Abstract
The interface between nickel oxide (NiOx) and self-assembled monolayers (SAMs) in perovskite solar cells (PSCs) often suffers from limited adsorption strength, poor energy-level alignment, and inadequate defect passivation, which hinder device performance and stability. To address these issues, we introduce a hybrid hole selective layer (HSL) combining atomic layer deposition (ALD)-fabricated NiOx with full-aromatic SAM molecules, creating a highly stable and efficient interface. ALD NiOx, enriched with hydroxyl groups, provides robust adsorption sites for the SAM molecule MeO-PhPACz, ensuring a strong, stable interaction. This hybrid HSL enhances energy-level alignment, hole selectivity, and defect passivation at the NiOx/perovskite interface. Devices utilizing this approach demonstrate significant performance improvements, achieving a power conversion efficiency (PCE) of 21.74%, with reduced voltage losses and minimal hysteresis. Furthermore, operational stability tests reveal enhanced durability under elevated humidity and temperature conditions. These findings highlight the potential of ALD NiOx and SAM hybrid HSL to overcome existing barriers, advancing the commercial viability of PSC technologies.
1. Introduction
Organic–inorganic hybrid perovskite solar cells (PSCs) have made remarkable strides in power conversion efficiency (PCE) in a short time [1,2,3,4,5]. Among these, the inverted p-i-n structure has attracted considerable attention for its low-temperature fabrication process, reduced hysteresis, enhanced stability, and ability to meet the structural demands of tandem solar cells [6,7]. In the fabrication of inverted PSCs, a hole selective layer (HSL) is first deposited onto the indium tin oxide (ITO) substrate. This layer not only ensures efficient hole collection but also minimizes charge recombination at the interface, which plays a critical role in the crystallization and nucleation of the perovskite material. Therefore, the development of high-performance HSL is essential for achieving high-efficiency inverted PSCs.
At present, a wide variety of HSLs have been employed in inverted PSCs. Among these, nickel oxide (NiOx) has garnered significant attention owing to its cost-effectiveness, facile manufacturability, outstanding hole extraction efficiency, and well-aligned energy levels [8,9,10,11,12,13]. The performance and stability of NiOx-based PSCs are frequently hindered by undesirable reactions between the perovskite layer and highly reactive Ni3+ species, as well as charge carrier recombination at the NiOx/perovskite interface [14,15,16]. To mitigate these issues, the combination of NiOx with self-assembled monolayers (SAMs) has emerged as a highly effective strategy [9,12,13,17,18]. Wang et al. employed bromobenzoic acid (Br-BA) to modify the surface of NiOx nanoparticles, reducing interfacial defects and trap states, enhancing perovskite crystallization quality, and optimizing energy-level alignment [19]. Yu et al. treated NiOx nanoparticles with H2O2, which enhanced the film’s conductivity and surface hydroxyl content, promoting SAM anchoring [20]. Zhao et al. incorporated sodium hexametaphosphate (SHMP) to modify NiOx, improving the surface hydroxyl density and film uniformity, thereby enhancing the anchoring of Me-4PACz and reducing the open-circuit voltage loss in wide bandgap perovskite solar cells [13].
In a previous work, we proposed a Ni vacancy defect modulate approach to leverage the conformal growth and surface self-limiting reaction characteristics of atomic layer deposition (ALD)-fabricated NiOx by varying the O2 plasma injection time to induce self-doping [21]. We fabricated ultrathin NiOx films with excellent conductivity, an optimal Ni3+/Ni2+ ratio, and stable surface states, serving as the HSL in p-i-n PSCs. However, interface defects at the NiOx/PVK interface can induce perovskite degradation, and a higher density of defects is typically present at the bottom perovskite interface. Building upon prior studies, the incorporation of SAMs with NiOx has been demonstrated to enhance the surface hydroxyl density, strengthen SAM anchoring, mitigate surface defects, and ultimately improve the film quality [13,19,20]. Consequently, we propose the introduction of a novel SAM at the NiOx/PVK interface to address the aforementioned detrimental characteristics.
Herein, we deposited a layer of MeO-PhPACz (hereafter referred to as MeO-Ph) [22] onto the ALD-deposited NiOx, which has a surface rich in -OH groups, thereby providing more adsorption sites for the SAM molecules. Compared to ITO/MeO-Ph, the binding energy between ALD-NiOx and MeO-Ph is increased, with stronger and more stable interactions and also improved hole extraction capability. In contrast to ALD-NiOx, MeO-Ph effectively passivates the surface defects of NiOx, significantly suppressing redox reactions at the HSL/PVK interface and notably enhancing the work function of the HSL. As a result, the hybrid HSL with well-aligned energy levels, stable surface states, and efficient hole extraction was achieved. The corresponding device showed a marked increase in PCE from 19.86% (ALD-NiOx) and 20.53% (MeO-Ph) to 21.74% (ALD-NiOx/MeO-Ph), with minimal hysteresis. Stability tests conducted on unencapsulated devices under various aging conditions demonstrated that devices based on ALD-NiOx/MeO-Ph exhibited excellent long-term stability.
2. Results and Discussion
Excellent optical transmittance significantly influences the short-circuit current of the device. We measured the transmittance of three thin films in the 350 nm–800 nm wavelength range. The results show that all three films exhibit transmittance above 90%, with MeO-Ph achieving an optical transmittance exceeding 95% due to its ultrathin nature. To investigate the interaction between MeO-Ph and NiOx (unless otherwise specified, NiOx refers to ALD-NiOx), we used XPS to evaluate the surface chemical properties of ITO/NiOx before and after MeO-Ph adsorption. Pure MeO-Ph adsorbed on an ITO substrate was used as a reference sample, with the elemental composition summarized in Table S1. The differences in phosphorus (P) in Figure 1a and nitrogen (N) in Table S1 confirm the successful adsorption of MeO-Ph on the ITO/NiOx surface. The concentration of P atoms in the ITO/NiOx/MeO-Ph sample is 4.45%, significantly higher than the 3.29% observed in the ITO/MeO-Ph sample, indicating that the presence of NiOx enhances the adsorption of MeO-Ph.
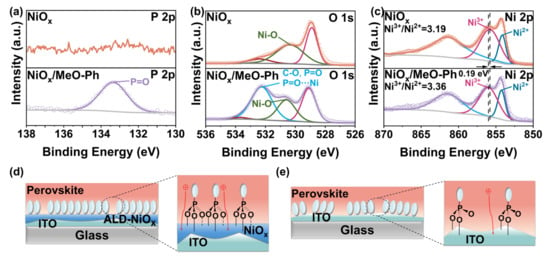
Figure 1.
XPS spectra of (a) P 2p, (b) O 1s, and (c) Ni 2p for the three films, and adsorption patterns of MeO-Ph on (d) NiOx and (e) ITO surfaces.
Furthermore, the concentration of nickel (Ni) in the NiOx substrate decreases after MeO-Ph adsorption, suggesting that the signal from the NiOx substrate is partially obscured by the adsorbed MeO-Ph layer. To more clearly observe the differences between the samples, we performed peak fitting on the high-resolution XPS spectra of Ni 2p and O 1s. In the Ni 2p spectrum, the binding energy of Ni3+ increased from 855.78 eV to 855.97 eV after the adsorption of MeO-Ph, with a similar shift observed for Ni2+, which increased from 854.14 eV to 854.33 eV. Comparable changes were also observed in the O 1s XPS signals of NiOx/MeO-Ph compared to pristine NiOx. These chemical shifts suggest that oxygen atoms in MeO-Ph donate lone pair electrons to the vacant orbitals of nickel atoms, leading to the formation of coordination covalent bonds (P=O-Ni) at the interface. This provides compelling evidence of a charge transfer between MeO-Ph and NiOx [23,24,25]. Additionally, in the O 1s spectrum, a signal corresponding to C-O at 532.25 eV was detected in the NiOx/MeO-Ph sample, which originates from the methoxy group of MeO-Ph. This further confirms the successful adsorption of MeO-Ph onto the NiOx surface [22]. Based on previous studies [23], we propose a bonding model between NiOx and MeO-Ph (Figure 1d,e). Compared to ITO, NiOx prepared via ALD exhibits metal vacancies (VNi) on its surface, which increase the number of highly reactive oxygen dangling bonds. This provides additional adsorption sites for MeO-Ph. This enables the formation of a robust tridentate adsorption configuration.
From the AFM images (Figure S2a–c), a noticeable increase in RMS roughness is observed for NiOx/MeO-Ph and MeO-Ph surfaces compared to bare NiOx. This indicates the successful attachment of MeO-Ph onto the substrate surface. Due to the limitations of the spin-coating process, the RMS of MeO-Ph is larger than that achieved with the ALD process. Following MeO-Ph adsorption, KPFM measurements reveal a decrease in CPD from −0.41 V to −0.50 V and −0.45 V, respectively. This significant shift in CPD serves as a reliable indicator of successful MeO-Ph adsorption. Moreover, the CPD of NiOx/MeO-2PACz is lower than that of NiOx, indicating that the adsorption of MeO-Ph leads to an increase in the WF of NiOx. This increase is attributed to the intrinsic dipole moment of MeO-Ph, oriented from the carbazole group to the phosphonic acid group, which generates an electric field at the surface. Additionally, since the EF of MeO-Ph is lower than that of NiOx, electron transfer occurs from NiOx to MeO-Ph upon contact, forming a space charge region. The combined effects of the dipole layer and the space charge region contribute to the enhanced WF of NiOx [26].
The WF values obtained from UPS measurements confirm that the introduction of MeO-Ph results in a more favorable alignment of the hole extraction energy levels. The WF values for NiOx, MeO-Ph, and NiOx/MeO-Ph are −4.52 eV, −4.87 eV, and −4.98 eV, respectively (Figure 2a,b). These changes are likely to contribute to improvements in VOC and JSC. Based on UV–Vis spectroscopy results, the optical band gaps (Eg) for NiOx, MeO-Ph, and NiOx/MeO-Ph, calculated via Tauc plots, are 3.87 eV, 3.19 eV, and 3.82 eV, respectively. The Eg value for MeO-Ph is consistent with previous literature [22]. The reduction in Eg for NiOx/MeO-Ph is attributed to changes in the Ni2+/Ni3+ ratio [24] (Figure S3a).
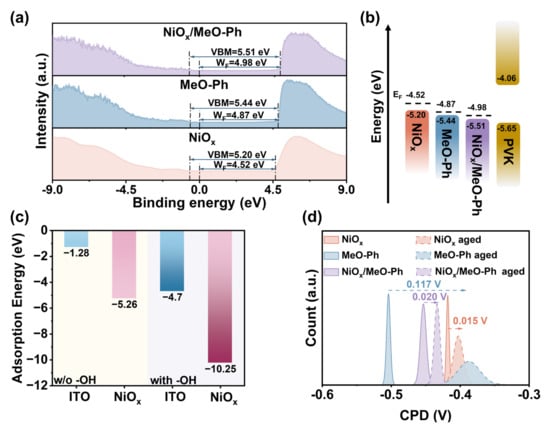
Figure 2.
(a) UPS plots and (b) energy band schematics of NiOx, MeO-Ph, and NiOx/MeO-Ph. MeO-Ph on hydroxyl-free ITO, NiOx, hydroxyl-containing ITO, and NiOx surfaces. (c) Specific adsorption energy results from theoretical calculations. (d) CPD changes before and after the aging of different substrates.
We designed the corresponding structural models for DFT calculations to accurately illustrate the ability of polyhydroxy NiOx to enhance the adsorption of self-assembled molecules (Figure S4). As shown in Figure 2c, when no -OH groups are present on the substrate surface, the binding energies of MeO-Ph on ITO and NiOx are −1.28 eV and −5.26 eV, respectively. However, when -OH groups are present at the substrate interface, the binding energy of MeO-Ph significantly increases to −4.7 eV and −10.25 eV. These results clearly demonstrate that the presence of NiOx significantly enhances the adsorption energy of MeO-Ph on the substrate, and the existence of -OH groups further promotes this increased adsorption strength. To more directly observe the stability of MeO-Ph on different substrates, we fabricated HSL structures of ITO/NiOx, ITO/MeO-Ph, and ITO/NiOx/MeO-Ph and subjected them to aging tests in air (60 °C, 50 ± 10% relative humidity). The changes in surface potential of the films before and after aging were recorded using KPFM (Oxford Jupiter XR, Oxfordshire, UK). As shown in the figure, prior to aging, all three films exhibit relatively uniform surface potential, indicating that MeO-Ph is evenly deposited on both ITO and NiOx surfaces. After 480 h of aging, the CPD of ITO/MeO-Ph significantly decreases (ΔCPD ≈ 0.117 V), whereas the CPD changes of ITO/NiOx and ITO/NiOx/MeO-Ph are only 0.015 V and 0.020 V, respectively. This suggests that the substrate with NiOx as a seed layer can securely anchor MeO-Ph to the surface, thereby enhancing the operational stability of the corresponding devices. We hypothesize that the CPD change in ITO/MeO-Ph is due to the hydrolysis of the coordinating metal of the hydroxyl groups linked to the SAM molecules in the atmosphere, leading to the desorption of the SAM molecules [27,28]. Additionally, it is likely that the tridentate binding strength between SAM and NiOx is stronger than the bidentate binding between SAM and ITO. Furthermore, the higher hydroxyl density on the NiOx surface compared to ITO further contributes to the improved stability.
To evaluate changes in the defect density, we fabricated p-type devices with a planar structure of ITO/HSL (NiOx, MeO-Ph, NiOx/MeO-Ph)/PVK/spiro-OMeTAD/Ag, and the J–V characteristics measured under dark conditions are shown in Figure 3a. The corresponding data are plotted in a logarithmic–logarithmic coordinate system. As the applied voltage increases, the current exhibits a linear increase in the ohmic contact region, followed by a rapid rise above the trap-filling limit voltage (VTFL). The VTFL values for NiOx/MeO-Ph and MeO-Ph are 0.36 V and 0.42 V, respectively, both lower than the 0.45 V for NiOx. Using the formula (Equation (S1)), the defect density is calculated to decrease from 8.13 × 10−15 cm−3 (NiOx) to 6.91 × 10−15 cm−3 (MeO-Ph) and 5.84 × 10−15 cm−3 (NiOx/MeO-Ph), indicating that the introduction of MeO-Ph effectively passivates the HSL/PVK interface defects [29]. To evaluate the impact of different HSL on charge extraction and transport, we performed PL spectroscopy measurements, as shown in Figure S5. Compared to the MeO-Ph/PVK sample, the NiOx/MeO-Ph/PVK sample exhibits a lower PL intensity, indicating that NiOx/MeO-Ph provides better hole extraction and transport capabilities than MeO-Ph [20]. The lowest PL intensity of NiOx is likely due to carrier recombination at the NiOx/PVK interface defects. The TRPL was further used to quantify the carrier dynamics, where the data were fitted to a double-exponential decay function, with the results shown in Table S2. The decay time constant τ1 is attributed to the charge extraction by the HSL, while τ2 corresponds to the radiative recombination of free carriers within the perovskite layer [24]. The device based on NiOx/MeO-Ph exhibits the shortest τ1 and the longest τ2, confirming that the mixed HSL not only enhances the hole extraction rate but also passivates the NiOx/PVK interface defects.
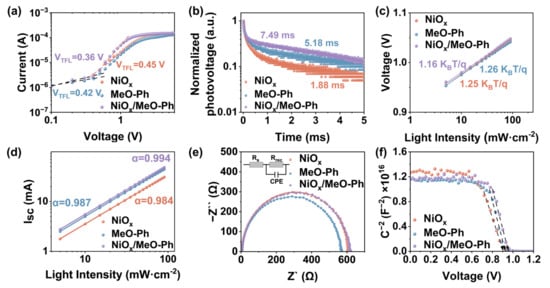
Figure 3.
NiOx, MeO-Ph, and NiOx/MeO-Ph based on (a) dark J–V characteristic plots for hole-only devices; (b) TPV plots of NiOx and MeO-Ph; (c,d) VOC and ISC plots of the corresponding PSCs as a function of light intensity; (e) EIS plots of the corresponding devices, the insets showing the equivalent circuits used to fit the Nyquist plots; and (f) Mott–Schottky plots of different PSCs.
TPV and TPC are direct methods to investigate carrier behavior (recombination or extraction) [30]. The characterization results, shown in Figure 3b and Figure S6, clearly indicate that, for devices based on NiOx/MeO-Ph, the photocurrent decay time is significantly reduced, while the photovoltage decay time is markedly increased. This suggests a reduction in intrinsic defects, faster charge extraction, and suppressed interfacial recombination. Additionally, to assess the charge recombination state in the devices, the dependence of VOC and ISC on light intensity was measured for the PSCs. As shown in Figure 3c, the device based on NiOx/MeO-Ph exhibits the smallest slope of 1.16 KB/q. The decrease in this slope can be attributed to the effective passivation of the NiOx/PVK interface by MeO-Ph [31]. However, we observed that the slope for devices based on MeO-Ph is slightly larger than that of NiOx-based devices. This may be due to the partial dissolution of the SAM during the perovskite deposition process caused by the DMF/DMSO solvents, which leads to a reduction in interface coverage and subsequent interfacial charge recombination as a result of direct contact between the perovskite and ITO [32]. Figure 3d shows the relationship between ISC and light intensity. The closer the α value is to 1, the less trap-assisted recombination occurs [33]. The results indicate that the device based on NiOx/MeO-Ph exhibits an α value closest to 1, which is consistent with the VOC light intensity dependence observed earlier. In EIS, the value of Rrec is proportional to the diameter of the semicircle, with a larger diameter indicating less charge recombination [24]. The EIS fitting results show that the Rrec values for NiOx, MeO-Ph, and NiOx/MeO-Ph are 598.1 Ω, 554.7 Ω, and 599.8 Ω, respectively. The smaller Rrec value for MeO-Ph may be due to the partial dissolution of the SAM by DMF/DMSO, leading to an increase in defects at the HSL/PVK interface. The Mott–Schottky curve (Figure 3f) is typically used to measure the built-in potential (Vbi) of PSCs [34]. The Vbi of the NiOx/MeO-Ph-based device is 0.95 V, higher than that of NiOx (0.92 V) and MeO-Ph (0.94 V). The higher Vbi is attributed to the optimization of energy-level alignment, which provides a stronger driving force for carrier extraction and separation, aiming for a higher VOC.
The crystallization and film formation of the perovskite layer are largely influenced by the HSL. According to the SEM images (Figure 4b and Figures S7 and S8), the perovskite grains based on MeO-Ph and NiOx/MeO-Ph are significantly larger, with an increase in film thickness as well. To explain these observations, we conducted water contact angle measurements (Figure S9). The results show that NiOx exhibits good surface wettability, which facilitates the formation of smaller perovskite grains. In contrast, the water contact angles for MeO-Ph and MeO-Ph/NiOx are significantly higher. Therefore, the changes in perovskite grain size can be attributed to an increase in the surface tension of the substrate, which enhances its hydrophobicity and reduces the mobility of grain boundaries, thereby favoring the growth of columnar grains [35]. The variation in thickness may be due to the introduction of MeO-Ph, which reduces the surface defects of NiOx and provides a more favorable environment for perovskite film growth [36]. XRD patterns (Figure S10) and Tauc plots (Figure S11) show that there are no significant changes in the crystallization quality, crystal orientation, or optical bandgap of perovskite films grown on different HSL.
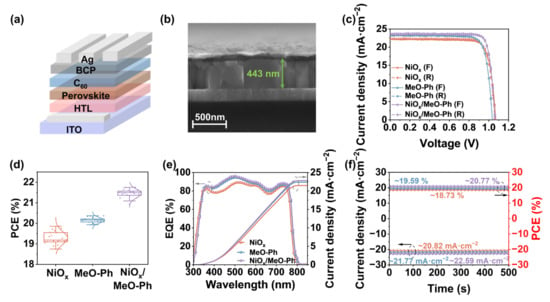
Figure 4.
(a) Device schematic of p-i-n-structured PSCs. (b) Cross-sectional thickness plots of perovskite deposited on NiOx/MeO-Ph surfaces. (c) Forward and reverse scanning J–V curves under simulated AM 1.5 G solar illumination (100 mW cm−2). (d) Extraction of PCE distributions from J–V measurements of NiOx, MeO-Ph, and NiOx/MeO-Ph-based PSCs (20 individual devices). (e) EQE spectra of the best-performing device with an active area of 0.1 cm2 for the integrated JSC, and (f) power output stabilized at the point of maximum power over 500 s (SPO).
To evaluate the performance of NiOx/MeO-Ph-mixed HSL in single-junction solar cells, we fabricated p-i-n-structured PSCs with the architecture of ITO/HSL/PVK/C60/BCP/Ag, as shown in Figure 4a. Devices based on NiOx and MeO-Ph as the HSL were prepared for comparison. The J–V characteristics of the optimized devices with different HSLs are shown in Figure 4b. Table S3 summarizes the photovoltaic parameters. The best-performing device with the NiOx/MeO-Ph-mixed HSL achieved a PCE of 21.74%, with VOC, JSC, and FF values of 1.07 V, 23.74 mA cm−2, and 84.43%, respectively. Compared to the pure NiOx device, the incorporation of MeO-Ph effectively passivated interface defects, leading to significant improvements in JSC, and FF.
In contrast to the pure MeO-Ph device, the NiOx/MeO-Ph device exhibited enhanced VOC, FF, and JSC due to stronger SAM adsorption, reduced shunt paths, and improved hole extraction efficiency. Figure 4d and Figure S12 present the statistical distribution of various parameters for devices fabricated with different HSLs. Devices based on the NiOx/MeO-Ph-mixed HSL exhibit improvements in all the parameters. Among them, MeO-Ph shows the lowest VOC, which is attributed to the perovskite deposition process, where some SAM molecules are washed away, leading to significant interface carrier recombination [32], consistent with the results from EIS and VOC light intensity dependence tests. The JSC value of the devices is in good agreement with the JSC calculated from the external quantum efficiency (EQE) measurements, with the improvement in JSC attributed to the enhanced hole transport capability of the HSL. Additionally, the devices with different HSL demonstrate stable photocurrent and PCE output at the maximum power point (Figure 4f).
The detrimental redox reactions at the NiOx/PVK interface represent a major challenge for the commercialization of PSCs. We aim to mitigate this issue by introducing MeO-Ph. Perovskite films were deposited on different HSLs, and their changes were monitored in an atmosphere at 60 °C and 60 ± 10% relative humidity. As shown in Figure S13, after 240 h of heating, the (100) crystallographic peak of the perovskite film deposited on the three different HSLs still predominated. To gain further insight into whether the adverse interfacial reactions were suppressed, we calculated the intensity ratio of PbI2(001) to PVK(100) over time. The results indicated that, for perovskites grown on MeO-Ph and NiOx/MeO-Ph, the PbI2(001)/PVK(100) ratio was significantly lower compared to those grown on NiOx. This phenomenon suggests that the introduction of MeO-Ph effectively alleviates the detrimental redox reactions at the NiOx/PVK interface.
To further demonstrate the advantages of the NiOx/MeO-Ph hybrid HSL, we investigated the operational stability of the corresponding devices under different environmental conditions. The results reveal that the hybrid HSL-based devices exhibit the highest stability, whether in an ambient atmosphere at 60 °C with a relative humidity of 60 ± 10% (Figure 5b) or in a nitrogen environment at 85 °C (Figure 5c). The efficiency degradation observed for the pure MeO-Ph device may be attributed to desorption or detachment of the SAM under high-humidity conditions [27], as no significant efficiency loss was observed in the nitrogen environment at 85 °C. To verify the applicability of the hybrid HSL, we fabricated devices using various perovskite compositions, including MAPbI3 (1.59 eV), Cs0.05(FA0.92MA0.08)0.95Pb(I0.92Br0.08)3 (1.59 eV), and Cs0.05FA0.8MA0.15Pb(I0.75Br0.25)3 (1.68 eV), and analyzed the variations in JSC and PCE. As shown in Figure 5d and Figure S14, the hybrid HSL consistently delivers significant enhancements in both JSC and PCE compared to NiOx and MeO-Ph, regardless of the same bandgap with different compositions or different bandgaps. This confirms that the hybrid HSL possesses broad applicability across various perovskite systems. Moreover, the MPP tracking under harsh conditions (unencapsulated, continuous illumination (1 sun), and negative electric bias) (Figure 5e) showed that the MPP of NiOx-based PSCs dropped quickly (~79% after 500 h), while the PSCs with NiOx/MeO-Ph as the HSL maintain over 95% of its initial PCE value after 500 h, indicating better operational stability.
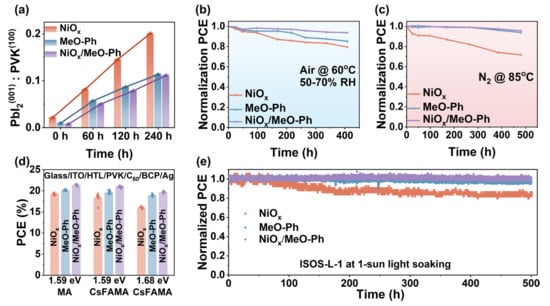
Figure 5.
(a) Plots of normalized PbI2(001):PVK(100) intensities vs. aging time for stability measurements were performed at 60 °C and 60 ± 10% relative humidity. Long-term stability tests of unencapsulated PSCs in (b) air at 60 ± 10% relative humidity and 60 °C. and (c) an N2 environment at 85 °C. (d) PCE performances of NiOx, MeO-Ph, and NiOx/MeO-Ph in different perovskites. (e) Stabilized power output at the MPP at RT and N2 under 1 sun illumination.
3. Materials and Methods
3.1. Materials and Synthesis
All reagents and solvents used were commercially available without further purification. Methylammonium iodide (MAI) was synthesized by the reaction of hydriodic acid (57 wt% in H2O, Aldrich, St. Louis, MO, USA) and methylamine (30–33 wt% in ethanol, Aladdin, Shanghai, China). Formamidinium iodide (FAI), methylammonium bromide (MABr), and methylammonium chloride (MACl) were synthesized according to the procedures in previous publications [22]. Lead iodide (PbI2), lead bromide (PbBr2), cesium iodide (99.9%), N,N-dimethylformamide (DMF, 99.8%, anhydrous), dimethyl sulfoxide (DMSO, 99.9%, anhydrous), N-methylpyrrolidone (NMP), chlorobenzene (CB, 99.8%, anhydrous), ethyl acetate (EA, 99.8%, anhydrous), 2-propanol (IPA, 99.8%, anhydrous), and nickel oxide (Ni2O3) were purchased from Sigma-Aldrich. Lead iodide (99.998%) was purchased from TCI. Fullerene-C60 and 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP) were purchased from Lumtec (Taiwan, China). Bis-methylcyclopentadienyl-nickel (Ni(MeCp)2, 99.9999%) was purchased from Nanjing Ai Mou Yuan Scientific Equipment (Nanjing, China). The synthesis procedure of MeO-PhPACz was reported in a previous work [22].
3.2. Computational Method
All the calculations based on the density functional theory (DFT) and the Projector Augmented Wave (PAW) potential were implemented with Device Studio (https://iresearch.net.cn/cloudSoftware, accessed on 22 March 2024, 2023A), which provides a number of functions for performing visualization, modeling, and simulation. The molecular geometry simulation used BDF [37,38,39,40] software (2024A) integrated in the Device Studio program. We used the B3LYP functional to calculate the molecules and optimized the molecular structure using the Def2-SVP basis set. For calculation of the doping mechanism, DFT calculations were performed by using the DS-PAW [41] package (2024A) in the Device Studio program. The generalized gradient approximation in the Perdew–Burke–Ernzerhof (PBE) format was used to compute exchange and correlation energies, and a plane wave basis set cutoff energy of 650 eV was adopted. Grimme’s DFT-D3 was used for dispersion correction. A mesh of 1 × 1 × 1 gamma-centered k-points was used for the Brillouin zone integration. The criterion of the electron self-consisting iteration was set as 1.0 × 10−5 eV, and the maximum force was relaxed down to 0.05 eV Å−1.
3.3. Device Fabrication
3.3.1. Preparation of the Hole Selective Layer
The preparation of ALD NiOx:indium tin oxide (ITO) glass (7–9 Ω m−2 square, AGC, Tokyo, Japan) was ultrasonically cleaned in deionized water and ethanol for 15 min each time and dried with N2 flow. High-temperature tape was applied to one side of the ITO to prevent back contact from being formed by NiOx deposited in this area. Next, the substrates were further cleaned with O2 plasma for 10 min. The ITO was transferred to the ALD reaction chamber (PEALD-200R, Kemin Electronic Equipment Technology Co., Jiaxing, China) with the substrate temperature set at 150 °C, chamber pressure below 10−3 torr, and reaction gas flow rate of 50 sccm. ALD NiOx was deposited using Ni(MeCp)2 as the precursor and O2 plasma as the co-reactant. The Ni(MeCp)2 bubbler was kept at 60 °C to ensure adequate vapor pressure and dosed using Ar carrier gas through a delivery line heated to 100 °C. Each ALD cycle consisted of 0.9 s Ni(MeCp)2 dose, 10 s purge time, 7.5 s O2 plasma exposure (200 W), and 10 s purge time. After deposition, the NiOx was quickly transferred to an N2 glove box, and the high-temperature tape was peeled off before proceeding to the next application.
MeO-Ph hole selective layer: The cleaned ITO substrates were treated with O2 plasma for further cleaning. A 2 mg/mL solution of MeO-PhPACz in ethanol was spin-coated onto the substrate at 3000 rpm for 30 s, followed by annealing on a hot plate at 100 °C.
NiOx/MeO-Ph hybrid hole selective layer: A 2 mg/mL solution of MeO-PhPACz in ethanol was spin-coated onto the ALD-deposited NiOx at 3000 rpm for 30 s, followed by annealing on a hot plate at 100 °C.
3.3.2. Fabrication Process of Perovskite Films
MAPbI3 perovskite films: The 1.3 M perovskite precursor solution was prepared by dissolving a mixture of MAI and PbI2 in a mixed solvent DMF/DMSO/NMP (835:75:90, v/v/v) and then magnetically stirring for several hours. Then, 60 μL solutions were spin-coated at 1000 rpm for 10 s and 5000 rpm for 20 s, and 140 μL of CB was dropped on the substrates in the last 5 s to form intermediate films. The resulting perovskite film was annealed at 100 °C for 10 min.
Cs0.05(FA0.92MA0.08)0.95Pb(I0.92Br0.08)3 perovskite films: The 1.47 M perovskite precursor solution was prepared by dissolving a mixture of PbI2, CsI, PbBr2, MABr, and FAI in a mixed solvent DMF/DMSO (4:1, v/v) and then magnetically stirring for several hours. Then, 60 μL solutions were spin-coated at 1000 rpm for 10 s and 5000 rpm for 25 s, and 110 μL of EA was dropped on the substrates in the last 10 s to form intermediate films. The resulting perovskite film was annealed at 100 °C for 20 min.
Cs0.05FA0.8MA0.15Pb(I0.75Br0.25)3 perovskite films: A 1.4 M wide bandgap (1.68 eV) perovskite precursor solution was prepared by dissolving a mixture of FAI, MABr, PbI2, PbBr2, and CsI in a mixed solvent DMF/DMSO (4:1, v/v) and then magnetically stirring for several hours. Then, 100 μL perovskite precursor solution was spread on the whole area of the HSLs/ITO substrates and spun using the spin-coating process (3500 rpm for 40 s, 5 s acceleration). According to the antisolvent method, 150 μL of ethyl acetate was dropped on the film in 10 s before the end of the program. The resulting perovskite film was then annealed at 100 °C for 30 min.
Finally, 25 nm of C60 was sequentially evaporated at rates of 0.2 Å/s. Next, 140 μL of BCP solution (0.5 mg/mL in IPA) was dynamically spin-coated at 6000 rpm for 30 s. Finally, a 120 nm Ag electrode was thermally evaporated under a vacuum of 5 × 10−4 Pa.
3.4. Characterizations and Measurements
The grazing incidence X-ray diffraction (GI-XRD) measured by X-ray diffraction (XRD, Rigaku TTRAXIII, Mito, Japan) at an incident angle of 0.5°. X-ray diffraction (XRD) was measured with a PANalytical X’Pert3 powder diffractometer (Malvern Panalytical, Shanghai, China) equipped with a Cu-sealed tube (λ = 1.541874 Å) at 40 kV and 40 mA. Ultraviolet–Visible (UV–Vis) absorption spectra and transmission spectra were recorded on a Cary 5000 spectrophotometer (Agilent, Santa Clara, CA, USA) The water contact angle was measured by a DataPhysics contact angle tester (DataPhysics, Filderstadt, Germany). The top view and cross-section morphologies of the perovskite films were investigated using field emission scanning electron microscopy (SEM) (Apreo S LoVac, Thermo, Waltham, MA, USA). The surface roughness and surface potential of perovskite films by atom force microscopy (AFM) and kelvin probe force microscopy (KPFM) were carried out using Oxford Jupiter XR (Oxfordshire, UK). The X-ray photoelectron spectrum (XPS) and ultraviolet photoelectron spectroscopy (UPS) were performed using an X-ray photoelectron spectroscopy system (Axis Supra, Shimadzu, Kyoto, Japan) with Al Kα X-ray radiation (1486.6 eV) as the X-ray source. Photoluminescence (PL) and time-resolved photoluminescence (TRPL) were carried out with a series of fluorescence spectrometers (FLS-980). The excitation and emission wavelengths were 460 nm and 770 nm for the TRPL measurements, respectively. The transient photovoltage (TPV), transient photocurrent (TPC), electrochemical impedance spectroscopy (EIS), and Mott–Schottky measurements were carried out using an electrochemical workstation (Zennium Zahner, Kronach, Germany). Space-charge limited current (SCLC) measurement was performed on a Keithley 2401 source meter ranging from 0 V to 5 V. The photovoltaic performance was measured on a source meter (Keithley 2420, Cleveland, OH, USA) under AM 1.5 G (0.1 W/cm2) illumination with a solar simulator (Enli Tech, Taiwan, China); the current density–voltage (J–V) characteristics were recorded by a source meter (Keithley 2420) with 100 mV/s scan speed. The external quantum efficiency (EQE) spectrum was measured by the EQE system (QE-R 3011, Enli Tech, Taiwan, China) in DC mode.
3.5. Stability Measurement
The long-term stability assessment of the perovskite solar cells was carried out by repeating the J–V characterizations over various times. For the thermal stability test, the devices were stored at 85 °C in N2 for 500 h, then 60 °C and 60% RH in the air for 400 h. For the storage stability test, unencapsulated devices were kept in dark conditions with N2 at room temperature for 960 h. Thereafter, they were placed in air at 25 °C with a relative humidity of 60% for 120 h. The long-term MPP tracking was carried out on the Multi-Channels Solar Cells Stability Test System (Wuhan 91PVKSolar Technology Co. Ltd., Wuhan, China). The solar cells were fixed at the Vmpp and under constant 1 sun illumination, and the current density variation was recorded every 10 s at room temperature in a nitrogen-flowing atmosphere.
4. Conclusions
In summary, the NiOx/MeO-Ph hybrid HSL strategy offers multiple advantages. On the one hand, compared to pure MeO-Ph, the incorporation of NiOx provides more adsorption sites, enhances the adsorption strength, facilitates energy band alignment at the HSL/PVK interface, prevents direct contact between the perovskite and ITO, and improves the hole selectivity of the HSL. On the other hand, compared to pure NiOx, the introduction of MeO-Ph achieves dual passivation of defects at the perovskite bottom surface and the HSL/PVK interface, effectively suppressing undesirable interfacial redox reactions. Devices based on the hybrid HSL exhibit superior performance and demonstrate the longest operational lifetimes under various environmental conditions. These findings confirm that the application of the hybrid HSL in single-junction PSCs is a highly advantageous approach. We believe this study provides a solid foundation for the development of high-performance and highly stable p-i-n PSCs and accelerates their path toward commercialization.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30061299/s1: Figure S1: Light transmittance of NiOx, MeO-Ph, and NiOx /MeO-Ph; Figure S2: Surface roughness of (a) NiOx, (b) MeO-Ph, and (c) NiOx/MeO-Ph. CPD distribution of (d,e) three films (from left to right: NiOx, MeO-Ph, and NiOx/MeO-Ph); Figure S3: (a,b) (αhv)x vs. hv plots (x = 2 or 1/2) of NiOx, MeO-Ph, and NiOx/MeO-Ph; Figure S4: Theoretical adsorption mode of MeO-Ph on surfaces without -OH (a) ITO and (b) NiOx, and with -OH (c) ITO and (d) NiOx; Figure S5: NiOx, MeO-Ph, and NiOx/MeO-Ph based on (a) PL spectra and (b) TRPL spectra; Figure S6: TPC plots of NiOx, MeO-Ph, and NiOx/MeO-Ph-based PSCs; Figure S7: Top view particle size distribution plots of perovskite deposited on (a) NiOx, (b) MeO-Ph, and (c) NiOx/MeO-Ph surfaces; Figure S8: Cross-sectional thickness plots of perovskite deposited on (a) NiOx and (b) MeO-Ph surfaces; Figure S9: Water contact angle plots of perovskite deposited on (a) NiOx, (b) MeO-Ph, and (c) NiOx/MeO-Ph surfaces; Figure S10: XRD spectra plots of perovskite deposited on NiOx, MeO-Ph, and NiOx/MeO-Ph surfaces; Figure S11: (αhν)2 vs. energy (eV) plots of perovskite deposited on NiOx, MeO-Ph, and NiOx/MeO-Ph surfaces; Figure S12: Extraction of (a) VOC, (b) JSC, and (c) FF distributions from J–V measurements of NiOx, MeO-Ph, and NiOx/MeO-Ph-based PSCs (20 individual devices); Figure S13: Stability measurements were performed at 60 °C and 60 ± 10% relative humidity. Includes the evolution of XRD patterns of perovskite deposited on (a) NiOx, (b) MeO-Ph, and (c) NiOx/MeO-Ph; Figure S14: JSC performances of NiOx, MeO-Ph, and NiOx/MeO-Ph in different perovskites; Figure S15: (a) JSC and (b) PCE performances of NiOx/(MeO-2PACz or MeO-Ph)-mixed hole transport layers; Table S1: Atomic concentrations of various major elements of NiOx, MeO-Ph, and NiOx/MeO-Ph; Table S2: TRPL fitting parameters based on NiOx, MeO-Ph, and NiOx/MeO-Ph devices; Table S3: NiOx, MeO-Ph, and NiOx/MeO-Ph as the photovoltaic parameters for the best HSL PSCs.
Author Contributions
P.G., F.G. and X.Y. designed the idea. F.G. and X.Y. fabricated the PSCs. F.G. and X.Y. conducted the characterizations. Y.L. helped with the theoretical calculations. Y.C. performed the synthesis. C.L. (Chi Li) and C.L. (Chunming Liu) helped with manuscript revision. C.L. (Chi Li) helped with the AFM measurements. F.G. wrote the first manuscript. P.G. provided financial support, guided the project, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
P.G. acknowledges the financial support from the National Natural Science Foundation of China (Grant nos. 22175180, 21975260, and 52311530673).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request.
Acknowledgments
HZWTECH are thanked for allowing use of the DS-PAW and BDF codes in Device Studio. The authors also gratefully acknowledge HZWTECH for supporting the computational resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A Mixed-Cation Lead Mixed-Halide Perovskite Absorber for Tandem Solar Cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Steirer, K.X.; Schulz, P.; Teeter, G.; Stevanovic, V.; Yang, M.; Zhu, K.; Berry, J.J. Defect Tolerance in Methylammonium Lead Triiodide Perovskite. ACS Energy Lett. 2016, 1, 360–366. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-Hole Diffusion Lengths > 175 Μm in Solution-Grown CH3NH3PbI3 Single Crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus Free Charges in Organo-Lead Tri-Halide Perovskites. Nat. Commun. 2014, 5, 3586. [Google Scholar] [CrossRef]
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual Defect Physics in CH3NH3PbI3 Perovskite Solar Cell Absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Saliba, M.; Stolterfoht, M.; Wolff, C.M.; Neher, D.; Abate, A. Measuring Aging Stability of Perovskite Solar Cells. Joule 2018, 2, 1019–1024. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-Less Inverted CH3NH3PbI3 Planar Perovskite Hybrid Solar Cells with 18.1% Power Conversion Efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Liu, X.; Ye, L.; Zhao, S.; Chen, Y.; Yan, H.; Han, J.; Lin, H. Superwetting Nanofluids of NiOx-Nanocrystals/CsBr Solution for Fabricating Quality NiOx-CsPbBr3 Gradient Hybrid Film in Carbon-Based Perovskite Solar Cells. Small Methods 2024, 8, 2400283. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic Perovskite/Silicon Tandem Solar Cell with >29% Efficiency by Enhanced Hole Extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Z.; Yu, L.; Yuan, H.; Zhang, J.; Liu, X.; Hu, Z.; Zhu, Y. Synthesis of Well Dispersed NiO Ink for Efficient Perovskite Solar Cells. J. Alloys Compd. 2021, 860, 157889. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Wang, Y.; Ma, Q.; Wang, Z.; Yang, Z.; Wan, M.; Mahmoudi, T.; Hahn, Y.-B.; Mai, Y. Hole-Transport Management Enables 23%-Efficient and Stable Inverted Perovskite Solar Cells with 84% Fill Factor. Nano-Micro Lett. 2023, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, F.; Wang, X.; Chen, R.; Zhang, H.; Zhan, L.; Jiang, X.; Li, Y.; Ji, X.; Liu, S.; et al. Minimizing Buried Interfacial Defects for Efficient Inverted Perovskite Solar Cells. Science 2023, 380, 404–409. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Kong, T.; Liu, Y.; Chen, W.; Gao, P.; Bi, D. Phosphates Modulated NiOx HTL toward a Lower Voc Loss in Wide Bandgap Perovskite Solar Cells. Adv. Funct. Mater. 2024, 2419393. [Google Scholar] [CrossRef]
- Boyd, C.C.; Shallcross, R.C.; Moot, T.; Kerner, R.; Bertoluzzi, L.; Onno, A.; Kavadiya, S.; Chosy, C.; Wolf, E.J.; Werner, J.; et al. Overcoming Redox Reactions at Perovskite-Nickel Oxide Interfaces to Boost Voltages in Perovskite Solar Cells. Joule 2020, 4, 1759–1775. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Sun, J.; Liu, F.; Chen, J.; Ding, L.; Chen, C. Perovskite Solar Cells with NiOx Hole-Transport Layer. J. Semicond. 2023, 44, 100201–100204. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Zhang, J.; Zhang, L.; Wu, H.; Zhou, Y.; Wang, Y.; Wang, Y.; Fu, W.; Chen, H. Interfacial Modification of NiOx for Highly Efficient and Stable Inverted Perovskite Solar Cells. Adv. Energy Mater. 2024, 14, 2400616. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.; Du, M.; Cao, Y.; Ren, X.; Zhang, L.; Wang, H.; Zhao, S.; Wang, K.; Liu, S. (Frank) Self-Assembled Amphiphilic Monolayer for Efficient and Stable Wide-Bandgap Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2202802. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xiao, W.; Chen, R.; Sun, Z.; Zhang, Y.; Lei, X.; Hu, S.; Kober-Czerny, M.; Wang, J.; et al. Buried Interface Molecular Hybrid for Inverted Perovskite Solar Cells. Nature 2024, 632, 536–542. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.; Zhao, T.; Cheng, J.; Eslamian, M.; Choy, W.C.H.; Jen, A.K.-Y. Effects of Self-Assembled Monolayer Modification of Nickel Oxide Nanoparticles Layer on the Performance and Application of Inverted Perovskite Solar Cells. ChemSusChem 2017, 10, 3794–3803. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, Z.; Zhou, H.; Zhang, Q.; Wang, Z.; Ma, F.; Qu, Z.; Zhao, Y.; Chu, X.; Zhang, X.; et al. Homogenized NiOx Nanoparticles for Improved Hole Transport in Inverted Perovskite Solar Cells. Science 2023, 382, 1399–1404. [Google Scholar] [CrossRef]
- Yu, X.; Liu, C.; Li, C.; Wang, C.; Li, Y.; Liang, L.; Yu, W.; Wang, Y.; Liu, C.; Liu, Y.; et al. Controlled NiOx Defect Engineering to Harnessing Redox Reactions in Perovskite Photovoltaic Cells via Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2024, 16, 31114–31125. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhang, H.; Yan, W.; Li, Y.; Liang, L.; Yu, W.; Yu, X.; Wang, Y.; Yang, Y.; et al. Fully Aromatic Self-Assembled Hole-Selective Layer toward Efficient Inverted Wide-Bandgap Perovskite Solar Cells with Ultraviolet Resistance. Angew. Chem. Int. Ed. 2024, 63, e202315281. [Google Scholar] [CrossRef]
- Sun, J.; Shou, C.; Sun, J.; Wang, X.; Yang, Z.; Chen, Y.; Wu, J.; Yang, W.; Long, H.; Ying, Z.; et al. NiOx-Seeded Self-Assembled Monolayers as Highly Hole-Selective Passivating Contacts for Efficient Inverted Perovskite Solar Cells. Solar RRL 2021, 5, 2100663. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, L.; Wang, C.; Huang, J.; Liu, S.; Liu, X.; Zhang, J.; Hu, Z.; Zhu, Y. Efficient Inverted Perovskite Solar Cells with a Low-Temperature Processed NiOx/SAM Hole Transport Layer. J. Mater. Chem. C 2024, 12, 1507–1515. [Google Scholar] [CrossRef]
- Mao, L.; Yang, T.; Zhang, H.; Shi, J.; Hu, Y.; Zeng, P.; Li, F.; Gong, J.; Fang, X.; Sun, Y.; et al. Fully Textured, Production-Line Compatible Monolithic Perovskite/Silicon Tandem Solar Cells Approaching 29% Efficiency. Adv. Mater. 2022, 34, 2206193. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Sato, A.; Ajiro, K.; Shiokawa, M.; Hashimoto, Y.; Maeda, T.; Sugiyama, M.; Gotanda, T.; Marumoto, K. Performance Improvement Mechanisms of Perovskite Solar Cells by Modification of NiOx Hole-Selectivecontacts with Self-Assembled-Monolayers. Sol. Energy Mater. Sol. Cells 2023, 258, 112428. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Ying, Z.; Chen, Y.; Wang, X.; Zhang, M.; Su, S.; Guo, X.; Sun, J.; Shou, C.; et al. Reconstruction of the Indium Tin Oxide Surface Enhances the Adsorption of High-Density Self-Assembled Monolayer for Perovskite/Silicon Tandem Solar Cells. Adv. Funct. Mater. 2023, 33, 2304708. [Google Scholar] [CrossRef]
- Armstrong, N.R.; Carter, C.; Donley, C.; Simmonds, A.; Lee, P.; Brumbach, M.; Kippelen, B.; Domercq, B.; Yoo, S. Interface Modification of ITO Thin Films: Organic Photovoltaic Cells. Thin Solid Films 2003, 445, 342–352. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Jia, X.; Guo, X.; Li, J.; Li, Z.; Sun, K.; Jiang, X.; Alvianto, E.; Shi, Z.; et al. Refining the Substrate Surface Morphology for Achieving Efficient Inverted Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2302280. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, G.; Hu, J.; Wang, G.; Chen, M.; Chen, Y.; Li, M.; Zhang, H.; Chen, Y. In Situ Polymer Network in Perovskite Solar Cells Enabled Superior Moisture and Thermal Resistance. J. Phys. Chem. Lett. 2022, 13, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Phung, N.; Verheijen, M.; Todinova, A.; Datta, K.; Verhage, M.; Al-Ashouri, A.; Köbler, H.; Li, X.; Abate, A.; Albrecht, S.; et al. Enhanced Self-Assembled Monolayer Surface Coverage by ALD NiO in P-i-n Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 14, 2166–2176. [Google Scholar] [CrossRef]
- Tang, H.; Shen, Z.; Shen, Y.; Yan, G.; Wang, Y.; Han, Q.; Han, L. Reinforcing Self-Assembly of Hole Transport Molecules for Stable Inverted Perovskite Solar Cells. Science 2024, 383, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface Optimization to Eliminate Hysteresis for Record Efficiency Planar Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Almora, O.; Aranda, C.; Mas-Marzá, E.; Garcia-Belmonte, G. On Mott-Schottky Analysis Interpretation of Capacitance Measurements in Organometal Perovskite Solar Cells. Appl. Phys. Lett. 2016, 109, 173903. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-Wetting Surface-Driven High-Aspect-Ratio Crystalline Grain Growth for Efficient Hybrid Perovskite Solar Cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, X.; Zhong, J.-X.; Feng, W.; Yang, M.; Tian, T.; Gong, L.; Wu, W.-Q. Chemical Linkage and Passivation at Buried Interface for Thermally Stable Inverted Perovskite Solar Cells with Efficiency over 22%. CCS Chem. 2023, 5, 1802–1814. [Google Scholar] [CrossRef]
- Liu, W.; Hong, G.; Dai, D.; Li, L.; Dolg, M. The Beijing Four-Component Density Functional Program Package (BDF) and Its Application to EuO, EuS, YbO and YbS. Theor. Chem. Acc. 1997, 96, 75–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Suo, B.; Wang, Z.; Zhang, N.; Li, Z.; Lei, Y.; Zou, W.; Gao, J.; Peng, D.; Pu, Z.; et al. BDF: A Relativistic Electronic Structure Program Package. J. Chem. Phys. 2020, 152, 064113. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, L. The Beijing Density Functional (BDF) Program Package: Methodologies and Applications. J. Theor. Comput. Chem. 2003, 2, 257–272. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, L. Relativistic Density Functional Theory: The Bdf Program Package. In Recent Advances in Relativistic Molecular Theory; Recent Advances in Computational Chemistry; World Scientific: Singapore, 2004; Volume 5, pp. 257–282. ISBN 978-981-238-709-7. [Google Scholar]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).