Abstract
Proteolysis-targeting chimeras (PROTACs) have emerged as a revolutionary therapeutic modality that enables degradation of therapeutically relevant proteins through the protein disposal machinery, the ubiquitin-proteasome system (UPS). Unlike traditional small-molecule inhibitors, PROTACs harness bifunctional molecules to induce targeted protein degradation, offering advantages such as increased specificity, catalytic activity, and the potential to address previously undruggable targets. Since their conception 20 years ago, PROTACs have made significant strides in target protein degradation (TPD), and today, PROTACs are on the verge of their first clinical approval. This review presents a detailed overview of PROTAC targets, clinical development progress, and the design and detailed synthesis of degrader molecules that have advanced to clinical trials.
1. Introduction
Targeted protein degradation has emerged as a transformative concept in modern drug discovery, offering novel strategies for intervention in cellular processes previously deemed “undruggable.” In 2001, Sakamoto et al. revolutionized the field of protein degradation with the first demonstration of the concept of PROTAC technology [1,2,3]. Over the next decade, this innovative approach captured the attention of biotech companies and academic institutions. Among the pioneering advancements were Bavdegalutamide (ARV-110) and Vepdegestrant (ARV-471), which were among the first compounds to enter clinical trials [4,5]. On 8 August 2025 FDA has accepted a New Drug Application (NDA) for Vepdegestrant for the treatment of ESR1-mutated ER+/HER2-advanced breast cancer patients based on the pivotal Phase 3 VERITAC-2 clinical trial (NCT05654623), demonstrating statistically significant and clinically meaningful improvement in median progression-free survival (PFS) compared to Fulvestrant [6,7].
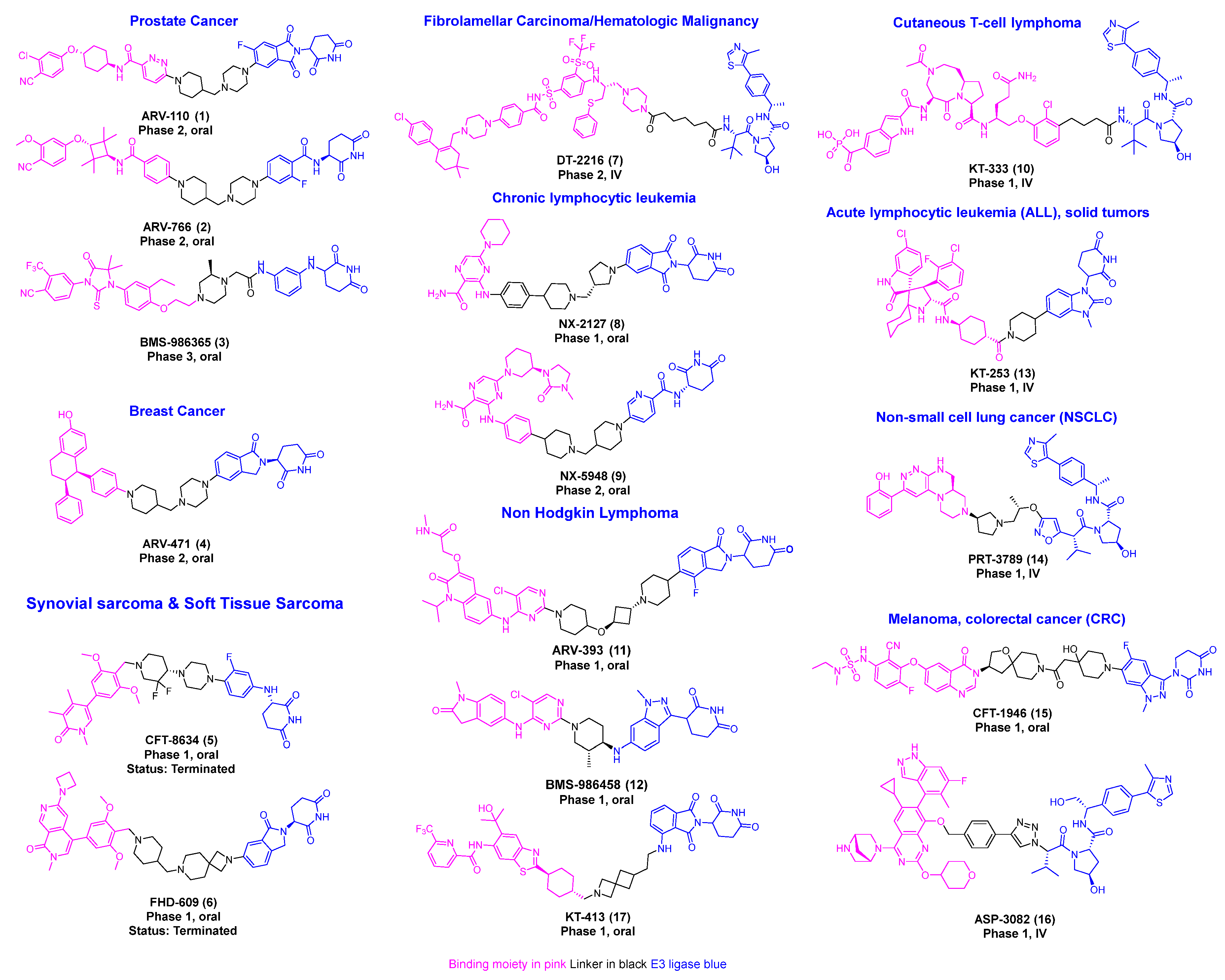
Recent research indicates a significant rise in the utilization of different E3 ligases in PROTAC development, highlighting the expanding scope of targeted protein degradation strategies [8,9,10]. Among these, E3 ligases Von Hippel–Lindau (VHL) and Cereblon (CRBN)-based PROTAC degraders have progressed to clinical development [11,12,13,14]. PROTAC technology has now been successfully used to target more than 100 proteins. This review offers a comprehensive overview of disclosed PROTAC degrader cancer targets, clinical development progress, and the design and detailed synthesis of degrader molecules (Figure 1) [13,15].
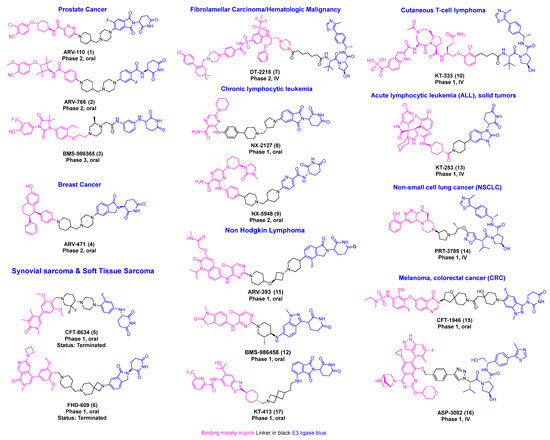
Figure 1.
Disclosed structures of PROTACs in the clinical trial.
2. Discussion of Targets, Clinical Development Progress, and the Design and Detailed Synthesis of Degrader Molecules
2.1. PROTACS Targeting Androgen Receptor (AR)
Prostate cancer is the second most common cancer in men, causing over 400,000 deaths annually, with new cases expected to rise from 1.4 million in 2020 to 2.9 million by 2040 [16,17,18]. The androgen receptor (AR), a nuclear hormone receptor, plays a central role in the biology of prostate cancer. The AR is a 110 kDa transcription factor composed of an N-terminal activation function-1 (AF1) domain, a DNA-binding domain, a hinge region, and a ligand-binding domain (LBD, also known as AF2). In its inactive state, AR exists as a monomer bound to heat shock proteins (HSPs). Upon binding dihydrotestosterone (DHT), the HSPs dissociate, allowing AR to dimerize, translocate to the nucleus, and bind androgen response elements (AREs), thereby activating the transcription of genes that promote tumor growth and survival [19,20].
Current treatment approaches include surgical removal of the prostate and androgen deprivation therapy (ADT). While antiandrogen therapies are initially effective in clinical settings, AR mutations frequently lead to resistance. These mutations can convert AR antagonists into partial agonists and increase the receptor’s sensitivity to other steroid hormones such as estradiol, progesterone, and hydrocortisone. This process contributes to the development of lethal castration-resistant prostate cancer (CRPC), which remains incurable to date [21].
Clinical Development and Outcomes of Bavdegalutamide (ARV-110), Luxdegalutamide (ARV-766) and Gridegalutamide (BMS-986365): AR degraders present a promising strategy to overcome acquired AR mutations, as they enable the elimination of the receptor regardless of whether a mutation shifts a ligand’s effect from antagonism to agonism. ARV-110 (Bavdegalutamide), developed by Arvinas, was the first PROTAC-based AR degrader to enter clinical trials in 2019 for mCRPC (NCT03888612). However, Phase II results, measured by the prostate-specific antigen 50% reduction (PSA50), revealed that its efficacy was confined mainly to a small subgroup of patients with AR T878A/S or H875Y mutations. Among 140 patients with biomarker and PSA follow-up, those with AR T878A/S and/or H875Y mutations showed PSA declines of 46% (PSA50) and 58% (PSA30), compared to 10% and 23%, respectively, in patients without these mutations [4,22]. In a group of 7 patients with these mutations assessed by RECIST (Response Evaluation Criteria in Solid Tumors) criteria, 6 showed tumor shrinkage, including two confirmed partial responses. Of those with only one prior novel hormonal agent (NHA) and no chemotherapy, 5 out of 19 patients (26%) achieved PSA50. Overall, response rates were 16% for PSA50 and 29% for PSA30 [23].
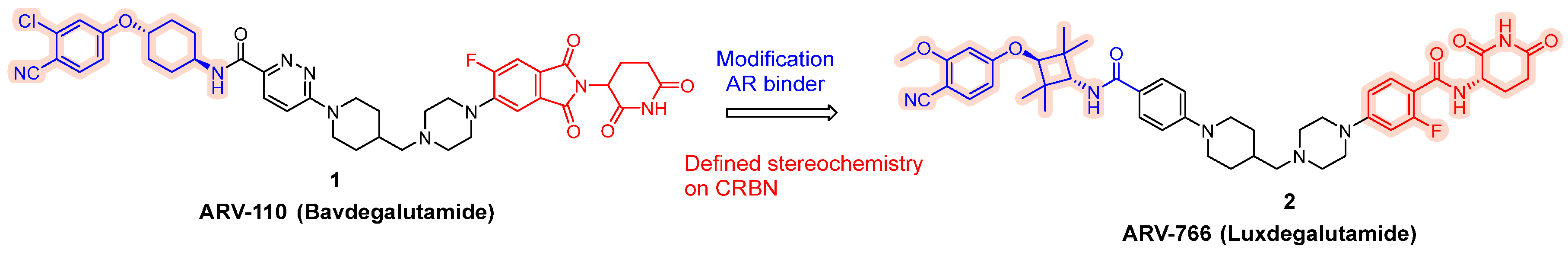
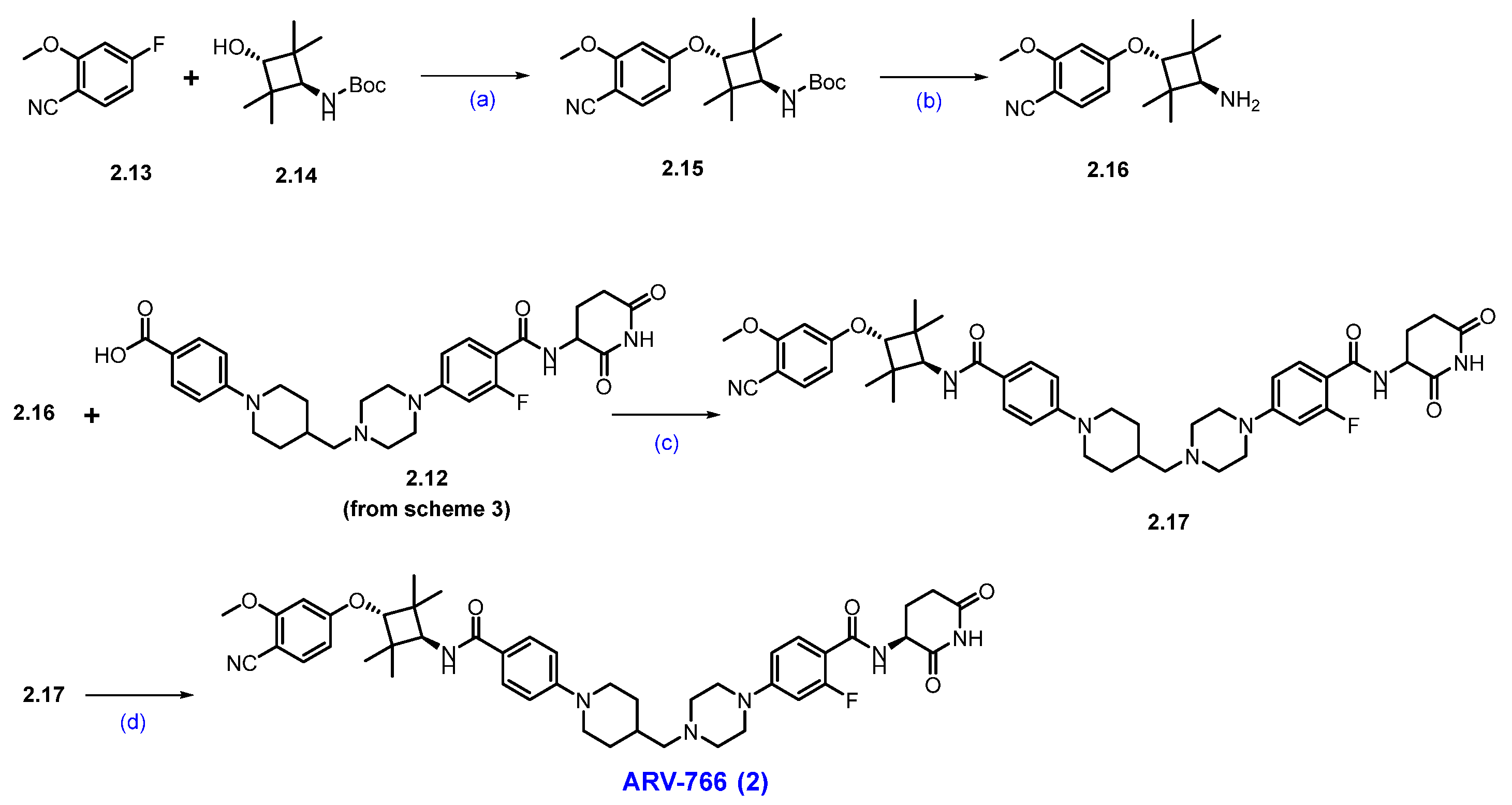
To expand its AR-targeting portfolio and treat a broader range of prostate cancer patients, Arvinas developed Luxdegalutamide (ARV-766), a second-generation AR degrader designed for enhanced potency and efficacy. The discovery of ARV-766 was presented at AACR 2023. Key structural optimizations leading to Luxdegalutamide included replacing the six-membered ring in the POI moiety of ARV-110 with a tetramethyl cyclobutane ring, substituting a chlorine atom with a methoxy group in the AR binder, and modifying the CRBN component with precise stereochemistry (ARV-766, 2, Figure 2).
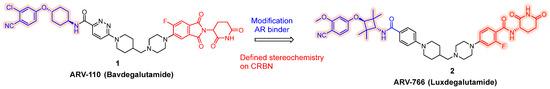
Figure 2.
Discoveries along the way to ARV-766 (2).
ARV-766 has demonstrated robust degradation activity against multiple clinically relevant AR mutants, including wild-type AR as well as L702H (which occurs in up to 24% of treated mCRPC patients), W742C, T878A/D891H, S889G, M750V, T878A/S889G, and the dual mutant F877L/T878A. This broad mutational coverage supports its potPential for clinical benefit across a broader patient population. Importantly, ARV-766 does not degrade key IMiD neosubstrates such as GSPT1, Aiolos, Ikaros, CK1α, and SALL4; an attribute that may translate into a more favorable safety profile [24].
As of 15 December 2023, a total of 103 patients had been treated with ARV-766 (34 in Phase 1 and 69 in Phase 2). Among 28 PSA-evaluable patients with AR LBD mutations, the PSA50 response rate was 50.0% [24]. Given the increasing prevalence of the L702H mutation, more patients stand to benefit from the robust efficacy profile that ARV-766 offers. Furthermore, ARV-766 demonstrated a significantly improved tolerability profile compared to ARV-110, with markedly lower rates of fatigue, nausea, vomiting, and diarrhea. In conjunction with its compelling efficacy data, this favorable safety profile positions ARV-766 as a “superior” PROTAC AR degrader for both mCRPC and metastatic castration-sensitive prostate cancer (mCSPC).
Another advanced AR-targeting agent, BMS-986365 (Gridegalutamide), developed by BMS, employs a dual mechanism of action that combines AR degradation with receptor antagonism (NCT06764485). This dual strategy provides a more robust and durable suppression of the AR pathway compared to traditional antagonists [25].
In early-phase studies, BMS-986365 was administered to heavily pretreated patients with mCRPC. The median radiographic progression-free survival (rPFS) reached an impressive 16.5 months among chemotherapy-naïve patients, compared to just 5.5 months in those who had undergone prior chemotherapy. Furthermore, clinical benefits were seen across a wide range of patients, regardless of their AR ligand-binding domain mutational status [25,26].
Building on this promising foundation, the ongoing Phase 3 study titled rechARge is set to investigate the efficacy and safety of BMS-986365 in comparison to the investigator’s choice of therapy, including docetaxel, abiraterone plus prednisone/prednisolone, or enzalutamide, in mCRPC patients. The primary goal of this pivotal trial is to assess rPFS, reinforcing our commitment to advancing treatment options for patients in need [27].
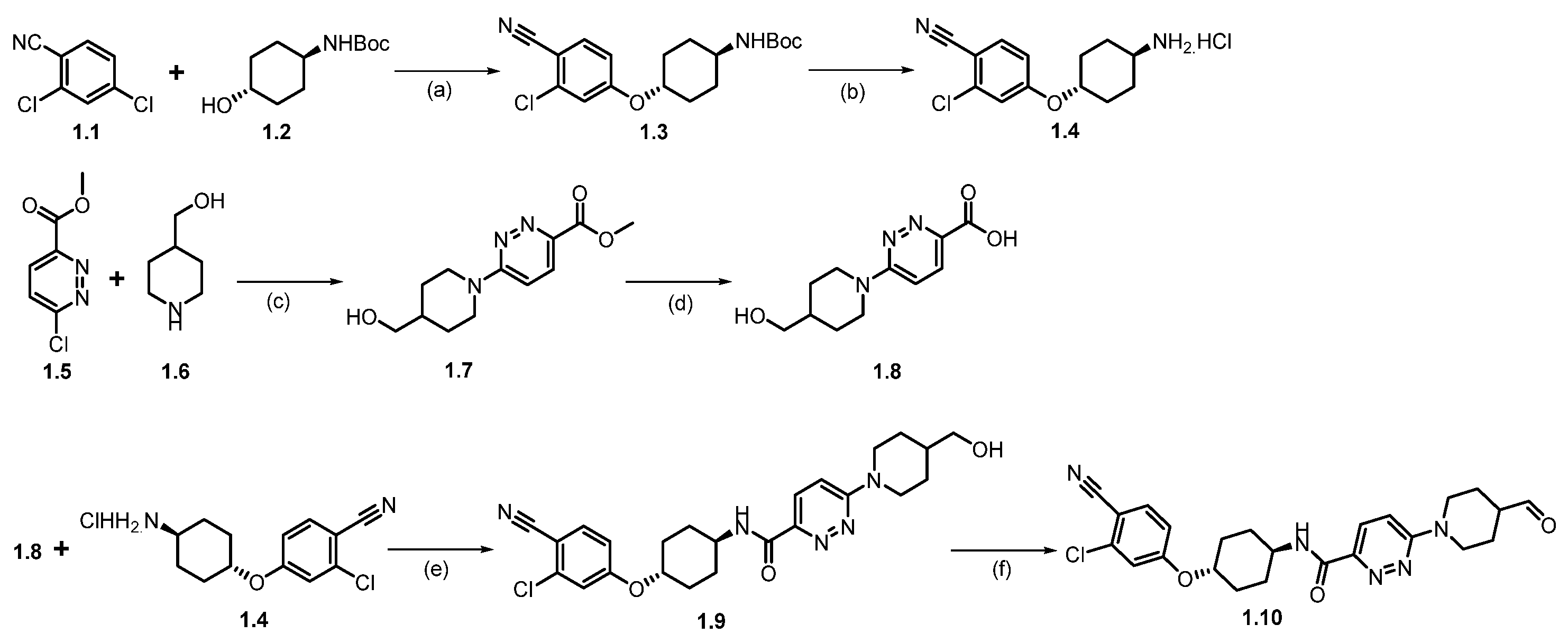
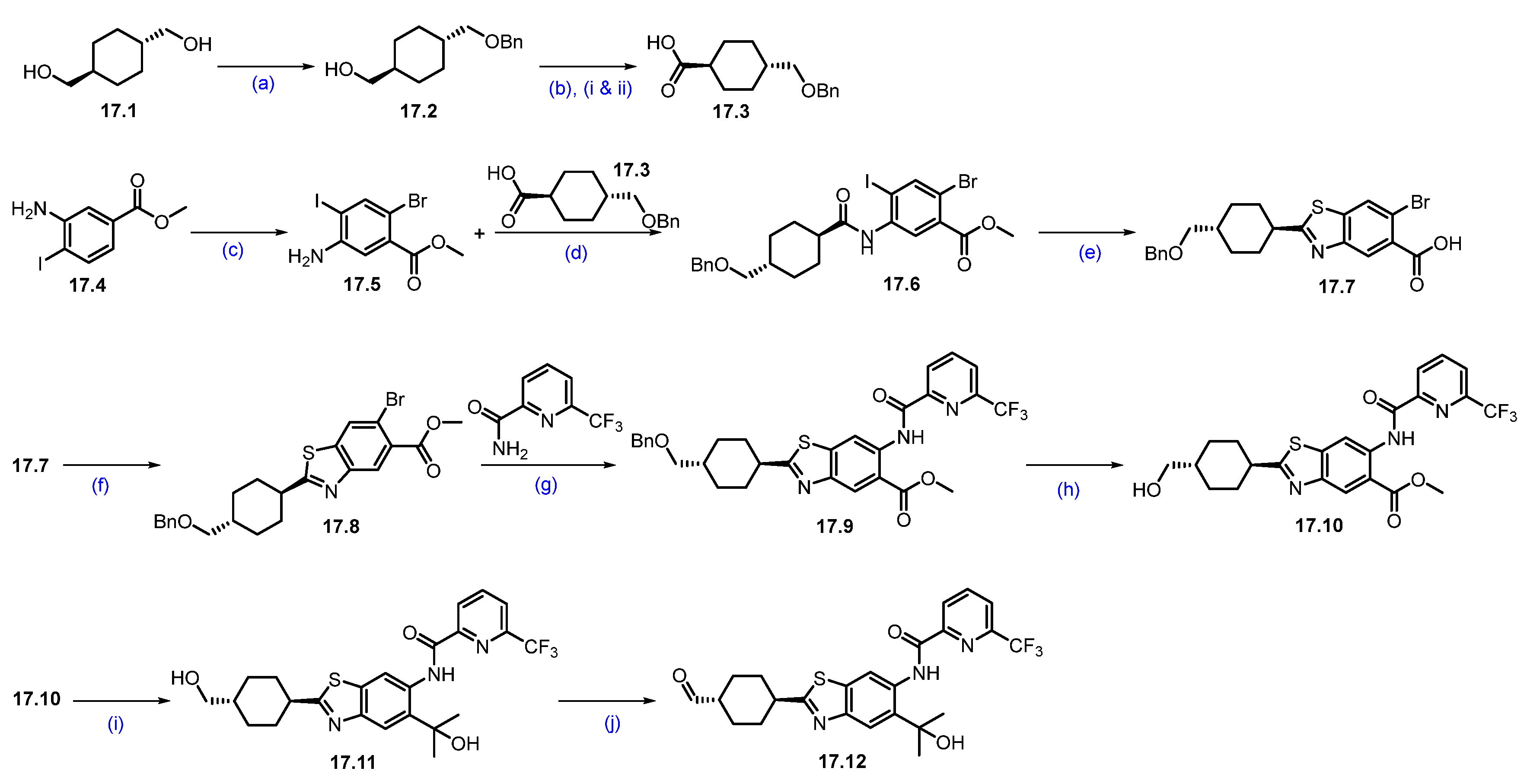
Synthesis of ARV-110 (1). The synthesis commenced with nucleophilic aromatic substitution (SNAr) reaction between commercially available 2,4-dichlorobenzonitrile (1.1) and tert-butyl((1R,4R)-4-hydroxycyclohexyl)carbamate (1.2) in dimethylacetamide (DMA), using sodium hydride (NaH), which afforded ether product 1.3 (Scheme 1). In the subsequent step, the deprotection of the -Boc group from 1.3 was achieved by treating with HCl in methanol at room temperature, which afforded 1.4. The resulting product was recrystallized from methyl tert-butyl ether, yielding pure product 1.4 as the HCl salt. Following this, a nucleophilic aromatic substitution (SNAr) reaction was performed between compounds 1.5 and 1.6 in dimethyl sulfoxide (DMSO) with diisopropyl ethylamine (DIPEA) to form substituted product 1.7, which was then hydrolyzed to give the acid 1.8. The amidation of acid 1.8 with amine 1.4, using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC.HCl) and 2-hydroxy pyridine oxide (HOPO), yields product 1.9. Finally, (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl (TEMPO) catalyzed oxidation of primary alcohol on 1.9 with sodium hypochlorite (NaOCl) and sodium bicarbonate (NaHCO3) in dichloromethane (DCM) produced the key aldehyde fragment 1.10 [28].
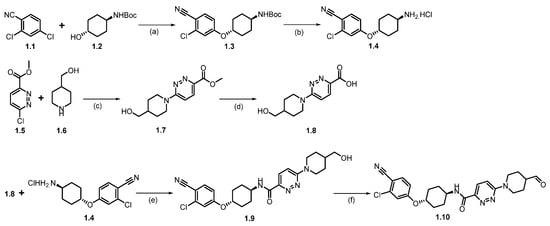
Scheme 1.
Synthesis of aldehyde fragment 1.10 of ARV-110: (a) NaH, DMA, 0 °C to 45 °C, 2 h; (b) HCl in MeOH, r.t., 2 h; (c) DIPEA, DMSO, 90 °C, 2 h; (d) LiOH.H2O, THF, MeOH, r.t., 1 h; (e) HOPO, EDC.HCl, DMA, DIPEA, 20 °C, 12 h; (f) TEMPO, NaOCl, NaHCO3, DCM, 20 °C, 12 h.
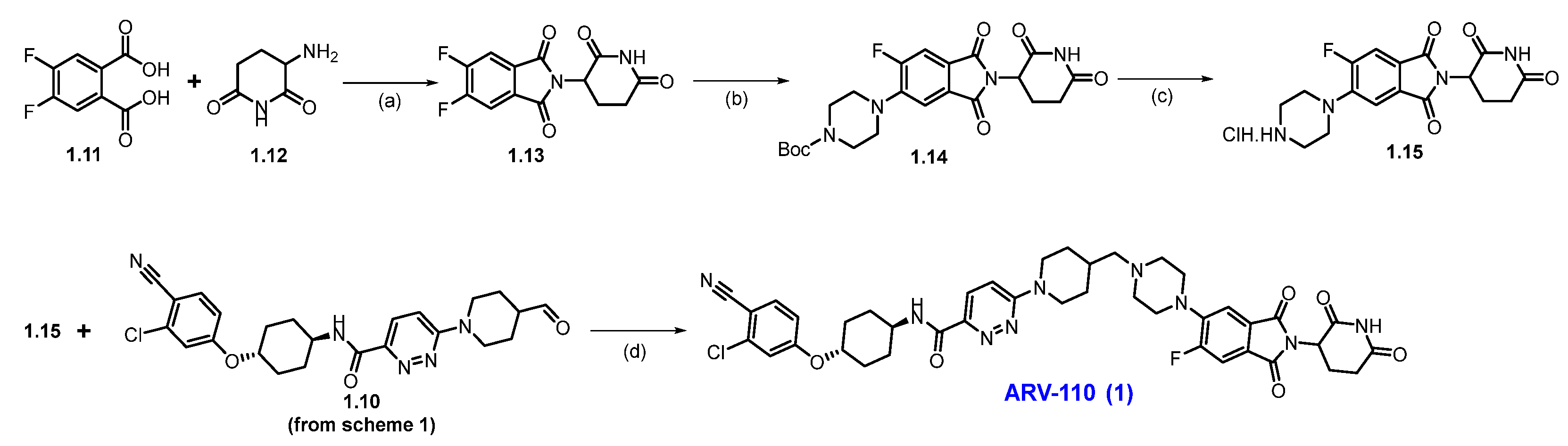
Scheme 2 outlines the final synthesis of ARV-110. Reaction of commercially available 4,5-difluorophthalic acid 1.11 and 3-amino piperidine-2,6-dione 1.12 in acetic acid and sodium acetate (NaOAc), afforded thalidomide-5,6-F 1.13. The next step involved an SNAr reaction with substituted N-boc piperazine scaffold 1.13 and commercially available Boc-piperazine in N-methyl-2-pyrrolidone (NMP) with NaHCO3 at 90 °C, producing N-Boc piperazine substituted product 1.14. Deprotection of the tert-butyloxycarbonyl (Boc) group on 1.14 was carried out with HCl in methanol, giving thalidomide product 1.15 as an HCl salt, which was then recrystallized from isopropanol (IPA). Reductive amination reaction of 1.15 with aldehyde 1.10 (Scheme 1) in DMA using sodium triacetoxyborohydride (Na(OAc)3BH) as a reductant, resulting in crude ARV-110. Crystallization from an ethanol solution afforded ARV-110 (1) as a light yellow crystalline solid [28].
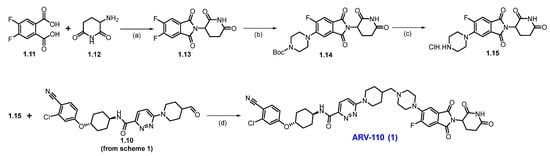
Scheme 2.
Synthesis of ARV-110 (1): (a) Ac2O, CH3COONa, 120 °C, 4 h; (b) Boc-piperazine, NaHCO3, NMP, 90 °C, 16 h; (c) 3M HCl in MeOH, DCM, 35 °C, 21 h; (d) Na(OAc)3BH, NMP, r.t., 3 h.
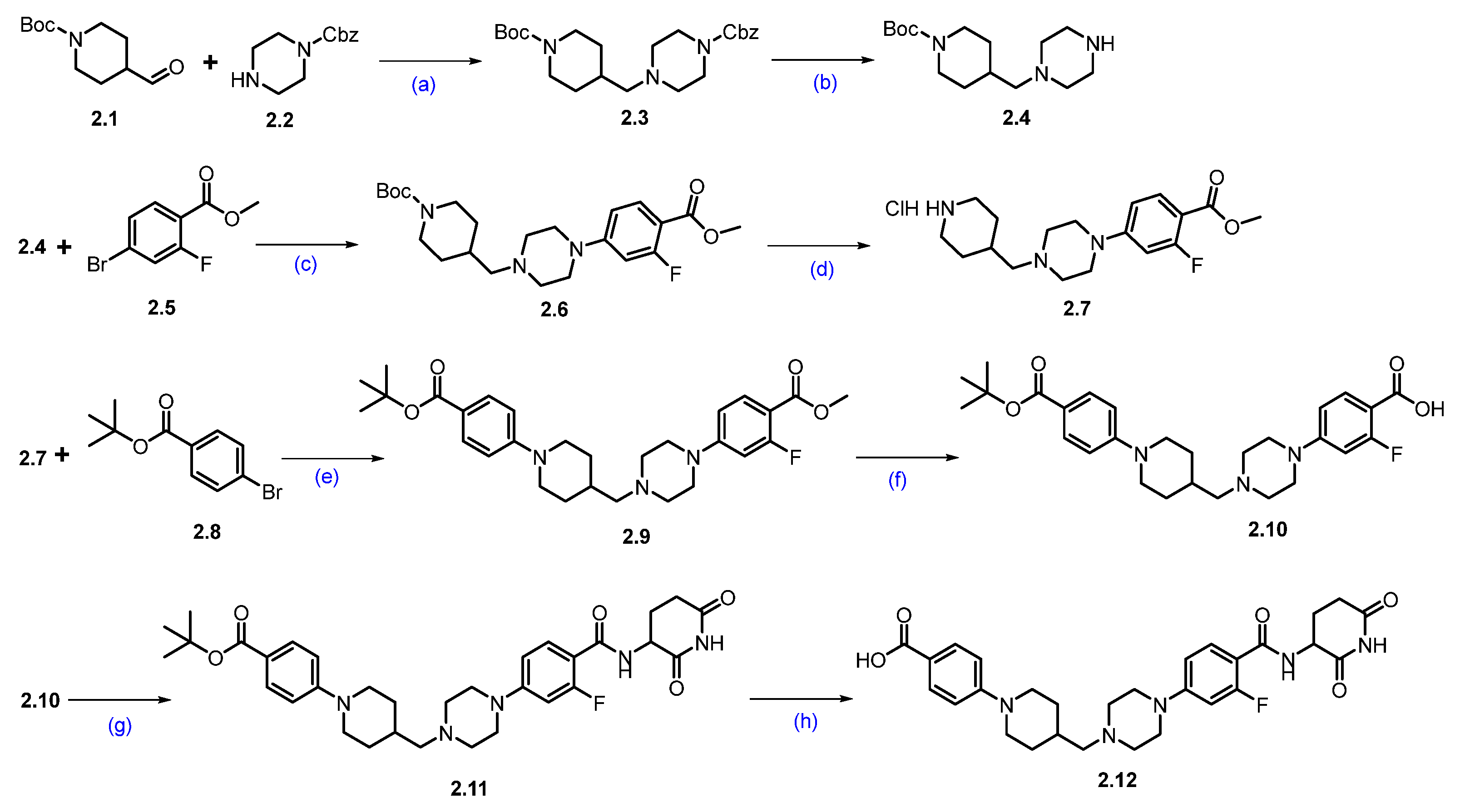
Synthesis of ARV-766 (2). Scheme 3 and Scheme 4 illustrate the synthesis of ARV-766. The synthesis started with commercially available aldehyde 2.1 and amine 2.2. The reductive amination of compounds 2.1 and 2.2 afforded reductive alkylated product 2.3, and subsequent deprotection of the benzyloxycarbonyl (Cbz) group using palladium on carbon (Pd/C) in ethanol (EtOH) afforded -boc protected piperazine amine compound 2.4. Under Buchwald coupling conditions, the reaction between amine 2.4 and aryl bromo derivative 2.5 afforded 2.6. Following this, Boc deprotection was carried out using 4N HCl in dioxane in ethyl acetate (EtOAc) to generate scaffold 2.7. A second Buchwald coupling reaction between piperidine amine, 2.7, and substituted bromobenzene, 2.8 resulted in the formation of the coupled compound 2.9, which, after being subjected to saponification with Lithium hydroxide (LiOH), produced the acid compound 2.10. Subsequently, acid-amine coupling of 2.10 and 3-aminopiperidine-2,6-dione in the presence of EDC.HCl in DMF yielded amide compound 2.11 and deprotection of the tert-butyl group using 4N HCl in dioxane in EtOAc to afford key carboxylic acid compound 2.12 (Scheme 3).
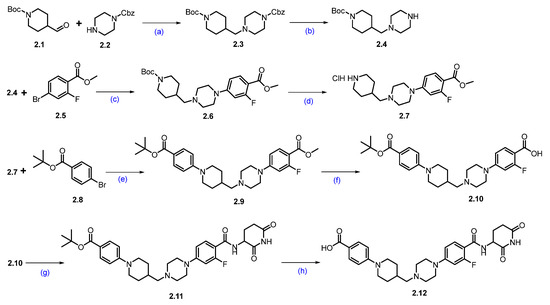
Scheme 3.
Synthesis of fragment 2.12 of ARV-766 (2): (a) NaCNBH3, MeOH, r.t., 10 h; (b) 10%Pd/C, H2, EtOH, 50 °C, 12 h; (c) Pd(OAc)2, BINAP, Cs2CO3, dioxane, 110 °C, 16 h; (d) 4N HCl in dioxane, EtOAc, 1 h; (e) Pd(OAc)2, BINAP, Cs2CO3, dioxane, 110 °C, 16 h; (f) LiOH.H2O, MeOH, H2O, 40 °C, 10 h; (g) 3-aminopiperidine-2,6-dione, EDC.HCl, HOBt, DIPEA, DMF, r.t., 10 h; (h) 4N HCl in dioxane, EtOAc, 1 h.
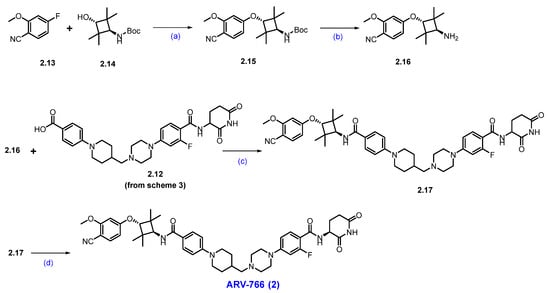
Scheme 4.
Synthesis of ARV-766 (2): (a) NaH, DMF, 0 °C to 20 °C, 4 h; (b) 4N HCl in dioxane, DCM, 1 h; (c) EDC.HCl, HOBt, DIPEA, DMF, r.t., 24 h; (d) SFC purification.
The final synthesis of ARV-766 is described in Scheme 4. The SNAr reaction of commercially available compounds 2.13 and 2.14, in the presence of NaH in dimethylformamide (DMF), produced ether product 2.15 in 64% yield. Boc deprotection of 2.15 using 4N HCl in dioxane-furnished amine 2.16 at a quantitative yield. In the final step of the synthesis, acid 2.12 (Scheme 3) was coupled with amine 2.16 in the presence of EDC.HCl in DMF to furnish ARV-766, as a diastereomeric mixture, it was separated by SFC to obtain enantiopure ARV-766 (2) [14].
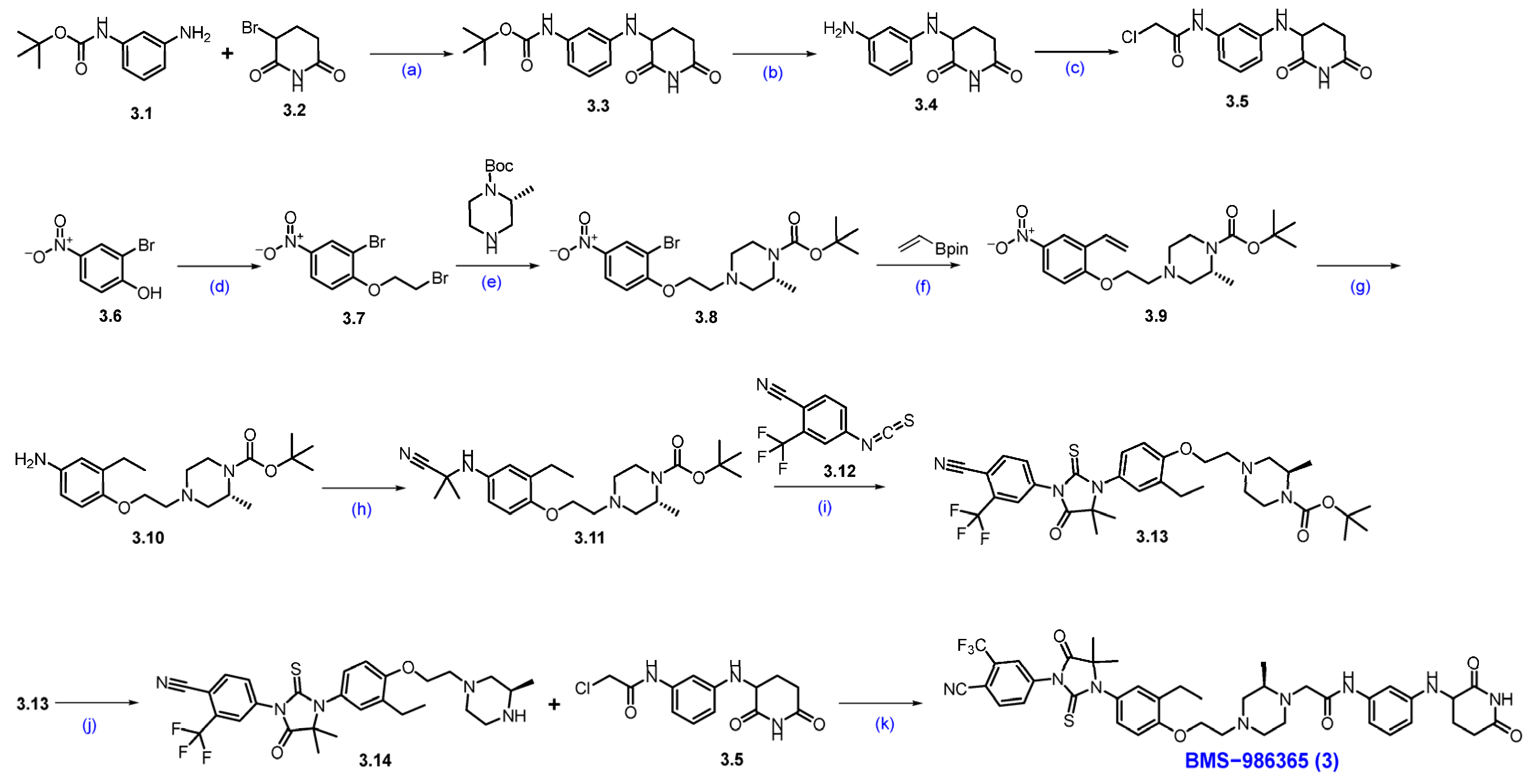
Synthesis of BMS−986365 (3). The synthesis started with commercially available compounds tert-butyl (3-aminophenyl)carbamate 3.1 and 3-bromopiperidine-2,6-dione 3.2 [29]. Nucleophilic substitution reaction of 3.1 with 3.2 gave amine substituted product 3.3. Deprotection of the Boc group of compound 3.3 with 4N HCl furnished compound 3.4 (Scheme 5). Subsequently, amidation reaction using 2-chloroacetic acid with 3.4 afforded the compound 3.5. The synthesis of another part of the molecule began with 2-bromo-4-nitrophenol 3.6, which was converted to compound 3.7 by following a substitution reaction with 1,2-dibromoethane. Compound 3.7 was then subjected to another substitution reaction with tert-butyl-(R)-2-methylpiperazine-1-carboxylate in the presence of DIPEA to furnish compound 3.8. Suzuki coupling reaction of compound 3.8 with vinyl boronic acid pinacol ester by using Pd(dppf)Cl2 and K3PO4 furnished compound 3.9. Nitro functional group and vinyl moiety in compound 3.9 were reduced in hydrogenation conditions using Pd/C in methanol to furnish compound 3.10. Compound 3.10 was next converted to compound 3.11 by an addition reaction with 2-hydroxy-2-methylpropanenitrile in the presence of MgSO4, which gave 3.11, which was immediately converted to compound 3.13 following another addition reaction with compound 3.12 in an acidic medium. Deprotection of the Boc group of compound 3.13 using 4N HCl furnished compound 3.14. A substitution reaction of compound 3.14 with compound 3.5 in the presence of DIPEA in DMF furnished the final compound BMS−986365 (3).
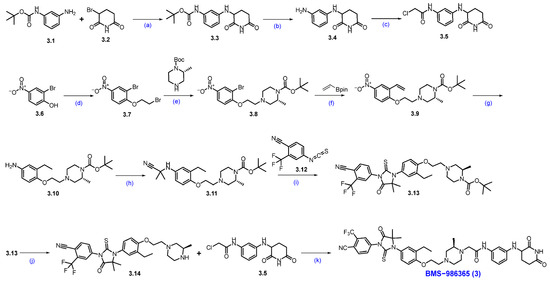
Scheme 5.
Synthesis of BMS−986365 (3): (a) NaHCO3, DMF, 80 °C, 16 h; (b) 4N HCl in dioxane, DCM, 25 °C, 30 min.; (c) 2-Cl-acetic acid, HATU, DIPEA, DMF, 15 °C, 1 h; (d) 1,2-dibromoethane, DIPEA, DMF, 60 °C, 12 h; (e) DIPEA, DMF, 60 °C, 12 h; (f) Pd(dppf)Cl2, K3PO4, dioxane, H2O, 90 °C, 12 h; (g) Pd/C, H2, MeOH, 50 psi, 30 °C, 12 h; (h) 2-hydroxy-2-methylpropanenitrile, MgSO4, 60 °C, 12 h; (i) DMF, r.t. for 1 h then HCl in MeOH, 80 °C, 1 h; (j) 4N HCl in dioxane, DCM, r.t., 2 h; (k) DIPEA, DMF, 60 °C, 12 h.
2.2. PROTACS Targeting Estrogen Receptor (ER)
Breast cancer is one of the most common cancers worldwide and the leading cause of cancer-related death among women. In 2023, about 297,790 U.S. women were expected to be diagnosed with invasive breast cancer, with an estimated 43,700 deaths. However, advances in treatment from 1989 to 2020 reduced mortality by 43%, saving around 460,000 lives [30]. Luminal A and luminal B classifications, accounting for 70% of cases, are defined by ER expression, making it a crucial diagnostic and therapeutic target [31]. Standard treatments for ER-positive breast cancers involve estrogen deprivation or direct ER inhibition with selective estrogen receptor modulators (SERMs) such as tamoxifen, or selective estrogen receptor degraders (SERDs), such as Fulvestrant. Tamoxifen is widely prescribed but exhibits tissue-specific effects and can act as a partial ER agonist in some contexts, potentially increasing the risk of secondary cancers with prolonged use [32]. Fulvestrant blocks ER function and promotes its degradation but is limited by poor pharmacokinetic properties and suboptimal target coverage [33].
Therapeutic resistance remains a significant obstacle, affecting 30–50% of patients with advanced metastatic breast cancer. Resistance can arise through various mechanisms, including ligand-independent ER activation via the PI3K/AKT/mTOR [34,35] and RAS/RAF/MEK/ERK [36] signaling pathways, or through gain-of-function mutations in the ESR1 gene. The most frequently observed ESR1 mutations Y537S and D538G occur in the ligand-binding domain and drive estrogen-independent ER activity [37,38]. These mutations reduce the effectiveness of aromatase inhibitors, SERMs, and SERDs. Endocrine resistance can also arise from altered interactions between ER and its coactivators or corepressors, or through compensatory signaling crosstalk between ER and growth factor receptor pathways [39,40].
Consequently, there is a growing interest in the development of next-generation therapies, including orally bioavailable PROTAC-based ER degraders. These agents aim to achieve more complete ER suppression and overcome resistance associated with receptor mutations that confer agonist activity [41,42,43,44]. Notable examples include Arvinas’s PROTAC-based degrader Vepdegestrant. Additional ER-targeting PROTACs, such as AC682 and HRS-1358, are currently in clinical trials, although their molecular structures have not been disclosed [45].
Clinical Development and Outcomes of Vepdegestrant (ARV-471, 4): Data from the 2025 ASCO Annual Meeting, derived from the Phase 3 VERITAC-2 trial (NCT05654623), indicate that Vepdegestrant (ARV-471) provides a statistically significant and clinically meaningful improvement in progression-free survival (PFS) compared to Fulvestrant in previously treated patients with ER+/HER2- breast cancer. The median follow-up was 7.4 months for the Vepdegestrant group (n = 136) and 6.0 months for the Fulvestrant group (n = 134). Median PFS was 5.0 months with Vepdegestrant and 2.1 months with Fulvestrant. At the time of analysis, 58% of PFS events had occurred in the Vepdegestrant arm compared to 71% in the Fulvestrant arm, with ongoing treatment in 33% and 12% of patients, respectively. The six-month PFS rates were 45.2% for Vepdegestrant and 22.7% for Fulvestrant. However, the difference in PFS was less pronounced among the overall study population, with a median PFS of 3.8 months for Vepdegestrant and 3.6 months for Fulvestrant. In the ESR1-mutant subgroup, Vepdegestrant (n = 121) more than doubled the clinical benefit rate (CBR) compared with Fulvestrant (n = 119) at 42.1% versus 20.2%, respectively. The objective response rate (ORR) was also higher with Vepdegestrant, at 18.6% (n = 97), compared to 4.0% with Fulvestrant. Safety data from all treated patients (Vepdegestrant, n = 312; Fulvestrant, n = 307) indicated that both treatments were generally well tolerated [5,46,47].
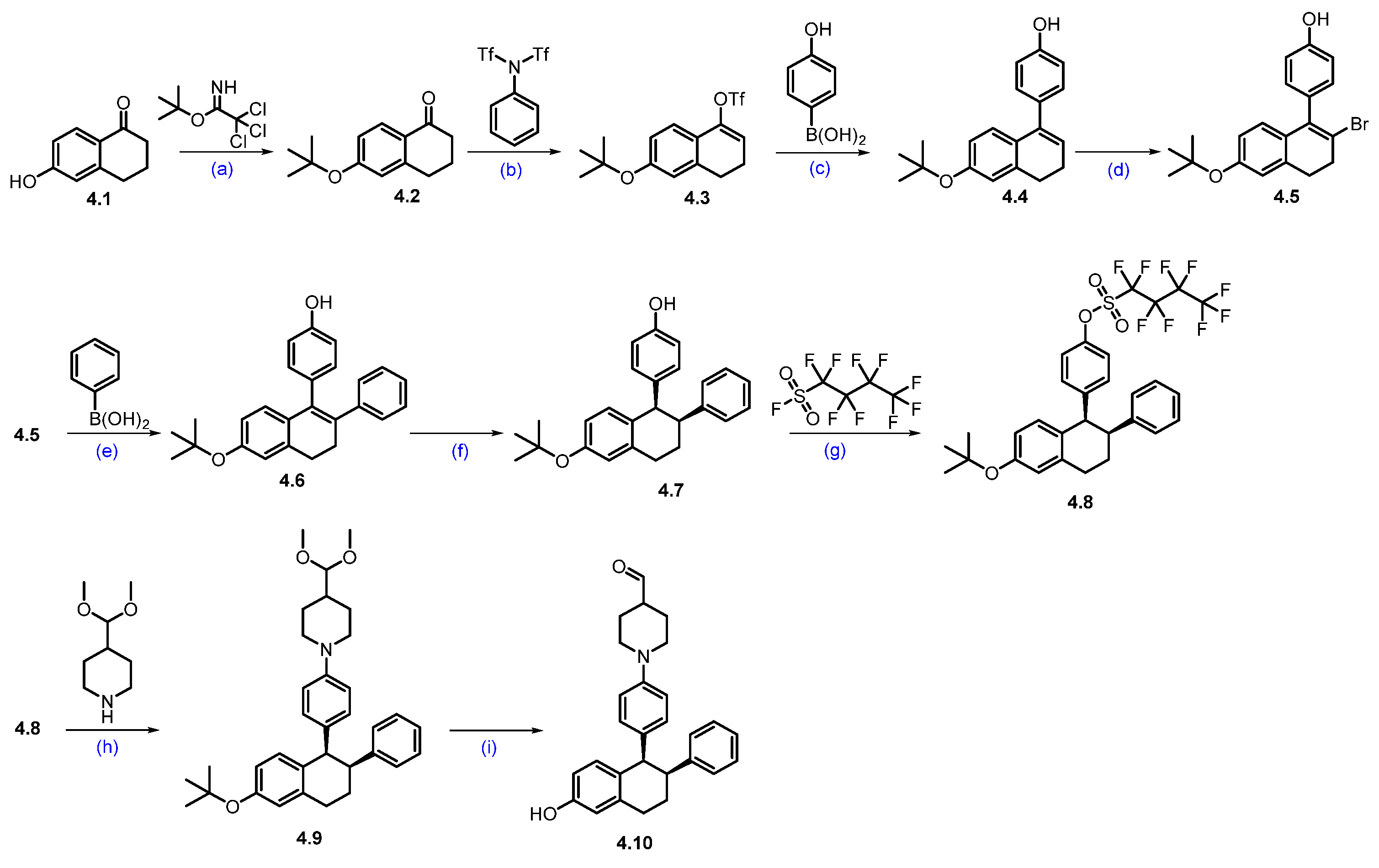
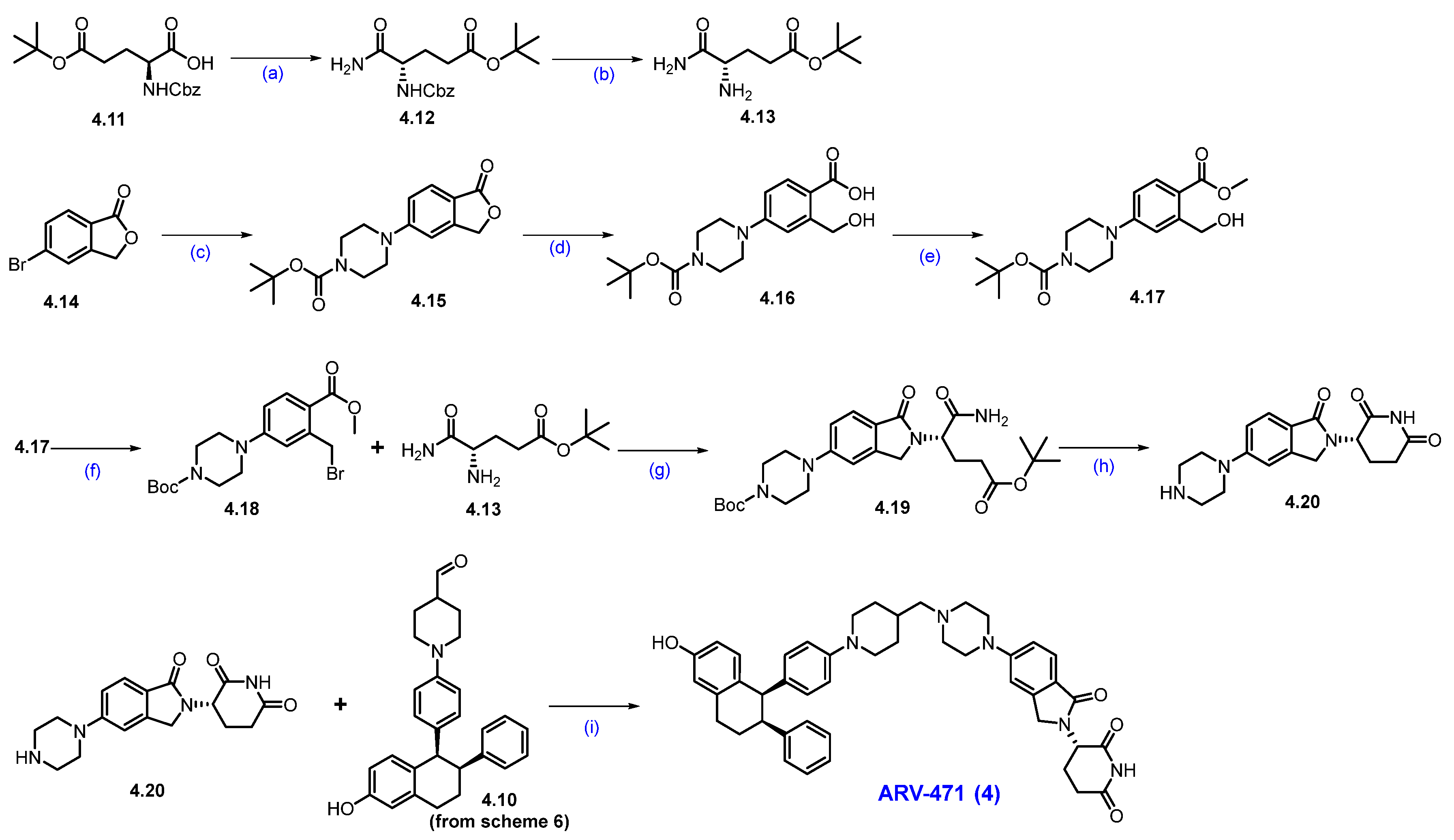
Synthesis of ARV-471 (4) [48]. The synthesis of ARV-471 was shown in Scheme 6 and Scheme 7. The commercially available phenolic hydroxyl group of 6-hydroxy-1-tetralone 4.1 was treated with tert-butyl 2,2,2-trichloroacetimidate in DCM, which afforded tert-butyl ether derivative 4.2. The ketone in 4.2 was converted into triflate 4.3 with LDA at low temperature to form an enolate, which then reacted with phenyl triflimide, yielding 4.3 in 78% yield. The reactive triflate scaffold underwent a palladium-catalyzed Suzuki–Miyaura coupling with (4-hydroxyphenyl)boronic acid to afford product 4.4 with an 88% yield. Vinylic bromination of 4.4 using NBS in acetonitrile produced the key bromo derivative 4.5. The resultant brominated compound 4.5 was subsequently used in a palladium-catalyzed Suzuki–Miyaura coupling using phenylboronic acid, generating coupled compound 4.6. Reduction of the double bond with Pd/C, followed by chiral SFC separation, yielded the desired syn-disubstituted tetrahydronaphthalene 4.7. The phenolic hydroxyl group in 4.7 was further activated with perfluorobutylsulfonyl fluoride in the presence of K2CO3 in a mixture of THF and acetonitrile, obtaining 4.8 with a 91% yield. Subsequently, the palladium-catalyzed Buchwald–Hartwig amination coupling of nonaflate 4.8 with 4-(dimethoxymethyl)piperidine proceeded efficiently using Pd(OAc)2 as the catalyst, XPhos as the ligand, and t-BuONa as the base in toluene, yielding acetal 4.9 in an excellent 87% yield. Acetal 4.9 was effectively deprotected with 2M H2SO4 in THF, affording aldehyde 4.10 in 92% yield [49,50].
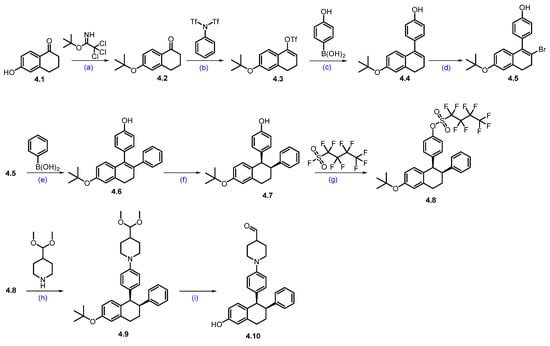
Scheme 6.
Synthesis of aldehyde fragment 4.10 of ARV-471 (4): (a) PPTS, DCM, 10 °C, 16 h; (b) LDA, THF, −70 °C, 1 h then 20 °C for 2 h; (c) Pd(dppf)Cl2, K2CO3, dioxane, H2O, 100 °C, 10 h; (d) NBS, ACN; r.t., 1.5 h; (e) Pd(dppf)Cl2, K2CO3, dioxane, H2O, 100 °C, 12 h; (f) H2, Pd/C, 50 psi, 30 °C, 36 h then chiral SFC; (g) K2CO3, THF, ACN, r.t., 16 h; (h) Pd(OAc)2, X-phos, NaOtBu, toluene, 90 °C, 16 h; (i) 2M H2SO4, THF, 70 °C, 1 h.
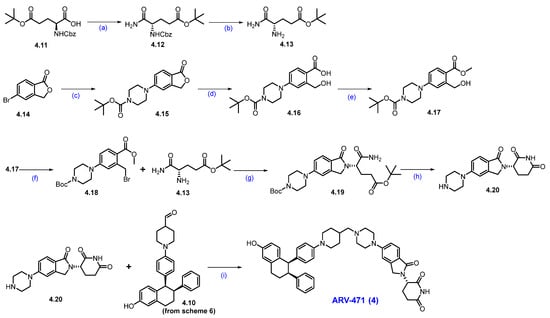
Scheme 7.
Synthesis of ARV-471 (4): (a) pyridine, (Boc)2O, NH4HCO3, 1,4-dioxane, 0 °C to 25 °C, 16 h; (b) 10%Pd/C, H2, MeOH, 50 psi, r.t., 16 h; (c) Pd2(dba)3, X-phos, K3PO4, 1,4-dioxane, 100 °C, 15 h; (d) NaOH, THF. MeOH, H2O, r.t., 16 h; (e) TMS-diazomethane, MeOH/EtOAc, −10 °C, 15 min; (f) CBr4, PPh3, THF; r.t., 1 h; (g) DIPEA, ACN, 80 °C, 12 h; (h) benzenesulfonic acid, ACN, 85 °C, 12 h; (i) NaCNBH3, NaOAc, MeOH, 1,2-DCE, 20 °C, 12 h.
Next, the commercially available acid 4.11 was converted into the amide derivative 4.12 using ammonium bicarbonate (NH4HCO3), Boc anhydride ((Boc)2O), and pyridine in 1,4-dioxane. The -Cbz group in 4.12 was then removed using Pd/C in MeOH, resulting in amine 4.13. Next, commercially available 5-bromophthalide 4.14 and Boc-piperazine underwent Buchwald coupling to produce compound 4.15. This compound was hydrolyzed with NaOH to yield carboxylic acid derivative 4.16, which was subsequently converted to ester 4.17 using TMS-diazomethane (Scheme 7).
The benzylic alcohol in compound 4.17 was subjected to an Appel reaction using CBr4 and PPh3 in THF, resulting in the formation of the benzylic bromide scaffold 4.18. Aliphatic amine derivative 4.13 was then reacted with 4.18 in acetonitrile in the presence of DIPEA to yield compound 4.19. In situ cyclization and -Boc deprotection of 4.19 were achieved by heating it in acetonitrile with benzenesulfonic acid, resulting in the key scaffold amine 4.20 as a benzenesulfonic acid salt. Finally, a reductive amination between 4.20 and 4.10 (Scheme 6) in the presence of NaOAc and sodium cyanoborohydride (NaCNBH3) produced ARV-471 (4) with a yield of 35% [48].
2.3. PROTACS Targeting Bromodomain-Containing Protein 9 (BRD9)
Synovial sarcoma is a rare, aggressive cancer mainly affecting young men under 30, with about 1000 cases annually. It usually arises near large joints in the limbs. While therapies targeting BRD9 have been explored, conventional inhibition has been ineffective due to BRD9′s role as a scaffolding protein in complex cellular processes. Targeted degradation of BRD9 presents a promising therapeutic alternative. While classical inhibitors that disrupt BRD9′s have yielded limited phenotypic responses, genetic knockdown has shown more profound effects, underscoring the limitations of traditional small-molecule inhibitors. A key challenge is that epigenetic regulators like BRD9 typically possess multi-domain architectures and function within multi-subunit chromatin remodeling complexes, which diminishes the impact of bromodomain inhibition alone. As a result, these inhibitors do not completely shut down the oncogenic transcription driven by BRD9 [51,52]. Importantly, both synovial sarcoma and SMARCB1-deficient tumors exhibit a critical dependence on BRD9, a core component of the noncanonical SWI/SNF complex that includes the SS18-SSX fusion oncoprotein. This creates a synthetic lethal vulnerability: cancer cells harboring the SS18-SSX fusion require BRD9 for oncogenic transcription and proliferation, whereas normal tissues show minimal dependence on BRD9 [53]. As a result, targeted degradation of BRD9 represents a promising therapeutic strategy, selectively eliminating cancer cells through exploitation of this synthetic lethal dependency [54,55,56].
Clinical Development and Outcomes of CFT8634 (5) and FHD-609 (6): Two promising BRD9 PROTACs, CFT8634 and FHD-609, have advanced into clinical trials. They are designed to degrade BRD9 specifically for the treatment of advanced and metastatic synovial sarcoma, ensuring selectivity over BRD7. However, in April 2023, Foghorn Therapeutics suspended patient enrollment in the FHD-609 trial after a participant experienced a serious grade 4 QTc prolongation at the second-highest dose. Patients affected by this event have been transitioned to a reduced dose, while the FDA has placed the study under a partial clinical hold, allowing those who are responding to the treatment to continue (NCT04965753) [57]. C4 Therapeutics has also introduced an oral BRD9 degrader, CFT8634, intended for patients with synovial sarcoma and SMARCB1-null solid tumors (NCT05355753). Despite achieving high levels of BRD9 degradation in the Phase 1 clinical trial, further investigation was halted in November 2023 due to inadequate efficacy as a standalone therapy [58,59].
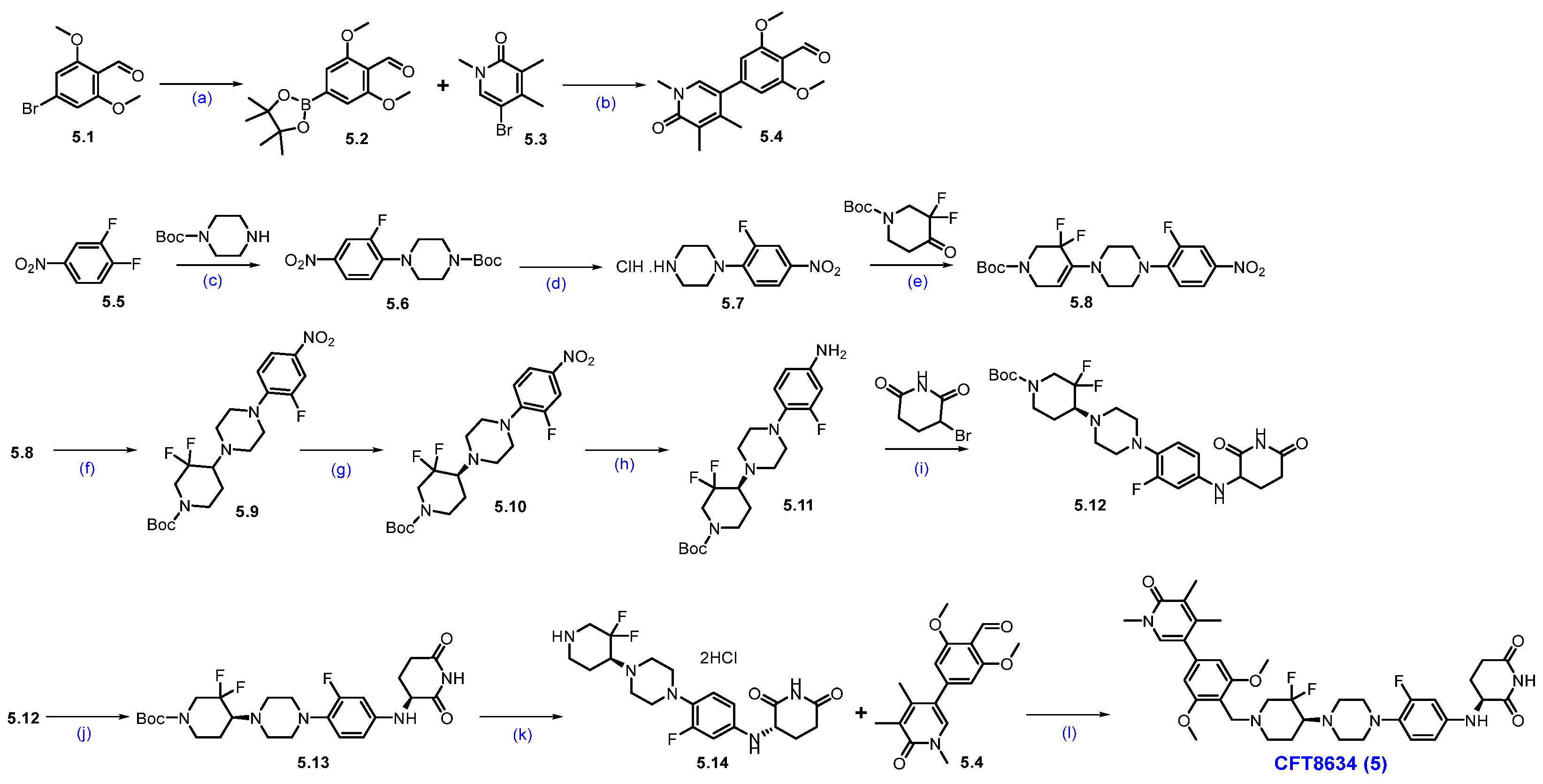
Synthesis of CFT-8634 (5): Scheme 8 outlines the synthesis of CFT8634 as disclosed in the patent application by C4 Therapeutics [60]. The synthetic route begins with aldehyde 5.1, which underwent a Suzuki coupling reaction with bis(pinacolato)diboron to yield boronate ester 5.2. The aryl boronate scaffold is then coupled with aryl bromo compound 5.3 via another Suzuki reaction to produce di-aryl compound 5.4. In parallel, 1,2-difluoro-4-nitrobenzene (5.5) reacted with Boc-piperazine in a nucleophilic substitution to give N-Boc-piperazine substituted product 5.6, which was subsequently Boc-deprotected to form amine 5.7 as a HCl salt. Reductive amination of amine 5.7 with tert-butyl 3,3-difluoro-4-oxopiperidine-1-carboxylate, with NaCHBH3, produces derivative 5.9. A chiral resolution of 5.9 yielded the enantiomerically pure compound 5.10. The nitro group in 5.10 was reduced using hydrogen gas and palladium on carbon, generating aryl amine 5.11. Subsequent nucleophilic substitution of amine 5.11 with a Bromo piperidinedione affords compound 5.12. Chiral chromatographic separation then isolates the desired diastereomer 5.13. Final Boc deprotection of 5.13 using HCl in isopropyl acetate generates amine 5.14, which underwent reductive amination with aldehyde 5.4 to yield the final product, CFT8634 (5).
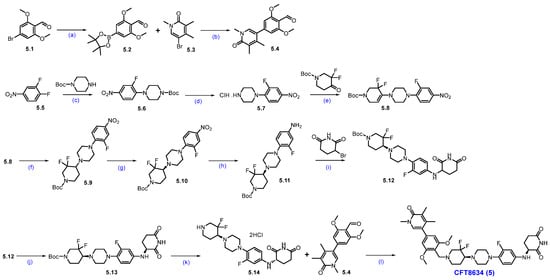
Scheme 8.
Synthesis of CFT8634 (5): (a) B2(Pin)2, KOAc, Pd(dppf)Cl2, dioxane, 140 °C, 40 min.; (b) K2CO3 (aq. sol.), 120 °C, MW irradiation, 30 min.; (c) Cs2CO3, DMF, r.t., 16 h; (d) 4M HCl in dioxane, r.t., 2 h; (e) NaOAc, AcOH, ACN, 135 °C, 12 h; (f) NaCNBH3, 1,2-DCE/MeOH, AcOH, r.t., 24 h; (g) SFC separation (h) 10% Pd/C/H2, EtOAc; (i) NaHCO3, DMF, 70 °C; (j) Chiral HPLC separation; (k) HCl in iPrOAc, r.t., 10 h; (l) Na(OAc)3BH, AcOH, DIPEA, DMAc, −5 °C to r.t., 16 h.
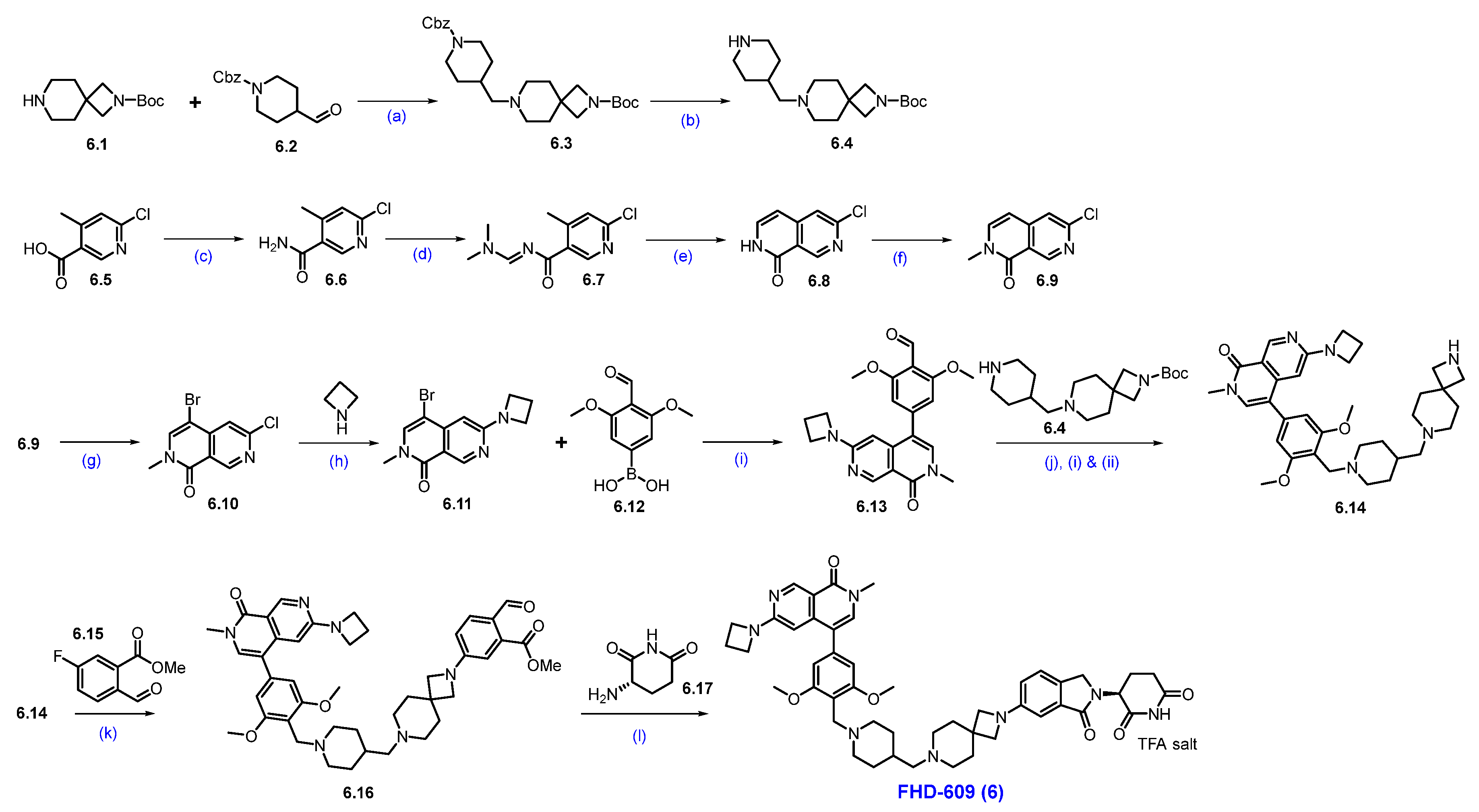
Synthesis of FHD-609 (6): Scheme 9 describes the synthesis of FHD-609 [61]. The synthesis started with a reductive amination reaction of amine 6.1 with aldehyde 6.2 to produce 6.3. The Cbz group in 6.3 was deprotected under hydrogenation conditions, affording free amine 6.4. Separately, acid 6.5 is converted to amide 6.6 through an amidation reaction using ammonium chloride. Formation of an imine 6.6 from compound 6.7, followed by cyclization under strongly basic conditions, yielded Cl-naphthyridone derivative 6.8. An N-methylation reaction of 6.8 with sodium hydride and methyl iodide yielded compound 6.9, which, upon bromination with NBS, furnished bromo derivative 6.10. Substitution reaction of 6.10 with cyclobutyl amine furnished compound 6.11. Subsequent Suzuki coupling reaction of 6.11 with the desired substituted phenylboronic acid 6.12 furnished product 6.13. Reductive amination of 6.13 with amine 6.4, followed by Boc deprotection, furnishes key scaffold 6.14. An SNAr reaction of 6.14 with compound 6.15 using Na2CO3 and DMA under heating conditions furnished compound 6.16. Finally, a reductive amination of aldehyde 6.16 with amine 6.17, followed by cyclization, completes the synthesis of FHD-609 (6).
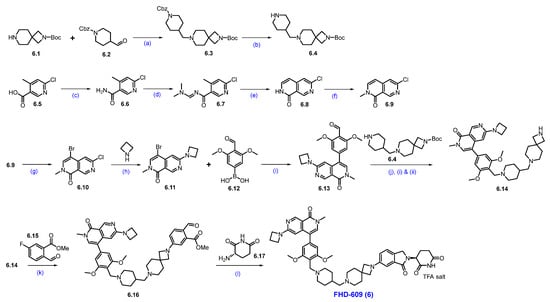
Scheme 9.
Synthesis of FHD-609 (6): (a) Na(OAc)3BH, NaOAc, MeOH, r.t.; (b) Pd/C, H2, MeOH, 40 psi, r.t., 16 h; (c) NH4Cl, HATU, DIPEA, DCM, r.t., 3 h; (d) DMF-DMA, 2-Me-THF, 80 °C, 1 h; (e) t-BuOK, THF, 60 °C, 30 min.; (f) NaH, MeI, THF, 0 °C to r.t., 12 h; (g) NBS, DMF, 90 °C, 2 h; (h) K2CO3, DMSO, 130 °C, 2 h; (i) Pd(dppf)Cl2, Cs2CO3, dioxane, H2O, 70 °C, 2 h; (j) (i) Na(OAc)3BH, NaOAc, NMP, MeOH; (ii) 5% H2SO4, heating; (k) Na2CO3, DMA, reflux, 16 h; (l) NaCNBH3, AcOH, THF/MeOH, 0 °C to r.t., 16 h.
2.4. PROTACs Targeting B-Cell Lymphoma–Extra Large (Bcl-xL)
T-cell lymphoma (TCL) is a rare and difficult-to-diagnose hematologic malignancy that originates from T lymphocytes. These cancerous cells evade apoptosis by overexpressing anti-apoptotic members of the Bcl-2 protein family, particularly Bcl-xL. Traditional small-molecule inhibitors such as ABT263 (navitoclax) have shown limited clinical success in advanced TCL due to dose-limiting thrombocytopenia, as Bcl-xL is also essential for platelet survival. To overcome this challenge, researchers developed DT2216, a VHL-recruiting PROTAC designed to selectively degrade Bcl-xL in tumor cells while sparing platelets. This tissue-selectivity arises from the differential expression of the E3 ligase VHL: platelets express very low levels of VHL, making them less vulnerable to Bcl-xL degradation, whereas tumor cells with higher VHL expression are effectively targeted [62,63]. DT2216 has received orphan drug designation for the treatment of TCL and small cell lung cancer, as well as fast track designation for adult patients with R/R peripheral TCL (PTCL) and cutaneous TCL (CTCL). DT2216 represents a therapeutic approach, demonstrating how targeted protein degradation can leverage differential E3 ligase expressions to enable tissue-selective cancer treatment with improved safety profiles [64,65].
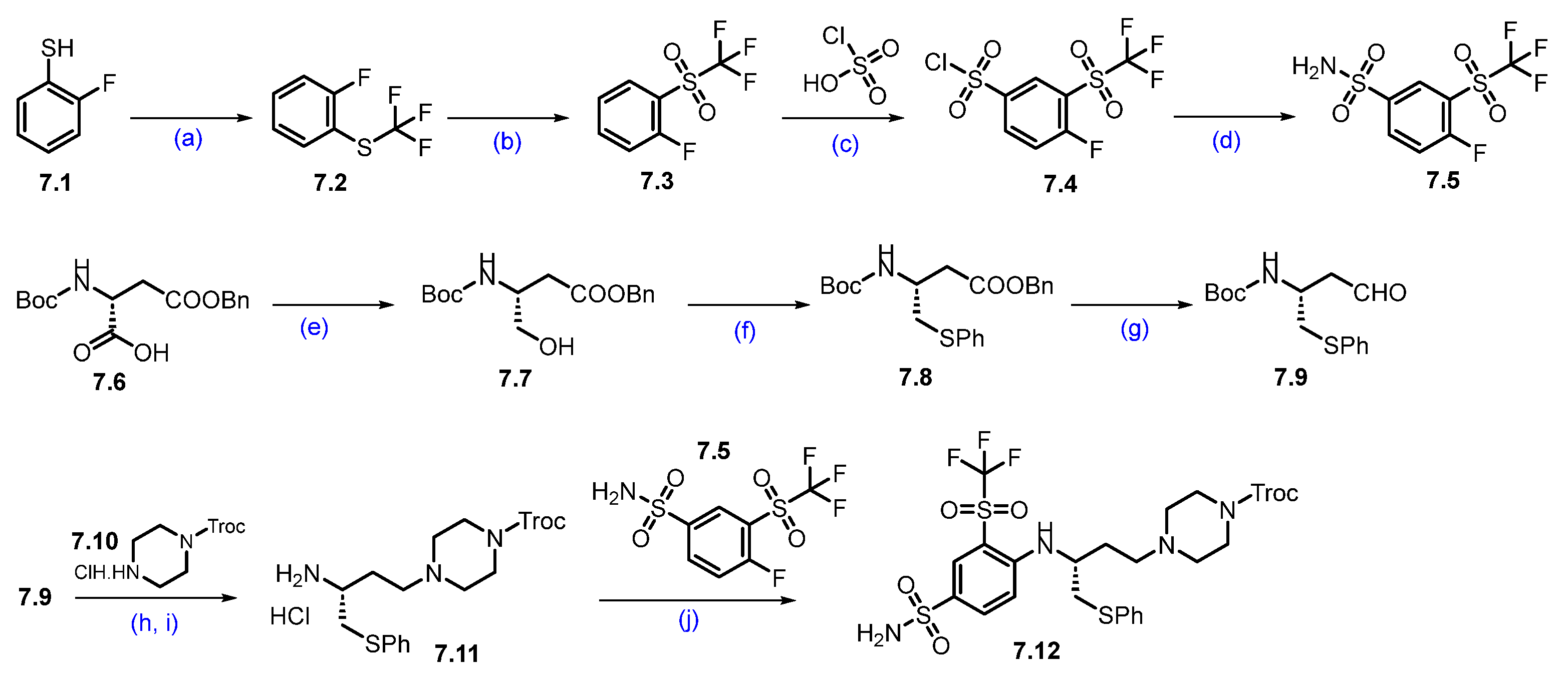
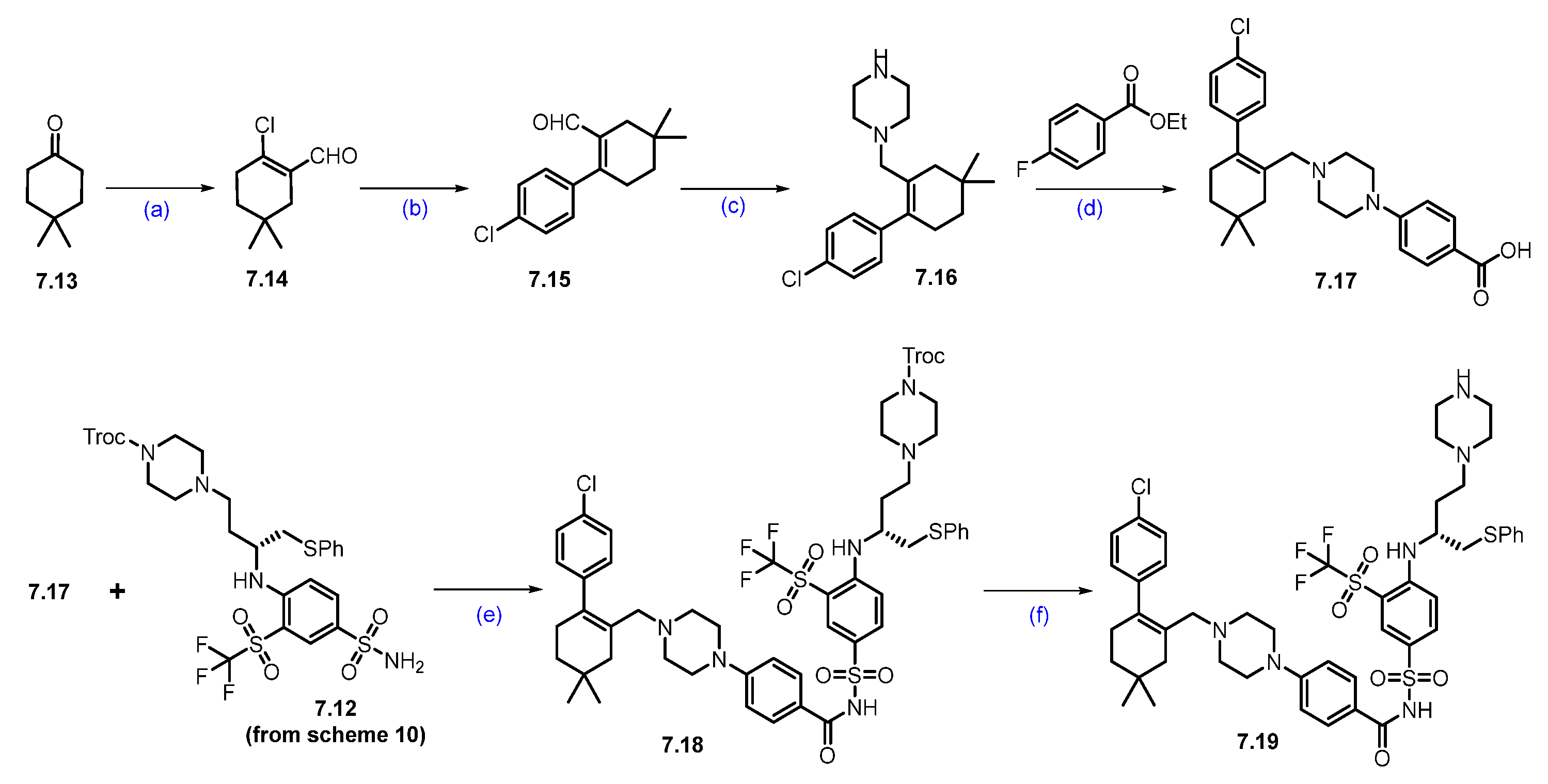
Synthesis of DT-2216 (7). The synthesis of DT-2216 was described in Scheme 10, Scheme 11 and Scheme 12 [66]. The synthesis was initiated from commercially available fluorothiophenol 7.1. The first step involves trifluoromethylation of 2-fluorobenzene-1-thiol 7.1 with trifluoromethyl iodide and methyl viologen dichloride, which afforded compound 7.2. The compound 7.2 was oxidized with periodic acid using ruthenium(III) chloride hydrate as a catalyst in a CH3CN:H2O solvent mixture, to yield compound 7.3. In the next step, (trifluoromethyl)sulfonylbenzene derivative 7.3 reacted with chlorosulfonic acid at an elevated temperature to give the compound 7.4, which further reacted with aqueous ammonia in isopropyl acetate (iPrOAc) to provide the compound 7.5 (Scheme 10).
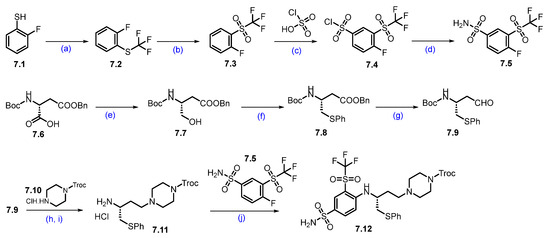
Scheme 10.
Synthesis of fragment 7.12 of DT-2216 (7): (a) CF3I, methyl viologen dichloride, NEt3, DMF, r.t., 16 h; (b) NaIO4; RuCl3.H2O, ACN, H2O, r.t., 3 h; (c) chlorosulfonic acid, 120 °C, 5 h; (d) Isopropyl acetate, aq. NH3; (e) N-methylmorpholine, isobutyl chloroformate, THF, −25 °C to −15 °C then NaBH4; (f) Bu3P, diphenyl disulfide, toluene, 80 °C, 16 h; (g) DIBAL-H, toluene, −78 °C, 2 h; (h) Na(OAc)3BH, NEt3, DCM, r.t., 1 h; (i) 4M HCl in dioxane, DCM, r.t., 1 h; (j) NEt3, ACN, reflux, 4 h.
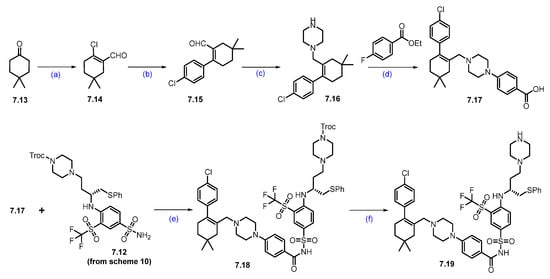
Scheme 11.
Synthesis of fragment 7.19 of DT-2216 (7): (a) POCl3, PBr3, DMF, 0 °C to 60 °C, 16 h; (b) 4-Cl-C6H4B(OH)2, PdCl2(PPh3)2, KOAc, 1,4-dioxane, H2O, 90 °C, 12 h; (c) NaCNBH3, TFA/DCM, (d) DABCO, LiOH; (e) EDC.HCl, DMAP, DCM, r.t., 16 h; (f) Zn, AcOH, THF, r.t., 16 h.
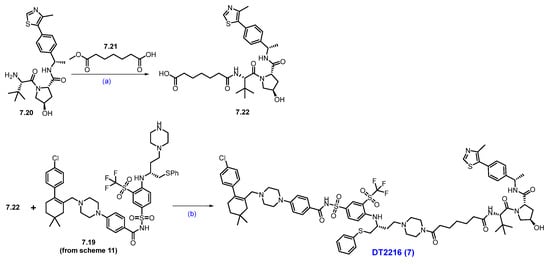
Scheme 12.
Synthesis of DT-2216 (7): (a) HATU, NEt3, DCM, 0 °C to r.t., 2 h; LiOH, MeOH, H2O, r.t., 3 h; (b) HATU, NEt3, DCM, r.t., 2 h.
Next, Boc-D-Asp(OBzl)-OH (7.6) was first converted to its corresponding acid chloride, which was then reduced with sodium borohydride (NaBH4) to afford alcohol 7.7. The alcohol 7.7 was converted to sulfide 7.8 using diphenyl disulfide. The ester functionality in compound 7.8 was treated with DIBAL-H to furnish aldehyde 7.9. Compound 7.9 was converted to amine 7.11 following a two-step protocol. In the first step, the aldehyde reacted with Troc-protected piperazine 7.10 under reductive amination conditions. In the next step, 4M HCl-mediated -Boc deprotection afforded compound 7.11 as a HCl salt. Amine 7.11 was reacted with 7.5 in an SNAr condition to furnish product 7.12.
As shown in Scheme 11, compound 7.19 was prepared from 4,4-dimethylcyclohexan-1-one (7.13) through a sequence of reactions. In the first step, the commercially available compound 4,4-dimethylcyclohexanone was treated with PBr3 and POCl3 in DMF to obtain aldehyde 7.14, which was coupled with 4-chloro phenylboronic acid using Suzuki conditions to give compound 7.15. The aldehyde functionality of 7.15 was reacted with Boc-piperazine under reductive amination conditions, followed by Boc-deprotection, to furnish 7.16. The scaffold 7.16 was added to 4-fluoro ethyl benzoate in an SNAr condition, followed by a saponification reaction, which furnished acid 7.17. An EDC.HCl-mediated coupling of acid 7.17 and sulfonamide 7.12 furnished compound 7.18. Finally, the Troc-protecting group was deprotected using Zinc (Zn) and acetic acid (AcOH) to obtain key scaffold 7.19 [66].
Next, commercially available amine 7.20 was converted to acid derivative 7.22 following a two-step protocol (Scheme 12). In the first step, HATU-mediated coupling of amine 7.20 with acid 7.21 formed an amide, and in the second step, the methyl ester was saponified using LiOH to furnish the acid 7.22. Finally, an acid–amine coupling reaction was carried out between acid 7.22 and amine 7.19 (Scheme 11) utilizing HATU and NEt3 in DCM to obtain DT-2216 (7) [66].
2.5. PROTACs Targeting Bruton’s Tyrosine Kinase (BTK)
BTK plays a vital role in B cell receptor signaling, which is essential for B cell development, function, and survival. B-cell malignancies such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) rely heavily on BTK signaling for proliferation and survival. CLL is the most prevalent form of leukemia among adults in Western countries, accounting for roughly 30% of all leukemia cases [67]. Covalent and non-covalent Bruton’s tyrosine kinase inhibitors (BTKis), such as ibrutinib and zanubrutinib, are widely used in the treatment of B-cell malignancies. However, disease progression during BTKi therapy is often driven by the emergence of resistance mutations [68]. The most common mechanism of resistance in chronic lymphocytic leukemia (CLL) during prolonged exposure to covalent BTK inhibitors is the BTK Cys481Ser (C481S) mutation. This mutation disrupts the covalent binding required for inhibitor efficacy, rendering these therapies ineffective [69,70,71].
To address this resistance, a new generation of non-covalent BTK inhibitors (ncBTKi), including pirtobrutinib and nemtabrutinib, has been developed. These inhibitors do not rely on binding to Cys481 and have shown encouraging efficacy. However, resistance to ncBTKi can still emerge through a variety of additional BTK mutations. These include gatekeeper mutations (T474I/F/S/L/Y), mutations at the C381 residue (C381S/R/Y), kinase-impaired variants such as L528W, and mutations near the ATP-binding pocket (e.g., D539A, V416L, Y545N, and A428D) [72]. These mutations not only interfere with inhibitor binding but may also promote the recruitment of alternative kinases or confer a neomorphic scaffolding function to BTK, thereby enabling sustained oncogenic signaling despite BTK inhibition [73,74,75].
Unlike traditional inhibitors, BTK degraders eliminate both the kinase activity and the resistance-associated scaffolding function, effectively killing inhibitor-resistant cancer cells. Currently, six BTK-targeting PROTACs are undergoing clinical evaluation for B-cell malignancies. Nurix Therapeutics is at the forefront of this effort, developing two BTK degraders: NX-2127 (NCT04830137), which simultaneously degrades BTK and the transcription factor Ikaros; and NX-5948 (NCT05131022), a next-generation compound designed to selectively degrade BTK [76,77,78].
Clinical Development and Outcomes of NX-2127 (8): As of 9 June 2023, a Phase 1a/b clinical trial had enrolled 47 patients with various B-cell malignancies, including chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), Waldenström macroglobulinemia (WM), and follicular lymphoma (FL). The treatment demonstrated a manageable safety profile, with common adverse events (AEs) including neutropenia, hypertension, anemia, and atrial fibrillation. In terms of efficacy, among the evaluable patients with CLL/SLL, nine achieved partial responses, eleven had stable disease, and four experienced disease progression at the time of data cutoff [79]. These early findings indicate that NX-2127 may offer therapeutic benefits in patients with heavily pretreated B-cell malignancies.
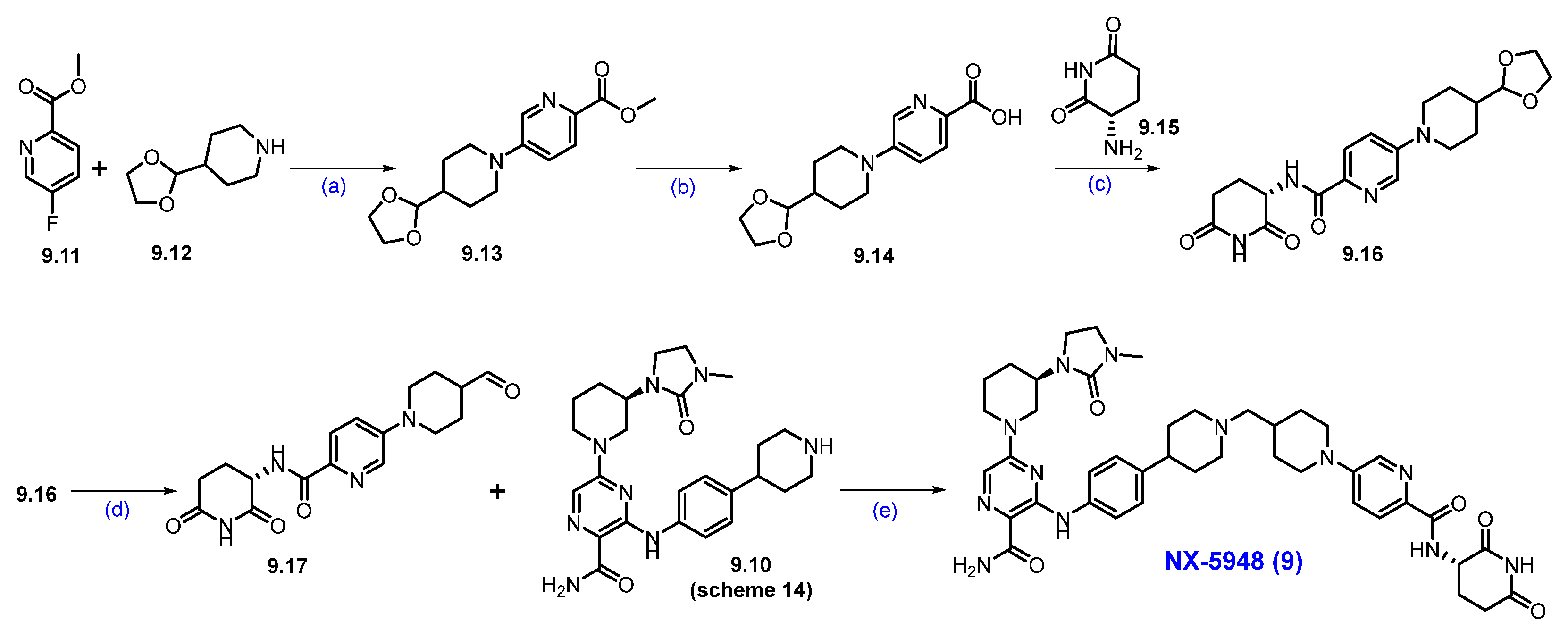
Clinical Development and Outcomes of NX-5948 (9): FDA has granted fast-track designation to NX-5948 for the treatment of adult patients with relapsed/refractory WM who have received at least two lines of therapy, including a BTK inhibitor [80,81,82].
Among 49 evaluable patients with relapsed/refractory CLL/small lymphocytic lymphoma (SLL), NX-5948 achieved an objective response rate (ORR) of 75.5% across all doses tested. Notably, with longer treatment duration, the ORR increased to 84.2% in patients with at least two response assessments. Regardless of prior treatments, baseline mutations, high-risk molecular features, or central nervous system (CNS) involvement, responses were observed. The treatment was well tolerated, with common adverse events including mild to moderate purpura/contusion, fatigue, petechiae, neutropenia, and rash. In the cohort of patients with relapsed/refractory Waldenström’s Macroglobulinemia (WM), NX-5948 demonstrated an ORR of 77.8%, with objective responses observed in 7 out of 9 evaluable patients. The treatment was generally well tolerated, with a safety profile consistent with previous findings. These preliminary findings suggest that NX-5948 is a promising therapeutic candidate for patients with B-cell malignancies, including those resistant to existing BTK inhibitors.
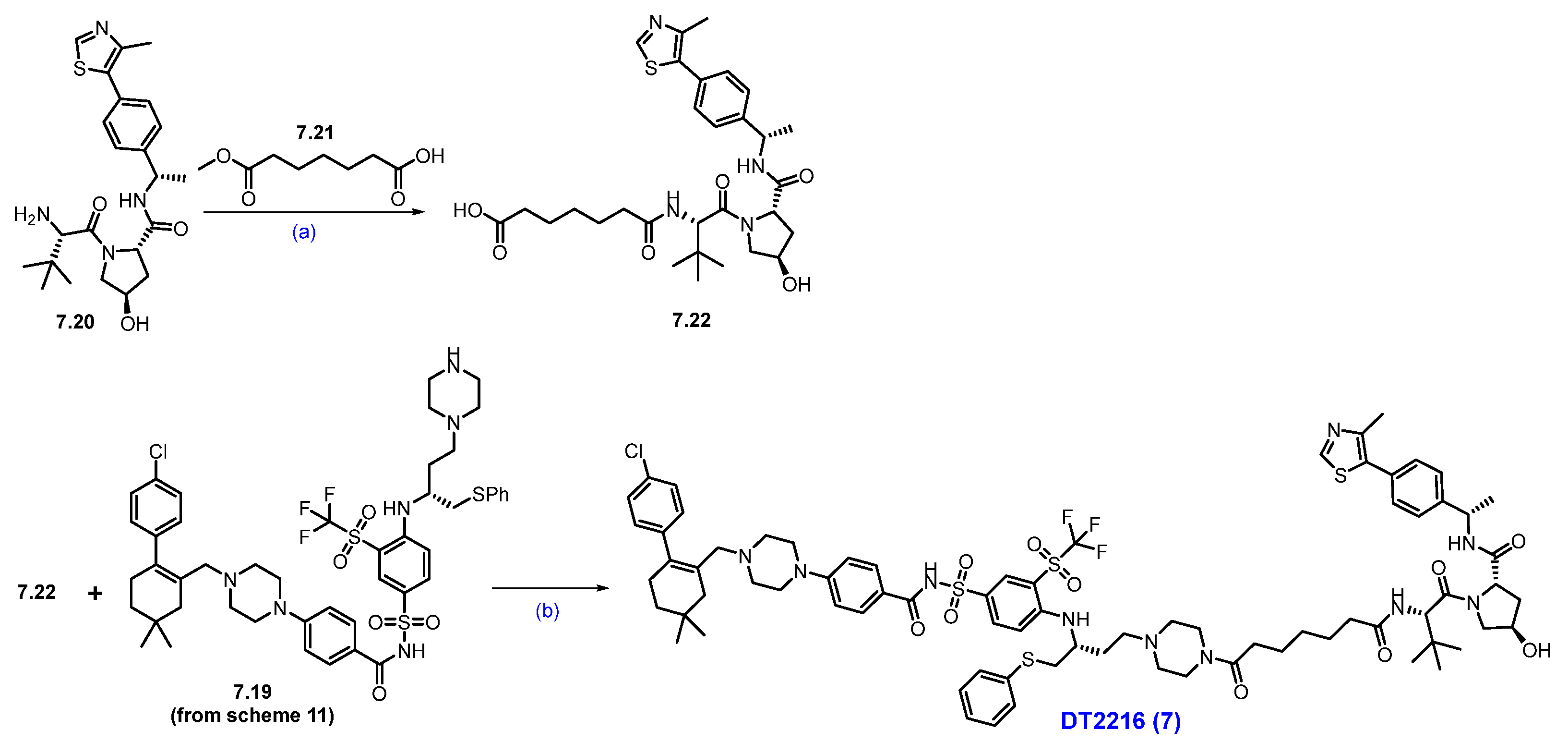
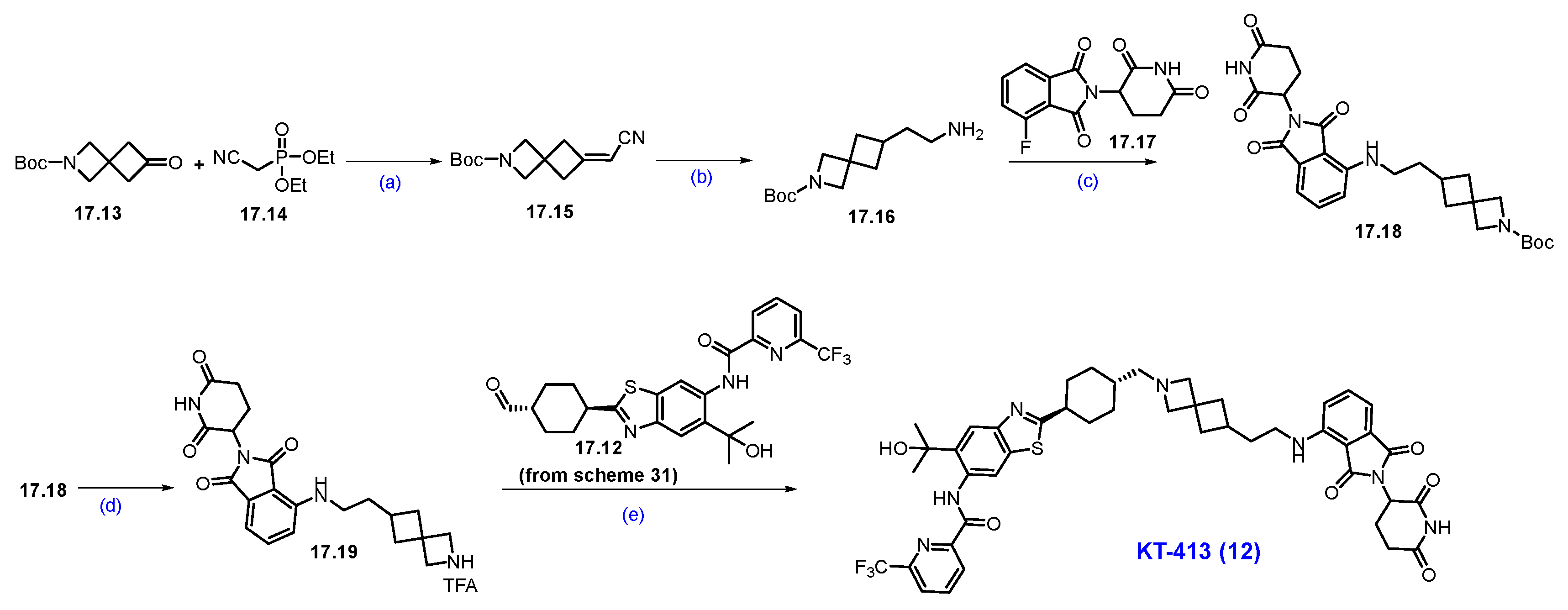
Synthesis of NX-2127 (8): The synthesis of NX-2127 was shown in Scheme 13 [78]. SNAr reaction of commercially available 3,5-dichloropyrazine-2-carbonitrile 8.1 and piperidine in DMF under heating conditions, which afforded product 8.2. It was then subjected to a Buchwald coupling reaction with compound 8.3 to furnish product 8.4. The cyano functionality on 8.4 was oxidized with H2O2/NaOH to afford 8.5. Deprotection of the Boc group in 8.5 using trifluoroacetic acid (TFA) in DCM yielded amine scaffold 8.6. In parallel, an SNAr reaction of commercially available 5-fluoro thalidomide 8.7 with alcohol 8.8 in the presence of DIPEA in NMP to furnish pyrrolidine substituted scaffold 8.9. Compound 8.9 was then oxidized to aldehyde 8.10 using Dess-Martin reagent (DMP) in DCM. Finally, a reductive amination reaction between aldehyde 8.10 and amine 8.6 furnished compound NX-2127 (8).
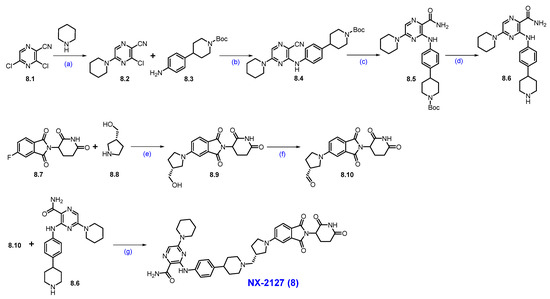
Scheme 13.
Synthesis of NX-2127 (8): (a) Piperidine, DIPEA, DMF, r.t., 2 h; (b) BINAP, Pd(OAc)2, Cs2CO3, dioxane, 90 °C, 1.5 h; (c) H2O2, NaOH, MeOH, DMSO, r.t, 30 min.; (d) TFA, DCM, r.t., 2 h; (e) DIPEA, NMP, 80 °C, 12 h; (f) DMP, DCM, r.t., 16 h; (g) Na(OAc)3BH, DIPEA, dioxane, r.t., 16 h.
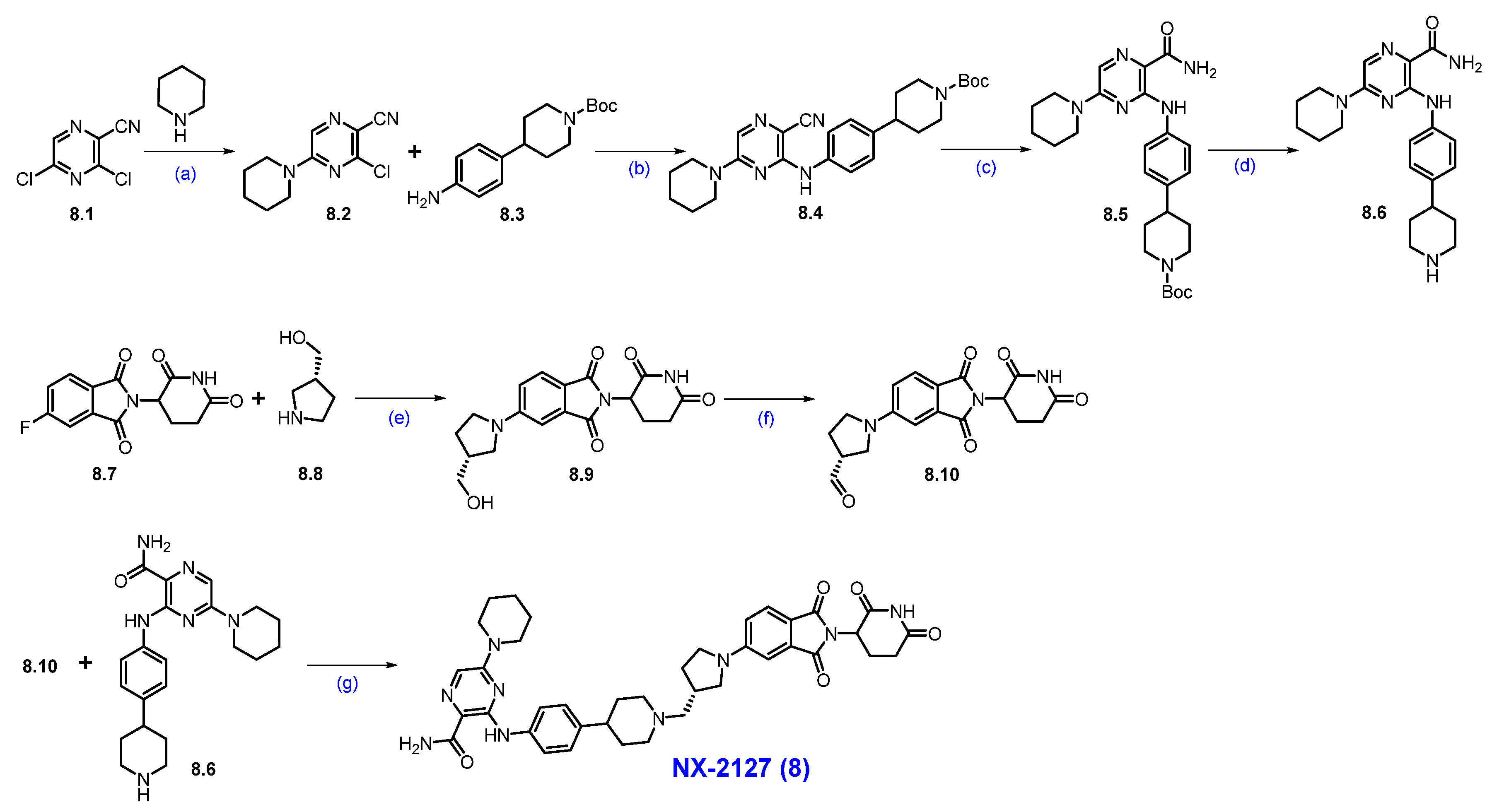
Synthesis of NX-5948 (9): The synthesis of NX-5948 was shown in Scheme 14 and Scheme 15 [83]. The first step, commercially available tert-butyl-(R)-3-aminopiperidine-1-carboxylate 9.1 and 1-chloro-2-isocyanatoethane 9.2 were reacted in the presence of triethylamine in DCM to furnish compound 9.3. Intramolecular SN2 reaction of compound 9.3 in the presence of NaH produced cyclized moiety 9.4. N-methylation of compound 9.4 with methyl iodide yielded compound 9.5. The Boc protecting group in 9.5 was removed using HCl in dioxane, yielding the free amine 9.6. Next, an aromatic nucleophilic substitution between compound 8.1 and amine 9.6 in the presence of diisopropylethylamine (DIPEA) in DMF yielded derivative 9.7. Subsequently, Buchwald amination of 9.7 with amine 8.3, using Pd(OAc)2 and BINAP as the catalytic system, provided compound 9.8. Oxidation of the cyano group in 9.8 with hydrogen peroxide and cesium carbonate (Cs2CO3) afforded compound 9.9. Finally, Boc deprotection of 9.9 using TFA in DCM furnished the key scaffold amine 9.10.
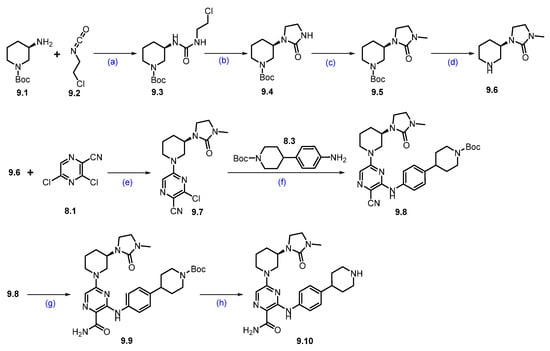
Scheme 14.
Synthesis of fragment 9.10 of NX-5948 (9): (a) NEt3, DCM, 0 °C to r.t., 3 h; (b) NaH, THF, 0 °C to r.t., 10 h; (c) NaH, MeI, THF, 0 °C to r.t., 16 h; (d) 4N HCl in dioxane, r.t., 2 h; (e) DIPEA, DMF, 0 °C to r.t., 16 h; (f) Pd(OAc)2, BINAP, Cs2CO3, 110 °C, 3 h; (g) H2O2, Cs2CO3, MeOH, DMSO, 0 °C to r.t., 2 h; (h) TFA in DCM, r.t., 30 min.
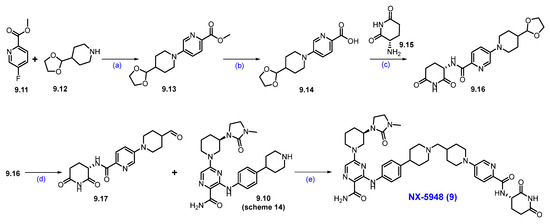
Scheme 15.
Synthesis of NX-5948 (9): (a) DIPEA, DMSO, 100 °C, 14 h; (b) NaOH, THF, H2O, r.t., 16 h; (c) HATU, DIPEA, DMF, r.t., 24 h; (d) 2M HCl in THF, r.t., (e) Na(OAc)3BH, AcOH, 1,2-DCE, r.t., 16 h.
An aromatic nucleophilic substitution reaction was carried out between commercially available methyl-5-fluoropicolinate 9.11 and 4-(1,3-dioxolan-2-yl)piperidine 9.12 at elevated temperatures in DMSO using DIPEA as the base, affording piperidine-substituted product 9.13. Saponification of 9.13 with sodium hydroxide in a THF/water mixture yielded carboxylic acid 9.14. This derivative was then coupled with commercially available (S)-3-aminopiperidine-2,6-dione 9.15 using HATU and DIPEA in DMF to generate compound 9.16. Subsequent acid-catalyzed hydrolysis of the acetal group in 9.16 with 2M HCl in THF afforded aldehyde 9.17. Finally, reductive amination between aldehyde 9.17 and amine 9.10 (Scheme 14) successfully furnished the final product, NX-5948 (9).
2.6. PROTACs Targeting Signal Transducer and Activator of Transcription 3 (STAT3)
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that serves as a promising therapeutic target for cancer and other diseases. Among all STAT proteins, STAT3 has attracted significant attention for its roles in cell growth, survival, differentiation, regeneration, immune response, and cellular respiration [84]. Persistent activation of STAT3 contributes to oncogenesis within the tumor microenvironment, and high Phospho-STAT3 activation is associated with worse outcomes in cancers such as non-small cell lung cancer, gastric cancer, and colorectal cancer. In addition to its involvement in cancer, STAT3 is also implicated in autoimmune and inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, atherosclerosis, and inflammatory bowel disease [85,86].
However, targeting STAT3 with inhibitors has proven challenging due to the highly charged phosphotyrosine binding site located in its SH2 domain. A significant challenge with SH2 domain inhibitors is the high degree of homology STAT3 shares with other STAT family members, making it challenging to achieve selectivity. Moreover, STAT3 can exhibit transcriptional activity in its monomeric form, so inhibitors that only block dimerization may provide only partial suppression of its gene-regulatory functions. To overcome these limitations, PROTACs have emerged as a promising strategy for targeting STAT3 [87,88,89,90]. For instance, the STAT3 degrader SD-36 has demonstrated over 1000-fold greater potency in suppressing STAT3 activity compared to its parent compound SI-109. Notably, while SI-109 binds STAT1 and STAT4 with similar affinity, SD-36 selectively degrades STAT3. This highlights a key advantage of degraders: cellular efficacy can be achieved even when biochemical affinity is modest [91].
Clinical Development and Outcomes of KT-333 (10): KT-333 is a first-in-class, potent, and highly selective PROTAC degrader of STAT3 developed by Kymera Therapeutics that has progressed into a clinical trial. It is undergoing Phase 1a/1b trials for relapsed/refractory classic Hodgkin’s lymphoma (cHL), cutaneous T-cell lymphoma (CTCL), and NK-cell lymphoma (NCT05225584). KT-333 has effectively and selectively degraded STAT3 in these trials, inhibiting the STAT3 pathway and demonstrating clinically significant responses in specific patient groups [92,93,94,95].
KT-333 achieved up to 95% mean maximum STAT3 degradation in peripheral blood mononuclear cells at higher dose levels. Early clinical results have also revealed signs of antitumor activity across various hematologic malignancies. Notably, two patients with relapsed/refractory Hodgkin’s lymphoma achieved complete responses and subsequently underwent potentially curative stem cell transplants. In addition, patients with cutaneous T-cell lymphoma (CTCL) experienced substantial tumor reductions. These preliminary outcomes suggest that KT-333 holds promise as a transformative treatment for STAT3-driven cancers [93].
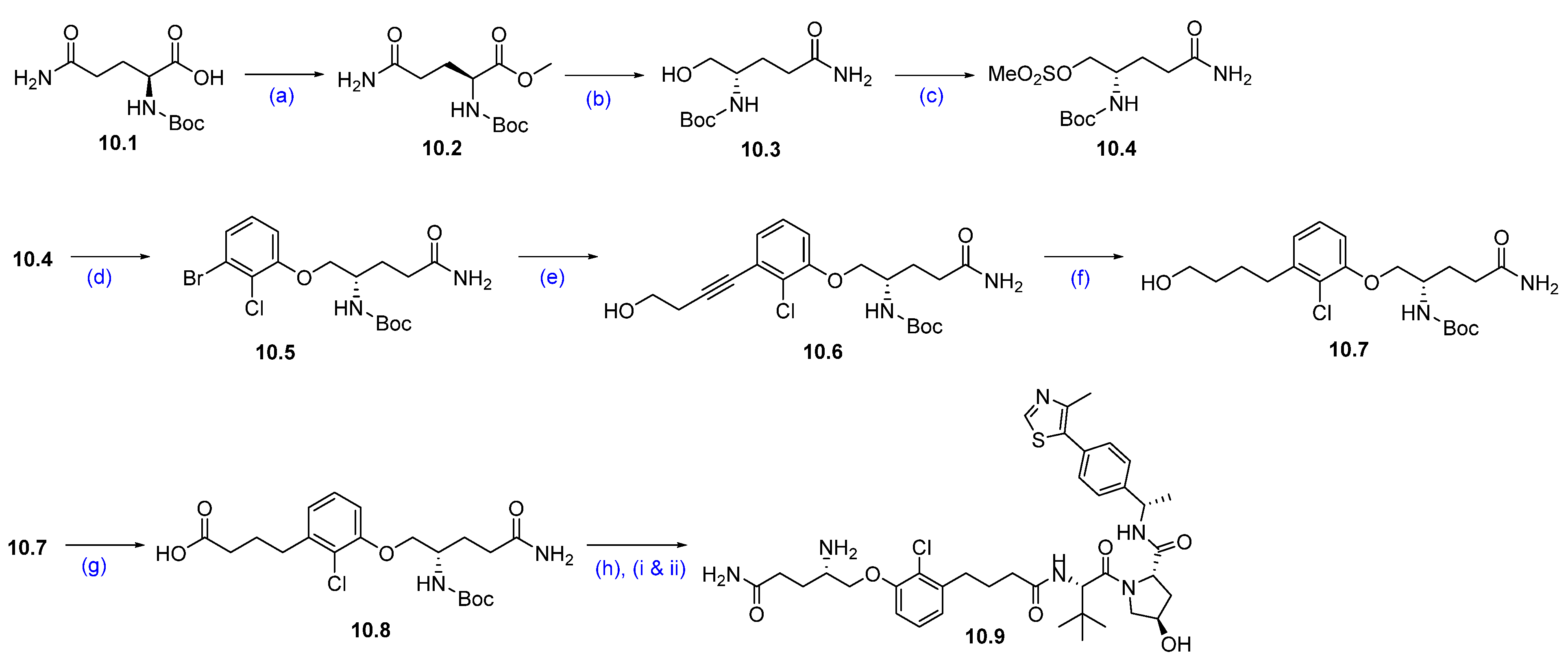
Synthesis of KT-333 (10): The synthesis of KT-333 is shown in Scheme 16, Scheme 17 and Scheme 18 [95], beginning with the commercially available starting material 10.1. Methyl (tert-butoxycarbonyl)-L-glutaminate 10.2 was prepared via esterification of (tert-butoxycarbonyl)-L-glutamine 10.1. The ester group in compound 10.2 was then reduced to the corresponding alcohol 10.3 using NaBH4 in THF. The resultant alcohol was subsequently converted to mesylate 10.4 by treatment with mesyl chloride and triethylamine.
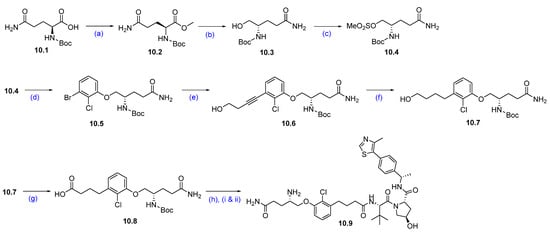
Scheme 16.
Synthesis of fragment 10.9 of KT-333 (10): (a) DCC, DMAP, DCM, MeOH, r.t., 16 h; (b) NaBH4, THF, MeOH, 0 °C to r.t., 16 h; (c) MsCl, NEt3, THF, 0 °C to r.t., 3 h; (d) 3-Br-2-Cl-phenol, NaI, Cs2CO3, DMF, 80 °C, 10 h; (e) but-3-yn-ol, Pd(PPh3)4, CuI, NEt3, DMSO, r.t., 16 h; (f) PtO2, H2, MeOH, r.t, 16 h; (g) PDC, DMA, r.t., 16 h; (h) (i) VHL-1, DMA, HATU, DIPEA, r.t., 16 h; (ii) 4M HCl in dioxane, THF, r.t., 2 h.
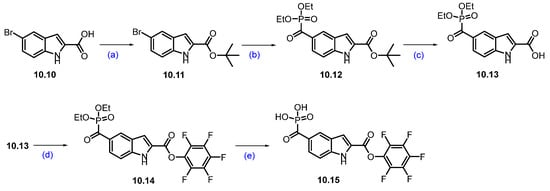
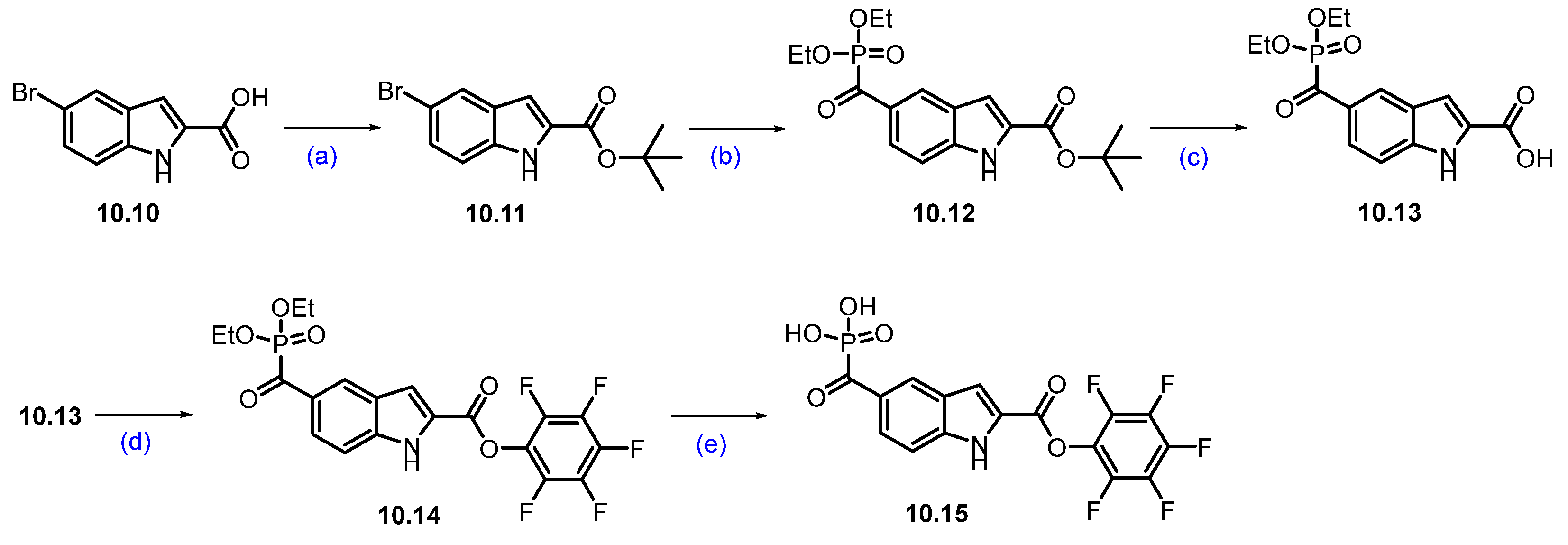
Scheme 17.
Synthesis of fragment 10.15 of KT-333 (10): (a) tert-butyl 2,2,2-trichloroacetimide, BF3·Et2O, THF, 0 °C to r.t., 16 h; (b) CO, Pd2(dba)3, Xantphos, diethylphosphonate, NEt3, toluene, 90 °C, 4 h; (c) TFA, DCM, r.t., 2 h; (d) 2,3,4,5,6-pentafluorophenol, DCC, DCM, r.t., 16 h; (e) TMSI, DCM, r.t., 30 min.
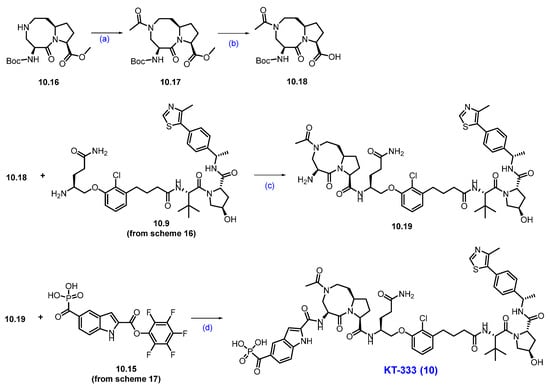
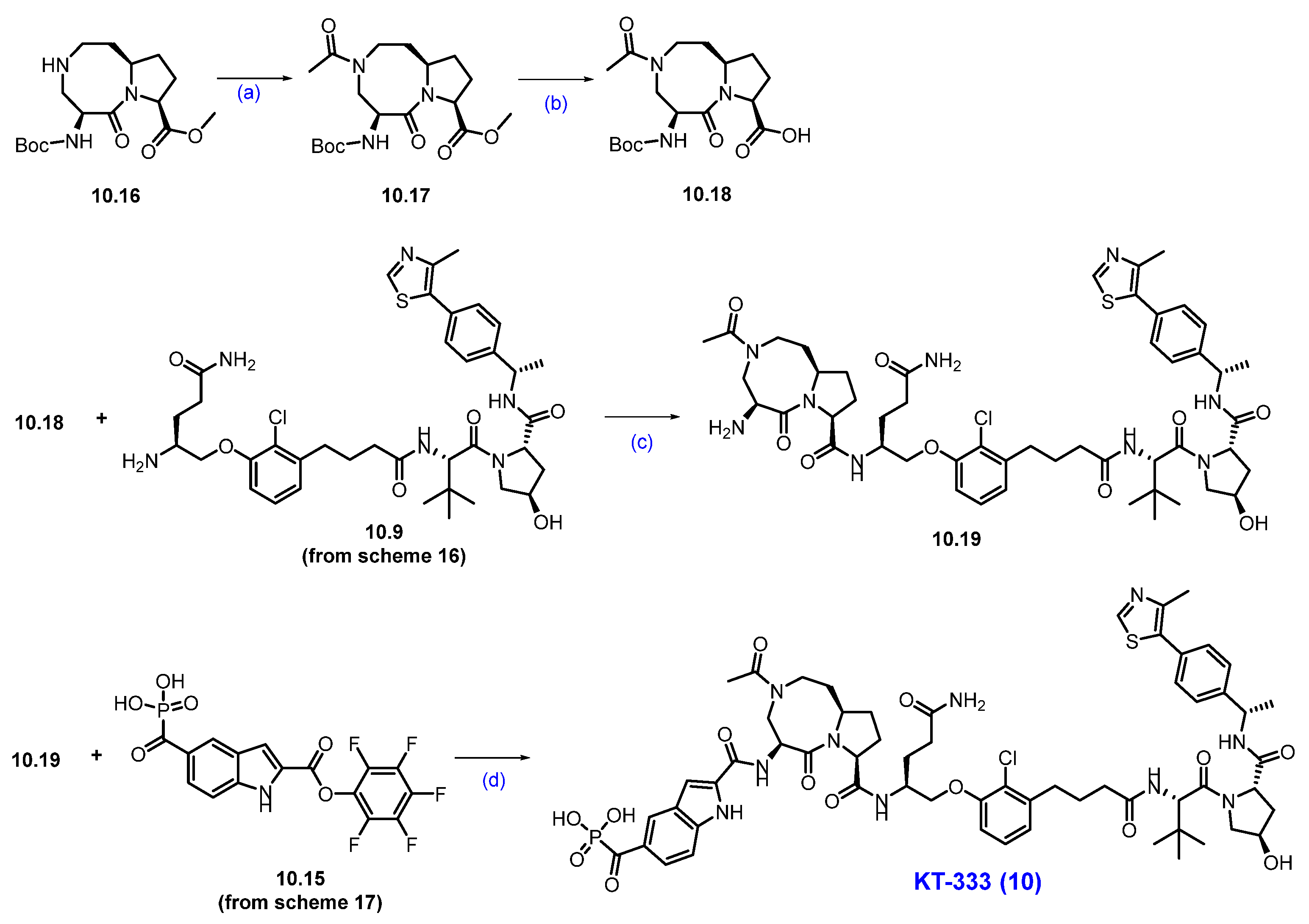
Scheme 18.
Synthesis of KT-333 (10): (a) AcCl, NEt3, DCM, 0 °C to r.t., 3 h; (b) LiOH, THF/H2O, r.t, 16 h; (c) HATU, DIPEA, DMA, 0 °C to r.t., 16 h; HCl in dioxane, DCM, r.t., 5 h; (d) DIPEA, NMP, ACN, r.t.
Next, a substitution reaction between mesylate 10.4 and 3-bromo-2-chlorophenol in the presence of Cs2CO3 afforded phenol ether 10.5. A chemo-selective Sonogashira coupling of the bromide of 10.5 with but-3-yn-1-ol furnished alkyne derivative 10.6. This alkyne was then subjected to hydrogenation using platinum oxide as a catalyst to yield the corresponding saturated alcohol 10.7.
Subsequent oxidation of compound 10.7 with pyridinium dichromate (PDC) produced carboxylic acid 10.8. Further compound 10.8 was coupled with the VHL ligand using HATU and DIPEA in dimethylacetamide (DMA) to form a Boc-protected coupled product. Finally, the Boc deprotection was carried out using HCl in dioxane/THF mixture at room temperature, furnished the target compound 10.9 (Scheme 16) [96].
The synthesis of compound 10.15 was started with commercially available bromo acid 10.10. tert-Butyl-5-bromo-1H-indole-2-carboxylate 10.11 was synthesized by esterifying commercially available starting material 5-bromo-1H-indole-2-carboxylic acid 10.10 with tert-butyl-2,2,2-trichloroacetimidate and BF3-etherate in THF. The bromo indole derivative 10.11 was then converted into a phosphate ester 10.12 using a palladium-catalyzed coupling with diethyl phosphonate in the presence of carbon monoxide. The tert-butyl group in compound 10.12 was then deprotected using TFA in DCM to furnish 10.13. The indole carboxylic acid derivative 10.13 was then coupled with pentafluorophenol using DCC and DMAP in DCM to afford compound 10.14. Phosphate ester hydrolysis of 10.14 using TMSI in DCM produced compound 10.15 in 78% yield (Scheme 17) [97].
The final synthesis of KT-333 is described in Scheme 18. The synthesis started with compound 10.16, which was converted to the corresponding acetamide 10.17 using acetyl chloride and triethylamine. Subsequently, 10.17 was subjected to ester hydrolysis with LiOH in THF and water to afford acid 10.18. The acid 10.18 was then coupled with previously synthesized amine derivative 10.9 (Scheme 16) using HATU and DIPEA to produce a Boc-protected compound, which was next subjected to Boc deprotection with HCl to afford amine 10.19. In the final step, compounds 10.19 and 10.15 (Scheme 17) were coupled in the presence of DIPEA in NMP to furnish KT-333 (10) in good yield [96].
2.7. PROTACs Targeting B Cell Lymphoma 6 (BCL6)
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma, originating from the uncontrolled proliferation of B cells. It accounts for approximately 25–30% of all non-Hodgkin’s lymphomas. This disease is highly heterogeneous, with 20–40% of patients exhibiting mutations in the B cell lymphoma 6 (BCL6) gene [98]. BCL6 plays a crucial role as a transcription factor, essential for regulating cell cycle checkpoints, differentiation, apoptosis, and the DNA damage response. Evidence has increasingly suggested that sustained expression of BCL6 is necessary for the survival of DLBCL cells and is associated with the occurrence of various cancers [99]. Over the past few decades, significant efforts have been directed toward developing BCL6 inhibitors to interrupt the protein–protein interactions between the BCL6 BTB domain and its corepressor. While some compounds have effectively blocked these interactions, most inhibitors have demonstrated suboptimal antiproliferative effects, and currently, none have entered clinical development [100,101,102].
Notably, Arvinas and Bristol Myers Squibb have independently announced two CRBN-based BCL6-targeting PROTACs, ARV-393 and BMS-986458, which have progressed into early clinical studies. ARV-393 is being tested in patients with relapsed/refractory non-Hodgkin lymphoma (NCT06393738). Meanwhile, BMS-986458 is undergoing Phase 1/2 clinical trials for the same condition, being evaluated as a standalone treatment and in combination with the monoclonal antibody rituximab (NCT06090539) [103].
Clinical Development and Outcomes of ARV-393 (11): Arvinas presented new preclinical combination data for ARV-393, their investigational PROTAC BCL6 degrader, at the 2025 AACR Annual Meeting [104]. The data showed synergistic antitumor activity, including complete regressions, when combined with standard-of-care (SOC) chemotherapy, SOC biologics, and investigational oral small-molecule inhibitors (SMIs) in models of high-grade B-cell lymphoma (HGBCL) and aggressive diffuse large B-cell lymphoma (DLBCL). The combination of ARV-393 and SOC chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) achieved significantly greater tumor growth inhibition compared to ARV-393 monotherapy, with all mice receiving the ARV-393 and R-CHOP combination exhibiting complete tumor regression. Furthermore, ARV-393 combined with SOC biologics targeting CD20 (rituximab), CD19 (tafasitamab), or CD79b (polatuzumab vedotin) resulted in tumor regressions and demonstrated significantly more potent tumor growth inhibition than each agent used alone. In preclinical models, ARV-393 also increased CD20 expression, supporting further exploration of combinations with CD20-targeted agents, particularly in cases of low or absent CD20 expression. Moreover, when combined with investigational small-molecule inhibitors targeting clinically validated oncogenic drivers of lymphoma, such as BTK (Acalabrutinib), Bcl2 (Venetoclax), or EZH2 (Tazemetostat), ARV-393 demonstrated superior tumor growth inhibition compared to each agent alone. All mice treated with these combinations showed tumor regressions [104]. These preclinical data suggest that ARV-393 holds promise as a novel therapeutic approach for lymphoma patients, particularly those who have not responded to existing treatments.
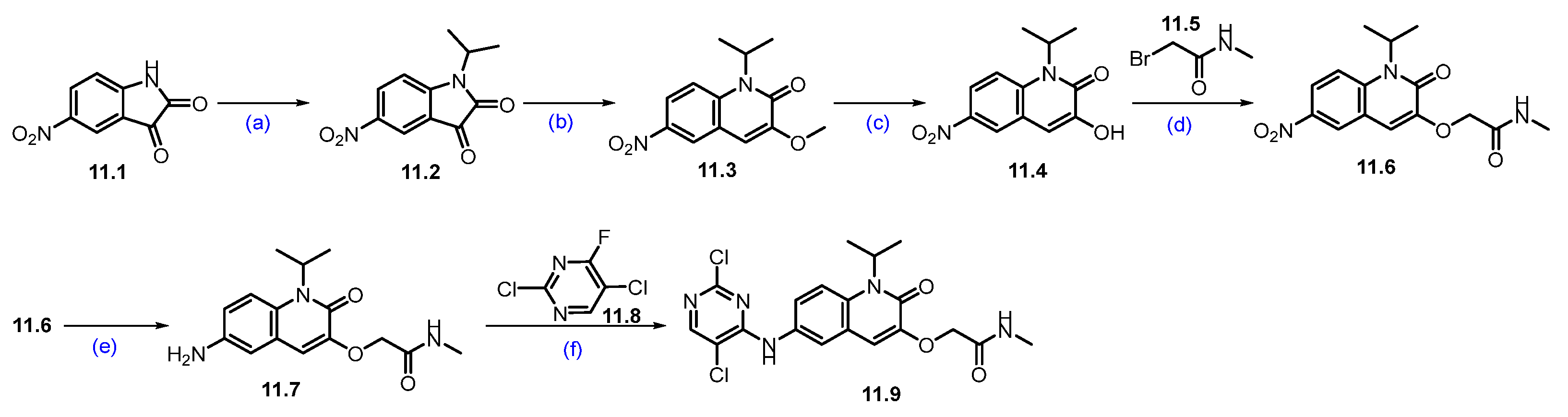
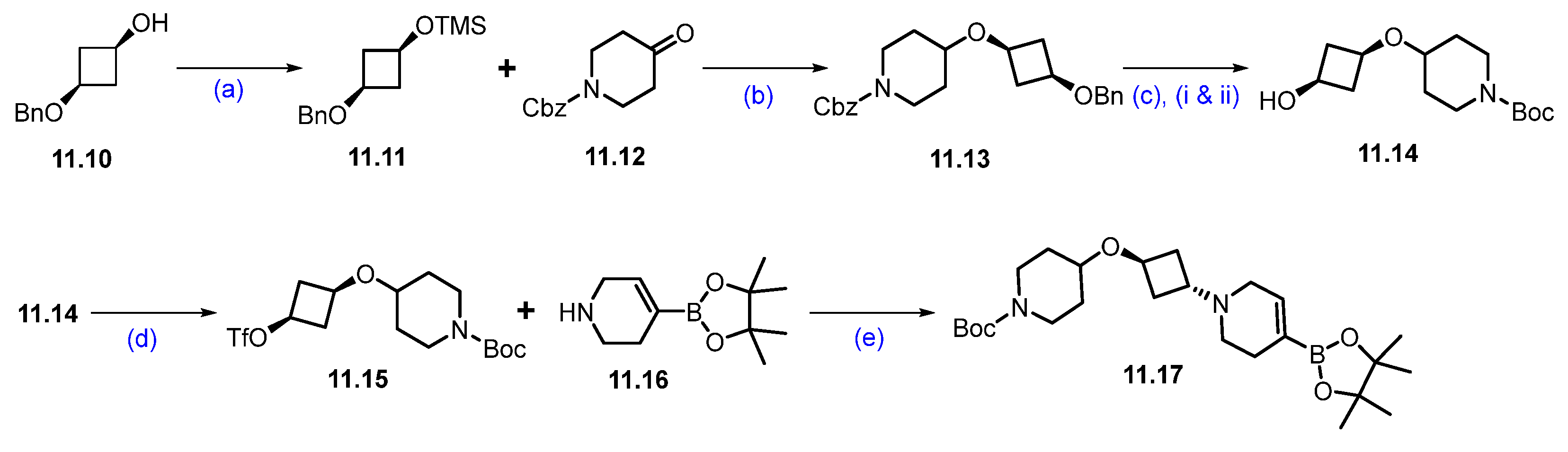
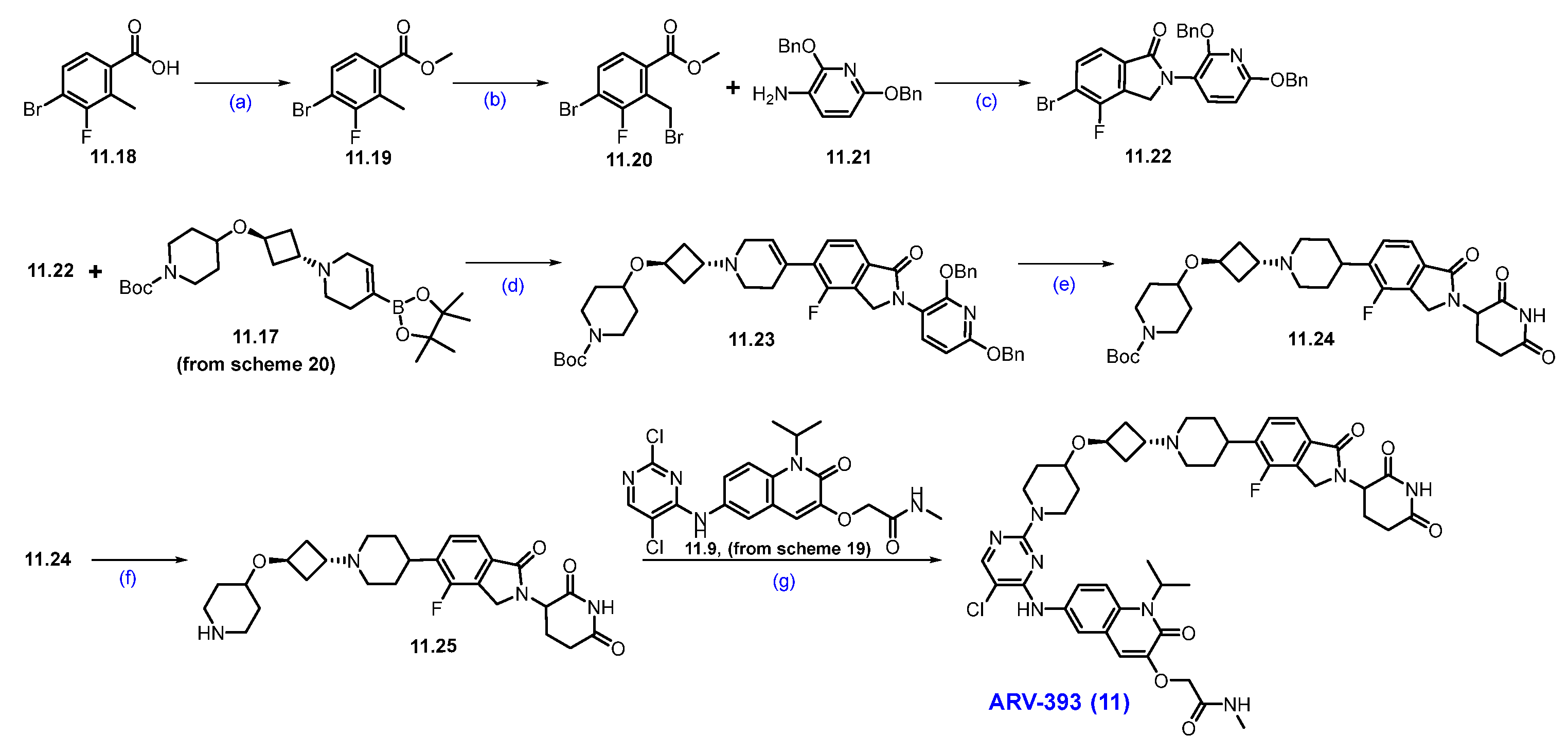
Synthesis of ARV-393 (11): The synthesis of ARV-393 is shown in Scheme 19, Scheme 20 and Scheme 21 [105]. The synthesis of ARV-393 requires three key building blocks and is completed in a total of 18 steps.
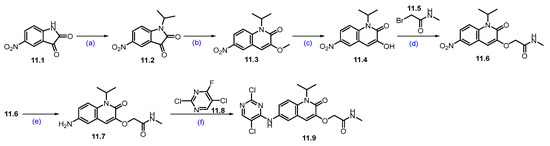
Scheme 19.
Synthesis of fragment 11.9 of ARV-393 (11): (a) 2-iodopropane, K2CO3, DMF, r.t., 48 h; (b) TMSCH2N2, NEt3, EtOH, r.t., 12 h; (c) BBr3, DCM, 0 °C, 2 h; (d) K2CO3, DMF, 20 °C, 4 h; (e) 10%Pd/C, H2, MeOH/DMF, r.t., 4 h; (f) DIPEA, DMSO, 100 °C, 2 h.
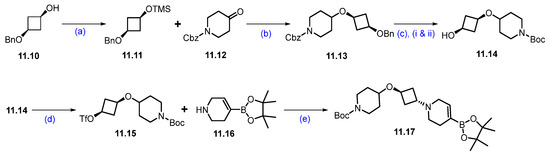
Scheme 20.
Synthesis of fragment 11.17 of ARV-393 (11): (a) TMSCl, NEt3, DCM, 0 °C to 2 h; (b) triethylsilane, DCM, TMS triflate, −78 °C to 0 °C, 2 h; (c) (i) 10%Pd/C, Pd(OH)2/C, 15 psi, 55 °C, H2, 48 h; (ii) (Boc)2O; (d) triflic anhydride, NEt3, −40 °C, 2 h; (e) DIPEA, ACN, r.t., 5 h.
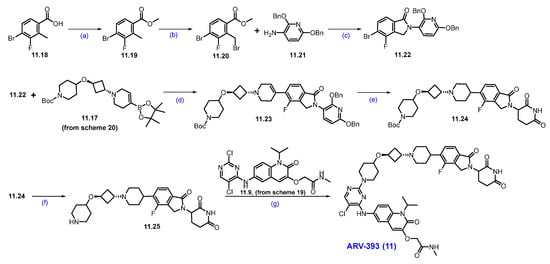
Scheme 21.
Synthesis of ARV-393 (11): (a) H2SO4, MeOH, 0 °C to 60 °C 16 h; (b) NBS, AIBN, 1,2-DCE, 80 °C, 6 h; (c) DIPEA, ACN, 70 °C, 16h; (d) Pd(dppf)Cl2, CsF, dioxane/H2O, 90 °C; (e) 10%Pd/C, H2, EtOH, r.t., 16 h; (f) HCl in dioxane, r.t., 2 h; (g) DIPEA, DMSO, 120 °C, 1 h.
The reaction commenced with the N-alkylation of 5-nitro-indoline-2,3-dione 11.1 with 2-iodopropane using potassium carbonate (K2CO3), which afforded alkylated product 11.2. The ring expansion reaction of 11.2 with TMS-diazomethane and triethylamine (NEt3) obtained quinoline derivative 11.3, which was further treated with boron tribromide (BBr3) to obtain hydroxy derivative 11.4. It was subjected to O-alkylation with K2CO3 to furnish the ether derivative 11.6. The nitro group reduction of 11.6 was carried out with Pd/C under hydrogenation conditions, which gave amino quinoline 11.7. SNAr reaction of amine on 11.7 with 11.8 using DIPEA at elevated temperature yielded regiomeric amine substituted product 11.9 (Scheme 19) [106].
The synthesis of the second fragment begins with the trimethylsilyl protection of commercially available cis-3-(benzyloxy)cyclobutane-1-ol, resulting in the formation of the di-protected cyclobutyl derivative 11.11. This scaffold is then subjected to Lewis acid-catalyzed etherification with triethylsilane, which yields the ether derivative 11.13. Subsequently, hydrogenation of compound 11.13 with Pd/C, followed by in situ treatment with Boc anhydride, facilitates the substitution of the -Cbz protection group with Boc protection, resulting in compound 11.14. This compound is then treated with trifluoromethanesulfonic anhydride in the presence of triethylamine to produce the reactive triflate 11.15. Finally, 11.15 was reacted with commercially available compound 11.16, which afforded N-alkylated derivative 11.17 (Scheme 20) [105].
Esterification of 4-bromo-3-fluoro-2-methylbenzoic acid 11.18 was carried out using MeOH and H2SO4 to obtain ester 11.19, which was further treated with NBS in the presence of a catalytic amount of AIBN in CCl4, affording benzyl bromide derivative 11.20. Condensation of 11.20 with 2,6-bis(benzyloxy)pyridine-3-amine using DIPEA and a catalytic amount of acetic acid in acetonitrile obtained substituted isoindolone derivative 11.22. Suzuki coupling reaction of 11.22 with 11.17 using Pd(dppf)Cl2, CsF in dioxane at 90 °C rendered coupling product 11.23. Furthermore, it was subjected to hydrogenation to remove dibenzyl functionalities, followed by Boc group deprotection using HCl in dioxane, which furnished the key scaffold 11.25. Finally, the nucleophilic substitution of the secondary amine of 11.25 on 2-Cl-pyrimidine of 11.9 (Scheme 19) resulted in ARV-393 (11) (Scheme 21) [106].
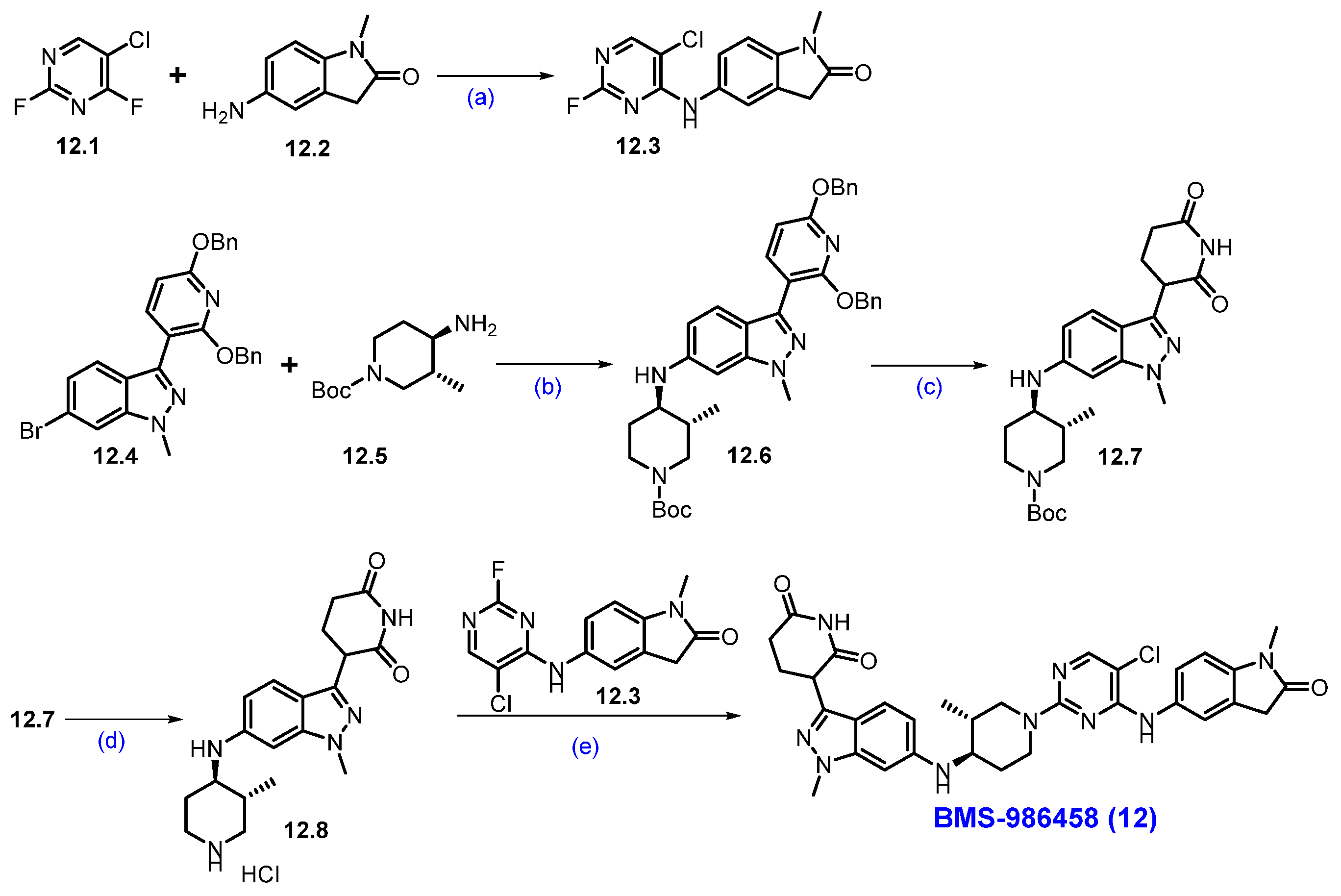
Synthesis of BMS-986458 (12): The synthesis of BMS-986458 is described in Scheme 22 [107]. The synthesis began with commercially available 5-chloro-2,4-difluoropyrimidine 12.1 and 5-amino-l-methylindolin-2-one 12.2. These two compounds reacted in THF in the presence of DIPEA at a lower temperature, yielding product 12.3. For the synthesis of cereblon ligand part, the synthesis began utilizing a literature-reported compound 12.4 [108], which was converted to intermediate 12.6 by following a Buchwald-Hartwig reaction with amine 12.5 in the presence of Ruphos-Pd-G3 and Cs2CO3 in 1,4-dioxane. Then, compound 12.6 was treated with Pd(OH)2/C under a hydrogen atmosphere to deprotect the benzyl protecting groups, which gave piperidine-2,6-dione derivative 12.7. Further deprotection of the Boc functionality on 12.7 in the presence of 4M HCl in EtOAc furnished amine 12.8. Finally, a SNAr reaction between amine 12.8 and 12.3 in DMSO, in the presence of DIPEA, produced the final degrader BMS-986458 (12).
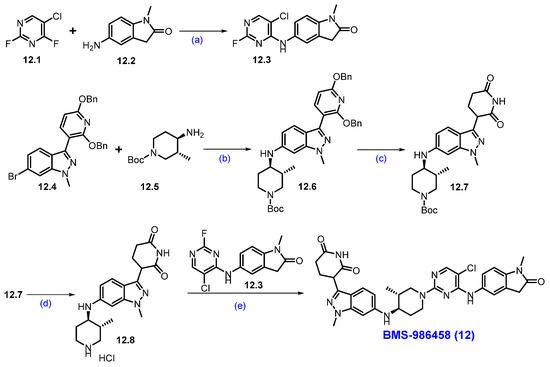
Scheme 22.
Synthesis of BMS-986458 (12): (a) DIPEA, THF, −40 °C to r.t., 16 h; (b) Ruphos-Pd-G3, Cs2CO3, 1,4-dioxane, 90 °C, 20 h; (c) Pd(OH)2/C, H2, MeOH, THF, 50 °C, 9 h; (d) 4M HCl in EtOAc, r.t., 16 h; (e) DIPEA, DMSO, 80 °C, 2 h.
2.8. PROTACs Targeting Murine Double Minute 2 (MDM2)
Addressing elevated protein levels resulting from off-target effects remains a significant challenge, particularly when targeting MDM2. A primary concern is the inadvertent activation of p53, a vital tumor suppressor, in healthy tissues. The MDM2 protein serves as a key cellular regulator of the tumor suppressor p53, often referred to as the “guardian of the genome.” p53 is essential for cancer prevention through its control of DNA repair, cell cycle progression, apoptosis, angiogenesis, and senescence [109]. Notably, mutations in the TP53 gene are found in approximately 50% of all human cancers, including those of the breast, colon, lung, liver, prostate, bladder, and skin [110]. As an E3 ligase, MDM2 targets p53 for degradation, making it an essential focus for cancer treatments. However, efforts to stabilize and enhance p53 expression using small molecules face challenges due to a negative feedback loop in which upregulated p53 increases the transcription of MDM2 messenger RNA. More than a dozen potent inhibitors designed to disrupt the MDM2–p53 interaction have entered clinical trials, but these agents often face setbacks [111,112]. Key challenges include dose-limiting toxicities (DLTs) from p53 activation in bone marrow, a narrow therapeutic window because of p53′s importance in normal cells, and resistance mechanisms involving TP53 mutations. Kymera Therapeutics has recently introduced KT-253, an MDM2 PROTAC degrader (NCT05775406). Unlike conventional small-molecule inhibitors, which may unintentionally upregulate MDM2 through feedback, KT-253 rapidly depletes MDM2 and triggers a sharp increase in p53 levels. This mechanism could support more robust and rapid therapeutic responses, enable intermittent dosing, mitigate toxicity, and give healthy tissues time to recover.
KT-253 was administered intravenously every three weeks to patients with advanced high-grade myeloid malignancies, such as acute myeloid leukemia (AML), acute lymphoblastic leukemia, lymphomas, and various solid tumors. Thus far, no treatment-related neutropenia or thrombocytopenia has been observed, and early signs of therapeutic efficacy have been noted at tolerated doses. However, on 31 May 2025, Kymera announced it would shift its focus and resources away from oncology to prioritize its growing immunology portfolio. As a result, further development of KT-253 beyond Phase 1 will proceed only through external partnerships [113]. The use of a PROTAC to target MDM2 illustrates an innovative strategy that employs one E3 ligase to degrade another E3 ligase.
Clinical Development and Outcomes of KT-253 (13): As of April 2024, an ongoing Phase 1a clinical trial has enrolled 24 patients: 16 with solid tumors and lymphomas (Arm A) and 8 with high-grade myeloid malignancies (Arm B) [114,115,116]. Preliminary data show encouraging activity. Arm A reported one confirmed partial response in a patient with Merkel cell carcinoma, along with four cases of stable disease in individuals with fibromyxoid sarcoma, adenoid cystic carcinoma, and renal cell carcinoma. Arm B showed one confirmed complete response and one partial response in post-myeloproliferative neoplasm acute myeloid leukemia patients. Treatment was associated with activation of the p53 pathway, demonstrated by elevated plasma levels of GDF-15 protein and increased expression of CDKN1A and PHLDA3 mRNA, even at the lowest dose levels, confirming successful MDM2 inhibition. KT-253 was generally well-tolerated; no cases of neutropenia or thrombocytopenia were observed. These early findings support KT-253′s potential to effectively inhibit MDM2, restore p53 function, and drive tumor regression in patients with p53 wild-type cancers [117].
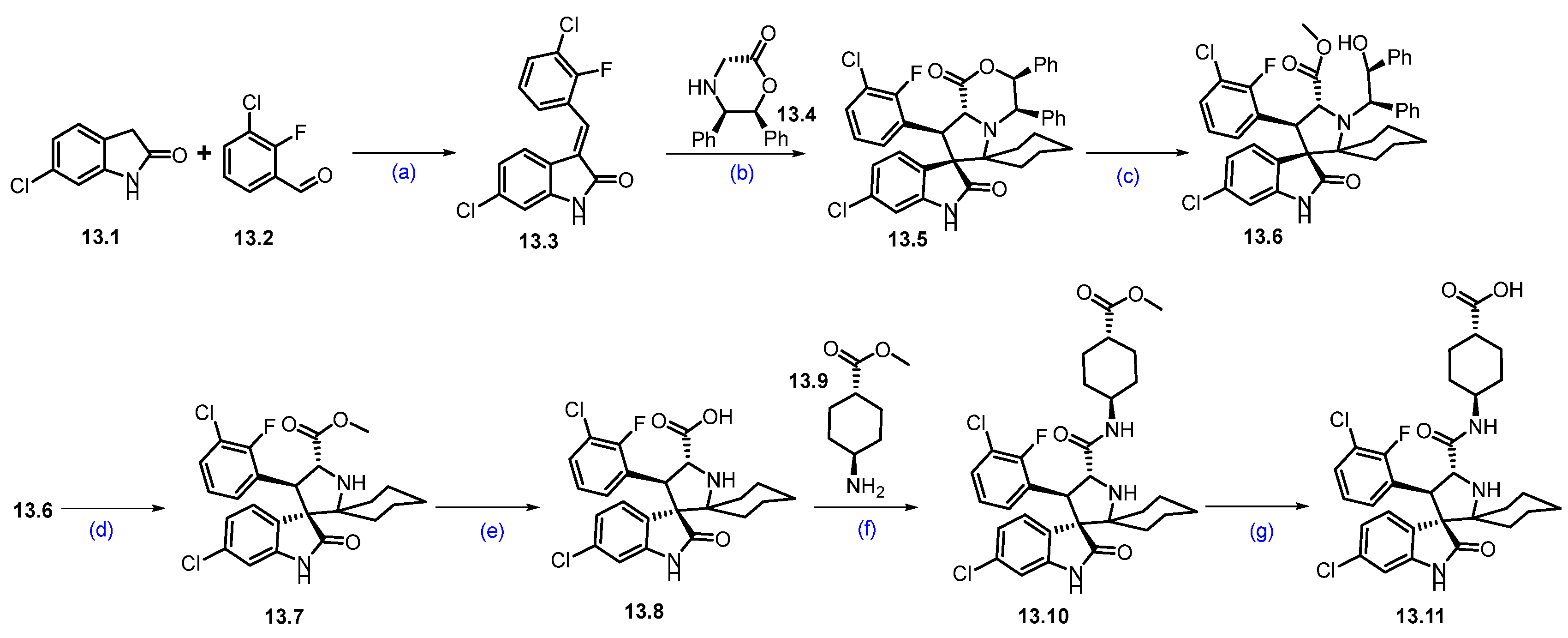
Synthesis of KT-253 (13): The synthesis of KT-253 is summarized in Scheme 23 and Scheme 24 [118]. Condensation of 3-chloro-2-fluoro benzaldehyde 13.2 with 6-chloroindolin-2-one 13.1 under basic conditions using piperidine in MeOH at reflux conditions afforded 13.3 as the exclusive desired E-isomer. Next, the asymmetric synthesis of the spirooxoindole core was achieved using 13.4 as a chiral auxiliary, with cyclohexanone at 140 °C, resulting in the cycloaddition product 13.5. It was treated with H2SO4 in MeOH, resulting in ring opening followed by in situ acid-catalyzed esterification product 13.6. Oxidative removal of the chiral auxiliary by ceric ammonium nitrate (CAN) rendered 13.7. Hydrolysis of 13.7 using aq. LiOH followed by amidation with trans cyclohexyl carboxylate 13.9 afforded 13.10. Next, ester hydrolysis of 13.10 with aq. LiOH resulted in key spirooxoindole scaffold 13.11 (Scheme 23).
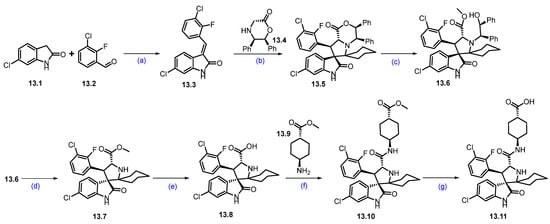
Scheme 23.
Synthesis of fragment 13.11 of KT-253 (13): (a) Piperidine, MeOH, 65 °C for 5 h then at r.t. for 12 h; (b) toluene, cyclohexanone, 140 °C, 12 h; (c) H2SO4, MeOH, 50 °C, 5 h; (d) CAN, ACN/H2O, r.t., 30 min.; (e) LiOH, THF/H2O, r.t., 15 min.; (f) TCFH, NMI, 0 °C to r.t., 2 min.; (g) LiOH, THF/H2O, r.t., 30 min.
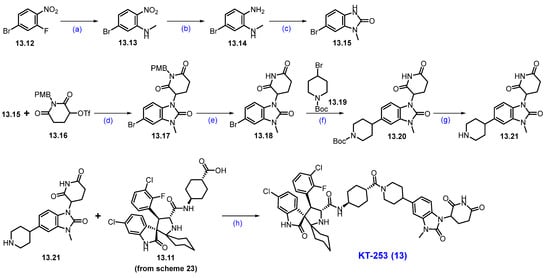
Scheme 24.
Synthesis of KT-253 (13): (a) MeNH2, THF, 15 °C, 10 min.; (b) Fe, AcOH, EtOAC, 80 °C, 6 h; (c) CDI, ACN, 80 °C, 6 h; (d) t-BuOK, THF, 0 °C to 10 °C, 2.5 h; (e) CH3SO3H, toluene, 120 °C, 2 h; (f) Ni/Ir photocatalysis, 50 W blue LED lamp, r.t., 14 h; (g) TFA, DCM, r.t., 30 min.; (h) TCFH, NMI, ACN, r.t., 2 min.
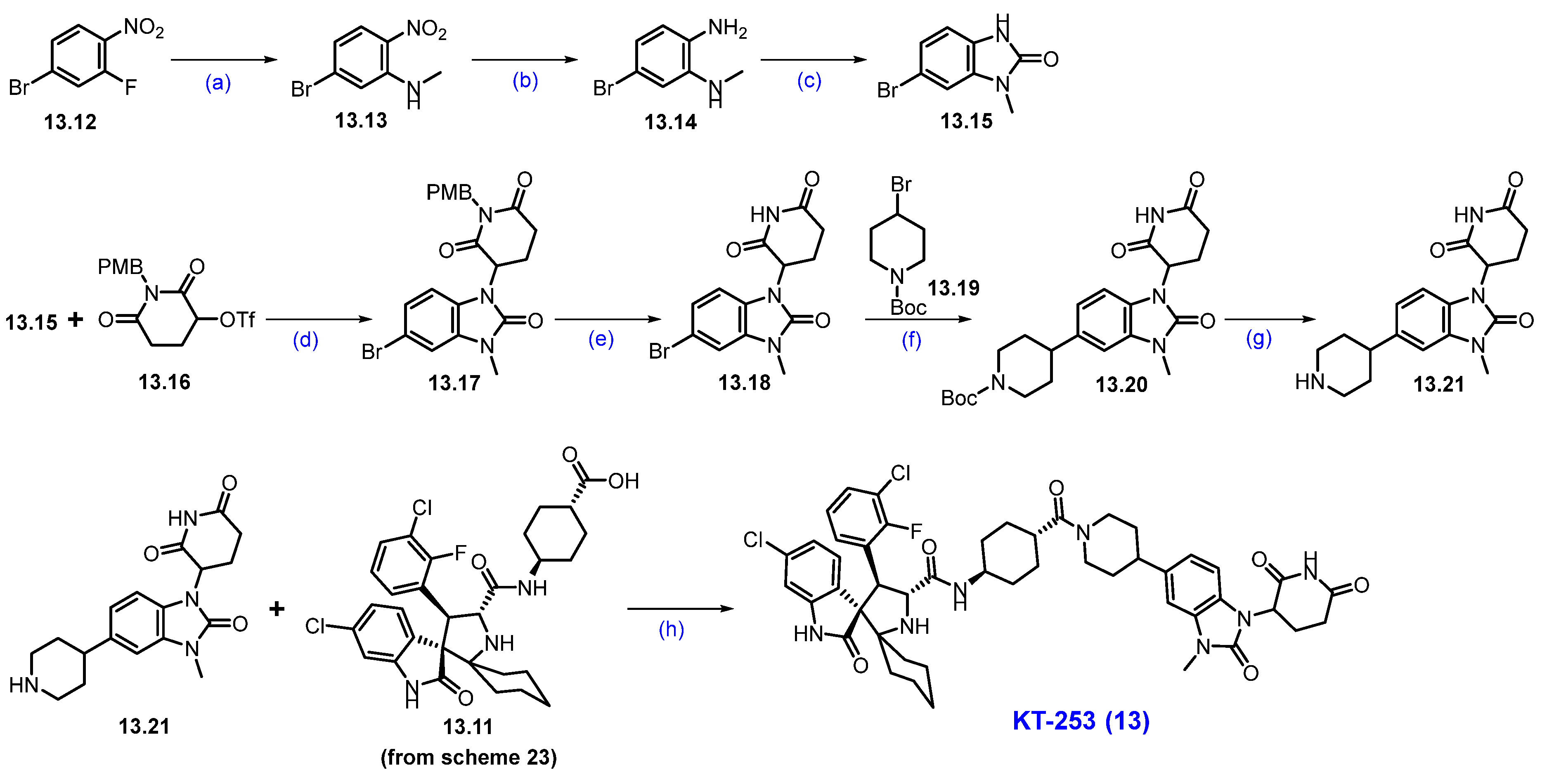
In parallel, substituted benzimidazole moiety 13.21 was synthesized in 7 steps (Scheme 24). An SNAr reaction of 13.12 with methylamine afforded 5-bromo-p-nitro-N-methylaniline derivative 13.13, which was subjected to reduction in the nitro moiety with Fe/AcOH, affording 1,2-diamino-bromobenzene 13.14. The cyclization of 13.14 with 1,1′-carbonyldiimidazole (CDI) at elevated temperature furnished benzimidazolone scaffold 13.15. Condensation of benzimidazole derivative 13.15 with 13.16 using potassium tert-butoxide (tBuOK) in THF conditions resulted in the formation of N-alkylated product 13.17. It was further treated with methanesulfonic acid (CH3SO3H) in toluene at 120 °C, resulting in 13.18. The compound then underwent a Ni/Ir photocatalyzed coupling between aryl bromide 13.18 and alkyl bromide 13.19 under irradiation using a 50W blue LED lamp, which afforded the newly formed C-C bond coupled product 13.20 in high yields. Finally, the deprotection of the Boc group in 13.20, using TFA, yielded 13.21. The resultant piperidine amine 13.21 was used in a coupling reaction with a previously synthesized carboxylic acid 13.11 (Scheme 23) under HATU and DIPEA conditions, furnishing KT-253 (13).
2.9. PROTACs Targeting Switch/Sucrose Non-Fermentable Related, Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily A, Member 2 (SMARCA2)
SMARCA2 and SMARCA4 are mutually exclusive, catalytically active components of the BAF chromatin remodeling complex and exhibit a synthetic lethal relationship [119]. Approximately 20% of human cancers harbor mutations in BAF complex subunits, with loss-of-function alterations in SMARCA4 particularly prevalent in malignancies such as non-small cell lung cancer (NSCLC). SMARCA2 and SMARCA4 share a high degree of sequence homology, including a conserved ATPase domain (93% similarity) and a bromodomain (96% similarity), both of which recognize acetylated chromatin [120]. Although small-molecule inhibitors have been developed to target the ATPase and bromodomains of SMARCA2/4, their high sequence similarity poses a significant challenge for achieving target selectivity. Most of these inhibitors fail to distinguish between SMARCA2 and SMARCA4. Moreover, bromodomain inhibition does not appear to affect cell viability in tumor models, suggesting that the bromodomain is not essential for cancer cell survival. As a result, a more promising strategy is the selective degradation of SMARCA2 in cancers where SMARCA4 is genetically inactivated [121,122,123].
PROTACs offer a unique advantage in this context. Despite originating from ligands that bind both SMARCA paralogs, PROTACs have demonstrated the ability to selectively degrade SMARCA2 while sparing SMARCA4. This selective degradation is especially compelling given the structural and functional similarities between the two proteins [124,125,126,127,128].
Prelude Therapeutics is developing two SMARCA2-selective PROTACs currently in Phase I clinical trials for patients with SMARCA4-deficient solid tumors: PRT3789, an intravenous agent (NCT05639751), and PRT7732, an oral compound with an undisclosed structure (NCT06560645). PRT3789 was specifically engineered with high aqueous solubility and an extended in vivo half-life—attributes well-suited for VHL-based heterobifunctional degraders [129,130]. A recent study by Shcherbakov et al. provides valuable insight into the basis of SMARCA2 selectivity through comprehensive biophysical characterization [131]. Using a VHL-recruiting PROTAC, the researchers examined ternary complex formation with both SMARCA2 and SMARCA4. HAP-NMR analysis revealed that the SMARCA2-containing ternary complex exhibited greater cooperativity and slower molecular tumbling in solution. Furthermore, isothermal titration calorimetry (ITC) using micelle-solubilized PROTACs demonstrated that SMARCA2 complexes were more enthalpically favorable and entropically efficient. These results are further corroborated by a related study from Wurz et al., which discovered that a single amino acid difference between SMARCA2 and SMARCA4 affects structural cooperativity during the formation of the ternary complex [132].
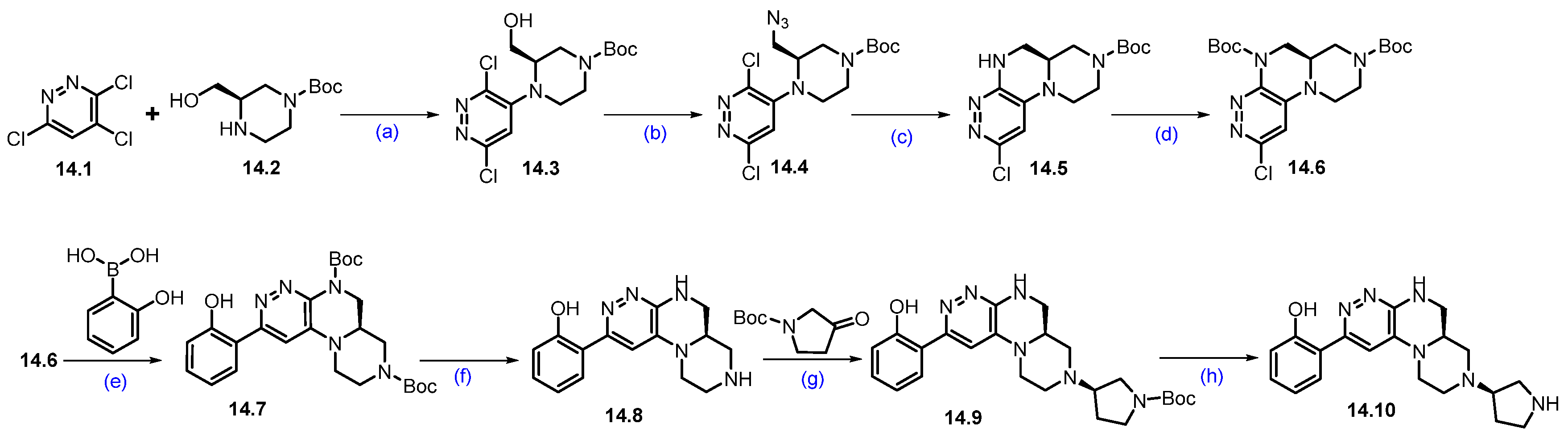
Synthesis of PRT3789 (14): The synthesis of PRT3789 is summarized in Scheme 25 and Scheme 26 [133]. Synthesis initiated with the SNAr reaction of commercially available 3,4,6-trichloropyridazine 14.1 and (R)-1-Boc-3-hydroxymethylpiperazine 14.2 using DIEPA in DMF afforded regiomeric piperazine-substituted scaffold 14.3. Conversion of hydroxymethyl on 14.3 under Mitsunobu conditions using diphenyl phosphoryl azide (DPPA) resulted in azidomethyl azide compound 14.4. Further reduction of azide with triphenylphosphine in THF, followed by cyclization with DIPEA, afforded cyclized scaffold 14.5. The latter was treated (TPP) with Boc anhydride in the presence of catalytic DMAP to yield Boc-protected scaffold 14.6. It was subjected to a Suzuki coupling reaction using 2-hydroxyphenylboronic acid in the presence of Pd(dppf)Cl2.DCM, K2CO3 conditions to give product 14.7. Deprotection of di-Boc functionalities in 14.7 was carried out with TFA to yield 14.8. Reductive amination of 14.8 with commercially available tert-butyl 3-oxopyrrolidine-1-carboxylate using Na(OAc)3BH followed by SFC purification and Boc deprotection under HCl/MeOH conditions to obtain key scaffold 14.10 (Scheme 25).
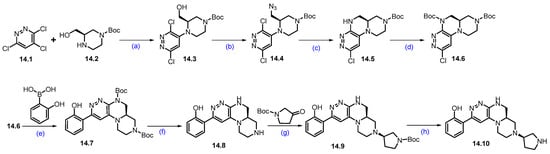
Scheme 25.
Synthesis of fragment 14.10 of PRT3789 (14): (a) DIPEA, DMF, 80 °C, 16 h; (b) TPP, DIAD, DPPA, THF, 0 °C to r.t., 16 h; (c) TPP, THF, 60 °C, 3 h then H2O, DIPEA, 60 °C, 20 h; (d) (Boc)2O, DMAP, DCM, r.t., 1 h; (e) Pd(dppf)Cl2.DCM, K2CO3, dioxane; 105 °C, 18 h; (f) TFA, DCM, r.t., 1 h; (g) HCl, MeOH, r.t., 1 h; (h) TFA, DCM, r.t., 16 h.
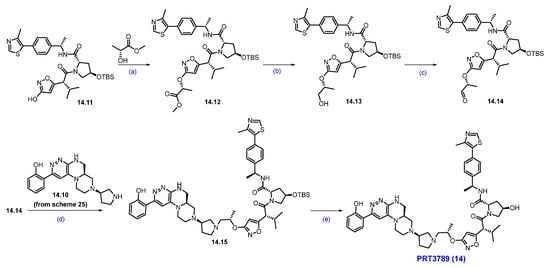
Scheme 26.
Synthesis of PRT3789 (14): (a) TPP, DIAD, THF, 0 °C to r.t., 16 h; (b) NaBH4, MeOH, r.t., 3 h; (c) IBX, ACN, 80 °C, 3 h; (d) Na(OAc)3BH, NaHCO3, DCM, rt.,18 h; (e) TFA, DCM, r.t., 1 h.
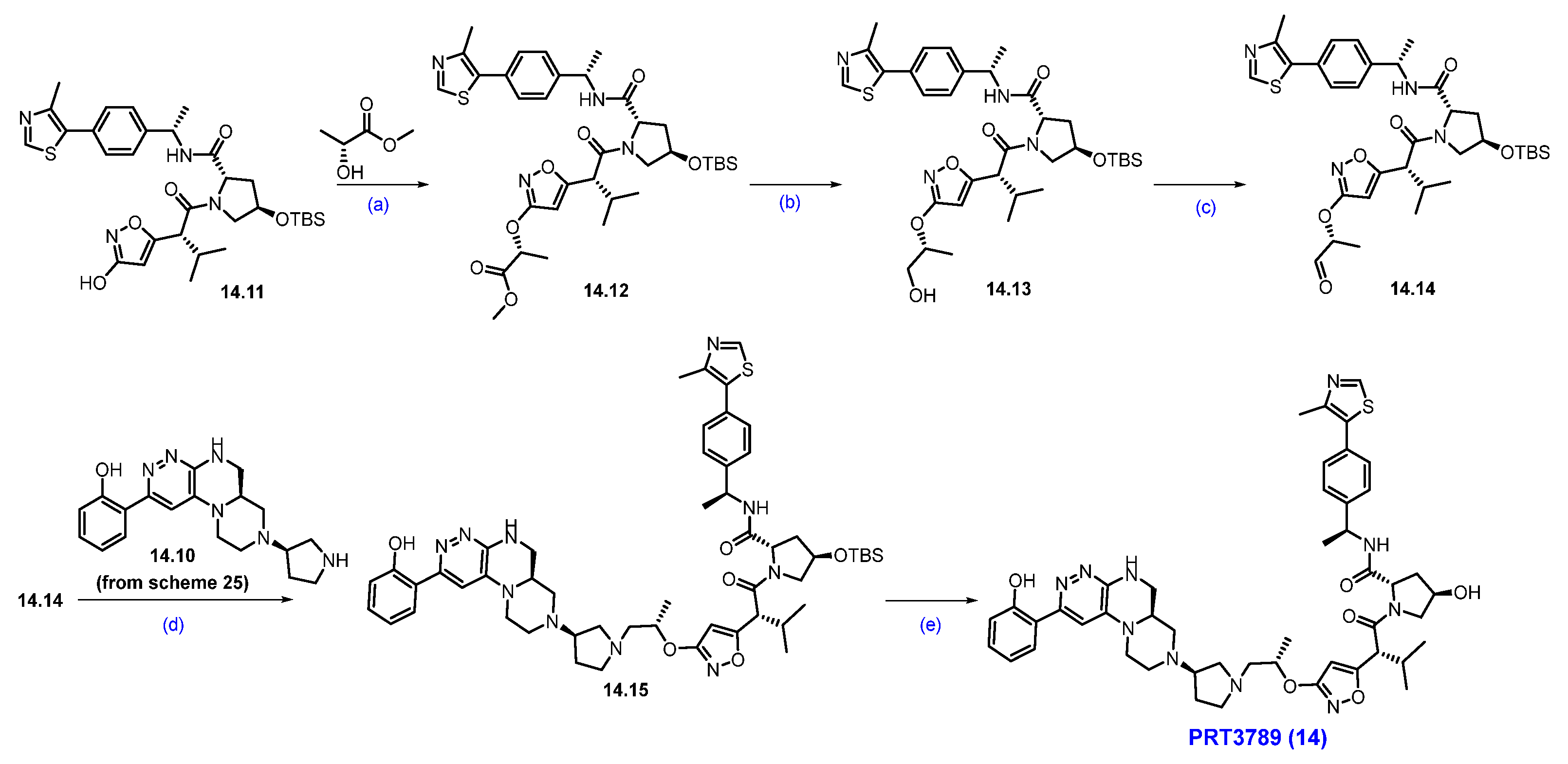
The Mitsunobu coupling reaction of 14.11 with commercially available methyl-(R)-2-hydroxypropanoate under TPP/DIAD conditions afforded scaffold 14.12 as shown in Scheme 26. It was subjected to ester reduction with NaBH4 in MeOH, followed by oxidation with IBX, which obtained the key aldehyde 14.14. Finally, the reductive amination of 14.10 (Scheme 25) with aldehyde 14.14 yielded the reductive amination scaffold 14.15. The silyl deprotection under acidic conditions furnished PRT3789 (14) [134].
2.10. PROTACs Targeting B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF)
The RAS-RAF-MEK-ERK signaling cascade plays a critical role in maintaining cellular homeostasis. Class 1 BRAF mutations, such as V600E and V600K, lead to hyperactivation of this pathway, driving uncontrolled cell growth. BRAF mutations are found in approximately 8% of all cancers, with high prevalence in melanoma (60%), colorectal cancer (10%), non-small cell lung cancer (NSCLC, 10%), and hairy cell leukemia (100%) [135]. RAF inhibitors like vemurafenib have improved PFS in patients harboring the BRAF V600E mutation. However, resistance to these inhibitors often emerges, along with paradoxical activation of RAF. Although some kinase inhibitors in clinical development aim to counter this effect, targeted protein degraders offer a potentially more effective strategy by eliminating both normal and mutant RAF activity [136]. CFT1946 is an oral, CRBN-based PROTAC developed by C4 Therapeutics to target and degrade mutant BRAF V600E. It is currently being investigated in a Phase 1/2 clinical trial (NCT05668585), both as a monotherapy and in combination with the MEK1/2 inhibitor trametinib, for patients with BRAF V600-driven cancers, including those resistant to standard BRAF inhibitors [137].
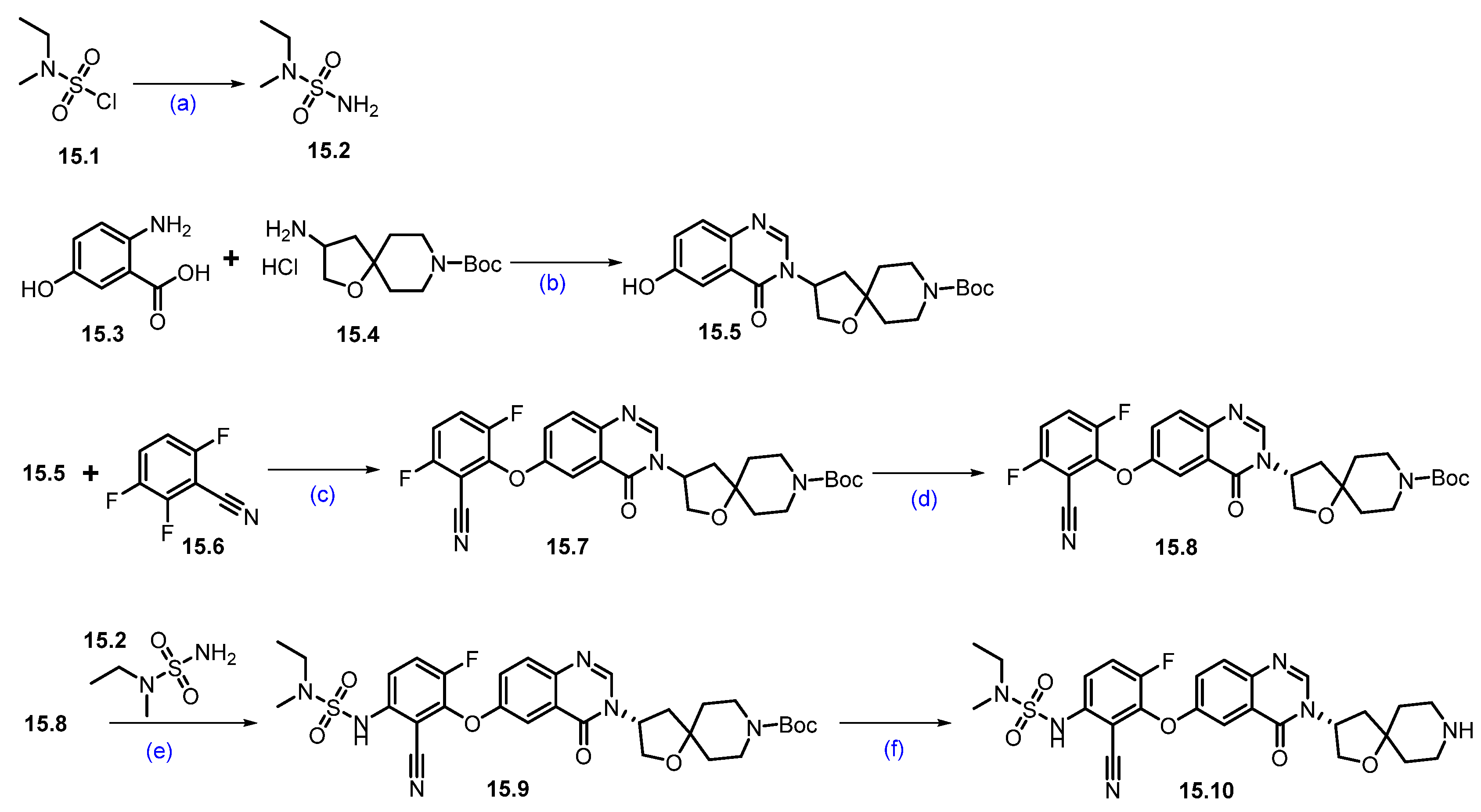
Clinical Development and Outcomes of CFT1946 (15): As of 12 April 2024, early results from an ongoing Phase 1/2 clinical trial of CFT1946 have been released. In the monotherapy dose-escalation phase, 25 patients with BRAF V600-mutant solid tumors were treated with CFT1946 at doses ranging from 20 mg to 320 mg twice daily. The patient population consisted of individuals with melanoma (40%), colorectal cancer (40%), non-small cell lung cancer (8%), and other tumor types (12%). CFT1946 exhibited dose-dependent bioavailability and effectively degraded the BRAF V600E protein. The treatment was generally well-tolerated, with most adverse events reported as mild to moderate. Importantly, signs of anti-tumor activity were observed, reinforcing the therapeutic potential of CFT1946 for patients with BRAF V600-mutant solid tumors [138,139].
Synthesis of CFT1946 (15): In Scheme 27 and Scheme 28, the synthesis of CFT1946 is presented as described by C4 therapeutics in their patents [140]. The quinazolinone scaffold 15.10 was synthesized starting from the 2-amino-5-hydroxy benzoic acid 15.3, as shown in Scheme 27. Briefly, a dehydrative coupling reaction of 15.3 and 15.4 in the presence of triethyl orthoformate resulted in quinazolinone framework 15.5. Aromatic nucleophilic substitution on 15.6 with the phenoxide generated from 15.5 afforded compound 15.7, furthering chiral purification, and resulting in the desired enantiopure nitrile 15.8. A second aromatic nucleophilic substitution of 15.8 with 15.2 furnished 15.9, which on Boc deprotection resulted in amine 15.10.
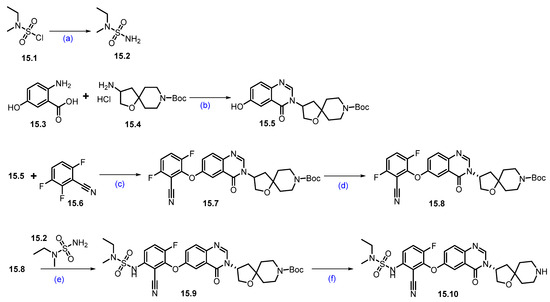
Scheme 27.
Synthesis of fragment 15.10 of CFT1946 (15): (a) 7N NH3 in MeOH, −10 °C to r.t., 14 h; (b) CH(OEt)3, toluene, THF, 110 °C, sealed tube, 18 h; (c) KOtBu, DMF/THF mixture, r.t., 14 h; (d) Chiral SFC; (e) Cs2CO3, DMF, 65 °C, 16 h; (f) 4N HCl in dioxane, r.t., 4 h.
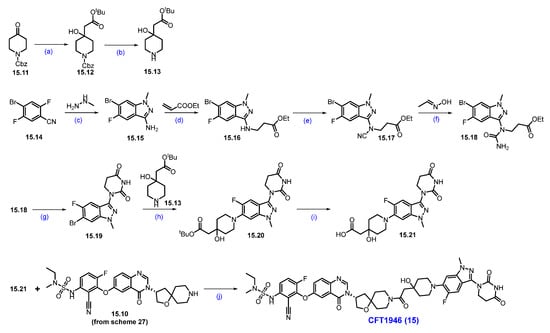
Scheme 28.
Synthesis of CFT1946 (15): (a) LDA, THF, −78 °C for 1 h then −78 °C to −10 °C for 2 h; (b) H2, 10% Pd/C, 1,4-dioxane; (c) EtOH, 80 °C, 12 h; (d) DBU-Lac, ionic liquid, 90 °C, 48 h; (e) CNBr, NaOAc, EtOH, 80 °C, 16 h; (f) InCl3 (cat.), toluene, reflux, 1 h; (g) triton-B, acetonitrile, r.t., 1 h; (h) Pd-PEPPSI-iHept(Cl), Cs2CO3, 1,4-dioxane, 110 °C, 2 h; (i) 4N HCl in dioxane, DCM, r.t., 6 h; (j) HATU, DIPEA, DMF, r.t., 12 h.
The nucleophilic addition of the carbanion generated from tert-butyl acetate to the carbonyl group of 15.11 generated alcohol 15.12, which, on Cbz deprotection with Pd/C and H2, furnished amine 15.13 as shown in Scheme 28. The indazole framework 15.15 was synthesized from substituted benzonitrile 15.14 and methyl hydrazine. Michael addition reaction of amine in 15.15 with ethyl acrylate in the presence of [DBU]·[Lac] ionic liquid furnished 15.16. N-cyanation of 15.16 with cyanogen bromide (CNBr) and NaOAc produced 15.17. An acid-catalyzed hydrolysis of the nitrile group followed by a cyclization reaction in the presence of Triton-B furnished compound 15.19. Subsequent Buchwald Hartwig coupling reaction of 15.19 with amine 15.13 furnished ester 15.20, which was converted to acid 15.21 by the deprotection of the tert-butyl group with HCl in dioxane. A final HATU-mediated acid–amine coupling reaction with amine 15.10 and previously prepared acid 15.21 (Scheme 27) resulted in CFT1946 (15).
2.11. PROTACs Targeting Kirsten Rat Sarcoma Virus (KRAS)
KRAS is a critical regulator of the MAPK signaling pathway, which governs essential cellular processes such as proliferation, migration, and gene expression. Activating mutations in KRAS are commonly found in several cancers, most notably pancreatic adenocarcinoma, non-small cell lung cancer (NSCLC), and colorectal cancer. These mutations result in constitutive activation of the MAPK pathway, driving uncontrolled tumor growth.
Historically, KRAS was deemed “undruggable” due to its strong binding affinity for GDP/GTP and the lack of suitable hydrophobic pockets for small-molecule targeting [141,142,143]. This perception shifted with the discovery of sotorasib and adagrasib, the first FDA-approved KRAS inhibitors [144,145,146]. These agents target the inducible switch II pocket and covalently bind to KRAS-GDP by exploiting the reactive cysteine residue introduced by the G12C mutation.
Expanding on this breakthrough, Mirati Therapeutics developed MRTX1133, the first selective and potent reversible inhibitor of KRASG12D, which was derived from an earlier irreversible G12C inhibitor [147]. While its entry into Phase 1 clinical trials in 2023 marked significant progress, the rapid emergence of resistance previously observed with KRAS G12C inhibitors remains a major concern [148]. Such resistance may arise from secondary mutations in KRAS, gene amplification, or compensatory reactivation of the MAPK pathway.
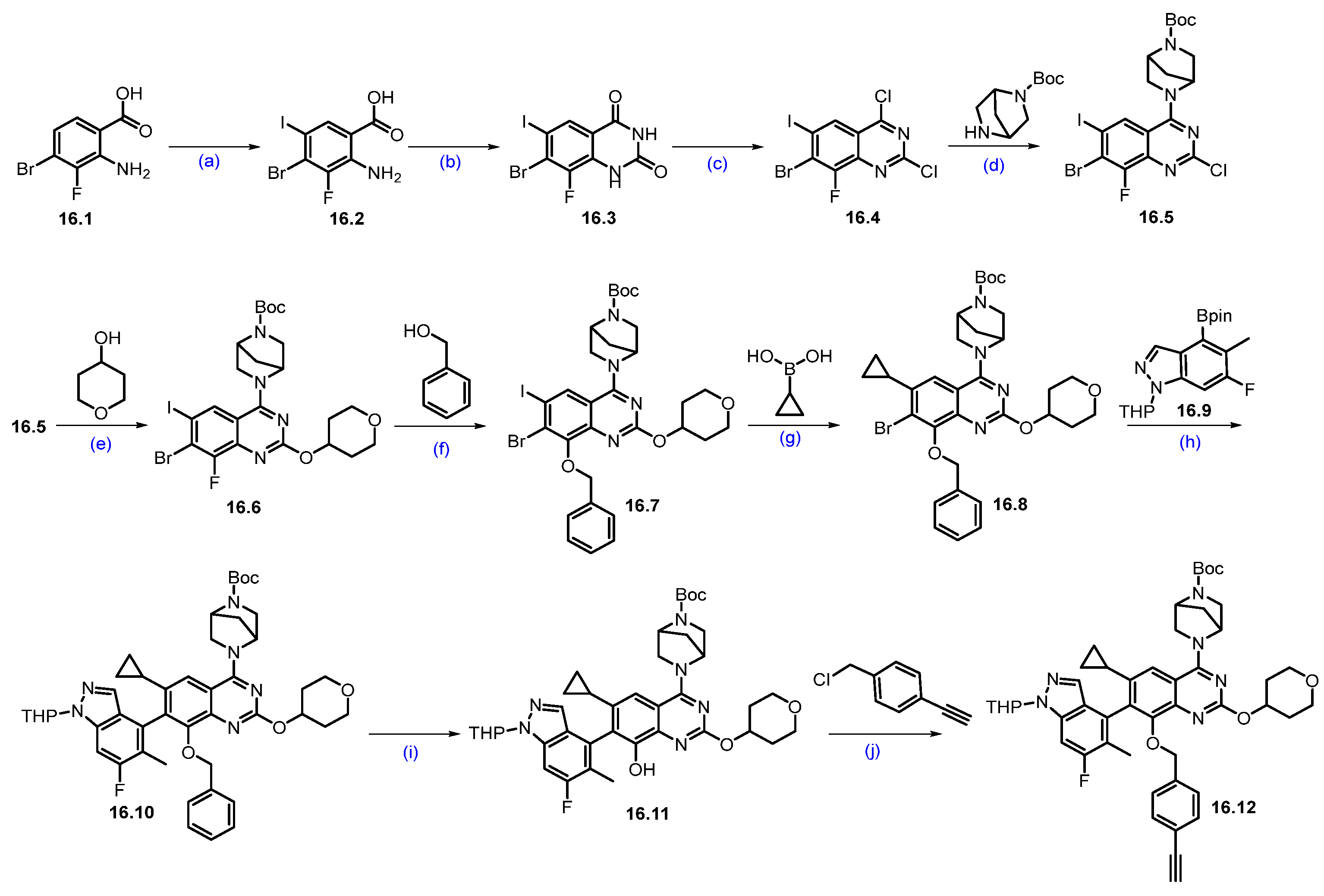
To address these limitations, targeted degradation of KRASG12D offers a promising alternative. ASP3082, developed by Astellas Pharma, is a KRASG12D selective degrader that employs VHL-mediated E3 ligase recruitment to eliminate the mutant protein [149,150]. Currently in Phase 1 clinical development, ASP3082 was granted FDA fast track designation in February 2023 for the treatment of pancreatic adenocarcinoma (NCT05382559).
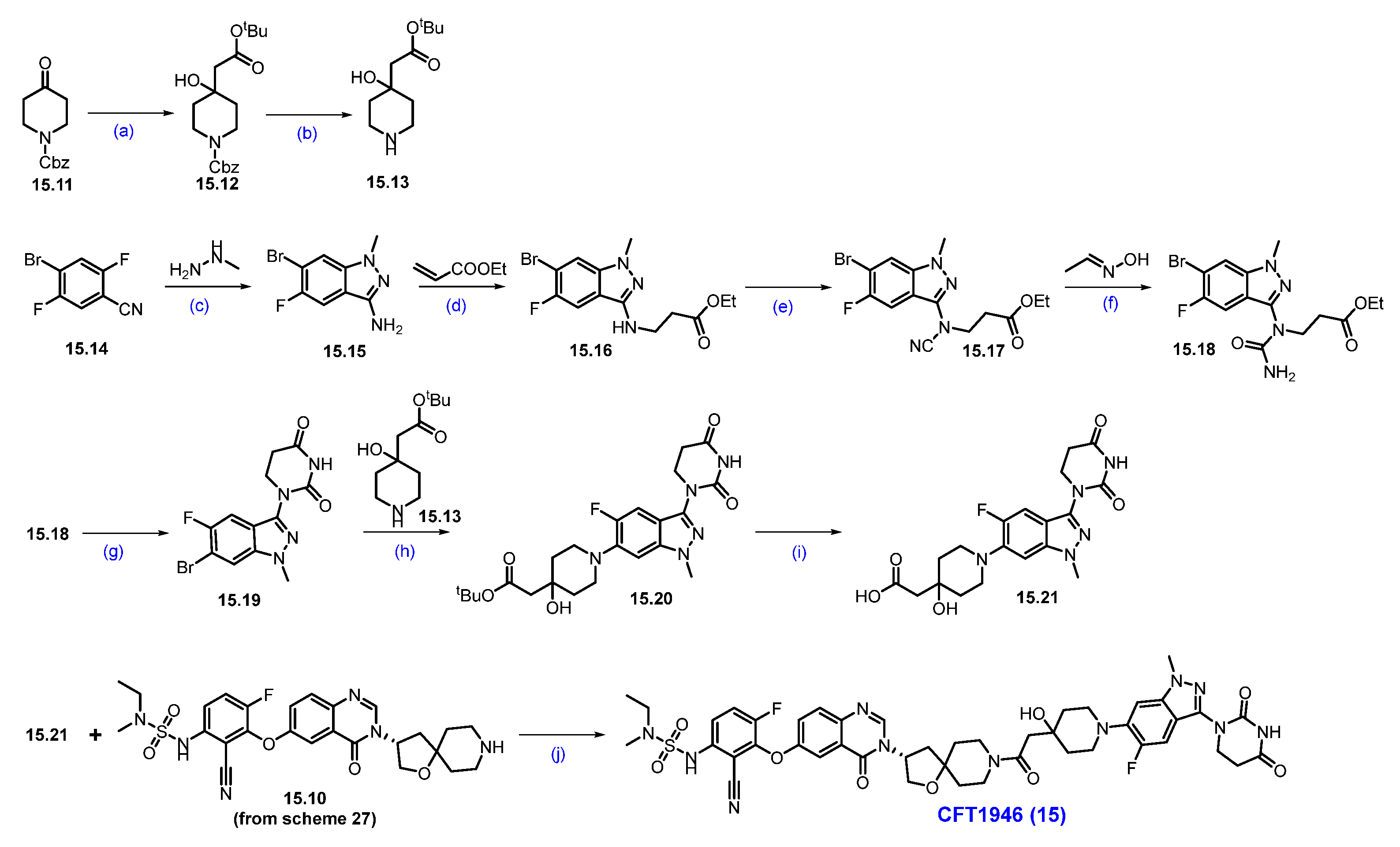
Synthesis of ASP3082 (16): The synthesis of ASP-3082 was described in Scheme 29 and Scheme 30 [151,152]. The synthesis started with commercially available substituted anthranilic acid 16.1, which was converted to compound 16.2 by iodination with NIS in DMF. Compound 16.2 was reacted with urea at elevated temperature to obtain quinazolinedione derivative 16.3. Compound 16.3 was treated with phosphorus oxychloride (POCl3) and DIPEA under heating conditions to obtain dichloroquinazoline derivative 16.4 in 50% yield in three steps. Aromatic nucleophilic substitution of more reactive chlorine in compound 16.4 was performed with tert-butyl (1R,4R)-2,5-diazabicyclo [2.2.1]heptane-2-carboxylate in THF in the presence of DIPEA as a base to produce compound 16.5. It was reacted with 4-hydroxy tetrahydropyran in an SNAr manner using Cs2CO3 as a base to furnish ether scaffold 16.6. The reactive fluorine in compound 16.6 was replaced with benzyl alcohol in another SNAr reaction using base-mediated potassium tert-butoxide to yield benzyloxy-substituted product 16.7. The chemo-selective Suzuki coupling reaction of compound 16.7 with cyclopropylboronic acid in the presence of Pd(dppf)Cl2 and K3PO4 produced iodo-substituted product 16.8. Another Suzuki reaction of compound 16.8 with THP-protected indazole boronic acid pinacol ester 16.9 in the presence of RuPhos Pd G3, RuPhos, and K3PO4 at 130 °C furnished scaffold 16.10 with moderate yields. Deprotection of the benzyl group on compound 16.10 was treated with Pd/C under a hydrogen atmosphere in methanol to afford compound 16.11. Final nucleophilic substitution of 1-(chloromethyl)-4-ethynylbenzene with compound 16.11 in the presence of Cs2CO3 produced the desired alkyne compound 16.12 in reasonable yield.
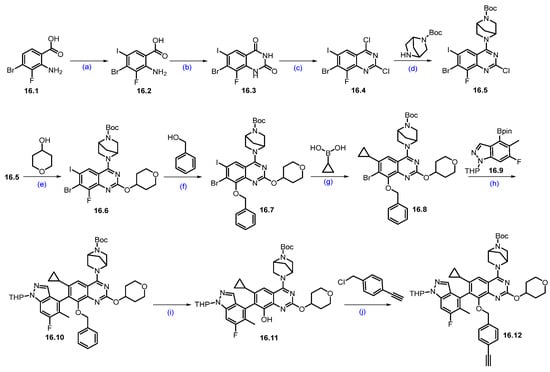
Scheme 29.
Synthesis of fragment 16.11 of ASP3082 (16): (a) NIS, DMF, 50 °C, 4 h; (b) Urea, 200 °C, 3 h; (c) POCl3, DIPEA, reflux, 16 h; (d) DIPEA, THF, 0 °C, 16 h; (e) Cs2CO3, DABCO, DMF, THF, r.t., 16 h; (f) t-BuOH, THF, 0 °C, 1.5 h; (g) Pd(dppf)Cl2, K3PO4, ACN, 1,4-dioxane, H2O, 100 °C, 3 h; (h) RuPhos PdG3, RuPhos, K3PO4, 1,4-dioxane, 100 °C, 2.5 h; (i) 10% Pd/C/H2, r.t., 2 h; (j) Cs2CO3, THF, 60 °C, 2 h.
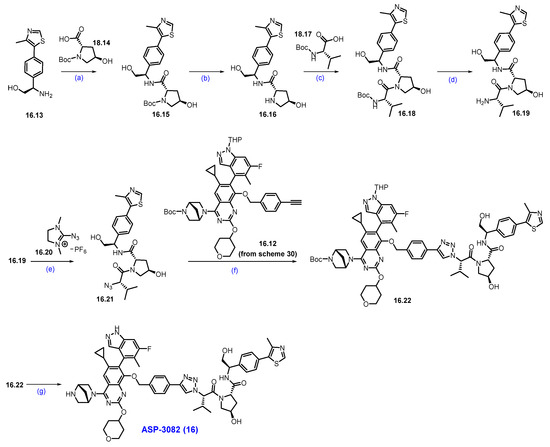
Scheme 30.
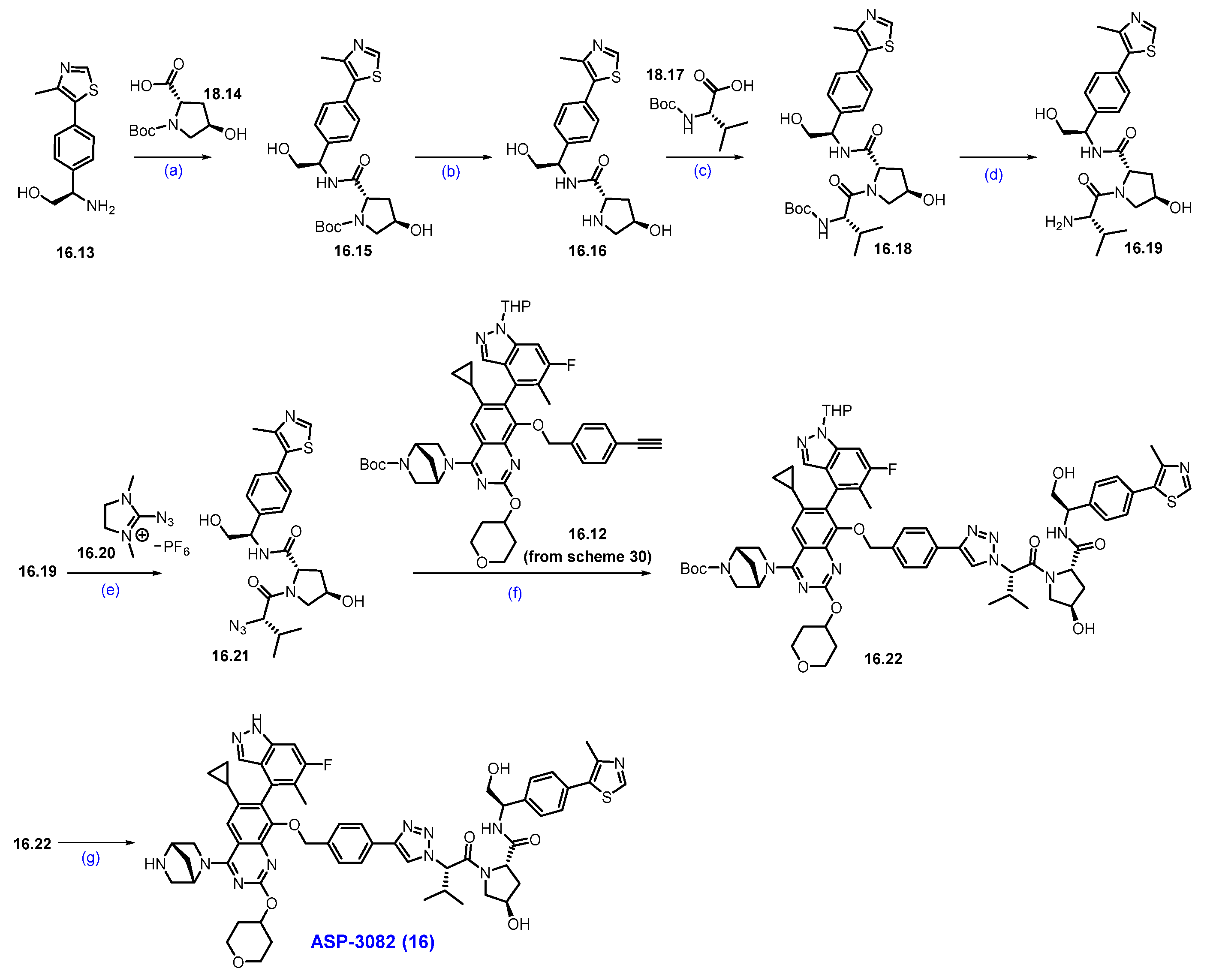
Synthesis of ASP-3082 (16): (a) HATU, DIPEA, DMF, 5 °C for 1 h then at r.t., for 1 h; (b) HCl in dioxane, MeOH/DCM mixture, 0 °C to r.t., 6 h; (c) HATU, DIPEA, DMF, 5 °C for 1 h then at r.t., for 1h; (d) HCl in dioxane, 0 °C to r.t., 5 h; (e) NEt3, ACN, THF, 0 °C, 5 h; (f) Sodium ascorbate, CuI, t-BuOH, THF, H2O, 50 °C, 3 h; (g) TFA, DCM, 0 °C to r.t., 2 h.
The final synthesis of ASP-3082 is described in Scheme 30.
The synthesis commenced with commercially available amine 16.13, which was converted to compound 16.15 through the amidation reaction with acid 16.14 in the presence of HATU and DIPEA in DMF, as mentioned in Scheme 30. Deprotection of -Boc group in compound 16.15 was removed with HCl in 1,4-dioxane, to obtain product 16.16. The amine 16.16 was reacted with N-boc-L-valine 16.17 under HATU and DIPEA conditions to give 16.18. Boc deprotection in compound 16.18 was achieved using HCl in dioxane to generate valyl amide 16.19. The free amine in compound 16.19 was converted to its corresponding azide utilizing reagent 16.20 in the presence of NEt3 in ACN and THF to furnish compound 16.21 at moderate yields. The click reaction of the azide 16.21 and previously synthesized alkyne 16.12 (from Scheme 29) in the presence of sodium ascorbate and copper iodide (CuI) in tert-butanol/water at 50 °C yielded triazole scaffold 16.22. Finally, the deprotection of the Boc and THP groups with TFA in DCM furnished the final compound, ASP-3082 (16), in excellent yield.
2.12. PROTACs Targeting Interleukin-1 Receptor-Associated Kinase 4 (IRAK4)
KT-413 is a novel dual-mechanism degrader developed to address the unmet medical need in treating the ABC subtype of diffuse large B-cell lymphoma (DLBCL), particularly in tumors driven by MYD88 mutations. It efficiently degrades IRAK4 and the transcription factors Ikaros and Aiolos by functioning both as a heterobifunctional degrader and a molecular glue. KT-413′s ability to simultaneously target these proteins results in potent inhibition of NF-κB signaling and activation of Type I interferon responses, restoring apoptosis and suppressing tumor growth. Supported by promising preclinical data, KT-413 is now in Phase 1 clinical trials (NCT05233033) for non-Hodgkin’s lymphoma, DLBCL, and MYD88-mutant indications [153].
Synthesis of KT-413 (17). Recently, Weiss et al. reported the development of KT-413 as a potent degrader that acts as a heterobifunctional degrader and molecular glue. Scheme 31 and Scheme 32 illustrate the synthesis of KT-413, as reported in the literature [154].
Scheme 31.
Synthesis of intermediate 17.12 of KT-413 (17): (a) BnBr, NaH, TBAI, THF, 45%; (b) (i) DMP, DCM; (ii) NaH2PO4, 2-Me-2-butene, NaClO2, t-BuOH, 90%; (c) NBS, DMF, 89%; (d) T3P, pyridine, DCM, 85%; (e) CuI, Na2S.9H2O, TFA, DMF, 95%; (f) MeI, K2CO3, DMF, 69%; (g) Pd2(dba)3, Xantphos, Cs2CO3, dioxane, 83%; (h) BCl3, DCM, 90%; (i) MeMgBr, THF, 66%; (j) DMP, DCM, 85%.
Scheme 32.
Synthesis of KT-413 (17): (a) tBuOK, THF, 66%; (b) Raney Ni, H2, NH3.H2O, 67%; (c) DIPEA, DMSO, 46%; (d) TFA, DCM, 58%; (e) TEA, AcOH, NaBH(OAc)3, THF, 59%.
The synthesis initiated with commercially available trans-1,4-cyclohexanedimethanol 17.1, which was converted to compound 17.2 by mono-benzyl protection using benzyl bromide and NaH. The primary alcohol of 17.2 was treated with Dess-Martin Periodinane (DMP) to furnish the aldehyde. Further oxidation of the aldehyde functionality under Pinnick oxidation (NaH2PO4 + NaClO2) afforded acid 17.3 in good yield. Next, commercially available substituted aniline 17.4 was subjected to selective bromination with NBS to furnish bromo aniline 17.5, which was coupled with carboxylic acid 17.3 under T3P conditions, to afford amide 17.6. Next, the reaction of the amide with sodium sulfide in the presence of CuI, followed by stirring with TFA, led to benzothiazole carboxylic acid intermediate 17.7. Esterification of acid intermediate 17.7 using MeI, K2CO3 afforded ester 17.8. Amination of 17.8 with 6-(trifluoromethyl)picolinamide under Buchwald-Hartwig conditions using Pd2(dba)3, Xantphos in dioxane furnished amide 17.9. Deprotection of the benzyl group with BCl3 followed by a reaction of methyl magnesium bromide (MeMgBr) afforded tertiary alcohol 17.11. Finally, oxidation of 17.11 with DMP afforded aldehyde intermediate 17.12 (Scheme 31).
The synthesis of the final degrader started with the commercially available spirocyclic ketone 17.13, which underwent a base-mediated Wittig homologation with 2-diethoxyphosphorylacetonitrile to produce 17.15. The resulting alkene 17.15 was subjected to Raney Ni-mediated hydrogenation to render amine 17.16. Next, intermediate 17.16 was subjected to SNAr reaction with 4-fluoro-thalidomide using diisopropylethylamine in DMSO at 130 °C, producing compound 17.18. It was treated with TFA to remove the Boc group to afford 17.19 as the TFA salt. Finally, reductive amination of 17.12 with amine 17.19 furnishes the final degrader KT-413 with excellent yield (Scheme 32).
3. Outlook and Perspectives
Since 2019, numerous PROTACs have entered clinical trials, primarily for cancer indications, but also for diseases such as Parkinson’s, inflammatory conditions, and chronic pain [154]. The field has advanced rapidly, with three leading candidates now in Phase III clinical trials—the final stage before potential regulatory approval. These late-stage programs target breast cancer, prostate cancer, and leukemia, underscoring the increasing clinical maturity of targeted protein degradation therapies.
Vepdegestrant, which targets estrogen receptors in breast cancer, is the first PROTAC to reach Phase III trials. It is followed by Bavdegalutamide, which aims to degrade the androgen receptor in metastatic prostate cancer, and NX-5948, which targets BTK, a key driver in chronic lymphocytic leukemia.
Despite their promise, PROTACs face several key challenges. These include difficulty targeting membrane-bound proteins, an inability to degrade extracellular proteins, uncertainties about the full biological impact of completely removing a protein, and the risk of off-target degradation.
In summary, targeted protein degradation (TPD) has rapidly emerged as both a robust research platform and a promising therapeutic strategy. As the field evolves, ongoing interdisciplinary collaboration will be crucial to overcoming current hurdles and expanding its clinical reach. Innovations such as the integration of novel E3 ligases, the identification of new binding sites, and the creation of diverse warheads are already setting degraders apart from conventional small molecules. With continued momentum in research and partnership, TPD is poised to drive even more groundbreaking advances in the future.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ACN—Acetonitrile; AR—Androgen Receptor; Bcl-xL—B cell lymphoma–extra-large; BCL6—B cell lymphoma 6; BRAF—B-Raf proto-oncogene, serine/threonine kinase; BRD9—Bromodomain-containing protein 9, BTK—Bruton’s tyrosine kinase; CLL—Chronic lymphocytic leukemia CRBN—Cereblon; CRPC—Castration-resistant prostate cancer; DHT—Dihydrotestosterone; DIAD—Diisopropyl Azodicarboxylate; DLBCL—Diffuse large B-cell lymphoma; EDC.HCl—Ethyl-3-(3-dimethylaminopropyl)carbodiimide; DPPA—Diphenylphosphoryl Azide; ER—Estrogen Receptor; GDP/GTP—Guanosine diphosphate/Guanosine triphosphate; HSPs—Heat shock proteins; IAP—Inhibitor of Apoptosis Proteins; IRAK4—Interleukin-1 receptor–associated kinase 4; KRAS—Kirsten rat sarcoma viral oncogene homolog; LRRK2—leucine-rich repeat kinase 2; MCL—Mantle cell lymphoma; MDM2—Murine double minute 2; ORR—objective response rate; PFS—Progression-free survival; Pd2(dba)3—Tris(dibenzylideneacetone)dipalladium(0); PROTAC—Proteolysis-targeting chimera; RECIST—Response Evaluation Criteria in Solid Tumors; SMARCA2—Switch/sucrose nonfermentable related, matrix associated, actin depended regulator of chromatin, subfamily A, member 2; STAT3—Signal inducer and activator of transcription 3; T3P—Propanephosphonic acid anhydride; TCL—T-cell lymphoma; TPD—Target protein degradation; TPL—Target protein ligand; TPP—Triphenylphosphine; Troc—Trichloroethyl chloroformate; ULL—Ubiquitin ligase ligand; UPS—Ubiquitin-proteasome system; VHL—Von Hippel–Lindau; X-phos—Dicyclohexyl [2′,4′,6′-tris(propan-2-yl)[1,1′-biphenyl]-2-yl]phosphane.
References
- Li, K.; Crews, C.M. PROTACs: Past, present and future. Chem. Soc. Rev. 2022, 51, 5214–5236. [Google Scholar] [CrossRef]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.B.; Neklesa, T.K.; Willard, R.R.; Gordon, D.A.; Pizzano, J.; Vitale, N.; Robling, K.; Dorso, M.A.; Moghrabi, W.; Landrette, S.; et al. Preclinical Evaluation of Bavdegalutamide (ARV-110), a Novel PROteolysis TArgeting Chimera Androgen Receptor Degrader. Mol. Cancer Ther. 2025, 24, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.M.; Flanagan, J.J.; Teh, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clin. Cancer Res. 2024, 30, 3549–3563. [Google Scholar] [CrossRef]
- Arnold, C. PROTAC protein degraders to drug the undruggable enter phase 3 trials. Nat. Med. 2024, 30, 3030–3031. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, J. NDA Submission of Vepdegestrant (ARV-471) to U.S. FDA: The Beginning of a New Era of PROTAC Degraders. J. Med. Chem. 2025, 68, 14129–14136. [Google Scholar] [CrossRef]
- Lu, J.; Qian, Y.M.; Altieri, M.; Dong, H.Q.; Wang, J.; Raina, K.; Hines, J.; Winkler, J.D.; Crew, A.P.; Coleman, K.; et al. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015, 22, 755–763. [Google Scholar] [CrossRef]
- Churcher, I. Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? J. Med. Chem. 2018, 61, 444–452. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Busino, L. Targeting the E3 ubiquitin ligases DCAF15 and cereblon for cancer therapy. Semin. Cancer Biol. 2020, 67, 53–60. [Google Scholar] [CrossRef]
- Humphreys, L.M.; Smith, P.; Chen, Z.; Fouad, S.; D’Angiolella, V. The role of E3 ubiquitin ligases in the development and progression of glioblastoma. Cell Death Differ. 2021, 28, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wu, Y.; Cheng, H.; Wang, X. The role of E3 ubiquitin ligases and deubiquitinases in bladder cancer development and immunotherapy. Front. Immunol. 2023, 14, 1202633. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Li, J.; Zhang, B.; Wang, Z.; Shaabani, S.; Ter Brake, F.; Essa, K.; Domling, A. PROTACs- a game-changing technology. Expert. Opin. Drug Discov. 2019, 14, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Rej, R.K.; Allu, S.R.; Roy, J.; Acharyya, R.K.; Kiran, I.N.C.; Addepalli, Y.; Dhamodharan, V. Orally Bioavailable Proteolysis-Targeting Chimeras: An Innovative Approach in the Golden Era of Discovering Small-Molecule Cancer Drugs. Pharmaceuticals 2024, 17, 494. [Google Scholar] [CrossRef]
- Vicente, A.T.S.; Moura, S.; Salvador, J.A.R. Synthesis, biological evaluation and clinical trials of Cereblon-based PROTACs. Commun. Chem. 2025, 8, 218. [Google Scholar] [CrossRef]
- Nierengarten, M.B. Cancer Statistics 2024: Deaths drop, incidences increase, prevention needed. Cancer-Am. Cancer Soc. 2024, 130, 1904. [Google Scholar] [CrossRef]
- Dizon, D.S.; Kamal, A.H. Cancer statistics 2024: All hands on deck. CA-Cancer J. Clin. 2024, 74, 8–9. [Google Scholar] [CrossRef]
- James, N.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Asangani, I.; Blair, I.A.; Van Duyne, G.; Hilser, V.J.; Moiseenkova-Bell, V.; Plymate, S.; Sprenger, C.; Wand, A.J.; Penning, T.M. Using biochemistry and biophysics to extinguish androgen receptor signaling in prostate cancer. J. Biol. Chem. 2021, 296, 100240. [Google Scholar] [CrossRef]
- Rathkopf, D.; Scher, H.I. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J. 2013, 19, 43–49. [Google Scholar] [CrossRef]
- Sobhani, N.; Neeli, P.K.; D’Angelo, A.; Pittacolo, M.; Sirico, M.; Galli, I.C.; Roviello, G.; Nesi, G. AR-V7 in Metastatic Prostate Cancer: A Strategy beyond Redemption. Int. J. Mol. Sci. 2021, 22, 5515. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Han, X. Targeting androgen receptor degradation with PROTACs from bench to bedside. Biomed. Pharmacother. 2023, 158, 114112. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Burris, H.; Vuky, J.; Dreicer, R.; Sartor, A.O.; Sternberg, C.N.; Percent, I.J.; Hussain, M.H.A.; Kalebasty, A.R.; Shen, J.; et al. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef]
- Petrylak, D.P.; McKean, M.; Lang, J.M.; Gao, X.; Dreicer, R.; Geynisman, D.M.; Stewart, T.F.; Gandhi, M.; Appleman, L.J.; Dorff, T.B.; et al. ARV-766, a proteolysis targeting chimera (PROTAC) androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC): Initial results of a phase 1/2 study. J. Clin. Oncol. 2024, 42, 5011. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Patel, M.R.; Choudhury, A.D.; Rasco, D.; Lakhani, N.; Hawley, J.E.; Srinivas, S.; Aparicio, A.; Narayan, V.; Runcie, K.D.; et al. Safety and clinical activity of BMS-986365 (CC-94676), a dual androgen receptor ligand-directed degrader and antagonist, in heavily pretreated patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2025, 36, 76–88. [Google Scholar] [CrossRef]
- Nayak, S.; Norris, J.D.; Ammirante, M.; Rychak, E.; Wardell, S.E.; Liao, D.; Toyama, B.; Kandimalla, R.; Christoforou, A.; Tsuji, T.; et al. Discovery of BMS-986365, a first-in-class dual androgen receptor ligand-directed degrader (AR LDD) and antagonist, for the treatment of advanced prostate cancer. Clin. Cancer Res. 2025, OF1–OF18. [Google Scholar] [CrossRef]
- Chi, K.N.; Mckay, R.R.; Sandhu, S.; Arranz, J.A.; Barthelemy, P.; Hadaschik, B.; Matsubara, N.; Shore, N.D.; Ye, D.W.; Cascella, T.; et al. rechARge: A randomized phase III trial of the androgen receptor ligand-directed degrader, BMS-986365, vs investigator’s choice in patients with mCRPC. Future Oncol. 2025, 21, 1771–1777. [Google Scholar] [CrossRef]
- Dong, H.; Duguid, R.J.; Jager, C.K.; Kaushal, A.M.; Kennedy, S.E.; Neeser, M.A.; Reeve, M.M.; Reo, J.P.; Zahedi, M.M.; Kattuboina, V.A.; et al. Methods of Manufacturing a Bifunctional Compound, Ultrapure Forms of the Bifunctional Compound, and Dosage Forms Comprising the Same. WO2021231174A1, 18 November 2021. [Google Scholar]
- Kandimalla, R.L.D. Combination of Substituted 3-((3-Aminophenyl)amino)Piperidine-2,6-Dione Compounds with Other Therapeutic Agents for Treating Androgen Receptor Mediated Diseases such as Prostate Cancer. WO2024102706A1, 23 May 2024. [Google Scholar]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA-Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Bergman, L.; Beelen, M.L.; Gallee, M.P.; Hollema, H.; Benraadt, J.; van Leeuwen, F.E. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 2000, 356, 881–887. [Google Scholar] [CrossRef]
- Bross, P.F.; Baird, A.; Chen, G.; Jee, J.M.; Lostritto, R.T.; Morse, D.E.; Rosario, L.A.; Williams, G.M.; Yang, P.; Rahman, A.; et al. Fulvestrant in postmenopausal women with advanced breast cancer. Clin. Cancer Res. 2003, 9, 4309–4317. [Google Scholar] [PubMed]
- Juric, D.; Janku, F.; Rodon, J.; Burris, H.A.; Mayer, I.A.; Schuler, M.; Seggewiss-Bernhardt, R.; Gil-Martin, M.; Middleton, M.R.; Baselga, J.; et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, e184475. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A.; Mayne, C.G.; Katzenellenbogen, B.S.; Greene, G.L.; Chandarlapaty, S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nat. Rev. Cancer 2018, 18, 377–388. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef]
- Rej, R.K.; Hu, B.; Chen, Z.X.; Acharyya, R.K.; Wu, D.M.; Metwally, H.; Mceachern, D.; Wang, Y.; Jiang, W.; Bai, L.C.; et al. Discovery of ERD-12310A as an Exceptionally Potent and Orally Efficacious PROTAC Degrader of Estrogen Receptor α (ERα). J. Med. Chem. 2024, 67, 20933–20965. [Google Scholar] [CrossRef]
- Chen, Z.X.; Hu, B.; Rej, R.K.; Wu, D.M.; Acharyya, R.K.; Wang, M.L.; Xu, T.F.; Lu, J.F.; Metwally, H.; Wang, Y.; et al. Discovery of ERD-3111 as a Potent and Orally Efficacious Estrogen Receptor PROTAC Degrader with Strong Antitumor Activity. J. Med. Chem. 2023, 66, 12559–12585. [Google Scholar] [CrossRef]
- Xu, G.; Havens, C.G.; Deng, Q.; Lowenstein, C.; Samanta, D.; Vidal, B.; Behshad, E.; Russell, M.; Orth, P.; Rice, C.T.; et al. Discovery and Characterization of PVTX-321 as a Potent and Orally Bioavailable Estrogen Receptor Degrader for ER+/HER2− Breast Cancer. J. Med. Chem. 2025, 68, 11299–11321. [Google Scholar] [CrossRef]
- Acharyya, R.K.; Rej, R.K.; Hu, B.; Chen, Z.; Wu, D.; Lu, J.; Metwally, H.; McEachern, D.; Wang, Y.; Jiang, W.; et al. Discovery of ERD-1233 as a Potent and Orally Efficacious Estrogen Receptor PROTAC Degrader for the Treatment of ER+ Human Breast Cancer. J. Med. Chem. 2024, 67, 19010–19037, Correction in J. Med. Chem. 2025, 68, 9014–9015. [Google Scholar] [CrossRef]
- Rej, R.K.; Thomas, J.I.I.; Acharyya, R.K.; Rae, J.M.; Wang, S.M. Targeting the Estrogen Receptor for the Treatment of Breast Cancer: Recent Advances and Challenges. J. Med. Chem. 2023, 66, 8339–8381. [Google Scholar] [CrossRef]
- Campone, M.; De Laurentiis, M.; Jhaveri, K.; Hu, X.C.; Ladoire, S.; Patsouris, A.; Zamagni, C.; Cui, J.W.; Cazzaniga, M.; Cil, T.; et al. Vepdegestrant, a PROTAC Estrogen Receptor Degrader, in Advanced Breast Cancer. N. Engl. J. Med. 2025, 393, 556–568. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P. Degradation of the Estrogen Receptor in Breast Cancer. N. Engl. J. Med. 2025, 393, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Crew, A.P.; Flanagan, J.; Gough, S.M.; Haskell, R.J., III; Moore, M.D.; Qian, Y.; Taylor, I.C.A.; Wang, J.; Chen, X. Methods of Treating Breast Cancer with Tetrahydronaphthalene Derivatives as Estrogen Receptor Degraders. WO2021041348A1, 4 March 2021. [Google Scholar]
- Avery, S.; Buske, J.M.; Chen, D.; Chen, H.; Chen, X.; Davidson, A.R.; Desrosiers, J.N.; Dong, H.Q.; Fellah, N.; Fernández, D.F.; et al. Development of a Commercial Manufacturing Process for Vepdegestrant, an Orally Bioavailable PROTAC Estrogen Receptor Degrader for the Treatment of Breast Cancer. Org. Process Res. Dev. 2024, 28, 4079–4090. [Google Scholar] [CrossRef]
- Chen, X.; Crew, A.P.; Flanagan, J.; Gough, S.M.; Haskell, R.J., III; Moore, M.D.; Qian, Y.; Taylor, I.C.A.; Wang, J. Methods of Treating Breast Cancer with Tetrahydronaphthalene Derivatives as Estrogen Receptor Degraders and Their Preparation. WO2022132652A1, 23 June 2022. [Google Scholar]
- Brien, G.L.; Remillard, D.; Shi, J.; Hemming, M.L.; Chabon, J.; Wynne, K.; Dillon, E.T.; Cagney, G.; Van Mierlo, G.; Baltissen, M.P.; et al. Targeted degradation of BRD9 reverses oncogenic gene expression in synovial sarcoma. eLife 2018, 7, e41305. [Google Scholar] [CrossRef]
- Webb, T.; Craigon, C.; Ciulli, A. Targeting epigenetic modulators using PROTAC degraders: Current status and future perspective. Bioorg Med. Chem. Lett. 2022, 63, 128653. [Google Scholar] [CrossRef]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef]
- Kadoch, C.; Crabtree, G.R. Reversible Disruption of mSWI/SNF (BAF) Complexes by the SS18-SSX Oncogenic Fusion in Synovial Sarcoma. Cell 2013, 153, 71–85. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, M.; Wang, Y.; Rej, R.K.; Aguilar, A.; Xu, T.F.; Bai, L.C.; Tosovic, J.; McEachern, D.; Li, Q.X.; et al. Discovery of CW-3308 as a Potent, Selective, and Orally Efficacious PROTAC Degrader of BRD9. J. Med. Chem. 2024, 67, 14125–14154. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.C.; D’Avino, A.R.; Cassel, S.H.; Mashtalir, N.; McKenzie, Z.M.; McBride, M.J.; Valencia, A.M.; Zhou, Q.H.; Bocker, M.; Soares, L.M.M.; et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat. Cell Biol. 2018, 20, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Foghorn Therapeutics Provides an Update on FHD-609. News Release. Foghorn®Therapeutics Inc. 24 April 2023. Available online: https://bit.ly/440mXs9 (accessed on 25 April 2023).
- A Study to Assess the Safety and Tolerability of CFT8634 in Locally Advanced or Metastatic SMARCB1-Perturbed Cancers, Including Synovial Sarcoma and SMARCB1-Null Tumors. Available online: https://clinicaltrials.gov/study/NCT05355753?term=CFT8634&rank=1 (accessed on 17 December 2024).
- Jackson, K.L.; Agafonov, R.V.; Carlson, M.W.; Chaturvedi, P.; Cocozziello, D.; Cole, K.; Deibler, R.; Eron, S.J.; Good, A.; Hart, A.A.; et al. discovery and characterization of CFT8634: A potent and selective degrader of BRD9 for the treatment of SMARCB1-perturbed cancers. Cancer Res. 2022, 82, ND09. [Google Scholar] [CrossRef]
- Jackson, K.L.; Nasveschuk, C.G.; Zeid, R.; Yin, N.; Veits, G.K.; Moustakim, M.; Yap, J.L.; He, M.; Yu, R.T.; Schnaderbeck, M.J.; et al. Selected Compounds for Targeted Degradation of brd9. WO2023/039208A1, 13 April 2023. [Google Scholar]
- Huang, L.; Terry, D.B.; Waetzig, J.D.; Gu, C.-H. Stability-Enhancing Compositions and Methods of Preparing Compounds. WO2023009719A2, 2 February 2023. [Google Scholar]
- Anuar, N.N.M.; Hisam, N.S.N.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar]
- Khan, S.; Zhang, X.; Lv, D.; Zhang, Q.; He, Y.; Zhang, P.; Liu, X.; Thummuri, D.; Yuan, Y.; Wiegand, J.S.; et al. A selective BCL-X(L) PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019, 25, 1938–1947. [Google Scholar] [CrossRef]
- Jaiswal, A.; Jaiswal, A.; Williamson, E.A.; Gelfond, J.; Zheng, G.R.; Zhou, D.H.; Hromas, R. Resistance to the BCL-XL degrader DT2216 in T-cell acute lymphoblastic leukemia is rare and correlates with decreased BCL-XL proteolysis. Cancer Chemoth. Pharm. 2023, 91, 89–95. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef]
- Zheng, G.; Zhou, D.; Zhang, X.; Khan, S.; He, Y.; Zhang, P. Preparation of Peptides as Bcl-2 Proteins Degraders for Cancer Treatment. WO2019144117A1, 25 July 2019. [Google Scholar]
- Gaballa, S.; Pinilla-Ibarz, J. BTK Inhibitors in Chronic Lymphocytic Leukemia. Curr. Hematol. Malig. Rep. 2021, 16, 422–432. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.H.; Steggerda, S.M.; Versele, M.; et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Lew, T.E.; Lin, V.S.; Cliff, E.R.; Blombery, P.; Thompson, E.R.; Handunnetti, S.M.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Huang, D.C.S.; et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021, 5, 4054–4058. [Google Scholar] [CrossRef]
- Woyach, J.A.; Brown, J.R.; Ghia, P.; Roeker, L.E.; Patel, K.; Eyre, T.A.; Munir, T.; Lech-Maranda, E.; Lamanna, N.; Tam, C.S.; et al. Pirtobrutinib in Post-cBTKi CLL/SLL: ~30 Months Follow-up and Subgroup Analysis With/Without Prior BCL2i from the Phase 1/2 BRUIN Study. Blood 2023, 142, 325. [Google Scholar] [CrossRef]
- Salvaris, R.T.; Brennan, J.; Lewis, K.L. BTK Is the Target That Keeps on Giving: A Review of BTK-Degrader Drug Development, Clinical Data, and Future Directions in CLL. Cancers 2025, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Desikan, S.P.; Nguyen, B.; Won, H.; Tantawy, S.I.; McNeely, S.; Marella, N.; Ebata, K.; Woyach, J.A.; Patel, K.; et al. Genomic Evolution and Resistance during Pirtobrutinib Therapy in Covalent BTK-Inhibitor (cBTKi) Pre-Treated Chronic Lymphocytic Leukemia Patients: Updated Analysis from the BRUIN Study. Blood 2023, 142, 326. [Google Scholar] [CrossRef]
- Wang, E.; Mi, X.L.; Thompson, M.C.; Montoya, S.; Notti, R.Q.; Afaghani, J.; Durham, B.H.; Penson, A.; Witkowski, M.T.; Lu, S.X.; et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N. Engl. J. Med. 2022, 386, 735–743. [Google Scholar] [CrossRef]
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kazmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef]
- Buhimschi, A.D.; Armstrong, H.A.; Toure, M.; Jaime-Figueroa, S.; Chen, T.L.; Lehman, A.M.; Woyach, J.A.; Johnson, A.J.; Byrd, J.C.; Crews, C.M. Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry 2018, 57, 3564–3575. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Noviski, M.; Lu, H.; Thompson, M.C.; Chirino, A.; Jahn, J.; Sondhi, A.K.; Gajewski, S.; Tan, Y.S.M.; et al. Kinase-impaired BTK mutations are susceptible to clinical-stage BTK and IKZF1/3 degrader NX-2127. Science 2024, 383, eadi5798. [Google Scholar] [CrossRef]
- Robbins, D.W.; Noviski, M.A.; Tan, Y.S.; Konst, Z.A.; Kelly, A.; Auger, P.; Brathaban, N.; Cass, R.; Chan, M.L.; Cherala, G.; et al. Discovery and Preclinical Pharmacology of NX-2127, an Orally Bioavailable Degrader of Bruton’s Tyrosine Kinase with Immunomodulatory Activity for the Treatment of Patients with B Cell Malignancies. J. Med. Chem. 2024, 67, 2321–2336. [Google Scholar] [CrossRef]
- Huynh, T.; Rodriguez-Rodriguez, S.; Roleder, C.; Whelan, S.; Tan, M.; Lee, E.; Munson, P.; Danilov, A.V. Nx-2127 and Nx-5948, Two Clinical Stage Cereblon-Recruiting BTK Degraders, Facilitate T Cell Functionality in Chronic Lymphocytic Leukemia. Blood 2024, 144, 77–78. [Google Scholar] [CrossRef]
- Searle, E.; Forconi, F.; Linton, K.; Danilov, A.; Mckay, P.; Lewis, D.; El-Sharkawi, D.; Gleeson, M.; Riches, J.; Injac, S.G.; et al. Initial Findings from a First-in-Human Phase 1a/b Trial of NX-5948, a Selective Bruton’s Tyrosine Kinase (BTK) Degrader, in Patients with Relapsed/Refractory B Cell Malignancies. Blood 2023, 142, 4473. [Google Scholar] [CrossRef]
- Noviski, M.A.; Brathaban, N.; Mukerji, R.; Yung, S.; Ye, J.; Bousquet, H.; de los Rios, M.S.G.; Bravo, B.; Chen, J.W.; Auger, P.; et al. NX-5948 promotes selective, sub-nanomolar degradation of inhibitor-resistant BTK mutants. Cancer Res. 2023, 83, 2850. [Google Scholar] [CrossRef]
- Flaherty, C. FDA Grants Fast Track Status to NX-5948 for Relapsed/Refractory CLL/SLL. Available online: https://www.onclive.com/view/fda-grants-fast-track-status-to-nx-5948-for-relapsed-refractory-cll-sll (accessed on 17 January 2024).
- Sands, A.T.; Kelly, A. Aileen Bifunctional Compounds for Degrading Btk via Ubiquitin Proteosome Pathway. WO2021113557A1, 10 June 2021. [Google Scholar]
- Hanlon, M.M.; Rakovich, T.; Cunningham, C.C.; Ansboro, S.; Veale, D.J.; Fearon, U.; McGarry, T. STAT3 Mediates the Differential Effects of Oncostatin M and TNFalpha on RA Synovial Fibroblast and Endothelial Cell Function. Front. Immunol. 2019, 10, 2056. [Google Scholar] [CrossRef] [PubMed]
- Mankan, A.K.; Greten, F.R. Inhibiting signal transducer and activator of transcription 3: Rationality and rationale design of inhibitors. Expert. Opin. Inv Drug 2011, 20, 1263–1275. [Google Scholar] [CrossRef]
- Zhou, J.; Tison, K.; Zhou, H.; Bai, L.; Acharyya, R.K.; McEachern, D.; Metwally, H.; Wang, Y.; Pitter, M.; Choi, J.E.; et al. STAT5 and STAT3 balance shapes dendritic cell function and tumour immunity. Nature 2025, 643, 519–528. [Google Scholar] [CrossRef]
- Zhou, H.B.; Bai, L.C.; Xu, R.Q.; Zhao, Y.J.; Chen, J.Y.; McEachern, D.; Chinnaswamy, K.; Wen, B.; Dai, L.P.; Kumar, P.; et al. Structure-Based Discovery of SD-36 as a Potent, Selective, and Efficacious PROTAC Degrader of STAT3 Protein. J. Med. Chem. 2019, 62, 11280–11300. [Google Scholar] [CrossRef]
- Zhou, H.B.; Bai, L.C.A.; Xu, R.Q.; McEachern, D.; Chinnaswamy, K.; Li, R.T.; Wen, B.; Wang, M.; Yang, C.Y.; Meagher, J.L.; et al. SD-91 as A Potent and Selective STAT3 Degrader Capable of Achieving Complete and Long-Lasting Tumor Regression. ACS Med. Chem. Lett. 2021, 12, 996–1004. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, H.; Bai, L.; McEachern, D.; Wu, D.; Acharyya, R.K.; Wang, M.; Tosovic, J.; Yang, C.Y.; Chinnaswamy, K.; et al. Discovery of SD-436: A Potent, Highly Selective and Efficacious STAT3 PROTAC Degrader Capable of Achieving Complete and Long-Lasting Tumor Regression. J. Med. Chem. 2024, 67, 20495–20513. [Google Scholar] [CrossRef]
- Liu, P.C.C.; Dixit, V.; Mayo, M.; Dey, J.; Yuan, K.; Karnik, R.; Walther, D.; Shi, Y.; Sharma, K.; Rong, H.J.; et al. A First-in-Class STAT3 Degrader KT-333 in Development for Treatment of Hematologic Cancers. Blood 2021, 138, 1865. [Google Scholar] [CrossRef]
- Bai, L.C.; Zhou, H.B.; Xu, R.Q.; Zhao, Y.J.; Chinnaswamy, K.; McEachern, D.; Chen, J.Y.; Yang, C.Y.; Liu, Z.M.; Wang, M.; et al. A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression. Cancer Cell 2019, 36, 498–511.e17. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, K. Unraveling the complexity of STAT3 in cancer: Molecular understanding and drug discovery. J. Exp. Clin. Cancer Res. 2024, 43, 23. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.; Gollerkeri, A.; De Savi, C.; Dey, J.; Agarwal, S.; Donohue, S.; Perea, R.; Klaus, C.; Gollob, J. Phase 1 study of KT-333, a targeted protein degrader, in patients with relapsed or refractory lymphomas, large granular lymphocytic leukemia, and solid tumors. J. Clin. Oncol. 2022, 40, TPS3171. [Google Scholar] [CrossRef]
- Smith, S.D.; Starodub, A.; Stevens, D.A.; Shastri, A.; Porcu, P.; Feldman, T.; Ewesuedo, R.; DeSavi, C.; Dey, J.; Agarwal, S.; et al. A Phase 1 Study of KT-333, a Targeted Protein Degrader of STAT3, in Patients with Relapsed or Refractory Lymphomas, Large Granular Lymphocytic Leukemia, and Solid Tumors. Blood 2022, 140, 12024–12025. [Google Scholar] [CrossRef]
- Mainolfi, N.; Ji, N.; Yang, B.; Zhang, Y. Preparation of Heterocycles as STAT Degraders and Uses Thereof. WO2020/206424A1, 8 October 2020. [Google Scholar]
- Ji, N.; Yang, B.; Zheng, X.; Zhu, X. STAT Degraders and Their Uses Thereof. WO2021188696A1, 23 September 2021. [Google Scholar]
- Yang, B.; Zheng, X.; Zhu, X. Stat Degraders and Uses Thereof. WO2022077010A1, 14 April 2022. [Google Scholar]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef]
- McLachlan, T.; Matthews, W.C.; Jackson, E.R.; Staudt, D.E.; Douglas, A.M.; Findlay, I.J.; Persson, M.L.; Duchatel, R.J.; Mannan, A.; Germon, Z.P.; et al. B-cell Lymphoma 6 (BCL6): From Master Regulator of Humoral Immunity to Oncogenic Driver in Pediatric Cancers. Mol. Cancer Res. 2022, 20, 1711–1723. [Google Scholar] [CrossRef]
- Kerres, N.; Steurer, S.; Schlager, S.; Bader, G.; Berger, H.; Caligiuri, M.; Dank, C.; Engen, J.R.; Ettmayer, P.; Fischerauer, B.; et al. Chemically Induced Degradation of the Oncogenic Transcription Factor BCL6. Cell Rep. 2017, 20, 2860–2875. [Google Scholar] [CrossRef]
- Mi, D.; Li, C.; Li, Y.; Yao, M.; Li, Y.; Hong, K.; Xie, C.; Chen, Y. Discovery of novel BCL6-Targeting PROTACs with effective antitumor activities against DLBCL in vitro and in vivo. Eur. J. Med. Chem. 2024, 277, 116789. [Google Scholar] [CrossRef]
- Bellenie, B.R.; Cheung, K.J.; Varela, A.; Pierrat, O.A.; Collie, G.W.; Box, G.M.; Bright, M.D.; Gowan, S.; Hayes, A.; Rodrigues, M.J.; et al. Achieving In Vivo Target Depletion through the Discovery and Optimization of Benzimidazolone BCL6 Degraders. J. Med. Chem. 2020, 63, 4047–4068. [Google Scholar] [CrossRef]
- Sherman, D. The discovery of ARV-393, a potent, orally bioavailable BCL6 targeting PROTAC® for the treatment of Non-Hodgkin’s Lymphoma. Cancer Res. 2024, 84, ND05. [Google Scholar] [CrossRef]
- Van Acker, A.; DeCarr, L.; Eaton, S.; Sherman, D.; Bortolon, E.; Bookbinder, M.; Pizzano, J.; Scopel, M.; Corwin, W.; Juncadella, I.; et al. ARV-393, a PROTAC B-cell lymphoma 6 (BCL6) degrader, combined with biologics or small molecule inhibitors (SMIs) induces tumor regressions in diffuse large B-cell lymphoma (DLBCL) models. Cancer Res. 2025, 85, 1655. [Google Scholar] [CrossRef]
- Berlin, M.; Dong, H.; Sherman, D.; Snyder, L.B.; Wang, J.; Zhang, W. Modulators of Bcl6 Proteolysis and Associated Methods of Use. WO2022/221673A1, 20 October 2022. [Google Scholar]
- Sherman, D. Modulators of Bcl6 Proteolysis and Associated Methods of Use. WO2024086759A1, 25 April 2024. [Google Scholar]
- Mortensen, D.S.; Huang, D.; Alexander, M.D.; Whitefield, B.W.; Shunatona, H.P.; Dodd, D.S.; Miseo, G.; Holmberg-Douglas, N.; Rhodes, J.; Griffin, J. Modulators of Bcl6 for Use in a Method of Treating a Cancer or an Autoimmune Disease. WO2025/059429A1, 20 March 2025. [Google Scholar]
- Acharyya, R.K.; Huang, L.; Aguilar, A.; Hu, B.; Bai, L.; Metwally, H.; McEachern, D.; Jiang, W.; Wang, Y.; Li, Q.; et al. MD-4251: A First-in-Class Oral MDM2 Degrader Inducing Complete Tumor Regression with Single-Dose Administration. J. Med. Chem. 2025, 68, 13249–13267. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Qin, J.J.; Srivenugopal, K.S.; Wang, M.H.; Zhang, R.W. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, X.; Yang, X.; Zhang, X. An overview of PROTACs targeting MDM2 as a novel approach for cancer therapy. Eur. J. Med. Chem. 2024, 272, 116506. [Google Scholar] [CrossRef]
- Aguilar, A.; Thomas, J.E.; Wang, S.M. Targeting MDM2 for the development of a new cancer therapy: Progress and challenges. Med. Chem. Res. 2023, 32, 1334–1344. [Google Scholar] [CrossRef]
- Mayo, M.; Chutake, Y.; Karnik, R.; McDonald, A.; Cho, P.S.; Filiatrault, J.; Chen, D.P.; Dixit, V.; Proctor, W.; Breitkopf, S.; et al. Development of KT-253, a Highly Potent and Selective Heterobifunctional MDM2 Degrader for the Treatment of Acute Myeloid Leukemia. Blood 2022, 140, 6239–6240. [Google Scholar] [CrossRef]
- Dumont, N.; Karnik, R.; Chutake, Y.; Dey, J.; Breitkopf, S.; Howarth, C.; Meeske, C.; Fasciano, A.; Ghilu, S.; Houghton, P.; et al. Predictive Markers of Response to the MDM2 Degrader KT-253. Eur. J. Cancer 2024, 211, S111–S112. [Google Scholar] [CrossRef]
- Lu, M.; Xia, L.J.; Chutake, Y.; Dey, J.; Hoffman, R. KT-253, a Highly Potent and Selective MDM2 Protein Degrader, Eliminates Malignant Myelofibrosis Stem/Progenitor Cells. Blood 2024, 144, 3588–3589. [Google Scholar] [CrossRef]
- Khawaja, M.R.R.U.H.; Naqash, A.R.; Schneider, R.; Shastri, A.; Stahl, M.; Moser, J.C.; Karim, N.F.A.; Madanat, Y.; Jonas, B.A.; Stein, E.; et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD) and efficacy of KT-253, a targeted protein degrader of MDM2, in patients with relapsed/refractory (R/R) solid tumors, lymphoma, high grade myeloid malignancies and acute lymphoblastic leukemia (ALL). J. Clin. Oncol. 2024, 42, 3084. [Google Scholar] [CrossRef]
- Chutake, Y.K.; Mayo, M.F.; Dumont, N.; Filiatrault, J.; Breitkopf, S.B.; Cho, P.; Chen, D.; Dixit, V.S.; Proctor, W.R.; Kuhn, E.W.; et al. KT-253, A Novel MDM2 Degrader and p53 Stabilizer, Has Superior Potency and Efficacy Than MDM2 Small Molecule Inhibitors. Mol. Cancer Ther. 2024, 24, 497–510. [Google Scholar] [CrossRef]
- Weiss, M.M. Mdm2 Degraders and Uses Thereof. WO2023/049790A2, 30 March 2023. [Google Scholar]
- Varga, J.; Kube, M.; Luck, K.; Schick, S. The BAF chromatin remodeling complexes: Structure, function, and synthetic lethalities. Biochem. Soc. Trans. 2021, 49, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Alfert, A.; Moreno, N.; Kerl, K. The BAF complex in development and disease. Epigenetics Chromatin 2019, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Gerstenberger, B.S.; Trzupek, J.D.; Tallant, C.; Fedorov, O.; Filippakopoulos, P.; Brennan, P.E.; Fedele, V.; Martin, S.; Picaud, S.; Rogers, C.; et al. Identification of a Chemical Probe for Family VIII Bromodomains through Optimization of a Fragment Hit. J. Med. Chem. 2016, 59, 4800–4811. [Google Scholar] [CrossRef] [PubMed]
- Sutherell, C.L.; Tallant, C.; Monteiro, O.P.; Yapp, C.; Fuchs, J.E.; Fedorov, O.; Siejka, P.; Müller, S.; Knapp, S.; Brenton, J.D.; et al. Identification and Development of 2,3-Dihydropyrrolo[1,2-a]quinazolin-5(1H)-one Inhibitors Targeting Bromodomains within the Switch/Sucrose Nonfermenting Complex. J. Med. Chem. 2016, 59, 5095–5101. [Google Scholar] [CrossRef]
- Vaswani, R.G.; Huang, D.S.; Anthony, N.; Xu, L.; Centore, R.; Schiller, S.; Li, Z.F.; Fan, H.; Setser, J.; Zawadzke, L.E.; et al. Discovery of FHD-286, a First-in-Class, Orally Bioavailable, Allosteric Dual Inhibitor of the Brahma Homologue (BRM) and Brahma-Related Gene 1 (BRG1) ATPase Activity for the Treatment of SWItch/Sucrose Non-Fermentable (SWI/SNF) Dependent Cancers. J. Med. Chem. 2025, 68, 1772–1792. [Google Scholar] [CrossRef]
- Cantley, J.; Ye, X.F.; Rousseau, E.; Januario, T.; Hamman, B.D.; Rose, C.M.; Cheung, T.K.; Hinkle, T.; Soto, L.; Quinn, C.; et al. Selective PROTAC-mediated degradation of SMARCA2 is efficacious in SMARCA4 mutant cancers. Nat. Commun. 2022, 13, 6814. [Google Scholar] [CrossRef]
- Previtali, V.; Bagnolini, G.; Ciamarone, A.; Ferrandi, G.; Rinaldi, F.; Myers, S.H.; Roberti, M.; Cavalli, A. New Horizons of Synthetic Lethality in Cancer: Current Development and Future Perspectives. J. Med. Chem. 2024, 67, 11488–11521. [Google Scholar] [CrossRef]
- Farnaby, W.; Koegl, M.; Roy, M.J.; Whitworth, C.; Diers, E.; Trainor, N.; Zollman, D.; Steurer, S.; Karolyi-Oezguer, J.; Riedmueller, C.; et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 2019, 15, 672–680. [Google Scholar] [CrossRef]
- Kofink, C.; Trainor, N.; Mair, B.; Wohrle, S.; Wurm, M.; Mischerikow, N.; Roy, M.J.; Bader, G.; Greb, P.; Garavel, G.; et al. A selective and orally bioavailable VHL-recruiting PROTAC achieves SMARCA2 degradation in vivo. Nat. Commun. 2022, 13, 5969. [Google Scholar] [CrossRef]
- Leng, L.Y.; Tu, W.B.; Yang, L.; Huang, L.Y.; Wang, M.; Meagher, J.L.; Chinnaswamy, K.; Allu, S.R.; Rej, R.K.; Tosovic, J.; et al. Discovery of High-Affinity SMARCA2/4 Bromodomain Ligands and Development of Potent and Exceptionally Selective SMARCA2 PROTAC Degraders. J. Med. Chem. 2025, 68, 1113–1133. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of PRT3789 in Combination with Pembrolizumab in Patients with Advanced or Metastatic Solid Tumors with a SMARCA4 Mutation. Available online: https://clinicaltrials.gov/study/NCT06682806 (accessed on 30 March 2025).
- ClinicalTrials.gov. PRT3789 Monotherapy and in Combo w/Docetaxel in Participants w/Advanced or Metastatic Solid Tumors w/SMARCA4 Mutation. Available online: https://clinicaltrials.gov/study/NCT05639751 (accessed on 30 March 2025).
- Shcherbakov, A.A.; Poppe, L.; Vaish, A. Biophysical Approaches for Investigating the Dynamics and Cooperativity of Ternary Complexes in Targeted Protein Degradation. J. Med. Chem. 2025, 68, 12904–12910. [Google Scholar] [CrossRef]
- Wurz, R.P.; Rui, H.; Dellamaggiore, K.; Ghimire-Rijal, S.; Choi, K.; Smither, K.; Amegadzie, A.; Chen, N.; Li, X.; Banerjee, A.; et al. Affinity and cooperativity modulate ternary complex formation to drive targeted protein degradation. Nat. Commun. 2023, 14, 4177. [Google Scholar] [CrossRef]
- Combs, A.P.; Lin, H.; Pitis, P.; Lu, L. Brm Targeting Compounds and Associated Methods of Use. WO2021/252666A1, 16 December 2021. [Google Scholar]
- Lu, L.; Combs, A.P.; Basch, C.H.; Shetty, R.; Dai, C.; Bersch, K.L.; Rose, J.A.; Beam, D.J.; Mei, S. Brm Targeting Compounds and Associated Methods of Use. WO2022099117A1, 12 May 2022. [Google Scholar]
- Alabi, S.; Jaime-Figueroa, S.; Yao, Z.; Gao, Y.; Hines, J.; Samarasinghe, K.T.G.; Vogt, L.; Rosen, N.; Crews, C.M. Mutant-selective degradation by BRAF-targeting PROTACs. Nat. Commun. 2021, 12, 920. [Google Scholar] [PubMed]
- Hanrahan, A.J.; Chen, Z.Y.; Rosen, N.; Solit, D.B. BRAF—A tumour-agnostic drug target with lineage-specific dependencies. Nat. Rev. Clin. Oncol. 2024, 21, 224–247. [Google Scholar] [PubMed]
- Vieito, M.; Mckean, M.; Spira, A.I.; Rosen, E.; Rodon, J.; Garcia, V.M.; Gambardella, V.; Saavedra, O.; Cousin, S.; Cassier, P.; et al. Preliminary results from a phase I study of CFT1946, a novel BIDAC degrader in mutant BRAF V600 solid tumors. Ann. Oncol. 2024, 35, S490–S491. [Google Scholar] [CrossRef]
- Villar, M.V.; McKean, M.; Spira, A.; Rosen, E.; Rodon, J.; Moreno, V.; Gambardella, V.; Gadea, O.S.S.; Cousin, S.; Cassier, P.; et al. A phase I/II study of CFT1946, a novel BIDAC degrader targeting mutant BRAF V600 solid tumors including metastatic colorectal cancer (CRC) in combination with cetuximab. Ann. Oncol. 2024, 35, S71–S72. [Google Scholar] [CrossRef]
- McKean, M.; Spira, A.I.; Rosen, E.; Subbiah, V.; Moreno, V.; Gambardella, V.; Vieito, M.; Saavedra, O.; Cousin, S.; Cassier, P.A.; et al. A phase 1/2 study of CFT1946, a novel, bifunctional degradation activating compound (BIDAC) degrader, of mutant BRAF V600 as monotherapy and in combination with trametinib, in mutant BRAF V600 solid tumors. J. Clin. Oncol. 2023, 41, TPS3163. [Google Scholar] [CrossRef]
- Nasveschuk, C.G.; Jackson, K.L.; Liang, Y.; Yu, R.T.; Duplessis, M.; Fitzgerald, M.E.; Garza, V.; Good, A.C.; Welzel, O.M.; Veits, G.K.; et al. Therapeutics for the Degradation of Mutant Braf. WO2022/261250A1, 15 December 2022. [Google Scholar]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- Goody, R.S.; Frech, M.; Wittinghofer, A. Affinity of Guanine-Nucleotide Binding-Proteins for Their Ligands—Facts and Artifacts. Trends Biochem. Sci. 1991, 16, 327–328. [Google Scholar]
- Tong, L.; Devos, A.M.; Milburn, M.V.; Kim, S.H. Crystal-Structures at 2.2 a Resolution of the Catalytic Domains of Normal Ras Protein and an Oncogenic Mutant Complexed with Gdp. J. Mol. Biol. 1991, 217, 503–516. [Google Scholar]
- FDA. Approves First KRAS Inhibitor: Sotorasib. Cancer Discov. 2021, 11, OF4. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Lanman, B.A.; Allen, J.R.; Allen, J.G.; Amegadzie, A.K.; Ashton, K.S.; Booker, S.K.; Chen, J.J.; Chen, N.; Frohn, M.J.; Goodman, G.; et al. Discovery of a Covalent Inhibitor of KRAS(G12C) (AMG 510) for the Treatment of Solid Tumors. J. Med. Chem. 2020, 63, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Allen, S.; Blake, J.F.; Bowcut, V.; Briere, D.M.; Calinisan, A.; Dahlke, J.R.; Fell, J.B.; Fischer, J.P.; Gunn, R.J.; et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor. J. Med. Chem. 2022, 65, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Park, W.; Kasi, A.; Spira, A.I.; Berlin, J.D.; Wang, J.S.; Herzberg, B.; Kuboki, Y.; Kitano, S.; Pelster, M.; Goldman, J.W.; et al. Preliminary safety and clinical activity of ASP3082, a first-in-class, KRAS G12D selective protein degrader in adults with advanced pancreatic (PC), colorectal (CRC), and non-small cell lung cancer (NSCLC). Ann. Oncol. 2024, 35, S486–S487. [Google Scholar] [CrossRef]
- Nagashima, T.; Inamura, K.; Nishizono, Y.; Suzuki, A.; Tanaka, H.; Yoshinari, T.; Yamanaka, Y. ASP3082, a First-in-class novel KRAS G12D degrader, exhibits remarkable anti-tumor activity in KRAS G12D mutated cancer models. Eur. J. Cancer 2022, 174, S30. [Google Scholar] [CrossRef]
- Nagashima, T. Pharmaceutical Composition Comprising a Quinazoline Compound. WO2023/119677A1, 29 June 2023. [Google Scholar]
- Yoshinari, T.; Nagashima, T.; Ishioka, H.; Inamura, K.; Nishizono, Y.; Tasaki, M.; Iguchi, K.; Suzuki, A.; Sato, C.; Nakayama, A.; et al. Discovery of KRAS(G12D) selective degrader ASP3082. Commun. Chem. 2025, 8, 254. [Google Scholar] [CrossRef]
- Weiss, M.M.; Zheng, X.Z.; Ji, N.; Browne, C.M.; Campbell, V.; Chen, D.P.; Enerson, B.; Fei, X.; Huang, X.; Klaus, C.R.; et al. Discovery of KT-413, a Targeted Protein Degrader of IRAK4 and IMiD Substrates Targeting MYD88 Mutant Diffuse Large B-Cell Lymphoma. J. Med. Chem. 2024, 67, 10548–10566. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, T.; Hu, Y. Global landscape of PROTAC: Perspectives from patents, drug pipelines, clinical trials, and licensing transactions. Eur. J. Med. Chem. 2025, 299, 118055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.