Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro
Abstract
1. Introduction
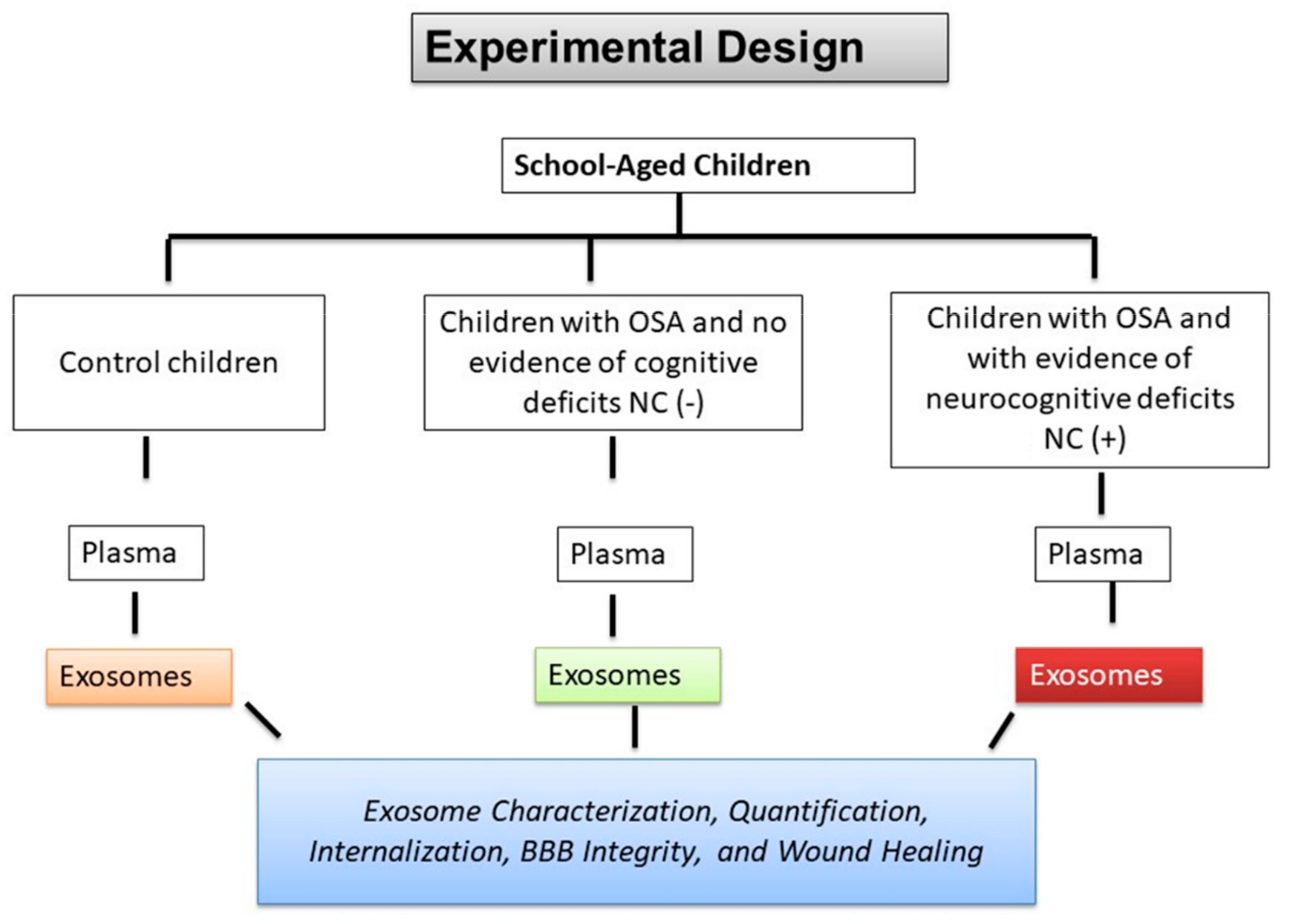
2. Results
2.1. Subject Characteristics
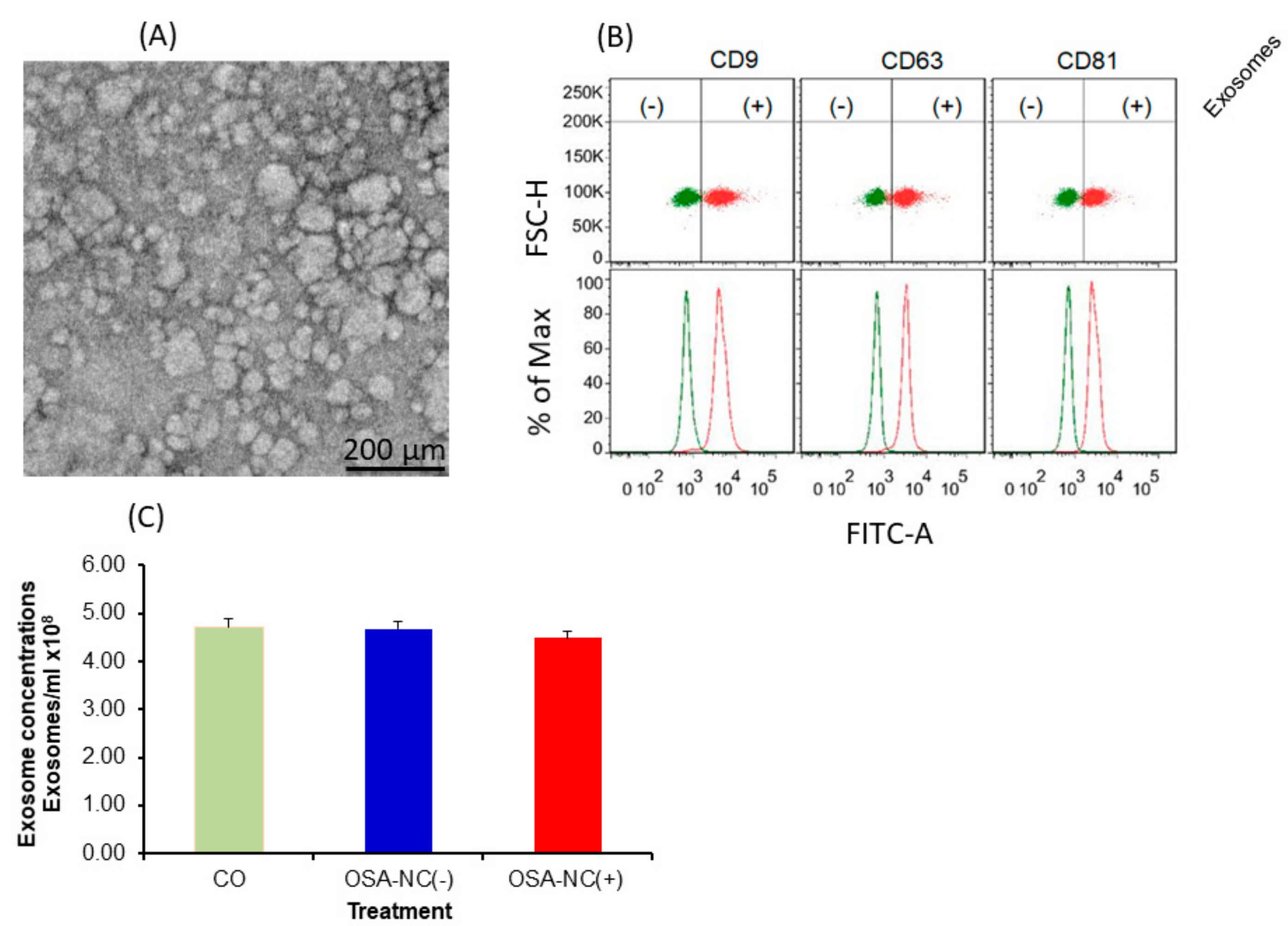
2.2. EVs Characterization and Quantification
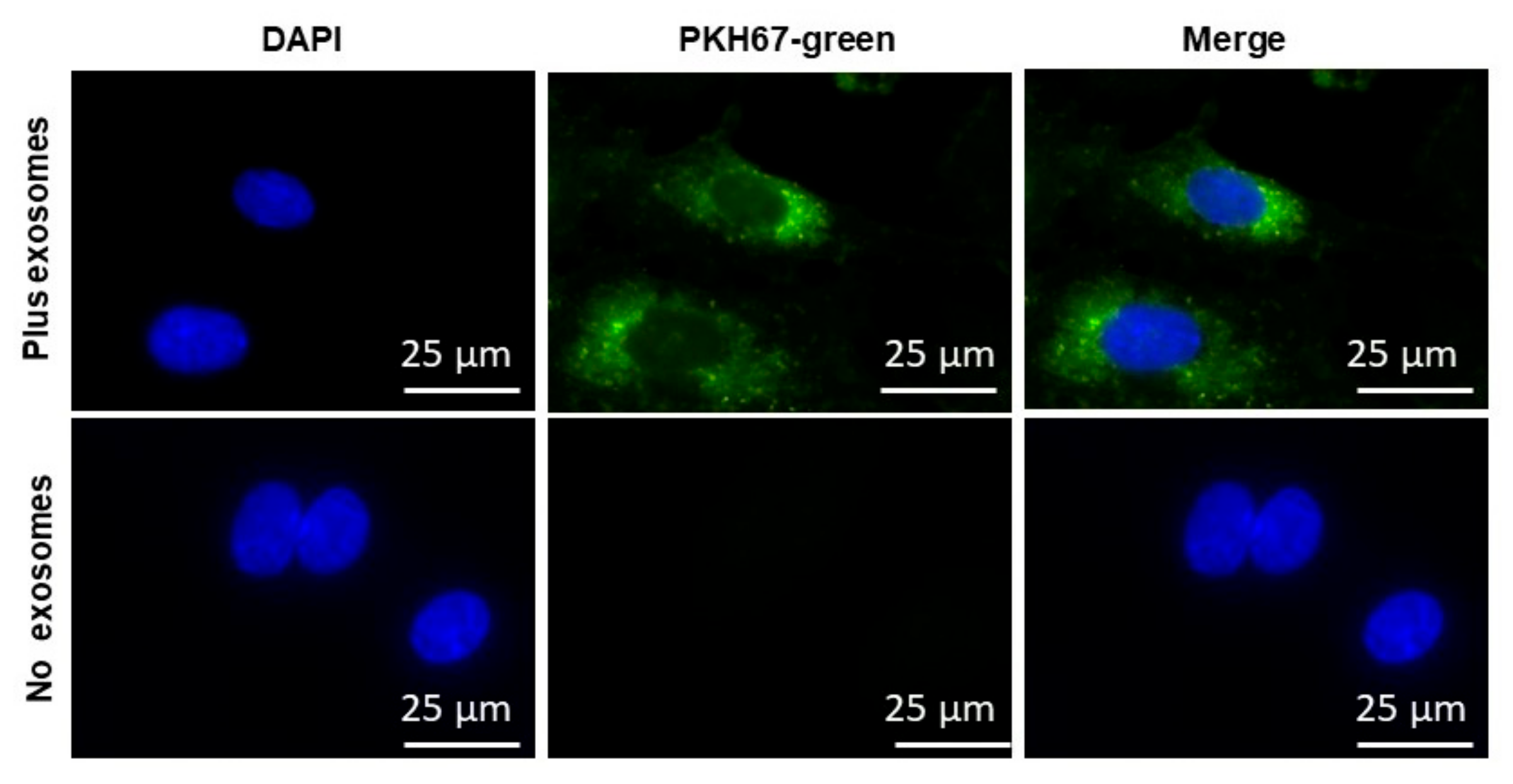
2.3. EVs Uptake
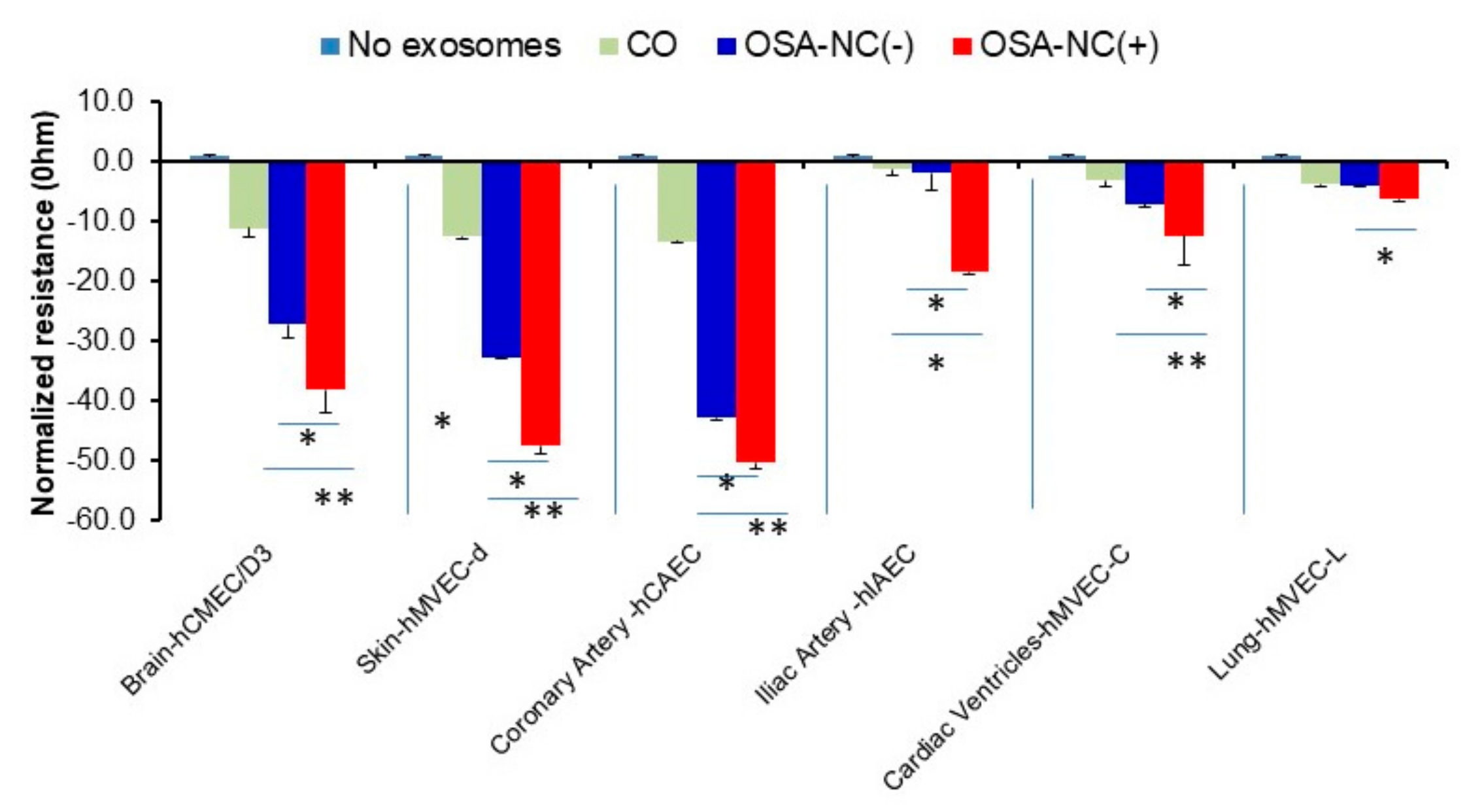
2.4. Effects of Circulating EVs on Endothelial Monolayer Barrier Integrity
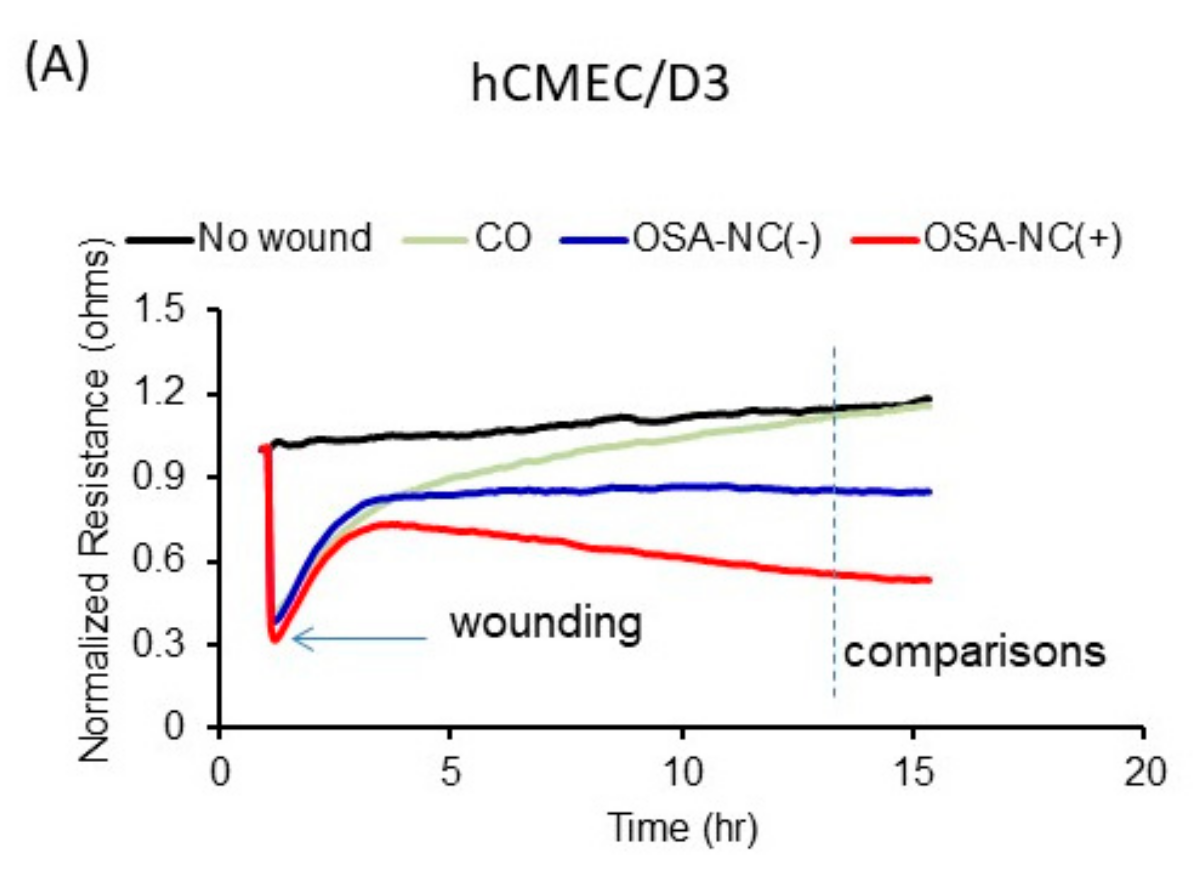
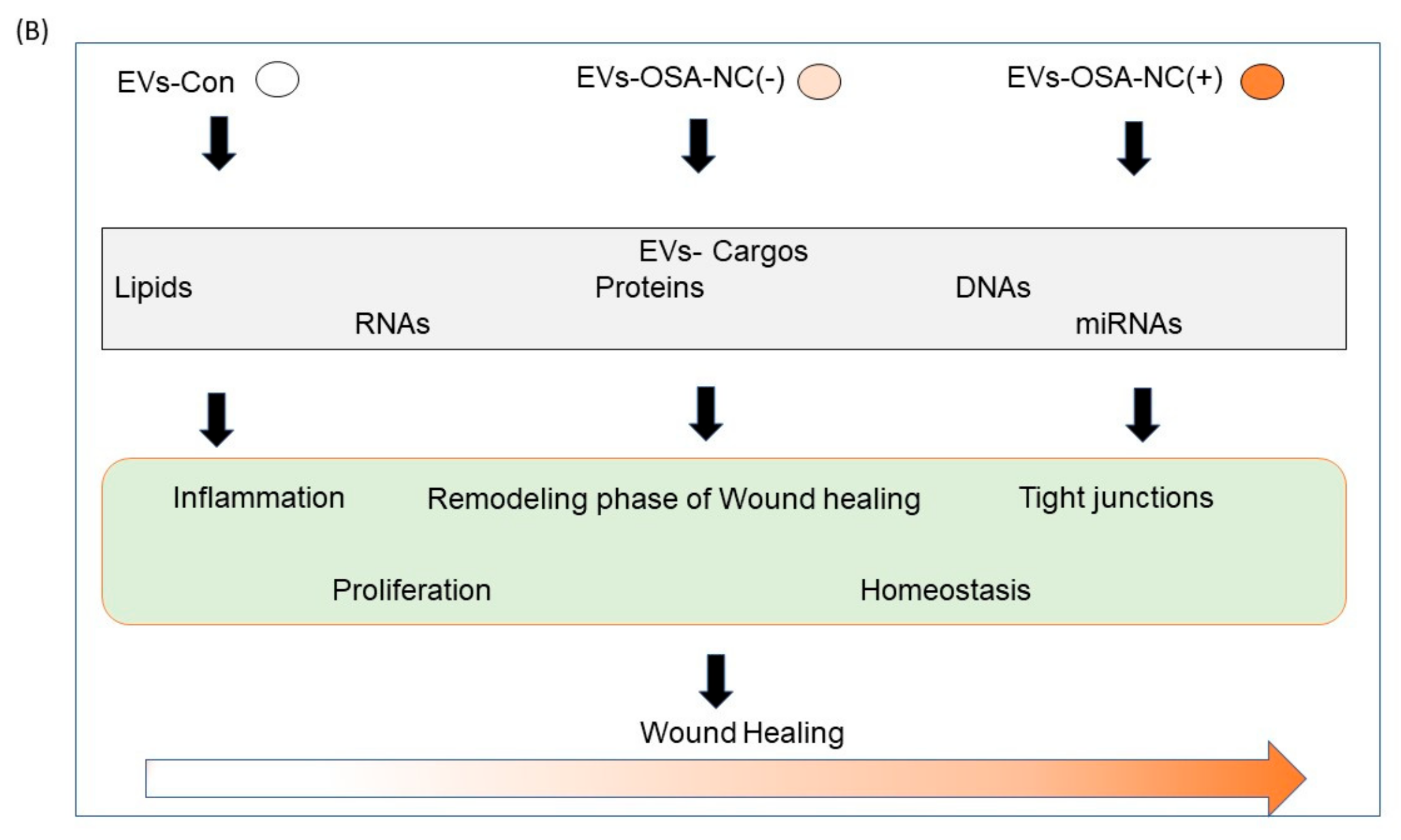
2.5. Effects of Circulating EVs on Wound Healing
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Subject Characteristics
5.2. Overnight Polysomnography
5.3. Neurocognitive Assessments
5.4. Plasma and EVs Isolation
5.5. EVs Characterization and Quantification
5.6. Cell Cultures
5.7. Plasma and Exosomes Isolation
5.8. EVs Endothelial Cell Uptake
5.9. Flow Cytometry
5.10. Electric Cell–Substrate Impedance Sensing (ECIS)
5.11. Wound-Healing Assay
5.12. Data Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OSA | Obstructive sleep apnea |
| BBB | Blood–brain barrier |
| PSG | Polysomnography |
| OSA-NC(−) | OSA without cognitive deficits |
| OSA-NC(+) | OSA with cognitive deficits |
| AHI | Apnea–hypopnea index |
| HCAEC | Human coronary artery endothelial cells |
| HIAEC | Human iliac artery endothelial cells |
| HMVEC-D | Human dermal microvascular endothelial cells |
| HMVEC-C | Human cardiac microvascular endothelial cells |
| HMVEC-L | Human lung microvascular endothelial cells |
| hCMEC/D3 | Human brain microvascular endothelial cell |
| ECIS | Electric cell–substrate impedance sensing |
| BMI | Body mass index |
| CNS | Central nervous system |
| FMI | Median, fluorescence intensity |
References
- Verstraeten, E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr. Neurol. Neurosci. Rep. 2007, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; De Gennaro, L.; Casagrande, M.; Bertini, M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology 2000, 37, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Auger, R.R.; Enders, F.T.; Felmlee-Devine, D.; Smith, G.E. The effects of poor sleep quality on cognitive function of patients with cirrhosis. J. Clin. Sleep Med. 2014, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.H.; Lee, D.Y.; Jhoo, J.H.; Woo, J.I. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am. J. Geriatr. Psychiatry 2011, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Shokoueinejad, M.; Fernandez, C.; Carroll, E.; Wang, F.; Levin, J.; Rusk, S.; Glattard, N.; Mulchrone, A.; Zhang, X.; Xie, A. Sleep apnea: A review of diagnostic sensors, algorithms, and therapies. Physiol. Meas. 2017, 38, R204–R252. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef]
- Archbold, K.H.; Pituch, K.J.; Panahi, P.; Chervin, R.D. Symptoms of sleep disturbances among children at two general pediatric clinics. J. Pediatrics 2002, 140, 97–102. [Google Scholar] [CrossRef]
- Lewin, D.S.; Rosen, R.C.; England, S.J.; Dahl, R.E. Preliminary evidence of behavioral and cognitive sequelae of obstructive sleep apnea in children. Sleep Med. 2002, 3, 5–13. [Google Scholar] [CrossRef]
- Owens, J.; Opipari, L.; Nobile, C.; Spirito, A. Sleep and daytime behavior in children with obstructive sleep apnea and behavioral sleep disorders. Pediatrics 1998, 102, 1178–1184. [Google Scholar] [CrossRef]
- Gozal, D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009, 10 (Suppl. 1), S12–S16. [Google Scholar] [CrossRef]
- Smith, D.L.; Gozal, D.; Hunter, S.J.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Impact of sleep disordered breathing on behaviour among elementary school-aged children: A cross-sectional analysis of a large community-based sample. Eur. Respir. J. 2016, 48, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.J.; Gozal, D.; Smith, D.L.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am. J. Respir. Crit. Care Med. 2016, 194, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Crabtree, V.M.; Sans Capdevila, O.; Witcher, L.A.; Kheirandish-Gozal, L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am. J. Respir. Crit. Care Med. 2007, 176, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Philby, M.F.; Macey, P.M.; Ma, R.A.; Kumar, R.; Gozal, D.; Kheirandish-Gozal, L. Reduced Regional Grey Matter Volumes in Pediatric Obstructive Sleep Apnea. Sci. Rep. 2017, 7, 44566. [Google Scholar] [CrossRef]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef]
- Row, B.W.; Kheirandish, L.; Neville, J.J.; Gozal, D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatric Res. 2002, 52, 449–453. [Google Scholar] [CrossRef]
- Row, B.W.; Liu, R.; Xu, W.; Kheirandish, L.; Gozal, D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am. J. Respir. Crit. Care Med. 2003, 167, 1548–1553. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar]
- Dotiwala, A.K.; Samra, N.S. Anatomy, Brain, Blood Brain Barrier; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Puhan, M.A.; Schlatzer, C.; Stradling, J.R.; Kohler, M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology 2015, 20, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.M.; Melehan, K.L.; Liu, P.Y.; Grunstein, R.R.; Phillips, C.L. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med. Rev. 2015, 20, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Trosman, I.; Trosman, S.J. Cognitive and Behavioral Consequences of Sleep Disordered Breathing in Children. Med. Sci. 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, L.; Gozal, D. Neurocognitive dysfunction in children with sleep disorders. Dev. Sci. 2006, 9, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Pack, A.I. Obstructive sleep apnea and cognitive impairment: Addressing the blood-brain barrier. Sleep Med. Rev. 2014, 18, 35–48. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Exosomes Disrupt the Blood-Brain Barrier in Children with Obstructive Sleep Apnea and Neurocognitive Deficits. Am. J. Respir. Crit. Care Med. 2018, 197, 1073–1076. [Google Scholar] [CrossRef]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Peris, E.; Qiao, Z.; Gileles-Hillel, A.; Li, R.C.; Yang, W.; Gozal, D. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 2014, 37, 1817–1824. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, Y.; Gozal, D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr. Physiol. 2012, 2, 1767–1777. [Google Scholar]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Masa, J.F.; Marin, J.M.; Qiao, Z.; Corral, J.; Mónica, G.; Sergi, M.; Leila, K.-G.; Carlos, E.; et al. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. Int. J. Obes. 2018, 42, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Youssefnia, N.; Foster, G.E.; Beaudin, A.E.; Qiao, Z.; Pialoux, V.; Matiram, P.; Patrick, J.H.; Leila, K.-G.; Marc, J.P.; et al. Plasma Exosomes and Improvements in Endothelial Function by Angiotensin 2 Type 1 Receptor or Cyclooxygenase 2 Blockade following Intermittent Hypoxia. Front. Neurol. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gozal, D.; Chan, W.C.; Andrade, J.; Prasad, B. Circulating Plasma Exosomes in Obstructive Sleep Apnea and Reverse-Dipping Blood Pressure. Eur. Respir. J. 2019, 138 (Suppl. 1), A12380. [Google Scholar] [CrossRef]
- Khalyfa, A.; Marin, J.M.; Qiao, Z.; Rubio, D.S.; Kheirandish-Gozal, L.; Gozal, D. Plasma Exosomes in OSA Patients Promote Endothelial Senescence: Effect of Long-Term Adherent Continuous Positive Airway Pressure. Sleep 2019. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Krisztina, B.; Enriqueta, C.; Francesco, C.; Joana, C.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Álvarez, M.L.; Mohammadi, M.; Rakesh, B.; Joaquin, T.-S.; Lei, H.; Jorge, A.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Patrick, J.H.; Marc, J.P.; David, G. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Martin-Burriel, I.; Gil, A.; Cubero, P.; Forner, M.; Khalyfa, A.; Marin, J.M. Stability of Circulating Exosomal miRNAs in Healthy Subjects. Sci. Rep. 2018, 8, 10306. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Khalyfa, A.A.; Gozal, D. Circulating microRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. Chest 2016, 149, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Poroyko, V.A.; Qiao, Z.; Gileles-Hillel, A.; Khalyfa, A.A.; Akbarpour, M.; Isaac, A.; Ramon, F.; David, G. Exosomes and Metabolic Function in Mice Exposed to Alternating Dark-Light Cycles Mimicking Night Shift Work Schedules. Front. Physiol. 2017, 8, 882. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gozal, D.; (Child Health Research Institute, Department of Child Health, University of Missouri School of Medicine, Columbia, MO 65201, USA). Personal communication, 2019.
- Blunden, S.; Lushington, K.; Kennedy, D.; Martin, J.; Dawson, D. Behavior and neurocognitive performance in children aged 5–10 years who snore compared to controls. J. Clin. Exp. Neuropsychol. 2000, 22, 554–568. [Google Scholar] [CrossRef]
- O’Brien, L.M.; Mervis, C.B.; Holbrook, C.R.; Bruner, J.L.; Klaus, C.J.; Rutherford, J.; Troy, J.R.; David, G. Neurobehavioral implications of habitual snoring in children. Pediatrics 2004, 114, 44–49. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.M.; Mervis, C.B.; Holbrook, C.R.; Bruner, J.L.; Smith, N.H.; McNally, N.; David, G. Neurobehavioral correlates of sleep-disordered breathing in children. J. Sleep Res. 2004, 13, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Downs, H.E.; Crabtree, V.M.; Gozal, D. Cognition, sleep and respiration in at-risk children treated for obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 336–342. [Google Scholar] [CrossRef]
- Gozal, D.; Capdevila, O.S.; Kheirandish-Gozal, L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am. J. Respir. Crit. Care Med. 2008, 177, 1142–1149. [Google Scholar] [CrossRef]
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 2008, 51, 84–91. [Google Scholar] [CrossRef]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Curr. Probl. Pediatric Adolesc Health Care 2016, 46, 19–26. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Spruyt, K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics 2010, 126, e1161–e1167. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Bosma, E.K.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Daneman, R. Formation and maintenance of the BBB. Mech. Dev. 2015, 138 Pt 1, 8–16. [Google Scholar] [CrossRef]
- Sonar, S.A.; Lal, G. Blood-brain barrier and its function during inflammation and autoimmunity. J. Leukoc. Biol. 2018, 103, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: A scaffolding and signalling center. Biochim. Biophys. Acta 2008, 1778, 601–613. [Google Scholar] [CrossRef]
- Wood, M.J.; O’Loughlin, A.J.; Samira, L. Exosomes and the blood-brain barrier: Implications for neurological diseases. Ther. Deliv. 2011, 2, 1095–1099. [Google Scholar] [CrossRef]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelium in health and disease. Pharmacol. Rep. 2008, 60, 139–143. [Google Scholar] [PubMed]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Joana, C.; Anabela, C.S.; Hernando, D.P.; El Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular transport of microRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Lo, C.M.; Keese, C.R.; Giaever, I. Monitoring motion of confluent cells in tissue culture. Exp. Cell Res. 1993, 204, 102–109. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef]
- Gozal, D.; Lipton, A.J.; Jones, K.L. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 2002, 25, 59–65. [Google Scholar] [CrossRef]
- Gozal, D. Sleep-disordered breathing and school performance in children. Pediatrics 1998, 102, 616–620. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; O’Brien, L.M.; Holbrook, C.R.; Gozal, D. Snoring and sleep-disordered breathing in young children: Subjective and objective correlates. Sleep 2004, 27, 87–94. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Carole, L.M.; Reena, M.; Sairam, P.; Stuart, F.Q.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jacqueline, J.; Christopher, L.; Michael, S.S.; Stephen, S.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
| Variable | CO (n = 6) | OSA-NC(−) (n = 12) | OSA-NC(+) (n = 12) |
|---|---|---|---|
| Age (years) | 6.3 ± 1.7 | 6.3 ± 1.6 | 6.5 ± 1.4 |
| Gender (male, %) | 50.0 | 50.0 | 50.0 |
| Ethnicity (African American, %) | 66.7 | 66.7 | 66.7 |
| BMI z-score | 1.02 ± 0.29 | 1.06 ± 0.25 | 1.04 ± 0.26 |
| Total sleep duration (min) | 473.2 ± 75.1 | 474.2 ± 66.3 | 478.8 ± 67.1 |
| REM sleep (%) | 20.2 ± 10.2 | 17.9 ± 9.5 | 18.2 ± 8.1 |
| Sleep latency (min) | 24.6 ± 15.2 | 20.7 ± 13.9 | 22.4 ± 15.6 |
| REM latency (min) | 118.7 ± 67.4 | 119.5 ± 52.4 | 117.7 ± 49.5 |
| Total Arousal Index (events/hour TST) | 12.5 ± 8.6 * | 20.9 ± 10.3 | 22.8 ± 11.4 |
| Respiratory Arousal Index (events/hour TST) | 0.4 ± 0.2 * | 8.2 ± 4.7 | 7.9 ± 4.2 |
| Obstructive Apnea Hypopnea Index (events/hour TST) | 0.5 ± 0.3 * | 19.4 ± 6.9 | 19.9 ± 7.1 |
| SpO2 Nadir (%) | 94.1 ± 1.3 * | 82.1 ± 9.0 | 80.3 ± 8.6 |
| ODI3% | 0.4 ± 0.2 * | 19.0 ± 8.4 | 18.4 ± 7.7 |
| Endothelial Cell Line | No Exosomes | CO | OSA-NC(−) | OSA-NC(+) |
|---|---|---|---|---|
| hCMEC/D3 | 100 | 97.6 ± 5.23 | 71.87 ± 5.37 | 45.08 ± 3.59 ** |
| hMVEC-d | 100 | 98.95 ± 6.63 | 82.97 ± 5.64 | 79.92 ± 4.25* |
| hCAEC | 100 | 93.07 ± 6.89 | 86.50 ± 6.78 | 83.82 ± 6.71 |
| hIAEC | 100 | 96.98 ± 7.21 | 95.15 ± 7.35 | 98.29 ± 7.11 |
| hMVEC-C | 100 | 97.02 ± 8.01 | 91.63 ± 6.86 | 86.83 ± 5.24 |
| hMVEC-L | 100 | 88.62 ± 6.84 | 86.07 ± 6.29 | 87.87 ± 6.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. Int. J. Mol. Sci. 2019, 20, 6233. https://doi.org/10.3390/ijms20246233
Khalyfa A, Gozal D, Kheirandish-Gozal L. Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. International Journal of Molecular Sciences. 2019; 20(24):6233. https://doi.org/10.3390/ijms20246233
Chicago/Turabian StyleKhalyfa, Abdelnaby, David Gozal, and Leila Kheirandish-Gozal. 2019. "Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro" International Journal of Molecular Sciences 20, no. 24: 6233. https://doi.org/10.3390/ijms20246233
APA StyleKhalyfa, A., Gozal, D., & Kheirandish-Gozal, L. (2019). Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. International Journal of Molecular Sciences, 20(24), 6233. https://doi.org/10.3390/ijms20246233






