Abstract
Tendon tissues have limited healing capacity. The incidence of tendon injuries and the unsatisfactory functional outcomes of tendon repair are driving the search for alternative therapeutic approaches envisioning tendon regeneration. Cellular therapies aim at delivering adequate, regeneration-competent cell types to the injured tendon and toward ultimately promoting its reconstruction and recovery of functionality. Mesenchymal stem cells (MSCs) either obtained from tendons or from non-tendon sources, like bone marrow (BM-MSCs) or adipose tissue (ASCs), have been receiving increasing attention over the years toward enhancing tendon healing. Evidences from in vitro and in vivo studies suggest MSCs can contribute to accelerate and improve the quality of tendon healing. Nonetheless, the exact mechanisms underlying these repair events are yet to be fully elucidated. This review provides an overview of the main challenges in the field of cell-based regenerative therapies, discussing the role of MSCs in boosting tendon regeneration, particularly through their capacity to enhance the tenogenic properties of tendon resident cells.
1. Introduction
Cells are Nature’s tissue engineers, intervening, through a spatiotemporal coordinated manner, in tissue homeostasis, disease development, tissue repair/healing, and ultimately regeneration. Within the Metazoa kingdom, regenerative abilities are unequally distributed [1]. Regeneration refers to post-natal restoration of a lost body part/structure and can occur through a variety of developmental mechanisms to originate structures that closely resemble the original prior to injury [1]. Replicating tissue development in mammals is a dramatic challenge, owing to the limited knowledge about signals involved in the initiation of regenerative processes. Upon injury, tissue healing typically occurs through a continuous process comprising three interdependent and overlapping stages: an initial inflammatory response, followed by a fibroblastic and/or proliferative stage and, finally, a prolonged remodeling phase [2,3]. In several cases, tissue healing is often associated with a pro-fibrotic scenario that leads to scar formation, rather than normal tissue regeneration [4].
Regenerative medicine, including tissue engineering, envisions the generation of adequate therapeutic strategies to restore the functionality of an injured tissue or organ. Central to these approaches, cells are key players in orchestrating regenerative responses and thus, are the main targets of any strategy envisioning tissue regeneration. Thus, despite the fact that different approaches can be considered, all rely on controlling to some extent cellular responses, which implies knowing the biology of the target tissue. Within the human body, tendons are highly prone to fibrotic healing through excessive and disorganized deposition of the extracellular matrix (ECM) [3]. This repair process is differentially regulated between fetal and adult tendon tissues [5,6,7].
Over the years, regenerative tendon approaches have been exploiting (i) cellular therapies [8,9]; (ii) injections of platelet rich-hemoderivatives (PRHd), like platelet-rich plasma (PRP) [10,11]; (iii) gene therapy; [12] and (iv) biomaterial-based strategies [13,14] aiming at promoting tissue regeneration and inhibiting peritendinous adhesions formation [3]. Given the biomechanical role of these musculoskeletal tissues, biomaterial-based strategies envision the development of tissue substitutes. However, cells have a primary role in maintaining tendon ECM dynamics and normal function and, thus, cell-based strategies are gaining attention, mainly through the combination of cells and biomaterials. In particular, mesenchymal stem cells (MSCs) have been extensively explored for musculoskeletal tissue engineering and regeneration, including for tendon applications [15]. In this review, we address the use of MSCs from tendon and non-tendon origin and their contributions to tendon healing. Firstly, the need for tendon regenerative therapies is tackled and the main characteristics of tendon cell populations and cellular niche are briefly addressed as the key environment providing insights on relevant signals to potentially direct tenogenic differentiation of stem cells. Finally, the use of cellular therapies envisioning a shift from pro-fibrotic tissue repair to tissue regeneration is discussed.
2. Tendon Pathophysiology
2.1. A Snapshot on Tendon Cellular Niche
Tendons are highly organized connective tissues comprising bottom-up assemblies of collagen molecules (Figure 1). This hierarchical arrangement of the extracellular matrix (ECM) guarantees the unique biomechanical performance of tendons, which is responsible for assuring effective loading transmission between muscles and bones and consequent body movements. Tendon ECM is comprised mainly of collagen type I, followed by collagen type III, as well as proteoglycans (decorin, fibromodulin, biglycan, lumican, aggrecan, and versican) and glycosaminoglycans (dermatan sulfate and chondroitin sulfate), as reviewed elsewhere [16,17].
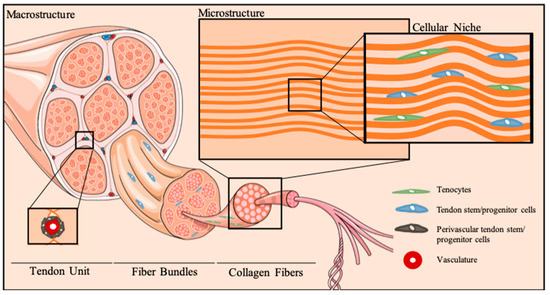
Figure 1.
Tendon cellular niche. Schematic representation of tendon hierarchical organization and micro-to-macro structural architecture of the cellular niche. Tenocytes reside between anisotropically-aligned collagen fibers. Multipotent stem cell populations, termed tendon stem/progenitor cells, can be found in several stem cell niches in tendons, particularly the perivascular niche, as well as other tendon areas, as the epi-, peri- and endotenon.
Tendons are relatively hypocellular tissues. Nonetheless, tendon cell populations assure ECM dynamics during tissue homeostasis. As a result of the stretching mechanical forces, tendon cells exhibit a characteristic elongated, spindle-shape morphology. Traditionally, tendons were believed to comprise a homogenous population of tenocytes, which are differentiated cells expressing common tendon markers, such as scleraxis (Scx), a basic loop-helix-loop transcription factor, tenomodulin (Tnmd), a transmembrane glycoprotein, as well as the tendon ECM component collagen type I (Col1) [18,19]. Nonetheless, tendon cell populations have been shown to include tendon stem/progenitor cells (TSPCs) [20], containing cells that fulfill the universal criteria of mesenchymal stem cells (MSCs)—clonogenecity, multipotency and self-renewal. Despite the knowledge gap on tendon biology, it is well recognized that both differentiated and TSPCs reside within a unique microenvironment of biophysical and biochemical signals [17,21]. Particularly, stretching mechanical forces and ECM topography are dominating factors governing tissue function and cellular processes [22]. Furthermore, a balance of biochemical factors tightly regulates the orchestration of biological processes in tendon. Specifically, tendon healing involves numerous cytokines (e.g., interleukin (IL)-6, IL-1β) and growth factors (e.g., basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-β, insulin-like growth factor (IGF)-1, platelet-derived growth factor (PDGF) and bone morphogenetic proteins (BMP)-12, -13, and -14), which are released in a temporally- and spatially-controlled manner [23,24,25]. Notwithstanding, it is worth mentioning that inflammatory cells play a crucial role in tendinopathies and tendon healing [3,26], intervening in the dynamics of tendon cellular niche.
Although the cellular and molecular mechanisms intervening in tendon development, homeostasis and repair are still to be fully uncovered, it is evident that an interplay between biophysical and biochemical signals coordinates cellular function and impacts tenogenic differentiation of stem cells.
2.2. Tendinopathies: A Painful Global Burden and the Need for Novel Therapies
Musculoskeletal conditions are the second largest contributor to disability, affecting people across the life-course worldwide. The Global Burden of Disease (GBD) study has estimated that 20–33% of people globally live with a painful musculoskeletal condition [27,28]. Among these, it is estimated that over 30 million human tendon/ligament-related procedures are taking place annually worldwide, representing an associated expenditure of over €150 billion euros in the USA and EU [8,29]. These treatments pose a considerable financial burden on healthcare systems and are expected to increase as a consequence of increased life expectancy and contemporary sedentary lifestyles [30], as well as of the rising prevalence of overweight, obesity and metabolic diseases [31].
Tendinopathies are commonly divided into two major classes: acute and chronic (degenerative) damage [32]. Acute tendinopathies are associated with traumatic damage of previously healthy tissue, whereas chronic tendinopathies (>3 months) are associated with overuse tendon injuries and frequently involve an unresolved inflammatory scenario and impaired performance [10]. Current therapeutic strategies frequently resort to surgical repair through suturing of the damaged tissues or the application of auto- or allo-grafts, whenever conservative treatments fail or are not appropriate. Furthermore, various artificial grafts/scaffolds for tendon/ligament substitution are commercially available, including both biological and synthetic scaffolds. However, a satisfactory functional outcome is still to be achieved given the drawbacks associated to these strategies, as reviewed elsewhere [33].
In general, the quality of repaired tendon tissue rarely returns to pre-injury levels, leading to high re-injury risk and the onset of degenerative changes related to the development of chronic tendinopathies [34,35]. Given the limited success of current clinical treatments, the management of tendon injuries and disorders requires the development of more effective strategies toward ultimately regenerating the injured tissue and recovering functionality.
3. Cell-Based Therapies for Tendon Applications
Cellular therapies envision the delivery of regeneration-competent cell populations, i.e., cells that are able to repopulate the injured tissue and/or of empowering resident cell populations with the ultimate goal of promoting tissue reconstruction and functional recovery. Tendon healing frequently encompasses the differentiation of resident TSPCs toward a phenotype resembling that of activated fibroblasts, leading to scar tissue formation, exacerbated inflammatory response and impaired functionality [7,36]. In this sense, the use of MSCs envisions a modulation of the inflammatory environment targeting a potential shift from pro-inflammatory and pro-fibrotic to pro-regenerative cellular responses, leading to a reduced infiltration of inflammatory cells and ordered deposition of ECM components [3,37]. Given the hypocellular nature of tendons, the application of cell-based therapies is quite intriguing and several challenges need to be addressed, as discussed in detail below.
3.1. General Considerations
MSCs have been long described to possess multilineage differentiation ability in vitro and have been widely used for applications in Tissue Engineering and Regenerative Medicine, including in the field of musculoskeletal repair [15]. Notwithstanding, the functional role of MSCs in regenerative therapies is being increasingly discussed and evidences exist that the therapeutic effects of these cells could be attributed mainly to their function of empowering resident cells [38,39,40], raising controversy over the terminology [41].
Adult stem cells of mesenchymal origin hold great interest in the field of tendon tissue engineering and regeneration as promising alternatives to the scarce population of tendon cells. Herein, we will discuss the main outcomes of strategies using MSCs from different sources for tendon repair. Nonetheless, pluripotent stem cells have also been explored in the field, namely embryonic stem cells and induced pluripotent stem cells (iPSCs) [42,43]. In particular, iPSCs can be easily obtained in an autologous setting, which facilitates clinical translation. The combination of iPSCs-derived MSCs with anisotropically-aligned fibrous biomaterial has proven beneficial for collagen production during tendon healing [44]. Notwithstanding, the use of iPSCs still faces several challenges and direct reprogramming of somatic cells has been attracting increasing interest, although very limited in the field of tendon applications owing to the lack of specific tendon markers. Alternatively, fibroblasts from different sources, including not only tendons and ligaments, but also skin, have been applied as well [45].
3.2. Main Challenges
Key issues to be considered when attempting to translate bioengineering strategies to tendon therapeutic approaches include (i) the lack of standardized isolation protocols, (ii) the inexistence of tendon-specific molecular markers and (iii) the definition of adequate differentiation protocols. Consequently, given the lack of well-established/standardized differentiation protocols and characterization markers for tenogenic differentiation, the selection of an ideal cell source represents a major challenge while trying to establish an effective cellular therapy.
• Isolation protocols
Several problems remain unsolved concerning the isolation of tendon cell populations. Isolation of TSPCs and tenocytes still lacks the tune of enrichment and selection approaches. Tendon cells were initially isolated following collagenase digestion of tendon explants [46,47]. However, over the years, several isolation methods have been described for obtaining TSPCs, also commonly termed tendon-derived stem cells (TDSCs), lacking a consensus between the type of digestion mix used, type of digestion mix used and time of digestion. Additionally, difficulties in characterizing tendon cells (as discussed below) have been limiting the definition of an optimal protocol. Table 1 presents examples of isolation protocols and main outcomes. Over the years, several protocols with slight variations have been described, but differences among cell characterization techniques and markers used have been challenging the field. Moreover, it is most likely that a mixed cell population is normally obtained, as it presents heterogeneous characteristics in terms of differentiation, clonogenecity and self-renewal capability [48]. Various studies assessed the multi-differentiation capacity of isolated cells, as well as the expression of tenogenic markers upon cell isolation. Nonetheless, TSPCs and tenocytes are commonly isolated in the same studies, and cell morphology is frequently used as the only aspect to identify and distinguish between cell populations. Tenocytes were traditionally described as small and fusiform cells [46,47], but over the years, different phenotypes have been reported based on nuclei morphology. Particularly, “resting” tenocytes within the tensional region of tendons exhibit spindle-shaped nuclei; “active” tenocytes possess a more elongated nucleus and “chondrocytic” tenocytes, which reside in compressed regions of tendons have round nuclei [49,50,51,52]. The first two types reside between collagen fibers and exhibit several cytoplasmic extensions allowing cell-cell communication through gap junctions, namely connexin (Cx)-32 and Cx-43 [53]. In turn, TSPCs are frequently described as large polygonal and star shaped cells, exhibiting round nuclei with prominent nucleoli, but acquiring a fibrobblast-like shape over passaging [20,49,54]. Therefore, some controversy may arise regarding the identity of these cell populations.

Table 1.
Protocols for tendon MSCs populations isolation and main characteristics.
• Molecular signature
Tendon cells are highly-specialized fibroblasts, being the main producers of tendon ECM components. Given their mesenchymal origin and the limitations of characterizing fibroblasts and distinguishing them from MSCs [45], the definition of an accurate molecular signature is still far from being achieved. Hence, the inexistence of molecular markers to discriminate between tendon cell populations, as well as to characterize every discrete step of cell lineage specification makes the purification and differentiation of TDSCs and TSPCs very complicated. In comparison to other MSCs, tendon stem cells are known to share common surface markers, to express identical genes and to respond in a similar manner to growth factor stimulation, however, it has been recognized that the expression profiles, although very alike, are not identical [20] and are species-dependent.
Strikingly, several factors such as age, donor variability, tendon type, and anatomic location, alongside with culture conditions, have also been reported to influence markers expression in these cell populations [20].
• Differentiation protocols
The establishment of adequate differentiation methods relies on the optimization of inductive protocols to commit stem cells toward a certain lineage. In opposition to chondrogenic and osteogenic differentiation, there is no standard induction protocol for tenogenesis. Generally, different growth factors can be added to culture medium to induce the desired phenotype, as these biomolecules are powerful tools in regulating biological responses. These regulatory effects have been demonstrated, for instance, after culturing human adipose-derived stem cells (ASCs) or human amniotic fluid stem cells (AFSCs) in the presence of growth factors associated with tendon development and healing, namely endothelial growth factor (EGF), PDGF-BB, bFGF, and TGF- β1 [68]. An upregulation of tendon-related genes demonstrated the potential use of biochemical molecules to induce cellular commitment toward the tenogenic lineage, with differential effects over the two cell types. Indeed, EGF and bFGF exerted more pronounced effects over AFSCs, whereas ASCs responded more evidently to EGF and PDGF-BB [68]. Furthermore, medium supplementation with TGF-β3 showed a potent regulatory influence of this growth factor over the temporal profile of tenogenic genes in both ASCs and bone marrow-derived mesenchymal stem cells (BM-MSCs) cultures [69]. In addition, studies investigating the effect of different combinations of growth factors in either 2D or 3D culture [70], as well as further combining with mechanical stimulation [71], are opening new avenues toward differentiating MSCs into the tenogenic lineage. Strategies targeting tenogenic differentiation through the addition of growth factors or by mechanical stimulation have been recently reviewed [72]. However, an ideal medium formulation is not yet available.
Even though several attempts have been made over the years to commit stem cells towards the tenogenic lineage, the identification of several major tenogenic biomarkers is of major importance. As the molecular signature of tendon cells is still to be uncovered, it is still difficult to clearly understand and clarify the differentiation process occurring in several cultures and in the proper tissue, posing a strong challenge to the development of effective cell-based therapies.
3.3. Mesenchymal Stem Cells for Tendon Regenerative Therapies
MSCs can be applied in either autologous or allogeneic settings and can be obtained from tendon or non-tendon tissues. In the following sections, the contribution of MSCs from different sources to tendon healing, and ultimately regeneration, is discussed.
3.3.1. Tendon Stem Cells
Tendon stem cells are frequently obtained as a heterogeneous population of stem and progenitor cells, as highlighted before. The purity of tendon cell populations is highly debatable, but these cells are believed to hold potential for improving tendon repair mechanisms. Autologous tenocyte implantation is currently under clinical studies (Phase 2-3 clinical trial, NCT01343836), but it is not clear whether the transplanted cells include only differentiated cells or a mixed population comprising also TDSCs or TSPCs.
Tendon stem cells represent 1–4% of the total number of nucleated cells in tendon tissues [20] and have been reported to differentiate into tenocytes, as well as along the chondrogenic, osteogenic and adipogenic lineages upon in vitro induction; and to originate tendon-, cartilage-, bone- and tendon-bone junction-like tissues in animal models [20,54,55,64,73]. Indeed, TDSCs have shown strong healing ability upon cell injection into a rat achilles tendon injury model [74]. These results highlight the possible contribution of tendon stem cell populations toward the generation of tendon-like tissue upon injury, but the mechanisms involved are still to be fully understood.
Nonetheless, the limited cell number obtained upon isolation from tendon tissues requires an additional step of in vitro expansion, which leads to phenotypic drift [75] and consequent reduction of healing capacity. Hence, recent technological advances propose the use of epigenomic approaches to maintain the tenogenic phenotype of TSPCs [76]. Indeed, the treatment of TSPCs with inhibitors of histone deacetylase activity enabled significant cell expansion without phenotypic alterations and treated TSPC sheets were able to accelerate tendon repair in an in-vivo rat model of pattelar tendon injury [76]. Although these outcomes open new avenues toward the potential application of tendon stem cells for regenerative therapies, additional steps of cellular manipulation in vitro make clinical translation more difficult.
3.3.2. Mesenchymal Stem Cells from Non-Tendon Tissues
Bone marrow-derived MSCs are the most commonly explored source of stem cells in tendon tissue engineering and regeneration approaches [52,77,78,79,80]. Nonetheless, adipose tissue-derived stem cells are also being increasingly explored for enhancing tendon repair owing to the easier accessibility for tissue harvesting and higher proliferative capacity, which renders increased cell numbers for clinical application. Although studies with MSCs have been targeting tenogenic differentiation of these cell sources, it is most likely that the therapeutic effects will be exerted by the empowering capacity of stem cells over tendon resident cell populations. Both bone marrow aspirate and adipose tissue constitute interesting stem cell sources for the translation of bioengineering approaches to the bedside, holding strong potential for managing tendinopathies in the clinics. Various clinical studies are currently being performed to better understand the clinical potential of MSCs in treating tendinopathies (Table 2). Recent evidences of the role of these cell types in tendon tissue engineering and regeneration strategies are illustrated below.

Table 2.
List of active clinical trials using MSCs to treat tendon injuries.
• Bone marrow-derived MSCs
BM-MSCs are commonly harvested through a minimally invasive aspiration procedure from the iliac crest and then isolated from the mononuclear cell fraction upon density centrifugation. BM-MSCs have been frequently used as a comparison in tendon stem cell characterization studies and are known to express several tendon-related markers, including scleraxis, tenomodulin, collagen types I, II and III, decorin, biglycan, although to a lower extent than TSPCs [20].
In vitro co-culture studies have been demonstrating the role of bi-directional crosstalk between tendon cells and BM-MSCs on the induction of a possible tenogenic phenotype through an up-regulation of tendon-related genes, including scleraxis and tenomodulin, and tendon ECM markers, like collagen type I, decorin and tenascin, together with significant ECM deposition [52,80]. Overall, these studies have been suggesting a role of BM-MSCs in enhancing the tenogenic properties of TDSCs and TSPCs, mostly through increased deposition of collagenous proteins and the recreation of a tendon-like ECM, favoring tenogenic differentiation.
Interestingly, BM-MSCs have been reported to generate embryonic tendon-like tissue in vitro through a process that is mediated by TGF-β3 signaling and requires a 3D environment [81]. In this study, BM-MSCs were cultured in fixed-length fibrin gels and were able to spontaneously generate collagen fibrils identical to those of an embryonic tendon [81]. These results highlight the potential contribution of BM-MSCs to tendon tissue engineering strategies.
Furthermore, BM-MSCs are being explored for their role in supporting tendon cells, particularly through the release of paracrine factors. Pre-conditioning tendon cells by in-vitro culture with BM-MSCs secretome and further combining those cells with an electrospun keratin-based scaffold resulted in improved biomechanical performance in an in-vivo rat model of chronic massive rotator cuff tear [82]. These outcomes suggest a beneficial effect of BM-MSCs secretome, which has been demonstrated to include several growth factors, cytokines and other soluble molecules intervening in cellular growth and/or maintenance and signal transduction processes [83].
Besides in-vitro tendon cell priming, other bioengineering approaches include the resort to scaffold-free cell sheet engineering. Cell sheets derived from co-culturing TDSCs and BM-MSCs have been reported to support tendon healing in a rat patellar tendon window injury model [80]. The combination of both cell types outperforms the effects of each cell population individually. It has been demonstrated that the ratio between both cell types is a crucial aspect for tissue repair; a 1:1 cell ratio led to the best therapeutic effect upon cell sheet implantation, resulting in a higher number of elongated cells and better orientation of collagen fibers, as well as the best mechanical performance [80]. Indeed, co-culture approaches constitute remarkable tools in overcoming some challenges of cellular therapies for tendon applications. For instance, TDSCs alone exhibited better regenerative ability than BM-MSCs alone upon cell injection into a rat achilles tendon injury model [74], but the limited number of TDSCs obtained upon isolation hinders clinical translation of TDSCs as a source for cellular therapies, further supporting the combination of tendon cell populations with BM-MSCs
In summary, BM-MSCs are paving their way in tendon regenerative therapies owing to their contribution to new tendon-like tissue formation and to boost the tenogenic properties of tendon cells populations.
• Adipose tissue-derived MSCs (ASCs)
ASCs constitute an interesting candidate cell source for tissue engineering and regenerative medicine applications. These cells can be isolated from subcutaneous adipose tissue and, more recently, from liposuction aspirates [84,85] and have been reported to improve tendon healing in in-vivo tendon injury models [72,86]. Similarly to BM-MSCs, the mechanisms behind such therapeutic effects have not been elucidated so far, but attempts to understand the role of ASCs have also been exploiting co-culture systems with tendon resident cells. Comparably to BM-MSCs, co-culture studies demonstrated that the cellular crosstalk leads to an up-regulation of tendon-related genes [87,88]. Nonetheless, difficulties in distinguishing between both cell types limit the interpretation of these outcomes as to whether MSCs are differentiating along the tenogenic lineage or boosting the tenogenic properties of tendon cells. We have recently addressed the influence of ASCs over tendon niche using indirect (transwell) co-cultures with tendon explants. Interestingly, ASCs seemed to aid in preserving the architecture of native tendon tissue over time in culture [89]. Additionally, collagenolytic activity of matrix metalloproteinases (MMPs) was increased in co-cultures, suggesting a fastened ECM remodeling [89]. This was further investigated in direct contact co-culture systems using ASCs and human tendon-derived cells, demonstrating an accelerated deposition of ECM components, particularly collagen type I, and improved COL1/COL3 (collagen type I to collagen type III) ratio [88]. Given the role of collagen type III in fibrotic healing and scar tissue formation, these results suggest that ASCs may have a beneficial effect toward shifting the repair microenvironment. Not only were ASCs able to support tendon cells, but they were also spontaneously more elongated in co-culture systems, demonstrating a possible commitment toward the tenogenic lineage [88,89]. Further studies using cellular labeling techniques may help shed light over the biological events involved.
Additional concerns that must be undoubtedly considered arise from the hypocellular nature of tendon tissues. ASCs exhibit a high proliferation rate, whereas tendons possess relatively low cell numbers and an overproliferative phase may lead to fibrotic tissue formation and scarring. Strikingly, co-culturing ASCs with tendon-derived cells enabled a control over the proliferation rate; indeed, lower cell numbers have been reported for co-culture systems in comparison to ASCs cultured alone [88].
Altogether, these results support a potential therapeutic effect for ASCs as they have been able to modulate the microenvironment of tendon niche in vitro, as well as to improve tendon healing in vivo, but more studies at the molecular level will be useful to help clarify the exact mechanisms behind these responses.
4. Conclusions and Perspectives
Evidences from both in vitro and in vivo studies suggest that tendon resident cells, including tendon MSCs (TSPCs or TDSCs), may be the main orchestrators directing tendon-regenerative processes. Nonetheless, such biological response upon injury may be further boosted by the administration of non-tendon MSCs through two distinct but synergistic mechanisms—“cell replacement” and “cell empowerment”. Indeed, MSCs include cellular populations with potential therapeutic contributions by homing to the target site and exhibiting the ability to reconstitute/repopulate the injured tissue, simultaneously exerting an immunomodulatory effect that may shift the inflammatory environment. These events are crucial in switching tendon repair from pro-fibrotic to tissue regeneration, but functional clinical outcomes are still to be investigated.
Future research in the field of cellular therapies for tendon regeneration must address several issues that still pose a huge obstacle to clinical translation, including the lack of standardized methods and the inexistence of an optimal panel of markers for characterizing tenogenic differentiation steps.
Furthermore, there is clear influence from the bi-directional crosstalk between tendon MSCs and BM-MSCs or ASCs in enhancing the tenogenic properties of native cell populations. Nonetheless, the cellular and molecular mechanisms involved in these processes need to be investigated in more detail. Notwithstanding, tendon tissue engineering and regeneration can evolve from the combination of MSCs of different origins with biomaterials’ support to improve tendon healing and simultaneously provide adequate mechanical performance at the tissue level.
Funding
The authors acknowledge the financial support from the European Union Framework Programme for Research and Innovation HORIZON2020, under the TEAMING Grant agreement No 739572—The Discoveries CTR, the ERC Grant CoG MagTendon nr 772817, and the Achilles Grant nr 810850, FCT–Fundação para a Ciência e a Tecnologia for the PhD grant of IC (PD/BD/128088/2016); and the Project NORTE-01-0145-FEDER-000021: “Accelerating tissue engineering and personalized medicine discoveries by the integration of key enabling nanotechnologies, marine-derived biomaterials and stem cells”, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AFSCs | Amniotic fluid stem cells |
| ASCs | Adipose-derived stem cells |
| bFGF | Basic Fibroblast Growth Factor |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| BMP | Bone Morphogenetic Protein |
| ECM | Extracellular matrix |
| EGF | Endothelial growth factor |
| GBD | Global Burden of Disease |
| IGF | Insulin-like Growth Factor |
| IL | Interleukin |
| iPSCs | Induced pluripotent stem cells |
| MMPs | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| PDGF | Platelet-derived Growth Factor |
| PRHd | Platelet-rich hemoderivatives |
| PRP | Platelet-rich plasma |
| TDSCs | Tendon-derived stem cells |
| TGF | Transforming Growth Factor |
| TSPCs | Tendon stem/progenitor cells |
References
- Bely, A.E.; Nyberg, K.G. Evolution of animal regeneration: Re-emergence of a field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Gómez-Florit, M.; Babo, P.S.; Domingues, R.; Reis, R.L.; Gomes, M.E. Blood derivatives awaken in regenerative medicine strategies to modulate wound healing. Adv. Drug Deliv. Rev. 2018, 12, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Irvine, D.J.; Moses, M.; Shaunak, S. Wound Healing Versus Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bull. 2010, 35. [Google Scholar] [CrossRef] [PubMed]
- Beredjiklian, P.K.; Favata, M.; Cartmell, J.S.; Flanagan, C.L.; Crombleholme, T.M.; Soslowsky, L.J. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 2003, 31, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.; Chien, C.; Bell, R.; Laudier, D.; Tufa, S.F.; Keene, D.R.; Andarawis-Puri, N.; Huang, A.H. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci. Rep. 2017, 7, 45238. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.I.; Costa-Almeida, R.; Gershovich, P.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Cell based approaches for tendon regeneration. In Tendon Regeneration: Understanding Tissue Physiology and Development to Engineer Functional Substitutes; Gomes, M.E., Rodrigues, M.T., Reis, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kaux, J.-F.; Crielaard, J.-M. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop. Belg. 2013, 79, 10–15. [Google Scholar] [PubMed]
- Filardo, G.; Di Matteo, B.; Kon, E.; Merli, G.; Marcacci, M. Platelet-rich plasma in tendon-related disorders: Results and indications. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1984–1999. [Google Scholar] [CrossRef]
- Tang, J.B.; Zhou, Y.L.; Wu, Y.F.; Liu, P.Y.; Wang, X.T. Gene therapy strategies to improve strength and quality of flexor tendon healing. Expert Opin. Biol. Ther. 2016, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Mooney, D.J. Biomaterials to Mimic and Heal Connective Tissues. Adv. Mater. 2019, 31, 1806695. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Gonçalves, A.I.; Costa-Almeida, R.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Fabrication of hierarchical and biomimetic fibrous structures to support the regeneration of tendon tissues. In Tendon Regeneration: Understanding Tissue Physiology and Development to Engineer Functional Substitutes; Gomes, M.E., Rodrigues, M.T., Reis, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–280. [Google Scholar]
- Loebel, C.; Burdick, J.A. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Gonçalves, A.I.; Gershovich, P.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Tendon stem cell niche. In Tissue Engineering and Stem Cell Niche; Vol. Stem Cell Niche; Turksen, K., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Spanoudes, K.; Gaspar, D.; Pandit, A.; Zeugolis, D.I. The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends Biotechnol. 2014, 32, 474–482. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The horizon of Materiobiology: A perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Evans, C.H. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999, 28, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Maak, T.; Walsh, C.; Rodeo, S.A.; Grande, D.; Dines, D.M.; Dines, J.S. Cytokines in rotator cuff degeneration and repair. J. Shoulder Elb. Surg. 2012, 21, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.C.; McInnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Briggs, A.M.; Woolf, A.D.; Dreinhöfer, K.; Homb, N.; Hoy, D.G.; Kopansky-Giles, D.; Åkesson, K.; March, L. Reducing the global burden of musculoskeletal conditions. Bull. World Health Organ. 2018, 96, 366–368. [Google Scholar] [CrossRef]
- Lomas, A.J.; Ryan, C.N.M.; Sorushanova, A.; Shologu, N.; Sideri, A.I.; Tsioli, V.; Fthenakis, G.C.; Tzora, A.; Skoufos, I.; Quinlan, L.R.; et al. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef]
- Abbah, S.A.; Spanoudes, K.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Assessment of stem cell carriers for tendon tissue engineering in pre-clinical models. Stem Cell Res. Ther. 2014, 5, 38. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. Metabolic Disease Epidemics: Emerging Challenges in Regenerative Medicine. Trends Endocrinol. Metab. 2019, 30, 147–149. [Google Scholar] [CrossRef]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for tendon and ligament repair: Review of the efficacy of commercial products. Expert Rev. Med. Devices 2009, 6, 61–73. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Longo, U.G.; Lamberti, A.; Maffulli, N.; Denaro, V. Tendon augmentation grafts: A systematic review. Br. Med. Bull. 2010, 94, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Galatz, L.M.; Gerstenfeld, L.; Heber-Katz, E.; Rodeo, S.A. Tendon regeneration and scar formation: The concept of scarless healing. J. Orthop. Res. 2015, 33, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.d.S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Lui, P.P.Y. Stem cell technology for tendon regeneration: Current status, challenges, and future research directions. Stem Cells Cloning Adv. Appl. 2015, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hindieh, J.; Leong, D.J.; Sun, H.B. Advances of stem cell based-therapeutic approaches for tendon repair. J. Orthop. Transl. 2017, 9, 69–75. [Google Scholar] [CrossRef]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of achilles tendon defect with autologous ascs engineered tendon in a rabbit model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Soares, R.; Granja, P.L. Fibroblasts as maestros orchestrating tissue regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Dehm, P.; Prockop, D.J. Synthesis and extrusion of collagen by freshly isolated cells from chick embryo tendon. Biochim. Biophys. Acta (BBA) Nucleic Acids Protein Synth. 1971, 240, 358–369. [Google Scholar] [CrossRef]
- Banes, A.J.; Donlon, K.; Link, G.W.; Gillespie, Y.; Bevin, A.G.; Peterson, H.D.; Bynum, D.; Watts, S.; Dahners, L. Cell populations of tendon: A simplified method for isolation of synovial cells and internal fibroblasts: Confirmation of origin and biologic properties. J. Orthop. Res. 1988, 6, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ—Update and future development. Stem Cell Res. Ther. 2015, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L.; Sinclair, C.; Goodship, A.E.; Smith, R.K. Tendon and ligament physiology. In Equine Sports Medicine and Surgery, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Saunders Elsevier: Amsterdam, The Netherlands, 2014; pp. 167–188. [Google Scholar]
- Bernard-Beaubois, K.; Hecquet, C.; Houcine, O.; Hayem, G.; Adolphe, M. Culture and characterization of juvenile rabbit tenocytes. Cell Biol. Toxicol. 1997, 13, 103–113. [Google Scholar] [CrossRef]
- Kryger, G.S.; Chong, A.K.; Costa, M.; Pham, H.; Bates, S.J.; Chang, J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J. Hand Surg. Am. 2007, 32, 597–605. [Google Scholar] [CrossRef]
- Luo, Q.; Song, G.; Song, Y.; Xu, B.; Qin, J.; Shi, Y. Indirect co-culture with tenocytes promotes proliferation and mrna expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology 2009, 61, 1–10. [Google Scholar] [CrossRef]
- Wagget, A.D.; Benjamin, M.; Ralphs, J.R. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur. J. Cell Biol. 2006, 85, 1145–1154. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.Y.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.-C. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Lui, P.P.; Rui, Y.F.; Wong, Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. Part A 2012, 18, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Lui, P.; Wong, Y.; Tan, Q.; Chan, K. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013, 22, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, I.; Wang, J.H.; Iwasaki, K.; Shimizu, T.; Okano, T. The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model. Acta Biomater. 2016, 42, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Q.; Wang, K.; Liu, H.; Ma, C.; Huang, H.; Liu, Y. Isolation and biological characterization of tendon-derived stem cells from fetal bovine. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chan, K.M.; Zhang, J.F.; Li, G. Tendon-derived stem cells undergo spontaneous tenogenic differentiation. Exp. Cell Res. 2016, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells. BMC Musculoskelet. Disord. 2018, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- de Mos, M.; Koevoet, W.J.L.M.; Jahr, H.; Verstegen, M.M.A.; Heijboer, M.P.; Kops, N.; van Leeuwen, J.P.T.M.; Weinans, H.; Verhaar, J.A.N.; van Osch, G.J.V.M. Intrinsic differentiation potential of adolescent human tendon tissue: An in-vitro cell differentiation study. BMC Musculoskelet. Disord. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.; Conforti, E.; Piccoli, M.; Ragone, V.; Creo, P.; Cirillo, F.; Masuzzo, P.; Tringali, C.; Cabitza, P.; Tettamanti, G.; et al. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am. J. Sports Med. 2013, 41, 1653–1664. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef]
- Ruzzini, L.; Abbruzzese, F.; Rainer, A.; Longo, U.G.; Trombetta, M.; Maffulli, N.; Denaro, V. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Nagura, I.; Kokubu, T.; Mifune, Y.; Inui, A.; Takase, F.; Ueda, Y.; Kataoka, T.; Kurosaka, M. Characterization of progenitor cells derived from torn human rotator cuff tendons by gene expression patterns of chondrogenesis, osteogenesis, and adipogenesis. J. Orthop. Surg. Res. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Vigano, M.; Perucca Orfei, C.; Colombini, A.; Stanco, D.; Randelli, P.; Sansone, V.; de Girolamo, L. Different culture conditions affect the growth of human tendon stem/progenitor cells (TSPCs) within a mixed tendon cells (TCs) population. J. Exp. Orthop. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.I.; Rodrigues, M.T.; Lee, S.J.; Atala, A.; Yoo, J.J.; Reis, R.L.; Gomes, M.E. Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS ONE 2013, 8, e83734. [Google Scholar] [CrossRef] [PubMed]
- Orfei, C.P.; Viganò, M.; Pearson, J.R.; Colombini, A.; Luca, P.D.; Ragni, E.; Santos-Ruiz, L.; de Girolamo, L. In vitro Induction of Tendon-Specific Markers in Tendon Cells, Adipose- and Bone Marrow-Derived Stem Cells is Dependent on TGF3, BMP-12 and Ascorbic Acid Stimulation. Int. J. Mol. Sci. 2019, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (Bmus), 3rd ed. 2014. Available online: http://www.Boneandjointburden.Org (accessed on 10 June 2019).
- Rinoldi, C.; Fallahi, A.; Yazdi, I.K.; Paras, J.C.; Kijeńska-Gawrońska, E.; Santiago, G.T.-D.; Tuoheti, A.; Demarchi, D.; Annabi, N.; Khademhosseini, A.; et al. Mechanical and biochemical stimulation of 3d multilayered scaffolds for tendon tissue engineering. ACS Biomater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Uysal, C.A.; Tobita, M.; Hyakusoku, H.; Mizuno, H. Adipose-derived stem cells enhance primary tendon repair: Biomechanical and immunohistochemical evaluation. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.; Corradetti, B.; Consiglio, A.L.; Recordati, C.; Bonacina, E.; Bizzaro, D.; Cremonesi, F. Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents 2011, 25, S75–S84. [Google Scholar] [PubMed]
- Al-ani, M.K.; Xu, K.; Sun, Y.; Pan, L.; Xu, Z.; Yang, L. Study of bone marrow mesenchymal and tendon-derived stem cells transplantation on the regenerating effect of achilles tendon ruptures in rats. Stem Cells Int. 2015, 2015, 984146. [Google Scholar] [CrossRef]
- Vermeulen, S.; Vasilevich, A.; Tsiapalis, D.; Roumans, N.; Vroemen, P.; Beijer, N.R.M.; Eren, A.D.; Zeugolis, D.; Boer, J.D. Identification of topographical architectures supporting the phenotype of rat tenocytes. Acta Biomater. 2019, 83, 277–290. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, E.; Yang, L.; Tu, W.; Lin, J.; Yuan, C.; Bunpetch, V.; Chen, X.; Ouyang, H. Histone deacetylase inhibitor treated cell sheet from mouse tendon stem/progenitor cells promotes tendon repair. Biomaterials 2018, 172, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Salamanna, F.; Tschon, M.; Maglio, M.; Aldini, N.N.; Fini, M. Mesenchymal stem cells for tendon healing: What is on the horizon? J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ekwueme, E.C.; Shah, J.V.; Mohiuddin, M.; Ghebes, C.A.; Crispim, J.F.; Saris, D.L.B.F.; Fernandes, H.A.M.; Freeman, J.W. Cross-talk between human tenocytes and bone marrow stromal cells potentiates extracellular matrix remodeling in vitro. J. Cell. Biochem. 2016, 117, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; Butler, D.L.; Boivin, G.P.; Smith, F.N.L.; Malaviya, P.; Huibregtse, B.; Caplan, A.I. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999, 5, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Wang, B.; Sun, Y.; Xu, J.; Lee, W.Y.W.; Xu, L.; Zhang, J.; Li, G. The use of co-cultured mesenchymal stem cells with tendon-derived stem cells as a better cell source for tendon repair. Tissue Engineer. Part A 2016, 19. [Google Scholar]
- Kapacee, Z.; Yeung, C.-Y.C.; Lu, Y.; Crabtree, D.; Holmes, D.F.; Kadler, K.E. Synthesis of embryonic tendon-like tissue by human marrow stromal/mesenchymal stem cells requires a three-dimensional environment and transforming growth factor β3. Matrix Biol. 2010, 29, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Sevivas, N.; Teixeira, F.G.; Portugal, R.; Direito-Santos, B.; Espregueira-Mendes, J.; Oliveira, F.J.; Silva, R.F.; Sousa, N.; Sow, W.T.; Luong, N.; et al. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am. J. Sports Med. 2018, 46, 449–459. [Google Scholar] [CrossRef]
- Baberg, F.; Geyh, S.; Waldera-Lupa, D.; Stefanski, A.; Zilkens, C.; Haas, R.; Schroeder, T.; Stühler, K. Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 434–441. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.; Ferraro, G. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef]
- de Mattos Carvalho, A.; Alves, A.L.G.; Gomes de Oliveira, P.G.; Álvarez, L.E.C.; Amorim, R.L.; Hussni, C.A.; Deffune, E. Use of adipose tissue-derived mesenchymal stem cells for experimental tendinitis therapy in equines. J. Equine Vet. Sci. 2011, 31, 26–34. [Google Scholar] [CrossRef]
- Veronesi, F.; Torricelli, P.; Bella, E.D.; Pagani, S.; Fini, M. In vitro mutual interaction between tenocytes and adipose-derived mesenchymal stromal cells. Cytotherapy 2015, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Calejo, I.; Reis, R.; Gomes, M. Crosstalk between adipose stem cells and tendon cells reveals a temporal regulation of tenogenesis by extracellular matrix deposition and remodeling. J. Cell. Physiol. 2017. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Berdecka, D.; Rodrigues, M.; Reis, R.; Gomes, M. Tendon explant cultures to study the communication between adipose stem cells and native tendon niche. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).