Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study
Abstract
:1. Introduction
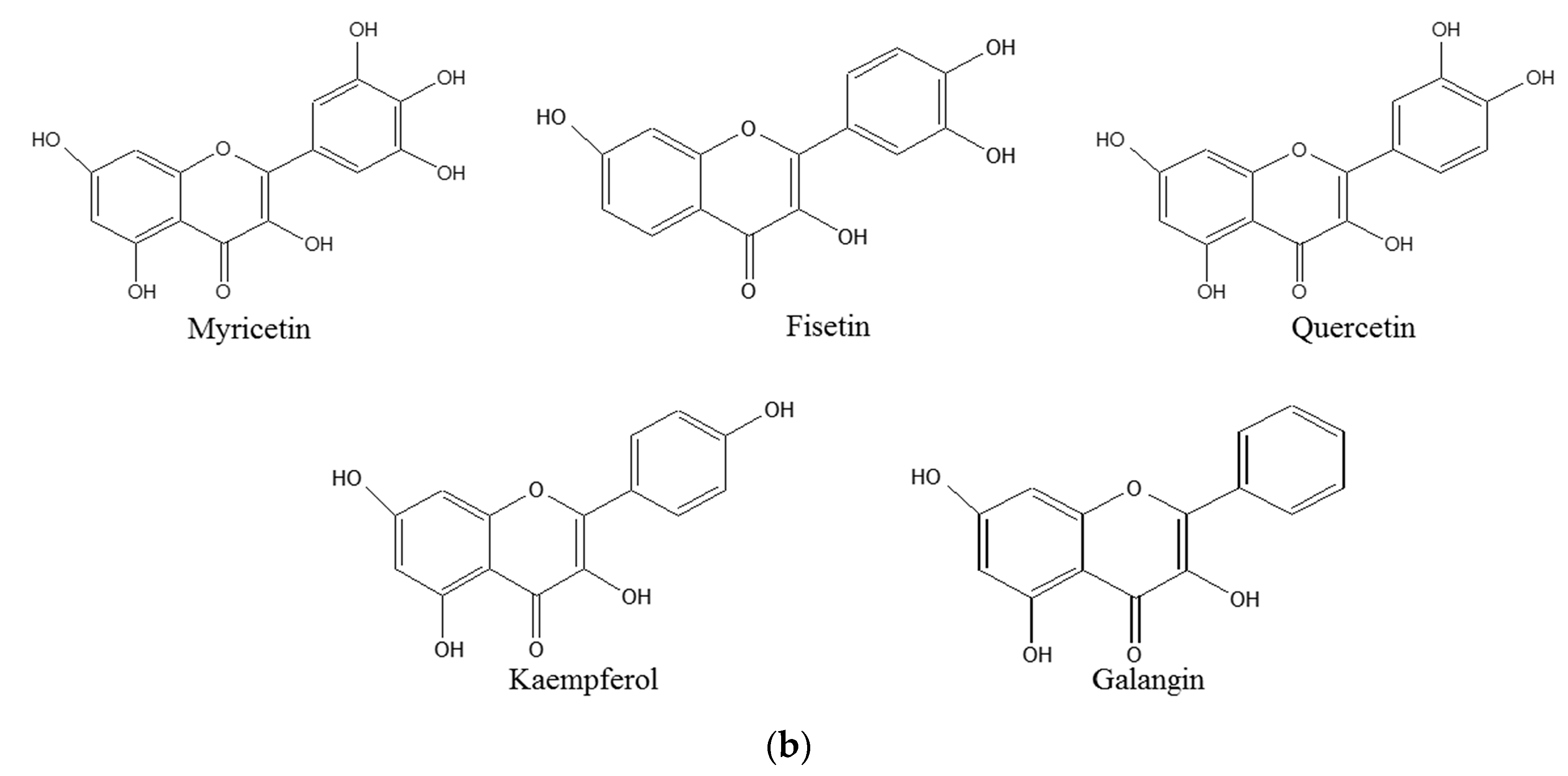
2. Results and Discussion
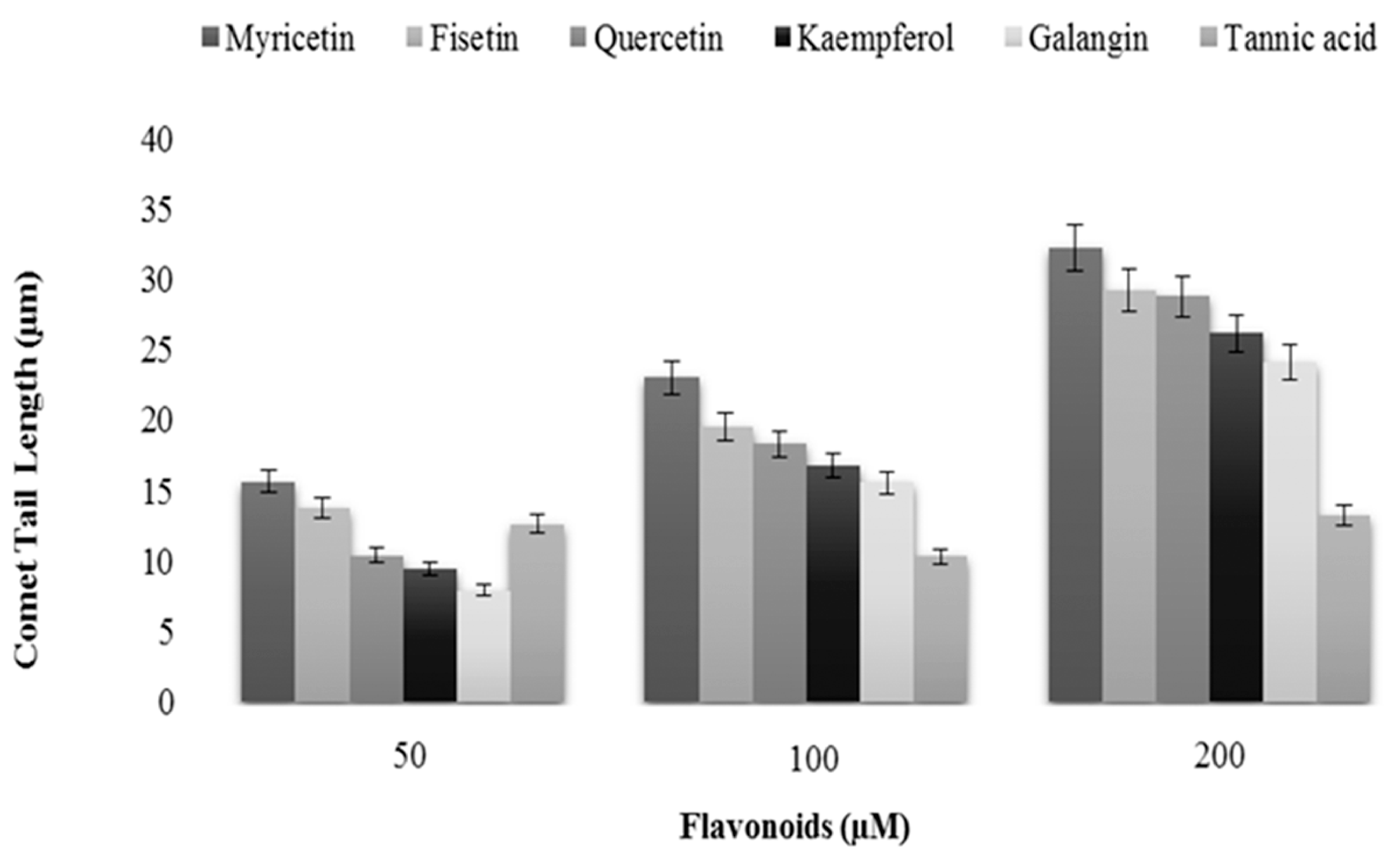
2.1. Cellular DNA Breakage by Flavonoids in Intact and Permeabilized Lymphocytes as Measured by Comet Assay
| Flavonoids (100 µM) | Comet Tail Length (µM) | |
|---|---|---|
| Intact Cells | Permeabilized Cells | |
| Control (No Flavonoid) | 3.95 ± 0.19 | 4.06 ± 0.20 |
| Myricetin | 25.06 ± 1.25 | 28.90 ± 1.44 |
| Fisetin | 20.45 ± 1.02 | 25.03 ± 1.25 |
| Quercetin | 17.74 ± 0.88 | 23.50 ± 1.17 |
| Kaempferol | 16.03 ± 0.80 | 20.66 ± 1.03 |
| Galangin | 14.85 ± 0.74 | 19.44 ± 0.97 |
2.2. Formation of Complexes of Flavonoids
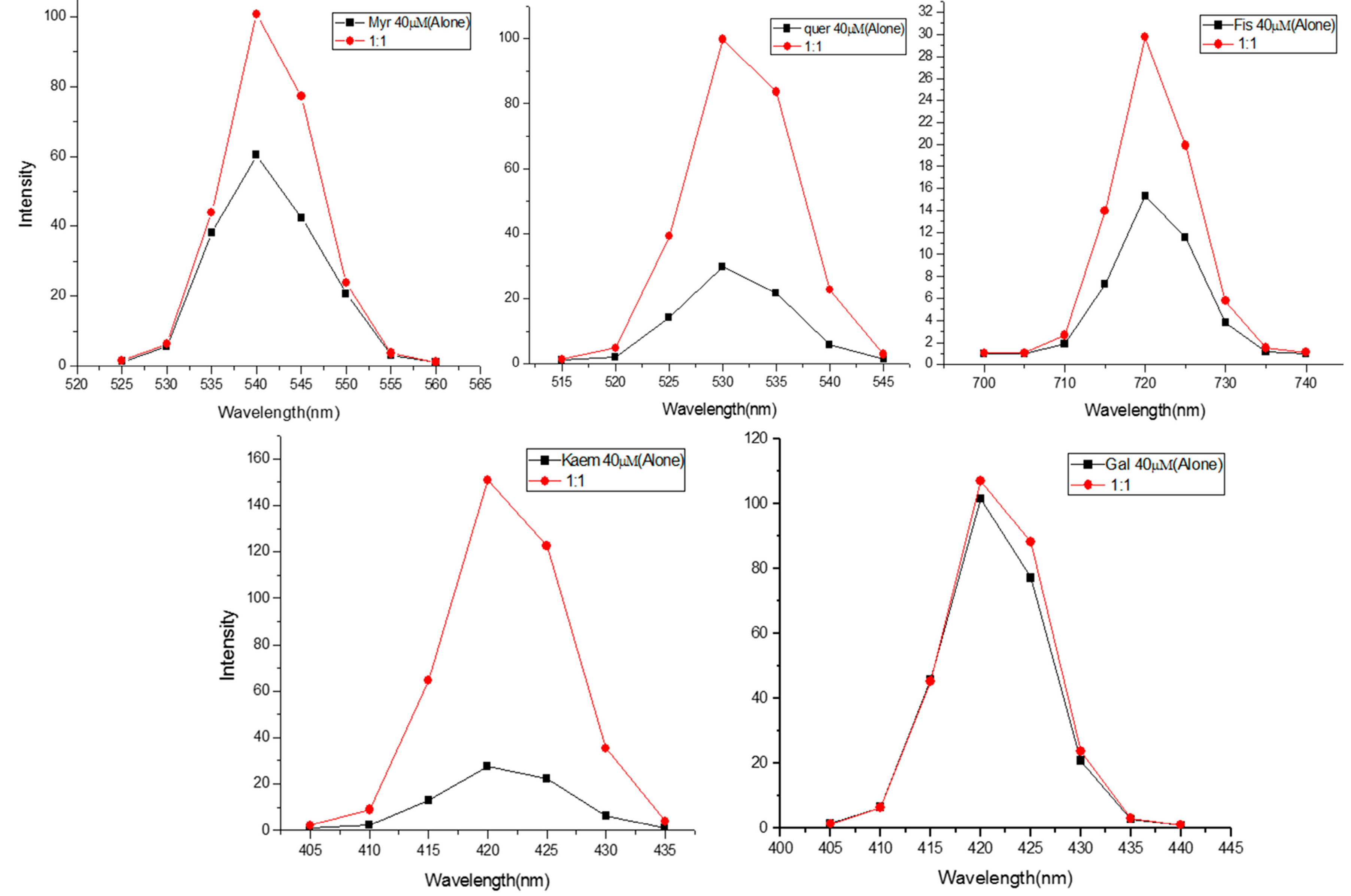
2.3. Effect of Reactive Oxygen Scavengers on the Flavonoids-Induced DNA Breakage in Permeabilized Lymphocytes
| Treatment | Intact Cells | Permeabilized Cells | ||
|---|---|---|---|---|
| Comet Tail Length % | Inhibition (µM) | Comet Tail Length % | Inhibition (µM) | |
| Control | 3.156 ± 0.17 | - | 3.25 ± 0.16 | - |
| Myricetin (100 µM) | 25.03 ± 1.25 | - | 31.56 ± 1.57 | - |
| +SOD (100 µg/mL) | 11.26 ± 0.56 * | 55.0 | 13.28 ± 0.76 * | 57.9 |
| +Catalase (100 µg/mL) | 12.46 ± 0.62 * | 50.2 | 15.05 ± 0.80 * | 52.3 |
| +Thiourea (1 mM) | 14.08 ± 0.70 * | 43.7 | 16.86 ± 0.84 * | 46.5 |
| Fisetin (100 µM) | 22.96 ± 1.14 | - | 27.04 ± 1.35 | - |
| +SOD (100 µg/mL) | 12.20 ± 0.61 * | 46.8 | 14.24 ± 0.71 * | 47.3 |
| +Catalase (100 µg/mL) | 12.76 ± 0.63 * | 44.4 | 14.64 ± 0.73 * | 45.8 |
| +Thiourea (1 mM) | 13.04 ± 0.65 * | 43.2 | 15.05 ± 0.75 * | 44.3 |
| Quercetin (100 µM) | 20.32 ± 1.01 | - | 24.54 ± 1.22 | - |
| +SOD (100 µg/mL) | 10.46 ± 0.52 * | 48.5 | 11.23 ± 0.56 * | 54.2 |
| +Catalase (100 µg/mL) | 11.06 ± 0.55 * | 45.5 | 13.42 ± 0.67 * | 45.3 |
| +Thiourea (1 mM) | 14.80 ± 0.74 * | 27.1 | 14.95 ± 0.74 * | 39.07 |
| Kaempferol (100 µM) | 17.63 ± 0.88 | - | 21.54 ± 1.07 | - |
| +SOD (100 µg/mL) | 9.50 ± 0.47 * | 46.1 | 10.36 ± 0.51 * | 51.9 |
| +Catalase (100 µg/mL) | 10.44 ± 0.52* | 40.7 | 10.45 ± 0.52 * | 51.4 |
| +Thiourea (1 mM) | 11.05 ± 0.55 * | 37.3 | 12.45 ± 0.62 * | 42.2 |
| Galangin (100 µM) | 14.40 ± 0.72 | - | 18.44 ± 0.92 | - |
| +SOD (100 µg/mL) | 8.55 ± 0.32 * | 40.6 | 9.65 ± 0.63 * | 47.6 |
| +Catalase (100 µg/mL) | 9.40 ± 0.37 * | 34.7 | 11.32 ± 0.59 * | 38.6 |
| +Thiourea (1 mM) | 10.15 ± 0.50* | 29.5 | 10.55 ± 0.53 * | 42.7 |
2.4. Effect of Specific Chelators of Metals on Flavonoid-Induced DNA Breakage in Intact and Permeabilized Lymphocytes
| Dose | Whole Lymphocytes | Permeabilized Lymphocytes | ||
|---|---|---|---|---|
| Comet Tail Length % | of Control (µM) | Comet Tail Length % | of Control (µM) | |
| Myricetin (200 µM) | 31.24 ± 1.56 * | - | 37.90 ± 1.89 ** | - |
| +Neocuproine (200 µM) | 21.06 ± 1.12 | 32.5 | 23.04 ± 1.15 | 39.2 |
| +Bathocuproine (200 µM) | 29.86 ± 1.50 | 4.41 | 22.50 ± 1.12 | 40.6 |
| +Histidine (200 µM) | 30.63 ± 1.55 | 1.95 | 37.73 ± 1.88 | 0.44 |
| +Desferioxaminemesylate (200 µM) | 30.04 ± 1.53 | 3.84 | 37.15 ± 1.85 | 1.97 |
| Fisetin (200 µM) | 29.80 ± 1.49 * | - | 36.04 ± 1.80 ** | - |
| +Neocuproine (200 µM) | 20.37 ± 0.91 | 31.6 | 23.54 ± 1.17 | 34.68 |
| +Bathocuproine (200 µM) | 28.84 ± 1.39 | 3.22 | 22.33 ± 1.11 | 38.04 |
| +Histidine (200 µM) | 29.40 ± 1.47 | 1.34 | 36.00 ± 1.80 | 0.11 |
| +Desferioxaminemesylate (200 µM) | 29.11 ± 1.45 | 2.31 | 35.38 ± 1.76 | 1.83 |
| Quercetin (200 µM) | 26.33 ± 1.40 * | - | 33.34 ± 1.66 ** | - |
| +Neocuproine (200 µM) | 18.54 ± 0.86 | 29.5 | 21.96 ± 1.09 | 34.13 |
| +Bathocuproine (200 µM) | 25.69 ± 1.31 | 2.43 | 21.43 ± 1.07 | 35.72 |
| +Histidine (200 µM) | 26.04 ± 1.35 | 1.10 | 33.30 ± 1.66 | 0.11 |
| +Desferioxaminemesylate (200 µM) | 26.00 ± 1.34 | 1.25 | 32.89 ± 1.64 | 1.34 |
| Kaempferol (200 µM) | 21.76 ± 1.18 * | - | 30.23 ± 1.51 ** | - |
| +Neocuproine (200 µM) | 16.53 ± 0.72 | 24.03 | 20.44 ± 1.02 | 32.38 |
| +Bathocuproine (200 µM) | 21.28 ± 1.15 | 2.20 | 19.70 ± 0.98 | 34.83 |
| +Histidine (200 µM) | 21.68 ± 1.17 | 0.36 | 30.19 ± 1.50 | 0.13 |
| +Desferioxaminemesylate (200 µM) | 21.52 ± 1.17 | 1.10 | 30.07 ± 1.50 | 0.52 |
2.5. Stoichiometry of Cu(II) Reduction by Flavonoids
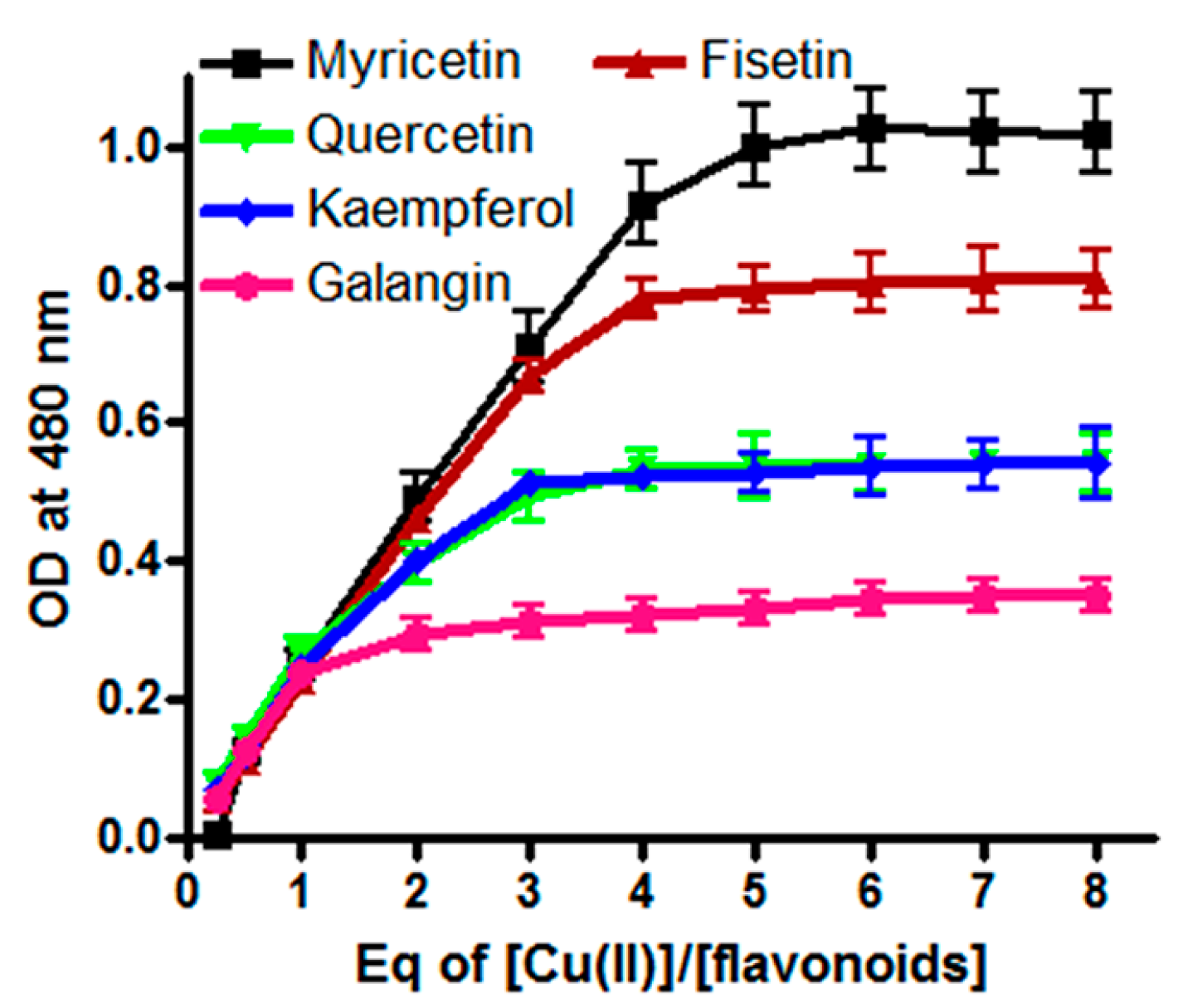
2.6. Intracellular Generation of H2O2/ROS by Flavonoids
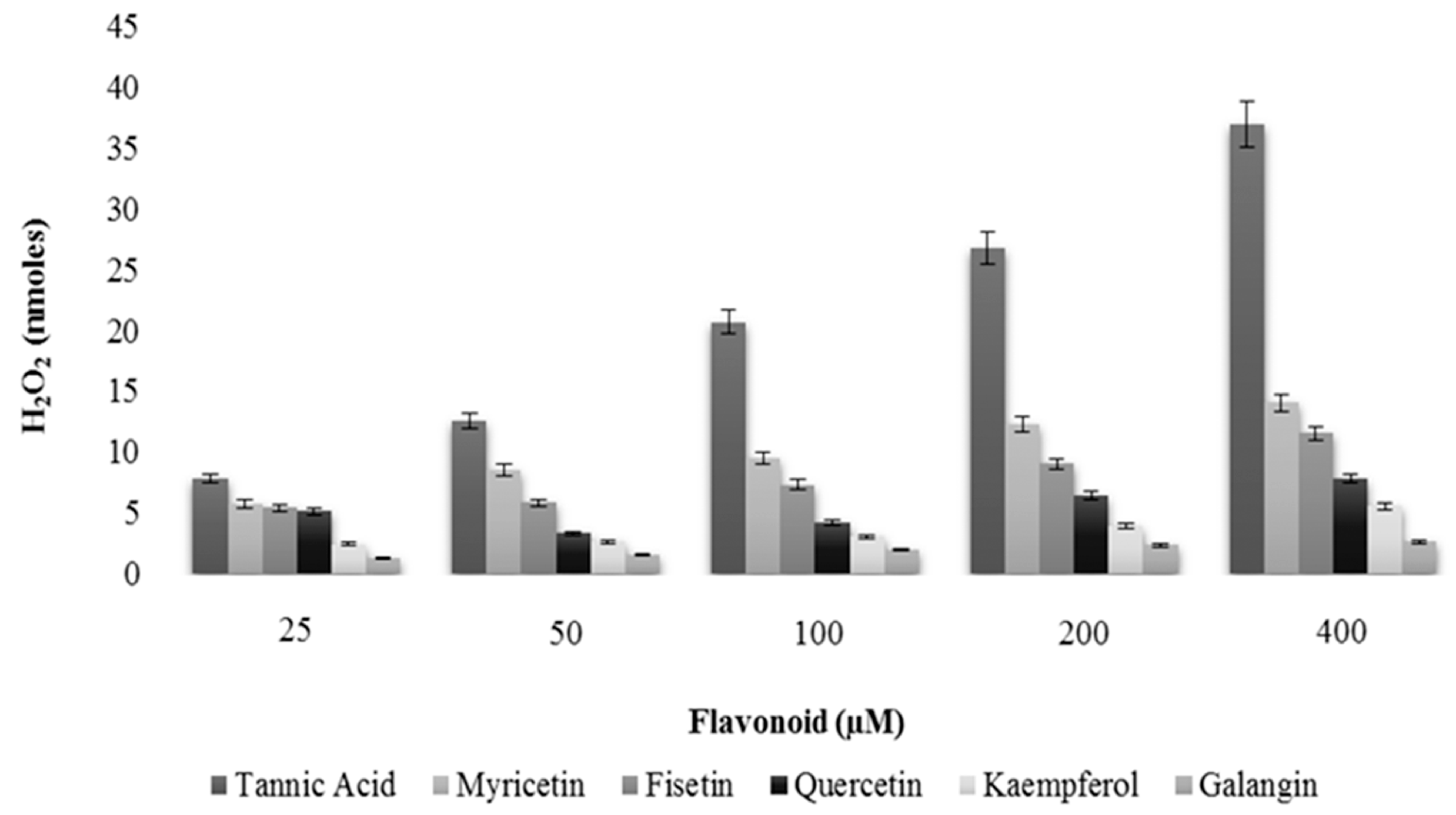
2.7. Thermodynamics of Flavonoid–ctDNA Binding-Iso Thermal Calorimetric Studies (ITC)
| Flavonoid | Ka( M−1) | n | ∆H (kJ/mol) | ∆S (J/mol/k) | ∆G (kJ/mol) |
|---|---|---|---|---|---|
| Myricetin | 1.9 × 102 | 74.62 | −49.21 | −64.43 | −30.01 |
| Fisetin | 0.9 × 102 | 66.50 | −43.01 | −58.18 | −25.67 |
| Quercetin | 0.8 × 102 | 63.32 | −39.81 | −49.76 | −24.98 |
| Kaempferol | 0.6 × 102 | 51.43 | −31.76 | −28.89 | −23.15 |
| Galangin | 0.3 × 102 | 44.67 | −22.25 | −7.11 | −20.13 |
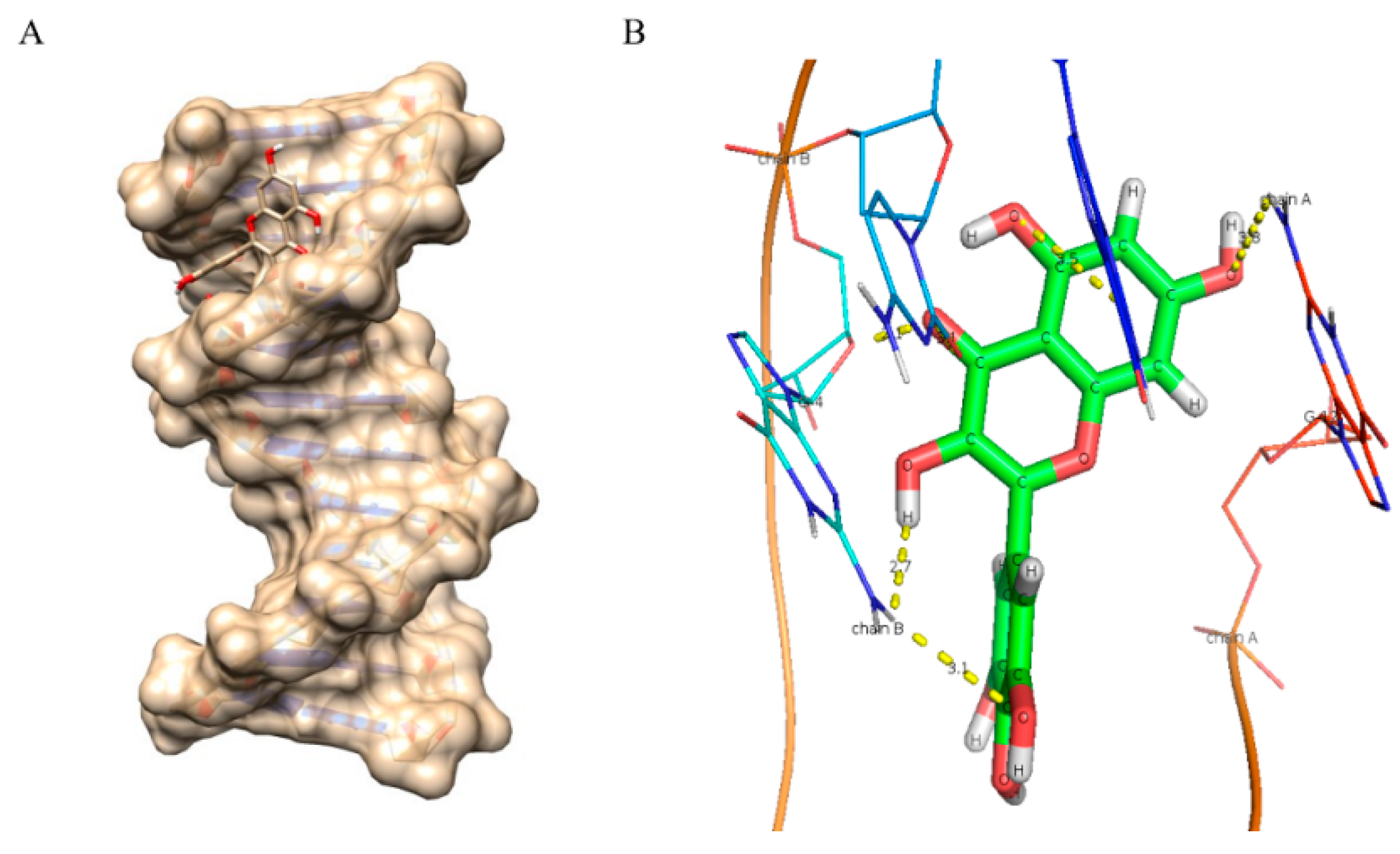
2.8. Molecular Docking Studies
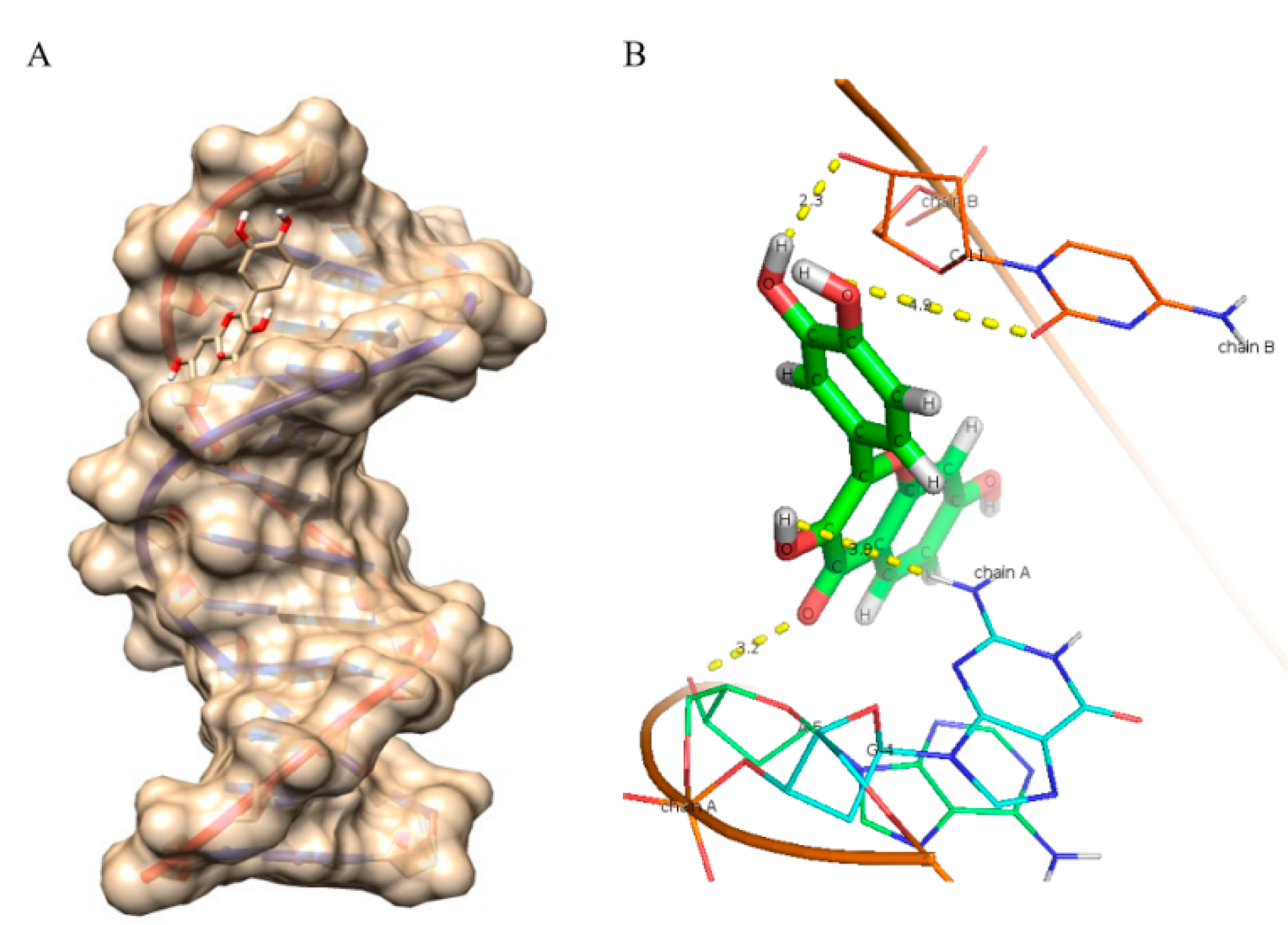
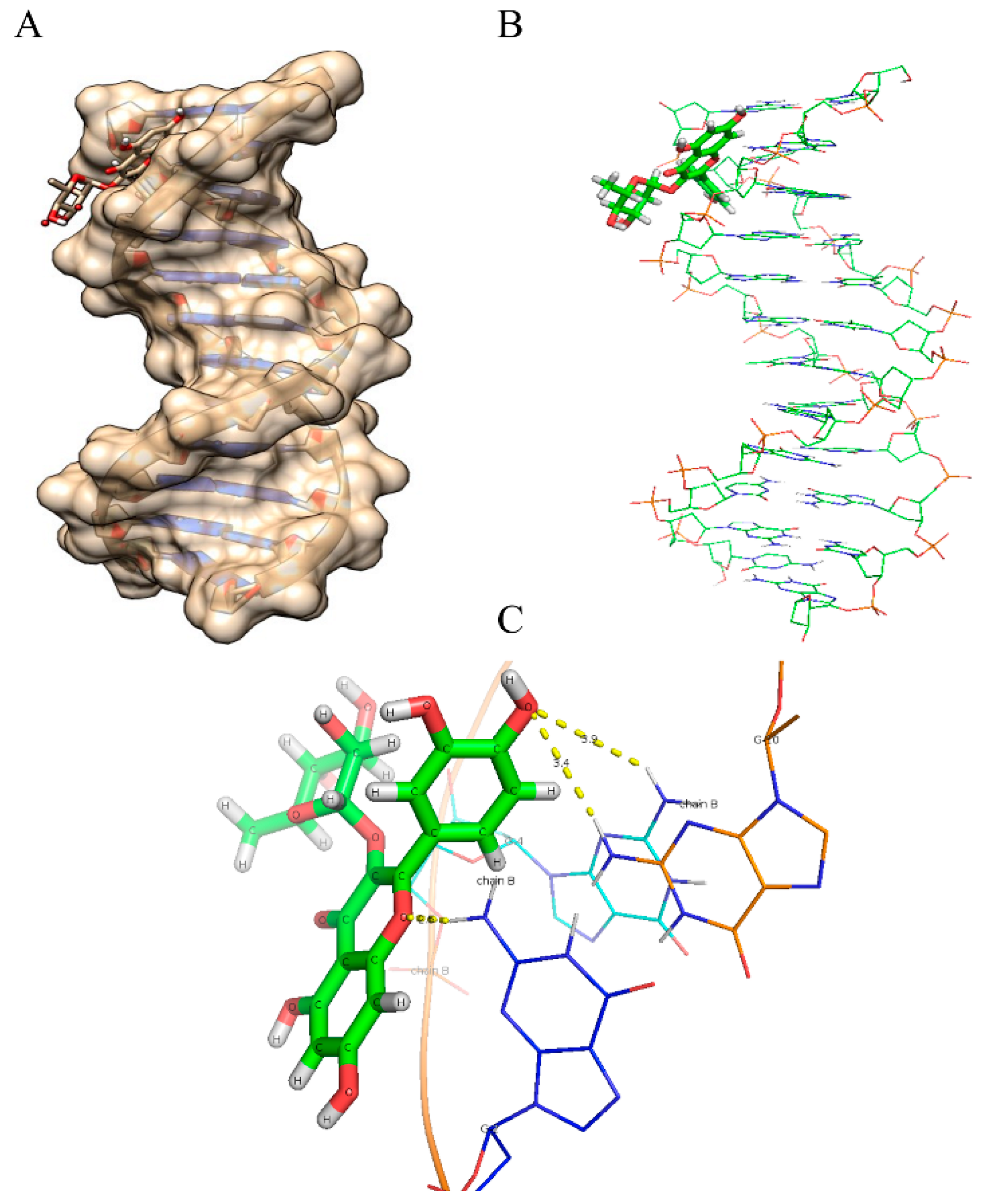
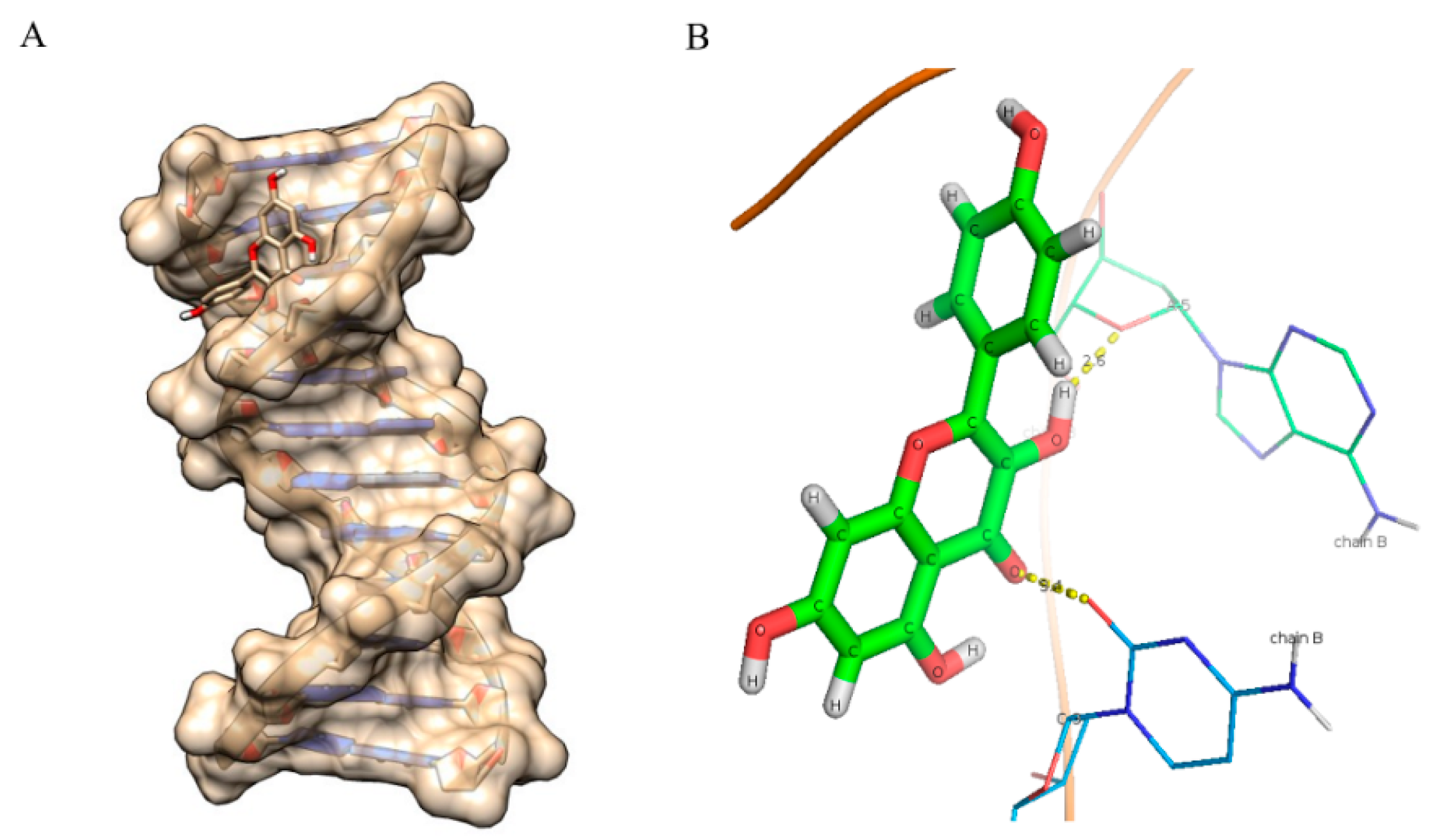
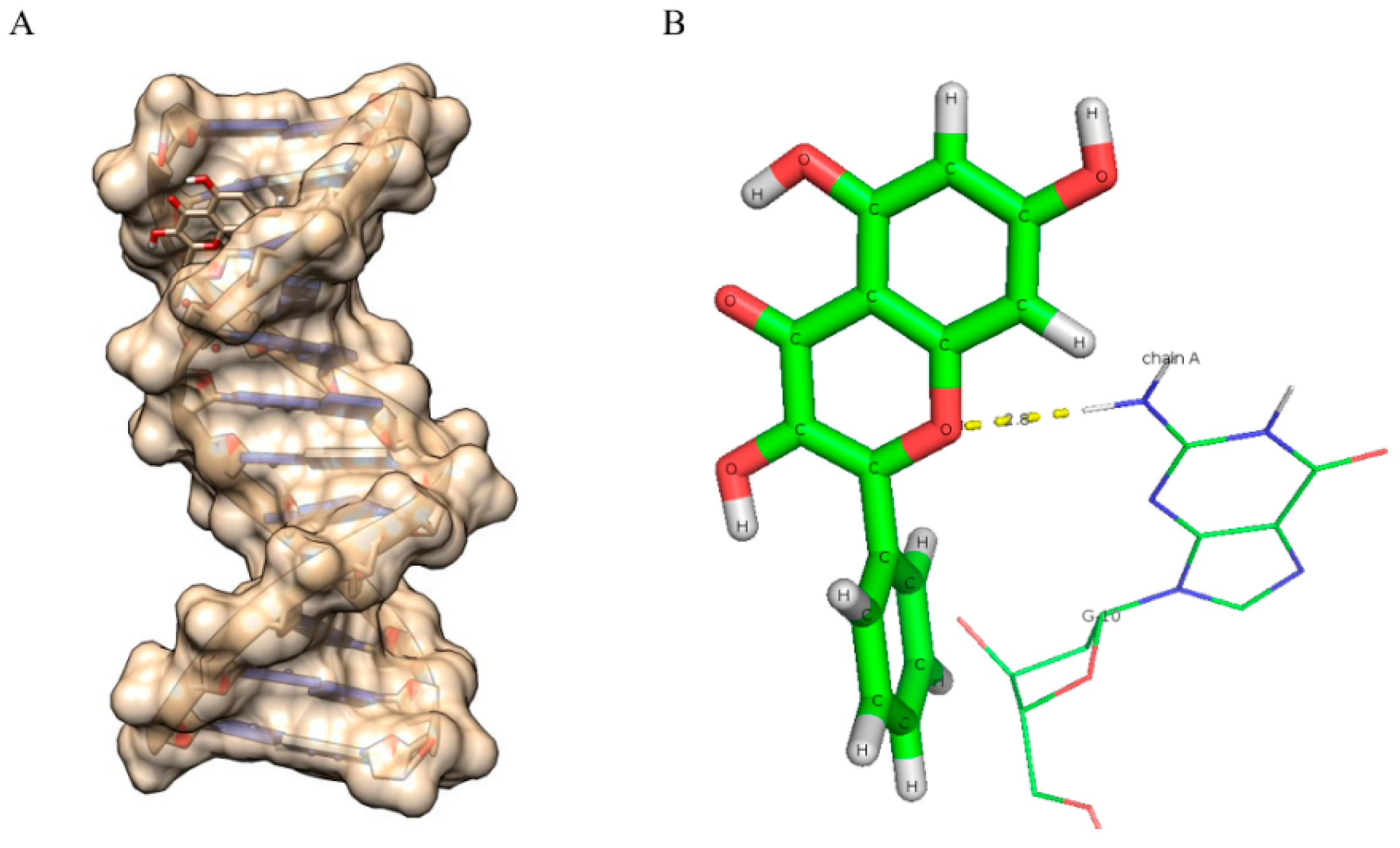
| Compounds | Binding Energy (kcal/mol) | Number of Hydrogen Bonds |
|---|---|---|
| Myricetin | −6.13 | 6 |
| Fisetin | −5.56 | 4 |
| Quercetin | −5.55 | 3 |
| Kaempferol | −5.24 | 2 |
| Galangin | −5.01 | 1 |
3. Experimental Section
3.1. Isolation of Lymphocytes
3.2. Viability Assessment of Lymphocytes
3.3. Lymphocytes Treatment and Assay for DNA Breakage Using Alkaline Single-Cell Gel Electrophoresis (Comet Assay)
3.4. Fluorescence Studies
3.5. Stoichiometric Titration of Cu(I) Production
3.6. Detection of H2O2 in the Incubation Medium by FOX Assay
3.7. Isothermal Titration Calorimetric Measurements (ITC)
3.8. Molecular Docking Studies
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, A.E.; Zeisel, S.H.; Groopman, J. Diet and carcinogenesis. Carcinogenesis 1993, 14, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Shamim, U.; Hanif, S.; Azmi, A.S.; Hadi, S.M. Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol. Nutr. Food Res. 2009, 53, 1376–1385. [Google Scholar] [PubMed]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via bcl-2 and bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [PubMed]
- Shamim, U.; Hanif, S.; Ullah, M.F.; Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Plant polyphenols mobilize nuclear copper in human peripheral lymphocytes leading to oxidatively generated DNA breakage: Implications for an anticancer mechanism. Free Radic. Res. 2008, 42, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Wattel, A.; Kamel, S.; Mentaverri, R.; Lorget, F.; Prouillet, C.; Petit, J.P.; Fardelonne, P.; Brazier, M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem. Pharmacol. 2003, 65, 35–42. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF κB and AP-1. J. Cell. Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, J.K.; Nirmala, P.; Praveen Kumar, B.A.; Kumar, A.P. Evaluation of protective effect of myricetin, a bioflavonoid in dimethyl benzanthracene-induced breast cancer in female wistar rats. South. Asian J. Cancer 2014, 3, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Ahmad, M.S.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bhat, S.H.; Hanif, S.; Hadi, S.M. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 2006, 580, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Khan, H.Y.; Zubair, H.; Sarkar, F.H.; Hadi, S.M. The prooxidant action of dietary antioxidants leading to cellular DNA breakage and anticancer effects: Implications for chemotherapeutic action against cancer. Cell Biochem. Biophys. 2013, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.F.; Geierstanger, B.H.; Wang, A.H.; Ho, P.S. Covalent modification of guanine bases in double-stranded DNA. The 1.2-a z-DNA structure of d(CGCGCG) in the presence of CuCl2. J. Biol. Chem. 1991, 266, 20175–20184. [Google Scholar] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Alrawaiq, N.S.; Abdullah, A. A review of flavonoid quercetin: Metabolism, bioactivity and antioxidant properties. Int. J. Pharm. Tech. Res. 2014, 6, 9. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [PubMed]
- Bryan, S.E. Metal Ions in Biological Systems; Marcel Dekker: New York, NY, USA, 1979. [Google Scholar]
- Burkitt, M.J.; Milne, L.; Nicotera, P.; Orrenius, S. 1,10-Phenanthroline stimulates internucleosomal DNA fragmentation in isolated rat-liver nuclei by promoting the redox activity of endogenous copper ions. Biochem. J. 1996, 313, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Swanson, S. The status of zinc, copper, and metallothionein in cancer patients. Prog. Clin. Biol. Res. 1988, 259, 161–175. [Google Scholar] [PubMed]
- Margalioth, E.J.; Udassin, R.; Cohen, C.; Maor, J.; Anteby, S.O.; Schenker, J.G. Serum copper level in gynecologic malignancies. Am. J. Obstet. Gynecol. 1987, 157, 93–96. [Google Scholar] [CrossRef]
- Yoshida, D.; Ikeda, Y.; Nakazawa, S. Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors. J. Neuro Oncol. 1993, 16, 109–115. [Google Scholar] [CrossRef]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A.; et al. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef]
- Leist, M.; Jaattela, M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Farhan Asad, S.; Singh, S.; Hadi, S.M. DNA breakage by resveratrol and Cu(II): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000, 154, 29–37. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Khan, H.Y.; Sohail, A.; Azim, S.; Ullah, M.F.; Ahmad, A.; Sarkar, F.H.; Hadi, S.M. Redox cycling of endogenous copper by thymoquinone leads to ros-mediated DNA breakage and consequent cell death: Putative anticancer mechanism of antioxidants. Cell Death Dis. 2013, 4, e660. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Khan, H.Y.; Ullah, M.F.; Ahmad, A.; Wu, D.; Hadi, S.M. Apogossypolone, derivative of gossypol, mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage. Eur. J. Pharm. Sci. 2012, 47, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophs. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Hanif, S.; Shamim, U.; Ullah, M.F.; Azmi, A.S.; Bhat, S.H.; Hadi, S.M. The anthocyanidin delphinidin mobilizes endogenous copper ions from human lymphocytes leading to oxidative degradation of cellular DNA. Toxicology 2008, 249, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cemeli, E.; Schmid, T.E.; Anderson, D. Modulation by flavonoids of DNA damage induced by estrogen-like compounds. Environ. Mol. Mutagen. 2004, 44, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Badwey, J.A.; Karnovsky, M.L. Active oxygen species and the functions of phagocytic leukocytes. Annu. Rev. Biochem. 1980, 49, 695–726. [Google Scholar] [CrossRef] [PubMed]
- Czene, S.; Tiback, M.; Harms-Ringdahl, M. pH-Dependent DNA cleavage in permeabilized human fibroblasts. Biochem. J. 1997, 323, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophs. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef]
- Bhat, R.; Hadi, S.M. DNA breakage by tannic acid and Cu(II): Sequence specificity of the reaction and involvement of active oxygen species. Mutat. Res. 1994, 313, 39–48. [Google Scholar] [CrossRef]
- Rahman, A.; Shahabuddin; Hadi, S.M.; Parish, J.H.; Ainley, K. Strand scission in DNA induced by quercetin and Cu(II): Role of Cu(I) and oxygen free radicals. Carcinogenesis 1989, 10, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, M. Ion permeability of the nuclear envelope. Physiological 1998, 13, 44–50. [Google Scholar]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [PubMed]
- Li, Y.; Sun, S.; Chang, Q.; Zhang, L.; Wang, G.; Chen, W.; Miao, X.; Zheng, Y. A strategy for the improvement of the bioavailability and antiosteoporosis activity of bcs iv flavonoid glycosides through the formulation of their lipophilic aglycone into nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L.; Guigas, C.; Klein, R.; Neudecker, C.; Renner, H.W.; Schmezer, P. Assessment of genotoxic effects by lindane. Food Chem. Toxicol. 1993, 31, 271–283. [Google Scholar] [CrossRef]
- Asad, S.F.; Singh, S.; Ahmad, A.; Khan, N.U.; Hadi, S.M. Prooxidant and antioxidant activities of bilirubin and its metabolic precursor biliverdin: A structure-activity study. Chem. Biol. Interact. 2001, 137, 59–74. [Google Scholar] [CrossRef]
- Hex Protein Docking. Available online: http://hex.loria.fr/hex.php (accessed on 15 August 2015).
- RSCB Protein Data Bank. Available online: http://www.rcsb.org./pdb/home/home.do (accessed on 15 August 2015).
- The PubChem Project. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 15 August 2015).
- Avogadro 1.0.1. Available online: http://freesoftware.site/download/avogadro-101-10749443.html (accessed on 15 August 2015).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754-26769. https://doi.org/10.3390/ijms161125992
Arif H, Rehmani N, Farhan M, Ahmad A, Hadi SM. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. International Journal of Molecular Sciences. 2015; 16(11):26754-26769. https://doi.org/10.3390/ijms161125992
Chicago/Turabian StyleArif, Hussain, Nida Rehmani, Mohd Farhan, Aamir Ahmad, and Sheikh Mumtaz Hadi. 2015. "Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study" International Journal of Molecular Sciences 16, no. 11: 26754-26769. https://doi.org/10.3390/ijms161125992
APA StyleArif, H., Rehmani, N., Farhan, M., Ahmad, A., & Hadi, S. M. (2015). Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. International Journal of Molecular Sciences, 16(11), 26754-26769. https://doi.org/10.3390/ijms161125992







