain the influence of sugar-derived carbonyl compounds on the yield and diastereoselectivity of the reaction, as well as the contribution of other components. To our satisfaction, Passerini reactions performed with ribofuranoside-derived aldehyde 3 gave the products 3a and 3d in high yields, i.e., 82% and 78%, respectively (Figure 3). Unlike aldehyde 2, the ratio of two diastereoisomers was the same for the two combination of reactants, i.e., 77:23 d.r., determined by 1H NMR spectroscopy of the reaction mixtures.
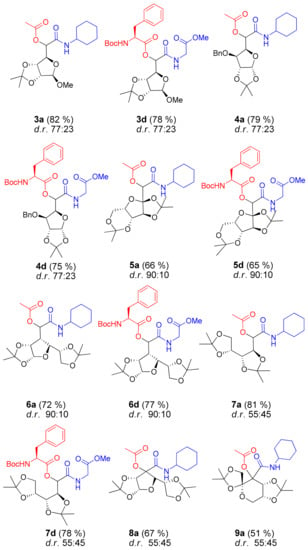
Figure 3.
Passerini products obtained with aldehydes and ketones 3–9. Isolated yields are given in parentheses; d.r. was determined by 1H NMR analysis of the reaction mixture.
Comparable results were obtained with xylofuranose-derived aldehyde 4, with high yields (79% for 4a and 75% for 4d) and moderate diastereoselectivity (77:23 d.r.). We then turned our attention to sorbofuranose-derived aldehyde 5, which can be considered a furanose equivalent of the fructose-derived aldehyde 1. The reaction turned out to be efficient with both groups of acid–isocyanide components, giving Passerini products 5a and 5d in satisfactory yields (~65%, Figure 3). More importantly, the reactions performed with aldehyde 5 were highly diastereoselective (90:10 d.r.), as previously observed with aldehyde 1 [12]. Further study was directed toward allofuranose-derived aldehyde 6, with a carbonyl group attached to the C3 position of the furanose ring. We were pleased to find that reactions proceeded with very good yields, i.e., 72% for 6a and 77% for 6d, and again with high diastereoselectivity, i.e., 90:10 d.r. Riva et al. reported Lewis acid catalyzed Passerini reactions on chiral aldehydes derived from desymmetrized erythritol with diastereoselectivities up to 92:8 [15]. This report prompted us to examine aldehyde 7, obtained by oxidation of 2,3:4,6-di-O-isopropylidene-d-arabinose, under our Passerini reaction conditions. The reactions proceeded smoothly, giving the products 7a and 7d in very good yields (~80%, Figure 3), but stereoselectivity was disappointing, corresponding to 55:45 d.r.. Riva et al. used 0.4 equiv. of Lewis acid (ZnBr2) to improve the diastereoselectivity with erythritol-derived aldehydes; however, our attempts to affect stereoselectivity by adding a Lewis acid were unsuccessful. A possible explanation can be the presence of only one dioxolane ring in erythritol-derived aldehydes, where chelation of the metal occurred by the carbonyl oxygen and one of the dioxolane oxygens [15], while bis isopropylidene-protected aldehyde 7 possesses multiple binding sites for the Lewis acid. Finally, to complete our pool of sugar-derived carbonyl compounds, Passerini reactions were performed with furanose ketone 8 and pyranose ketone 9. Although ketones are generally less reactive in the Passerini reaction, products 8a and 9a were obtained in satisfactory yields (67% and 51%, respectively), while isolation and purification of products 8d and 9d were inefficient, so their data are not included in this paper. As with aldehyde 7, there was virtually no stereoselectivity (55:45 d.r.).
At this point, we were able to observe that bis-isorpopylidene-protected compounds bearing an aldehyde group attached to the tetrasubstituted carbon atoms (1 and 5) are efficient chiral inductors, supplying Passerini products with 90:10 d.r. The same diastereoselectivity was observed with compound 6, in which an aldehyde group is attached to the trisubstituted carbon atom (C3 position of the furanose ring). Other compounds with an aldehyde group attached to the trisubstituted carbon atom (2, 3, and 4) were less efficient chiral inductors, giving Passerini products with up to 77:23 d.r. A possible explanation can be the increased sterically hindrance of aldehyde 6 compared with 2, 3, or 4, where the aldehyde group is adjacent to the ring oxygen. Acyclic aldehyde 7 and ketones 8 and 9 gave practically racemic mixtures of Passerini products.
There are many examples of synthetic glycopeptide structures exploited for the generation of new biomaterials based on weak noncovalent interactions [1]. For example, sulfated glycopeptide supramolecular nanostructures were demonstrated to amplify the signaling of bone morphogenetic protein 2 and promote the regeneration of bone [16], while glycosaminoglycan-like nanofibers were found to mimic the bioactive functions of natural hyaluronic acid molecules [17]. Due to the fact that different supramolecular architectures can be accessed by varying peptide length and composition, we considered further functionalization of Passerini products, especially those derived from N-terminally protected amino acids. Although the presence of two different acid-labile groups, Boc and isopropylidene, seems an aggravating factor, we found a smooth deprotection/coupling approach and confirmed it on two examples presented in Scheme 1. The removal of the Boc group in product 2d and the subsequent coupling with Boc–Pro–OH afforded the tetradepsipeptide 2j in 78% yield. Following the same methodology, in situ deprotection of 5d and coupling with Boc–Phe–OH gave the partially deprotected product 5j in 65% yield. It seems that the six-membered dioxolane ring is more susceptible to acid cleavage under the applied conditions compared to the five-membered ring. Additionally, we prepared Passerini product 2k (Scheme 1) comprising Fmoc-protected phenylalanine to confirm that selective Fmoc-deprotection in the presence of other base-labile groups is feasible with 20% piperidine in dimethylformamide (DMF) to yield 77% of the free amine 2l.
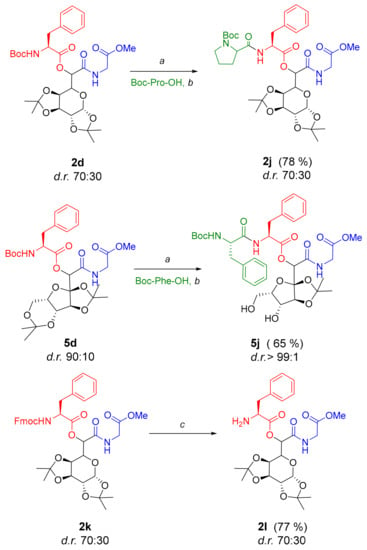
Scheme 1.
Post-condensation modification of selected Passerini products: a trifluoroacetic acid (TFA)/ dichloromethane (DCM) = 1:1, room temperature (RT), 2 min; b benzotriazol-1-yloxytris(dimethylamino)phosphonium (BOP, 1.5 eq), hydroxybenzotriazole (HOBt,1.2 eq), N-methylmorpholine (NMM, 2 eq), dimethylformamide (DMF)/DCM = 1:1, RT, overnight; c 20% piperidine in DMF, RT, 15 min.
The presence of a base-labile ester group in Passerini products can be of interest for another post-condensation modification, namely, the synthesis of highly valuable α-hydroxy amides or acids [18,19,20]. Therefore, we attempted the NaOH-mediated removal of acetyl groups in Passerini products 1c, 2c, 5c, and 6c (Scheme 2). All reactions worked efficiently affording the C-terminally protected dimers 1m, 2m, 5m, and 6m, comprising α-hydroxy C-glycosyl acid and glycine. Moreover, NMR analysis revealed the preservation of the chiral information derived from the diastereoselective Passerini reaction. C-glycosyl amino acids are widely exploited for the synthesis of glycomimetics, peptidomimetics, and foldamers [21]. The conformational bias imposed by the presence of a furanoid or pyranoid ring, adjustable hydrophilicity, and tunable properties in terms of substituents and stereochemistry are highly desirable features, making C-glycosyl amino acids and their derivatives versatile building blocks for multiple purposes.
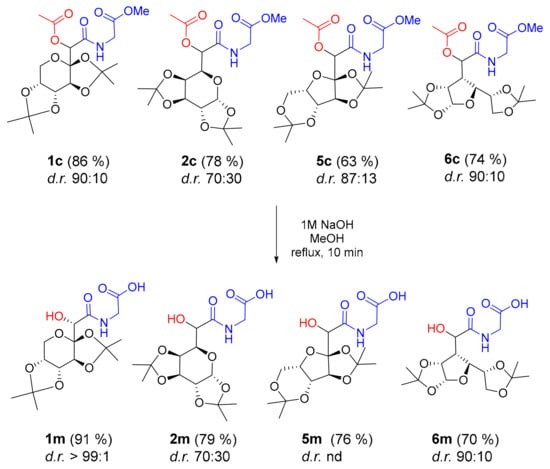
Scheme 2.
Base-mediated deprotection of selected Passerini products; nd: not determined.
With a rich portfolio of different glycosylated structures in hands and an elaborated methodology to access more complex glycopeptide systems, we are currently focused toward building up multivalent glycosylated structures, exploring and understanding the functions of the obtained molecules. We are particularly interested in understanding how the selection of carbohydrate scaffolds as well as their number and distribution within the “carrier” molecule can help in solving highly challenging problems, like targeting carbohydrate–peptide or carbohydrate–carbohydrate interactions [22].
3. Materials and Methods
All experiments were monitored by analytical thin-layer chromatography (TLC) on Silica Gel 60 F254 plates (Merck; Darmstadt, Germany) after spraying with 10% H2SO4 and heating. Flash column chromatography was performed on silica gel (Merck, 40–63 μm particle size) by standard techniques, eluting with solvents as indicated. All NMR experiments were carried out by using a Bruker Avance 600 spectrometer (600.13 MHz, 1H; 150.91 MHz, 13C). Samples in CDCl3 solutions were recorded in 5 mm NMR tubes at 298 K. Chemical shifts in parts per million were referenced to tetramethylsilane (TMS) as internal standard. Spectra were assigned on the basis of 2D homonuclear (COSY) and heteronuclear (HMQC, HMBC) experiments. 1H chemical shifts were assigned to the particular starting component, and the following abbreviations were used: cyclohexyl = CyHex; tetrabutyl = tBu; methyl cyanoacetate = Gly; fructopyranose-derived aldehyde 1 = Fru; galactose-derived aldehyde 2 = Gal; ribose-derived aldehyde 3 = Rib; xylose-derived aldehyde 4 = Xyl; sorbose-derived aldehyde 5 = Sor; allose-derived aldehyde 6 = Alo; arabinose-derived aldehyde 7 = Ara; glucofuranose-derived ketone 8 = GluF; fructopyranose-derived ketone 9 = FruP. High-resolution mass spectrometry (HRMS) was performed with a MALDI–TOF/TOF spectrometer in positive ionization mode. The calibration type was internal, with calibrants produced by matrix ionization dissolved in α-cyano-4-hydroxycinnamic acid matrix. Accurately measured spectra were internally calibrated, and elemental analysis was performed on Data Explorer v. 4.9 software with mass accuracy better than 5 ppm. NMR spectra of all compounds are given in Supplementary Materials.
DS1,2 refers to a product isolated as an inseparable mixture of two diastereoisomers, while DS1 and DS2 refer to isolated diastereoisomer 1 and diastereoisomer 2.
3.1. General Procedure for Passerini Reactions
To a glass vial containing 1 M solution of oxo-compound (0.1 mmol) in DCM under nitrogen were added acid (0.11 mmol) and the isocyanide component (0.11 mmol) dissolved in 100 µL DCM. With all reactants added, the reaction mixture was stirred at room temperature until the consumption of the aldehyde/ketone component, which typically required 6–24 h. The reactions were concentrated under reduced pressure, and the reaction mixtures were purified by flash column chromatography.
3.1.1. Methyl 2-(2-acetoxy-2-((3aR,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-3a-yl)acetamido)acetate (1c)
Yield 86% (36 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 2:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.95 (s, 0.9H, NH), 6.91 (s, 0.1H, NH), 5.50 (s, 0.1H, H-1 Fru), 5.18 (s, 0.9H, H-1 Fru), 4.69 (d, J = 2.6 Hz, 0.1H, H-3 Fru), 4.58 (dd, J = 7.9, 2.8 Hz, 0.9H, H-4 Fru), 4.54 (dd, J = 8.0, 2.6 Hz, 0.1H, H-4 Fru), 4.36 (d, J = 2.8 Hz, 0.9H, H-3 Fru), 4.22 (dd, J = 7.9, 1.3 Hz, 0.9H, H-5 Fru), 4.17 (dd, J = 8.0, 1.6 Hz, 0.1H, H-5 Fru), 4.09 (dd, J = 18.5, 5.0 Hz, 1H, CH2 Gly), 4.02 (dd, J = 18.5, 4.9 Hz, 1H, CH2 Gly) 3.96 (dd, J = 18.5, 4.6 Hz, 0.1H, H-6 Fru), 3.92 (dd, J = 13.0, 1.9 Hz, 0.9H, H-6 Fru), 3.83 (d, J = 13.0 Hz, 1H, H-6 Fru), 3.73 (s, 0.3H, CH3 Gly), 3.72 (s, 2.7H, CH3 Gly), 2.19 (s, 0.3H, CH3 Ac), 2.17 (s, 2.7H CH3 Ac), 1.51 (s, 3H, CH3 isop), 1.48 (s, 3H, CH3 isop), 1.44 (s, 3H, CH3 isop), 1.31 (s, 3H, CH3 isop). 13C NMR (CDCl3) δ = (170.2, 169.2, 165.7) (CO), (109.9, 109.6) (CH3 isop), (102.1, 101.5) (C-2 Fru), (75.7, 73.8, 71.6, 70.8, 70.8, 70.4, 70.3) (C-1,3,4,5 Fru), (62.0, 61.8) (C-6 Fru), (52.6, 52.4) (CH3 Gly), (41.6, 41.3) (CH2 Gly), (26.7, 26.7, 26.2, 26.0, 25.5, 25.2, 24.3, 24.2) (CH3 isop), (21.1, 20.9) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10Na [M + Na]+ 440.1533; found 440.1528.
3.1.2. 2-(Cyclohexylamino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-5-yl)ethyl acetate (2a)
Yield 81% (35 mg); white solid mp = 111–112 °C; Rf (DS1) = 0.52, Rf (DS2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 80:20. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 6.18 (d, J = 7.9 Hz, 1H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 4.95 (d, J = 9.7 Hz, 1H, H-6 Gal), 4.60 (dd, J = 7.9, 2.6 Hz, 1H, H-3 Gal), 4.31 (dd, J = 4.8, 2.6 Hz, 1H, H-2 Gal), 4.28 (dd, J = 8.0, 1.8 Hz, 1H, H-4 Gal), 4.17 (dd, J = 9.7, 1.7 Hz, 1H, H-5 Gal), 3.84–3.70 (m, 1H, CH-1 CyHex), 2.13 (s, 3H, CH3 Ac), 1.88 (m, 2H, CyHex), 1.67 (m, 2H, CyHex), 1.56 (m, 1H, CyHex), 1.50 (s, 3H, CH3 isop), 1.41 (s, 3H, CH3 isop), 1.36–1.32 (m, 2H, CyHex), 1.31 (s, 3H, CH3 isop), 1.30 (s, 3H, CH3 isop), 1.16 (d, J = 11.9 Hz, 3H, CyHex). 13C NMR (CDCl3): δ = (169.9, 167.0) (CO), (109.8, 109.7) (C isop), 96.4 (C-1 Gal), (71.0, 70.7, 70.6, 70.6, 67.2) (C-2,3,4,5,6 Gal), 48.6 (CH CyHex), (33.04, 32.99) (CyHex), (26.29, 26.17) (CH3 isop), 25.78 (CyHex), 25.23 (CH3 isop), (24.95, 24.91) (CyHex), 24.63 (CH3 isop), 20.95 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2291.
3.1.3. 2-(tert-Butylamino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-5-yl)ethyl acetate (2b)
Yield 78% (31 mg); colorless oil; Rf (DS1) = 0.58, Rf (DS2) = 0.60 (EtOAc/petroleum ether 1:1, v/v); d.r. 80:20. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.19 (s, 0.8H, NH), 6.09 (s, 0.2H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.14 (d, J = 6.5 Hz, 0.2H, H-6 Gal), 4.87 (d, J = 9.8 Hz, 0.8H, H-6 Gal), 4.61–4.55 (m, 1H, H-3 Gal), 4.32–4.23 (m, 2H, H-2,4 Gal), 4.18 (d, J = 6.4 Hz, 0.2H, H-5 Gal), 4.11 (dd, J = 9.8, 1.4 Hz, 0.8H, H-5 Gal), 2.13 (s, 3H, CH3 Ac), 1.49 (s, 3H, CH3 isop), 1.40 (s, 3H, CH3 isop), 1.36–1.25 (m, 15H, CH3 isop, CH3 tBu). 13C NMR (CDCl3) δ = (170.5, 169.7, 167.2, 166.3) (CO), (109.8, 109.7, 109.7, 109.3) (C isop), (96.6, 96.4) (C-1 Gal), (73.9, 71.6, 71.1, 71.0, 70.9, 70.6, 70.6, 67.4, 67.4) (C-2,3,4,5,6 Gal), 51.7 (C tBu), (28.9, 28.7) (CH3 tBu), (26.3, 26.2, 26.2, 26.1, 25.2, 24.6, 24.3) (CH3 isop), (21.2, 20.9) (CH3 OAc). HRMS (MALDI–TOF/TOF) m/z: calcd. for C19H31NO8 [M + H]+ 402.2128; found 402.2146.
3.1.4. Methyl 2-(2-acetoxy-2-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)acetamido)acetate (2c)
Yield 78% (32 mg); colorless oil; Rf (DS1, DS2) = 0.45 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.88 (s, 0.3H, NH), 6.79 (s, 0.7H, NH), 5.50 (d, J = 4.7 Hz, 1H, H-1 Gal), 5.37 (d, J = 6.8 Hz, 0.3H, H-6 Gal), 5.08 (d, J = 9.5 Hz, 0.7H, H-6 Gal), 4.61 (m, 1H, H-3 Gal), 4.41 (d, J = 8.0 Hz, 0.3H, H-4 Gal), 4.32–4.26 (m, 2H, H-4 Gal, H-2 Gal, H-5 Gal), 4.20 (dd, J = 16.5, 8.4 Hz, 1H, H-5 Gal, CH2 Gly), 4.04 (m, 1.4H, CH2 Gly), 3.91 (dd, J = 18.3, 4.3 Hz, 0.3H, CH2 Gly), 3.72 (s, 3H, CH3 Gly), 2.15 (s, 0.9H, CH3 Ac), 2.12 (s, 2.1H, CH3 Ac), 1.53 (s, 0.9H, CH3 isop), 1.51 (s, 2.1H, CH3 isop), 1.44 (s, 0.9H, CH3 isop), 1.42 (s, 2.1H, CH3 isop), 1.31 (s, 6H, CH3 isop). 13C NMR (CDCl3) δ = (170.5, 170.0, 169.9, 169.8, 168.3, 167.6) (CO), (110.0, 109.8, 109.6, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (72.9, 71.3, 71.1, 70.91, 70.8, 70.7, 70.7, 70.5, 67.2, 67.0) (C-2,3,4,5,6 Gal), (52.5, 52.5) (CH3 Gly), (41.6, 41.4) (CH2 Gly)), (26.3, 26.2, 26.1, 26.1, 25.2, 25.2, 24.7, 24.4) (CH3 isop), (21.1, 20.8) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10 [M + H]+ 418.1713; found 418.1711.
3.1.5. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (2d)
Yield 80% (49 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.27–6.92 (m, 6H, Ph, NH), 5.50 (d, J = 5.0 Hz, 1.3H, H-1,6 Gal), 5.19 (d, J = 8.9 Hz, 0.7H, H-6 Gal), 4.97 (t, J = 8.0 Hz, 1H, NH), 4.67–4.45 (m, 2H, H-3 Gal, α-Phe), 4.30 (dd, J = 6.8, 4.4 Hz, 1.3H, H-2,4 Gal), 4.25–4.09 (m, 1.7H, H-5, H-4 Gal), 4.07–3.87 (m, 2H, CH2 Gly), 3.72 (d, J = 4.6 Hz, 3H, CH3 Gly), 3.22 (dd, J = 14.0, 6.2 Hz, 1H, β-Phe), 3.08 (dd, J = 14.8, 6.5 Hz, 1H, β-Phe), 1.54 (d, J = 3.8 Hz, 3H, CH3 isop), 1.46 (s, 1H, CH3 isop), 1.37 (bs, 11H, CH3 Boc, CH3 isop), 1.30 (dd, J = 8.0, 3.5 Hz, 6H, CH3 isop). 13C NMR (CDCl3) δ = (171.5, 170.8, 169.8, 169.8, 167.8, 167.3) (CO), (155.5, 155.4) (CO Boc), (136.5, 136.4) (C Ph), (129.8, 129.7, 128.7, 128.6, 127.1, 126.9) (CH Ph), (109.9, 109.8, 109.6, 109.3) (C isop), (96.4, 96.4) (C-1), (80.3, 80.1) (C Boc), (73.3, 71.6, 71.43, 71.03, 70.9, 70.6, 70.3) (C-2,3,4,6), (67.2, 67.0) (C-5 Gal), (54.9, 54.5) (α-Phe), 52.5 (CH3Gly), (41.5, 41.3) (CH2 Gly), (38.3, 37.5) (β-Phe), 28.5 (CH3 Boc), (26.3, 26.2, 26.2, 26.0, 25.2, 24.5, 24.5) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C30H42N2O12Na [M + Na]+ 645.2635; found 645.2615.
3.1.6. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-4-methylpentanoate (2e)
Yield 58% (34 mg); colorless oil; Rf (DS1,2) = 0.55, (EtOAc/petroleum ether 1:1, v/v); ¡ 75:25. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 7.33 (s, 0.25H, NH), 6.93 (s, 0.75H, NH), 5.50 (0.25, H-6 Gal), 5.50 (d, J = 4.8 Hz, 0.75H, H-1 Gal), 5.44 (d, J = 4.8 Hz, 0.25H, H-1 Gal), 5.19 (d, J = 8.9 Hz, 0.75H, H-6 Gal), 4.90 (dd, J = 16.4, 7.7 Hz, 1H, NH Leu), 4.63–4.60 (m, 0.75H, H-3 Gal), 4.57 (dd, J = 7.9, 2.1 Hz, 0.25H, H-3 Gal), 4.50 (d, J = 7.6 Hz, 0.25H, α-Leu), 4.36 (m, 0.75H, α-Leu), 4.33–4.16 (m, 3H, H-2,4,5 Gal), 4.02 (ddd, J = 41.5, 18.2, 5.3 Hz, 2H, CH2 Gly), 3.70 (d, J = 6.9 Hz, 3H, CH3 Gly), 1.74 (s, 3H, β-Leu, γ-Leu), 1.51 (d, J = 7.5 Hz, 3H, CH3 isop), 1.45–1.37 (m, 15H, CH3 Boc, CH3 isop), 1.31–1.27 (m, 3H, CH3 isop), 0.94–0.91 (m, 6H, CH3 Leu). 13C NMR (CDCl3) δ = (172.8, 172.0, 169.9, 169.8, 167.8, 167.5) (CO), (156.0, 155.7) (CO Boc), (109.9, 109.6, 109.1) (C isop), 96.4 (C-1 Gal), (80.3, 80.0) (C Boc), (72.7, 71.6, 71.0, 71.0, 70.6, 70.4) (C-2,3,4,6 Gal), (67.4, 67.0) (C-5 Gal), (52.9, 52.4, 52.3) (CH3 Gly, α-Leu), (41.8, 41.4, 41.3, 41.0) (CH2 Gly, β-Leu), 29.9, (28.5, 28.5) (Boc), (26.3, 26.2, 26.1) (CH3 isop), (25.3, 25.2) (γ-Leu), (24.9, 24.8, 24.6, 23.1, 23.0) (CH3 isop), (22.3, 22.0) (δ-Leu). HRMS (MALDI–TOF/TOF) m/z: calcd. for C27H44N2O12Na [M + Na]+ 611.2792; found 611.2805.
3.1.7. 1-tert-Butyl 2-(2-((2-methoxy-2-oxoethyl) amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl)pyrrolidine-1,2-dicarboxylate (2f)
Yield 67% (40 mg); colorless oil; Rf (DS1,2) = 0.35, (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ = 7.38 (dd, J = 19.5, 14.3 Hz, 0.45H, NH), 6.74 (t, J = 4.7 Hz, 0.55H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.28 (d, J = 8.0 Hz, 0.55H, H-6 Gal), 5.05 (d, J = 9.7 Hz, 0.45H, H-6 Gal), 4.60 (ddd, J = 25.8, 7.9, 2.3 Hz, 1H, H-3 Gal), 4.38–4.23 (m, 4H, H-2, H-4, H-5 Gal, CH2 Gly), 4.10 (dd, J = 17.9, 6.0 Hz, 0.55H, CH2 Gly), 4.02 (t, J = 5.7 Hz, 1H, α-Pro), 3.88 (dd, J = 17.9, 5.3 Hz, 0.45H, CH2 Gly), 3.71 (s, 1.6H, CH3 Gly), 3.68 (s, 1.4H, CH3 Gly), 3.55–3.46 (m, 1H, δ-Pro), 3.42–3.30 (m, 1H, δ-Pro), 2.33–2.26 (m, 0.55H, β-Pro), 2.22–2.13 (m, 1.5H, β-Pro), 1.88 (ddd, J = 25.6, 11.5, 8.4 Hz, 2H, γ-Pro), 1.36 (ddd, J = 36.5, 29.4, 26.3 Hz, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ = (171.8, 171.5, 169.9, 168.3, 167.8) (CO), (155.3, 154.1) (CO Boc), (109.8, 109.7, 109.6, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (80.2, 80.1) (C Boc), (71.9, 71.2, 70.9, 70.8, 70.7, 70.5, 70.4) (C-2,3,4,6 Gal), (67.1, 66.9) (C-5 Gal), (59.2, 58.9) (α-Pro), (52.4, 52.3) (CH3 Gly), (47.0, 46.5) (δ-Pro), (41.5, 41.2) (CH2 Gly), (30.9, 30.2) (β-Pro), (28.7, 28.5) (CH3 Boc), (26.3, 26.2, 26.2, 25.2, 25.2, 24.7, 24.4) (CH3 isop), (24.4, 23.4) (γ-Pro). HRMS (MALDI–TOF/TOF) m/z: calcd. for C26H40N2O12 [M + H]+ 595.2479; found 595.2453.
3.1.8. 1-Benzyl 2-tert-butyl 1-(1-(2-((2-methoxy-2-oxoethyl) amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethoxy)-1-oxo-3-phenylpropan-2-yl) hydrazine-1,2-dicarboxylate (2g)
Yield 74% (57 mg); colorless oil; Rf (DS1,2) = 0.44 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.27 (d, J = 16.6 Hz, 11H, CH Ph, NH), 5.50 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.25–5.16 (m, 4H, H-6 Gal, CH2 Cbz, NH), 5.07 (m, 1H, α-hPhe), 4.55 (d, J = 10.2 Hz, 1H, H-3 Gal), 4.46 (dd, J = 8.0, 1.3 Hz, 1H, H-4 Gal), 4.30 (dd, J = 4.7, 2.5 Hz, 1H, H-2 Gal), 4.15–3.98 (m, 3H, H-5 Gal, CH2 Gly), 3.72 (s, 1.8H, CH3 Gly), 3.70 (s, 1.2H, CH3 Gly), 3.31 (dd, J = 12.8, 6.8 Hz, 1H, β-hPhe), 3.24–3.07 (m, 1H, β-hPhe), 1.54–1.12 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (169.8, 169.1, 168.5, 167.6) (CO), 155.3 (CO Boc), (137.9, 136.0) (C Ph), (129.6, 128.5, 128.4, 128.2, 127.9, 126.6) (CH Ph), (110.1, 109.8, 109.6, 109.6) (C isop), (96.4, 96.3) (C-1 Gal), (81.2, 81.2) (C Boc), (70.9, 70.8, 70.8, 70.4, 70.2) (H-2,3,4,6 Gal), (68.5, 68.3) (CH2 Cbz), (67.7, 67.1) (C-5 Gal), (62.7, 62.2) (α-Phe), 52.5 (CH3 Gly), (41.5, 41.2) (CH2 Gly), (34.6, 34.3) (β-Phe), (28.3, 28.1) (CH3 Boc), (26.2, 26.1, 26.0, 25.9, 25.1, 24.4, 24.4, 24.2) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C38H49N3O12Na [M + Na]+ 794.3112; found 794.3111.
3.1.9. 1-Benzyl 2-tert-butyl 1-(1-(2-((2-methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethoxy)-4-methyl-1-oxopentan-2-yl)hydrazine-1,2-dicarboxylate (2h)
Yield 53% (39 mg); colorless oil; Rf (DS1,2) = 0.54 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.31 (m, 5H, Ph Cbz), 6.91 (s, H, NH), 6.72 (s, H, NH), 5.49 (dd, J = 13.6, 4.7 Hz, 1H, H-1 Gal), 5.15 (ddd, J = 33.8, 24.8, 12.0 Hz, 4H, H-6 Gal, CH2 Cbz, NH), 4.79 (t, J = 10.2 Hz, 1H, α-hLeu), 4.63–4.48 (m, 1H, H-3 Gal), 4.39–3.86 (m, 5H, H-2,4,5 Gal, CH2 Gly), 3.72 (d, J = 3.1 Hz, 3H, CH3 Gly), 1.94 (m, 1H, β-hLeu), 1.63 (m, 1H, β-hLeu), 1.56–1.19 (m, 21H, CH3 Boc, CH3 isop), 1.06–0.80 (m, 6H, δ-hLeu). 13C NMR (CDCl3) δ = (169.8, 169.7, 167.7) (CO), (156.4, 155.1) (CO Boc), 136.1 (C Cbz), (128.8, 128.8, 128.3) (CH Cbz), (109.9, 109.86, 109.8, 109.8) (C isop), (96.4, 96.3) (C-1 Gal), (81.5, 81.4) (CO Boc), (74.4, 71.6, 71.4, 70.9, 70.6, 70.4, 70.2) (C-2,3,4,6), (68.8, 68.7) (CH2 Cbz), (67.2, 67.1, 66.9) (C-5 Gal), (60.3, 59.3) (α-Leu), 52.5 (CH3 Gly), (41.6, 39.9) (CH2 Gly), (37.0, 36.8) (β-Leu), 28.4 (CH3 Boc), (26.2, 26.2, 26.1, 26.0) (CH3 isop), (25.2, 25.2) (γ-Leu), (24.5, 24.4, 24.3, 23.9, 23.5) (CH3 isop), (21.7, 21.7) (δ-Leu).HRMS (MALDI–TOF/TOF) m/z: calcd. for C35H51N3O14 [M + Na]+ 760.3269; found 760.3243.
3.1.10. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethyl 1-((tert-butoxycarbonyl)amino)pyrrolidine-2-carboxylate (2i)
Yield 66% (39 mg); colorless oil; Rf (DS1,2) = 0.38 (EtOAc/petroleum ether 2:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.03 (s, 0.3H), 6.95 (s, 0.7H), 6.71 (d, J = 13.8 Hz, 1H), 5.52 (d, J = 4.8 Hz, 0.7H), 5.49 (d, J = 4.9 Hz, 0.3H), 5.37 (d, J = 8.0 Hz, 0.3H), 5.19 (d, J = 9.3 Hz, 0.7H), 4.61 (dd, J = 7.9, 2.4 Hz, 1H), 4.47 (dd, J = 8.0, 1.7 Hz, 0.3H), 4.32 (dd, J = 4.9, 2.6 Hz, 0.7H), 4.28 (dd, J = 7.8, 1.8 Hz, 1H), 4.21 (dd, J = 9.3, 1.8 Hz, 0.3H), 4.18–4.13 (m, 0.7H), 4.03 (dd, J = 7.4, 5.4 Hz, 2H), 3.92 (dd, J = 18.3, 4.8 Hz, 0.3H), 3.71 (s, 0.9H), 3.71 (s, 2.1H), 3.29 (td, J = 14.2, 7.6 Hz, 1H, δ-Pro), 3.13–3.06 (m, 1H, δ-Pro), 2.21 (dq, J = 16.4, 8.2 Hz, 1H, β-Pro), 2.13–2.03 (m, 1H, β-Pro), 1.93–1.84 (m, 2H, γ-Pro), 1.38 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ = (173.0, 172.0, 170.0, 169.9, 168.3, 167.1) (CO), (110.1, 110.0, 109.7, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (80.2, 80.0) (CO Boc), (72.8, 71.1, 70.9, 70.8, 70.7, 70.6, 70.4) (C-2,3,4,6 Gal), (67.5, 67.2) (C-5 Gal), (64.8, 64.0) (α-Pro), (53.4, 53.2) (δ-Pro), (52.5, 52.4) (CH3 Gly), (41.5, 41.3) (CH2 Gly), (28.6, 28.5) (CH3 Boc), (27.9, 27.6) (β-Pro), (26.2, 26.2, 26.1, 25.2, 25.1, 24.6, 24.5) (CH3 isop), (22.3, 22.2) (γ-Pro). HRMS (MALDI–TOF/TOF) m/z: calcd. for C26H41N3O12Na [M + Na]+ 610.2588; found 610.2571.
3.1.11. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethyl 2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-phenylpropanoate (2k)
Yield 74% (55 mg); colorless oil; Rf (DS1,2) = 0.39, (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.73 (d, J = 7.5 Hz, 2H, Fmoc), 7.54–7.49 (m, 2H, Fmoc), 7.37 (t, J = 7.4 Hz, 2H, Fmoc), 7.30–7.19 (m, 7H, Ph), 6.96 (s, 0.3H, NH), 6.87 (s, 0.7H, NH), 5.52 (d, J = 4.7 Hz, 0.3H, H-1 Gal), 5.50 (d, J = 4.8 Hz, 0.7H, H-1 Gal), 5.44 (d, J = 7.6 Hz, 0.3H, H-6 Gal), 5.31 (d, J = 8.3 Hz, 0.7H, NH), 5.29–5.24 (m, 0.3H, NH), 5.17 (d, J = 9.2 Hz, 0.7H, H-6 Gal), 4.78–4.69 (m, 1H, α-Phe), 4.58 (ddd, J = 32.1, 7.8, 2.2 Hz, 1H, H-3 Gal), 4.38 (dt, J = 18.2, 9.1 Hz, 1H, CH2 Fmoc), 4.30 (dd, J = 4.9, 2.6 Hz, 1H, H-2 Gal), 4.27–4.00 (m, 6H, CH2 Gly, CH Fmoc, CH2 Fmoc, H-5,4 Gal), 3.72 (s, 2.1H, CH3 Gly), 3.68 (s, 0.9H, CH3 Gly), 3.26 (dd, J = 14.0, 6.2 Hz, 1H, β-Phe), 3.13 (dd, J = 14.0, 6.4 Hz, 1H, β-Phe), 1.55–1.21 (m, 12H, CH3 isop). 13C NMR (CDCl3) δ (171.1, 170.5, 169.8, 167.7) (CO Fmoc), (155.9, 155.8) (CO Fmoc), (144.0, 143.9, 141.5) (C Fmoc), (136.4, 136.1) (C Ph), (129.8, 129.8, 128.8, 128.6, 127.9, 127.2, 127.1) (CH Ph), (125.3, 125.3, 125.2) (CH Fmoc-Ph), (120.2, 120.1) (CH Fmoc-Ph), (110.0, 109.9, 109.6, 109.3) (C isop), (96.4, 96.4) (C-1 Gal), (73.5, 71.5, 71.3, 71.0, 70.9, 70.8, 70.5, 70.3) (C-2,3,4,6 Gal), 67.4 (C-5 Gal), 67.2 (CH2 Fmoc), 67.0 (C-5 Gal), (55.1, 54.9) (α-Phe), (52.5, 52.4) (CH3 Gly), (47.4, 47.3) (CH Fmoc), (41.5, 41.3) (CH2 Gly), (38.3, 37.6) (β-Phe), (26.3, 26.2, 26.2, 26.0, 25.2, 24.5, 24.4) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C40H44N2O12 [M + H]+ 745.2972; found 745.2990.
3.1.12. 2-(Cyclohexylamino)-1-((3aR,4S,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3] dioxol-4-yl)-2-oxoethyl acetate (3a)
Yield 82% (30 mg); colorless oil; Rf = 0.63 (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 5.97 (d, J = 7.5 Hz, 0.77H, NH), 5.90 (d, J = 7.7 Hz, 0.23H, NH), 5.11 (d, J = 7.6 Hz, 0.23H, H-5 Rib), 5.07 (d, J = 6.9 Hz, 0.77H, H-5 Rib), 4.95 (s, 0.23H, H-1 Rib), 4.94 (s, 0.77H, H-1 Rib), 4.87 (dd, J = 6.0, 1.4 Hz, 0.23H, H-2 Rib), 4.71 (dd, J = 6.2, 0.5 Hz, 0.77H, H-2 Rib), 4.60 (d, J = 6.9 Hz, 0.77H, H-4 Rib), 4.55 (d, J = 6.0 Hz, 0.77H, H-3 Rib), 4.53 (d, J = 6.0 Hz, 0.23H, H-3 Rib), 4.50 (dd, J = 7.6, 1.4 Hz, 0.23H, H-4 Rib), 3.79–3.72 (m, 1H, CH-1 CyHex), 3.32 (s, 2.3H, OCH3), 3.28 (s, 0.7H, OCH3), 2.15 (s, 0.7H, CH3 Ac), 2.13 (s, 2.3H, CH3 Ac), 1.89 (m, 2H, CyHex), 1.66 (m, 2H, CyHex), 1.58 (m, 1H, CyHex), 1.45 (s, 3H, CH3 isop), 1.34 (m, 2H, CyHex), 1.29 (s, 2.3H, CH3 isop), 1.28 (s, 0.7H, CH3 isop), 1.16–1.14 (m, 3H, CyHex). 13C NMR (CDCl3) δ = (170.0, 166.1) (CO), 112.9 (C isop), (111.1, 110.3) (C-1 Rib), (86.2, 86.0) (C-4 Rib), (85.4, 85.3) (C-3 Rib), (81.7, 81.4) (C-2 Rib), (74.8, 73.6) (C-5 Rib), (56.2, 55.5) (OCH3), 48.5 (CH CyHex), 48.35 (CH CyHex), (33.1, 33.1) (CyHex), (26.8, 26.7) (CH3 isop), (25.7, 25.6, 25.3, 24.9, 24.9) (CyHex), (21.4, 21.1) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H29NO7Na [M + Na]+ 394.1842; found 394.1843.
3.1.13. 1-((3aR,4S,6R,6aR)-6-Methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (3d)
Yield 78% (44 mg); colorless oil; Rf (DS1) = 0.53, (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) δ = 7.36–7.08 (m, 5H, CH Ph), 7.15 (s, 1H, NH), 5.36 (d, J = 4.6 Hz, 1H, NH), 5.19 (d, J = 4.8 Hz, 0.23H, H-5 Rib), 5.04 (d, J = 7.6 Hz, 0.77H, H-5 Rib), 4.96 (s, 0.77H, H-1 Rib), 4.94 (s, 0.23H, H-1 Rib), 4.74–4.53 (m, 4H, H-2,3,4 Rib, α-Phe), 4.09 (d, J = 5.8 Hz, 0.23H, CH2 Gly), 4.03 (d, J = 5.8 Hz, 0.77H, CH2 Gly), 3.94 (d, J = 5.4 Hz, 0.77H, CH2 Gly), 3.88 (d, J = 5.4 Hz, 0.23H, CH2 Gly), 3.71 (d, J = 5.2 Hz, 3H, CH3 Gly), 3.33–3.30 (m, 3.77H, CH3 Rib, β-Phe), 2.98 (dd, J = 14.3, 8.8 Hz, 1.23H, β-Phe), 1.46 (s, 2.31H, CH3 isop), 1.42 (s, 0.69H, CH3 isop), 1.39 (s, 2.31H, Boc), 1.37 (s, 6.9H, Boc), 1.31 (s, 2.1H, CH3 isop), 1.27 (s, 0.69H, CH3 isop). 13C NMR (CDCl3) δ = (170.5, 169.6, 167.2) (CO), 156.2 (CO Boc), 136.4 (C Ph), (129.6, 129.3, 129.0, 128.9, 127.6, 127.3) (CH Ph), 112.8 (C isop), (111.9, 109.9) (C-1 Rib), (86.4, 86.2) (C-4 Rib), (85.7, 85.6) (C-3 Rib), (81.2, 81.0) (C-2 Rib), (80.9, 80.7) (C Boc), (75.0, 74.5) (C-5 Rib), (56.3, 55.9) (α-Phe), (55.3, 54.9) (CH3 Rib), (52.5, 52.3) (CH3 Gly), (41.2, 41.1) (CH2 Gly), (37.6, 37.6) (β-Phe), (28.5, 28.4) (CH3 Boc), (26.9, 26.7) (CH3 isop), (25.7, 25.3) CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C27H38N2O11Na [M + Na]+ 589.2373; found 589.2399.
3.1.14. 1-((3aR,5S,6S,6aR)-6-(Benzyloxy)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)-2-(cyclohexylamino)-2-oxoethyl acetate (4a)
Yield 79% (36 mg); colorless oil; Rf (DS1) = 0.40, Rf (DS2) = 0.26 (EtOAc/petroleum ether 1:2, v/v); d.r. 77:23. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 7.41–7.14 (m, 5H, Ph), 6.01 (d, J = 7.9 Hz, 1H, NH), 5.92 (d, J = 3.7 Hz, 1H, H-1 Xyl), 5.11 (d, J = 9.2 Hz, 1H, H-5 Xyl), 4.62 (d, J = 11.6 Hz, 1H, CH2a-Ph), 4.60 (d, J = 3.7 Hz, 1H, H-2 Xyl), 4.45–4.38 (m, 2H, H-4 Xyl, CH2b-Ph), 3.99 (d, J = 3.1 Hz, 1H, H-3 Xyl), 3.79–3.70 (m, 1H, CH-1 CyHex), 1.98 (s, 3H, CH3 Ac), 1.84 (m, 2H, CyHex), 1.63 (m, 2H), 1.55–1.50 (m, 1H, CyHex), 1.45 (s, 3H, CH3 isop), 1.30 (bs, 5H, CH3 isop, CyHex), 1.16–1.06 (m, 3H, CyHex). 13C NMR (CDCl3) δ (169.70, 166.78) (CO), 136.97 (C Ph), (128.84, 128.45, 128.35) (CH Ph), 112.61 (C isop), 105.65 (C-1 Xyl), (81.79, 81.30, 78.64, 72.47, 70.22) (C-2,3,4,5-Xyl, CH2-Ph), 48.52 (CH CyHex), (32.89, 32.83) (CyHex), (27.01, 26.58) (CH3 isop), (25.73, 24.79, 24.75) (CyHex), 20.78 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C24H33NO7 [M + H]+ 448.2335; found 448.2346.
3.1.15. 1-((3aR,5S,6S,6aR)-6-(Benzyloxy)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)-2-((2-methoxy-2-oxoethyl) amino)-2-oxoethyl 2-((tert-butoxycarbonyl) amino)-3-phenylpropanoate (4d)
Yield 75% (48 mg); colorless oil; Rf (DS1) = 0.52, (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) δ = 7.42–7.11 (m, 10H, Bn, Ph), 6.86 (s, 1H, NH), 5.94 (d, J = 3.6 Hz, 1H, H-1 Xyl), 5.65 (d, J = 7.2 Hz, 0.23H, H-5 Xyl), 5.43 (d, J = 9.0 Hz, 0.77H, H-5 Xyl), 4.91 (m, 1H, α-Phe), 4.67–4.39 (m, 5.77H, H-2, H-4, H-3 Xyl, NH, CH2 OBn), 4.24 (d, J = 3.9 Hz, 0.23H, H-3 Xyl), 4.02 (d, J = 5.3 Hz, 0.77H, CH2 Gly), 3.99 (m, 0.77H, CH2 Gly), 3.92 (d, J = 5.7 Hz, 0.46H, CH2 Gly), 3.71 (s, 2.31H, CH3 Gly), 3.69 (s, 0.69H, CH3 Gly), 3.28 (dd, J = 14.1, 5.6 Hz, 0.77H, β-Phe), 3.12 (dd, J = 14.2, 5.3 Hz, 0.23H, β-Phe), 3.00 (dd, J = 14.1, 7.7 Hz, 0.77H, β-Phe), 2.88 (dd, J = 14.1, 8.3 Hz, 0.23H, β-Phe), 1.48 (s, 3H, CH3 isop), 1.35 (s, 3H, CH3 isop), 1.30 (s, 9H, CH3 Boc). 13C NMR (CDCl3) δ (171.0, 169.8, 167.7) (CO), 155.4 (CO Boc), (137.1, 136.5, 136.1) (C Ph), (129.7, 129.5, 128.8, 128.7, 128.6, 128.4, 128.3, 128.2, 127.2, 127.0) (CH Ph), (112.8, 112.4) (C isop), (105.8, 105.2) (C-1 Xyl), (83.0, 83.0) (C-2 Xyl), (81.9, 81.4) (C-3 Xyl), (80.6, 80.0) (C Boc), (79.0, 78.5) (C-4 Xyl), (72.8, 72.6) (CH2-OBn), (72.1, 70.0) (C-5 Xyl), (55.0, 54.3) (α-Phe), (52.5, 52.4) (CH3 Gly), (41.5, 41.1) (CH2 Gly), (37.9, 37.1) (β-Phe), 28.3 (CH3 Boc), (28.35, 27.2, 27.0, 26.6, 26.5) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C33H42N2O11Na [M + Na]+ 665.2686; found 665.2682.
3.1.16. 2-(Cyclohexylamino)-2-oxo-1-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxin-8a-yl)ethyl acetate (5a)
Yield 66% (28 mg); colorless oil; Rf (DS1) = 0.50, Rf (DS2) = 0.40 (toluen/aceton 5:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 6.30 (d, J = 7.6 Hz, 1H, NH), 5.26 (s, 1H, H-1 Sor), 4.41 (s, 1H, H-5 Sor), 4.31 (d, J = 2.2 Hz, 1H, H-4 Sor), 4.17 (d, J = 1.5 Hz, 1H, H-3 Sor), 4.07 (dd, J = 13.6, 2.2 Hz, 1H, H-6a Sor), 3.98 (d, J = 13.5 Hz, 1H, H-6b Sor), 3.85–3.75 (m, 1H, CH-1 CyHex), 2.17 (s, 3H, CH3 Ac), 1.89 (m, 2H, CyHex), 1.63 (s, 3H, CyHex), 1.54 (m, 1H, CyHex), 1.46 (s, 3H, CH3 isop), 1.41 (d, 6H, CH3 isop), 1.38 (bs, 3H, CH3 isop), 1.34 (m, 1H, CyHex), 1.24–1.17 (m, 3H, CyHex). 13C NMR (CDCl3): δ = (169.6, 164.6) (CO), (113.8, 113.2) (C isop), 97.8 (C-2 Sor), 85.66 (C-5 Sor), 73.7 (C-3 Sor), 72.9 (C-4 Sor), 72.7 (C-1 Sor), 60.6 (C-6 Sor), 48.4 (CH CyHex), 32.9 (CyHex), (32.8, 29.2) (CyHex), (27.8, 26.5, 25.9) (CH3 isop), (24.7, 24.7) (CyHex), 21.0 (CH3 isop), 18.9 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2272.
3.1.17. Methyl 2-(2-acetoxy-2-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo [3,2-d][1,3] dioxin-8a-yl)acetamido)acetate (5c)
Yield 63% (26 mg); colorless oil; Rf (DS1) = 0.30, Rf (DS2) = 0.27 (EtOAc/petroleum ether 1:1, v/v); d.r. 87:13. 1H NMR (CDCl3) δ = 7.10 (s, 0.87H, NH), 6.84 (s, 0.13H, NH), 5.68 (s, 0.13H, H-1 Sor), 5.39 (s, 0.87H, H-1 Sor), 4.87 (s, 0.13H, H-5 Sor), 4.41 (s, 0.87H, H-5 Sor), 4.33 (d, J = 1.7 Hz, 0.87H, H-4 Sor), 4.29 (d, J = 2.3 Hz, 0.13H, H-4 Sor), 4.23–3.96 (m, 5H, H-3,6 Sor, CH2 Gly), 3.73 (d, J = 1.8 Hz, 3H, CH3 Gly), 2.22 (s, 0.39H, CH3 Ac), 2.18 (s, 2.61H, CH3 Ac), 1.47–1.30 (m, 12H, CH3 isop). 13C NMR (CDCl3) δ (170.2, 169.4, 165.6) (CO), 114.0 (C isop), 112.9 (C isop), 97.8 (C-2 Sor), 85.6 (C-5 Sor), (74.5, 74.0, 73.5, 73.2, 72.8, 72.4) (C-1,3,4 Sor), 60.4 (C-6 Sor), 52.5 (CH3 Gly), (41.7, 41.3) (CH2 Gly), (29.1, 28.5, 27.9, 27.7, 26.4) (CH3 isop), (19.2, 18.8) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10 [M + H]+ 418.1713; found 418.1697.
3.1.18. 2-((2-Methoxy-2-oxoethyl) amino)-2-oxo-1-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxin-8a-yl)ethyl 2-((tert-butoxycarbonyl) amino)-3-phenylpropanoate (5d)
Yield 65% (40 mg); colorless oil; Rf (DS1,2) = 0.34 (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) δ = 7.35–7.05 (m, 6H, CH Ph, NH), 5.50 (s, 1H, H-1 Sor), 4.94 (d, J = 8.4 Hz, 1H, NH Phe), 4.65 (dd, J = 12.9, 8.0 Hz, 1H, α-Phe), 4.42 (s, 1H, H-5 Sor), 4.36 (s, 1H, H-4 Sor), 4.25 (s, 1H, H-3 Sor), 4.17 (dd, J = 18.5, 4.9 Hz, 1H, CH2 Gly), 4.14–4.10 (m, 2H, H-6 Sor), 4.07 (dd, J = 18.5, 4.7 Hz, 1H, CH2 Gly), 3.76 (s, 3H, CH3 Gly), 3.42 (dd, J = 14.2, 4.7 Hz, 1H, β-Phe), 3.06 (dd, J = 14.2, 8.2 Hz, 1H, β-Phe), (1.51, 1.44, 1.40, 1.36) (21H, CH3 isop, CH3 Boc). 13C NMR (CDCl3) δ (170.8, 170.1, 165.3) (CO), 155.4 (CO Boc), 136.6 (C Ph), (129.8, 128.7, 126.9) (CH Ph), (114.1, 112.8) (C isop), 97.8 (C-2 Sor), 85.5 (C-5 Sor), 80.0 (C Boc), 74.2 (C-3 Sor), 72.7 (C-4 Sor), 60.5 (C-6 Sor), 54.6 (α-Phe), 52.5 (CH3 Gly), 41.8 (CH2 Gly), 38.4 (β-Phe), (29.1, 28.4, 27.7, 26.5) (CH3 isop), 18.9 (CH3 Boc). HRMS (MALDI–TOF/TOF) m/z: calcd. for C30H42NO12Na [M + Na]+ 645.2635; found 645.2641.
3.1.19. 2-(Cyclohexylamino)-1-((3aR,5S,6R,6aR)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)-2-oxoethyl acetate (6a)
Yield 72% (32 mg); colorless oil; Rf (DS1, DS2) = 0.37, (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.04 (d, J = 7.9 Hz, 1H, NH), 5.75 (d, J = 3.6 Hz, 0.9H, H-3’ Alo), 5.73 (d, J = 3.7 Hz, 0.1H, H-3’ Alo), 5.37 (d, J = 6.4 Hz, 0.1H, H-1 Alo), 5.21 (d, J = 8.7 Hz, 0.9H, H-1 Alo), 4.69–4.62 (m, 1H, H-2 Alo), 4.27–4.16 (m, 2H, H-4,5 Alo), 4.03 (dd, J = 8.4, 6.5 Hz, 1H, H-6 Alo), 3.95 (dd, J = 8.4, 6.6 Hz, 0.9H, H-6 Alo), 3.88 (dd, J = 8.1, 5.4 Hz, 0.1H, H-6 Alo), 3.76 (m, 1H, CH-1 CyHex), 2.51 (m, 0.1H, H-3 Alo), 2.40–2.28 (m, 0.9H, H-3 Alo), 2.11 (s, 2.7H, CH3 Ac), 2.09 (s, 0.3H, CH3 Ac), 1.86 (s, 2H, CyHex), 1.70–1.54 (m, 3H, CyHex), 1.50 (s, 3H, CH3 isop), 1.40 (s, 3H, CH3 isop), 1.38–1.33 (m, 2H, CyHex), 1.32 (s, 3H, CH3 isop), 1.27 (s, 3H, CH3 isop), 1.20–1.11 (m, 3H, CyHex). 13C NMR (CDCl3) δ (170.2, 167.2) (CO), (112.7, 109.8) (C isop), (105.1, 104.6) (C-1 Alo), (81.6, 81.4, 80.3, 78.5, 76.9, 71.4, 71.3) (C-2,3’,4,5), (66.9, 65.8) (C-6 Alo), (48.6) (C-3 Alo), (48.4, 48.3) (CH CyHex), 32.9 (CyHex), (27.0, 26.6, 26.5, 25.7) (CH3 isop), (25.7, 24.8) (CyHex), 21.1 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C22H35NO8Na [M + Na]+ 464.2260; found 464.2254.
3.1.20. Methyl 2-(2-acetoxy-2-((3aS,5S,6R,6aS)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)acetamido) acetate (6c)
Yield 74% (32 mg); colorless oil; Rf (DS1, DS2) = 0.38, (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.77 (s, 0.9H, NH), 6.74 (s, 0.1H, NH), 5.76 (d, J = 3.6 Hz, 0.9H, H-3’ Alo), 5.73 (d, J = 3.7 Hz, 0.1H, H-3’ Alo), 5.48 (d, J = 6.0 Hz, 0.1H, H-1 Alo), 5.38 (d, J = 8.2 Hz, 0.9H, H-1 Alo), 4.82 (t, J = 4.1 Hz, 0.9H, H-2 Alo), 4.77 (t, J = 4.1 Hz, 0.1H, H-2 Alo), 4.26 (dd, J = 9.7, 5.0 Hz, 0.9H, H-4 Alo), 4.22–4.06 (m, 2.1H, H-4,5 Alo, CH2 Gly), 4.05–4.00 (m, 1H, H-6 Alo), 3.94 (dd, J = 8.2, 6.9 Hz, 0.9H, H-6 Alo), 3.87 (dd, J = 18.3, 4.4 Hz, 1.1H, H-6 Alo, CH2 Gly), 3.74 (s, 0.3H, CH3 Gly), 3.72 (s, 2.7H, CH3 Gly), 2.52–2.45 (m, 0.1H, H-3 Alo), 2.37–2.30 (m, 0.9H, H-3 Alo), 2.12 (s, 2.7H, CH3 Ac), 2.10 (s, 0.3H, CH3 Gly), 1.77 (s, 0.3H, CH3 isop), 1.50 (s, 0.3H, CH3 isop), 1.47 (s, 2.7H, CH3 isop), 1.39 (s, 2.7H, CH3 isop), 1.30 (s, 3H, CH3 isop), 1.28 (s, 3H, CH3 isop). 13C NMR (CDCl3) δ (170.1, 170.0, 168.8) (CO), (112.7, 109.8) (C isop), (105.1, 104.6) (C-1 Alo), (81.3, 81.2, 79.9, 78.4, 77.3, 76.9, 71.0, 70.7) (C-2,3’,4,5), (67.2, 66.0) (C-6 Alo), (52.7, 52.5) (CH3 Gly), (48.8, 48.7, 48.3) (CH CyHex, C-3 Alo)), (41.3, 41.2) (CH2 Gly), (26.9, 26.8, 26.7, 26.5, 26.4, 25.6, 25.2) (CH3 isop), (21.0, 20.9) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C19H29NO10Na [M + Na]+ 454.1689; found 454.1696.
3.1.21. 1-((3aS,6aS)-5-(2,2-Dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)-2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (6d)
Yield 77% (49 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) δ 7.33–7.16 (m, 5H, Ph), 6.92 (s, 1H, NH), 5.75 (d, J = 3.7 Hz, 1H, H-3’ Alo), 5.49 (d, J = 7.6 Hz, 1H, H-1 Alo), 5.01 (d, J = 7.4 Hz, 1H, NH Phe), 4.78 (t, J = 4.0 Hz, 1H, α-Phe), 4.59 (dd, J = 13.3, 6.7 Hz, 1H, H-2 Alo), 43.1 (dd, J = 9.7, 4.4 Hz, 1H, H-5 Alo), 4.12 (ddd, J = 22.6, 11.3, 5.5 Hz, 2H, H-4 Alo, CH2 Gly), 3.98 (d, J = 6.5 Hz, 2H, H-6 Alo), 3.86 (dd, J = 18.0, 4.9 Hz, 1H, CH2 Gly), 3.72 (s, 3H, CH3 Gly), 3.22 (dd, J = 14.1, 5.7 Hz, 1H, β-Phe), 3.07 (dd, J = 14.0, 7.1 Hz, 1H, β-Phe), 2.35 (ddd, J = 9.9, 7.8, 4.6 Hz, 1H, H-3 Alo), 1.57–1.28 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (171.1, 169.9, 168.6) (CO), 155.7 (CO Boc), 136.2 (C Ph), (129.6, 128.8, 127.3) (CH Ph), (112.7, 110.0) (C isop), 104.6 (C-1 Alo), 81.3 (C-2 Alo), 80.5 (C Boc), 79.5 (C-4 Alo), 76.6 (C-5 Alo), 71.3 (C-3’ Alo), 65.8 (C-6 Alo), 54.8 (α-Phe), 52.5 (CH3 Gly), 48.2 (C-3 Alo), 41.3 (CH2 Gly), 37.9 (β-Phe), 28.49 (CH3 Boc), (26.86, 26.62, 26.39, 25.44) (CH3 isop).HRMS (MALDI–TOF/TOF) m/z: calcd. for C31H44N2O12Na [M + Na]+ 659,2792; found 659,2790.
3.1.22. 2-(Cyclohexylamino)-2-oxo-1-((4R,4’R,5S)-2,2,2’,2’-tetramethyl-[4,4’-bi(1,3-dioxolan)]-5-yl)ethyl acetate (7a)
Yield 81% (32 mg); colorless oil; Rf (DS1) = 0.50, Rf (DS2) = 0.48 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ = 5.95 (d, J = 7.9 Hz, 0.55H, NH), 5.77 (d, J = 8.1 Hz, 0.45H, NH), 5.37 (d, J = 2.7 Hz, 0.55H, H-1 Ara), 5.28 (d, J = 2.3 Hz, 0.45H, H-1 Ara), 4.37 (dd, J = 7.5, 2.3 Hz, 0.45H, H-2 Ara), 4.32 (t, J = 7.8 Hz, 0.55H, H-3 Ara), 4.10 (dd, J = 8.0, 2.7 Hz, 0.55H, H-2 Ara), 4.08–3.99 (m, 2H, H-4, H-5 Ara), 3.94 (dd, J = 8.4, 4.9 Hz, 0.55H, H-5 Ara), 3.89 (m, 0.45H, H-5 Ara), 3.78–3.70 (m, 1.45H, H-3 Ara, CH-1 CyHex), 2.15 (s, 1.35H, CH3 Ac), 2.14 (s, 1.65H, CH3 Ac), 1.85 (m, 2H, CyHex), 1.64 (m, 2H, CyHex), 1.56 (m, 1H, CyHex), 1.45 (s, 2H, CH3 isop), 1.36–1.26 (m, 12H, CH3 isop, CyHex), 1.17–1.07 (m, 3H, CyHex). 13C NMR (CDCl3) δ = (169.3, 169.13, 166.7, 165.9) (CO), (110.5, 110.2, 110.2, 110.1) (C isop), (80.1, 79.4) (C-2 Ara), (77.4, 77.2) (C-3 Ara), (77.1, 76.9) (C-4 Ara), (73.7, 72.7) (C-1 Ara), 67.4 (C-5 Ara), (48.4, 48.3) (CH CyHex), (33.2, 33.1, 33.1, 33.0) (CyHex), (27.4, 27.4, 27.0, 27.0, 26.7) (CH3 isop), (25.7, 25.7) (CyHex), (25.6, 25.3) (CH3 isop), (24.9, 24.8) (CyHex), (21.1, 21.0) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C20H33NO7Na [M + Na]+ 422.2155; found 422.2138.
3.1.23. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((4R,4’R,5S)-2,2,2’,2’-tetramethyl-[4,4’-bi(1,3-dioxolan)]-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (7d)
Yield 78% (46 mg); colorless oil; Rf (DS1) = 0.48 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ 7.60 (m, 0.55H, NH), 7.36–7.25 (m, 5H, CH Ph), 7.11 (m, 0.45H, NH), 5.50 (d, J = 2.7 Hz, 0.55H, H-1 Ara), 5.44 (d, J = 1.6 Hz, 0.45H, H-1 Ara), 5.12 (d, J = 6.0 Hz, 0.45H, α-Phe), 4.97 (d, J = 7.1 Hz, 0.55H, α-Phe), 4.65–4.45 (m, 1.45H, H-2 Ara, NH), 4.29 (t, J = 7.4 Hz, 0.55H, H-3 Ara), 4.15–3.92 (m, 5.45H, H-2, H-3, H-4, H-5, CH2 Gly), 3.81 (td, J = 7.9, 3.4 Hz, 0.55H, H-5 Ara), 3.73 (s, 1.65H, CH3 Gly), 3.71 (s, 1.35H, CH3 Gly), 3.21–3.05 (m, 2H, β-Phe), 1.47–1.27 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (171.7, 170.6, 169.8, 169.6, 168.0, 167.0) (CO), 156.0 (CO Boc), (135.9, 135.5) (C Ph), (129.4, 129.3, 129.2, 129.0, 127.6, 127.4) (CH Ph), (110.5, 110.3, 110.0, 109.9) (C isop), (81.0, 80.8) (C Boc), (79.6, 79.2) (C-2 Ara), 77.1 (C-3 Ara), (76.8, 76.6) (C-4 Ara), (74.2, 72.9) (C-1 Ara), (67.47, 67.2) (C-5 Ara), (55.9, 54.8) (α-Phe), (52.4, 52.3) (CH3 Gly), (41.3, 41.1) (CH2 Gly), (37.8, 37.7) (β-Phe), (28.4, 28.4) (CH3 Boc), (27.4, 27.4, 26.9, 26.9, 26.7, 26.6, 25.5, 25.1) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C29H42N2O11Na [M + Na]+ 617.2686; found 617.2687.
3.1.24. (3aR,5S,6aR)-6-(Cyclohexylcarbamoyl)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl acetate (8a)
Yield 67% (29 mg); colorless oil; Rf (DS1) = 0.70, Rf (DS2) = 0.50 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) Major isomer: δ = 7.40 (d, J = 7.5 Hz, 1H, NH), 5.88 (d, J = 3.6 Hz, 1H, H-1 GlcF), 4.87 (d, J = 3.6 Hz, 1H, H-2, GlcF), 4.45 (t, J = 4.7 Hz, 1H, H-5, GlcF), 4.42 (d, J = 2.8 Hz, 1H, H-4 GlcF), 4.13 (dd, J = 8.4, 6.5 Hz, 1H, H-6a GlcF), 4.06 (dd, J = 8.4, 6.9 Hz, 1H, H-6b, GlcF), 3.80–3.73 (m, 1H, CH-1 CyHex), 2.09 (m, 3H, CH3 Ac), 1.93–1.82 (m, 2H, CyHex), 1.67–1.60 (m, 2H, CyHex), 1.59 (m, 5H, CH3 isop, CyHex), 1.41 (s, 3H, CH3 isop), 1.36 (m, 4H, CH3 isop, CyHex), 1.33 (s, 3H, CH3 isop), 1.27–1.15 (m, 3H, CyHex). Minor isomer: δ = 6.17 (d, J = 8.1 Hz, 1H, NH), 5.85 (d, J = 3.8 Hz, 1H, H-1 GlcF), 5.33 (d, J = 3.8 Hz, 1H, H-2 GlcF), 4.10–4.06 (m, 1H, H-6a GlcF), 4.02 (d, J = 9.3 Hz, 1H, H-5 GlcF), 3.97–3.91 (m, 2H, H-4, H-6b GlcF), 3.83–3.77 (m, 1H, CH-1 CyHex), 2.11 (s, 3H, CH3 Ac), 1.85 (ddd, J = 27.8, 12.4, 3.5 Hz, 2H, CyHex), 1.69–1.54 (m, 4H, CyHex), 1.50 (s, 3H, CH3 isop), 1.46 (s, 3H, CH3 isop), 1.41–1.33 (m, 1H, CyHex), 1.33 (s, 3H, CH3 isop), 1.31 (s, 3H, CH3 isop), 1.22–1.08 (m, 3H, CyHex). 13C NMR (CDCl3) Major isomer: δ = (170.0, 163.9) (CO), (114.0, 108.7) (C isop), 105.4 (C-1 GlcF), 85.6 (C-3 GlcF), 82.8 (C-2 GlcF), 82.3 (C-4 GlcF), 73.5 (C-5 GlcF), 65.0 (C-6 GlcF), 48.4 (CH CyHex), (32.7, 32.7) (CyHex), (27.0, 26.7, 26.5) (CH3 isop), 25.8 (CyHex), 25.5 (CH3 isop), (24.6, 24.5) (CyHex), 21.5(CH3 Ac). Minor isomer: δ = (169.0, 166.6) (CO), (112.8, 110.1) (C isop), 105.7 (C-1 GlcF), 85.3 (C-3 GlcF), 80.9 (C-2 GlcF), 80.4 (C-4 GlcF), 74.6 (C-5 GlcF), 68.4 (C-6 GlcF), 48.2 (CH CyHex), (33.1, 32.7) (CyHex), (27.1, 27.0, 26.8, 25.8) (CH3 isop), (25.7, 24.7, 24.7) (CyHex), 21.0 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2302.
3.1.25. (3aR,4′R,7aR)-7-(Cyclohexylcarbamoyl)-2,2,2′,2′-tetramethyltetrahydrospiro[[1,3]dioxolo[4,5-c]pyran-6,4′-[1,3]dioxolan]-7-yl acetate (9a)
Yield 51% (22 mg); colorless oil; Rf (DS1) = 0.80, Rf (DS2) = 0.40 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) Major isomer: δ = 5.99 (d, J = 7.7 Hz, 1H, NH), 5.30 (d, J = 7.0 Hz, 1H, H-4 FruP), 4.60 (dt, J = 7.3, 3.7 Hz, 1H, H-5 FruP), 4.57 (d, J = 9.6 Hz, 1H, H-1a FruP), 4.07 (m, 1H, H-1b FruP), 4.05 (m, 1H, H-6a FruP), 3.80–3.74 (m, 1H, CH-1 CyHex), 3.68 (dd, J = 12.8, 3.4 Hz, 1H, H-6b FruP), 2.11 (s, 3H, CH3 Ac), 1.86 (t, J = 17.8 Hz, 2H, CyHex), 1.65–1.57 (m, 4H, CyHex), 1.47 (s, 6H, CH3 isop), 1.46 (s, 3H, CH3 isop), 1.38 (s, 1H, CyHex), 1.30 (s, 3H, CH3 isop), 1.21–1.06 (m, 3H, CyHex). Minor isomer: δ = 6.66 (d, J = 7.3 Hz, 1H, NH), 5.08 (d, J = 7.2 Hz, 1H, H-4 FruP), 4.31 (d, J = 9.9 Hz, 1H, H-1a FruP), 4.30–4.24 (m, 1H, H-5 FruP), 4.20 (dd, J = 12.6, 3.3 Hz, 1H, H-6a FruP), 3.93 (d, J = 9.9 Hz, 1H, H-1b FruP), 3.79 (dd, J = 12.6, 0.9 Hz, 1H, H-6b FruP), 3.77–3.73 (m, 1H, CyHex CH-1), 2.12 (s, 3H, CH3 Ac), 1.92 (dd, J = 33.6, 9.8 Hz, 2H, CyHex), 1.66 (m, 2H, CyHex), 1.58 (m, 2H, CyHex), 1.51 (s, 3H, CH3 isop), 1.49 (s, 3H, CH3 isop), 1.43 (s, 3H, CH3 isop), 1.36 (s, 3H, CH3 isop), 1.33 (m, 1H, CyHex), 1.23–1.12 (m, 3H, CyHex). 13C NMR (CDCl3) Major isomer: δ = 168.8, 166.6 (CO), 110.6, 109.5 (C isop), 104.5 (C-2 FruP), 80.5 (C-3 FruP), 73.1 (C-4 FruP), 72.8 (C-5 FruP), 72.3 (C-1 FruP), 64.7 (C-6 FruP), 48.3 (CH-1 CyHex), 32.9, 32.7 (CyHex), 26.9, 26.6, 26.1, 25.8, 25.0, 24.7, 24.6 (CH3 isop), 21.6 (CH3 Ac). Minor isomer: δ = 169.3, 164.6 (CO), 111.6, 110.8 (C isop), 103.9 (C-2 FruP), 81.0 (C-3 FruP), 73.60 (C-1 FruP), 73.4 (C-4 FruP), 73.0 (C-5 FruP), 64.4 (C-6 FruP), 48.9 (CH-1 CyHex), 32.9, 32.7 (CyHex), 26.4, 26.3, 26.2 (CH3 isop), 25.8 (CyHex), 25.3 (CH3 isop), 24.9 (CyHex), 21.7 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8Na [M + Na]+ 450.2104; found 450.2107.
3.2. Post-Condensation Modification of Selected Passerini Products
Boc deprotection and coupling of amino acid: Passerini product 2d (10 mg, 0.016 mmol) was dissolved in trifluoroacetic acid (TFA)/DCM (1:1, v/v, 100 µL), the reaction was stirred at room temperature for 2 min, and the reaction mixture was evaporated with toluene. Boc–Pro–OH (4 mg, 0.018 mmol, 1.2 eq), benzotriazol-1-yloxytris(dimethylamino)phosphonium (BOP, 10 mg, 0.023 mmol, 1.5 eq), and hydroxybenzotriazole (HOBt, 3.5 mg, 0.023 mmol, 1.5 eq) were dissolved in 1 mL DCM/DMF (1:1), followed by the addition of the Boc-deprotected compound (8 mg, 0.015 mmol) and N-methylmorpholine (NMM, 2eq). The reaction mixture was stirred for 24 h. The product was purified by flash column chromatography (petrol ether/ethyl acetate, v/v = 1:6).
3.2.1. tert-Butyl 2-((1-(2-((2-methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethoxy)-1-oxo-3-phenylpropan-2-yl)carbamoyl) pyrrolidine-1-carboxylate (2j)
Yield 78% (9 mg); colorless oil; Rf (DS1,2) = 0.55 (EtOAc/petroleum ether 6/1, v/v); d.r. 70:30. 1H NMR (MeOD) δ = 7.36–7.18 (m, 5H, Ph), 5.52 (d, J = 4.9 Hz, 0.3H, H-1 Gal), 5.49 (d, J = 4.7 Hz, 0.7H, H-1 Gal), 5.44 (m, 0.3H, H-6 Gal) 5.13 (m, 0.7H, H-6 Gal), 4.84 (s, 1H, α-Phe), 4.66 (dd, J = 8.0, 2.3 Hz, 1H, H-3 Gal), 4.39 (ddd, J = 11.1, 4.7, 2.4 Hz, 1H, H-2 Gal), 4.28–4.18 (m, 2H, H-4,5 Gal), 4.15 (bs, 1H, α-Pro), 4.06–3.92 (m, 2H, CH2 Gly), 3.74 (d, J = 2.7 Hz, 3H, CH3 Gly), 3.44 (m, 1H, δ-Pro), 3.39–3.34 (m, 2H, δ-Pro, β-Phe), 3.01 (bs, 1H, β-Phe), 2.17 (m, 1H, β-Pro), 1.91 (m, 1H, β-Pro), 1.78 (m, 2H, γ-Pro), 1.55 (d, J = 11.6 Hz, 3H, CH3 isop), 1.46 (d, J = 16.2 Hz, 6H, CH3 isop), 1.37–1.24 (m, 12H, CH3 isop, Boc). 13C NMR (MeOD) δ (170.0, 169.8, 169.3, 169.1, 168.5, 167.7, 167.4) (CO), 136.4 (C Ph), (128.4, 127.6, 125.9) (CH Ph), (108.9, 108.5, 108.3) (C isop), (95.9, 95.7) (C-1 Gal), (72.5, 71.1, 70.3, 70.3, 70.2, 70.0, 69.5, 67.2, 66.5) (C-2,3,4,5,6 Gal), 59.7 (α-Pro), (53.2, 52.9) (α-Phe), (50.7, 50.7) (CH3 Gly), 45.9 (δ-Pro), (40.1, 39.9) (CH2 Gly), (36.3, 36.0) (β-Phe), 30.3 (Boc), (26.7, 24.5, 24.4, 23.3) (CH3 isop), (22.8, 22.5) (γ-Pro). HRMS (MALDI–TOF/TOF) m/z: calcd. for C35H49N3O13Na [M + Na]+ 742.3163; found 742.3190.
Boc deprotection and coupling of amino acid: Passerini product 5d (13 mg, 0.02 mmol) was dissolved in TFA/DCM (1:1, v/v, 100 µL), the reaction was stirred at room temperature for 2 min, and then the solvent was evaporated. Boc–Phe–OH (6.4 mg, 0.024 mmol, 1.2 eq), BOP (13 mg, 0.03 mmol, 1.5 eq), and HOBt (4.6 mg, 0.03 mmol, 1.5 eq) were dissolved in 1 mL DCM/DMF (1:1) followed by the addition of the Boc-deprotected compound and NMM (2 eq). The reaction mixture was stirred for 24 h, the solvent was evaporated, and the product was extracted with DCM. The product was purified by flash column chromatography (petrol ether/ethyl acetate, v/v = 1/1).
3.2.2. (S)-(S)-1-((3aR,5S,6R,6aS)-6-Hydroxy-5-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-3a-yl)-2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl 2-((S)-2-((tert-butoxycarbonyl)amino)-3-phenylpropanamido)-3-phenylpropanoate (5j)
Yield 65% (9.5 mg); colorless oil; Rf = 0.30 (EtOAc/petroleum ether 1/1, v/v). 1H NMR (CDCl3): δ = 7.68 (s, 1H, NH), 7.38–7.16 (m, 8H, CH Ph), 6.91 (dd, J = 6.4, 2.9 Hz, 2H, CH Ph), 6.00 (d, J = 5.4 Hz, 1H, NH Phe), 5.77 (s, 1H, H-1 Sor), 5.16 (d, J = 7.5 Hz, 1H, NH Phe), 4.93 (s, 1H, H-5 Sor), 4.60 (dt, J = 8.8, 5.3 Hz, 1H, α-Phe), 4.35–4.12 (m, 4H, α-Phe, CH2 Gly, H-3,4 Sor), 3.91–3.78 (m, 3H, CH2 Gly, H-6 Sor), 3.75 (s, 3H, CH3 Gly), 3.14 (dd, J = 14.3, 5.1 Hz, 1H, β-Phe), 3.02 (d, J = 7.1 Hz, 2H, β-Phe), 2.94–2.81 (m, 1H, β-Phe), 1.91 (bs, 2H, OH), 1.49 (s, 3H, CH3 isop), 1.34–1.23 (m, 12H, CH3 isop, Boc). 13C NMR (CDCl3): δ = (173.0, 170.0, 169.30, 168.3) (CO), (137.1, 135.2) (C Ph), (129.9, 129.2, 129.1, 129.1, 127.72, 127.5) (CH Ph), 112.7 (C isop), 112.0 (C-2 Sor), (88.0, 82.7, 80.6, 76.7, 75.2) (C-1,3,4,5 Sor), 61.2 (C-6 Sor), (55.9, 54.3) (α-Phe), 52.8 (CH3 Gly), 41.1 (CH2 Gly), (38.6, 36.9) (β-Phe), 28.5 (Boc), (27.4, 26.3) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C35H49N3O13Na [M + Na]+ 742.3163; found 742.3190.
Fmoc deprotection: A solution of 20% piperidine in DMF (0.2 mL) was added to the Passerini product 2k (20 mg, 0.03 mmol), the mixture was stirred at room temperature for 15 min, and then the solvent was evaporated. The product was purified by flash column chromatography (petrol ether/ethyl acetate, v/v = 1/1).
3.2.3. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethyl 2-amino-3-phenylpropanoate (2l)
Yield 77% (12 mg); colorless oil; Rf (DS1,2) = 0.40 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3): δ = 7.25 (m, 5H, Ph), 5.45 (d, J = 4.9 Hz, 0.7H, H-1 Gal), 5.41 (d, J = 4.6 Hz, 0.3H, H-1 Gal), 4.91–4.84 (m, 0.7H, H-6 Gal), 4.75 (td, J = 8.3, 4.9 Hz, 0.3H, H-6 Gal), 4.62 (ddd, J = 14.4, 8.0, 2.5 Hz, 1H, H-3 Gal), 4.46 (dd, J = 8.0, 1.6 Hz, 0.3H, α-Phe), 4.40 (dd, J = 7.9, 1.7 Hz, 0.7H, α-Phe), 4.31 (dd, J = 4.9, 2.5 Hz, 1H, H-2 Gal), 4.28–3.87 (m, 4H, CH2 Gly, H-5,4 Gal), 3.72 (m, 3H, CH3 Gly), 3.29 (ddd, J = 37.2, 14.3, 5.2 Hz, 1H, β-Phe), 3.15–3.10 (m, 1H, β-Phe), 2.95 (s, 1H, NH), 2.88 (s, 1H, NH), 1.51–1.25 (m, 12H, CH3 isop). 13C NMR (CDCl3): δ 171.7, 170.4, 170.3, 170.1, 169.8, 169.5 (CO), 136.1, 135.8 (C Ph), 128.8, 128.8, 128.2, 126.5, 126.4 (CH isop), 109.7, 109.1, 109.1, 108.8 (CH3 isop), 95.9, 95.4 (C-1 Gal), 73.5, 73.5, 70.3, 70.2, 70.2, 70.0, 69.8, 68.8, 67.8, 66.5 (C-2,3,4,5,6 Gal), 53.6, 53.2 (α-Phe), 51.7, 51.5 (CH3 Gly), 40.7, 40.6 (CH2 Gly), 36.9, 36.1 (β-Phe), 25.5, 25.4, 25.3, 25.2, 24.6, 24.3, 23.7, 23.3 (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C25H34N2O10Na [M + Na]+ 545.2111; found 545.2109.
3.3. Base-Mediated Deprotection of Selected Passerini Products
General procedure: the Passerini product (0.035 mmol) was dissolved in MeOH (0.5 mL), and 1N NaOH (2 eq) was added. The reaction was stirred under reflux (10 min) or at room temperature (30 min), followed by TLC (EtOAc/EtOH/HOAc/H2O 70:10:2:2, v/v). The solvent was evaporated, and the product was purified by flash column chromatography.
3.3.1. 2-((S)-2-Hydroxy-2-((3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-3a-yl)acetamido)acetic acid (1m)
Yield 91% (11.5 mg); colorless oil; Rf = 0.74 (EtOAc/EtOH/HOAc/H2O 70:10:2:2, v/v). 1H NMR (CDCl3) δ = 7.36–7.29 (m, 1H, NH), 5.52 (bs, 2H, OH), 4.66 (d, J = 2.6 Hz, 1H, H-3 Fru), 4.61 (dd, J = 7.8, 2.6 Hz, 1H, H-4 Fru), 4.22 (d, J = 8.2 Hz, 1H, H-5 Fru), 4.16–4.09 (m, 2H, H-1 Fru, CH2 Gly), 3.99 (dd, J = 18.1, 4.6 Hz, 1H, CH2 Gly), 3.90 (d, J = 11.7 Hz, 1H, H-6 Fru), 3.80 (d, J = 12.9 Hz, 1H, H-6 Fru), 1.47 (s, 3H, CH3 isop), 1.46 (s, 3H, CH3 isop), 1.38 (s, 3H, CH3 isop), 1.33 (s, 3H, CH3 isop). 13C NMR (CDCl3) δ 171.3 (CO), 109.7, 109.4 (C isop), 103.5 (C-2 Fru), 71.6, 70.9, 70.5, 70.4 (C-1,3,4,5 Fru), 61.9 (C-6 Fru), 42.4 (CH2 Gly), 26.6, 26.2, 25.7, 24.2 (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: Calcd. for C15H23NO9Na [M + Na]+ 384.1271; found 384.1269.
3.3.2. 2-(2-Hydroxy-2-((3aR,5R,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)acetamido)acetic acid (2m)
Yield 79% (10 mg); colorless oil; Rf (DS1,2) = 0.58 (EtOAc/EtOH/HOAc/H2O 70:10:2:2, v/v); d.r. 70:30. 1H NMR (CDCl3) δ 7.44 (s, 1H, NH), 5.54 (dd, J = 16.6, 4.7 Hz, 1H, H-1 Gal), 5.13 (bs, 2H, OH), 4.61 (dt, J = 8.0, 4.0 Hz, 1H, H-3 Gal), 4.48 (d, J = 8.1 Hz, 1H, H-4 Gal), 4.38–4.23 (m, 2H, H-2,6 Gal), 4.12–3.96 (m, 3H, CH2 Gly, H-5 Gal), 1.53–1.26 (m, 12H, CH3 isop). 13C NMR (CDCl3) δ 173.5, 171.8 (CO), 110.1, 109.8, 109.60, 109.6 (C isop), 96.6, 96.5 (C-1 Gal), 73.7, 72.9, 71.3, 71.2, 70.9, 70.8, 70.7, 70.4, 67.8, 66.7 (C-2,3,4,5,6), 41.78 (CH2 Gly), 26.1, 26.1, 26.0, 25.2, 25.1, 24.4, 24.2 (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C15H23NO9Na [M + Na]+ 384.1271; found 384.1272.
3.3.3. 2-((S)-2-Hydroxy-2-((3aS,3bR,7aS,8aS)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxin-8a-yl)acetamido)acetic acid (5m)
Yield 76% (9.6 mg); colorless oil; Rf (DS1,2) = 0.75 (EtOAc/EtOH/HOAc/H2O 70:10:2:2, v/v); d.r. nd. 1H NMR (CDCl3) δ 7.35 (d, J = 5.2 Hz, 1H), 4.63 (s, 1H), 4.32 (d, J = 8.0 Hz, 2H), 4.16 (s, 1H), 4.13–3.98 (m, 4H), 1.40 (m, 12H). 13C NMR (CDCl3) δ 171.1 (CO), 114.5, 113.7 (C isop), 97.9 (C-2 Sor), 85.3 (C-5), 73.7, 73.0, 70.8 (C-1,3,4), 60.5 (C-6), 42.5 (CH2 Gly), 29.2, 27.7, 26.6, 18.8 (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C15H23NO9Na [M + Na]+ 384.1271; found 384.1281.
3.3.4. 2-(2-((3aS,5S,6R,6aS)-5-((R)-2,2-Dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)-2-hydroxyacetamido)acetic acid (6m)
Yield 70% (0.11 mmol, 29 mg); colorless oil; Rf (DS1,2) = 0.62 (EtOAc/EtOH/HOAc/H2O 70:10:2:2, v/v); d.r. 90:10. 1H NMR (CDCl3): δ 7.44 (t, J = 5.3 Hz, 0.9H, NH), 7.35–7.31 (m, 0.1H, NH), 5.76 (d, J = 3.4 Hz, 0.1H, H-3 Alo), 5.74 (d, J = 3.5 Hz, 0.9H, H-3 Alo), 5.58 (s, 2H, OH), 4.86 (t, J = 4.0 Hz, 0.9H, H-2 Alo), 4.83 (t, J = 4.0 Hz, 0.1H, H-2 Alo), 4.63 (d, J = 3.3 Hz, 0.1H, H-4 Alo), 4.51 (d, J = 7.0 Hz, 0.9H, H-4 Alo), 4.28–4.22 (m, 0.1H, H-5 Alo), 4.20 (dd, J = 9.5, 6.7 Hz, 0.9H, H-5 Alo), 4.12–4.01 (m, 3H, H-1 Alo, H-6, CH2 Gly), 4.00–3.90 (m, 2H, H-6 Alo, CH2 Gly), 2.66 (dt, J = 10.2, 3.8 Hz, 0.1H, H-3 Alo), 2.28 (ddd, J = 9.7, 7.1, 4.7 Hz, 0.9H, H-3 Alo), 1.54 (s, 0.3H, CH3 isop), 1.52 (s, 2.7H, CH3 isop), 1.43 (s, 0.3H, CH3 isop), 1.41 (s, 2.7H, CH3 isop), 1.33 (s, 2.7H, CH3 isop), 1.31 (s, 2.7H, CH3 isop), 1.28 (s, 0.6H, CH3 isop). 13C NMR (CDCl3): δ 173.2 (CO), (112.7, 110.4) (C isop), 104.7 (C1 Alo), (82.9, 79.5, 77.0, 69.3) (C-2,4,5,3 Alo), 67.2 (C-6 Alo), 51.1 (CH3 Gly), 47.6 (C-3 Alo), 41.6 (CH2 Gly), (27.0, 26.5, 26.4, 25.5) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C16H25NO9Na [M + Na]+ 398.1427; found 398.1445
4. Conclusions
In summary, we systematically validated the reactivity of eight different isopropylidene-protected carbohydrate-derived aldehydes and ketones in the Passerini-type multicomponent reaction. Simple post-condensation modifications allowed to gain more complex structures or versatile building blocks to access new types of biomimetics. The here described methodology can be also applied to other classes of biomolecules, like lipids and peptides, and used as a platform for generating new nature-mimicking systems.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/24/6236/s1.
Author Contributions
Conceptualization, I.J.; Formal analysis, K.V.-K., J.S.S.; Investigation, K.V.-K.; Methodology, K.V.-K.; Writing—review & editing, I.J.
Funding
This research was funded by the Croatian Science Foundation, grant number 3102.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| MCRs | multicomponent reactions |
| DCM | dichloromethane |
| NMR | nuclear magnetic resonance |
| DMF | dimethylformamide |
| BOP | benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate |
| HOBt | hydroxybenzotriazole |
| TFA | trifluoroacetic acid |
| NMM | N-methylmorpholine |
| RT | room temperature |
| MALDI TOF/TOF | matrix-assisted laser desorption/ionization with time-of-flight mass spectrometer |
References
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tejada, A.; Cañada, F.J.; Jiménez-Barbero, F.J. Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef] [PubMed]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Shigemitsu, H.; Hamachi, I. Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches. Acc. Chem. Res. 2017, 50, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Menchi, G.; Guarna, A.; Trabocchi, A.J. Skeletal diversity from carbohydrates: Use of mannose for the diversity-oriented synthesis of polyhydroxylated compounds. J. Org. Chem. 2015, 80, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.S.; Bower, J.F.; Galan, M.C. Carbohydrates as enantioinduction components in stereoselective catalysis. Org. Biomol. Chem. 2016, 14, 4008–4017. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F. Carbohydrate drugs: Current status and development prospect. Drug Discov. Ther. 2015, 9, 79–87. [Google Scholar] [CrossRef]
- Dwek, R.A.; Butters, T.D.; Platt, F.M.; Zitzmann, N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 2002, 1, 65–75. [Google Scholar] [CrossRef]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef]
- Movsisyan, L.D.; Schäfer, E.; Nguyen, A.; Ehrmann, F.R.; Schwab, A.; Rossolini, T.; Zimmerli, D.; Wagner, B.; Daff, H.; Heine, A.; et al. Sugar Acetonides are a Superior Motif for Addressing the Large, Solvent-Exposed Ribose-33 Pocket of tRNA-Guanine Transglycosylase. Chem. Eur. J. 2018, 24, 9957–9967. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivsatava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Vlahoviček-Kahlina, K.; Vazdar, M.; Jakas, A.; Smrečki, V.; Jerić, I. Synthesis of Glycomimetics by Diastereoselective Passerini Reaction. J. Org. Chem. 2018, 83, 13146–13156. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Yousuf, R.; Khan, S.; Shafiullah, S. Recent advances in multicomponent reactions involving carbohydrates. RSC Adv. 2015, 5, 57883–57905. [Google Scholar] [CrossRef]
- Suć, J.; Barić, D.; Jerić, I. Multicomponent synthesis of hydrazino depsipeptides. RSC Adv. 2016, 6, 99664–99675. [Google Scholar] [CrossRef]
- Moni, L.; Banfi, L.; Basso, A.; Martino, E.; Riva, R. Diastereoselective Passerini Reaction of Biobased Chiral Aldehydes: Divergent Synthesis of Various Polyfunctionalized Heterocycles. Org. Lett. 2016, 18, 1638–1641. [Google Scholar] [CrossRef]
- Lee, S.S.; Fyrner, T.; Chen, F.; Álvarez, Z.; Sleep, E.; Chun, D.S.; Weiner, J.A.; Cook, R.W.; Freshman, R.D.; Schallmo, M.S.; et al. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat. Nanotechnol. 2017, 12, 821–829. [Google Scholar] [CrossRef]
- Ustun Yaylaci, S.; Sardan Ekiz, M.; Arslan, E.; Can, N.; Kilic, E.; Ozkan, H.; Orujalipoor, I.; Ide, S.; Tekinay, A.B.; Guler, M.O. Supramolecular GAG-like Self-Assembled Glycopeptide Nanofibers Induce Chondrogenesis and Cartilage Regeneration. Biomacromolecules 2016, 17, 679–689. [Google Scholar] [CrossRef]
- Brust, A.; Wang, C.I.; Daly, N.L.; Kennerly, J.; Sadeghi, M.; Christie, M.J.; Lewis, R.J.; Mobli, M.; Alewood, P.F. Vicinal Disulfide Constrained Cyclic Peptidomimetics: A Turn Mimetic Scaffold Targeting the Norepinephrine Transporter. Angew. Chem. Int. Ed. 2013, 52, 12020–12023. [Google Scholar] [CrossRef]
- Han, M.-L.; Hu, X.P.; Huang, J.D.; Chen, L.G.; Zheng, Z. New chiral amino alcohol ligands derived from 1-phenylethylamine for efficient Ru-catalyzed asymmetric transfer hydrogenation. Tetrahedron Asymmetry 2011, 22, 222–225. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Marco-Aleixandre, A.; Pedro, J.R. Enantioselective Synthesis of Vicinal Halohydrins via Dynamic Kinetic Resolution. Org. Lett. 2006, 8, 127–130. [Google Scholar] [CrossRef]
- Gruner, S.A.W.; Locardi, E.; Lohof, E.; Kessler, H. Carbohydrate-Based Mimetics in Drug Design: Sugar Amino Acids and Carbohydrate Scaffolds. Chem. Rev. 2002, 102, 491–514. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Blanco, J.L.; Ortiz Mellet, C.; García Fernández, J.M. Multivalency in heterogeneous glycoenvironments: Hetero-glycoclusters, -glycopolymers and -glycoassemblies. Chem. Soc. Rev. 2013, 42, 4518–4531. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).