Abstract
An approach to the synthesis of the isochromane moiety embodying the AC-ring system of the stephaoxocanes, by the use of an Oxa-Pictet Spengler type cyclization strategy, is reported.
Stephania excentrica and Stephania cepharantha (Menispermaceae) are herbs employed since ancient times in traditional Chinese medicine. They are the source of many interesting natural products, among them the stephaoxocanes, a small family of tetracyclic isoquinoline alkaloids with only a few known members [1].
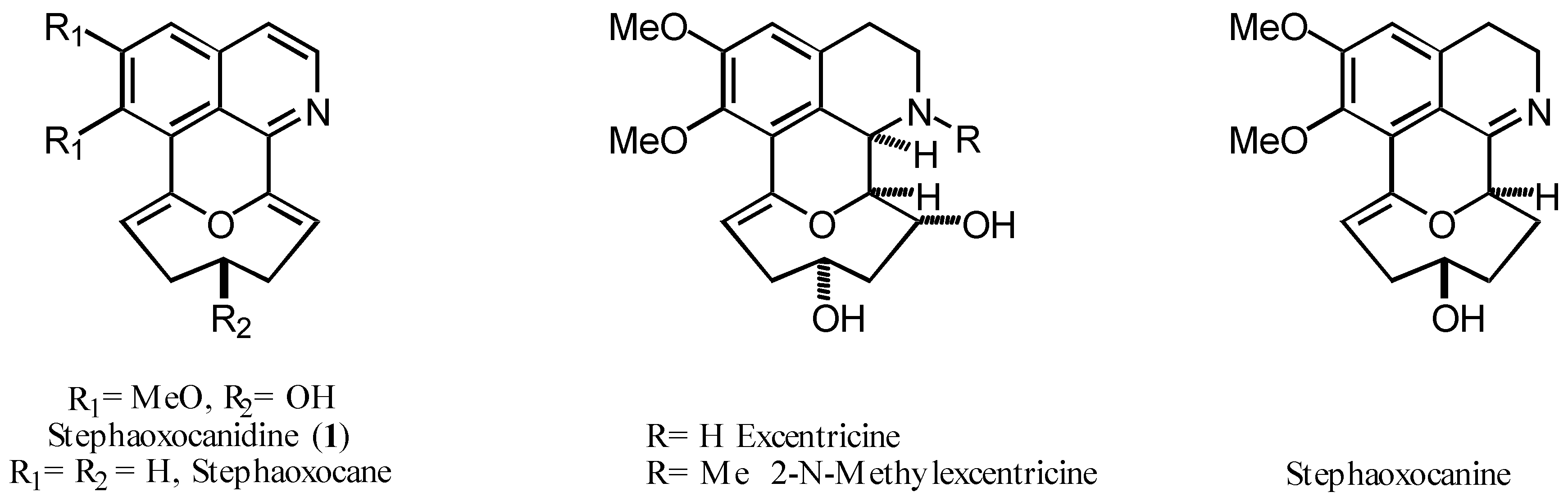

The interesting structural characteristics of these natural products in relationship with our research work [2] prompted us to study the elaboration of models for the total synthesis of natural stephaoxocanes, particularly stephaoxocanidine (1).
In this communication, we report the elaboration of isochromanone 2 starting from commercially available m-anisaldehyde (3), following an Oxa-Pictet-Spengler type strategy [3].
As shown in the Scheme, bromination of 3 with bromine in AcOH provided bromoaldehyde 4, which olefination under Wittig conditions gave olefins 5. Next, dihydroxylation of the double bond followed by transacetalization of the resulting diol with acetal furnished acetal 7, which TiCl4 promoted cyclization [4] yielded isochromanol 8. Finally, Swern oxidation of 8 allowed the obtention of ketone 2.
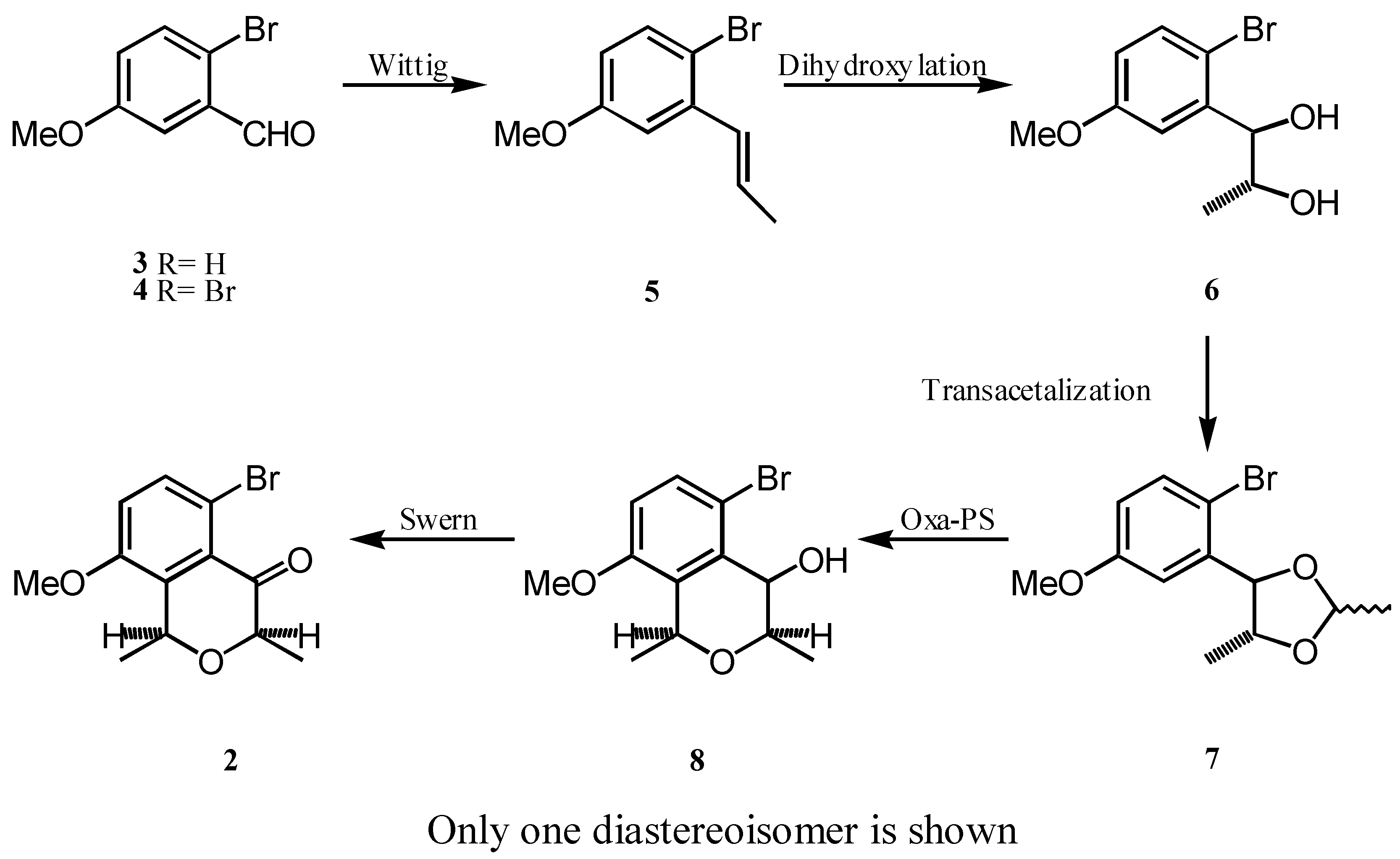

The Wittig reaction resulted in a mixture of olefins the isomerization of which was not studied in this preliminary approach; therefore, products 6-8 as well as 2 were obtained as diastereomeric mixtures, keeping the product the proportion found in the starting material.
Details of the synthesis, the cyclization reaction course with different model molecules and synthetic potential of this strategy will be discussed.
Acknowledgements
To Fundación Antorchas, CONICET, SECyT-UNR, AUGM and ANPCyT for grants received.
References and Notes
- (a) Kashiwaba, N.; Morooka, S.; Kimura, M.; Ono, M.; Toda, J.; Suzuki, H.; Sano, T. Nat. Prod. Rep. 1997, 9, 177. (b) Deng, J.-Z.; Zhao, S.-X.; Miao, Z.-C. Nat. Prod. Lett. 1993, 2, 283.
- (a) Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1 1993, 403. (b) Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1 1996, 2497. (c) Ponzo, V. L.; Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1 1997, 3131.
- Wünsch, B.; Zott, M. Liebigs Ann. Chem. 1992, 39.
- Giles, R. G. F.; Rickards, R. W.; Senanayake, B. S. J. Chem. Soc., Perkin Trans. 1 1996, 2241.