Abstract
A new method for the in situ elaboration of B-alkoxyoxazaborolidines is presented. Their use in the enantioselective reduction of prochiral aromatic ketones provides excellent chemical and optical yields of chiral alcohols.
Since the development of Corey [1], the B-alkyloxazaborolidines (OAB) have gained reputation as efficient catalysts in the enantioselective reduction of prochiral ketones. In addition to provide alcohols in high optical purity [2], they can be employed in small quantities and their reaction mechanism allows the prediction of the stereochemistry of the newly generated chiral center.
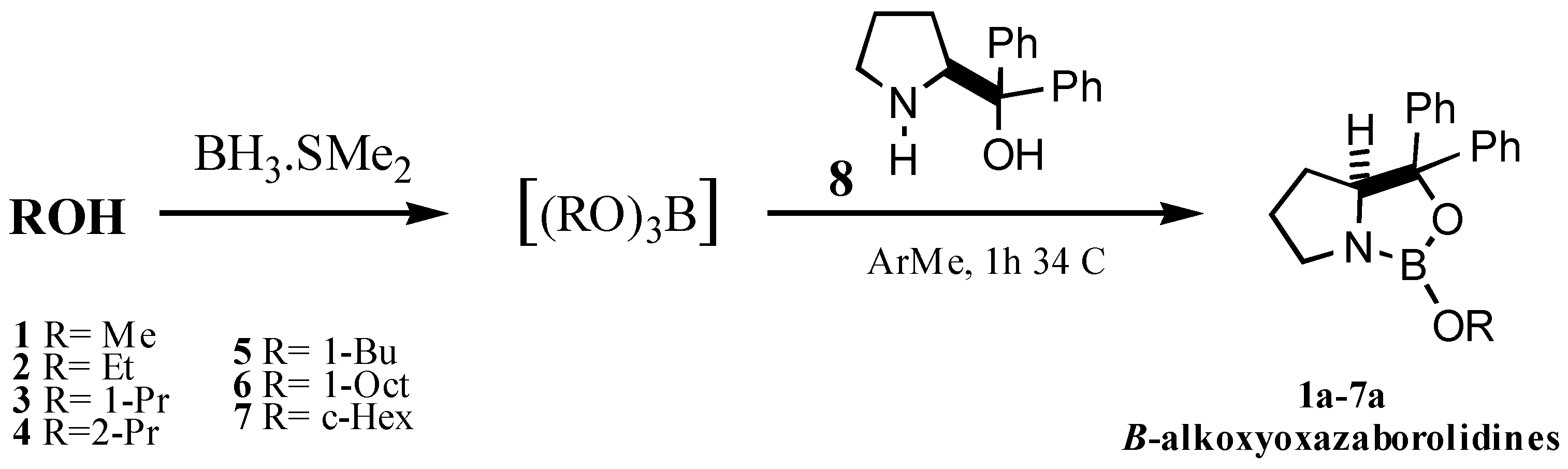
Numerous OAB synthesized from different aminoalcohols have been reported [3], however the most used OAB is that derived from α,α-diphenylpyrrolidinemethanol (8) developed by Corey.
In spite of the advantages of this new type of catalysts, the various methods described for their obtention, many times discourage their use, being time consuming [4] or requiring extensive separation steps prior to their use [5].
In order to avoid these inconvenients, we decided to study the synthesis of B-alkoxyoxazaborolidines, reacting alkyl borates with 8 by analogy with the strategies reported for the elaboration of alkyl-OAB, and then to evaluate the ability of the product to enantioselectively reduce prochiral aromatic ketones.
In this communication we introduce a new, practical and efficient method for the in situ elaboration of B-alkoxyoxazaborolidines employing inexpensive reagents and avoiding separation steps which could alter the optical quality of the reduction.
We also demonstrate the efficiency and capability of the B-alkoxyoxazaborolidines as catalysts through the reduction of several substituted acetophenones. The enantioselectivity obtained is generally comparable to that observed with the B-methyloxazaborolidine developed by Corey.

| Product | B-alkoxy-OAB | ee(%) | Yield (%) |
| R-1-(3,4-dimethoxyphenyl)ethanol | 1a-7a | >95 | >93 |
| R-1-(4-acetoxy-3-methoxyphenyl)ethanol | 5a | 97 | ≈100 |
| R-1-(4-hydroxy-3-methoxyphenyl)ethanol | 6a | >98 | 98 |
| R-1-(2,4-dimethoxyphenyl)ethanol | 6a | 90 | ≈100 |
| R-1(4-nitrophenyl)ethanol | 6a | >95 | ≈100 |
| R-1(4-aminophenyl)ethanol | 6a | 95 | ≈100 |
| R-1(4-bromophenyl)ethanol | 6a | 97 | 98 |
Acknowledgements
To Fundación Antorchas, CONICET, SECyT-UNR, AUGM and ANPCyT for grants received. VLP thanks CONICET for a fellowship.
References and Notes
- Corey, E. J.; Shibata, S.; Bakshi, R. J. Am. Chem. Soc. 1987, 109, 5551.
- Corey, E. J.; Helal, C. Angew. Chem. Int. Ed. Eng. 1998, 37, 1986.
- Wallbaum, S.; Martens, J. Tetrahedron: Asymmetry 1992, 3, 1475.
- Corey, E. J.; Bakshi, R. Tetrahedron Lett. 1990, 31, 611.
- Mathre, D.; Jones, T.; Xavier, L.; Blacklock, L. T.; Reamer, R.; Mohan, J.; Turner Jones, T.; Hoogsteen, K.; Baum, M.; Grabowski, E. J. J. Org. Chem. 1991, 56, 751.
- Masui, M.; Shiori, T. Synlett 1997, 273.