Physicochemical Properties and Cytoprotective Effects on PC12 Cells of Polysaccharides from Belamcanda chinensis (L.) DC. Obtained via a Gradient Ethanol Precipitation Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of the Physicochemical Characteristics
2.1.1. Determination of Chemical Composition
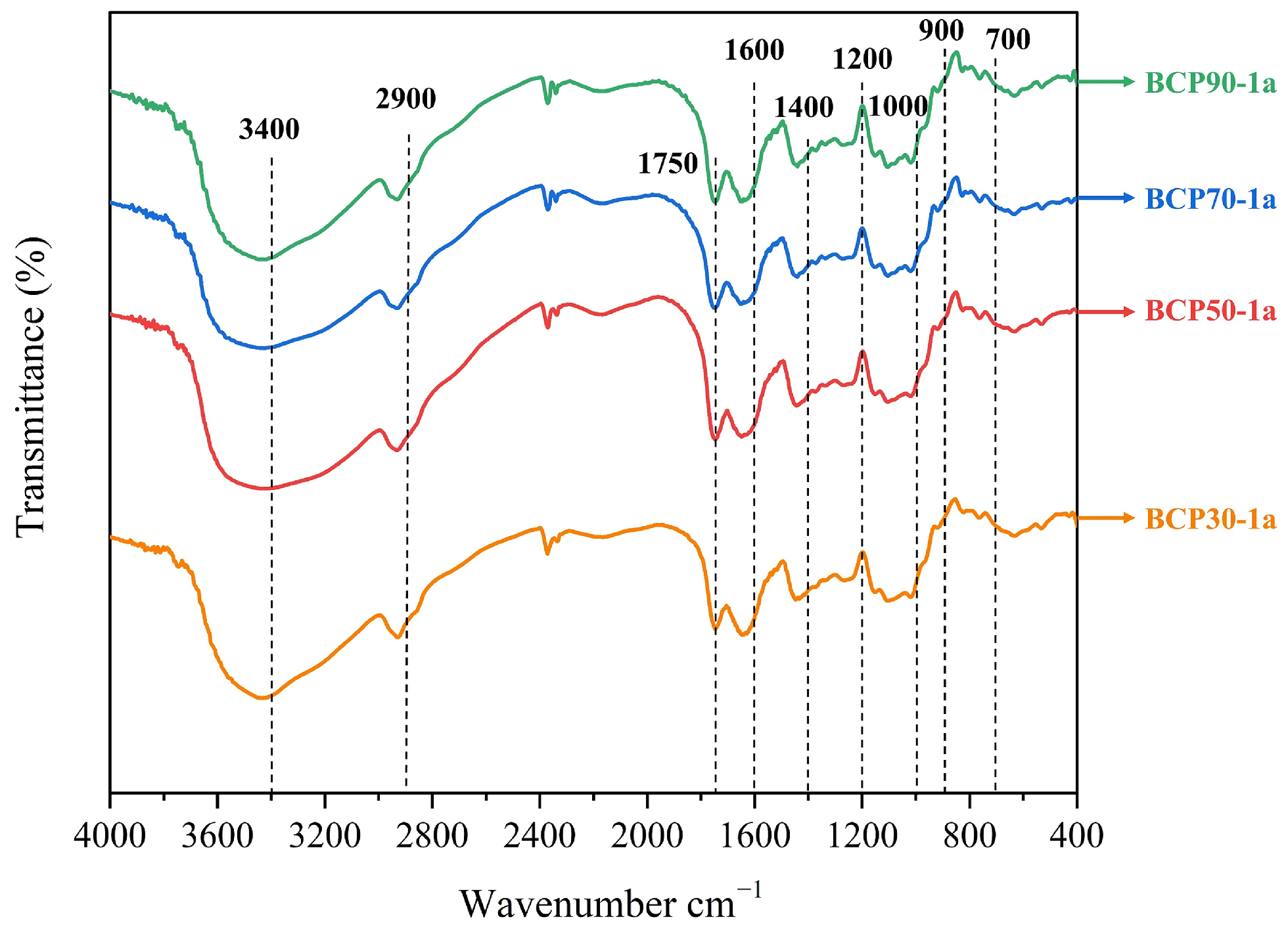
2.1.2. Determination of FT-IR Spectroscopy
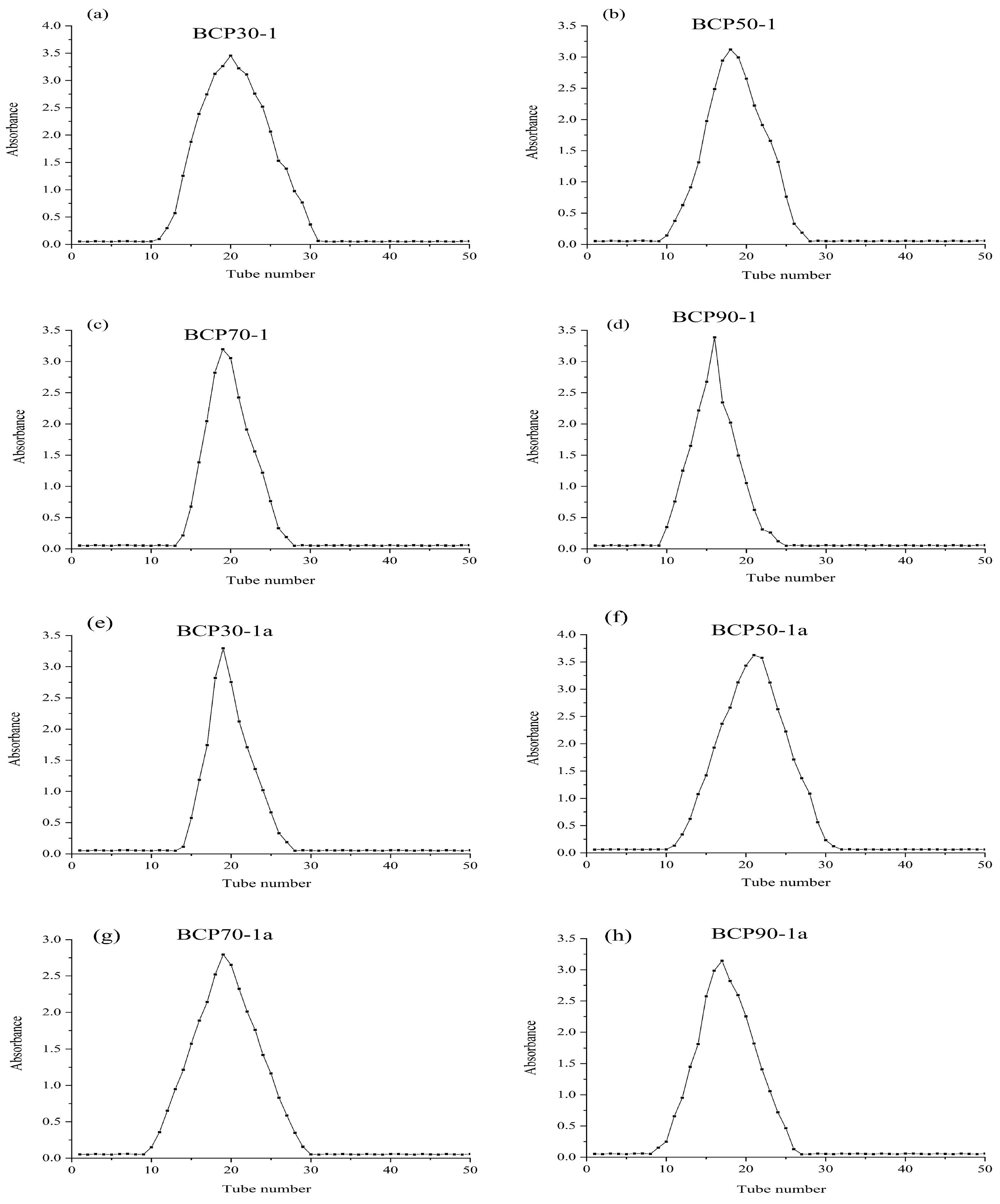
2.1.3. Determination of Molecular Weight and Polydispersity
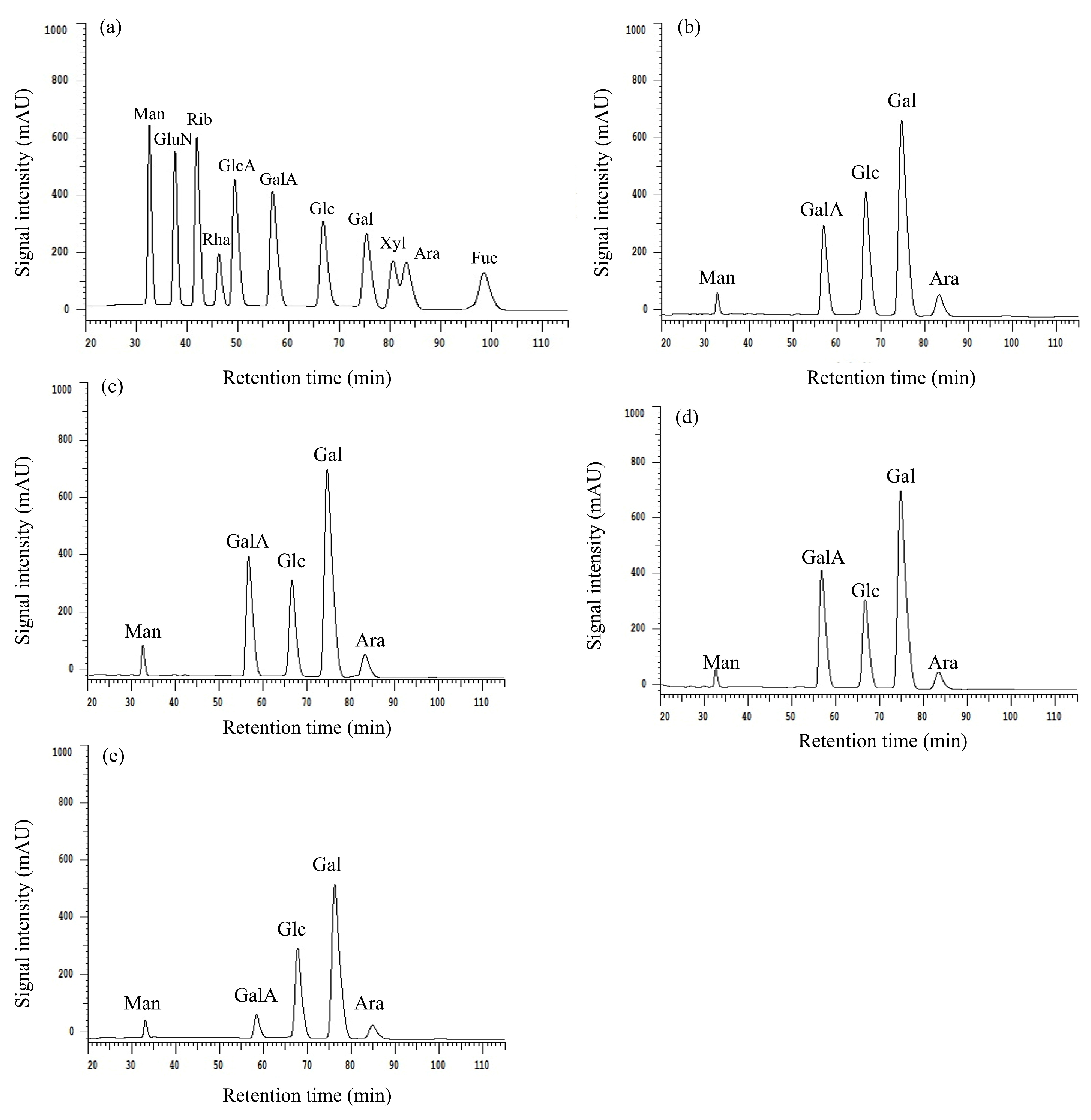
2.1.4. Determination of the Monosaccharide Composition
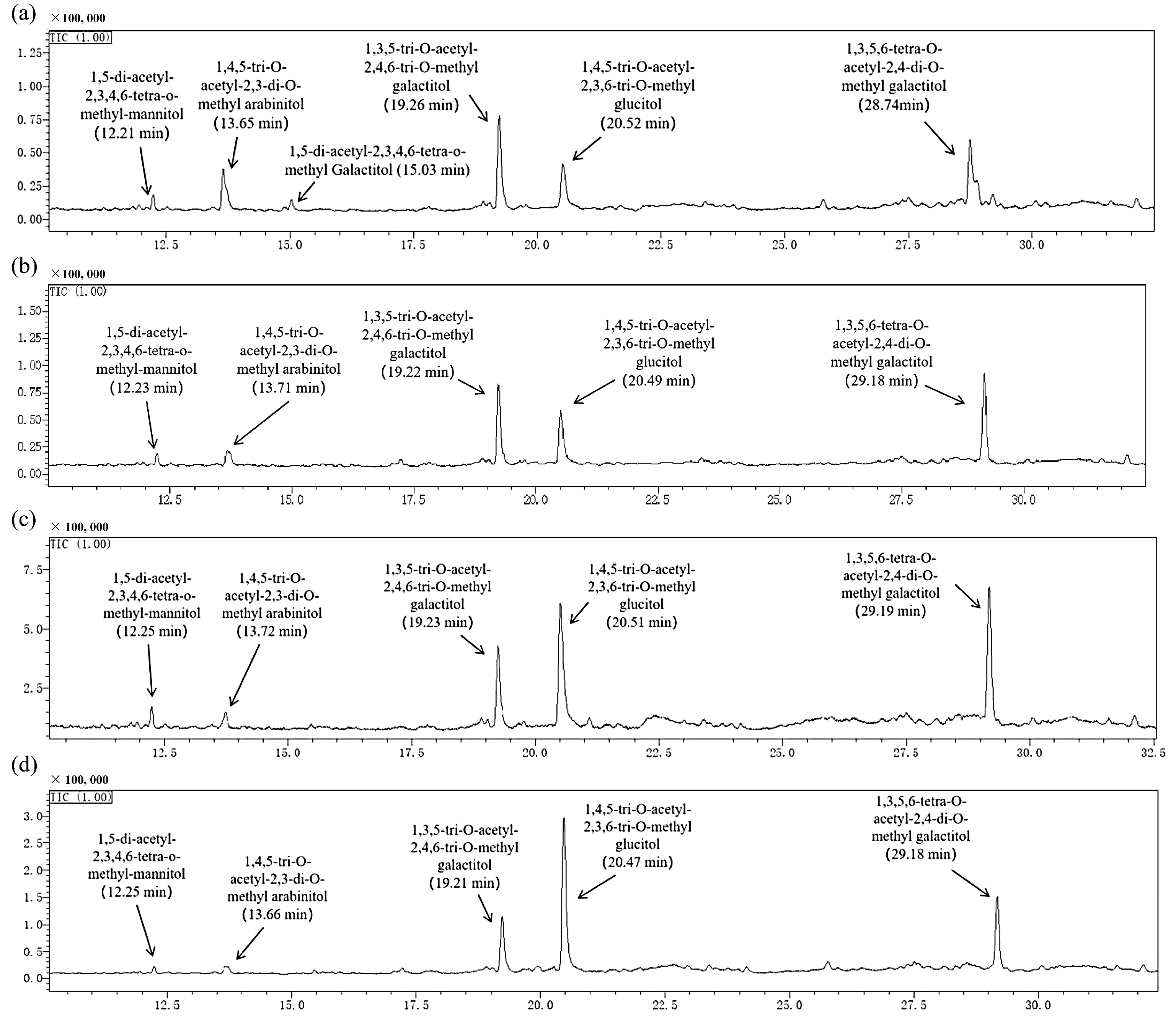
2.1.5. Methylation Analysis
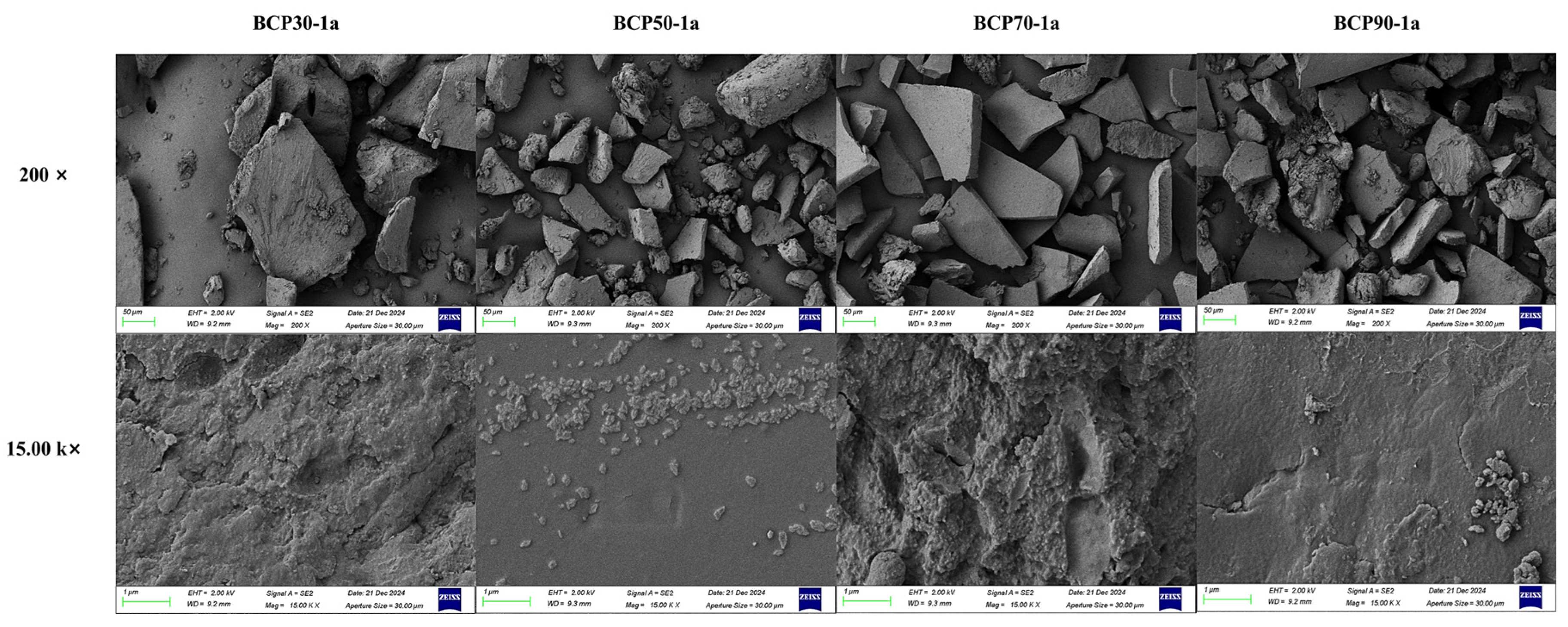
2.1.6. Scanning Electron Microscopy Analysis
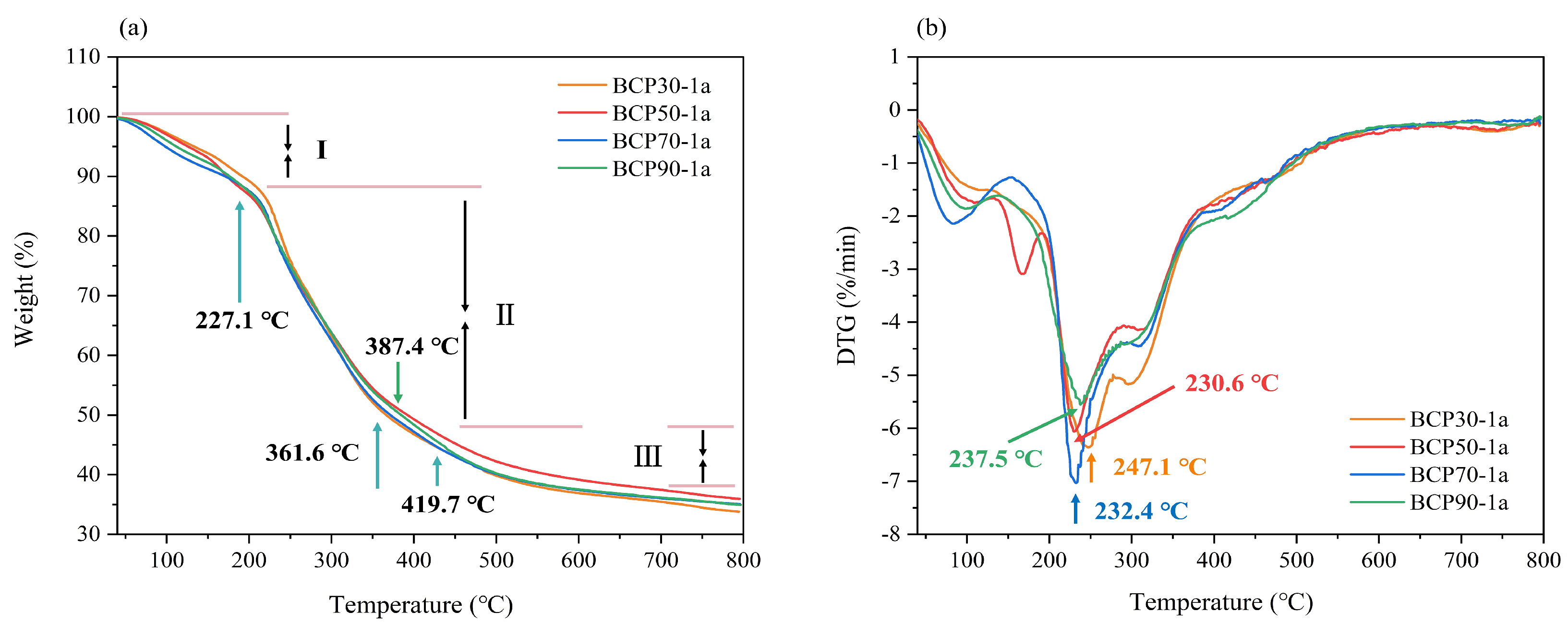
2.1.7. Thermal Gravimetric Analysis
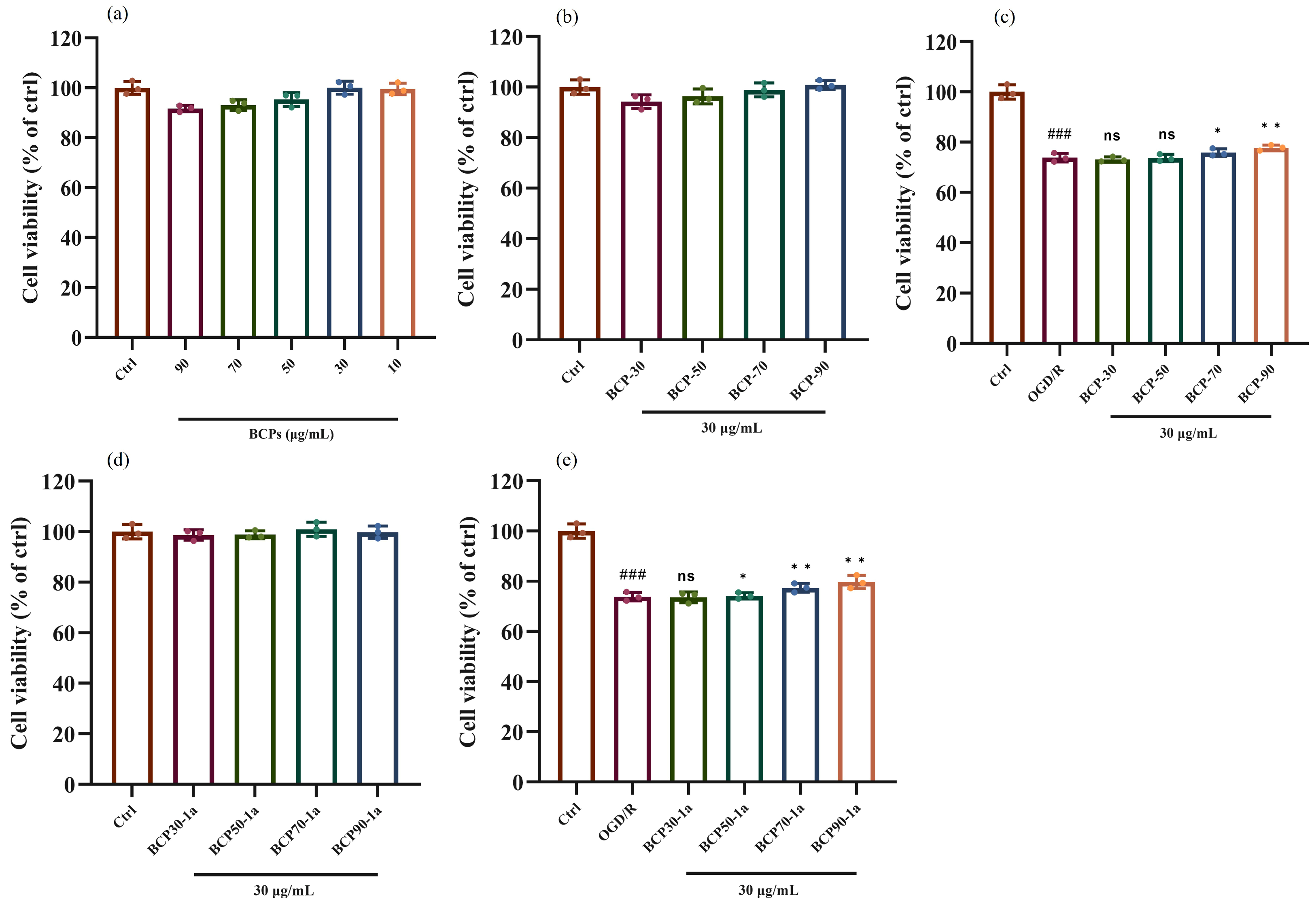
2.2. Biological Avtivity and Structure–Activity Relationship Analysis
3. Materials and Methods
3.1. Materials and Chemicals
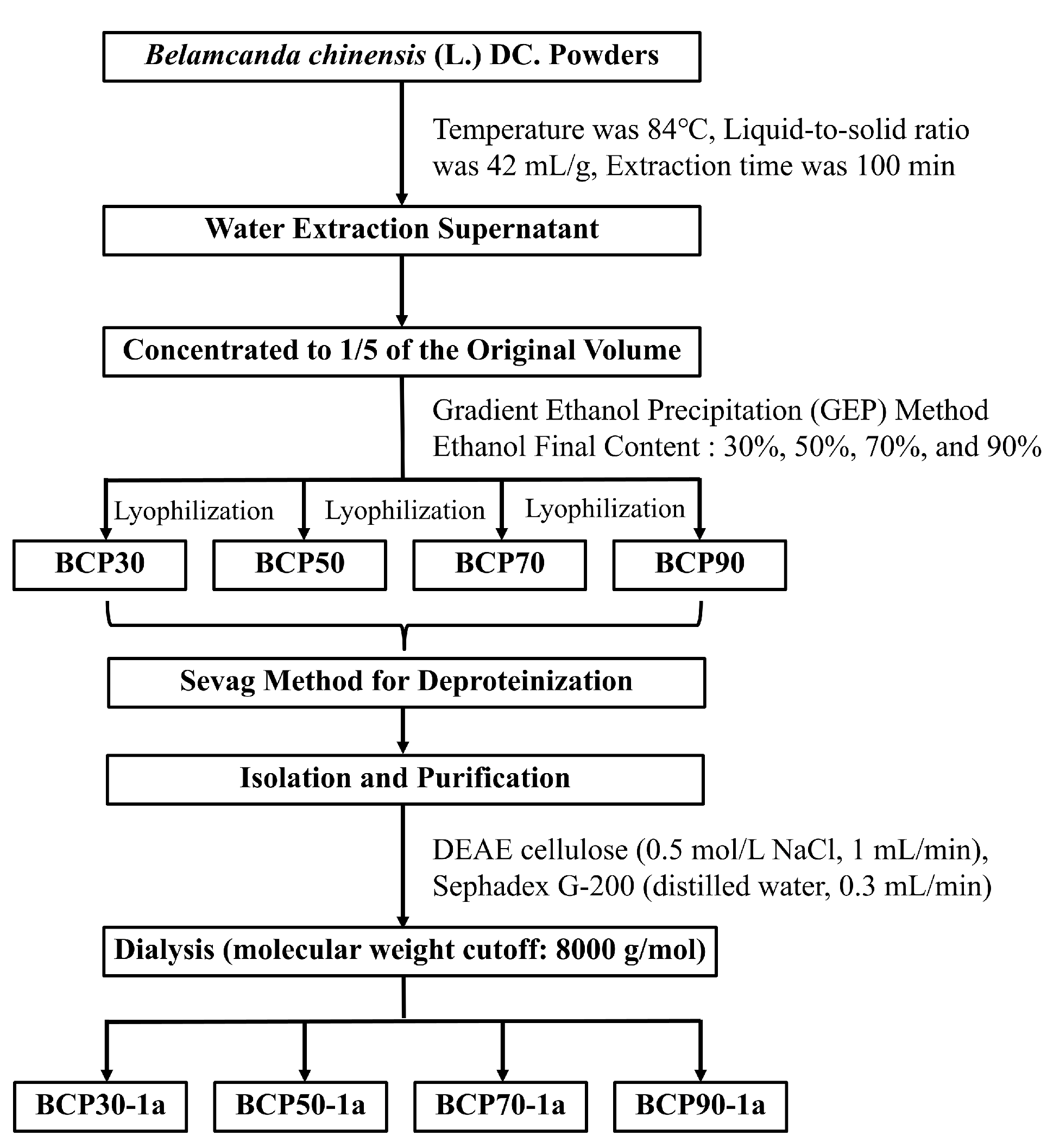
3.2. Preparation of Polysaccharides
3.3. Determination of the Physicochemical Characteristics
3.3.1. Determination of the Chemical Composition
3.3.2. Determination of FT-IR Spectroscopy
3.3.3. Determination of Molecular Weight and Polydispersity
3.3.4. Determination of Monosaccharide Composition
3.3.5. Methylation Analysis
3.3.6. Scanning Electron Microscopy Analysis
3.3.7. Thermal Gravimetric Analysis
3.4. Biological Activity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ping, Y.K.; Li, J.Y.; Xie, L.L.; Zhao, J.; Chen, X.Y.; Chen, D.N.; Wang, Y.M.; Jiang, C.; Li, X. GPNMB attenuates neuroinflammation and improves ischemic stroke via modulation of PI3K/Akt and p38 MAPK signaling pathways. Brain Res. 2025, 1849, 149381. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Y.; Li, X.; Zhang, R.; Zhang, J.J.; Ren, J.; Du, J.; Su, Z.; Tian, X.J.; Wang, Y.M.; Xiang, F.; et al. S100A4 exerts neuroprotective effects by attenuating blood-brain barrier disruption and oxidative stress via the PI3K/Akt/Nrf2 axis in ischemic stroke. Biochem. Biophys. Res. Commun. 2025, 742, 151099. [Google Scholar] [CrossRef]
- Yu, M.L.; Xiong, Y.J.; He, H.Y.; Deng, Y.H. The mechanism of acetylation-mediated fusion of lysosomes with autophagosomes in neurons after ischemic stroke. Life Sci. 2025, 362, 123305. [Google Scholar]
- Zeng, X.; Zhang, Y.D.; Ma, R.Y.; Chen, Y.J.; Xiang, X.M.; Hou, D.Y.; Li, X.H.; Huang, H.; Li, T.; Duan, C.Y. Activated Drp1 regulates p62-mediated autophagic flux and aggravates inflammation in cerebral ischemia-reperfusion via the ROS-RIP1/RIP3-exosome axis. Mil. Med. Res. 2022, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Li, Y.; Chen, J.P.; Li, D.Z.; Jiang, Q.; Wu, T.; Zhou, X.Z. Oxygen glucose deprivation/re-oxygenation-induced neuronal cell death is associated with Lnc-D63785 m6A methylation and miR-422a accumulation. Cell Death Dis. 2020, 11, 816. [Google Scholar]
- Hu, Z.Z.; Yuan, Y.; Zhang, X.; Lu, Y.F.; Dong, N.; Jiang, X.; Xu, J.J.; Zheng, D. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxid. Med. Cell. Longev. 2021, 2021, 6219715. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Li, J.; Zhang, W. Chrysin, which targets PLAU, protects PC12 cells from OGD/R-stimulated damage through repressing the NF-κB signaling pathway. Regen. Ther. 2022, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, G.; Zhou, B. TSPO knockdown attenuates OGD/R-induced neuroinflammation and neural apoptosis by decreasing NLRP3 inflammasome activity through PPARγ pathway. Brain Res. Bull. 2022, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, X.; Cheng, B.; Jia, Z.; Hua, H.; Xin, Y. Chemical characterization of polysaccharides isolated from Scrophularia ningpoensis and its protective effect on the cerebral ischemia/reperfusin injury in rat model. Int. J. Biol. Macromol. 2019, 139, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Wang, R.; Ding, B.; Zhong, W.; Cao, H. Protective and antioxidant effect of Danshen polysaccharides on cerebral ischemia/reperfusion injury in rats. Int. J. Biol. Macromol. 2013, 60, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.; Chen, L.; Liu, Z.; Zhang, H.; Wang, J.; Sun, X.; Zhong, W.; Wang, N.; Tian, K.; et al. Therapeutic effect of Ginkgo biloba polysaccharide in rats with focal cerebral ischemia/reperfusion (I/R) injury. Carbohyd. Polym. 2013, 98, 1383–1388. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Hu, Z.Y.; Jin, L.; Zong, T.Q.; Zhang, X.H.; Liu, Y.N.; Yang, P.C.; Sun, S.F.; Zhou, W.; Li, G. Efficient degradation and enhanced anticomplementary activity of Belamcanda chinensis (L.) DC. polysaccharides via trifluoroacetic acid treatment with different degrees. Int. J. Biol. Macromol. 2024, 276, 134117. [Google Scholar] [CrossRef]
- Ha, M.T.; Gal, M.; Kim, J.A.; Lee, J.H.; Min, B.S. Sucrosephenylpropanoid esters and isoflavonoids isolated from Belamcanda chinensis roots and their potential anti-osteoclastogenic activity. Bioorg. Chem. 2024, 143, 107066. [Google Scholar] [CrossRef]
- Liu, M.C.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Dorota, W.; Bogdan, J.; Ireneusz, K.; Wieslaw, O.; Adam, M. Antimutagenic and anti-oxidant activities of isoflavonoids from Belamcanda chinensis (L.) DC. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 696, 148–153. [Google Scholar]
- Duan, Y.Q.; Hu, Z.Y.; Jin, L.; Zong, T.Q.; Huang, Y.Y.; Sun, J.F.; Zhou, W.; Li, G. Isolation, characterization and anticomplementary activity of polysaccharides from the rhizomes of Belamcanda chinensis (L.) DC. Chem. Biodivers. 2022, 19, e202200525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Hou, J.T.; Liu, Y.H.; Xu, J.; Guo, Y.Q. An arabinose-rich heteropolysaccharide isolated from Belamcanda chinensis (L.) DC treats liver cancer by targeting FAK and activating CD40. Carbohyd. Polym. 2024, 331, 121831. [Google Scholar] [CrossRef]
- Tang, Y.; Miao, Y.Z.; Tan, M.; Ma, Q.Q.; Liu, C.Y.; Yang, M.; Su, Y.Q.; Li, Q. Ultrasound assisted wall-breaking extraction and primary structures, bioactivities, rheological properties of novel Exidia yadongensis polysaccharide. Ultrason. Sonochem. 2023, 101, 106643. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.G.; You, G.; Zhou, X.Y.; Fan, H.L.; Liu, X.L. Physicochemical properties and in vitro hypoglycemic activities of hsian-tsao polysaccharide fractions by gradient ethanol precipitation method. Int. J. Biol. Macromol. 2023, 231, 123274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.W.; Jia, R.B.; Ou, Z.R.; Li, Z.R.; Zhao, M.M.; Luo, D.H.; Lin, L.Z. Comparative study on the structural characterization and α-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, R.; Zhang, Y.; Xu, X.; Pan, S.; Liu, F. Effect of H2O2/ascorbic acid degradation and gradient ethanol precipitation on the physicochemical properties and biological activities of pectin polysaccharides from Satsuma mandarin. Int. J. Biol. Macromol. 2024, 280, 135843. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, M.; Chen, X.; You, L.; Ma, Y.; Hileuskaya, K. Effects of UV/H2O2 degradation and step gradient ethanol precipitation on Sargassum fusiforme polysaccharides: Physicochemical characterization and protective effects against intestinal epithelial injury. Food Res. Int. 2022, 155, 111093. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, L.; Zhu, Y.; Zhang, X.; Zhao, H.; Zhang, B. In vitro fermentation characteristics and modulation effects of polysaccharide fractions from Schisandra sphenanthera on intestinal microflora. Int. J. Biol. Macromol. 2025, 289, 138771. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Cao, T.Z.; Zhang, T.T.; Liu, Y.Q.; Yan, Y.Z. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Arab, K.; Ghanbarzadeh, B.; Ayaseh, A.; Jahanbin, K. Extraction, purification, physicochemical properties and antioxidant activity of a new polysaccharide from Ocimum album L. seed. Int. J. Biol. Macromol. 2021, 180, 643–653. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.B.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, J.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Huang, G.L.; Huang, H.L. Ultrasonic/enzymatic extraction, characteristics and comparison of leechee peel polysaccharide. Ultrason. Sonochem. 2024, 108, 106948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Xu, Y.; Lv, J.J.; Cheng, M.X.; Wu, Y.; Cao, K.; Zhang, X.F.; Mou, X.N.; Fan, Q. Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory. Int. J. Biol. Macromol. 2018, 113, 195–204. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, tructure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Gao, W. Physicochemical features and antioxidant activity of polysaccharides from Herba patriniae by gradient ethanol precipitation. Arab. J. Chem. 2022, 15, 103770. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Yu, Y.; Wu, L.; Chen, T.; Wang, Z. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Goff, H.D. Fractionation of polysaccharides by gradient non-solvent precipitation: A review. Trends Food Sci. Tech. 2018, 81, 108–115. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, X.Y.; Zhu, C.H. Physicochemical properties and Pb2+ adsorption capacity of freeze-dried hawthorn pectin fractions by gradient ethanol precipitation. Int. J. Biol. Macromol. 2023, 245, 125581. [Google Scholar] [CrossRef]
- Yang, X.; Cao, D.L.; Ji, D.L.; Xu, H.J.; Feng, Y.Y.; Liu, A.J. Physicochemical characterization, rheological properties, and hypolipidemic and antioxidant activities of compound polysaccharides in Chinese herbal medicines by fractional precipitation. Int. J. Biol. Macromol. 2023, 242, 124838. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Wang, H.Y.; Zhang, T.; Zhang, X.Y.; Zhu, C.H. Characterization, antioxidant activity and in vitro digestion of hawthorn pectin prepared by gradient ethanol precipitation. Int. J. Biol. Macromol. 2024, 267, 131278. [Google Scholar] [CrossRef]
- Gu, J.Y.; Zhang, H.H.; Yao, H.; Zhou, J.; Duan, Y.Q.; Ma, H.L. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohyd. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xue, Z.H.; Wang, Y.J.; Lu, Y.P.; Li, R.L.; Li, N.N.; Wang, Q.R.; Zhang, M.; Chen, H.X. Chemical structure and inhibition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohyd. Polym. 2021, 252, 117185. [Google Scholar] [CrossRef]
- Wang, K.J.; Guo, J.T.; Cheng, J.X.; Zhao, X.H.; Ma, B.H.; Yang, X.B.; Shao, H.J. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.F.; Zhang, C.; Lyu, X.M.; Hua, X.; Zhao, W.; Zhang, W.B.; Yang, R.J. Structure and physicochemical properties of arabinan-rich acidic polysaccharide from the by-product of peanut oil processing. Food Hydrocoll. 2021, 117, 106743. [Google Scholar] [CrossRef]
- Guo, Y.F.; Deng, R.X.; Wang, Y.H.; Qu, M.H.; Liu, P.; Gao, J.Y. Extraction, separation, and antioxidant activity of polysaccharides from peony seed shell. Ind. Crops Prod. 2024, 222, 119843. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zeng, Z.; Lin, Y.; Xiong, B.; Zheng, B.; Zhang, Y.; Pan, L. Structural characterization of polysaccharide from an edible fungus Dictyophora indusiata and the remodel function of gut microbiota in inflammatory mice. Carbohyd. Polym. 2025, 351, 123141. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, H.; Zuo, Y.; Sun, J.; Wu, D.; Shu, X. Structural and functional properties of polysaccharides extracted from three Dioscorea species. Int. J. Biol. Macromol. 2024, 281, 136469. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Wen, H.; Xie, J. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2019, 126, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohyd. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiu, W.; Wang, X.; Yu, S.; Luo, Y.; Gu, X. Structural characterization and in vitro antioxidant and hypoglycemic activities of degraded polysaccharides from sweet corncob. J. Cereal Sci. 2022, 108, 103579. [Google Scholar] [CrossRef]
- Hang, H.; Chen, R.Z.; Wang, C.B.; Sun, Y.R.; Du, D.S. A review of the extraction processes and biological characteristics of Chrysanthemum polysaccharides. Int. J. Biol. Macromol. 2025, 285, 138224. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.H.M.; Shafie, M.H. A review of in vitro antioxidant and antidiabetic polysaccharides: Extraction methods, physicochemical and structure-activity relationships. Int. J. Biol. Macromol. 2024, 282, 137143. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sun, Y.X.; Dai, X.L. A review of the preparation, analysis and biological functions of chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef]
- Su, J.P.; Fang, J.Q.; Liu, C.; Liu, S.P.; Chen, C.; Tan, C.; Wang, P.P.; Peng, Y.P.; Fu, X. Advances in structure-hypoglycemic activity relationship and mechanisms of berry polysaccharides. Food Biosci. 2024, 62, 105472. [Google Scholar]
- Xue, H.K.; Hao, Z.T.; Gao, Y.C.; Cai, X.; Tang, J.T.; Liao, X.J.; Tan, J.Q. Research progress on the hypoglycemic activity and mechanisms of natural polysaccharides. Int. J. Biol. Macromol. 2023, 252, 126199. [Google Scholar] [CrossRef]
- Tang, J.; He, Z.H.; Zhang, B.H.; Cheng, J.J.; Qiu, W.X.; Chen, X.Y.; Chang, C.; Wang, Q.; Hu, J.J.; Cai, C.; et al. Structural properties, bioactivities, structure-activity relationships and bio-applications of polysaccharides from Auricularia auricula: A review. Int. J. Biol. Macromol. 2024, 280, 135941. [Google Scholar] [CrossRef]
- Yuan, G.X.; Wang, Y.T.; Niu, H.M.; Ma, Y.; Song, J.X. Isolation, purification, and physicochemical characterization of Polygonatum polysaccharide and its protective effect against CCl4-induced liver injury via Nrf2 and NF-κB signaling pathways. Int. J. Biol. Macromol. 2024, 261, 129863. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohyd. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
| BCP30 | BCP50 | BCP70 | BCP90 | |
|---|---|---|---|---|
| Yield (%) | 0.83 ± 0.42 d | 21.47 ± 0.73 a | 17.55 ± 0.81 b | 2.97 ± 0.55 c |
| Total sugar content (%) | 36.33 ± 0.77 a | 32.98 ± 0.61 b | 21.14 ± 0.73 c | 19.65 ± 0.66 d |
| Protein content (%) | 2.33 ± 0.46 c | 3.18 ± 0.73 bc | 3.96 ± 0.67 ab | 4.54 ± 0.62 a |
| Uronic acid content (%) | 12.25 ± 0.63 d | 21.71 ± 0.69 a | 19.87 ± 0.72 b | 3.65 ± 0.72 c |
| BCP30-1a | BCP50-1a | BCP70-1a | BCP90-1a | |
| Total sugar content (%) | 87.52 ± 0.69 a | 85.86 ± 0.73 b | 86.78 ± 0.92 ab | 87.46 ± 0.73 a |
| Protein content (%) | — | — | — | — |
| Uronic acid content (%) | 18.13 ± 0.88 c | 27.61 ± 0.72 a | 25.06 ± 0.82 b | 8.74 ± 0.70 d |
| BCP30-1a | BCP50-1a | BCP70-1a | BCP90-1a | |
|---|---|---|---|---|
| Molecular weight (Da) | 198.398 | 184.690 | 184.556 | 184.217 |
| MW/Mn | 1.13 | 1.08 | 1.12 | 1.10 |
| Monosaccharide composition (molar ratio) | ||||
| Man | 1.00 | 1.00 | 1.00 | 1.00 |
| GalA | 7.35 | 8.39 | 12.23 | 2.21 |
| Glc | 15.14 | 7.60 | 11.85 | 13.04 |
| Gal | 20.26 | 15.72 | 24.04 | 18.74 |
| Ara | 1.79 | 1.38 | 1.70 | 1.46 |
| Linkage Patterns | Retention Time (min) | Molar Percentage Ratio (%) |
|---|---|---|
| BCP30-1a | ||
| Manp-(1→ | 12.21 | 5.24 |
| →5)-Araf-(1→ | 13.65 | 15.24 |
| Galp-(1→ | 15.03 | 3.33 |
| →3)-Galp (or GalAp)-(1→ | 19.26 | 30.31 |
| →4)-Glcp-(1→ | 20.52 | 19.92 |
| →3,6)-Galp-(1→ | 28.74 | 25.96 |
| BCP50-1a | ||
| Manp-(1→ | 12.23 | 3.82 |
| →5)-Araf-(1→ | 13.71 | 7.53 |
| →3)-Galp (or GalAp)-(1→ | 19.22 | 36.82 |
| →4)-Glcp-(1→ | 20.49 | 21.54 |
| →3,6)-Galp-(1→ | 29.18 | 30.29 |
| BCP70-1a | ||
| Manp-(1→ | 12.25 | 2.76 |
| →5)-Araf-(1→ | 13.72 | 3.03 |
| →3)-Galp (or GalAp)-(1→ | 19.23 | 20.53 |
| →4)-Glcp-(1→ | 20.51 | 38.13 |
| →3,6)-Galp-(1→ | 29.19 | 35.55 |
| BCP90-1a | ||
| Manp-(1→ | 12.25 | 0.64 |
| →5)-Araf-(1→ | 13.66 | 1.06 |
| →3)-Galp (or GalAp)-(1→ | 19.21 | 22.45 |
| →4)-Glcp-(1→ | 20.47 | 49.43 |
| →3,6)-Galp-(1→ | 29.18 | 26.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Sun, J.; Xue, Y.; Xu, W.; Jiang, Y.; Zong, T.; Zhou, W.; Hu, Z.; Li, G. Physicochemical Properties and Cytoprotective Effects on PC12 Cells of Polysaccharides from Belamcanda chinensis (L.) DC. Obtained via a Gradient Ethanol Precipitation Method. Molecules 2025, 30, 998. https://doi.org/10.3390/molecules30050998
Duan Y, Sun J, Xue Y, Xu W, Jiang Y, Zong T, Zhou W, Hu Z, Li G. Physicochemical Properties and Cytoprotective Effects on PC12 Cells of Polysaccharides from Belamcanda chinensis (L.) DC. Obtained via a Gradient Ethanol Precipitation Method. Molecules. 2025; 30(5):998. https://doi.org/10.3390/molecules30050998
Chicago/Turabian StyleDuan, Yuanqi, Jinfeng Sun, Yongkang Xue, Weiwei Xu, Yuxin Jiang, Tieqiang Zong, Wei Zhou, Zhengyu Hu, and Gao Li. 2025. "Physicochemical Properties and Cytoprotective Effects on PC12 Cells of Polysaccharides from Belamcanda chinensis (L.) DC. Obtained via a Gradient Ethanol Precipitation Method" Molecules 30, no. 5: 998. https://doi.org/10.3390/molecules30050998
APA StyleDuan, Y., Sun, J., Xue, Y., Xu, W., Jiang, Y., Zong, T., Zhou, W., Hu, Z., & Li, G. (2025). Physicochemical Properties and Cytoprotective Effects on PC12 Cells of Polysaccharides from Belamcanda chinensis (L.) DC. Obtained via a Gradient Ethanol Precipitation Method. Molecules, 30(5), 998. https://doi.org/10.3390/molecules30050998






