Abstract
The elaboration of a 2,3,7-9a-tetrahydro-1H-8-oxa-1-aza-phenalen-9-one derivative, as a potential key intermediate for the synthesis of stephaoxocanes, employing Jackson’s tosylamidoacetal cyclization, is presented.
Stephania excentrica and S. cepharantha (Menispermaceae) have been used since ancient times in traditional Chinese medicine. Stephania cepharantha, which is widely cultivated in Japan, is native of Taiwan where its tuberous root is known as ber-yao-zi. Phytochemical studies on the methanolic extract of the tubers of S. cepharanta allowed the isolation of new bisbenzylisoquinolines, hasubananes, and morphinanes, as well as a host of known alkaloids. More detailed investigations carried out since 1992 exposed novel and interesting tetracyclic isoquinoline derivatives bearing an oxocane ring system (Figure), the first in their class, for which the term stephaoxocanes was coined [1].
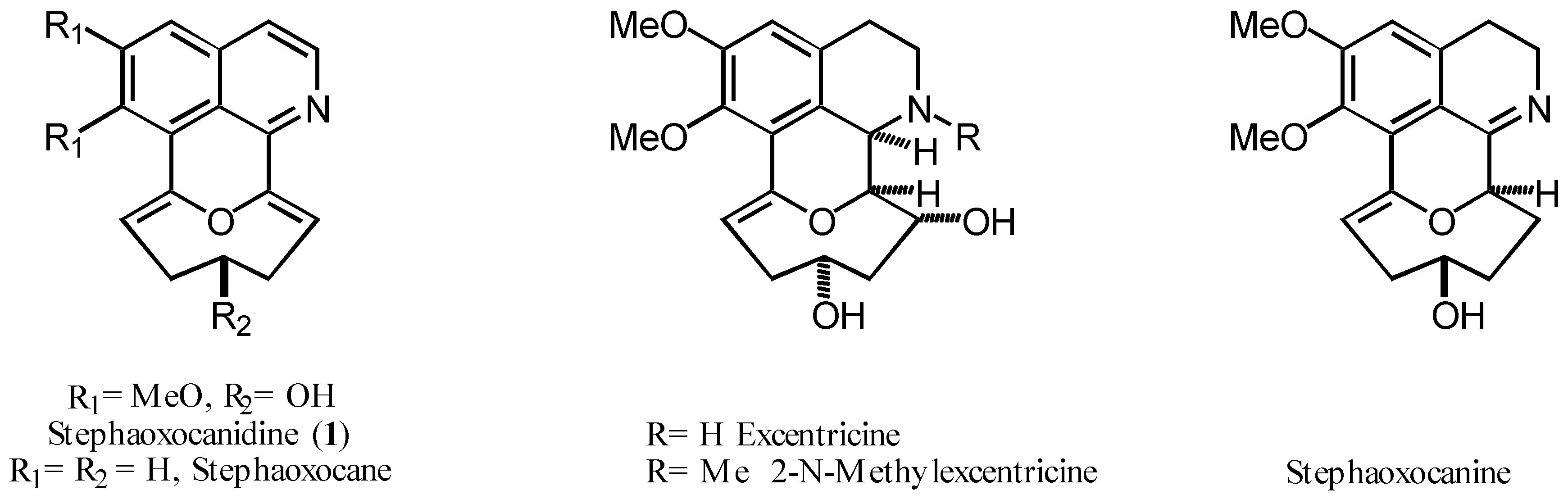
This rare family of natural products has very few members and the tiny amounts of them found in the natural sources constitute a serious obstacle in better defining their biological activity and usefulness, if any, and making new developments. As part of our research projects on interesting isoquinoline type natural products synthesis by the use of Jackson’s cyclization [1], we recently started to study the elaboration of model molecules for the total synthesis of natural stephaoxocanes, particularly stephaoxocanidine (1), displaying less structural complexity.
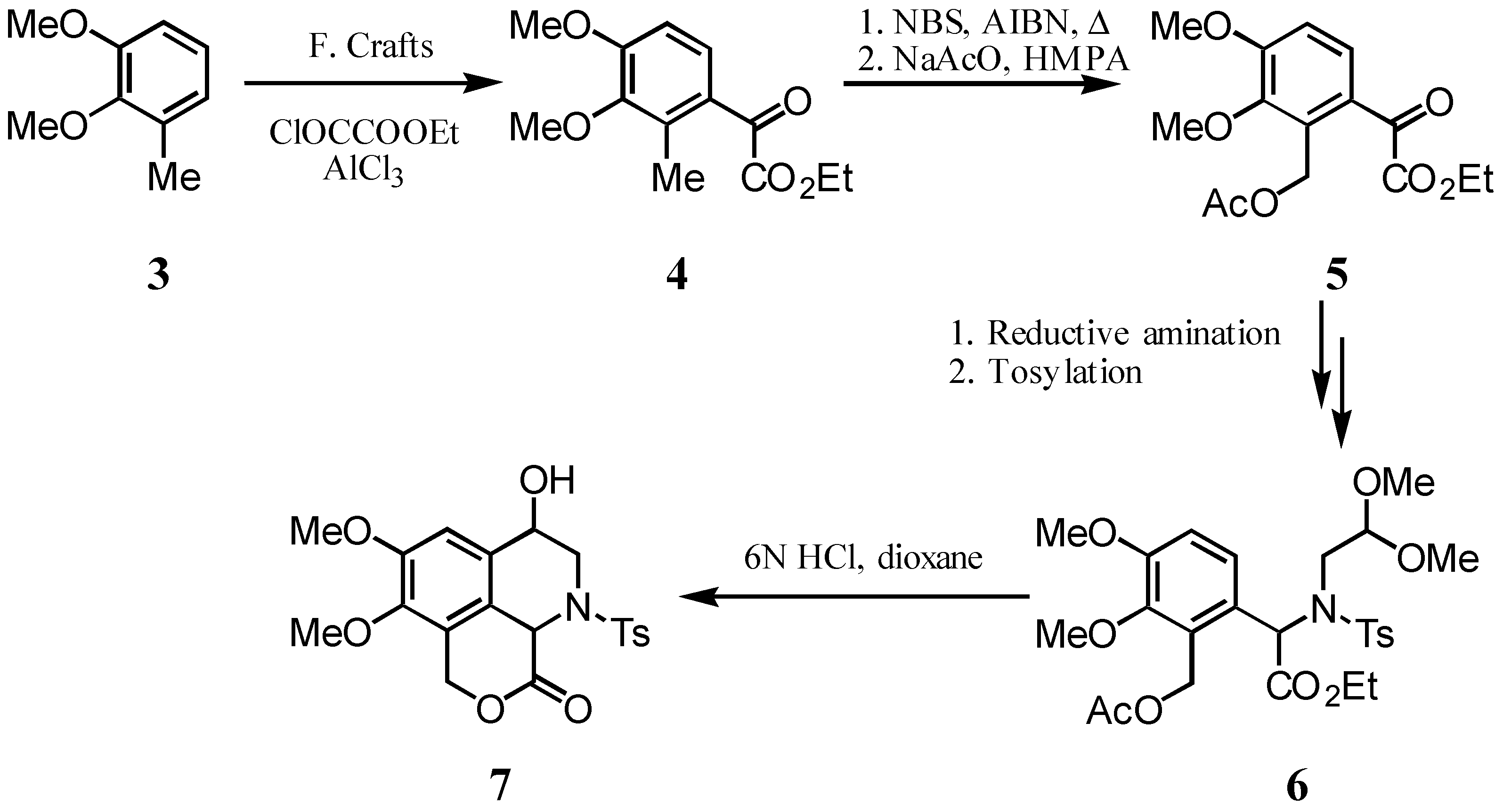
In this communication, the elaboration of tricyclic lactone 2, a potential key intermediate for the synthesis of stephaoxocanes, bearing their characteristic tetrahydrooxazaphenalene skeleton, is reported. The lactone was obtained by Friedel Crafts acylation of toluene derivative 3, followed by functionalization of the resulting intermediate 4 to ketoester 5 and application of Jackson’s sequence on the latter, which allowed the simultaneous construction of rings B and C of 2, by cyclization of sulfonamidoacetal 6 (Scheme). The chemical transformations involved in this synthetic sequence as well as their outcome will be discussed.
Acknowledgments
To Fundación Antorchas, CONICET, SECyT-UNR and ANPCyT for grants received.
References and Notes
- Kashiwaba, N.; Morooka, S.; Kimura, M.; Ono, M.; Toda, J.; Suzuki, H.; Sano, T. Nat. Prod. Rep. 1997, 9, 177. Deng, J.-Z.; Zhao, S.-X.; Miao, Z.-C. Nat. Prod. Lett. 1993, 2, 283.
- Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1 1993, 403. Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1 1996, 2497. Ponzo, V. L.; Kaufman, T. S. J. Chem. Soc., Perkin Trans. 1. 1997, 3131.
- Birch, A. J.; Jackson, A. H.; Shannon, P. V. R. J. Chem. Soc., Perkin Trans. 1. 1974, 2190. Recent modification: Ponzo, V. L.; Kaufman, T. S. Can. J. Chem. 1995, 73, 1348.