Abstract
Quantum mechanical calculations were carried out to study the molecular geometry and electronic structure of 2-amino-2-desoxyglucopyranose (AG) and the N-acetyl-, N-ethanoyl-, series of N-phthalimidoalkanoyl-AG. The total charge density, electrostatic potential, spatial distribution and positions of HOMO and LUMO of N-acyl-AGs with respect to their substitutes yield information on the reactivity of the molecules.
Introduction
2-Amino-2-desoxyglucopyranoses (AG) containing N-acyl functional group residues display unique chemical and biological behavior Experimentally, the structure of N- substituents on 2-amido-AG have been shown to significantly affect the reactivity of the glucopyranose skeleton. In some cases, their reactivity is significantly enhanced, allowing for selective reactions, which usually cause chemical or conformational rearrangements [1,2,3,4,5]. In other cases, the N-acyl-AG molecule becomes unreactive or the glucopyranose ring is destroyed, which is synthetically disastrous [1,2,3,4,5,6]. It would be a significant benefit to synthetic carbohydrate chemists to have a theoretical protocol for the quantum mechanical estimation of N-acyl-AG for an initial recognition of the reactivity of given compounds.
Results and Discussion
Quantum mechanical calculations were carried out to study the molecular geometry and electronic structure of compounds 2 using the HyperChem 5.0 package [7].
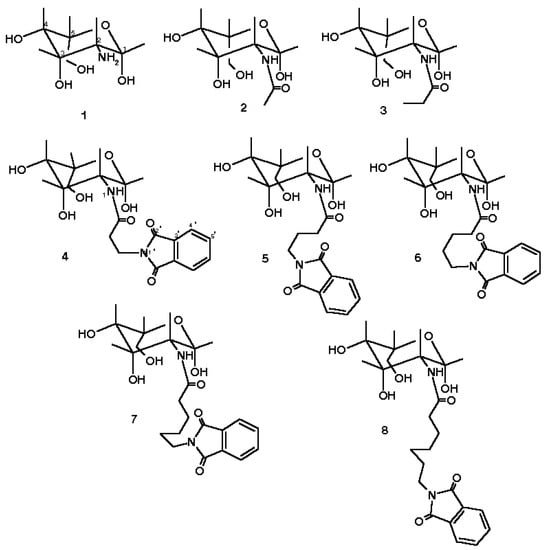
Scheme 1.
Structure of N-Acyl-2-amino-desoxigliukopyranoses 1-8.
Full geomety optimization was performed by the semi-empirical MNDO method at the RHF level of theory. The binding energy and the energy of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), bond lengths, angles and the charges of atoms finally were calculated. The energy calculations of conformers 1-8 show that α -anomers are lower in energy than β -anomers. This result is in agreement with the C13-NMR spectra, which show an increase of α - anomers at the expense of the β-forms [5]. Further calculations of substituted and of unsubstituted AG 1-8 are based on α -anomers’ conformations since these are lower in energy than the β -forms. The glucopyranose skeleton of N-acyl-AG adopts the chair conformation with a planar conformation of Nphthalimide rings.

Table 1.
HOMO and LUMO Energies, HOMO-LUMO Energy Gaps (ΔE) in eV, Heat of Formation, Binding Energy, Total Energy for Compounds 1-8.
| HOMO | LUMO | E | Heat of Formation kcal/mol | Binding Energy kcal/mol | Total Energy kcal/mol | |
|---|---|---|---|---|---|---|
| 1 | -10.531 | 2.639 | 13.170 | -221.67 | -2335.13 | -63900.96 |
| 2 | -10.345 | 0.888 | 11.233 | -253.48 | -2872.48 | -77875.57 |
| 3 | -10.332 | 0.878 | 11.210 | -286.43 | -3180.53 | -81128.65 |
| 4 | -10.109 | -1.084 | 9.024 | -288.57 | -4938.22 | -125656.11 |
| 5 | -10.128 | -1.105 | 9.023 | -301.06 | -5225.79 | -128810.07 |
| 6 | -10.188 | -1.171 | 9.017 | -307.95 | -5507.78 | -132403.79 |
| 7 | -10.102 | -1.076 | 9.026 | -300.99 | -5775.92 | -136477.83 |
| 8 | -10.173 | -1.150 | 9.022 | -304.79 | -6054.81 | -140084.72 |
The total charge density (Table 2), electrostatic potential (Figure 1 and Figure 2), spatial distribution and positions of HOMO (Figure 3, Figure 4, Table 1) and LUMO (Figure 5, Figure 6, Table 1) of Nacyl-AGs 1-8 with respect to their substituents provide information on the reactivity of the molecules in actual reactions with electrophiles or nucleophiles.
The representative plot of electron charge density indicates a build up of positive charge density on 2-C carbon atoms of N-acyl substituted pyranose skeleton 2-8 compared to unsubstituted 2-amino-AG 1. Figure 1 and Figure 2 presents the electrostatic potentials (ESP) of the N-alkanoyl and Nphthalimidoalkanoyl substituted AG.

Table 2.
Atomic charges of N-acyl-2-aminodesoxyglucopyranoses 1-8.
| 2-Aminodesoxyglucopyranose skeleton | C-α(O) | O(Cα) | N-phthalimide | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | C-4 | C-5 | O-1 | O-2(H) | H-1 | N-1 | N-1′ | C-2′ | O-1′ | |||
| 1 | 0.305 | 0.016 | 0.129 | 0.096 | 0.137 | -0.379 | -0.324 | 0.018 | -0.249 | |||||
| 2 | 0.289 | 0.110 | 0.151 | 0.073 | 0.110 | -0.371 | -0.330 | 0.027 | -0.378 | 0.343 | -0.328 | |||
| 3 | 0.289 | 0.108 | 0.151 | 0.073 | 0.110 | -0.371 | -0.328 | 0.028 | -0.378 | 0.353 | -0.323 | |||
| 4 | 0.269 | 0.073 | 0.119 | 0.090 | 0.104 | -0.375 | -0.343 | 0.031 | -0.380 | 0.350 | -0.318 | -0.452 | 0.413 | -0.305 |
| 5 | 0.272 | 0.076 | 0.120 | 0.088 | 0.135 | -0.376 | -0.340 | 0.030 | -0.376 | 0.341 | -0.313 | -0.454 | 0.403 | -0.301 |
| 6 | 0.289 | 0.108 | 0.152 | 0.074 | 0.110 | -0.372 | -0.330 | 0.025 | -0.378 | 0.354 | -0.329 | -0.451 | 0.402 | -0.301 |
| 7 | 0.290 | 0.109 | 0.152 | 0.074 | 0.109 | -0.330 | -0.330 | 0.027 | -0.381 | 0.353 | -0.322 | -0.452 | 0.402 | -0.306 |
| 8 | 0.289 | 0.111 | 0.151 | 0.074 | 0.110 | -0.371 | -0,330 | 0.025 | -0.380 | 0.355 | -0.329 | 0.450 | 0.402 | -0.303 |
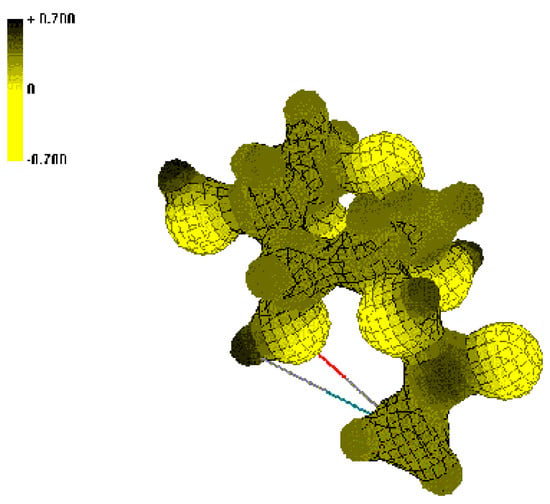
Figure 1.
Isosurface of the electrostatic potential near N-acyl-2-AG (2).
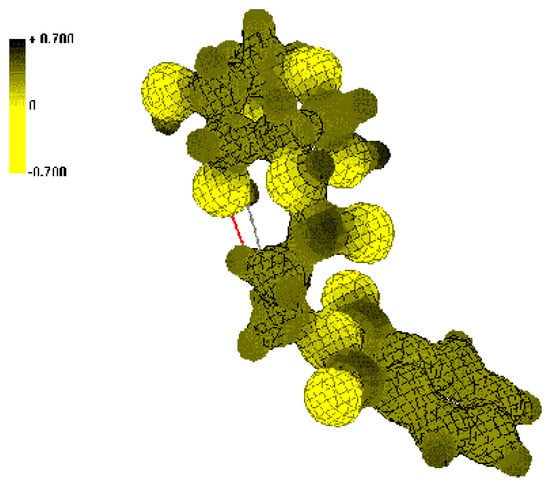
Figure 2.
Isosurface of the electrostatic potential in the spatial vicinity of N-acyl-2-AG (4).
Dark (black) colors indicate positive ESP regions and light (yellow) colors indicate negative ESP regions. Comparison of ESP of the N-alkanoyl-AG 2, 3 (Figure 1) with the N-phthalimidoalkanoyl-AG 4-8 (Figure 2) shows that for compounds 2, 3 increased positive charge regions located on the carbon atom of the amide bond, while derivatives 4-8 gets more positive ESP regions on the carbon atoms of the CO-N-CO fragment of the phthalimide ring.
Substitution on glucopyranose skeleton of N-acyl-AG 1-8 affects HOMO and LUMO energies (Table 1). All N-acyl substituents for 2-8 lead to decreased HOMO-LUMO gaps compared to the unsubstituted 2-amino-AG 1. Energy gap reduction is caused by a strong decrease of LUMO energies while HOMO energies increase slightly. The N-phthalimidoalkanoyl substitution for 4-8 leads to smaller band gap than N-acetyl, N-ethanoyl substitution for 2 and 3 respectively, since the LUMO energy for 4-8 is lowered more than for compounds with N-acetyl, N-ethanoyl moieties 2, 3. Sella et al and Sawicka et al proposed arguments to explain the observed easier nucleophilic ring opening and cleavage reactions [8,9,10]. One can therefore identify for N-acyl-AGs 2-8 the low-lying LUMO, as a site will be most likely involved in reactions with nucleophiles. Since the N-phthalimidoalkanoyl substitution 4-8 leads to HOMO-LUMO energy gap reduction with strong decrease of LUMO energies comparing to 1-3 the strongest reactivity effect was predicted for the N-phthalimidoalkanoyl substitution. This result reflects the experimental data [1,2,3,4,5,6,11,12].
The spatial distribution of LUMO in N-acetyl, N-ethanoyl substituted AG 2, 3 are concentrated around C atom of amide that reflects electron density is small. This site of the molecule therefore most likely participates in reactions with nucleophilic moieties. In the case of compounds 4-8, substituted with a series of N-alkanoylphthalimides, LUMO are located at the site of the phthalimide ring on the CO atoms. It shows that reaction with nucleophiles most likely occur close to the CO on the ring with subsequent cleavage of the CO-N-CO fragment.
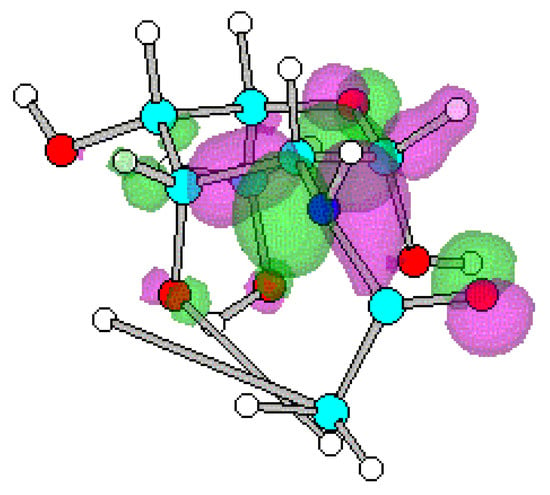
Figure 3.
Optimized geometry and spatial distribution of HOMO for N-acyl-AG (2).
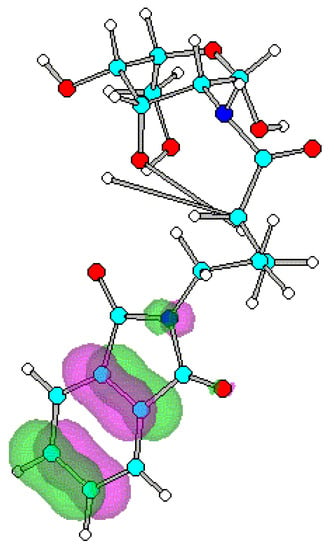
Figure 4.
Optimized geometry and spatial distribution of HOMO for N-acyl-AG (6).
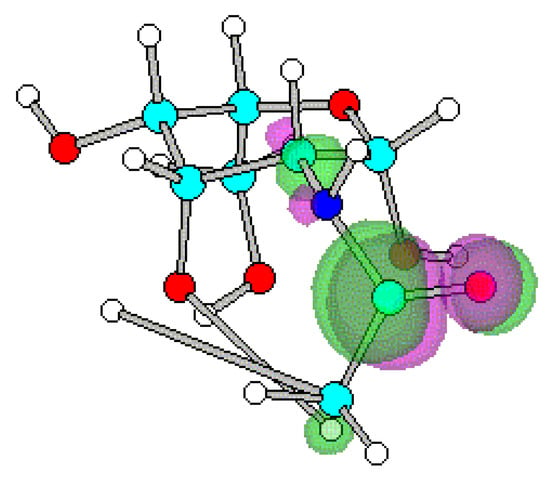
Figure 5.
Optimized geometry and spatial distribution of LUMO for N-acyl-AG (2).
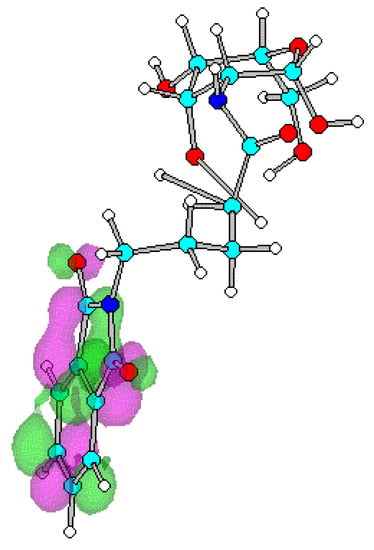
Figure 6.
Optimized geometry and spatial distribution of LUMO for N-acyl-AG (6).
The molecular orbital analysis of the electronic structure of the N-acyl-AGs 2-8 and 2-amino-AG 1 correlated with experimental results. N-acyl-AG are involved in reactions with nucleophiles in most cases causing decomposition of the amide bond. N-alkanoyl-AGs are reactive under forcing conditions, which sometimes cause destruction of glucopyranose skeleton [1,5,11,12]. AG substituted with Nphthalimide moieties display selective reactivity under mild condition. They can act with hydrazine leading to rearrangement of the CO-N-CO fragment of the phthalimide skeleton with the formation of ammonium salts bearing phthaloylhydrazide while the amide on the glucopyranose skeleton remains unreactive [1,2,3,4,5].
Conclusions
Results of quantum chemical calculations provide information about the reactivity of N-acyl-2-amino-2-desoxyglucopyranoses. Substitution on the glucopyranose skeleton affects HOMO and LUMO energies. N-phthalimidoalkanoyl substitution leads to a smaller HOMO-LUMO energy gap than in case of N-alkanoyl moieties. Energy gap reduction is caused by the strong decrease of LUMO energies. The strongest reactivity effect with nucleophiles was predicted for the 2-amino-2-desoxyglucopyranoses bearing N-phthalimidoalkanoyl substituents.
The spatial distribution of LUMO in the alkanoyl substituted 2-amino-2-desoxyglucopyranoses is located on the CO fragment of the amide. In the case of N-alkanoylphthalimides LUMO are located on the CO atoms at the site of phthalimide ring. This explains why different sites of N-acyl-2-amino-2-desoxyglucopyranoses will be most likely involved in reactions with nucleophiles.
Acknowledgments
The authors thank Dr. A. Stoncius, Department of Organic Chemistry, Vilnius University for providing access to HyperChem 5.0.
References and Notes
- Op Den Kamp, J. A. F.; Bonsen, P. P. M.; Van Deenen, L. L. M. Structural Investigation on Glucosaminyl Phosphatidyl-glicerol from Bacillus Megaterium. Biochim Biophys. Acta 1969, 176, 298–305. [Google Scholar] [CrossRef]
- Redlich, H.; Roy, W. Synthesen einiger β-Desosaminglycoside. Liebigs Ann. Chem. 1981, 7, 1215–1222. [Google Scholar] [CrossRef]
- Tang, P. W.; Williams, J.M. Further Studies of the Hydrazinolysis of 2-acetamido-1-N-acyl-2-desoxy-D-glucopyranosylamines. Carbohydr. Res. 1983, 121, 89–97. [Google Scholar] [CrossRef]
- Juodviršis, A.; Butenas, S.; Astrauskas, V. Derivatives of 2-N-(ω-phthalimidealkanoyl)amino-desoxy-1,3,4,6-tetra-O-acetyl-D-glucopyranoses Bearing Antiviral Activity. A. s. 1139148 (SU). 1984. [Google Scholar]
- Butenas, S. Synthesis and Investigation of N-acylated 2-amino-2-desoxyglucopyranoses and their Derivatives. Summary of doctoral thesis, Kaunas Politechnical Institute, Kaunas, 1985. [Google Scholar]
- Tang, P. W.; Williams, J.M. Degradation During the Hydrazinolysis of 2-acetamido-1-N-acyl-2-deoxy-b-D-glucopyranosylamines. Carbohydr.Res. 1983, 113, 13–15. [Google Scholar]
- Hyperchem 5.0, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA.
- Sawicka, D.; Houk, K. Aromaticity and Antiaromaticity in Small Ring Transition States, Assessed by NICS Values and Energetics. J. Mol. Model. 2000, 6, 158–165. [Google Scholar] [CrossRef]
- Sella, A.; Basch, H.; Hoz, S. Reactivity of Strained Compounds: Is Ground State Destabilization the Major Cause for Rate Enhancement. J. Am. Chem. Soc. 1996, 118, 416. [Google Scholar] [CrossRef]
- Jager, R.; Debowski, M.; Manners, I.; Vancso, G. J. Study of the Molecular Geometry, Electronic Structure, and Thermal Stability of Phosphazene and Heterophosphasene Rings with ab Initio Molecular Orbital Calculations. Inorg. Chem. 1999, 38, 1153–1159. [Google Scholar] [CrossRef]
- Harrison, R.; Fletcher, H. G. Synthesis with Partially Benzylated Sugars. IV. A Route to Some 1-O-Acyl-2-acylamido-2-deoxy-D-glucopyranoses and -D-galactopyranoses. J. Org. Chem. 1965, 30, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Jeanloz, R.W. 3,6-Di-O-methyl-D-glucosamide Hydrochloride (2-Amino-2-deoxy-3,6-di-O-methyl-D-glucose Hydrochloride). J. Org. Chem. 1961, 26, 905–908. [Google Scholar] [CrossRef]
- Sample Availability: Samples not available.
© 2000 by MDPI (http://www.mdpi.org).