Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane
Abstract
1. Introduction
2. Results
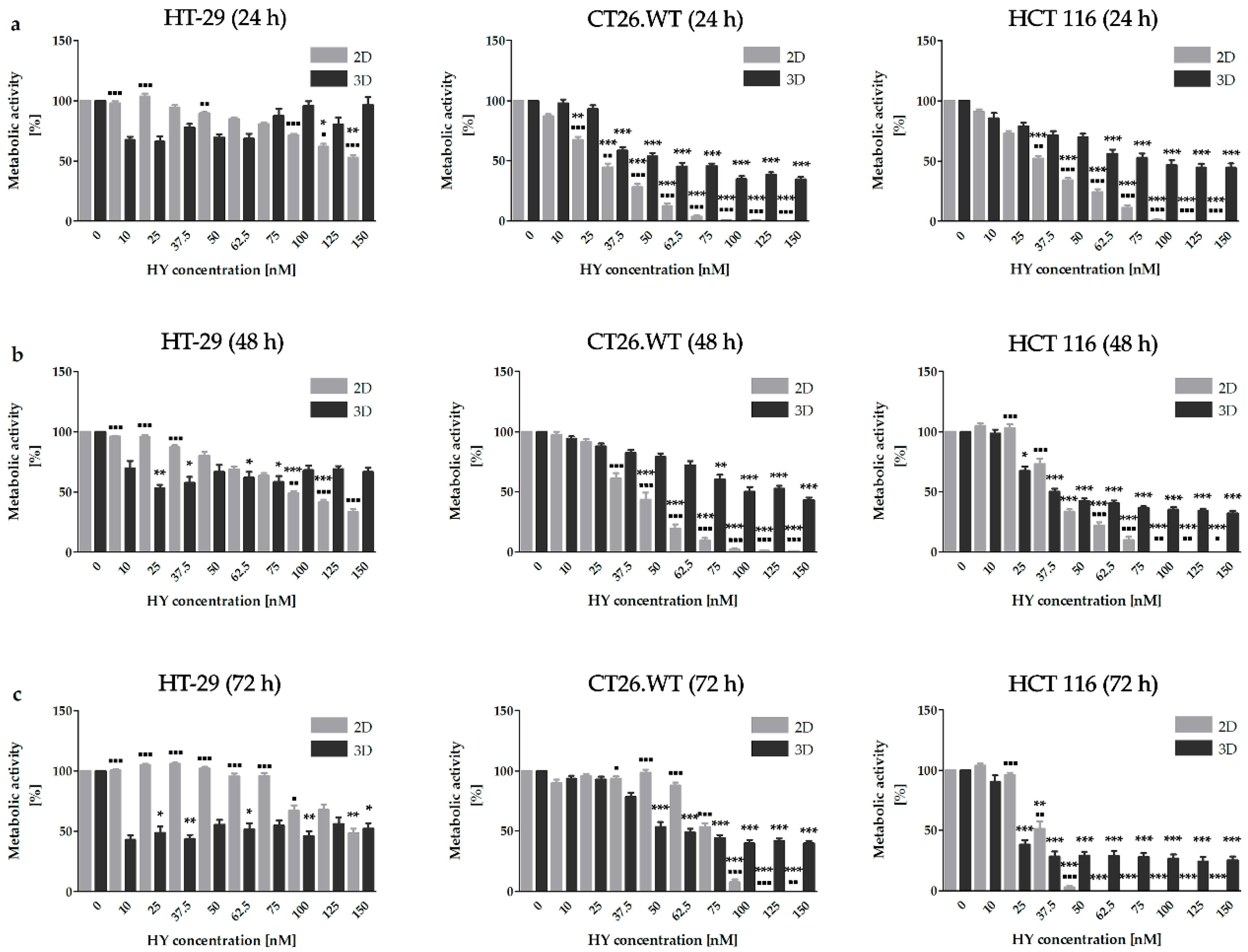
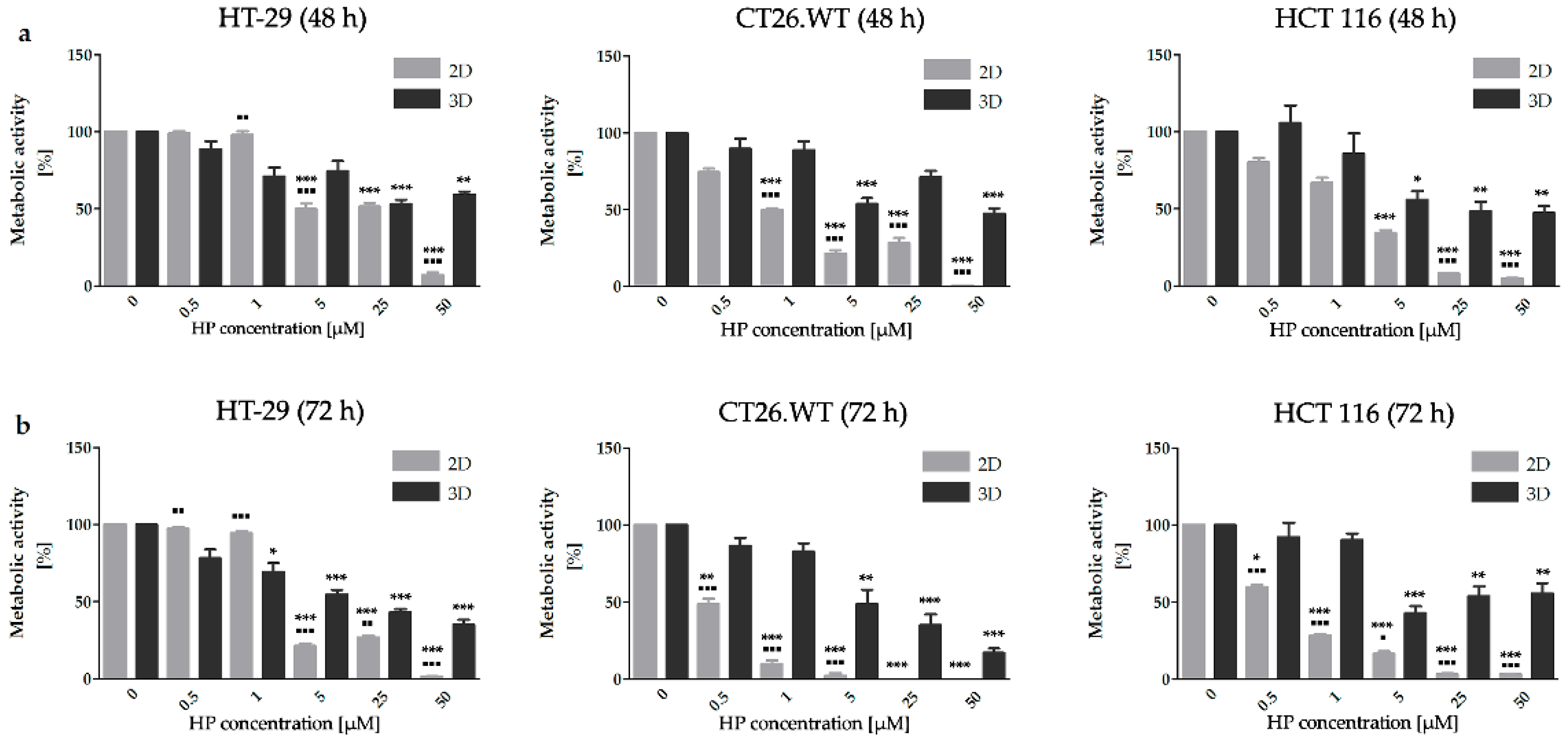
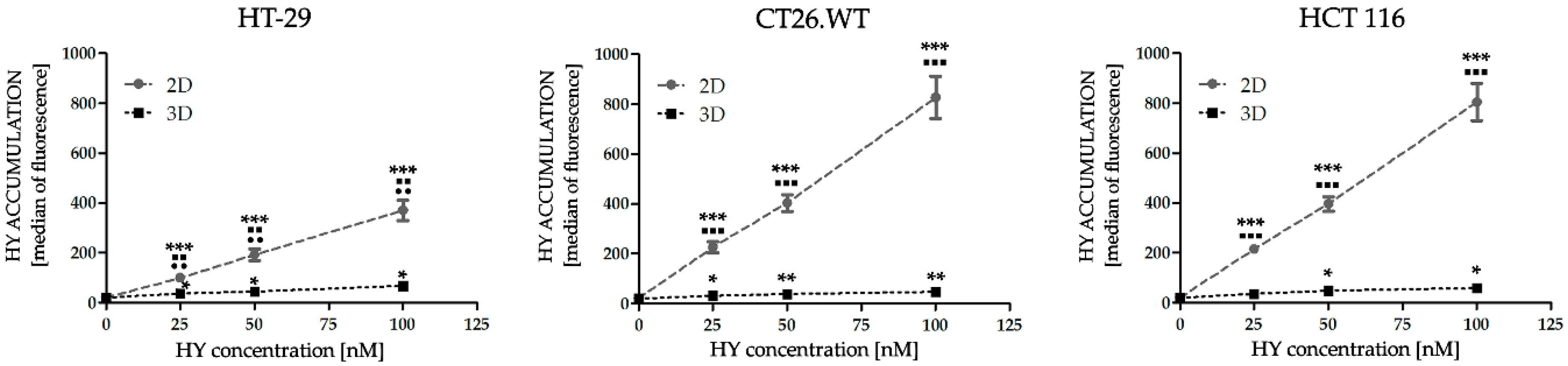
2.1. Dimensionality of Experimental Models as A Key Factor Affecting Accumulation Properties of HY and Metabolic Activity after HY-PDT and HP Treatment
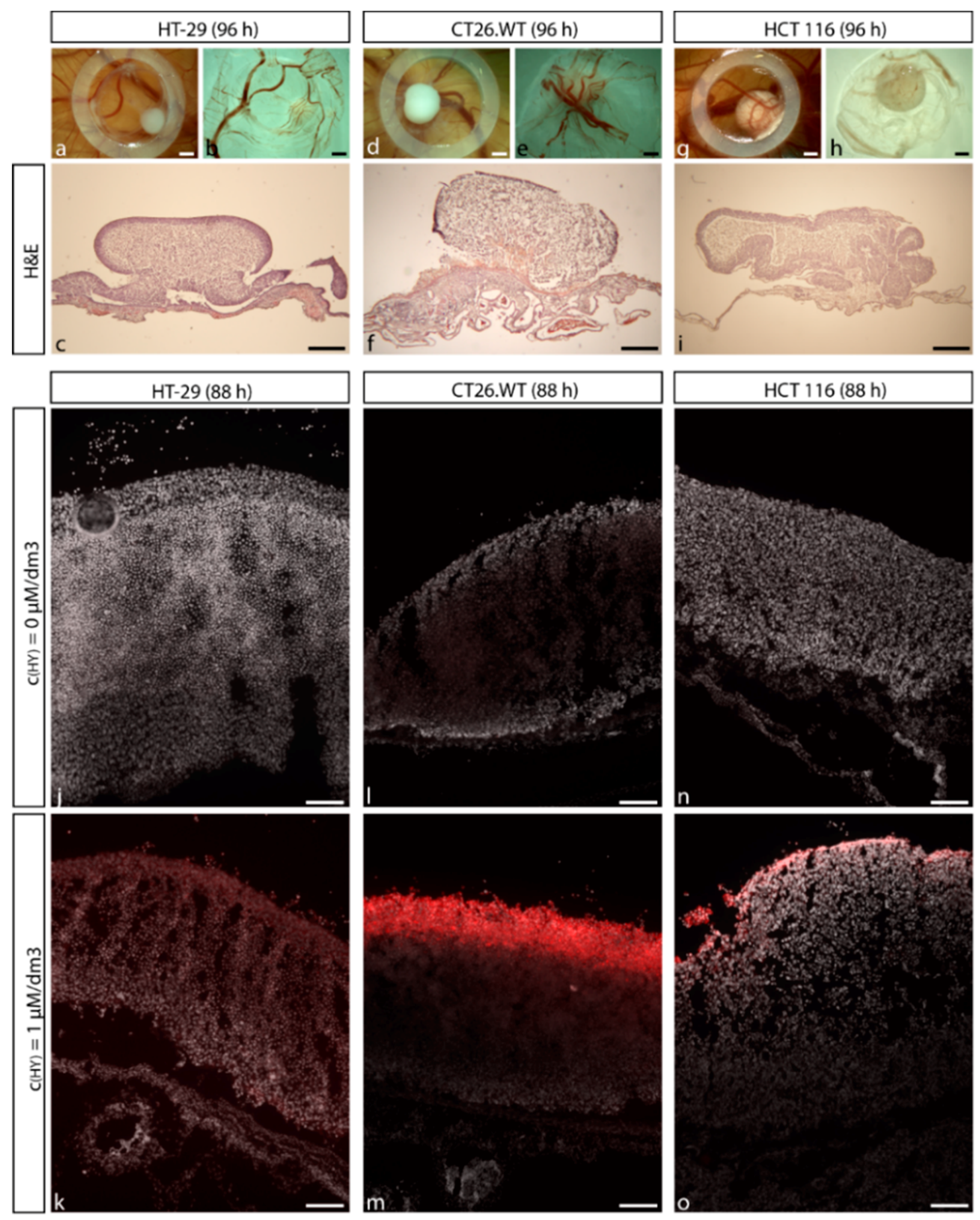
2.2. Establishment of CRC Micro-Tumors on CAM
2.3. Effect of HY-PDT and HP on Expression of Pro-Angiogenic Factors in Early Stages after Treatment Incidence
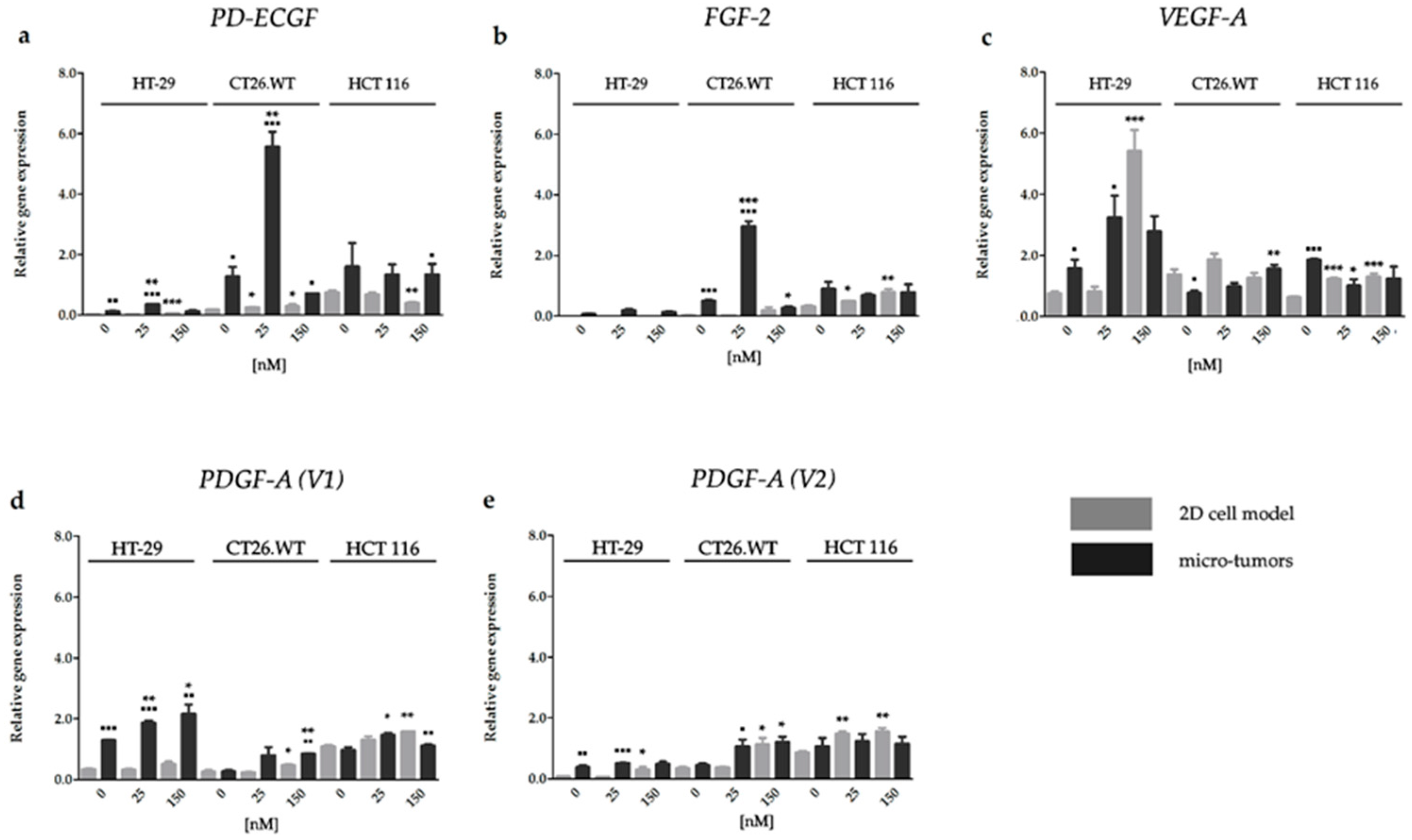
2.3.1. HY-PDT targeted at the Cellular Portion of CRC Micro-Tumors Upregulated Gene Expression of Pro-Angiogenic Factors
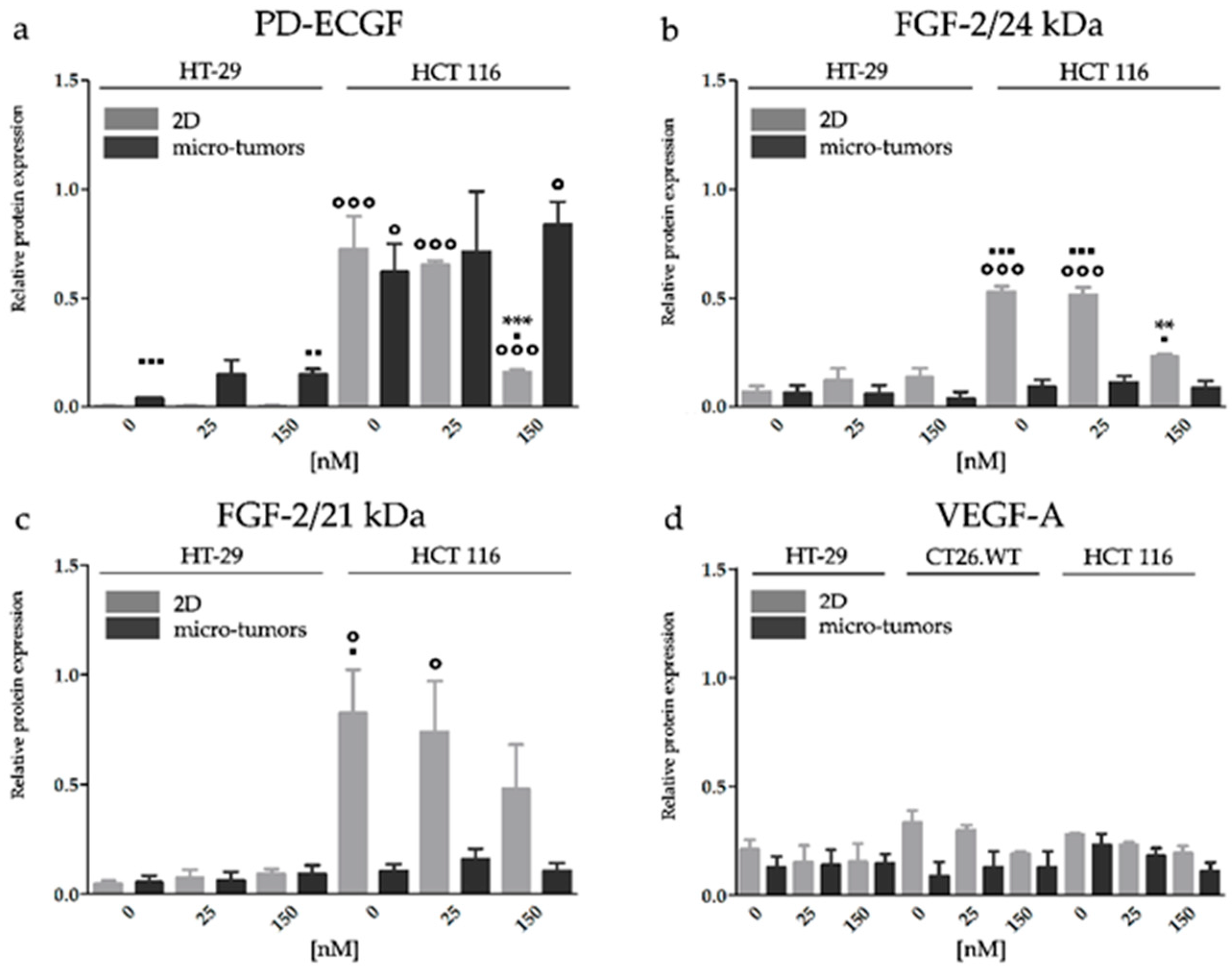
2.3.2. HY-PDT Targeted at the Cellular Portion of CRC Micro-Tumors did not Upregulate Pro-Angiogenic Protein Expression
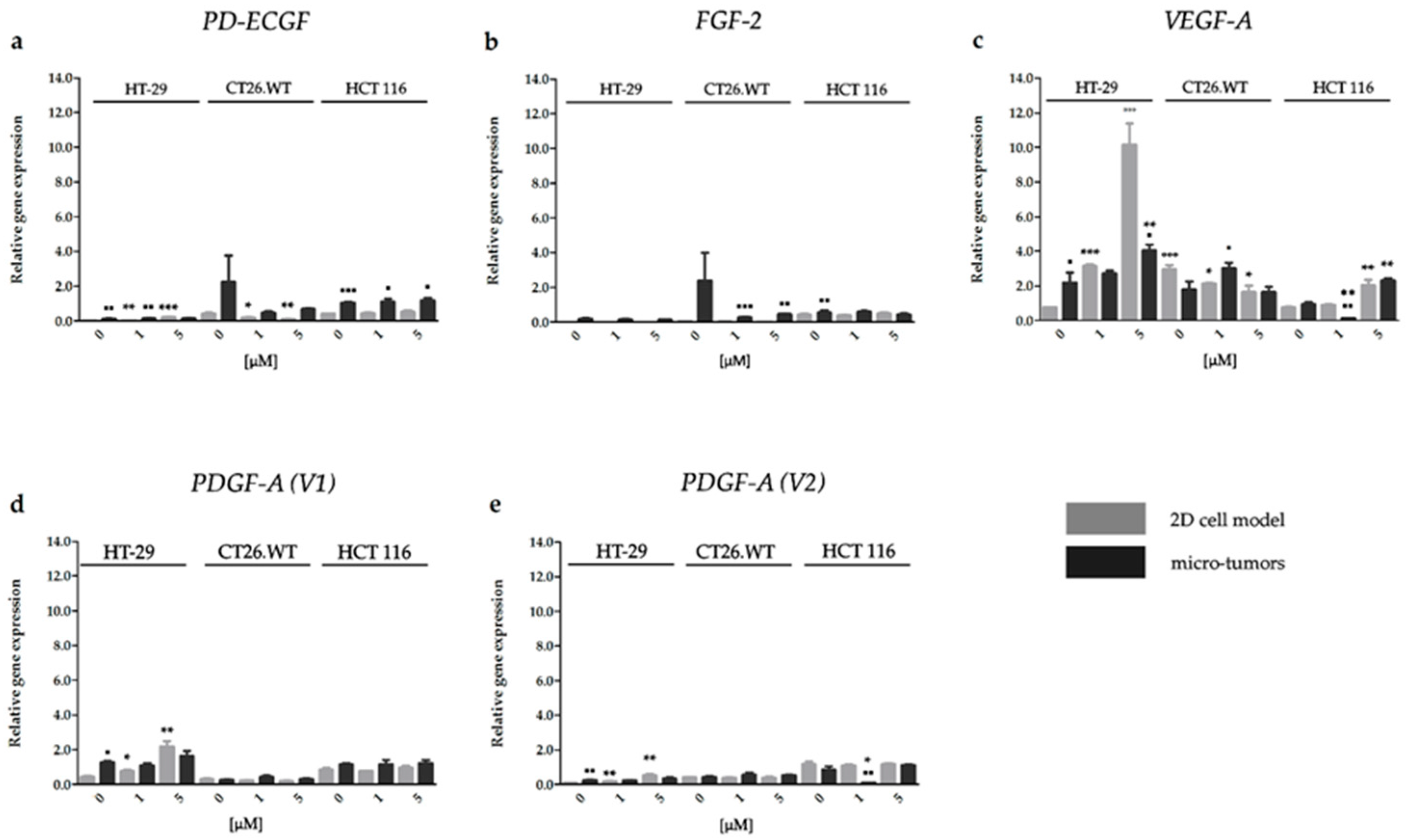
2.3.3. Effect of HP on the Gene Expression of Factors Associated with CRC Angiogenesis
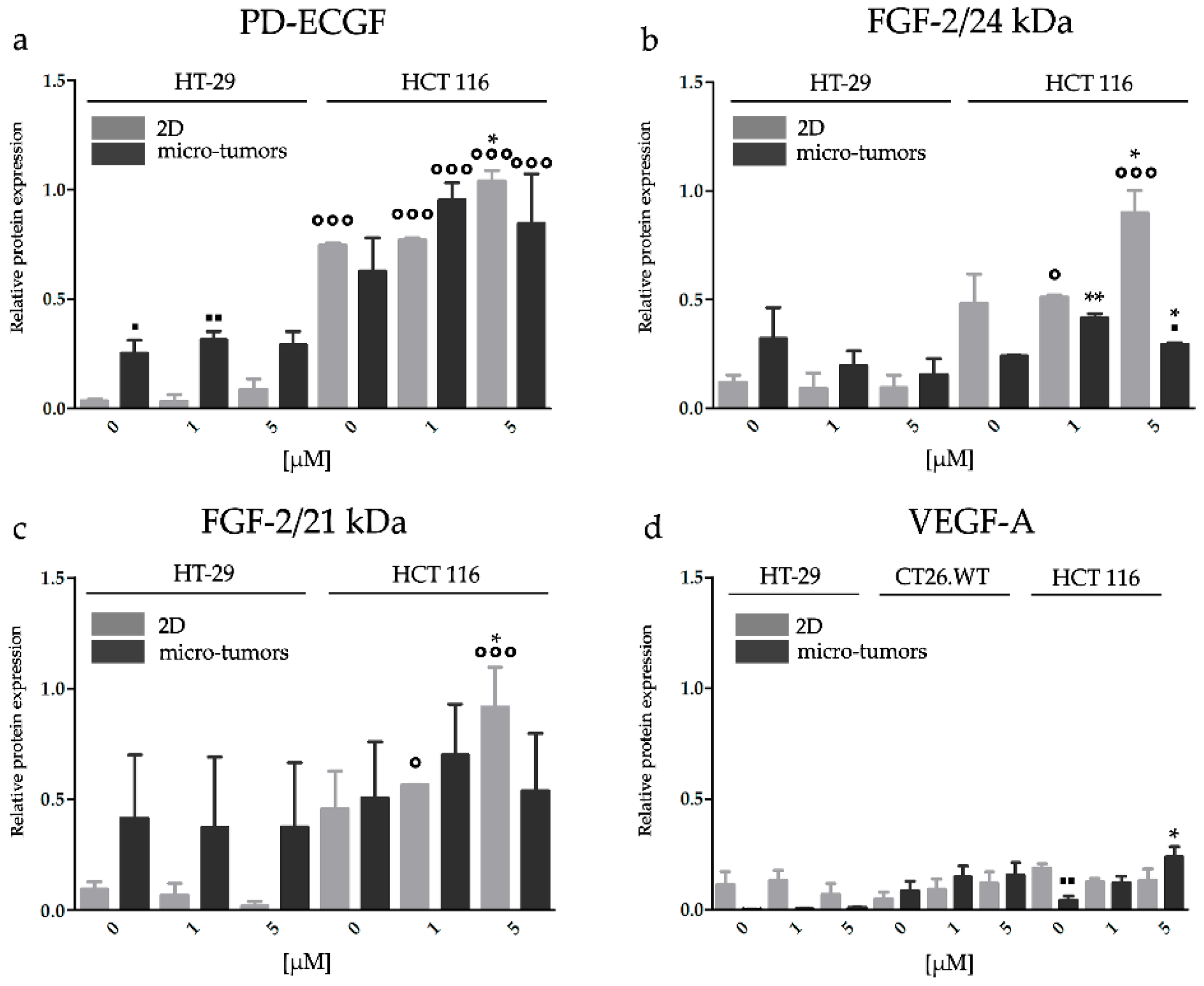
2.3.4. HP Increased Angiogenic Potential of CRC Micro-Tumors by Upregulation of Proteins Associated with a Pro-Angiogenic Phenotype
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
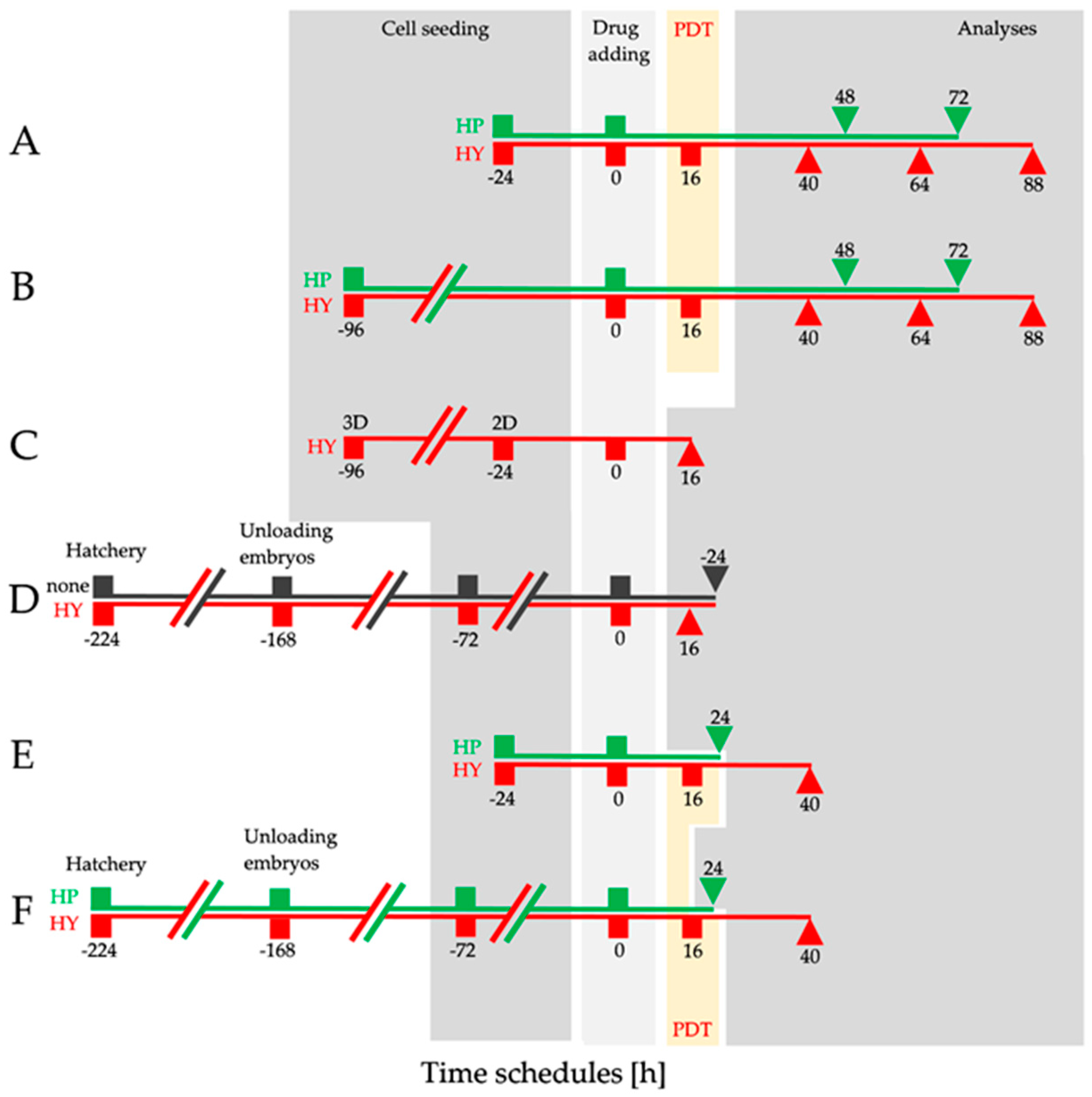
4.3. Experimental Design
4.4. HY Activation
4.5. Spheroids (3D Cell Model) Formation Assay
4.6. In vivo Experimental Micro-Tumor Creation
4.7. MTT Assay
4.8. Intracellular Content of HY
4.9. Western Blot Analysis
4.10. Real Time RT-PCR (RT-qPCR)
4.11. Tissue Processing
4.12. Histology, Immunolabeling, and HY Detection
4.13. Brightfield and Confocal Microscopy
4.14. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAM | Chorioallantoic membrane |
| CRC | Colorectal cancer |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| ECs | Endothelial cells |
| FGF-2 | Fibroblast growth factor 2 (protein) |
| FGF-2 | Fibroblast growth factor 2 (gene) |
| H&E | Hematoxylin and eosin |
| HP | Hyperforin |
| HY | Hypericin |
| HY-PDT | Photodynamic therapy with hypericin |
| PD-ECGF | Platelet derived endothelial cell growth factor (protein) |
| PD-ECGF | Platelet derived endothelial cell growth factor (gene) |
| PDGF-A | Platelet derived growth factor subunit A (protein) |
| PDGF-A (V1) | Platelet derived growth factor subunit A isoform 1 (gene) |
| PDGF-A (V2) | Platelet derived growth factor subunit A isoform 2 (gene) |
| PMM1 | Phosphomannomutase 1 (gene) |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| SJW | St. John’s wort |
| VEGF-A | Vascular endothelial growth factor subunit A (protein) |
| VEGF-A | Vascular endothelial growth factor subunit A (gene) |
References
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 319, 1374–1378. [Google Scholar]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatioid and other disease. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, R.; Grothey, A. Antiangiogenic therapy for refractory colorectal cancer: Current options and future strategies. Ther. Adv. Med. Oncol. 2017, 9, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, A.; Delle Monache, S.; Cortellini, A.; Di Padova, M.; Ficorella, C. “Vessels in the Storm”: Searching for Prognostic and Predictive Angiogenic Factors in Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Gago, F.; Balzarini, J.; Liekens, S. The Dual Role of Thymidine Phosphorylase in Cancer Development and Chemotherapy. Med. Res. Rev. 2014, 29, 1–69. [Google Scholar] [CrossRef]
- Schrader, E. Equivalence of St John’s wort extract (Ze 117) and fluoxetine: A randomized, controlled study in mild-moderate depression. Int. Clin. Psychopharmacol. 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Solomon, D.; Ford, E.; Adams, J.; Graves, N. Potential of St John’s Wort for the Treatment of Depression: The Economic Perspective. Aust. N. Z. J. Psychiatry 2011, 45, 123–130. [Google Scholar] [CrossRef]
- Bálintová, M.; Bruňáková, K.; Petijová, L.; Čellárová, E. Targeted metabolomic profiling reveals interspecific variation in the genus Hypericum in response to biotic elicitors. Plant Physiol. Biochem. 2019, 135, 348–358. [Google Scholar] [CrossRef]
- Nigutová, K.; Kusari, S.; Sezgin, S.; Petijová, L.; Henzelyová, J.; Bálintová, M.; Spiteller, M.; Čellárová, E. Chemometric evaluation of hypericin and related phytochemicals in 17 in vitro cultured Hypericum species, hairy root cultures and hairy root-derived transgenic plants. J. Pharm. Pharmacol. 2019, 71, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Delaey, E.; Vandenheede, J.R.; Agostinis, P.; Xu, Y.; de Witte, P.; Roskams, T. Efficacy of antitumoral photodynamic therapy with hypericin: Relationship between biodistribution and photodynamic effects in the RIF-1 mouse tumor model. Int. J. Cancer 2001, 93, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Roskams, T.; Xu, Y.; Agostinis, P.; De Witte, P.A.M. Photodynamic therapy with hypericin induces vascular damage and apoptosis in the RIF-1 mouse tumor model. Int. J. Cancer 2002, 98, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Sanovic, R.; Verwanger, T.; Hartl, A.; Krammer, B. Low dose hypericin-PDT induces complete tumor regression in BALB/c mice bearing CT26 colon carcinoma. Photodiagn. Photodyn. Ther. 2011, 8, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tassone, E.; Maran, C.; Masola, V.; Bradaschia, A.; Garbisa, S.; Onisto, M. Antidepressant hyperforin up-regulates VEGF in CNS tumour cells. Pharmacol. Res. 2011, 63, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Billard, C.; Kolb, J.-P.; Fourneron, J.-D.; Mirshahi, P. Hyperforin inhibits MMP-9 secretion by B-CLL cells and microtubule formation by endothelial cells. Leukemia 2006, 20, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.; Appendino, G.; Borsarini, A.; Sartor, L.; Pezzato, E.; Calabrese, F.; Donella-Deana, A.; Barbera, M.; Caniato, R.; Garbisa, S.; et al. Hyperforin Inhibits Cancer Invasion and Metastasis. Cancer Res. 2005, 64, 6225–6232. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Kiss, J.; Kirkin, V.; Averbeck, M.; Simon-Haarhaus, B.; Kremer, B.; Termeer, C.C.; Sleeman, J.; Simon, J.C. Hyperforin acts as an angiogenesis inhibitor in vitro and in vivo. Planta Med. 2005, 71, 999–1004. [Google Scholar] [CrossRef]
- Čavarga, I.; Bilčík, B.; Výboh, P.; Záškvarová, M.; Chorvát, D.; Kasák, P.; Mlkvý, P.; Mateašík, A.; Chorvátová, A.; Miškovský, P. Photodynamic Effect of Hypericin after Topical Application in the Ex Ovo Quail Chorioallantoic Membrane Model. Planta Med. 2014, 80, 56–62. [Google Scholar] [CrossRef]
- Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Hyperforin, a bio-active compound of St. John’s Wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int. J. Cancer 2005, 117, 775–780. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.K.; Lucky, S.S.; Chin, W.W.L.; Ali, S.M.; Soo, K.C.; Olivo, M. Molecular profiling of angiogenesis in hypericin mediated photodynamic therapy. Mol. Cancer 2008, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.Y.; Yee, K.K.L.; Soo, K.C.; Olivo, M. Effect of hypericin-mediated photodynamic therapy on the expression of vascular endothelial growth factor in human nasopharyngeal carcinoma. Int. J. Mol. Med. 2007, 20, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Hait, W.N. Anticancer drug development: The grand challenges. Nat. Rev. Drug Discov. 2010, 9, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Kola, I. The State of Innovation in Drug Development. Clin. Pharmacol. Ther. 2008, 83, 227–230. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Thomas, C.; Pardini, R.S. Oxygen Dependence of Hypericin-Induced Phototoxicity to Emt6 Mouse Mammary Carcinoma Cells. Photochem. Photobiol. 1984, 39, 831–837. [Google Scholar] [CrossRef]
- Vandenbogaerde, A.L.; Cuveele, J.F.; Proot, P.; Himpens, B.E.; Merlevede, W.J.; De Witte, P.A. Differential cytotoxic effects induced after photosensitization by hypericin. J. Photochem. Photobiol. B Biol. 1997, 38, 136–142. [Google Scholar] [CrossRef]
- Miadokova, E.; Chalupa, I.; Vlckova, V.; Sevcovicova, A.; Nadova, S.; Kopaskova, M.; Hercegova, A.; Gasperova, P.; Alfoldiova, L.; Komjatiova, M.; et al. Genotoxicity and antigenotoxicity evaluation of non-photoactivated hypericin. Phyther. Res. 2010, 24, 90–95. [Google Scholar] [CrossRef]
- Jendželovská, Z.; Jendželovský, R.; Hiľovská, L.; Kovaľ, J.; Mikeš, J.; Fedoročko, P. Single pre-treatment with hypericin, a St. John’s wort secondary metabolite, attenuates cisplatin- and mitoxantrone-induced cell death in A2780, A2780cis and HL-60 cells. Toxicol. Vitr. 2014, 28, 1259–1273. [Google Scholar] [CrossRef]
- Feruszová, J.; Imreová, P.; Bodnárová, K.; Ševčovičová, A.; Kyzek, S.; Chalupa, I.; Gálová, E.; Miadoková, E. Photoactivated hypericin is not genotoxic. Gen. Physiol. Biophys. 2016, 35, 223–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blank, M.; Mandel, M.; Keisari, Y.; Meruelo, D.; Lavie, G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003, 63, 8241–8247. [Google Scholar]
- Blank, M.; Mandel, M.; Hazan, S.; Keisari, Y.; Lavie, G. ANTI-cancer Activities of Hypericin in the Dark. Photochem. Photobiol. 2004, 74, 120. [Google Scholar] [CrossRef]
- Berlanda, J.; Kiesslich, T.; Engelhardt, V.; Krammer, B.; Plaetzer, K. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J. Photochem. Photobiol. B Biol. 2010, 100, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Besic Gyenge, E.; Forny, P.; Lüscher, D.; Laass, A.; Walt, H.; Maake, C. Effects of hypericin and a chlorin based photosensitizer alone or in combination in squamous cell carcinoma cells in the dark. Photodiagn. Photodyn. Ther. 2012, 9, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Barliya, T.; Mandel, M.; Livnat, T.; Weinberger, D.; Lavie, G. Degradation of HIF-1alpha under Hypoxia Combined with Induction of Hsp90 Polyubiquitination in Cancer Cells by Hypericin: A Unique Cancer Therapy. PLoS ONE 2011, 6, e22849. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Yuen, G.Y.; Chee, S.K.; Olivo, M. Hypericin-mediated photodynamic therapy in combination with Avastin (bevacizumab) improves tumor response by downregulating angiogenic proteins. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zupkó, I.; de Witte, P.A. Photodynamic therapy with hypericin in a mouse P388 tumor model: Vascular effects determine the efficacy. Int. J. Oncol. 2001, 18, 737–742. [Google Scholar] [CrossRef]
- Matsuura, T.; Kuratate, I.; Teramachi, K.; Osaki, M.; Fukuda, Y.; Ito, H. Thymidine phosphorylase expression is associated with both increase of intratumoral microvessels and decrease of apoptosis in human colorectal carcinomas. Cancer Res. 1999, 59, 5037–5040. [Google Scholar]
- Toi, M.; Rahman, M.A.; Bando, H.; Chow, L.W. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005, 6, 158–166. [Google Scholar] [CrossRef]
- Inokuchi, M.; Uetake, H.; Shirota, Y.; Yamada, H.; Tajima, M.; Sugihara, K. Gene expression of 5-fluorouracil metabolic enzymes in primary colorectal cancer and corresponding liver metastasis. Cancer Chemother. Pharmacol. 2004, 53, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008, 15, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Ueda, J.; Ishiwata, T. Fibroblast Growth Factor Receptor 2: Expression, Roles, and Potential as a Novel Molecular Target for Colorectal Cancer. Patholog. Res. Int. 2012, 2012, 574768. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, V.P.; Shein, S.A.; Korchagina, A.A.; Gurina, O.I. VEGF in tumor progression and targeted therapy. Curr. Cancer Drug Targets 2013, 13, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Eswarappa, S.M.; Potdar, A.A.; Koch, W.J.; Fan, Y.; Vasu, K.; Lindner, D.; Willard, B.; Graham, L.M.; DiCorleto, P.E.; Fox, P.L. Programmed Translational Readthrough Generates Antiangiogenic VEGF-Ax. Cell 2014, 157, 1605–1618. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Ge, J.; Hu, F.; Zhao, Y. Can Vascular Endothelial Growth Factor and Microvessel Density Be Used as Prognostic Biomarkers for Colorectal Cancer? A Systematic Review and Meta-Analysis. Sci. World J. 2014, 2014, 102736. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kitadai, Y.; Bucana, C.D.; Cleary, K.R.; Ellis, L.M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995, 55, 3964–3968. [Google Scholar]
- Uthoff, S.M.S.; Duchrow, M.; Schmidt, M.H.H.; Broll, R.; Bruch, H.-P.; Strik, M.W.; Galandiuk, S. VEGF isoforms and mutations in human colorectal cancer. Int. J. Cancer 2002, 101, 32–36. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue architecture: The ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 2003, 15, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Brugge, J.S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 2005, 5, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.-C.; Zhang, C.; Zhang, J.; Khong, Y.M.; Chang, S.; Samper, V.D.; van Noort, D.; Hutmacher, D.W.; Yu, H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab. Chip 2007, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Pickl, M.; Ries, C.H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009, 28, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Morizane, A.; Doi, D.; Kikuchi, T.; Nishimura, K.; Takahashi, J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res. 2011, 89, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, G.; Di Lorenzo, D.; Steimberg, N. Modelling tissues in 3D: The next future of pharmaco-toxicology and food research? Genes Nutr. 2009, 4, 13–22. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Goldwirt, L.; Beccaria, K.; Carpentier, A.; Idbaih, A.; Schmitt, C.; Levasseur, C.; Labussiere, M.; Milane, A.; Farinotti, R.; Fernandez, C. Preclinical impact of bevacizumab on brain and tumor distribution of irinotecan and temozolomide. J. Neurooncol. 2015, 122, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cesca, M.; Morosi, L.; Berndt, A.; Nerini, I.F.; Frapolli, R.; Richter, P.; Decio, A.; Dirsch, O.; Micotti, E.; Giordano, S.; et al. Bevacizumab-Induced Inhibition of Angiogenesis Promotes a More Homogeneous Intratumoral Distribution of Paclitaxel, Improving the Antitumor Response. Mol. Cancer Ther. 2016, 15, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Torok, S.; Rezeli, M.; Kelemen, O.; Vegvari, A.; Watanabe, K.; Sugihara, Y.; Tisza, A.; Marton, T.; Kovacs, I.; Tovari, J.; et al. Limited tumor tissue drug penetration contributes to primary resistance against angiogenesis inhibitors. Theranostics 2017, 7, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.K.L.; Soo, K.C.; Olivo, M. Anti-angiogenic effects of Hypericin-photodynamic therapy in combination with Celebrex in the treatment of human nasopharyngeal carcinoma. Int. J. Mol. Med. 2005, 16, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, G.; Garbisa, S.; Sogno, I.; Vannini, N.; Noonan, D.M.; Generoso, L.; Albini, A. Mechanisms of Hyperforin as an anti-angiogenic angioprevention agent. Eur. J. Cancer 2009, 45, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aica, I.; Lorusso, G.; Colalto, C.; Brunetta, E.; Niero, R.; Garbisa, S.; Albini, A.; Benelli, R.; Calabrese, F.; Agostini, C.; et al. Hyperforin Blocks Neutrophil Activation of Matrix Metalloproteinase-9, Motility and Recruitment, and Restrains Inflammation-Triggered Angiogenesis and Lung Fibrosis. J. Pharmacol. Exp. Ther. 2007, 321, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pérez, J.; Ballesteros, P.; Cerdán, S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. Magn. Reson. Mater. Phys. Biol. Med. 2005, 18, 293–301. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Jendželovská, Z.; Jendželovský, R.; Kuchárová, B.; Fedoročko, P. Hypericin in the Light and in the Dark: Two Sides of the Same Coin. Front. Plant Sci. 2016, 7, 560. [Google Scholar] [CrossRef]
- Curcio, E.; Salerno, S.; Barbieri, G.; De Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Arii, S.; Furutani, M.; Isigami, S.; Mori, A.; Onodera, H.; Chiba, T.; Imamura, M. Implication of vascular endothelial growth factor and p53 status for angiogenesis in noninvasive colorectal carcinoma. Cancer 2000, 88, 1820–1827. [Google Scholar] [CrossRef]
- Kondo, Y.; Arii, S.; Mori, A.; Furutani, M.; Chiba, T.; Imamura, M. Enhancement of angiogenesis, tumor growth, and metastasis by transfection of vascular endothelial growth factor into LoVo human colon cancer cell line. Clin. Cancer Res. 2000, 6, 622–630. [Google Scholar] [PubMed]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Durupt, F.; Koppers-Lalic, D.; Balme, B.; Budel, L.; Terrier, O.; Lina, B.; Thomas, L.; Hoeben, R.C.; Rosa-Calatrava, M. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 2012, 19, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Buriková, M.; Bilčík, B.; Mariana, M.; Výboh, P.; Bizík, J.; Mateašík, A.; Miškovský, P.; Čavarga, I. Hypericin fluorescence kinetics in the presence of low density lipoproteins: Study on quail CAM assay for topical delivery. Gen. Physiol. Biophys. 2016, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumor Biol. 2017, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thong, P.S.-P.; Watt, F.; Tan, P.H.; Olivo, M.; Soo, K.C.; Ren, M.Q. Hypericin-photodynamic therapy (PDT) using an alternative treatment regime suitable for multi-fraction PDT. J. Photochem. Photobiol. B Biol. 2005, 82, 1–8. [Google Scholar] [CrossRef]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A.M. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Ali, S.; Olivo, M. Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int. J. Oncol. 2002, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Galanou, M.C.; Theodossiou, T.A.; Tsiourvas, D.; Sideratou, Z.; Paleos, C.M. Interactive Transport, Subcellular Relocation and Enhanced Phototoxicity of Hypericin Encapsulated in Guanidinylated Liposomes via Molecular Recognition. Photochem. Photobiol. 2008, 84, 1073–1083. [Google Scholar] [PubMed]
- Mikeš, J.; Hýžďalová, M.; Kočí, L.; Jendželovský, R.; Kovaľ, J.; Vaculová, A.; Hofmanová, J.; Kozubík, A.; Fedoročko, P. Lower sensitivity of FHC fetal colon epithelial cells to photodynamic therapy compared to HT-29 colon adenocarcinoma cells despite higher intracellular accumulation of hypericin. Photochem. Photobiol. Sci. 2011, 10, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Jendželovský, R.; Jendželovská, Z.; Hiľovská, L.; Kovaľ, J.; Mikeš, J.; Fedoročko, P. Proadifen sensitizes resistant ovarian adenocarcinoma cells to cisplatin. Toxicol. Lett. 2016, 243, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kleban, J.; Mikeš, J.; Szilárdiová, B.; Kovaľ, J.; Sačková, V.; Solár, P.; Horváth, V.; Hofmanová, J.; Kozubík, A.; Fedoročko, P. Modulation of Hypericin Photodynamic Therapy by Pretreatment with 12 Various Inhibitors of Arachidonic Acid Metabolism in Colon Adenocarcinoma HT-29 Cells. Photochem. Photobiol. 2007, 83, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Dowling, C.M.; Walsh, D.; Coffey, J.C.; Kiely, P.A. The importance of selecting the appropriate reference genes for quantitative real time PCR as illustrated using colon cancer cells and tissue. F1000Research 2016, 5, 99. [Google Scholar] [CrossRef]
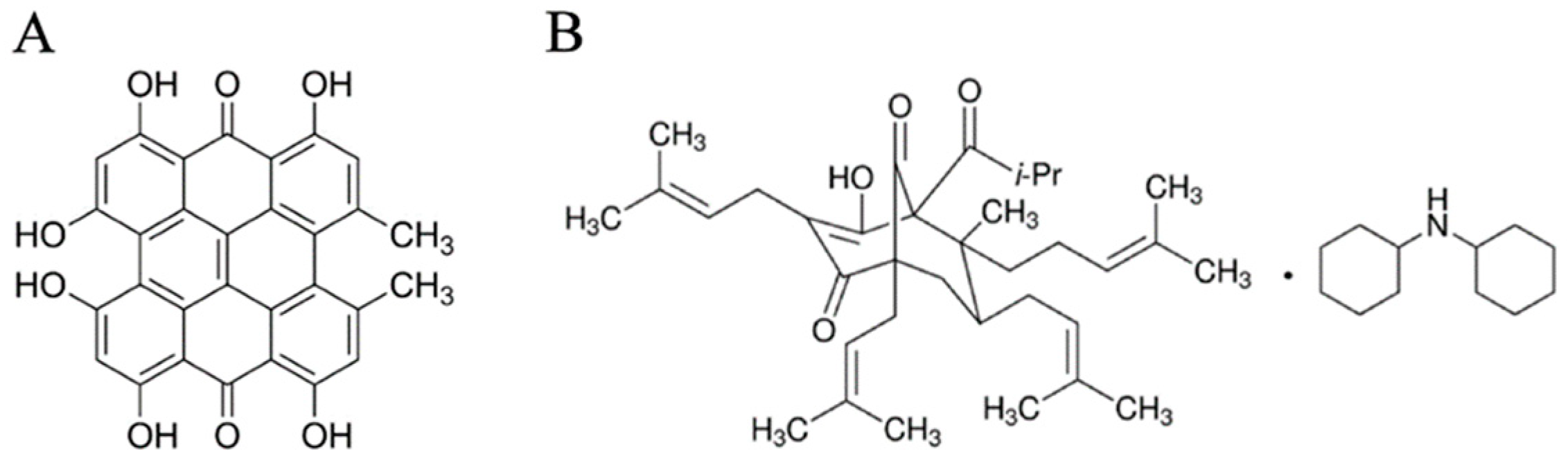
| Gene (GenBank Ref.seq.) | Cell lines | Primer Name | Sequence | Ta and Product Length |
|---|---|---|---|---|
| hPD-ECGF (NM_001113755) | HT-29, HCT 116 | hTymp_for | TCAATGTCATCCAGAGCCCAG | 60 °C 188 bp |
| hTymp_rev | CCCCTCCACGAGTTTCTTACT | |||
| mPD-ECGF (NM_138302) | CT26.WT | mTymp_for | ATCGCACAGCCCTAAGTCTC | 58 °C 71 bp |
| mTymp_rev | CCCTAGAGCCAGTAGCATCG | |||
| hVegfA (NM_001025366) | HT-29, HCT 116 | hVegfA_for | AGGAGGGCAGAATCATCACGA | 60 °C 138 bp |
| hVegfA_rev | ACACAGGATGGCTTGAAGATGT | |||
| mVegfA (NM_001287056) | CT26.WT | mVegfA_for | TCTTCAAGCCGTCCTGTGTGC | 61 °C 127 bp |
| mVegfA_rev | CTTTGGTGAGGTTTGATCCG | |||
| hPdgfA (V1) (NM_002607) | HT-29, HCT 116 | hPdgfA_for1 | AAGCAGCCAACCAGATGTGA | 61 °C 133 bp |
| PdgfA_rev1/2 | GGAGGAGAACAAAGACCGCA | |||
| mPdgfA (V1) (NM_008808) | CT26.WT | mPdgfA_for1 | GGAGGAGACAGATGTGAGGTG | 61 °C 134 bp |
| PdgfA_rev1/2 | GGAGGAGAACAAAGACCGCA | |||
| PdgfA (V2) (NM_033023) | HT-29, HCT 116, CT26.WT | PdgfA_for2 | AGGACACGGATGTGAGGTGA | 60 °C 128 bp |
| PdgfA_rev1/2 | GGAGGAGAACAAAGACCGCA | |||
| FGF2 (NM_001361665) | HT-29, HCT 116, CT26.WT | FGF2_for | GAAGAGCGACCCTCACATCAA | 61 °C 265 bp |
| FGF2_rev | CTGCCCAGTTCGTTTCAGTG | |||
| PMM1 (NM_002676) | HT-29, HCT 116, CT26.WT | PMM1_for | GCTCGCCAGAAAATTGACCCT | 61 °C 177 bp |
| PMM1_rev | ATACTGCACCGTCCCGTTCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majerník, M.; Jendželovský, R.; Babinčák, M.; Košuth, J.; Ševc, J.; Tonelli Gombalová, Z.; Jendželovská, Z.; Buríková, M.; Fedoročko, P. Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane. Int. J. Mol. Sci. 2019, 20, 3004. https://doi.org/10.3390/ijms20123004
Majerník M, Jendželovský R, Babinčák M, Košuth J, Ševc J, Tonelli Gombalová Z, Jendželovská Z, Buríková M, Fedoročko P. Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane. International Journal of Molecular Sciences. 2019; 20(12):3004. https://doi.org/10.3390/ijms20123004
Chicago/Turabian StyleMajerník, Martin, Rastislav Jendželovský, Marián Babinčák, Ján Košuth, Juraj Ševc, Zuzana Tonelli Gombalová, Zuzana Jendželovská, Monika Buríková, and Peter Fedoročko. 2019. "Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane" International Journal of Molecular Sciences 20, no. 12: 3004. https://doi.org/10.3390/ijms20123004
APA StyleMajerník, M., Jendželovský, R., Babinčák, M., Košuth, J., Ševc, J., Tonelli Gombalová, Z., Jendželovská, Z., Buríková, M., & Fedoročko, P. (2019). Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane. International Journal of Molecular Sciences, 20(12), 3004. https://doi.org/10.3390/ijms20123004






