Gene Therapy for Pancreatic Diseases: Current Status
Abstract
1. Introduction
2. Pancreatic Cancer
3. Pancreatitis
4. Pancreatic Pain
5. Diabetes Mellitus
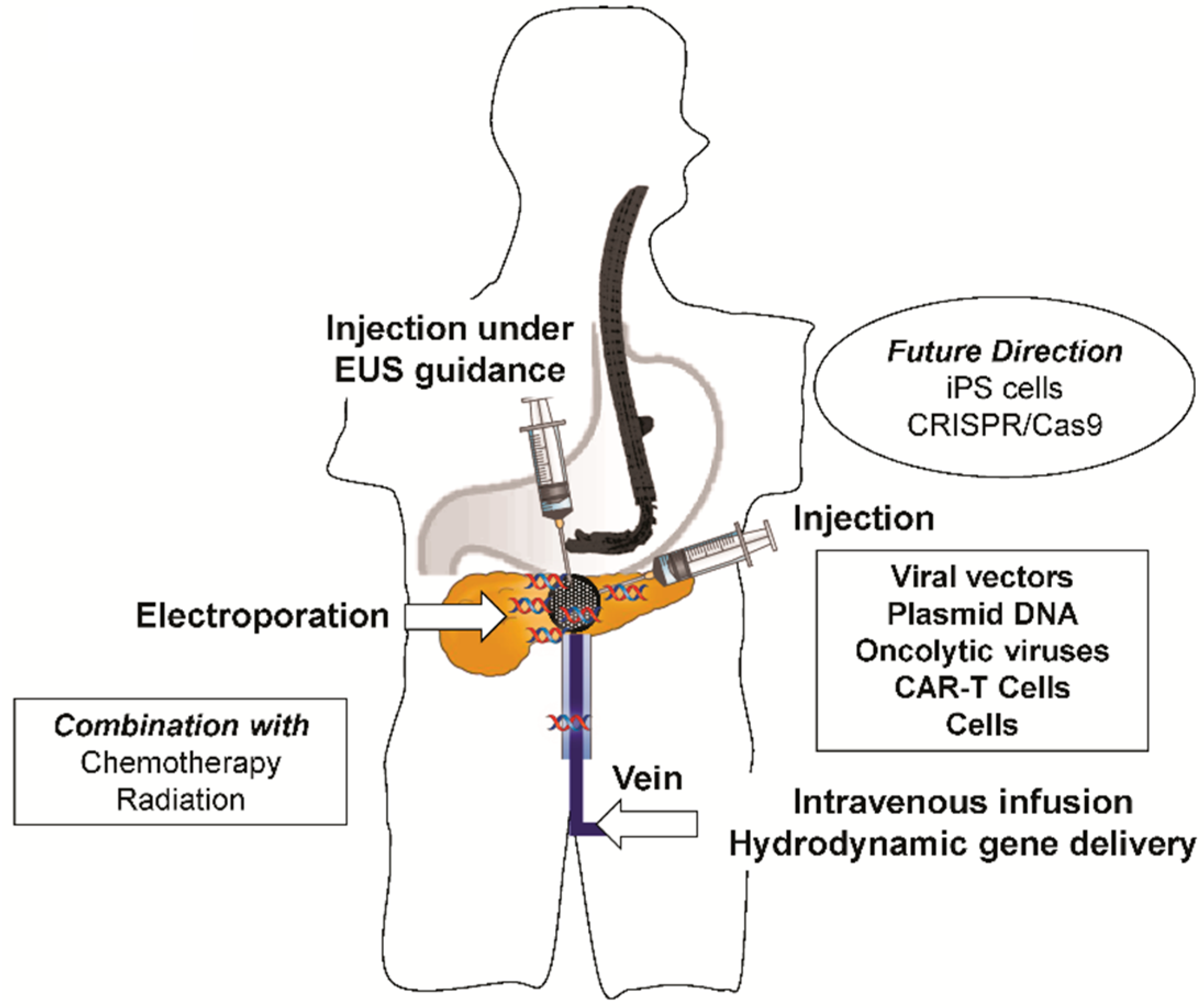
6. Delivery Methods for Pancreatic Gene Therapy
7. Clinical Trials
8. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Susan, S. Gray’s Anatomy, the Anatomical Basis of Clinical Practice London, 41st ed.; Stringer, M.D., Ed.; Churchill Livingstone: London, UK, 2015. [Google Scholar]
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Outani, H.; Akita, H.; Nakai, T.; Takada, R.; Imura, Y.; Tanaka, T.; Tamiya, H.; Oshima, K.; Takahashi, H.; Ohkawa, K.; et al. Clinical features and prognosis of patients With the bone metastasis of pancreatic cancer: A single-institutional cohort study. Pancreas 2018, 47, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.M.; Meier, C.R.; Jick, S.S.; Schneider, C. The potential of glycemic control and body weight change as early markers for pancreatic cancer in patients with long-standing diabetes mellitus: A case-control study. Pancreas 2018, 47, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Danai, L.V.; Babic, A.; Rosenthal, M.H.; Dennstedt, E.A.; Muir, A.; Lien, E.C.; Mayers, J.R.; Tai, K.; Lau, A.N.; Jones-Sali, P.; et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018, 558, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.L.; Rehman, A.A.; Gondi, C.S. Therapeutic options for the management of pancreatic cancer. World J. Gastroenterol. 2014, 20, 11142–11159. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Aldaoud, A.; Wolf, T.; Krammer-Steiner, B.; Linde, H.; Scheiner-Sparna, R.; Hamm, D.; Jänicke, M.; Marschner, N. Results from the prospective German TPK clinical cohort study: Treatment algorithms and survival of 1174 patients with locally advanced, inoperable or metastatic pancreatic ductal adenocarcinoma. Int. J. Cancer 2018. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Bender, R.J.; Halverson, D.; Rahib, L.; Hendifar, A.E.; Mikhail, S.; Chung, V.; Picozzi, V.J.; Sohal, D.; Blais, E.M.; et al. Molecular profiling of pancreatic cancer patients: Initial results from the know your tumor initiative. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, M.; Lebrin, M.; Gross, F.; Bournet, B.; Cordelier, P.; Buscail, L. Gene therapy for pancreatic cancer: Specificity, issues and hopes. Int. J. Mol. Sci. 2017, 18, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Di Magliano, M.P.; Logsdon, C.D. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology 2013, 144, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, X.; Chen, Q.; Liu, T.; Lu, C.; Yu, J.; Miao, Y.; Wei, J. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J. Exp. Clin. Cancer Res. 2018, 37, 130. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.L.; Zhang, J.J.; Chandimali, N.; Ghosh, M.; Gera, M.; Kim, N.; Park, Y.H.; Kwon, T.; Jeong, D.K. SALL4 suppresses reactive oxygen species in pancreatic ductal adenocarcinoma phenotype via FoxM1/Prx III axis. Biochem. Biophys. Res. Commun. 2018, 503, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, H.; Taher, L.; Denz, A.; Grützmann, R.; Pilarsky, C.; Weber, G.F. Identification of Prognostic Biomarkers by Combined mRNA and miRNA Expression microarray Analysis in Pancreatic Cancer. Transl. Oncol. 2018, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Liu, L.; Xu, H.X.; Wu, C.T.; Xiang, J.F.; Xu, J.; Liu, C.; Long, J.; Ni, Q.X.; Yu, X.J. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br. J. Surg. 2016, 103, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Kato, Y.; Mizukami, Y.; Widholz, S.; Boukhali, M.; Revenco, I.; Grossman, E.A.; Ji, F.; Sadreyev, R.I.; Liss, A.S.; et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat. Cell. Biol. 2018, 20, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Gilly, F.N.; Beaujard, A.; Bienvenu, J.; Trillet Lenoir, V.; Glehen, O.; Thouvenot, D.; Malcus, C.; Favrot, M.; Dumontet, C.; Lombard-Bohas, C.; et al. Gene therapy with Adv-IL-2 in unresectable digestive cancer: Phase I-II study, intermediate report. HepatoGastroenterology 1999, 46, 1268–1273. [Google Scholar] [PubMed]
- Mulvihill, S.; Warren, R.; Venook, A.; Adler, A.; Randlev, B.; Heise, C.; Kirn, D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: A phase I trial. Gene Ther. 2001, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Salmons, B.; Löhr, M.; Günzburg, W.H. Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: Results of a phase I/II clinical trial. J. Gastroenterol. 2003, 38, 78–84. [Google Scholar] [PubMed]
- Pecher, G.; Häring, A.; Kaiser, L.; Thiel, E. Mucin gene (MUC1) transfected dendritic cells as vaccine: Results of a phase I/II clinical trial. Cancer Immunol. Immunother. 2002, 51, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bedford, R.; Abbruzzese, J.L.; Lahoti, S.; Reid, T.R.; Soetikno, R.M.; Kirn, D.H.; Freeman, S.M. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin. Cancer Res. 2003, 9, 555–561. [Google Scholar] [PubMed]
- Gordon, E.M.; Cornelio, G.H.; Lorenzo, C.C., 3rd; Levy, J.P.; Reed, R.A.; Liu, L.; Hall, F.L. First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int. J. Oncol. 2004, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Mazzolini, G.; Ruiz, J.; Herraiz, M.; Quiroga, J.; Herrero, I.; Benito, A.; Larrache, J.; Pueyo, J.; Subtil, J.C.; et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. 2004, 22, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Senzer, N.; Mani, S.; Rosemurgy, A.; Nemunaitis, J.; Cunningham, C.; Guha, C.; Bayol, N.; Gillen, M.; Chu, K.; Rasmussen, C.; et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: A phase I study in patients with solid tumors. J. Clin. Oncol. 2004, 22, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, G.; Alfaro, C.; Sangro, B.; Feijoó, E.; Ruiz, J.; Benito, A.; Tirapu, I.; Arina, A.; Sola, J.; Herraiz, M.; et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 2005, 23, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kim-Schulze, S.; Manson, K.; DeRaffele, G.; Mitcham, J.; Seo, K.S.; Kim, D.W.; Marshall, J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J. Transl. Med. 2007, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Laheru, D.; Lutz, E.; Burke, J.; Biedrzycki, B.; Solt, S.; Onners, B.; Tartakovsky, I.; Nemunaitis, J.; Le, D.; Sugar, E.; et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin. Cancer Res. 2008, 14, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Chua, V.S.; Fernandez, L.; Quon, D.; Blackwelder, W.C.; Gordon, E.M.; Hall, F.L. Advanced phase I/II studies of targeted gene delivery in vivo: Intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol. Ther. 2010, 18, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Kasuya, H.; Sahin, T.T.; Nomura, N.; Kanzaki, A.; Misawa, M.; Shirota, T.; Yamada, S.; Fujii, T.; Sugimoto, H.; et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011, 18, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kubuschok, B.; Pfreundschuh, M.; Breit, R.; Hartmann, F.; Sester, M.; Gärtner, B.; König, J.; Murawski, N.; Held, G.; Zwick, C.; et al. Mutated Ras-transfected, EBV-transformed lymphoblastoid cell lines as a model tumor vaccine for boosting T-cell responses against pancreatic cancer: A pilot trial. Hum. Gene Ther. 2012, 23, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Ohana, P.; Konikoff, F.M.; Leichtmann, G.; Hubert, A.; Appelbaum, L.; Kopelman, Y.; Czerniak, A.; Hochberg, A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Farrell, J.J.; Senzer, N.; Nemunaitis, J.; Rosemurgy, A.; Chung, T.; Hanna, N.; Chang, K.J.; Javle, M.; Posner, M.; et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: A phase I/II study. Gastrointest. Endosc. 2012, 75, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A live-attenuated listeria vaccine (ANZ-100) and a live-attenuated listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Hardacre, J.M.; Mulcahy, M.; Small, W.; Talamonti, M.; Obel, J. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J. Gastrointest. Surg. 2013, 17, 94–100; discussion 100–101. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Wild, A.T.; Wang, H.; Tran, P.T.; Chang, K.J.; Taylor, G.E.; Donehower, R.C.; Pawlik, T.M.; Ziegler, M.A.; Cai, H.; et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J. Clin. Oncol. 2013, 31, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.M.; Haas, S.L.; Kröger, J.C.; Friess, H.M.; Höft, R.; Goretzki, P.E.; Peschel, C.; Schweigert, M.; Salmons, B.; Gunzburg, W.H. Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: Data from two clinical trials in pancreatic cancer. Pharmaceutics 2014, 6, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.K.; Shirley, L.A.; Chung, V.M.; Marsh, C.L.; Walker, J.; Coyle, W.; Marx, H.; Bekaii-Saab, T.; Lesinski, G.B.; Swanson, B.; et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol. Immunother. 2015, 64, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Khvalevsky, E.Z.; Hubert, A.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; David, E.B.; Raskin, S.; et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015, 6, 24560–24570. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized Phase 2 trial of the oncolytic virus Pelareorep (Reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Inoko, K.; Hiraoka, K.; Inagaki, A.; Takahashi, M.; Kushibiki, T.; Hontani, K.; Takano, H.; Sato, S.; Takeuchi, S.; Nakamura, T.; et al. Therapeutic activity of retroviral replicating vector-mediated prodrug activator gene therapy for pancreatic cancer. Cancer Gene Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Buscail, L.; Bournet, B.; Vernejoul, F.; Cambois, G.; Lulka, H.; Hanoun, N.; Dufresne, M.; Meulle, A.; Vignolle-Vidoni, A.; Ligat, L.; et al. First-in-man phase 1 clinical trial of gene therapy for advanced pancreatic cancer: Safety, biodistribution, and preliminary clinical findings. Mol. Ther. 2015, 23, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Carlson, S.K.; Foster, N.R.; Lowe, V.; Quevedo, F.; McWilliams, R.R.; Grothey, A.; Jatoi, A.; Alberts, S.R.; Rubin, J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol. Ther. 2008, 16, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Lutz, E.; Yeo, C.J.; Lillemoe, K.D.; Biedrzycki, B.; Kobrin, B.; Herman, J.; Sugar, E.; Piantadosi, S.; Cameron, J.L.; Solt, S.; et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011, 253, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013, 36, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.; Hu, Y.; Morreale, M.; Lerner, L.; Ying Lin, W.; Sen, R.; Cai, Y.; Karunasena, E.; Thakar, M.; Saggi, S.; et al. A novel epigenetic modulating agent sensitizes pancreatic cells to a chemotherapy agent. PLoS ONE 2018, 13, e0199130. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Rabb, M.; Madureira, P.A.; Clements, D.; Gujar, S.A.; Waisman, D.M.; Giacomantonio, C.A.; Lee, P.W. Gemcitabine-mediated tumour regression and p53-dependent gene expression: Implications for colon and pancreatic cancer therapy. Cell Death Dis. 2013, 4, e791. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, H.; Pilarsky, C.; Weber, G.F. The Effect of GPRC5a on the Proliferation, Migration Ability, Chemotherapy Resistance, and Phosphorylation of GSK-3β in Pancreatic Cancer. Int. J. Mol. Sci. 2018, 19, 1870. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef] [PubMed]
- Vassaux, G.; Angelova, A.; Baril, P.; Midoux, P.; Rommelaere, J.; Cordelier, P. The Promise of gene therapy for pancreatic cancer. Hum. Gene Ther. 2016, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tysome, J.R.; Briat, A.; Alusi, G.; Cao, F.; Gao, D.; Yu, J.; Wang, P.; Yang, S.; Dong, Z.; Wang, S.; et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009, 16, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Sicard, F.; Gayral, M.; Lulka, H.; Buscail, L.; Cordelier, P. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. 2013, 21, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Lossie, S.L.; Kasik, E.P.; Channon, A.M.; Ni, S.; Kennedy, M.A. A mouse model study of toxicity and biodistribution of a replication defective adenovirus serotype 5 virus with its genome engineered to contain a decoy hyper binding site to sequester and suppress oncogenic HMGA1 as a new cancer treatment therapy. PLoS ONE 2018, 13, e0192882. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Maeng, K.; Nawab, A.; Francois, R.A.; Bray, J.K.; Reinhard, M.K.; Boye, S.L.; Hauswirth, W.W.; Kaye, F.J.; Aslanidi, G.; et al. Efficient gene delivery and expression in pancreas and pancreatic tumors by capsid-optimized AAV8 vectors. Hum. Gene Ther. Methods 2017, 28, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Benihoud, K.; Vigant, F.; Schmidt, C.Q.; Wortmann, A.; Bachem, M.G.; Simmet, T.; Kochanek, S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS ONE 2015, 10, e0117254. [Google Scholar] [CrossRef] [PubMed]
- Gayral, M.; Lulka, H.; Hanoun, N.; Biollay, C.; Sèlves, J.; Vignolle-Vidoni, A.; Berthommé, H.; Trempat, P.; Epstein, A.L.; Buscail, L.; et al. Targeted oncolytic herpes simplex virus type 1 eradicates experimental pancreatic tumors. Hum. Gene Ther. 2015, 26, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Rejiba, S.; Bigand, C.; Parmentier, C.; Masmoudi, A.; Hajri, A. Oncosuppressive suicide gene virotherapy “PVH1-yCD/5-FC” for pancreatic peritoneal carcinomatosis treatment: NF-κB and Akt/PI3K involvement. PLoS ONE 2013, 8, e70594. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.L.; Tabebordbar, M.; Cheng, J.K.; Mali, P.; Wu, E.Y.; Ng, A.H.; Zhu, K.; Wagers, A.J.; Church, G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods 2016, 13, 868–874. [Google Scholar] [CrossRef]
- Chiou, S.H.; Winters, I.P.; Wang, J.; Naranjo, S.; Dudgeon, C.; Tamburini, F.B.; Brady, J.J.; Yang, D.; Grüner, B.M.; Chuang, C.H.; et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev. 2015, 29, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.D.; Pillarisetty, V.G. T-cell programming in pancreatic adenocarcinoma: A review. Cancer Gene Ther. 2017, 24, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Sukumaran, S.; Bajgain, P.; Watanabe, N.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; Fisher, W.E.; Leen, A.M.; Vera, J.F. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol. Ther. 2017, 25, 249–258. [Google Scholar] [CrossRef] [PubMed]
- DeSelm, C.J.; Tano, Z.E.; Varghese, A.M.; Adusumilli, P.S. CAR T-cell therapy for pancreatic cancer. J. Surg. Oncol. 2017, 116, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gonska, T. Genetic predisposition in pancreatitis. Curr. Opin. Pediatr. 2018, 30, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Stroes, E.S.; Méthot, J.; Brisson, D.; Tremblay, K.; Bernelot Moens, S.J.; Iotti, G.; Rastelletti, I.; Ardigo, D.; Corzo, D.; et al. Long-term retrospective analysis of gene therapy with alipogene tiparvovec and its effect on lipoprotein lipase deficiency-induced pancreatitis. Hum. Gene Ther. 2016, 27, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, R.; Reddy, D.N. Pain in chronic pancreatitis: Managing beyond the pancreatic duct. World J. Gastroenterol. 2013, 19, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, Y.; Noë, M.; Li, Q.; Pasricha, P.J. Neuronal transforming growth factor beta signaling via SMAD3 contributes to pain in animal models of chronic pancreatitis. Gastroenterology 2018, 154, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; McNearney, T.A.; Lin, W.; Wilson, S.P.; Yeomans, D.C.; Westlund, K.N. Treatment of inflamed pancreas with enkephalin encoding HSV-1 recombinant vector reduces inflammatory damage and behavioral sequelae. Mol. Ther. 2007, 15, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; McNearney, T.A.; Chu, R.; Lu, Y.; Ren, Y.; Yeomans, D.C.; Wilson, S.P.; Westlund, K.N. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol. Pain 2008, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, H.; Hatipoglu, N.; Gul, U.; Tatli, Z.U.; Ellard, S.; Flanagan, S.E.; De Franco, E.; Kurtoglu, S. Permanent neonatal diabetes mellitus and neurological abnormalities due to a novel homozygous missense mutation in NEUROD1. Pediatr. Diabetes 2018, 19, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Sollinger, H.W.; Alam, T. Insulin gene therapy for type 1 diabetes mellitus. Exp. Clin. Transplant. 2015, 13, 37–45. [Google Scholar] [PubMed]
- Sponton, C.H.; Kajimura, S. AAV-mediated gene therapy as a strategy to fight obesity and metabolic diseases. EMBO Mol. Med. 2018, 10, e9431. [Google Scholar] [CrossRef] [PubMed]
- Tasyurek, M.H.; Altunbas, H.A.; Canatan, H.; Griffith, T.S.; Sanlioglu, S. GLP-1-mediated gene therapy approaches for diabetes treatment. Expert Rev. Mol. Med. 2014, 16, e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dorrell, C.; Naugler, W.E.; Heskett, M.; Spellman, P.; Li, B.; Galivo, F.; Haft, A.; Wakefield, L.; Grompe, M. Long-term correction of diabetes in mice by in vivo reprogramming of pancreatic ducts. Mol. Ther. 2018, 26, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Hao, H.; Liu, J. Induction of hepatocytes-derived insulin-producing cells using small molecules and identification of microRNA profiles during this procedure. Biochem. Biophys. Res. Commun. 2018, 498, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Zickri, M.B.; Aboul-Fotouh, G.I.; Omar, A.I.; El-Shafei, A.A.; Reda, A.M. Effect of stem cells and gene transfected stem cells therapy on the pancreas of experimentally induced Type 1 diabetes. Int. J. Stem Cells 2018. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Jambrina, C.; Casana, E.; Sacristan, V.; Muñoz, S.; Darriba, S.; Rodó, J.; Mallol, C.; Garcia, M.; León, X.; et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Suda, T.; Zhang, G.; Liu, D. Advances in gene delivery systems. Pharmaceut. Med. 2011, 25, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kamimura, K.; Kobayashi, Y.; Abe, H.; Yokoo, T.; Sakai, N.; Nagoya, T.; Sakamaki, A.; Abe, S.; Hayashi, K.; et al. Efficacy and safety of pancreas-targeted hydrodynamic gene delivery in rats. Mol. Ther. Nucleic Acids 2017, 9, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.F.; Kowalski, P.S.; Doloff, J.C.; Tsosie, J.K.; Bakthavatchalu, V.; Winn, C.B.; Haupt, J.; Jamiel, M.; Langer, R.; Anderson, D.G. Endothelial siRNA delivery in nonhuman primates using ionizable low-molecular weight polymeric nanoparticles. Sci Adv. 2018, 4, eaar8409. [Google Scholar] [CrossRef] [PubMed]
- Gorgulu, K.; Diakopoulos, K.N.; Ai, J.; Schoeps, B.; Kabacaoglu, D.; Karpathaki, AF.; Ciecielski, K.J.; Kaya-Aksoy, E.; Ruess, D.A.; Berninger, A.; et al. Levels of the autophagy related 5 protein affect progression and metastasis of pancreatic tumors in mice. Gastroenterology 2018. [Google Scholar] [CrossRef] [PubMed]
| No. | Ref. | Conditions | Title | Carrier | Interventions | Phases | Enrollment |
|---|---|---|---|---|---|---|---|
| 1 | [19] | Pancreatic Cancer | Gene therapy with Adv-IL-2 in unresectable digestive cancer: phase I-II study, intermediate report. | Adenovirus | AdV/Interleukin 2 | Phase 1/Phase 2 | 7 |
| 2 | [20] | Pancreatic Cancer | Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. | Adenovirus | AdV/ONYX-015 | Phase 1 | 23 |
| 3 | [21] | Pancreatic Cancer | Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: results of a phase I/II clinical trial. | Lipofectamine (Plasmid DNA) | Lipofectamine/Cyto. P450 | Phase 1/Phase 2 | 14 |
| 4 | [22] | Pancreatic Cancer | Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. | Cationic liposome (Plasmid DNA) | Cationic liposome/dendritic cells transfected with MUC1 cDNA | Phase 1/Phase 2 | 10 |
| 5 | [23] | Pancreatic Cancer | A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. | Adenovirus | AdV/ONYX-015 + gemcitabine | Phase 1/Phase 2 | 21 |
| 6 | [24] | Pancreatic Cancer | First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. | Retrovirus | Rv/Rexin-G | Phase 1 | 3 |
| 7 | [25] | Pancreatic Cancer | Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. | Adenovirus | AdV/Interleukin 12 | Phase 1 | 7 |
| 8 | [26] | Pancreatic Cancer | TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. | Adenovirus | TNFerade | Phase 1 | 30 |
| 9 | [27] | Pancreatic Cancer | Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. | Adenovirus | Adv encoding interleukin-12 gene | Phase 1 | 11 |
| 10 | [28] | Pancreatic Cancer | Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. | Vaccinia and Pox virus | Vaccinia and Pox virus expressing CEA MUC-1 and co-stimulatory molecules | Phase 1 | 10 |
| 11 | [29] | Pancreatic Cancer | Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. | Plasmid DNA | Plasmid/GVAX | Phase 1 | 50 |
| 12 | [30] | Pancreatic Cancer | Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. | Retrovirus | Rv/Rexin-G at two dosages | Phase 1/Phase 2 | 9 |
| 13 | [31] | Pancreatic Cancer | A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. | Herpes virus | HF10 oncolytic herpes virus | Phase 1 | 6 |
| 14 | [32] | Pancreatic Cancer | Mutated Ras-transfected, EBV-transformed lymphoblastoid cell lines as a model tumor vaccine for boosting T-cell responses against pancreatic cancer: a pilot trial. | Immunotherapy | Lymphocytes modified with an episomal EBV expressing Ras mutant | Phase 1 | 7 |
| 15 | [33] | Pancreatic Cancer | Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. | Plasmid DNA | Plasmid/expression of diphtheria-toxin gene | Phase 1 | 9 |
| 16 | [34] | Pancreatic Cancer | EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. | Adenovirus | AdV/TNFerade with chemoradiation | Phase 1 | 50 |
| 17 | [35] | Pancreatic Cancer | A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. | Cancer vaccine | Attenuated listeria vaccine | Phase 1 | 26 |
| 18 | [36] | Pancreatic Cancer | Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. | Immunotherapy | Algenpantucel-L + gemcitabine + 5FU | Phase 2 | 70 |
| 19 | [37] | Pancreatic Cancer | Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. | Adenovirus | AdV/TNFerade + Chemoradiation Vs chemoradiation | Phase 3 | 304 |
| 20 | [38] | Pancreatic Cancer | Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: data from two clinical trials in pancreatic cancer. | Lipofectamine (Plasmid DNA) | Lipofectamine/Cyto. P450 | Phase 2 | 13 |
| 21 | [39] | Pancreatic Cancer | Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. | Adenovirus | AdV/HSV thymidine kinase | Phase 1 | 24 |
| 22 | [40] | Pancreatic Cancer | RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. | RNAi | SiG12-LODER® + gemcitabine | Phase 1/Phase 2 | 15 |
| 23 | [41] | Pancreatic Cancer | Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. | Cancer vaccine | GVAX + CRS 2017 | Phase 2 | 90 |
| 24 | [42] | Pancreatic Cancer | Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. | Reovirus | Reolysin + paclitaxel + carboplatin | Phase 2 | 73 |
| 25 | [43] | Pancreatic Cancer | AdV/Theragene + Chemotherapy | Adenovirus | AdV/Theragene + Chemotherapy | Phase 1 | 9 |
| No. | Ref. | NCT Number | Conditions | Title | Carrier | Phases | Enrollment | Study Start | Study Completion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT00415454 | Pancreatic Cancer | Study Combining Suicide Gene Therapy with Chemoradiotherapy in the Treatment of Non-Metastatic Pancreatic Adenocarcinoma | Adenovirus | Phase 1 | 8 | November 2006 | N/A | |
| 2 | [44] | NCT01274455 | Pancreatic Cancer | Gene Therapy of Pancreatic Ductal Adenocarcinoma | Plasmid DNA | Phase 1 | 22 | December 2010 | March 2013 |
| 3 | NCT02806687 | Pancreatic Cancer | Effect of Intratumoral Injection of Gene Therapy for Locally Advanced Pancreatic Cancer | Plasmid DNA | Phase 2 | 100 | January 2017 | June 2019 | |
| 4 | NCT02894944 | Pancreatic Cancer | Clinical Trial Phase I for Theragene in Combination with Chemotherapy for the Locally Advanced Pancreatic Cancer | Adenovirus | Phase 1 | 9 | August 2016 | July 2018 | |
| 5 | [45] | NCT00121745 | Pancreatic Cancer | Evaluation of Safety of Rexin-G Gene Transfer for Advanced Pancreatic Cancer | Retrovirus | Phase 1 | 12 | July 2005 | July 2007 |
| 6 | NCT03165188 | Pancreatic Cancer | Long Term Follow-Up Study for Subjects Previously Treated With Algenpantucel-L (HyperAcute-Pancreas) Immunotherapy | Immunotherapy | Not Applicable | 500 | September 2017 | June 2031 | |
| 7 | NCT01583686 | Pancreatic Cancer | CAR T Cell Receptor Immunotherapy Targeting Mesothelin for Patients With Metastatic Cancer | CAR-T | Phase 1/Phase 2 | 136 | April 2012 | December 2028 | |
| 8 | NCT02830724 | Pancreatic Cancer | Administering Peripheral Blood Lymphocytes Transduced With a CD70-Binding Chimeric Antigen Receptor to People With CD71 Expressing Cancers | CAR-T | Phase 1/Phase 2 | 113 | April 2017 | January 2028 | |
| 9 | NCT00638612 | Pancreatic Cancer | AdV-tk Therapy With Surgery and Chemoradiation for Pancreas Cancer (PaTK02) | Adenovirus | Phase 1 | 27 | August 2008 | June 2015 | |
| 10 | NCT02465983 | Pancreatic Cancer | Pilot Study of Autologous T-cells in Patients With Metastatic Pancreatic Cancer | CAR-T | Phase 1 | 4 | May 2015 | November 2017 | |
| 11 | NCT03190941 | Pancreatic Cancer | Administering Peripheral Blood Lymphocytes Transduced With a Murine T-Cell Receptor Recognizing the G12V Variant of Mutated RAS in HLA-A*1102 Patients | Immunotherapy | Phase 1/Phase 2 | 110 | September 2017 | June 2028 | |
| 12 | NCT03225989 | Pancreatic Cancer | Trial Investigating an Immunostimulatory Oncolytic Adenovirus for Cancer | Adenovirus | Phase 1/Phase 2 | 50 | March 2018 | December 2022 | |
| 13 | NCT03192462 | Pancreatic Cancer | TAA Specific Cytotoxic T Lymphocytes in Patients With Pancreatic Cancer | Immunotherapy | Phase 1/Phase 2 | 45 | January 2018 | November 2025 | |
| 14 | NCT00004178 | Pancreatic Cancer | Gene Therapy in Treating Patients With Cancer | Immunotherapy | Phase 1 | null | April 1998 | December 2001 | |
| 15 | [46] | NCT00084383 | Pancreatic Cancer | Vaccine Therapy Combined With Adjuvant Chemoradiotherapy in Treating Patients With Resected Stage I or Stage II Adenocarcinoma (Cancer) of the Pancreas | Cancer vaccine | Phase 2 | 60 | January 2002 | July 2006 |
| 16 | [47] | NCT00836407 | Pancreatic Cancer | Ipilimumab +/- Vaccine Therapy in Treating Patients With Locally Advanced, Unresectable or Metastatic Pancreatic Cancer | Cancer vaccine | Phase 1 | 30 | February 2009 | July 2012 |
| 17 | NCT00305760 | Pancreatic Cancer | Vaccine Therapy, Cyclophosphamide, and Cetuximab in Treating Patients With Metastatic or Locally Advanced Pancreatic Cancer | Cancer vaccine | Phase 2 | 60 | December 2005 | N/A | |
| 18 | NCT02750657 | Pancreatic Cancer | Study of Changes and Characteristics of Genes in Patients With Pancreatic Cancer for Better Treatment Selection | Genetic Profiling | Not Applicable | 180 | December 2015 | December 2021 | |
| 19 | NCT00303927 | Pancreatic Cancer | Capecitabine as Second-Line Therapy in Treating Patients With Stage IV Pancreatic Cancer Who Have the Thymidylate Synthase Gene | Genetic Profiling | Phase 2 | 65 | December 2005 | N/A | |
| 20 | NCT01188109 | Pancreatic Cancer | Gemcitabine/Cisplatin for Resected Pancreas Cancer: Establishing the Role of ERCC2 in Treatment Decision | Genetic Profiling | Phase 2 | 25 | July 2010 | July 2015 | |
| 21 | NCT00389610 | Pancreatic Cancer | Vaccine Therapy in Treating Patients With Pancreatic Cancer That Has Been Removed by Surgery | Cancer vaccine | Phase 2 | 56 | September 2006 | December 2018 | |
| 22 | NCT01394120 | Pancreatic Cancer | Chemotherapy Selection Based on Therapeutic Targets for Advanced Pancreatic Cancer | For Targeted and Tailored Treatment | Phase 2 | 60 | August 2011 | December 2013 | |
| 23 | NCT00066404 | Pancreatic Cancer | Intrapleural BG00002 in Treating Patients With Malignant Pleural Mesothelioma or Malignant Pleural Effusions | Recombinant adenovirus | Phase 1 | null | April 2003 | N/A | |
| 24 | NCT01474564 | Pancreatic Cancer | Feasibility of Obtaining and Characterizing Circulating Tumorigenic Cells in Patients With Pancreatic Adenocarcinoma | Genetic Profiling | Not Applicable | 60 | November 2011 | November 2019 | |
| 25 | NCT02405585 | Pancreatic Cancer | Immunotherapy and SBRT Study in Borderline Resectable Pancreatic Cancer | Immunotherapy | Phase 2 | 10 | April 2015 | N/A | |
| 26 | NCT02705196 | Pancreatic Cancer | LOAd704 Oncolytic Virus Therapy for Pancreatic Cancer | Adenovirus | Phase 1/Phase 2 | 26 | November 2016 | August 2019 | |
| 27 | NCT00669734 | Pancreatic Cancer | Vaccine Therapy and Sargramostim in Treating Patients With Pancreas Cancer That Cannot Be Removed By Surgery | Cancer vaccine | Phase 1 | 18 | February 2010 | N/A | |
| 28 | NCT00727441 | Pancreatic Cancer | Vaccine Therapy With or Without Cyclophosphamide in Treating Patients Undergoing Chemotherapy and Radiation Therapy for Stage I or Stage II Pancreatic Cancer That Can Be Removed by Surgery | Cancer vaccine | Not Applicable | 87 | July 2008 | March 2018 | |
| 29 | NCT00947102 | Pancreatic Cancer | Influence of Gemcitabine Treatment on Immunological and Serological Profile in Patients With Pancreatic Cancer | Observation | Not Applicable | null | February 2009 | December 2011 | |
| 30 | NCT00051467 | Pancreatic Cancer | A Study of TNFerade Biologic With 5-FU and Radiation Therapy for First-Line Treatment of Unresectable Locally Advanced Pancreatic Cancer | Adenovirus | Phase 3 | null | N/A | N/A | |
| 31 | NCT03531125 | Pancreatic Cancer | Gene Expression in Resectable Pancreatic Cancer | Procedure of Endoscopic Ultrasound | Not Applicable | 10 | June 2018 | December 2019 | |
| 32 | NCT00429858 | Pancreatic Cancer | Gemcitabine and S-2 for Locally Advanced Unresectable or Metastatic Pancreatic Cancer | Chemotherapy | Phase 2 | 21 | January 2007 | October 2010 | |
| 33 | NCT02568267 | Pancreatic Cancer | Basket Study of Entrectinib (RXDX-101) for the Treatment of Patients With Solid Tumors Harboring NTRK 1/2/3 (Trk A/B/C), ROS2, or ALK Gene Rearrangements (Fusions) | Genetic Profiling | Phase 2 | 300 | November 2015 | October 2020 | |
| 34 | NCT00159471 | Pancreatic Cancer | Genes as Predictors of Response to Gemcitabine, Docetaxel, and Capecitabine (GTX) in Metastatic or Unresectable Pancreatic Cancer. | Genetic Profiling | Not Applicable | 1 | February 2005 | July 2006 | |
| 35 | NCT00386399 | Pancreatic Cancer | Study of Mitomycin-C in Patients With Advanced or Recurrent Pancreatic Cancer With Mutated BRCA3 Gene | Genetic Profiling | Phase 2 | 0 | October 2006 | February 2008 | |
| 36 | NCT01836432 | Pancreatic Cancer | Immunotherapy Study in Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer | Immunotherapy | Phase 3 | 302 | May 2013 | June 2017 | |
| 37 | NCT00255827 | Pancreatic Cancer | Vaccine Treatment for Surgically Resected Pancreatic Cancer | Cancer vaccine | Phase 1/Phase 2 | 7 | November 2005 | September 2007 | |
| 38 | NCT01938716 | Pancreatic Cancer | Gemcitabine Pharmacokinetics After Preoperative Chemoradiation Therapy | Chemotherapy | Not Applicable | 40 | March 2012 | March 2019 | |
| 39 | NCT03193190 | Pancreatic Cancer | A Study of Multiple Immunotherapy-Based Treatment Combinations in Participants With Metastatic Pancreatic Ductal Adenocarcinoma (Morpheus-Pancreatic Cancer) | Immunotherapy | Phase 1/Phase 2 | 185 | July 2017 | September 2020 | |
| 40 | NCT00089024 | Pancreatic Cancer | Combination Chemotherapy, and Radiation Therapy in Treating Patients With Locally Advanced Pancreatic Cancer | Chemotherapy | Phase 2 | 50 | February 2004 | N/A | |
| 41 | NCT02465060 | Pancreatic Cancer | Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial) | Genetic Profiling | Phase 2 | 6452 | August 2015 | N/A | |
| 42 | NCT01191684 | Pancreatic Cancer | Vaccine Therapy in Treating Patients With Colorectal, Stomach, or Pancreatic Cancer | Cancer vaccine | Phase 1 | 12 | October 2011 | August 2013 | |
| 43 | NCT01088789 | Pancreatic Cancer | A Trial of Boost Vaccinations of Pancreatic Tumor Cell Vaccine | Plasmid DNA | Phase 2 | 72 | April 2010 | April 2023 | |
| 44 | NCT02514421 | Pancreatic Cancer | Evaluation of Safety and Efficacy of Electrochemotherapy in the Treatment of Pancreatic Adenocarcinoma | Device of Electroporation | Not Applicable | 24 | July 2015 | July 2017 | |
| 45 | NCT02414100 | Pancreatic Cancer | Patient Derived Cancer Cell Lines in Identifying Molecular Changes in Patients With Previously Untreated Pancreatic Cancer Receiving Gemcitabine Hydrochloride-Based Chemotherapy | Genetic Profiling | Not Applicable | 0 | December 2013 | December 2016 | |
| 46 | NCT00936104 | Pancreatic Cancer | Side Population in Pancreatic Ductal Adenocarcinoma (PDAC) | Not Applicable | 20 | August 2008 | July 2012 | ||
| 47 | NCT03302637 | Pancreatic Cancer | Oral Microbiome and Pancreatic Cancer | Genetic Profiling | Not Applicable | 732 | December 1992 | December 2010 | |
| 48 | NCT03602079 | Pancreatic Cancer | Study of A166 in Patients With Relapsed/Refractory Cancers Expressing HER2 Antigen or Having Amplified HER3 Gene | Genetic Profiling | Phase 1/Phase 2 | 82 | July 2018 | May 2021 | |
| 49 | NCT03337087 | Pancreatic Cancer | Liposomal Irinotecan, Fluorouracil, Leucovorin Calcium, and Rucaparib in Treating Patients With Metastatic Pancreatic, Colorectal, Gastroesophageal, or Biliary Cancer | Chemotherapy | Phase 1/Phase 2 | 110 | August 2018 | December 2022 | |
| 50 | NCT00128622 | Pancreatic Cancer | Denileukin Diftitox Followed by Vaccine Therapy in Treating Patients With Metastatic Cancer | Cancer vaccine | Phase 1 | 24 | September 2005 | May 2009 | |
| 51 | NCT02592395 | Pancreatic Cancer | Study of FOLFIRINOX Electrochemotherapy in the Treatment of Pancreatic Adenocarcinoma | Device of Electroporation | Phase 1 | 0 | October 2015 | October 2017 | |
| 52 | NCT02432963 | Pancreatic Cancer | Vaccine Therapy and Pembrolizumab in Treating Patients With Solid Tumors That Have Failed Prior Therapy | Cancer vaccine | Phase 1 | 19 | November 2015 | February 2019 | |
| 53 | NCT00959946 | Pancreatic Cancer | Study Of Bosutinib With Capecitabine In Solid Tumors And Locally Advanced Or Metastatic Breast Cancer | Chemotherapy | Phase 1/Phase 2 | 32 | September 2009 | March 2011 | |
| 54 | NCT01643499 | Pancreatic Cancer | Genotype-guided Dosing of mFOLFIRINOX Chemotherapy in Patients With Previously Untreated Advanced Gastrointestinal Malignancies | Genetic Profiling | Phase 1 | 79 | March 2012 | August 2020 | |
| 55 | NCT02576665 | Pancreatic Cancer | A Study of Toca 511, a Retroviral Replicating Vector, Combined With Toca FC in Patients With Solid Tumors or Lymphoma (Toca 7) | Retrovirus | Phase 1 | 30 | July 2016 | November 2019 | |
| 56 | NCT00711997 | Pancreatic Cancer | Phase 1/2a DTA-H19 in Patients With Unresectable Pancreatic Cancer | Plasmid DNA | Phase 1/Phase 2 | 9 | August 2009 | December 2010 | |
| 57 | NCT02239861 | Pancreatic Cancer | TAA-Specific CTLS for Solid Tumors (TACTASOM) | Immunotherapy | Phase 1 | 16 | April 2015 | December 2018 | |
| 58 | NCT03281382 | Metastatic Pancreatic Cancer | Phase 1 Trial of Interleukin 13 Gene Therapy for Metastatic Pancreatic Cancer | Adenovirus | Phase 1 | 9 | July 2017 | June 2021 | |
| 59 | NCT02340117 | Metastatic Pancreatic Cancer | Study of Combined SGT-54 Plus Gemcitabine/Nab-Paclitaxel for Metastatic Pancreatic Cancer | Immunotherapy | Phase 2 | 28 | January 2015 | December 2020 | |
| 60 | NCT00868114 | Metastatic Pancreatic Cancer | Direct Tumor Injection KLH-Pulsed Dendritic Cells in Unresectable Pancreatic Cancer | Cell | Phase 2 | 35 | July 2006 | December 2015 | |
| 61 | NCT01437007 | Metastatic Pancreatic Cancer | TKM 080302 for Primary or Secondary Liver Cancer | RNAi | Phase 1 | 1 | August 2011 | June 2012 | |
| 62 | NCT02416466 | Metastatic Pancreatic Cancer | CAR-T Hepatic Artery Infusions and Sir-Spheres for Liver Metastases | CAR-T | Phase 1 | 8 | April 2015 | January 2019 | |
| 63 | NCT01116791 | Peritoneal Carcinomatosis | Cytoreductive Surgery (CRS) Plus Hyperthermic Intraoperative Peritoneal Chemotherapy(HIPC) With Cisplatin to Treat Peritoneal Carcinomatosis From Upper Gastrointestinal Cancer | Procedure of Cytoreductive Surgery plus Hyperthermic Intraoperative Peritoneal Chemotherapy | Phase 2 | 34 | July 2010 | December 2015 | |
| 64 | NCT02315625 | Neuroendocrine Tumors of the Pancreas | Study of Mutation-Targeted Therapy With Sunitinib or Everolimus in People With Advanced Low- or Intermediate-Grade Neuroendocrine Tumors of the Gastrointestinal Tract and Pancreas With or Without Cytoreductive Surgery | Genetic Profiling | Phase 2 | 120 | April 2015 | December 2025 | |
| 65 | NCT00444444 | Pancreatitis | Genetic Analysis for Predicting of Relapse During Steroid Treatment for Autoimmune Pancreatitis (AIP) | 40 | February 2002 | June 2007 |
| Gene Transfer Methods | Functional Component | Targeted Genes | Features | Features |
|---|---|---|---|---|
| Viral Vectors | Tumor suppressor genes, Pro-apoptotic genes, Suicide genes, siRNA, miRNA, | |||
| Oncoretrovirus | RNA | High efficiency | Random integration, low titer | |
| Lentivirus | RNA | High efficiency, sustained gene expression | Random integration, low titer | |
| Foamy virus | RNA | High efficiency, sustained gene expression | Random integration, low titer | |
| Adenovirus | Double stranded DNA | High efficiency, sustained gene expression, infect non-dividing cells | Host innate immune response | |
| Adeno-associated virus | Single stranded DNA | No pathogenic, sustained gene expression, infect to non-dividing cells | Integration may occur, small capacity of transgene, low titer | |
| Herpes simplex virus | Double stranded DNA | No integration, sustained gene expression | Low transduction efficiency | |
| Non-viral Vectors (Chemicals) | ||||
| Lipids | Cationic lipids | High efficiency in vitro, ease to prepare | Low efficiency in vivo, acute immune response | |
| Polymers | Cationic polymers | Highly effective in vitro, ease to prepare | Toxic to cells, acute immune response | |
| Proteins | Natural or chemically modified proteins in cationic nature | Highly effective in vitro, less toxic, can be target specific | Low activity in vivo | |
| Peptides | Lysine or arginine residues in peptides | Highly effective in vitro, less toxic, can be target specific | Low activity in vivo | |
| Non-viral Vectors (Physical Methods) | ||||
| Needle injection | Mechanic force | Simple | Low efficiency, expression limited to needle track | |
| Gene gun | Pressure | Good efficiency | Limited to target area, need surgical procedure for internal organ | |
| Electroporation | Electric pulse | High efficiency | Tissue damage, limited target area, need surgical procedure for internal organ | |
| Sonoporation | Ultrasound | Site specific | Low efficiency, tissue damage | |
| Magnetofection | Magnetic field | Site specific | Low efficiency, limited target area, need surgical procedure for internal organ | |
| Hydrodynamic delivery | Hydrodynamic pressure | Simple, high efficiency, site specific | Need catheter insertion technique in large animals | |
| Immunotherapy | Cytokines | Require ex vivo cell culture | ||
| Adoptive Immunotherapy | CAR-T | Require ex vivo cell culture | ||
| Vaccination | Antigen-pulsed dendritic cells | Intravenous or subcutaneous or local administration |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimura, K.; Yokoo, T.; Terai, S. Gene Therapy for Pancreatic Diseases: Current Status. Int. J. Mol. Sci. 2018, 19, 3415. https://doi.org/10.3390/ijms19113415
Kamimura K, Yokoo T, Terai S. Gene Therapy for Pancreatic Diseases: Current Status. International Journal of Molecular Sciences. 2018; 19(11):3415. https://doi.org/10.3390/ijms19113415
Chicago/Turabian StyleKamimura, Kenya, Takeshi Yokoo, and Shuji Terai. 2018. "Gene Therapy for Pancreatic Diseases: Current Status" International Journal of Molecular Sciences 19, no. 11: 3415. https://doi.org/10.3390/ijms19113415
APA StyleKamimura, K., Yokoo, T., & Terai, S. (2018). Gene Therapy for Pancreatic Diseases: Current Status. International Journal of Molecular Sciences, 19(11), 3415. https://doi.org/10.3390/ijms19113415





